Abstract
Objectives
Exact wound diagnosis is essential for successful wound management and a holistic care of the patient suffering from a wound. Wound management has been traditionally seen as a nursing area, but this can lead to considerable delays in wound diagnostics. A diagnostic delay has been recognised as an element of diagnostic error, which, in turn, affects patient safety. The aim of this cohort study was to examine diagnostic delays of chronic wound within primary care.
Setting
A specialised diagnostic unit, a wound care team, was established in the primary healthcare with the objective of reducing diagnostic and treatment delays in primary care.
Participants
The data consists of 197 consecutive patients attending their first appointment with the wound care team in 2016. The collected data included basic demographics, information about the clinical pathway, including doctor’s appointments in primary and specialised care, as well as the International Classification of Diseases 10th Revision (ICD-10) diagnostic codes.
Primary and secondary outcome measures
The diagnostic delays were calculated in days and divided into three groups: (1) patient-related delay, (2) diagnostic delay and (3) organisational delay.
Results
The median duration of a patient-related delay was 2 days (IQR 0–14), whereas a physician’s first evaluation was performed at a median of 8 (1–32) days from wound appearance and the correct diagnosis by the wound care team was established in a median of 57 (33–100) days. The organisational delay from first contact to diagnosis was a median of 41 (22–80) days. Only one in three patients had a diagnostic delay of less than 4 weeks.
Conclusions
According to this study, the diagnostic delay occurs within primary care, as an organisational delay from first contact to correct diagnosis. It is possible to arrange an optimal pathway of care in which a holistic wound care process starts within primary care.
Keywords: WOUND MANAGEMENT, VASCULAR SURGERY, Diabetic foot, Health & safety, Quality in health care, PRIMARY CARE
Strengths and limitations of this study.
Strengths of the study include a unique data which contain primary care patients suffering from chronic wounds.
Strengths include also systematic and detailed data review and collection.
Limitations include the possibility of interpretation bias.
Limitations include also the possibility of error in defining the moment of ‘the right diagnosis’.
There is no input for patient and public involvement in the study design, which could be seen as a limitation.
Introduction
Chronic wounds pose a significant burden to healthcare, constituting roughly 2%–6% of all healthcare costs.1–3 According to a recent study in the UK, the annual prevalence of wounds increased by 71% between 2012/2013 and 2017/2018 and the patient management costs increased by 48% in real terms.4 In addition to costs, chronic wounds cause substantial suffering at the individual level, leading to an impaired quality of life, social isolation and mental health problems.5–7 Wound management can be successful only when the wound is correctly diagnosed and treated accordingly.8 9 Wound care has been traditionally viewed as measures related to the assessment of the wound bed, which can obscure the importance of the holistic care of the patient.10
In many European countries, wound patients are first seen in primary care by general practitioners (GPs) or nurses.11 This poses a significant challenge to primary care: wound patients should receive a timely evaluation by a qualified healthcare professional who can make the correct diagnosis, plan holistic treatment and make the necessary referrals.12 This process should aim to avoid diagnostic error, which has been recognised by the WHO as a global challenge to patient safety.13 Diagnostic error includes an incorrect or delayed diagnosis, which leads to patient harm or to inappropriate or delayed treatment. The diagnostic errors mainly occur within primary care or at the emergency department, where physicians lack the appropriate tools and sufficient time to make accurate decisions.14 15
In 2013, a special wound care team was established in the primary healthcare system of the Helsinki area. The wound care team consists of a wound care nurse and a GP specialised in wound care. This team has the possibility to consult a podiatrist and/or vascular surgeon. Patients are referred to a wound care team consultation from all primary care units: health centres, home care units and nursing homes. The instructions for the primary care personnel were to react early and refer patients suffering from a non-healing wound within (2–)4 weeks of wound appearance in order to have the wound appropriately diagnosed. The main focus of the wound care team was to discover the correct diagnosis as early as possible and, thereafter, to initiate proper treatment accordingly.
The purpose of this study was to evaluate the delay in the diagnostic process in the clinical pathway of wound patients who were referred for a consultation by the wound care team during 2016. The delays were divided into system-related and patient-related delays.
Material and methods
This prospective cohort study analysed the characteristics and medical history of 197 consecutive patients who visited the wound care team in primary healthcare during 2016. The information was collected at the first wound care team appointment.
Data were collected from electronic patient records. The collected data consisted of patients’ background factors (sex, age, comorbidities, medication, previous wounds, state of mobility and living standards, need of home care, smoking, blood sugar levels and lipids), as well as the date of wound appearance, the date of the patient’s first contact with a primary care unit and physician’s appointment, the date of consulting the wound care team and the date of admission to a specialist care unit if needed. In order to analyse the diagnostic process, additional information was collected on signs of infection and bacterial swab results, on whether the Ankle Brachial Index (ABI) was measured, or pulse palpation occurred, or whether neuropathy was detected with a monofilament test. Observations of oedema and any blood test analyses regarding the wound were also recorded. Furthermore, information was gathered from radiological examinations, if performed, as well as any further investigations within specialist care, such as toe pressure and angiography results. The treatment plan was evaluated and compared with the diagnostic methods and the International Classification of Diseases 10th Revision (ICD-10) code. (online supplemental table 1).
bmjopen-2022-062673supp001.pdf (30.1KB, pdf)
Delays were calculated at different points of the care pathway, starting from wound appearance. The different types of delays included: (1) patient-related delay (time from wound appearance to the patient’s first contact with healthcare providers), (2) diagnostic delay (time from the onset of the wound to the first physician’s appointment where the initial diagnosis was made) and (3) organisational delays within primary care in arriving at the correct diagnosis and treatment (from the first contact with the primary healthcare unit to the wound care team consultation). Some patients needed a referral to a specialist consultation, his delay was also considered.
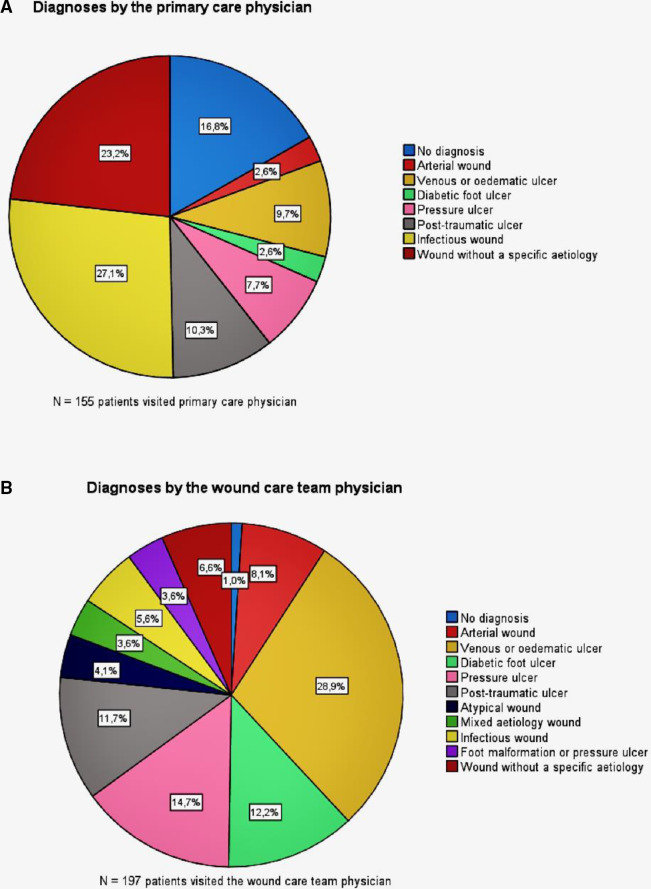
Diagnostic codes were collected as ICD-10 codes and were compared with the diagnoses made by the primary care physician, by the wound care team physician and by the specialist. As the number of different diagnostic codes was high, we categorised the diagnoses into 10 groups (table 1, figure 1A, B). In the grouping process, we also included, in addition to the diagnostic code, information on how the wound had appeared and which diagnostic tools had been used to arrive at the specific conclusion and treatment plan.
Table 1.
Categorisation and diagnostic variation in the clinical pathway of a patient with a wound
| Diagnostic categories | Primary care physician (n=155) | Wound care team physician (n=197) | Specialist care physician (n=111) |
| No diagnosis* | 26 | 2 | 1 |
| Arterial wound | 4 | 16 | 26 |
| Venous or oedematous ulcer | 15 | 57 | 17 |
| Diabetic foot ulcer | 4 | 24 | 15 |
| Pressure ulcer | 12 | 29 | 9 |
| Post-traumatic wound | 16 | 23 | 3 |
| Atypical wound | 0 | 8 | 7 |
| Mixed-aetiology ulcer | 0 | 7 | 3 |
| Infectious wound | 42 | 11 | 10 |
| Foot malformation or pressure ulcer | 0 | 7 | 1 |
| Wound of unspecified aetiology | 36 | 13 | 19 |
*The category was defined as ‘no diagnosis’ when a patient had been seen by a physician but there was no ICD-10-coded diagnosis in the patient records.
ICD-10, International Classification of Diseases 10th Revision.
Figure 1.
Diagnostic differentiation between the primary care physician and the wound care team physician.
In order to avoid bias caused by outliers, 16 patients whose wound had persisted for over 365 days prior to the wound team consultation were excluded from the delay analysis.
Patient and public involvement
No patient or public involvement has occurred in planning, managing, designing or carrying out this research.
Results
A total of 197 patients were included in this study. The mean age was 71 years, and 106 (53.5%) of the patients were women. The basic demographics and risk factors of the patients are reported in tables 2–3.
Table 2.
Basic demographics
| Female (n) | % | Male (n) | % | Total (n) | % | |
| Sex | 106 | 53.8 | 91 | 46.2 | 197 | 100 |
| Age (years) | ||||||
| Mean | 78 | 39.6 | 69 | 97.2 | 71 | 36.0 |
| Median | 80 | 40.6 | 73 | 37.1 | 41 | 20.8 |
| Mobility (n) | ||||||
| Walking | 38 | 19.3 | 56 | 28.4 | 94 | 47.7 |
| Walking with assistance device | 33 | 16.8 | 20 | 10.2 | 53 | 26.9 |
| Walking with device only indoors | 16 | 8.1 | 5 | 2.5 | 21 | 10.7 |
| Wheelchair | 11 | 5.6 | 8 | 4.1 | 19 | 9.6 |
| Bedridden | 8 | 4.1 | 2 | 1.0 | 10 | 5.1 |
| Residence (n) | ||||||
| Home | 57 | 28.9 | 73 | 37.1 | 130 | 66.0 |
| Home with home care | 30 | 15.2 | 12 | 6.1 | 42 | 21.3 |
| Assisted living facility | 14 | 7.1 | 5 | 2.5 | 19 | 9.6 |
| 24/7 care nursing home | 5 | 2.5 | 1 | 0.5 | 6 | 3.0 |
Table 3.
Description of the sample (n=197, % of total) in individuals; comorbidities and risk factors
| Female (n) | % | Male (n) | % | Total (n) | % | P value* | |
| Comorbidities | |||||||
| Hypertension | 60 | 30.5 | 50 | 25.4 | 110 | 55.8 | |
| Heart failure | 25 | 12.7 | 11 | 5.6 | 36 | 18.3 | p=0.044 |
| Ischaemic heart disease | 13 | 6.6 | 10 | 5.1 | 23 | 11.7 | |
| Atrial fibrillation | 37 | 18.8 | 24 | 12.2 | 61 | 31.0 | |
| Respiratory condition | 27 | 13.7 | 11 | 5.6 | 38 | 19.3 | |
| Cancer | 14 | 7.1 | 14 | 7.1 | 28 | 14.2 | |
| Mental health condition | 9 | 4.6 | 9 | 4.6 | 18 | 9.1 | |
| Dementia/memory disorder | 25 | 12.7 | 9 | 4.6 | 34 | 17.3 | p=0.013 |
| Diabetes | 30 | 15.2 | 47 | 23.9 | 77 | 39.1 | p<0.001 |
| Peripheral arterial disease | 16 | 8.1 | 24 | 12.2 | 40 | 20.3 | p=0.042 |
| Kidney malfunction | 12 | 6.1 | 15 | 7.6 | 27 | 13.7 | |
| Rheumatoid arthritis | 15 | 7.6 | 6 | 3.0 | 21 | 10.7 | p=0.091 |
| Liver malfunction | 0 | 0.0 | 6 | 3.0 | 6 | 3.0 | p=0.007 |
| Spinal stenosis | 5 | 2.5 | 3 | 1.5 | 8 | 4.1 | |
| Gout | 3 | 1.5 | 8 | 4.1 | 11 | 5.6 | p=0.064 |
| Haematological condition | 5 | 2.5 | 6 | 3.0 | 11 | 5.6 | |
| Chronic pain disorder | 2 | 1.0 | 0 | 0.0 | 2 | 1.0 | |
| Urinary condition | 4 | 2.0 | 10 | 5.1 | 14 | 7.1 | p=0.045 |
| Cerebrovascular disorder | 15 | 7.6 | 13 | 6.6 | 28 | 14.2 | |
| Dermatological disease | 3 | 1.5 | 2 | 1.0 | 5 | 2.5 | |
| Musculoskeletal disorder | 21 | 10.7 | 7 | 3.6 | 28 | 14.2 | p=0.018 |
| No comorbidities | 3 | 1.5 | 4 | 2.0 | 7 | 3.6 | |
| Risk factors | |||||||
| Previous wounds | 46 | 23.4 | 50 | 25.4 | 96 | 48.7 | |
| Previous deep venous thrombosis | 9 | 4.6 | 1 | 0.5 | 10 | 5.1 | p=0.020 |
| Venous insufficiency | 9 | 4.6 | 7 | 3.6 | 16 | 8.1 | |
| Chronic oedema | 3 | 1.5 | 2 | 1.0 | 5 | 2.5 | |
| Chronic cellulitis | 10 | 5.1 | 5 | 2.5 | 15 | 7.6 | |
| Previous amputation | 4 | 2.0 | 1 | 0.5 | 5 | 2.5 | |
| Smoking (n) | 13 | 6.6 | 28 | 14.2 | 41 | 20.8 | p=0.001 |
| Drug misuse | 2 | 1.0 | 5 | 2.5 | 7 | 3.6 | p=0.010 |
| Alcohol abuse | 3 | 1.5 | 11 | 5.6 | 14 | 7.1 | |
| Overweight (BMI 24–30) | 59 | 29.9 | 62 | 31.5 | 121 | 61.4 | |
| Obesity (BMI over 30) | 26 | 13.2 | 29 | 14.7 | 55 | 27.9 | |
| High cholesterol (diagnosis) | 22 | 11.2 | 23 | 11.7 | 45 | 22.8 | |
| LDL over 3.0 | 23 | 11.7 | 19 | 9.6 | 42 | 21.3 | |
| Joint malformation | 8 | 4.1 | 3 | 1.5 | 11 | 5.6 | p=0.010 |
| Neuropathy (diagnostic coded) | 8 | 4.1 | 18 | 9.1 | 26 | 13.2 | p<0.001 |
| Neuropathy (monofilament test posit.) | 32 | 16.2 | 54 | 27.4 | 86 | 43.7 | |
| MRSA | 2 | 1.0 | 6 | 3.0 | 8 | 4.1 | p=0.048 |
| Hemiplegia | 2 | 1.0 | 2 | 1.0 | 4 | 2.0 | |
| HbA1c (n=153) | |||||||
| Mean (SD) | 43 (12.9) | 49 (16.8) | 46 (15.1) | p=0.018† | |||
| Median (IQR) | 40 | 43 | 41(37–52) | ||||
| BMI (n=178) | |||||||
| Mean (SD) | 27.4 (8.2) | 29.0 (6.2) | 28 (7.4) | ||||
| Median | 26 | 28 | 26 (23–32) | ||||
| fP-Kol-LDL (n=169) | |||||||
| Mean (SD) | 2.5 (0.83) | 2.4 (0.86) | 2.5 (0.84) | ||||
| Median | 2.4 | 2.3 | 2.4 (1.8–3.0) | ||||
| fP-Gluk (n=173) | |||||||
| Mean (SD) | 6.0 (1.8) | 7.3 (3.4) | 6.6 (2.7) | p=0.002† | |||
| Median | 5.8 | 6.7 | 5.9 (5.3–6.9) |
*Pearson’s χ2, difference between female and male patients.
†One-way analysis of variance test.
BMI, body mass index; fP-Gluk, fasting plasma glucose; fP-Kol-LDL, fasting plasma low-density lipoprotein Cholesterol; HbA1c, glycated hemoglobin; LDL, low-density lipoprotein Cholesterol; MRSA, Methicillin-resistant Staphylococcus aureus.
The majority of the patients were living at home (n=172; 86.9%) either without any support (n=130) or with home care (n=42). The patients’ living status is presented in table 2. Almost half of the patients had had wounds earlier (48.7%). As regards comorbidities, 39.1% had diabetes, 11.7% had ischaemic heart disease, 17.3% had memory disorders and 9.1% had a mental health condition. Only 3.6% of the patients had no comorbidities. Overweight (61.4%), obesity (27.9%) and neuropathy (43.7%) were relatively common. Venous insufficiency or a previous deep venous thrombosis had been diagnosed in only 13.2%. As can be seen in tables 3 and 4, the patients had several comorbidities and heterogenous medications. Almost half of the patients used analgesics (44.7% used non-steroidal anti-inflammatory drugs or paracetamol and 15.2% used opioids), whereas different psychopharmaceuticals were used by 11.7%–17.8% of the patients.
Table 4.
Description of the sample (n=197) in individuals; medication
| Medication | Female (n) | % | Male (n) | % | Total (n) | % | Χ2 |
| Cardiac and vessel medicine | |||||||
| Anticoagulant | 41 | 20.8 | 26 | 13.2 | 67 | 34.0 | p=0.164 |
| Antithrombotic | 29 | 14,7 | 28 | 14.2 | 57 | 28.9 | |
| Beta blocker | 57 | 28.9 | 41 | 20.8 | 98 | 49.7 | |
| Diuretic | 45 | 22.8 | 32 | 16.2 | 77 | 39.1 | |
| Calcium channel blocker | 27 | 13.7 | 31 | 15.7 | 58 | 29.4 | |
| Angiotensin-converting enzyme-blocker | 38 | 19.3 | 47 | 23.9 | 85 | 43.1 | p=0.018 |
| Statin | 40 | 20.3 | 35 | 17.8 | 75 | 38.1 | |
| Diabetes medicine | |||||||
| Oral diabetes medicine | 16 | 8.1 | 21 | 10.7 | 37 | 18.8 | p=134 |
| Insulin | 12 | 6.1 | 30 | 15.2 | 42 | 21.3 | p<0.001 |
| Psychopharmaceuticals | |||||||
| Dementia medicine | 16 | 8.1 | 6 | 3.0 | 22 | 11.2 | p=0.066 |
| Antidepressant | 14 | 7.1 | 11 | 5.6 | 25 | 12.7 | |
| Benzodiazepine | 13 | 6.6 | 10 | 5.1 | 23 | 11.7 | |
| Sleeping pills | 23 | 11.7 | 12 | 6.1 | 35 | 17.8 | p=0.135 |
| Analgesia (mild) | 57 | 28.9 | 31 | 15.7 | 88 | 44.7 | p=0.008 |
| Opiates | 18 | 9.1 | 12 | 6.1 | 30 | 15.2 | |
| Immune system medicine | |||||||
| Cancer medicine | 2 | 1.0 | 3 | 1.5 | 5 | 2.5 | |
| Immunosuppressive | 11 | 5.6 | 4 | 2.0 | 15 | 7.6 | p=0.124 |
| Cortisone per oral | 12 | 6.1 | 4 | 2.0 | 16 | 8.1 | p=0.083 |
| Cortisone cream | 4 | 2.0 | 0 | 0.0 | 4 | 2.0 | p=0.064 |
| Supplements | |||||||
| Thyroxin | 16 | 8.1 | 3 | 1.5 | 19 | 9.6 | p=0.006 |
| Calcium supplement | 46 | 23.4 | 16 | 8.1 | 62 | 31.5 | p<0.001 |
| Folic acid | 7 | 3.6 | 3 | 1.5 | 10 | 5.1 | |
| B12 supplement | 11 | 5.6 | 12 | 6.1 | 23 | 11.7 | |
| Vitamin D supplement | 44 | 22.3 | 22 | 11.2 | 66 | 33.5 | p=0.013 |
| Nutrition supplement | 6 | 3.0 | 2 | 1.0 | 8 | 4.1 | |
| Magnesium supplement | 7 | 3.6 | 4 | 2.0 | 11 | 5.6 | |
| Vitamin K supplement | 11 | 5.6 | 9 | 4.6 | 20 | 10.2 | |
| Other | |||||||
| Inhaler/nebuliser | 26 | 13.2 | 16 | 8.1 | 42 | 21.3 | |
| Proton pump inhibitor | 39 | 19.8 | 20 | 10.2 | 59 | 29.9 | p=0.030 |
| Urine medicine | 8 | 4.1 | 12 | 6.1 | 20 | 10.2 | |
| Skin cream | 19 | 9.6 | 10 | 5.1 | 20 | 10.2 | p=0.190 |
mild painkiller, non-steroidal anti-inflammatory drugs and paracetamol.
Diagnostics
Forty-two (21.3%) patients were not seen by a primary care physician before they visited the wound care team, meaning that the diagnostic process was not even started before this visit. Of the 155 patients who had been seen by a physician prior to the wound care team, 129 (83.2%) had a recorded diagnosis code (ICD-10), while 26 (16.8%) patients remained undiagnosed. Thus, 34.5% of the patients (n=68) received their first diagnosis by the wound care team.
Out of the patients who were seen by a primary care physician, 85 (58.8%) had no delay (median 0 days), meaning that the patients visited an emergency room and were seen by a physician immediately. The diagnoses for these patients mainly comprised infectious wounds (n=30, 35.3%) and wounds with no specific cause (n=21, 24.7%), while a diagnostic code was not recorded for 10.6% (n=9). Hence, 15 patients had traumatic wounds and saw the physician at an acute appointment.
Of those patients who saw a primary care physician (n=155), 36.2% (n=56) had clinical signs of infection according to the patient records. However, as many as 94 (60.6%) patients were treated with antibiotics, and 82 (52.9%) had a bacterial swab taken.
Of the 129 patients who had a diagnosis before the first appointment with the wound care team, the same diagnosis was made by the team and the primary care physician in 59 (45.7%) of the cases. The concordant diagnoses most often entailed pressure ulcers, infectious ulcers, as well as venous and post-traumatic ulcers. Specialist care was received by 111 patients. The same diagnosis (ICD-10) was made by all three points in the clinical pathway in only 24 (12.2%) cases, and the majority of these comprised infectious ulcers, followed by a venous aetiology and wounds without a specific diagnosis. (Table 5)
Table 5.
Differentiation of the diagnoses when they remained unchanged or were revised over the clinical pathway
| Categorised diagnostic groups | Diagnoses that remained unchanged | Diagnoses that were revised | |||
| Within primary care | Throughout the entire clinical pathway | By wound care team and specialist care | Primary care physician’s diagnosis | Wound care team physician’s diagnosis | |
| Arterial wound | 0 | 0 | 16 | 4 | 7 |
| Venous or oedematous ulcer | 13 | 5 | 17 | 2 | 24 |
| Diabetic foot ulcer | 2 | 1 | 13 | 2 | 11 |
| Pressure ulcer | 11 | 3 | 9 | 1 | 8 |
| Post-traumatic wound | 15 | 3 | 3 | 0 | 2 |
| Atypical wound | 0 | 0 | 7 | 0 | 4 |
| Mixed-aetiology wound | 0 | 0 | 2 | 0 | 3 |
| Infectious wound | 10 | 8 | 9 | 32 | 1 |
| Foot malformation or pressure ulcer | 0 | 0 | 1 | 0 | 5 |
| Wound of unspecified aetiology | 8 | 4 | 6 | 27 | 3 |
Of the patients who visited a specialist, the same diagnosis was made by the wound care team physician and the specialist in 75.5% of the cases (tables 5 and 6). The concordant diagnoses most often comprised diabetic foot ulcers (20.5%), arterial ulcers (19.3%) and venous or oedematous ulcers (15.7%). In the remaining 24.6% (n=27) of the patients, the diagnosis made by the wound care team was revised in specialist care, mostly comprising arterial ulcers (40.7%) that were usually referred for further investigations with a suspicion of an arterial wound. The ulcers that turned out to be arterial wounds were diagnosed by the wound care team as diabetic foot ulcers (14.8%), wounds of mixed aetiology (18.5%), foot malformations (14.8%) and oedematous ulcers (37.0%).
Table 6.
Diagnostic differentiation through the treatment pathway
| The same diagnosis* throughout entire treatment pathway | The same diagnosis within primary care† | The same diagnosis by wound care team and specialist | |
| 1=the same | 24 | 60 | 83 |
| 0=different | 47 | 67 | 27 |
| Total | 71 | 127‡ | 110 |
| % of diagnosed§ ¶ patients | 21.8 | 47.2 | 75.5 |
| % of 197 (whole sample) | 12.2 | 30.5 | 42.1 |
*Treatment pathway: primary care physician, wound care team (physician) and specialist care.
†Primary care physician and wound care team (physician).
‡Two patients were undiagnosed in the wound care team. They were of those of the 129 diagnosed in the primary care.
§One hundred and fifty-five patients visited a doctor in primary care before the appointment with the wound care team, but only 129 patients were diagnosed.
¶One hundred and ten patients were diagnosed in specialist care.
Post-traumatic wounds were categorised into one category. In the primary care setting, there were 16 post-traumatic ulcers, 15 of which were assessed in the emergency room (ER).
Delays
The median time from wound appearance to the first healthcare contact was 2 days (IQR 0–14 days, range 0–351 days) and from wound appearance to the first evaluation by a physician 8 days (IQR 1–32 days, range 0–314 days). The majority of the patients had their first healthcare contact at the emergency department where a physician also examined the patient, or at the district nurse’s office at a health centre with the possibility of an immediate physician’s consultation. The median time from the onset of the wound to the first wound care team appointment was 57 days (IQR 33–100 days, range 2–358 days). The median time between the first healthcare contact and the wound care team appointment was 41 days (IQR 22–80 days: range 1–484 days). Only one in three patients (n=61) had an organisational delay of less than 4 weeks between the first contact with health services and the appointment with the wound care team.
Half of the patients (n=113, 57.4%) were referred to a secondary healthcare unit to be seen by a specialist, most often by a vascular surgeon (n=67), followed by a plastic surgeon (n=43) and a dermatologist (n=13). Twenty-one (18.6%) patients were referred to two or more specialists. The median delay from the first appointment with the wound care team to the appointment with the secondary healthcare specialist was 21 days (IQR 8–55, min–max −58 to 235; range 293 days).
The median time from the appearance of the wound to the final diagnosis was 57 days (IQR 33–101; min–max 2 to 358; range 356 days).
The delays in different subgroups are presented in table 7.
Table 7.
Delays in different subgroups. Figures are presented as medians (IQR; min–max; range)
| Delays are calculated and analysed with the inclusion criterion ‘wound appearance within 365 days prior to wound care team appointment’. | |||||||
| n | Wound appearance - first contact to healthcare | Wound appearance to first physician evaluation | Wound appearance to wound care team | Delay from first contact to wound care team (organisational delay within primary care) | Delay wound care team to specialist care | Mann-Whitney U | |
| All patients | 182 | 2 (0–14; 0 to 351; 351) | 8 (1–32; 0 to 314; 314) | 57 (33–101; 2 to 358; 356) | 42 (22–80; 1 to 484; 483) | 21 (7–52; –58 to 252; 414) | |
| Male | 81 | 3 (0–24; 0 to 351; 351) | 9 (1–37; 0 to 314; 314) | 69 (37–111; 2 to 358; 356)* | 44 (23–85; 2 to 484; 482) | 23 (3–48; –58 to 235; 293) | *p=0.058 |
| Female | 101 | 1 (0–8; 0 to 295; 295) | 8 (1–24; 0 to 295; 295) | 54 (30–96; 2 to 306; 304)* | 41 (22–76; 1 to 264; 263) | 20 (8–58; 0 to 176; 176) | |
| Age under 65 years1 | 46 | 6 (1–27; 0 to 298; 298)* | 9 (3–30; 0 to 142; 142) | 62 (36–100; 11 to 320; 309) | 37 (22–76; 6 to 382; 376) | 28 (14–48; 1 to 182; 181) | *1 vs 2; p=0.005 |
| Age 65–80 years2 | 62 | 0 (0–14; 0 to 258; 258)* | 10 (0–38; 0 to 314; 314) | 61(41–106; 10 to 337; 327) | 49 (25–88; 4 to 264; 260) | 16 (2–50; 0 to 235; 235) | *1 vs 3; p=0.003 |
| Age over 80 y3 | 74 | 1 (0–8; 0 to 351; 351)* | 7 (1–32; 0 to 295; 295) | 53.5 (30–98; 2 to 358; 356) | 40 (22–78; 1 to 484; 483) | 16 (6–56; –58 to 167; 225) | |
| DM+ | 72 | 1 (0–15; 0 to 142; 142) | 10 (1–37; 0 to 142; 142) | 59 (36–102; 2 to 324; 322) | 45 (26–75; 2 to 245; 243) | 14 (3–48; 0 to 235; 235) | No statistical |
| DM– | 110 | 2 (0–13; 0 to 351; 351) | 7 (1–32; 0 to 314; 314) | 56 (31–101; 4 to 358; 354) | 40 (22–84; 1 to 482; 483) | 26 (8–56; –58 to 167; 225) | Difference |
| Living at home | 160 | 2 (0–15; 0 to 351; 351) | 9 (1–33; 0 to 314; 314) | 57 (33–102; 2 to 358; 356) | 42 (22–83; 1 to 382; 381) | 20 (5–48; 0 to 235; 235)* | *p=0.010 |
| Living in institution | 22 | 1 (0–8; 0 to 295; 295) | 3 (0–14; 0 to 295; 295) | 55 (34–86; 7 to 306; 299) | 43 (24–72; 7 to 484; 477) | 56 (16–143; –58 to 176; 234)* | |
| Walking; outdoors | 134 | 3 (0–15; 0 to 351; 351)* | 8 (1–27; 0 to 246; 246) | 56 (33–97; 4 to 358; 354) | 40 (22–76; 1 to 382; 381) | 22 (6–56; −58 to 235; 293) | *p=0.047 |
| Not-walking; staying indoors | 48 | 0 (0–5; 0 to 298; 298) | 9 (1–55; 0 to 314; 314) | 71 (33–108; 2 to 337; 335) | 54 (24–94; 2 to 484; 482) | 16 (7–46; 0 to 182; 182) | |
| Delay before wound care team under 28 days | 61 | 6 (0–20; 0 to 351; 351)* | 7 (3–24; 0 to 295; 295) | 26 (19–41; 2 to 358; 356)* | 18 (12–22; 1 to 28; 27) | 22 (4–42; 0 to 167; 167) | *p<0.001 |
| Delay before wound care team over 28 days | 121 | 0 (0–8; 0 to 258; 258) | 9 (0–34; 0 to 314; 314) | 73 (51–112; 29 to 337; 308) | 70 (42–101; 29 to 484; 455) | 20 (7–55; –58 to 235; 293) | |
| Unchanged diagnosis (PC–WT–spec.) | 20 | 3 (2–12; 0 to 246; 246) | 7 (3–19; 0 to 246; 246) | 51 (32–80; 5 to 267; 262) | 35 (20–74; 2 to 186; 184) | 23 (8–45; 0 to 89; 89) | |
| Different diagnosis | 43 | 1 (0–17; 0 to 258; 258) | 8 (0–42; 0 to 314; 314) | 71 (37–111; 4 to 337; 333) | 57 (30–89;1 to 245; 244) | 18 (7–98; 0 to 235; 235) | |
| The same diagnosis within primary care | 55 | 2 (0–7; 0 to 246; 246) | 3 (1–18; 0 to 246; 246) | 52 (31–78; 5 to 267; 262)* | 40 (20–71; 2 to 186; 184) | 33 (9–56; 0 to 176; 176) | *p=0.042 |
| Different diagnosis | 61 | 1 (0–24; 0 to 258; 258) | 17 (1–56; 0 to 314; 314) | 73 (37–126; 4 to 337; 333) | 53 (31–98; 1 to 245; 244) | 15 (7–64; 0 to 235; 235) | |
Age categories are 1 under 65 years, 2 between 65-80 years, 3 over 80 years. There is a significancy in patient-related delay in seeking health services after onset of the wound between age group under 65 year older than 65 years.
DM, Diabetes mellitus; PC, Primary care; spec, specialist care; WT, Wound care Team.
Discussion
It is well-known that an early diagnosis of the underlying cause of a chronic wound is essential for wound healing and for avoiding amputations.8 16 17 However, there are only a few studies describing the importance of wound diagnosis and the deleterious effects of diagnostic delays.18–20 In the current study, we investigated the diagnostic processes and delays in wound care in the Helsinki area. The patients’ first contact with healthcare services after wound appearance was prompt, the median delay being only 2 days. In stark contrast, it took 57 days from the appearance of the wound before the patient was seen by the wound care team for the first time. In our material, only 31.0% of the patients visited the wound care team within 4 weeks of the first healthcare appointment. This caused a significant delay in reaching the correct diagnosis of the wound. We also discovered that only 65.5% of all patients had a recorded wound diagnosis before the first wound care team appointment and that this diagnosis matched the final diagnosis in approximately 50% of the cases. Accordingly, in European countries, the delay in diagnosing diabetic foot ulcers (DFU) was over 3 weeks in 21%–34% of the patients. The shortest average time from event to diagnosis was 10 days in the UK, 14 days in Spain and France and 20 days in Germany.12
In Finland, wound patients are evaluated and treated mainly during primary care appointments, including home care and nursing homes.21 In the mentioned European countries, the healthcare professionals who participate in the diagnostic process vary considerably. In the UK, only 6% of GPs completely agreed that the care and management of DFUs is the GP’s responsibility, and 22% did not diagnose DFUs. Instead, in 27% of the cases, district nurses made the diagnosis. In the UK, the approach seems more often to be multidisciplinary, as 49% of the GPs referred patients to a podiatrist if needed. In all four countries, GPs were able to refer patients with DFU to specialised multidisciplinary clinics.22
In the UK, DFUs were diagnosed by a GP in only 45% of the cases, and most of the wounds were diagnosed by a district nurse or practice nurse.12
The optimal treatment pathways for wound patients include patient surveillance and an early detection of wound healing problems. Errors in the pathway may lead to delays and, consequently, even fatal errors, such as amputations, in some patients.17
We took a closer look at the ICD-10 codes assessed by the primary care physicians, wound care team physicians and specialists and found that they differed from each other significantly. This highlights the complexity of wound diagnostics. Surprisingly, only 12.2% of the patients had the same diagnosis throughout the whole pathway of care, and these mostly comprised infectious wounds. Based on our data, we assume that there was a significant overdiagnosis of infections in primary care, since an infectious wound was diagnosed in 32.6% of the cases and antibiotics were prescribed for 63.2% of the patients in the primary care setting. Similar results have been obtained in Sweden, where the use of a national wound register diminished the use of antibiotics.23 Evidence of difficulties in the diagnostics of wound infections is also found in a study of GPs recognising and treating wound infections—according to the results, GPs mostly desired further knowledge about when to start or stop treatment (81%–82%), about topical antimicrobials (80%–68%) and about when to prescribe antibiotics (82%–95%).24
Another diagnostic challenge was the diagnosis of an ischaemic wound and DFU in primary care. Only four diabetic and arterial ulcers were diagnosed in primary care. In contrast, the wound care team diagnosed a DFU in 54 patients, and 26 patients were referred to a specialist when an ischaemic wound was suspected. This problem is also detected in a multicentre study performed in four European countries. The researchers found that, even though GPs described neuropathy and peripheral arterial disease as cofactors in the DFU development, they investigated DFUs with additional tests in only half of the cases; this entailed monofilament tests in 21%–43% and, more often, pulse palpation or the measurement of the ABI in 78%–90% of the cases, but diabetic foot infection was tested in only 7%–20% of the investigated cases.11
Our main finding was that there was an organisational delay in reaching a timely diagnosis. The time between wound appearance and the first contact with a physician was adequate, the median being 8 days and two-thirds (58.8%, n=85) of the patients had their first evaluation at the first contact with health services. This means that a physician’s examination was mostly available at the ER or as a rapid consultation during a nurse’s appointment at the health centre. However, our study shows that, among these patients, the initial physician’s evaluation very seldom leads to a correct diagnosis and treatment.
Regarding wound patients, ER assessment should include the three most important and acute aetiologies, such as infection, ischaemia and diabetes,25 but otherwise the ER might not be the optimal setting for diagnosing wounds.
Post-traumatic ulcers could be a subgroup of oedematous leg ulcers due to the same management approach, namely compression therapy, which should be assessed immediately after vascular/arterial causes have been ruled out. Hence, according to the present data, oedematous and post-traumatic ulcers accounted for 40.6% of all the ulcers and were treated with compression.
We found that the wound diagnostic process was good enough in the wound care team, as the diagnosis did not change for 75.5% of the patients who were referred by the team to specialist care. In the remaining 24.5%, in whom the diagnosis made by a specialist differed from the diagnosis made by the wound care team, the final diagnosis was confirmed using diagnostic tools that were not available in the health centre. In these cases, however, the wound care team often had a default diagnosis to base the referral on, and specialist care then responded to this idea, most often leading to the correct treatment. Indeed, the referral was very useful for these patients.
On the other hand, there were ordinary delays in receiving a specialist evaluation, as the median delay was 21 days, where the largest differences were between the subgroups of patients living in institutions and those living at home. This might be explained by the advance care planning among nursing home residents. However, previous studies propose delays of less than 2 weeks in diagnosing arterial ulcers and DFUs to avoid leg losses.17 As a response to the challenge of timely referral to the correct department for treatment there are globally several multidisciplinary wound clinics to have only one place to send a patient for a consultation.26–28
Previous studies suggest that diagnostic errors are often preceded by common symptoms, followed by common diagnoses.29 30 Studies have shown that the most frequent error is ‘premature closure’, meaning ‘the tendency to stop considering other possibilities after reaching a diagnosis’.31 32 In the UK, diagnosis-related and assessment-related incidents were the highest causes of patient harm.29 As a conclusion, the ability to use differential diagnostic methods is key when diagnosing wound aetiologies. There is always a danger of diagnosing a wound incorrectly, if possibilities to perform differential diagnostics are lacking.19 30
As a solution, a broader range of differential diagnostic possibilities as regards the origin of the wound would probably help in the first evaluation and in avoiding diagnostic error and delay in the treatment.33 34 Our study shows that the problem for wound management lies in the primary care; that is, wounds that should be referred to multidisciplinary care are not recognised. The solution is continuous education of primary care physicians and nurses focusing on basic differential diagnostics of chronic wounds instead of wound management per se. Education is needed also to bring up the awareness of the triage—remembering that also an acute wound may turn into a chronic wound which needs quick response and treatment. One practical tool to tackle these diagnostic challenges could be the use of checklists35–37 and digitalised wound diagnostic tools (38, submitted for publication). It has been determined in other contexts that there are tools for avoiding fatal errors in differential diagnostics, such as existing guidelines and, to be regarded with a grain of salt, electronic aids in decision-making.39–41
Limitations
The limitations of this study are related to the variety of aetiologies behind chronic wounds. There are no generally agreed-upon diagnostic codes to be used for chronic wounds, and the differentiation potential is enormous. Most often wounds are coded as merely a wound of unspecified aetiology (L97, L98), or they are S-coded, which refers to a traumatic wound. Therefore, it is difficult to define when a diagnosis is correct or not.
Outliers also constituted a limitation of the study, as we could not include them in the data analysis. Some patients in the material had suffered from a wound for several years. Despite this, they were referred to the wound care team when it was established in 2013. In our delay analyses, we tried to avoid this bias by selecting patients whose wounds had appeared less than 1 year prior to the appointment with the wound care team.
Conclusion
It seems that the diagnostic delay of wound patients occurs within primary care. It is an organisational delay and causes patient harm, as the patients are not receiving a timely and correct diagnosis and treatment. Infectious wounds seem to be easy to detect, but there is a risk of overdiagnosis, leading to an overuse of antibiotics. However, primary care physicians seem to pay little attention to distinguishing arterial insufficiency or DFU.
The delay before seeing a primary care physician was not substantial, but the physicians’ differential diagnostic approaches did not cover peripheral arterial disease or DFU. Consequently, the delay before being seen by the wound care team was over 1 month, which is a long time when treating DFU, especially those of vascular origin.
Based on our results, we propose that it is possible to arrange an optimal treatment pathway within a primary care setting, where a holistic wound care process is initiated, provided that there is organisational support, knowledge, skills and a multidisciplinary team available. It has been demonstrated that such an approach does not even require any additional resources, but rather a rearrangement of the patient care.16 42 We also suggest that the specialist care clinics could play a supportive role in the treatment of complex wounds, while the primary care system could take responsibility for the holistic wound care.
Ethics
Not applicable/no human participants included. This is a retrospective registry-based study. Data were anonymised before the authors assessed them for the purpose of the study. Due to the nature of the study ethical approval was not required for the study. Study was approved in the Institutional Review Board (IRB) of the Abdominal Centre, Helsinki University Hospital and in the IRB of the city of Helsinki.
Supplementary Material
Footnotes
Twitter: @KirsiIsoherran1
Contributors: KA is the responsible guarantor of the content and has contributed to the designing of the study, conducting, collecting and analysing the data and reporting of the work described in the article. KI has contributed to the following parts of the study: designing of the study and reporting, reviewing and editing of the final manuscript. MV has contributed to the following parts in this study: designing of the study, data analysis and interpretation, revisions to scientific content of the manuscript, reviewing and editing of the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: KA has received a personal working grant from the University of Helsinki, The Finnish Wound Association and The Finnish Association for General Practice. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at Helsinki and declare: KI and MV have no support from any organisation for the submitted work, KA has received a working grant from the University of Helsinki, The Finnish Wound Association and The Finnish Association for General Practice; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J 2016;13 Suppl 2:5–15. 10.1111/iwj.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips CJ, Humphreys I, Fletcher J, et al. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J 2016;13:1193–7. 10.1111/iwj.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that different wound types impose on the UK's National health service. Int Wound J 2017;14:322–30. 10.1111/iwj.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open 2020;10:e045253. 10.1136/bmjopen-2020-045253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen CK. Human wound and its burden: updated 2020 compendium of estimates. Adv Wound Care 2021;10:281–92. 10.1089/wound.2021.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health Qual Life Outcomes 2007;5:44. 10.1186/1477-7525-5-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green J, Jester R, McKinley R, et al. The impact of chronic venous leg ulcers: a systematic review. J Wound Care 2014;23:601–12. 10.12968/jowc.2014.23.12.601 [DOI] [PubMed] [Google Scholar]
- 8.Mooij MC, Huisman LC. Chronic leg ulcer: does a patient always get a correct diagnosis and adequate treatment? Phlebology 2016;31:68–73. 10.1177/0268355516632436 [DOI] [PubMed] [Google Scholar]
- 9.Dean S. Leg ulcers - causes and management. Aust Fam Physician 2006;35:480–4. [PubMed] [Google Scholar]
- 10.Atkin L, Bućko Z, Conde Montero E, et al. Implementing timers: the race against hard-to-heal wounds. J Wound Care 2019;23:S1–50. 10.12968/jowc.2019.28.Sup3a.S1 [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Klepzig JL, Sánchez-Ríos JP, Manu C, et al. Perception of diabetic foot ulcers among general practitioners in four European countries: knowledge, skills and urgency. J Wound Care 2018;27:310–9. 10.12968/jowc.2018.27.5.310 [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Ríos JP, García-Klepzig JL, Manu C, et al. Referral of patients with diabetic foot ulcers in four European countries: patient follow-up after first GP visit. J Wound Care 2019;28:S4–14. 10.12968/jowc.2019.28.Sup8.S4 [DOI] [PubMed] [Google Scholar]
- 13.Sheikh A, Donaldson L, Dhingra-Kumar N. Diagnostic errors: technical series on safer primary care. Licence: CC BY-NC-SA 3.0 IGO2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 14.Kostopoulou O, Delaney BC, Munro CW. Diagnostic difficulty and error in primary care--a systematic review. Fam Pract 2008;25:400–13. 10.1093/fampra/cmn071 [DOI] [PubMed] [Google Scholar]
- 15.Irving G, Neves AL, Dambha-Miller H, et al. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open 2017;7:e017902. 10.1136/bmjopen-2017-017902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laakso M, Honkasalo M, Kiiski J, et al. Re-organizing inpatient care saves legs in patients with diabetic foot infections. Diabetes Res Clin Pract 2017;125:39–46. 10.1016/j.diabres.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 17.Noronen K, Saarinen E, Albäck A, et al. Analysis of the elective treatment process for critical limb ischaemia with tissue loss: diabetic patients require rapid revascularisation. Eur J Vasc Endovasc Surg 2017;53:206–13. 10.1016/j.ejvs.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 18.Walker CM, Bunch FT, Cavros NG, et al. Multidisciplinary approach to the diagnosis and management of patients with peripheral arterial disease. Clin Interv Aging 2015;10:1147–53. 10.2147/CIA.S79355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliassen A, Vandy F, McHugh J, et al. Marjolin’s ulcer in a patient with chronic venous stasis. Ann Vasc Surg 2013;27:1182.e5–1182.e8. 10.1016/j.avsg.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 20.Janowska A, Oranges T, Chiricozzi A, et al. Synergism of therapies after postoperative autograft failure in a patient with melanoma of the foot misdiagnosed as a pressure ulcer. Wounds 2018;30:E41–3. [PubMed] [Google Scholar]
- 21.Ahmajärvi KM, Isoherranen KM, Mäkelä A, et al. A change in the prevalence and the etiological factors of chronic wounds in Helsinki metropolitan area during 2008-2016. Int Wound J 2019;16:522–6. 10.1111/iwj.13077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manu C, Lacopi E, Bouillet B, et al. Delayed referral of patients with diabetic foot ulcers across Europe: patterns between primary care and specialised units. J Wound Care 2018;27:186–92. 10.12968/jowc.2018.27.3.186 [DOI] [PubMed] [Google Scholar]
- 23.Öien RF, Forssell HW. Ulcer healing time and antibiotic treatment before and after the introduction of the registry of ulcer treatment: an improvement project in a national quality registry in Sweden. BMJ Open 2013;3:e003091. 10.1136/bmjopen-2013-003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo KY. Physicians' knowledge and attitudes in the management of wound infection. Int Wound J 2016;13:600–4. 10.1111/iwj.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salava A, Isoherranen K. Rule of three I's: a mnemonic aid in undergraduate teaching of lower limb wounds. J Wound Care 2021;30:1030. 10.12968/jowc.2021.30.12.1030 [DOI] [PubMed] [Google Scholar]
- 26.Gottrup F. A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am J Surg 2004;187:S38–43. 10.1016/S0002-9610(03)00303-9 [DOI] [PubMed] [Google Scholar]
- 27.de Leon J, Bohn GA, DiDomenico L, et al. Wound care centers: critical thinking and treatment strategies for wounds. Wounds 2016;28:S1–23. [PubMed] [Google Scholar]
- 28.Kim PJ, Attinger CE, Steinberg JS, et al. Building a multidisciplinary hospital-based wound care center: nuts and bolts. Plast Reconstr Surg 2016;138:241S–7. 10.1097/PRS.0000000000002648 [DOI] [PubMed] [Google Scholar]
- 29.Carson-Stevens A, Hibbert P, Williams H, et al. Characterising the nature of primary care patient safety incident reports in the England and wales national reporting and learning system: a mixed-methods agenda-setting study for general practice. Health Serv Deliv Res 2016;4:1–76. 10.3310/hsdr04270 [DOI] [PubMed] [Google Scholar]
- 30.Ely JW, Kaldjian LC, D'Alessandro DM. Diagnostic errors in primary care: lessons learned. J Am Board Fam Med 2012;25:87–97. 10.3122/jabfm.2012.01.110174 [DOI] [PubMed] [Google Scholar]
- 31.Norman GR, Eva KW. Diagnostic error and clinical Reasoning. Med Educ 2010;44:94–100. 10.1111/j.1365-2923.2009.03507.x [DOI] [PubMed] [Google Scholar]
- 32.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med 2005;165:1493–9. 10.1001/archinte.165.13.1493 [DOI] [PubMed] [Google Scholar]
- 33.Schiff GD, Hasan O, Kim S, et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med 2009;169:1881–7. 10.1001/archinternmed.2009.333 [DOI] [PubMed] [Google Scholar]
- 34.Maude J. Differential diagnosis: the key to reducing diagnosis error, measuring diagnosis and a mechanism to reduce healthcare costs. Diagnosis 2014;1:107–9. 10.1515/dx-2013-0009 [DOI] [PubMed] [Google Scholar]
- 35.Snyder RJ, Jensen J, Applewhite AJ, et al. A standardized approach to evaluating lower extremity chronic wounds using a checklist. Wounds 2019;31:S29–44. [PubMed] [Google Scholar]
- 36.Kapp S, Santamaria N. The "self-treatment of wounds for venous leg ulcers checklist" (STOW-V Checklist V1.0): part 1-development, pilot and refinement of the checklist. Int Wound J 2022;19:705–13. 10.1111/iwj.13666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiseman JT, Fernandes-Taylor S, Gunter R, et al. Inter-rater agreement and checklist validation for postoperative wound assessment using smartphone images in vascular surgery. J Vasc Surg Venous Lymphat Disord 2016;4:320–8. 10.1016/j.jvsv.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaari S, Vähätalo M, Ahmajärvi K. A digital wound management checklist to support clinical decision-making: a qualitative validation study
- 39.Bond WF, Schwartz LM, Weaver KR, et al. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med 2012;27:213–9. 10.1007/s11606-011-1804-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riches N, Panagioti M, Alam R, et al. The effectiveness of electronic differential diagnoses (DDX) generators: a systematic review and meta-analysis. PLoS One 2016;11:e0148991. 10.1371/journal.pone.0148991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Kareh R, Hasan O, Schiff GD. Use of health information technology to reduce diagnostic errors. BMJ Qual Saf 2013;22 Suppl 2:ii40–51. 10.1136/bmjqs-2013-001884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders AP, Stoeldraaijers LGMC, Pero MWM, et al. Patient and professional delay in the referral trajectory of patients with diabetic foot ulcers. Diabetes Res Clin Pract 2013;102:105–11. 10.1016/j.diabres.2013.09.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062673supp001.pdf (30.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request.