Abstract
Aims
To test the hypothesis that reseeded anterior cruciate ligament (ACL)-derived cells have a better ability to survive and integrate into tendon extracellular matrix (ECM) and accelerate the ligamentization process, compared to adipose-derived mesenchymal stem cells (ADMSCs).
Methods
Acellularized tibialis allograft tendons were used. Tendons were randomly reseeded with ACL-derived cells or ADMSCs. ACL-derived cells were harvested and isolated from remnants of ruptured ACLs during reconstruction surgery and cultured at passage three. Cell suspensions (200 µl) containing 2 × 106 ACL-derived cells or ADMSCs were prepared for the purpose of reseeding. At days 1, 3, and 7 post-reseeding, graft composites were assessed for repopulation with histological and immunohistochemical analysis. Matrix protein contents and gene expression levels were analyzed.
Results
In the graft reseeded with ACL-derived cells, a large number of elongated cells that integrated into the matrix were evident at day 3 and day 7. However, in the graft reseeded with ADMSCs, only a small number of elongated cells were found integrated into the matrix. Immunofluorescence for Ki-67 and type I collagen confirmed the pronounced production of type I collagen by Ki-67-positive ACL-derived cells integrated into the ECM. A messenger RNA (mRNA) expression assay demonstrated significantly higher gene expression levels of types I (p = 0.013) and III (p = 0.050) collagen in the composites reseeded with ACL-derived cells than ADMSCs.
Conclusion
ACL-derived cells, when reseeded to acellularized tendon graft, demonstrated earlier better survival and integration in the tendon ECM and resulted in higher gene expression levels of collagen, which may be essential to the normal ligamentization process compared to ADMSCs.
Cite this article: Bone Joint Res 2022;11(11):777–786.
Keywords: ACL-derived cells, ADMSC, Ligamentization, Acellularized tendon allograft, Recellularization, adipose-derived mesenchymal stem cells, tendon allografts, anterior cruciate ligament (ACL), tendons, tendon grafts, collagen, type I collagen, gene expression, mRNA, RNA
Article focus
To explore the ligamentization potential of anterior cruciate ligament (ACL)-derived cells reseeded to acellularized tendon grafts.
Key messages
In the graft reseeded with ACL-derived cells, a large number of elongated cells that integrated into the matrix were evident.
We found that immunofluorescence for Ki-67 and type I collagen confirmed the pronounced production of type I collagen by Ki-67-positive ACL-derived cells that integrated into the matrix of the graft.
Messenger RNA (mRNA) expression analysis using quantitative polymerase chain reaction demonstrated significantly higher gene expression levels of types I and III collagen in the grafts reseeded with ACL-derived cells compared to those reseeded with adipose-derived mesenchymal stem cells.
Strengths and limitations
ACL-derived cells, which have great potential for clinical use as a source of autologous stem cell therapy, were assessed.
The study is limited by its in vitro nature. In vivo studies are required in the future.
Introduction
Anterior cruciate ligament reconstruction (ACLR) is one of the most commonly performed surgical procedures in the field of sports medicine. 1-3 Surgical techniques have evolved in recent years; however, graft failure occurs with an incidence of 0.7% to 10%. 4,5 For surgical success, ACLR requires biological maturation and remodelling of the tendon graft into a ligamentous ACL-like structure, which is often referred to as the ligamentization process. 6-10 It has been reported that the implanted tendon graft undergoes ligamentization beyond one year and does not reach full maturity until two years after surgery. 11,12 However, full restoration of either the biological or mechanical properties of the normal ACL has never been achieved. 11-13
Animal studies have shown that during the ligamentization process, cellular invasion consisting of mesenchymal stem cells and fibroblasts integrating into the tendon graft occurs after around six weeks, and remodelling of the graft toward the morphology and mechanical strength of an intact ACL occurs from this point onward. 13-15 However, the predominant synthesis of type III collagen, which is normally found in scar tissue and has a lower mechanical strength than type I collagen, continues to be sustained in higher concentrations than in the normal ACL tissue. 16 To overcome this time-consuming healing process, which usually leads to a weaker reparative scar tissue, a tissue-engineering strategy that involves reseeding of proliferative cells possessing a tenogenic differentiation potential into the tendon extracellular matrix (ECM) has been attempted. 17,18 Various cell types including tenocytes, fibroblasts, mesenchymal stem cells (MSCs), adipose-derived stem cells (ASCs), and fibroblasts have been studied; however, most studies have focused on the tenogenic differentiation potential of seeded cells, rather than their ability to survive and integrate into the tendon ECM and induce the ligamentization process of the tendon graft toward the properties of normal ACL tissue. 17-21
Several reports have shown that ruptured human ACL tissue possesses numerous cells that can promote healing and have high expansion and multilineage differentiation. 22-24 Indeed, autologous ACL-derived cells possess great potential for clinical use as a source of autologous stem cell therapy. Accordingly, we performed experiments to test the hypothesis that reseeded ACL-derived stem cells have a better ability to survive and integrate into tendon ECM and accelerate the ligamentization process compared to mesenchymal stem cells (MSCs), which infiltrate the implanted tendon grafts during the normal proliferative phase of tendon healing. 13 To test our hypothesis, cell survival and integration, gene expression, and matrix protein contents following reseeding of ACL-derived stem cells into acellularized tendon allograft were assessed and compared to adipose-derived mesenchymal stem cells (ADMSCs) in vitro.
Methods
Patients
This study was conducted according to institutional guidelines and approval from the Ethics Committee of Hanyang University Hospital, with written informed consent from all subjects (IRB-2018-05-001). Between March 2018 and December 2020, ruptured ACL remnant tissues were separated from 14 patients (11 males, three females) with a mean age of 22.7 years (standard deviation (SD) 4.25; 18 to 31) who had undergone primary ACL reconstruction within four weeks following injury.
Cell isolation and preparation
Methods for ACL-derived stem cell isolation were previously described and reported by Lee et al. 24 Briefly, torn ACL tissues were harvested and cut into 1 cm or smaller segments with scissors. The segments were enzymatically dissolved with 1 mg/ml collagenase type I (17100-017, Thermo Fisher Scientific, USA; Sigma-Aldrich, USA, C0130) in serum-free Dulbecco’s Modified Eagle Medium (DMEM) containing penicillin-streptomycin antibiotics (15140122, Thermo Fisher Scientific), followed by incubation with shaking at 37°C for 16 to 20 hours. Cell suspensions were filtered through a nylon mesh, washed in serum-free DMEM several times, and resuspended with 4 ml of phosphate-buffered saline (PBS) in a sterile tube for the subsequent reseeding experiment. After centrifugation at 1,500 rpm for three minutes, the cells were stored in a deep freezer at -80°C. Human ADMSCs were obtained from the cell bank (CEFO, South Korea) and prepared and stored in the same manner. The ADMSCs were isolated from fat tissue harvested from a 25-year-old female donor (Cefobio, CB-ADMSC-001). All cells were checked for mycoplasma using a polymerase chain reaction (PCR)-based method (6601, Takara, Japan) before long-term storage in liquid nitrogen. Passage 3 for both cell groups was used for further experiments, and cells from a single donor were used for each set of experiments.
Cell adhesion assay
To compare adhesion potency, a total of 1 × 105 cells were seeded in each well of six-well plates with six replications and incubated for a day. Non-adherent cells were carefully washed out with DMEM growth medium three times, fixed with 10% formalin for ten minutes, and stained with Giemsa staining solution (Sigma, 48900) for 15 minutes. The adherent cells were imaged using an upright microscope (Leica, Germany) and counted. To conduct F-actin (Phalloidins) staining, the cells were fixed with 10% formalin for ten minutes, permeabilized with 3% Triton X-100 for 15 minutes, washed with diluted PBS two times, stained with F-actin solution for 20 minutes at room temperature, and washed two times with diluted PBS. Stained cells were visualized using a fluorescence microscope (Leica) for analysis.
Cell proliferation assay
Cell proliferation was evaluated using water-soluble tetrazolium salt (WST) (DoGen, EZ-1000) following the manufacturer’s instructions. Cells were seeded at a density of 1 × 103 cells/well in 96-well plates in triplicate. On the indicated days, 10 μl of WST solution was added to the wells and incubated for one hour, allowing the WST to metabolize to formazan. Absorbance was measured using a microplate reader at 450 nm.
Fluorescence-activated cell sorting
Cells were fixed with 2% formalin and stained with CD29-PE (303004, Biolegend, USA), CD34-APC (343607, Biolegend), CD44-PE (338807, Biolegend), CD45-APC (368511, Biolegend), CD90-PE (328109, Biolegend), or CD105-PE (400112, Biolegend) for ten minutes at 4°C. After staining, the cells were washed with 1 X PBS containing 0.5% bovine serum albumin (BSA) and 0.1% sodium azide. Flow cytometry was performed using FACS Canto II (BD Biosciences, USA), and the data were analyzed using FlowJo software (Tracstar, USA).
Cell differentiation and assessment
Reagents for differentiation induction and analysis methods for chondrocyte, osteoblast, and adipocyte differentiation markers have been previously reported. 24-26 Cells were differentiated into mature osteoblasts, chondrocytes, or adipocytes for the indicated days. The differentiation medium was replaced every three days. On the indicated days, differentiated cells were analyzed following the standard protocols. 26-28
For osteoblast differentiation, ADMSCs and ACL-derived cells were seeded in 96-well plates at a density of 8 × 103 cells and replaced with osteogenic medium containing 50 μM ascorbic acid, 10 mM β-glycerolphosphate, and 100 nM dexamethasone. For chondrocyte differentiation, ADMSCs and ACL-derived cells were seeded in 15 ml tubes at a density of 5 × 105 cells to form a pellet. Cells were replaced with chondrogenic medium containing 10% Insulin-Transferrin-Selenium (ITS)+ premix tissue culture supplement (I3146, Sigma-Aldrich), 10 μM dexamethasone (D2915, Sigma-Aldrich), 1 μM ascorbate-2-phosphate (49752, Sigma-Aldrich), 1% sodium pyruvate (10302, Gibco), and 10 ng/ml TGF-β1 (100-21, Peprotech, UK). After centrifugation at 1,500 rpm for three minutes, the cells were placed in a CO2-enriched incubator. For adipocyte differentiation, ADMSCs and ACL-derived cells were seeded in 96-well plates at a density of 8 × 103 cells and replaced with adipogenic medium containing StemXVivo Adipogenic Supplement (CCM011, R&D Systems, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed to quantify the messenger RNA (mRNA) expression level of osteoblast-specific genes (alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), and osteocalcin), chondrocyte-specific genes (collagen type II, SOX9, and aggrecan), and adipocyte-specific genes (lipoprotein lipase, fatty acid-binding protein 4 (FABP4), and peroxisome proliferator-activated receptor-gamma), respectively.
Experiment design
A total of 14 strips of donated human acellularized tibialis tendon allografts were obtained from the Korea Public Tissue Bank; all tendon allografts were processed following their standard procedure. 15,29,30 Briefly, tendon allografts were completely removed of muscle and washed with sterile distilled water for five minutes, treated with 3% hydrogen peroxide for five minutes, and with 70% ethyl alcohol for 15 minutes. All washing procedures were repeated three times. Low-dose (25 kGy) gamma irradiation was performed for sterilization, and the tendon allografts were kept frozen at -80°C. For the experiment, grafts were thawed at room temperature for 15 minutes and cut into small segments with lengths of 1.5 cm. Grafts (e.g. tibialis tendon of both legs) from a single donor were used for each experiment set. Cell suspensions (200 µl) containing 2 × 106 cells of ACL-derived cells or ADMSCs were prepared for the purpose of reseeding. Each tendon segment was reseeded either with ACL-derived cells or ADMSCs by injecting a cell-concentrated suspension directly into the graft using a 25-gauge needle. The injection was performed meticulously into the core of the graft and carefully monitored for any leakages. Reseeded tendon segments were initially placed in a 5% CO2 incubator for 20 minutes and then replenished with DMEM (high glucose, SH30243.01, Hyclone, USA) medium containing 10% foetal bovine serum (FBS) and 1% penicillin-streptomycin. The DMEM growth medium was changed every three days. On the harvest days (1, 3, and 7 days), reseeded tendon segments were completely embedded in the Tissue-Tek O.C.T. compound (4583, Sakura, Japan) and kept frozen at -80°C. Then, the frozen tissue blocks were cryosectioned longitudinally at 6 µm thickness. Sections were used for histological and immunochemistry examination purposes, and were stored on slide glass in a deep freezer at -80°C.
Histological analysis
The staining procedures were followed in accordance with standard protocols or with the manufacturer’s instructions. The slides were stained with haematoxylin and eosin (H&E) for histological, Masson’s trichrome (ab150686, Abcam, UK) for total collagen, and Picrosirius red (ab150681, Abcam) for types I and III collagen analysis. All slides were mounted with a permanent mounting medium (H-5000, Vector Lab, USA). The cell-injected areas in each slide were observed in high-power fields at ×200 magnification. The number of positive cells in three visual fields was counted on five consecutive slides. Two blinded and independent observers (JP and SJ) performed the measurements, and the mean value was used for analysis.
Immunohistochemistry
Immunohistochemical staining was conducted as previously described by Jo et al. 31 Briefly, the tissue slides were fixed with cold acetone and washed with distilled water and 1 X PBS three times, followed by incubation with 1 X PBS, including 0.3% Triton X-100, for ten minutes and with BLOXALL (SP-6000, Vector Lab) for 30 minutes. The tissues slides were then immunostained with 1:100 primary antibodies in antibody diluent (S3022, DAKO, USA) at room temperature for one hour, followed by incubation with biotinylated anti-rabbit immunoglobulin G (IgG) secondary antibodies (BA-1000, Vector Lab) mixed with normal horse serum (S-2012, Vector Lab) for one hour, incubation with ABC kit components (PK-6102, Vector Lab) for 30 minutes as specified by the manufacturer, and incubation with DAB substrate kit (sk4100, Vector Lab) for one to five minutes. Finally, the immunostained slides were washed five times with distilled water and then counterstained with haematoxylin (1.05174.0500, Merck, Germany) for ten seconds, followed by dehydration by passage through a graded series of ethanol solutions (50% to 100% ethanol) and were finally mounted with permanent mounting medium (H-5000, Vector Lab). Images for stained cells were collected with a Nikon Eclipse Ti-U microscope. The antibodies for IHC were as follows: COL1A1 (Santa Cruz Biotechnology, USA, 8784; dilution, 1:200) and COL3 (ABclonal Technology, USA, a3795; dilution, 1:200).
Immunofluorescence
The tissue slides were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 1 X PBS containing 0.3% Triton X-100 (Sigma-Aldrich) and 1% BSA (Rocky Mountain Biologicals, USA) for an hour, and stained with primary antibody overnight at 4°C. After incubation, the slides were washed twice with 1 X PBS and incubated with Cy3 or Alexa 488-conjugated secondary antibody for an hour at room temperature. The slides were washed with distilled water and mounted with 4′,6-diamidino-2-phenylindole (DAPI; Vector Lab, USA). Images were obtained using a confocal microscope (Leica). The antibodies for immunofluorescence (IF) were Ki-67 (14-5698-82, Thermo Fisher Scientific) or type 1 collagen (Santa, sc-8784 and 66948, Cell Signalling Technology, USA).
RT-qPCR
For mRNA analysis, the frozen section slides were collected with a scraper, and total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific), following the conventional methods as previously described by Jo et al. 32 A total of 1 μg RNA for complementary DNA (cDNA) was conducted using reverse transcriptase (EP0442, Thermo Fisher Scientific). The cDNA was then used as the template for RT-qPCR. The RT-qPCR primers used were as follows: COL1 forward, 5’-AGTGGTTTGGATGGTGCCAA-3’; COL1 reverse, 5’-GCACCATCATTTCCACGAGC-3’; COL2 forward, 5′-CAACCAGGACCAAAGGGACA-3′; COL2 reverse, 5′-ACCTTTGTCACCACGATCCC-3′; COL3 forward, 5′-CTTCTCTCCAGCCGAGCTTC-3′; COL3 reverse, 5′-CCAGTGTGTTTCGTGCAACC-3′; tenascin C (TNC) forward, 5′-GGTTGCTGGAGACTGTGGAA-3′; TNC reverse, 5′-AGGTTTTCCAGAAGGGGCAG-3′; tenomodulin (TNMD) forward, 5’-AATGAACAGTGGGTGGTCCC-3’; TNMD reverse, 5’-TTGCCTCGACGGCAGTAAAT-3’; smooth muscle actin (SMA) forward, 5’-GTGATGGTGGGAATGGGACAA; SMA reverse, 5’-AGTGGTGCCAGATCTTTTCCA-3’; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5’-CAAGATCATCAGCAATGCC-3’; and GAPDH reverse, 5’-CTGTGGTCATGAGTCCTTCC-3’.
Statistical analysis
GraphPad Prism 7.0 (USA) was used to form quantitative data and statistical analysis. Statistical significance was tested using the Mann-Whitney U test and one-way analysis of variance (ANOVA) as appropriate. The values are shown as the mean and standard deviation (SD) from a minimum of three independent experiments. The asterisks represent the level of statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Characteristics of ACL-derived cells and ADMSCs
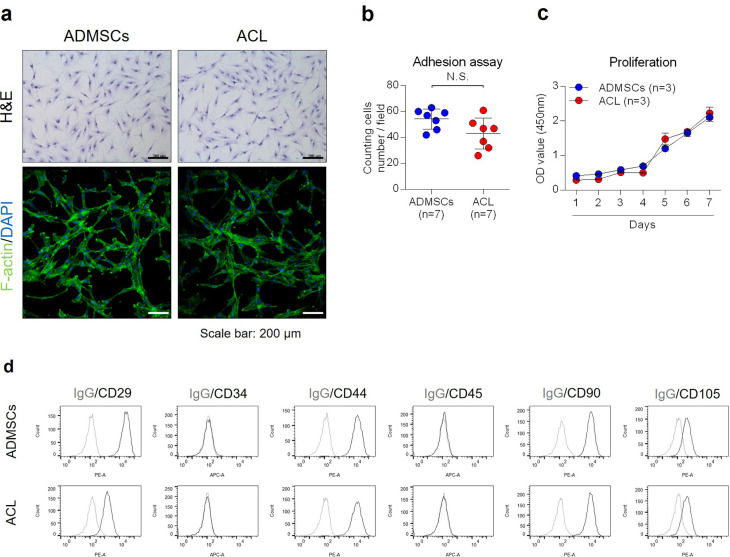
Histological features exhibited on H&E and F-actin staining revealed the typical elongated spindle shape morphology in both cell groups (Figure 1a). The adhesion and proliferation assay demonstrated comparable characteristics in terms of adhesion potency and proliferation rate between ACL-derived cells and ADMSCs during the seven days of analysis (Figures 1b and 1c). Fluorescence-activated cell sorting (FACS) analysis showed positive expression for mesenchymal stromal cell markers (CD29, CD44, CD90, and CD105) in both cell groups but negative expression for haematopoietic origin stem cell markers (CD34 and CD45) (Figure 1d). Multilineage differentiation potency was compared between the cell groups. There was no significant difference in physiological changes and expression of related differentiation markers in osteoblasts and chondrocytes; however, ADMSCs demonstrated higher adipocyte differentiation activities than ACL-derived cells (Supplementary Figure a).
Fig. 1.
a) Morphology of anterior cruciate ligament (ACL)-derived cells and adipose-derived mesenchymal stem cells (ADMSCs) was observed by Giemsa staining and F-actin staining. Scale bar 200 µm. b) ACL-derived cells and ADMSCs were incubated in a growth medium for a day, and the number of cells was counted for an adhesion assay (n = 6). c) ACL-derived cells and ADMSCs were incubated in a growth medium for the number of days indicated, and the cell proliferation rate was evaluated using a WST assay (n = 3). d) CD29, CD34, CD44, CD45, CD90, or CD105 expression of cell surface was analyzed by fluorescence-activated cell sorting (FACS). Data are presented as the mean and standard deviation. DAPI, 4′,6-diamidino-2-phenylindole; H&E, haematoxylin and eosin; IgG, immunoglobulin G; N.S., not significant; OD, optical density.
Cell integration and survival capacity of reseeded cells in the tendon graft
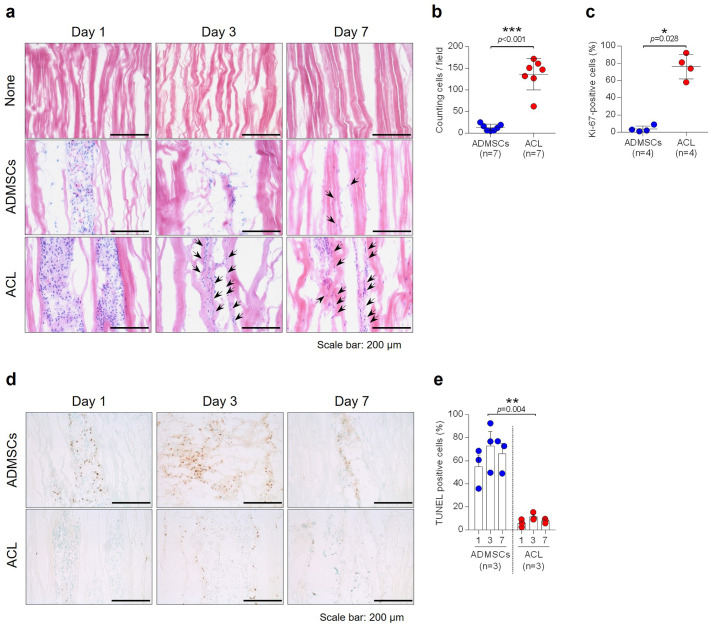
Histological features observed by H&E staining showed no cell fragments retained in the matrix and no substantial disruption of the overall ECM structure of the control tendon allograft (Figure 2a). On day 1, after reseeding of cells, a cluster of cells was found along the intrafascicular openings where the injection was performed (Figure 2a). In the graft reseeded with ACL-derived cells, a large number of elongated cells that integrated into the matrix were evident at day 3 and day 7; however, in the graft reseeded with ADMSCs, only a small number of cells that integrated into the matrix were found (Figure 2a). The number of cells that integrated into the matrix was counted at day 7, and a significantly higher number of integrated cells was confirmed in the graft reseeded with ACL-derived cells compared to the graft reseeded with ADMSCs (Figure 2b) (p < 0.001, Mann-Whitney U test). Furthermore, Ki-67 immunohistochemical staining was performed to confirm the proliferative capacity of reseeded cells, and grafts with ACL-derived cells demonstrated a significantly higher number of Ki-67 positive cells compared to grafts reseeded with ADMSCs at day 7 (Figure 2c and Supplementary Figure b) (p = 0.028, Mann-Whitney U test). Conversely, the cell death rate, assessed by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay, was significantly higher in grafts reseeded with ADMSCs compared to grafts reseeded with ACL-derived cells (Figures 2d and 2e) (p = 0.004, one-way ANOVA).
Fig. 2.
a) Each specimen was analyzed by haematoxylin and eosin (H&E) staining. Magnification ×200, scale bar 200 µm. Black arrows indicate the elongated cells that integrated into the matrix of tendon allografts. b) The number of elongated cells that integrated into the matrix was counted (n = 7). c) Proliferative cells were stained by Ki-67 and counted (n = 4). d) Apoptotic cells within cell-injected tendon allografts were stained by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay. Magnification ×200, scale bar 200 µm. e) Quantification of Figure 2d (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001, Mann-Whitney U test and one-way analysis of variance. Data are presented as the mean and standard deviation. ACL, anterior cruciate ligament; ADMSC, adipose-derived mesenchymal stem cell.
Matrix protein contents and gene expression
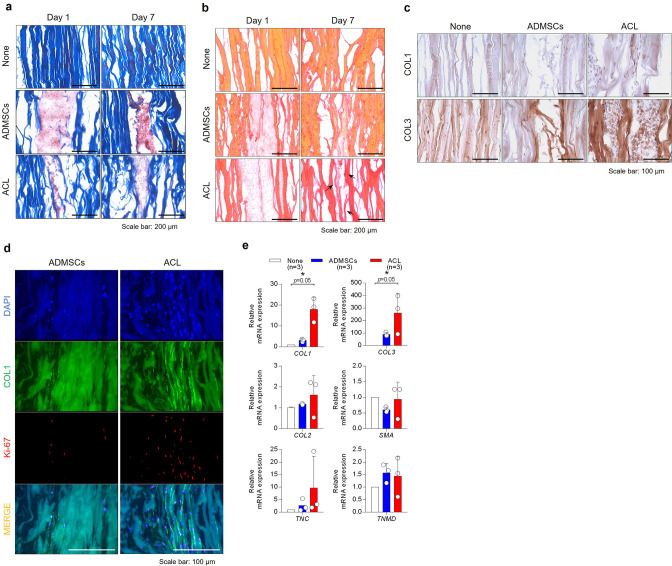
Masson’s trichrome staining and Sirius Red staining showed more consistent staining for collagen deposition in grafts reseeded with ACL-derived cells compared to those reseeded with ADMSCs at day 7 (Figures 3a and 3b). Types I and III collagen protein expression were more prominent in the grafts reseeded of ACL-derived cells than ADMSCs, as evaluated by immunohistochemical analysis (Figures 3c and 3d). Immunofluorescence for Ki-67 and type I collagen confirmed the pronounced production of type I collagen by Ki-67-positive ACL-derived cells integrated into the graft matrix (Figure 3d). A mRNA expression analysis performed at day 7 demonstrated significantly higher gene expression levels of types I and III collagen in the grafts reseeded with ACL-derived cells compared to ADMSCs (Figure 3e). However, other ligament-specific markers, including tenascin C (TNC), tenomodulin (TNMD), and smooth muscle actin (SMA) showed no significant difference.
Fig. 3.
Tendon allograft injected with anterior cruciate ligament (ACL)-derived cells, or adipose-derived mesenchymal stem cells (ADMSCs) was stained and observed by a) Masson’s trichrome staining for total collagen and b) Sirius Red staining for collagen types I and III. Magnification ×200, scale bar 200 µm. c) Immunohistochemical staining of the tendon allograft reseeded with ACL-derived cells or ADMSCs was conducted at seven days for type I collagen (upper) and type III collagen (lower). Magnification ×200, scale bar 100 µm. d) Immunofluorescence of the tendon allograft injected with ACL-derived cells or ADMSCs was conducted at day 7 for Ki-67 (red) and type I collagen (green). e) mRNA expression of ligament-specific markers (COL1, COL3, tenascin C (TNC), and tenomodulin (TNMD)), chondrogenic marker (COL2), and smooth muscle actin (SMA) was analyzed by quantitative reverse transcription polymerase chain reaction (n = 3). DAPI, 4′,6-diamidino-2-phenylindole; mRNA, messenger RNA.
Discussion
In this study, ACL-derived cells, when reseeded to acellularized tendon graft, demonstrated greater ability to survive and integrate into tendon ECM and resulted in higher gene expression levels of types I and III collagen compared to ADMSCs. In vivo studies are required to confirm the enhanced ligamentization process of these neotendon constructs following ACL reconstruction.
ACL reconstruction is often performed using the tendon graft; however, it must be noted that tendon tissue has low regenerative capacity, requiring a long time to produce a dense, collagen-rich matrix consisting of type I collagen, which is predominantly found in normal ACL tissue. 33 Studies have found that mesenchymal stem cell invasion occurs around six weeks following tendon graft implantation and, despite the invasion of multipotent activated cells, predominant synthesis of type III collagen continues to be sustained in higher concentrations, even at two years after graft implantation. 13-15 To overcome this time-consuming healing process that often leads to reparative scar tissue, reseeding of autologous tenogenic cells into acellular tendon ECM has been attempted. 17-21,34-36 Omae et al 34 reseeded acellularized tendon grafts with bone marrow stromal cells and implanted the composite into a rabbit patellar tendon defect, and found enhanced expression of a tendon phenotype. Ingram et al 35 performed ACL reconstruction in a xenogeneic model using acellular sonicated human tendon graft reseeded with tenocytes, and reported that reseeded cells remained detectable on the ECM for several weeks in vitro, although the cell number in the centre of the graft decreased. However, other studies have found limited infiltration of reseeded cells, as the tendon ECM is dense and does not allow sufficient cell integration. Chong et al 36 reseeded tenocytes into acellularized rabbit flexor tendon and found limited cell adhesion to the surface of the tendon. Furthermore, Pridgen et al 17 reseeded dermal fibroblasts on acellularized human flexor tendons and found that cell attachment was observed only on the surface of the tendon. Various cell types, including tenocytes, fibroblasts, BMSCs, and ADMSCs have been studied for reseeding purposes. 17-21,26,37-40 Stem cells derived from other tissues, such as BMSCs and ADMSCs, are much easier to acquire than tenocytes or tendon progenitor cells, and they have been proven efficient for tendon repair. 26,37 While BMSCs may carry the risk of nontendon differentiation and of forming ectopic bone during tendon repair, ADMSCs have been reported to enhance the production of collagen I and tenascin-C during healing of tendon. 38-40
Ruptured human ACL tissue possesses numerous cells that have the potential for high expansion and multilineage differentiation. 22-24 Autologous ruptured ACL tissue is easy to obtain during the arthroscopic procedure, 24 and assuming that these cells possess positional memory, 41 autologous ACL-derived cells have great potential for clinical use as a source of autologous stem cell therapy during ACLR. 24 Furthermore, by recolonizing the mature ligament-derived cells into the ECM, the time-consuming differentiation and maturation process of MSCs towards tenogenic lineage that often leads to reparative scar tissue (e.g. ligamentization) could be shortened, or omitted. 6,7,11,12 Our efforts primarily focused on assessing the ability of ACL-derived cells to survive and integrate into acellularized tendon ECM without the involvement of any other stimulation (e.g. growth factors), as Durgam et al 21 reported that stimulating reseeded cells with growth factors leads to higher ECM synthesis. In our study, an average of 937 mg (598 to 1,431) of ACL remnant tissue was collected from a single patient depending on the status of the ruptured ACL, and an average of 9.5 × 105 (6.2 × 105 to 1.4 × 106) primary ACL-derived cells were obtainable, thus supporting the clinical use of ACL-derived cells as a potential source of autologous stem cell therapy during ACL reconstruction. Our results demonstrated the limited survival and integration of reseeded ADMSCs in the tendon ECM, resulting in a significant decrease of Ki-67-positive cells by the end of day 7. This result was consistent with previous studies reporting that cells seeded on ECM remain detectable, but cell numbers decrease significantly with time. 35,42 On the other hand, ACL-derived cells showed better survival and integration in the matrix compared to ADMSCs, resulting in significantly higher gene expression of collagen, which is essential for the normal healing process of the tendon. We presume that such a difference in the ability to survive and integrate into tendon ECM between cell groups occurs due to differences in their cellular microenvironment, as ligaments and tendons share a similar microenvironment that determines cells’ adhesion, proliferation, and differentiation. 43,44 Histological sections obtained at day 7 also showed a large number of ACL-derived cells that aligned and integrated into the tendon matrix, whereas only a limited number of ADMSCs were found. Furthermore, a mRNA expression assay demonstrated significantly higher gene expression levels of types I and III collagen in the grafts reseeded with ACL-derived cells compared to ADMSCs.
Reseeding of acellularized tendon ECM remains a challenge because the ECM is dense and does not allow sufficient cell adhesion and infiltration. 17,36 Studies have suggested different strategies to improve recolonization of the tendon ECM, including static culture, injection, pulsatile perfusion, porcine superflexor tendon, extracorporeal shock wave, plasmin, and ultrasonification. 17,20,35,36,42,45-48 In this study, the direct injection technique was adopted for reseeding the cells into tendon ECM because it is easy to perform and allows direct intratendon pooling of cells. However, some cell and tissue damage (e.g. leakages) may occur within the ECM. 42,45
This study has several limitations. First, it is limited by its in vitro nature. However, as it has been observed that recellularized tendons are better colonized in vivo in the animal xenograft model compared to the in vitro design, 49 and stimulating reseeded cells with growth factors leads to higher ECM synthesis, 21 it could be expected that an in vivo study would also show better colonization of ACL-derived cells and higher collagen production. Second, the study is limited by the short follow-up, as reseeded grafts were observed for up to seven days (three timepoints). In vivo studies with longer follow-up are required to determine if our early study findings translate into improved later ligamentization healing processes in an in vivo environment. Third, consent forms did not mention harvesting fat tissue from patients during ACL surgery; ADMSCs were obtained from the cell bank. Furthermore, individual and sex differences may have affected the outcome; ACL donors mainly were young males, and therefore the findings of the study cannot be generalized to all patient populations. Fourth, although different stainings (e.g. Masson’s trichrome, Picrosirius red, and IHC) qualitatively showed more pronounced staining patterns for collagen deposition in the tendon grafts reseeded with ACL-derived cells than ADMSCs, the actual quantification of newly produced collagen was not performed in this study. Finally, the inhomogeneous distribution of cells, along with the potential for leakages, may result in sampling biases for certain study measures such as histological analyses.
In conclusion, acellularized tendon grafts seeded with ACL-derived cells demonstrated better survival and integration in the matrix and resulted in higher gene expression levels of types I and III collagen at seven days, compared to similar grafts seeded with ADMSCs. These findings may be essential to the ACL ligamentization process.
Author contributions
J. Park: Writing – original draft, Formal analysis, Investigation.
S. Jo: Writing – original draft, Writing – review & editing, Formal analysis, Methodology, Data curation, Visualization.
M-K. Lee: Resources, Methodology.
T-H. Kim: Validation, Funding acquisition.
I-H. Sung: Conceptualization, Resources.
J. K. Lee: Writing – original draft, Writing – review & editing, Validation, Resources, Funding acquisition, Project administration.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: this work was supported by the National Research Foundation of Korea (grant number: 2020R1F1A1049719, 2020R1A2C1102386, and 2021R1A6A1A03038899).
ICMJE COI statement
All authors declare no conflicts of interest.
Data sharing
Data are available from the corresponding author upon reasonable request.
Ethical review statement
The present study was authorized by the Ethics Committee of the Hanyang University Hospital (IRB-2018-05-001). All patients with anterior cruciate ligament reconstruction who participated in the present study signed a written informed consent.
Open access funding
The open access fee for this study was covered by the National Research Foundation of Korea funding mentioned above.
Supplementary material
The results of multidifferentiation potency and proliferative capacity comparison between adipose-derived mesenchymal stem cells and anterior cruciate ligament cells.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Jinsung Park, Email: ddochi0501@gmail.com.
Sungsin Jo, Email: joejo0517@gmail.com.
Myung-Kyu Lee, Email: mklee@kptb.kr.
Tae-Hwan Kim, Email: thkim@hanyang.ac.kr.
Il-Hoon Sung, Email: sungih@hanyang.ac.kr.
Jin K. Lee, Email: jklee77@hanyang.ac.kr.
References
- 1. Duquin TR, Wind WM, Fineberg MS, Smolinski RJ, Buyea CM. Current trends in anterior cruciate ligament reconstruction. J Knee Surg. 2009;22(1):7–12. 10.1055/s-0030-1247719 [DOI] [PubMed] [Google Scholar]
- 2. Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A(10):1387–1397. 10.2106/00004623-200010000-00004 [DOI] [PubMed] [Google Scholar]
- 3. Lee DW, Shim JC, Yang SJ, Cho SI, Kim JG. Functional effects of single semitendinosus tendon harvesting in anatomic anterior cruciate ligament reconstruction: comparison of single versus dual hamstring harvesting. Clin Orthop Surg. 2019;11(1):60–72. 10.4055/cios.2019.11.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ménétrey J, Duthon VB, Laumonier T, Fritschy D. “Biological failure” of the anterior cruciate ligament graft. Knee Surg Sports Traumatol Arthrosc. 2008;16(3):224–231. 10.1007/s00167-007-0474-x [DOI] [PubMed] [Google Scholar]
- 5. Tashiro Y, Gale T, Sundaram V, et al. . The graft bending angle can affect early graft healing after anterior cruciate ligament reconstruction: in vivo analysis with 2 years’ follow-up. Am J Sports Med. 2017;45(8):1829–1836. 10.1177/0363546517698676 [DOI] [PubMed] [Google Scholar]
- 6. Falconiero RP, DiStefano VJ, Cook TM. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14(2):197–205. 10.1016/s0749-8063(98)70041-6 [DOI] [PubMed] [Google Scholar]
- 7. Sánchez M, Anitua E, Azofra J, Prado R, Muruzabal F, Andia I. Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy. 2010;26(4):470–480. 10.1016/j.arthro.2009.08.019 [DOI] [PubMed] [Google Scholar]
- 8. Ahn GY, Nam IH, Lee YH, et al. . Factors affecting the extent of graft tendon synovialization after double-bundle anterior cruciate ligament reconstruction: based on second-look arthroscopic findings. Clin Orthop Surg. 2018;10(4):413–419. 10.4055/cios.2018.10.4.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim SG, Jung JH, Song JH, Bae JH. Evaluation parameters of graft maturation on second-look arthroscopy following anterior cruciate ligament reconstruction: a systematic review. Knee Surg Relat Res. 2019;31(1):2. 10.1186/s43019-019-0005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kyung HS. Graft considerations for successful anterior cruciate ligament reconstruction. Knee Surg Relat Res. 2019;31(1):1. 10.1186/s43019-019-0003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janssen RPA, Scheffler SU. Intra-articular remodelling of hamstring tendon grafts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2102–2108. 10.1007/s00167-013-2634-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheffler SU, Unterhauser FN, Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(9):834–842. 10.1007/s00167-008-0560-8 [DOI] [PubMed] [Google Scholar]
- 13. Janssen RPA, van der Wijk J, Fiedler A, Schmidt T, Sala HAGM, Scheffler SU. Remodelling of human hamstring autografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1299–1306. 10.1007/s00167-011-1419-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Unterhauser FN, Bail HJ, Höher J, Haas NP, Weiler A. Endoligamentous revascularization of an anterior cruciate ligament graft. Clin Orthop Relat Res. 2003;414(414):276–288. 10.1097/01.blo.0000079442.64912.51 [DOI] [PubMed] [Google Scholar]
- 15. Weiler A, Förster C, Hunt P, et al. . The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):881–891. 10.1177/0363546503261711 [DOI] [PubMed] [Google Scholar]
- 16. Petersen W, Laprell H. Insertion of autologous tendon grafts to the bone: a histological and immunohistochemical study of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc. 2000;8(1):26–31. 10.1007/s001670050006 [DOI] [PubMed] [Google Scholar]
- 17. Pridgen BC, Woon CYL, Kim M, et al. . Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng Part C Methods. 2011;17(8):819–828. 10.1089/ten.tec.2010.0457 [DOI] [PubMed] [Google Scholar]
- 18. Kryger GS, Chong AKS, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am. 2007;32(5):597–605. 10.1016/j.jhsa.2007.02.018 [DOI] [PubMed] [Google Scholar]
- 19. Zhang AY, Bates SJ, Morrow E, Pham H, Pham B, Chang J. Tissue-engineered intrasynovial tendons: optimization of acellularization and seeding. J Rehabil Res Dev. 2009;46(4):489–498. 10.1682/jrrd.2008.07.0086 [DOI] [PubMed] [Google Scholar]
- 20. Petri M, Kruppa C, Haasper C, et al. . Effects of continuous perfusion on human bone marrow stromal cells seeded on a decellularized bovine Achilles tendon matrix. Technol Health Care. 2011;19(4):223–231. 10.3233/THC-2011-0621 [DOI] [PubMed] [Google Scholar]
- 21. Durgam SS, Stewart AA, Pondenis HC, Gutierrez-Nibeyro SM, Evans RB, Stewart MC. Comparison of equine tendon- and bone marrow-derived cells cultured on tendon matrix with or without insulin-like growth factor-I supplementation. Am J Vet Res. 2012;73(1):153–161. 10.2460/ajvr.73.1.153 [DOI] [PubMed] [Google Scholar]
- 22. Mifune Y, Matsumoto T, Takayama K, et al. . Tendon graft revitalization using adult anterior cruciate ligament (ACL)-derived CD34+ cell sheets for ACL reconstruction. Biomaterials. 2013;34(22):5476–5487. 10.1016/j.biomaterials.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 23. Mifune Y, Matsumoto T, Ota S, et al. . Therapeutic potential of anterior cruciate ligament-derived stem cells for anterior cruciate ligament reconstruction. Cell Transplant. 2012;21(8):1651–1665. 10.3727/096368912X647234 [DOI] [PubMed] [Google Scholar]
- 24. Lee JK, Jo S, Lee YL, et al. . Anterior cruciate ligament remnant cells have different potentials for cell differentiation based on their location. Sci Rep. 2020;10(1):3097. 10.1038/s41598-020-60047-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JK, Jo S, Lee YL, et al. . Effect of muscle cell preservation on viability and differentiation of hamstring tendon graft in vitro. Cells. 2021;10(4):740. 10.3390/cells10040740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation☆. Methods. 2008;45(2):115–120. 10.1016/j.ymeth.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grässel S, Stöckl S, Jenei-Lanzl Z. Isolation, culture, and osteogenic/chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Methods Mol Biol. 2012;879:203–267. 10.1007/978-1-61779-815-3_14 [DOI] [PubMed] [Google Scholar]
- 28. Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5(7):1294–1311. 10.1038/nprot.2010.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vangsness CT, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2008;31(3):474–481. 10.1177/03635465030310032701 [DOI] [PubMed] [Google Scholar]
- 30. Yu YN, Ding C, Cai ZN, Chen XR. Cell cycle effects on the basal and DNA-damaging-agent-stimulated ADPRT activity in cultured mammalian cells. Mutation Research Letters. 2008;174(3):233–239. 10.1016/0165-7992(86)90157-0 [DOI] [PubMed] [Google Scholar]
- 31. Jo S, Wang SE, Lee YL, et al. . IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther. 2018;20(1):115. 10.1186/s13075-018-1582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jo S, Nam B, Lee YL, et al. . The TNF-NF-κB-DKK1 axis promoted bone formation in the enthesis of ankylosing spondylitis. J Rheum Dis. 2021;28(4):216–224. 10.4078/jrd.2021.28.4.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am J Sports Med. 2000;28(4):499–505. 10.1177/03635465000280040901 [DOI] [PubMed] [Google Scholar]
- 34. Omae H, Sun YL, An KN, Amadio PC, Zhao C. Engineered tendon with decellularized xenotendon slices and bone marrow stromal cells: an in vivo animal study. J Tissue Eng Regen Med. 2012;6(3):238–244. 10.1002/term.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ingram JH, Korossis S, Howling G, Fisher J, Ingham E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007;13(7):1561–1572. 10.1089/ten.2006.0362 [DOI] [PubMed] [Google Scholar]
- 36. Chong AKS, Riboh J, Smith RL, Lindsey DP, Pham HM, Chang J. Flexor tendon tissue engineering: acellularized and reseeded tendon constructs. Plast Reconstr Surg. 2009;123(6):1759–1766. 10.1097/PRS.0b013e3181a65ae7 [DOI] [PubMed] [Google Scholar]
- 37. Zhang B, Luo Q, Halim A, Ju Y, Morita Y, Song G. Directed differentiation and paracrine mechanisms of mesenchymal stem cells: potential implications for tendon repair and regeneration. Curr Stem Cell Res Ther. 2017;12(6):447–454. 10.2174/1574888X12666170502102423 [DOI] [PubMed] [Google Scholar]
- 38. Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22(5):998–1003. 10.1016/j.orthres.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 39. Lee SY, Kwon B, Lee K, Son YH, Chung SG. Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 2017;45(6):1429–1439. 10.1177/0363546517689874 [DOI] [PubMed] [Google Scholar]
- 40. Norelli JB, Plaza DP, Stal DN, Varghese AM, Liang H, Grande DA. Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J Tissue Eng. 2018;9:2041731418811183. 10.1177/2041731418811183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Birch HL. Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol. 2007;88(4):241–248. 10.1111/j.1365-2613.2007.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tischer T, Vogt S, Aryee S, et al. . Tissue engineering of the anterior cruciate ligament: a new method using acellularized tendon allografts and autologous fibroblasts. Arch Orthop Trauma Surg. 2007;127(9):735–741. 10.1007/s00402-007-0320-0 [DOI] [PubMed] [Google Scholar]
- 43. Petrigliano FA, McAllister DR, Wu BM. Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy. 2006;22(4):441–451. 10.1016/j.arthro.2006.01.017 [DOI] [PubMed] [Google Scholar]
- 44. Silva M, Ferreira FN, Alves NM, Paiva MC. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J Nanobiotechnology. 2020;18(1):23. 10.1186/s12951-019-0556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martinello T, Bronzini I, Volpin A, et al. . Successful recellularization of human tendon scaffolds using adipose-derived mesenchymal stem cells and collagen gel. J Tissue Eng Regen Med. 2014;8(8):612–619. 10.1002/term.1557 [DOI] [PubMed] [Google Scholar]
- 46. Bakirci E, Tschan K, May RD, Ahmad SS, Kleer B, Gantenbein B. The importance of plasmin for the healing of the anterior cruciate ligament. Bone Joint Res. 2020;9(9):543–553. 10.1302/2046-3758.99.BJR-2020-0048.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hexter AT, Hing KA, Haddad FS, Blunn G. Decellularized porcine xenograft for anterior cruciate ligament reconstruction: A histological study in sheep comparing cross-pin and cortical suspensory femoral fixation. Bone Joint Res. 2020;9(6):293–301. 10.1302/2046-3758.96.BJR-2020-0030.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu CC, Chou SH, Shen PC, Chou PH, Ho ML, Tien YC. Extracorporeal shock wave promotes activation of anterior cruciate ligament remnant cells and their paracrine regulation of bone marrow stromal cells’ proliferation, migration, collagen synthesis, and differentiation. Bone Joint Res. 2020;9(8):458–468. 10.1302/2046-3758.98.BJR-2019-0365.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schulze-Tanzil G, Al-Sadi O, Ertel W, Lohan A. Decellularized tendon extracellular matrix-a valuable approach for tendon reconstruction? Cells. 2012;1(4):1010–1028. 10.3390/cells1041010 [DOI] [PMC free article] [PubMed] [Google Scholar]