Abstract
Aims
There is a lack of biomaterial-based carriers for the local delivery of rifampicin (RIF), one of the cornerstone second defence antibiotics for bone infections. RIF is also known for causing rapid development of antibiotic resistance when given as monotherapy. This in vitro study evaluated a clinically used biphasic calcium sulphate/hydroxyapatite (CaS/HA) biomaterial as a carrier for dual delivery of RIF with vancomycin (VAN) or gentamicin (GEN).
Methods
The CaS/HA composites containing RIF/GEN/VAN, either alone or in combination, were first prepared and their injectability, setting time, and antibiotic elution profiles were assessed. Using a continuous disk diffusion assay, the antibacterial behaviour of the material was tested on both planktonic and biofilm-embedded forms of standard and clinical strains of Staphylococcus aureus for 28 days. Development of bacterial resistance to RIF was determined by exposing the biofilm-embedded bacteria continuously to released fractions of antibiotics from CaS/HA-antibiotic composites.
Results
Following the addition of RIF to CaS/HA-VAN/GEN, adequate injectability and setting of the CaS/HA composites were noted. Sustained release of RIF above the minimum inhibitory concentrations of S. aureus was observed until study endpoint (day 35). Only combinations of CaS/HA-VAN/GEN + RIF exhibited antibacterial and antibiofilm effects yielding no viable bacteria at study endpoint. The S. aureus strains developed resistance to RIF when biofilms were subjected to CaS/HA-RIF alone but not with CaS/HA-VAN/GEN + RIF.
Conclusion
Our in vitro results indicate that biphasic CaS/HA loaded with VAN or GEN could be used as a carrier for RIF for local delivery in clinically demanding bone infections.
Cite this article: Bone Joint Res 2022;11(11):787–802.
Keywords: Antimicrobial resistance, Biofilm, Biomaterial, Bone infections, Calcium sulphate, Hydroxyapatite, Rifampicin, gentamicin, rifampicin, vancomycin, antibiotics, biofilms, composites, biomaterials, strains, hydroxyapatite
Article focus
We evaluated a clinically used, biphasic bioresorbable calcium sulphate/hydroxyapatite (CaS/HA) biomaterial containing vancomycin (VAN)/gentamicin (GEN) as a carrier for the local delivery of rifampicin (RIF).
In vitro experiments focused on the antibiotic release profile, antibacterial and antibiofilm characteristics, and development of RIF resistance.
Key messages
CaS/HA biomaterial containing VAN or GEN could be used as a carrier for RIF.
A sustained release of RIF above the minimum inhibitory concentration of Staphylococcus aureus was observed until day 35.
CaS/HA-VAN/GEN combined with RIF showed excellent antibacterial and antibiofilm effects, and did not cause resistance development.
Strengths and limitations
CaS/HA biomaterial used in the present study is a clinically proven and approved material, which potentially makes it easy for future clinical studies and clinical translation of CaS/HA as a carrier of RIF.
Dual antibiotic delivery using CaS/HA biomaterial provides extended antibiotic release and improved bacterial eradication without development of RIF resistance.
The in vitro findings need to be validated in more mature biofilms and relevant animal models. However, being a proof-of-concept study, we believe our findings are valuable and aid the ongoing in vivo experiments.
Introduction
Bone infections are some of the most serious complications in orthopaedic surgery. In the poorly vascularized infected bone tissue, achieving an effective and sustained local concentration of systemically administered antibiotics is a challenge. 1,2 The current treatment methods involving long-term systemic antibiotics, following local debridement and filling of the remaining dead space, may also lead to unwanted toxicities and selection of antibiotic-resistant bacteria. In addition, multiple bacterial species associated with bone infections use biofilms as a defence mechanism, which further limits conventional treatment efficacy. 2,3
To achieve high local antibiotic levels, exceeding minimum inhibitory concentration (MIC) or minimum biofilm eradication concentration (MBEC) in bone infections, locally implanted polymethylmethacrylate (PMMA) containing antibiotics have been used clinically for the last few decades. However, the mechanism of antibiotic release from PMMA is a passive surface diffusion phenomenon. Hence, the material as a carrier does not provide sustained and optimal antibiotic release. 4 Furthermore, PMMA lacks bone-regenerating properties and is non-biodegradable. There are recently clinically approved biphasic ceramic carriers that have been shown to provide high local release of gentamicin (GEN) or vancomycin (VAN) reporting good outcomes in debrided bone infections. 5-7 However, no osteoconductive biomaterial currently contains rifampicin (RIF), considered to be a cornerstone second defence antibiotic in the treatment of severe longstanding bone infections. 8,9 Since PMMA combined with RIF results in incomplete setting and altered mechanical properties, PMMA is not suitable as a carrier for local delivery of RIF. 10,11
Commercially available, biphasic, bioresorbable calcium sulphate/hydroxyapatite (CaS/HA) bone void filler, composed of 60 wt% α-CaS hemihydrate and 40 wt% HA, has the ability to deliver biologically active molecules, hormones, and anti-cancer drugs. 12-15 Additionally, various clinical studies have reported its potential as a carrier for antibiotics such as GEN and VAN for the treatment of bone infections, with fewer side effects as compared to the commonly used systemic treatment mode. 5,16 Previously, Sebastian et al 17 showed that CaS/HA is a potential carrier for local delivery of RIF and reported that a maximum of 300 mg RIF was possible to mix with 10 ml CaS/HA composite, eluting concentrations several times higher than the MIC of most common pathogens of bone infections. 18 However, RIF monotherapy is not recommended due to rapid development of bacterial resistance. 19
In order to overcome the limitations stated above, we addressed the following research question in the present study: can a biphasic CaS/HA biomaterial be used as a carrier for combined local delivery of a HA-binding antibiotic (RIF) and CaS-mediated passively diffusing antibiotics (VAN/GEN) to exert an extended antibacterial effect?
Composites containing RIF/GEN/VAN, either alone or in combination, were first prepared and physiochemically characterized. To test the feasibility of using the biomaterial carrier delivering the antibiotic combinations in a clinical setting, important material characteristics such as setting time, material degradation, and the in vitro antibiotic elution profile were studied in detail. Finally, the antibacterial effect was tested on both planktonic bacteria and biofilm-embedded bacteria aged 48 hours.
Methods
Materials used
Medical-grade RIF (Rifampicin Ebb, 600 mg; Sanofi, Italy) was purchased from the local pharmacy (Apoteket AB, Sweden). CaS/HA composites containing GEN (Cerament G) or VAN (Cerament V) were purchased from Bonesupport AB (Sweden).
Antibiotic loaded CaS/HA composite synthesis
The following combinations of CaS/HA and antibiotics were prepared — CaS/HA-VAN, CaS/HA-VAN + RIF, CaS/HA-GEN, CaS/HA-GEN + RIF, and CaS/HA-RIF. For the CaS/HA-VAN and CaS/HA-VAN + RIF composites, 500 mg of CaS/HA powder (Cerament V; Bonesupport AB) was hand-mixed in a 48-well plate with either only VAN (24.57 mg) or in combination with RIF (8.11 mg) dissolved in 215 μl iodine-based mixing solution (Iohexol, C-TRU; Bonesupport AB) using a sterile wooden spatula for 30 seconds. The paste was transferred to a 1 ml syringe connected to an 18 G needle (Ø = 1.2 mm) and extruded into a mould with hemispherical wells (Ø = 4.8 mm) to obtain uniform-sized pellets for evaluating antibiotic release profile, material degradation, and continuous Kirby-Bauer disk diffusion assay. CaS/HA-GEN or CaS/HA-RIF composites were prepared by mixing 500 mg of CaS/HA powder (Cerament G; Bonesupport AB) and GEN (10.35 mg) or RIF (8.11 mg) dissolved in 283.5 μl of saline (Bonesupport AB). For the CaS/HA-GEN + RIF composites, preparation was the same but with the difference of intermittent mixing technique. Briefly, 500 mg of CaS/HA powder (Cerament G) was first hand-mixed with GEN (10.35 mg) dissolved in 200 μl mixing solution (Bonesupport AB) and 30 seconds later, RIF (8.11 mg) dissolved in 83.5 μl mixing solution (Bonesupport AB) was added and mixed again. This intermittent mixing technique was adopted to prevent the rapid setting of the composite when GEN and RIF were mixed simultaneously. The details of the material and their concentrations used for pellet preparation are given in Supplementary Table i.
Scanning electron microscopy analysis
To compare the structure and surface of the CaS/HA-VAN/GEN with or without RIF, hemispherical discs of the composites were sputter-coated and analyzed using a scanning electron microscope (SEM) (JSM-7800F; JEOL, Sweden) at an operating voltage of 10 kV.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) was used to verify the presence of RIF in CaS/HA-VAN/GEN composites. A fine powder of the composites was made, after which they were loaded on a Bruker Alpha FT-IR spectrometer (Bruker Optics, Germany). The % transmittance values were plotted against wavelength to identify vibrations from RIF.
Injectability and setting time
CaS/HA composites were prepared as mentioned previously. The injectability of the composite was then evaluated by extruding 250 μl of the paste manually through a graduated 1 ml syringe connected to an 18 G needle into a mould with hemispherical wells (Ø = 4.8 mm). The total weight of the syringe with paste before and after extrusion was measured. Each test was repeated three times at various timepoints (GEN: four, six, and eight minutes; VAN and RIF: three, five, and seven minutes). To confirm the setting of the tested composites, after 20 minutes of extrusion the consistency of the pellets was evaluated manually by pressing the pellets with a sterile spatula, and also the pellets were allowed to fall from a height of 50 cm to check whether they crumbled or not.
Antibiotic release profile and material degradation
For each combination, pellets in triplets were placed in 2 ml microcentrifuge tubes containing 1 ml of sterile phosphate-buffered saline (PBS) and placed at 37°C. At different timepoints (day 1, 3, 7, 14, 21, 28, and 35), PBS in the test tube was collected and tubes were supplemented with fresh 1 ml PBS. The amount of VAN and RIF released from the pellets was quantified using the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (Thermo Fisher Scientific, USA), and GEN quantification was performed by cloned enzyme donor immunoassay (CEDIA) on Roche Cobas, C501 (Roche Diagnostics, Switzerland). The degradation profile of the preweighed pellets at each timepoint (day 1, 3, 7, 14, 21, 28, and 35) was analyzed by the protocol described by Liu et al. 15
Cell viability analysis via MTT assay
The effect of released antibiotic fractions in the cell viability of MC3T3-e1 cells was tested via MTT assay.
Testing of efficacy on planktonic bacteria by continuous Kirby-Bauer disk diffusion assay
The antimicrobial efficacy of composites on planktonic bacteria was tested by continuous Kirby-Bauer disk diffusion assay using Staphylococcus aureus standard strain obtained from American Type Culture Collection (S. aureus ATCC 25923), and S. aureus clinical strain P3, isolated from a case of periprosthetic joint infection (PJI) at our hospital. For tested combinations of CaS/HA-antibiotics, pellets in triplets were used for each strain of S. aureus. For GEN and RIF pellets, using Clinical and Laboratory Standards Institute (CLSI) guidelines, zone of inhibitions (ZOIs) of ≥ 15 and ≥ 20 mm, respectively, were taken as strong antibacterial effects, and ZOIs of less than these were represented as moderate antibacterial effects. 20 For VAN, ZOI of ≥ 17 mm was used as a surrogate for strong antibacterial effect. 20
Antibacterial effect of pellets after day-35
To mimic the in vivo conditions, after day 35 the antibacterial effect of the pellets that had been used for antibiotic release assay was tested by continuous Kirby-Bauer disk diffusion assay.
Efficacy on preformed biofilms
Using a modified biofilm quantification method, 21 the antibiotics released at different timepoints (day 1, 3, 7, 14, 21, 28, and 35) from the pellets of CaS/HA-antibiotic composites were tested for their ability to disrupt the preformed biofilm. Briefly, S. aureus biofilms were allowed to form on the wells of 96-well flat-bottom tissue culture treated polystyrene microtitre plate (Costar, USA) coated with poly-L-lysine (0.2 mg/ml) (MilliporeSigma, Germany) for 48 hours with a change of media after 24 hours of incubation. Thereafter, 200 µl of tryptic soy broth supplemented with 1.0% glucose (TSBG) and TSBG-antibiotic fractions mixture (1:1) were added to the control and test wells, respectively, and incubated at 37°C for 24 hours. The wells were then stained with 1% (w/v) crystal violet solution (MilliporeSigma) and their optical density was measured at 590 nm using a microplate reader (iMark; Bio-Rad Laboratories, Japan). The absorbance from the test wells, which correlates to the amount of biofilm that remained after exposure to the antibiotic fractions, was compared with the absorbance of untreated control wells. The efficacy of the antibiotic fractions was tested on both S. aureus ATCC 25923 and S. aureus clinical strain P-3. For each tested group of antibiotic fractions, five separate wells were used (n = 5).
Viable bacteria after continuous treatment with antibiotic fractions, and development of resistance to VAN, GEN, and RIF
Viable bacteria after continuous treatment with antibiotic fractions, and the development of bacterial resistance to VAN, GEN, and RIF were determined by exposing the biofilm embedded bacteria continuously to released fractions of antibiotics from CaS/HA-VAN/GEN pellets with or without RIF from predetermined time points (day 1, 3, 7, 14, 21, 28, and 35) in 96-well microtitre plate. The development of resistance was tested on both S. aureus ATCC 25923 and S. aureus clinical strain P-3 whose MIC values against VAN, GEN, and RIF were predetermined using MALDI-TOF MS system (Vitek MSTM; bioMérieux, France). Strains showing resistance to RIF were further confirmed using MALDI-TOF MS system. For each tested group of antibiotic fractions, four separate wells were used (n = 4).
Statistical analysis
All data were presented as mean and standard deviation (SD). An independent-samples t-test was used to analyze injectability of CaS/HA-VAN/GEN with or without RIF, material degradation of the pellets, MTT assay, and to compare the differences in biofilm destruction. An independent-samples t-test or one-way analysis of variance (ANOVA) with Dunn’s multiple comparisons test was used to analyze the cumulative release profile of VAN, GEN, or RIF. For comparison of the difference in ZOI between different groups, ANOVA with Tukey’s multiple comparison test was used. A p-value of < 0.05 was considered statistically significant. Details of statistical analysis are indicated in each figure legend. All data processing was carried out on GraphPad Prism version 9.1.2 for MacOS (GraphPad Software, USA). For detailed information about the methods used in the study, please refer to the Supplementary Material.
Results
Preparation and characterization of antibiotic-loaded CaS/HA composites
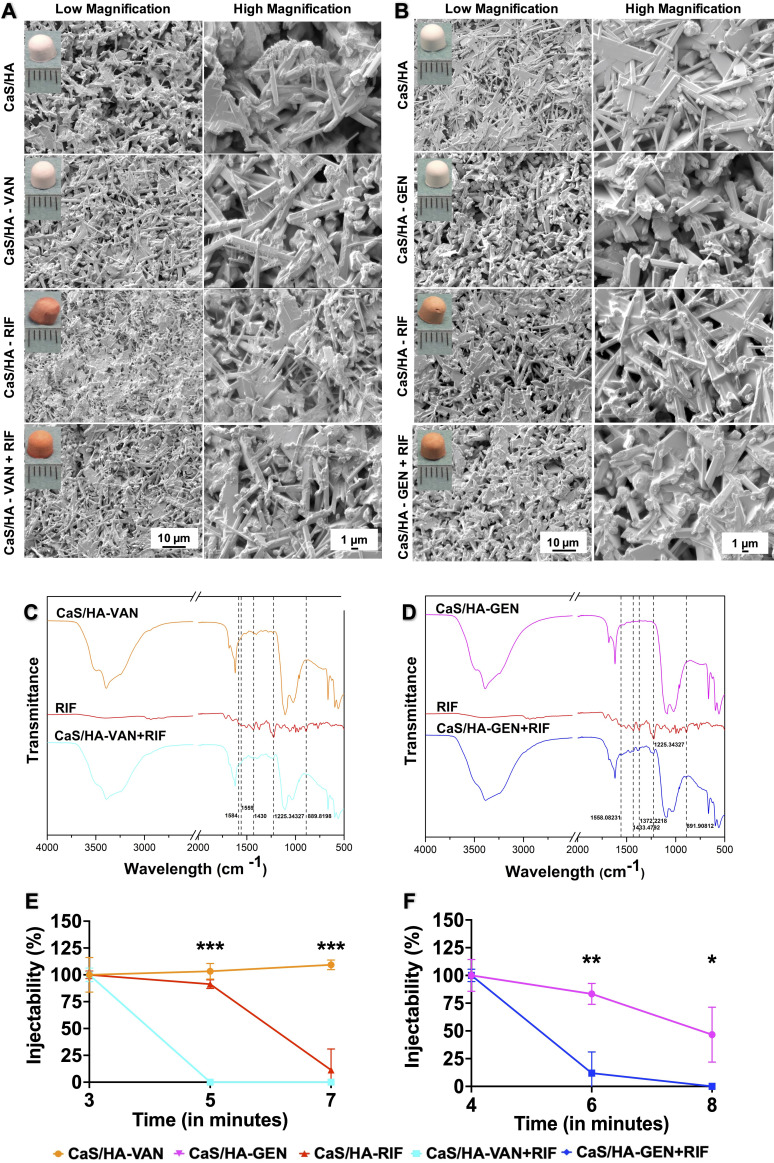
Pellets of all tested combinations of antibiotic-loaded CaS/HA composites were characterized regarding the surface morphology and physiochemical properties. CaS/HA pellets with either GEN or VAN were white in colour while combinations of RIF had dark orange colour (Figures 1a and 1b). SEM revealed that the addition of RIF to CaS/HA-VAN/GEN composites appeared to have no effect either on the surface morphology or the structure (Figures 1a and 1b). FTIR characterization further confirmed that the RIF was loaded onto CaS/HA-VAN/GEN composites. CaS/HA-VAN + RIF showed the same transmittance peaks at 1,584 cm-1, 1,559 cm-1, 1,430 cm-1, 1,225.3 cm-1, and 889 cm-1 as RIF, whereas CaS/HA-GEN + RIF showed the same transmittance peaks as those of RIF at 1,558 cm-1, 1,433.4 cm-1, 1,372.2 cm-1, 1,225.3 cm-1, and 891 cm-1, which could not be seen in only CaS/HA-VAN/GEN composites (Figures 1c and 1d).
Fig. 1.
Morphological and physiochemical characterization of calcium sulphate/hydroxyapatite-vancomycin/gentamicin (CaS/HA-VAN/GEN) with or without rifamipicin (RIF). a) and b) Scanning electron microscopy (SEM) images showing surface characteristics and pore distribution of CaS/HA-VAN/GEN pellets with or without RIF. Hemispherical pellets casted using an elastic mould of 4.8 mm diameter are shown in inset of a) and b). c) and d) Fourier transform infrared spectroscopy (FTIR) spectra from: c) CaS/HA-VAN, pure RIF, or CaS/HA-VAN + RIF; and d) CaS/HA-GEN, pure RIF, or CaS/HA-GEN + RIF. e) and f) Injectability of tested CaS/HA-VAN/GEN composites with or without RIF. An independent-samples t-test was used to compare the injectability of CaS/HA having VAN/GEN alone or in combination with RIF. *p < 0.05, **p < 0.01, ***p < 0.001.
Injectability and setting time
CaS/HA-VAN composites could be completely extruded from the syringes at all three tested timepoints (three, five, and seven minutes) whereas addition of RIF to CaS/HA-VAN significantly reduced the injectability, and by five minutes the composite became uninjectable (Figure 1e). The CaS/HA-GEN composites could be completely extruded from the syringes at four minutes without blocking the syringe outlet or causing obvious pressure filtration effects, and by eight minutes it dropped to almost 47% (Figure 1f). By dropping the injectability to almost 12% by six minutes, a significant injectability reduction trend was observed with the addition of RIF to CaS/HA-GEN as well. At both three and five minutes, CaS/HA-RIF composites maintained the injectability and by seven minutes it reduced to almost 11%. All the CaS/HA-antibiotic composites were completely set within 15 minutes of injection, and they subjectively seemed durable and did not crumble while handled.
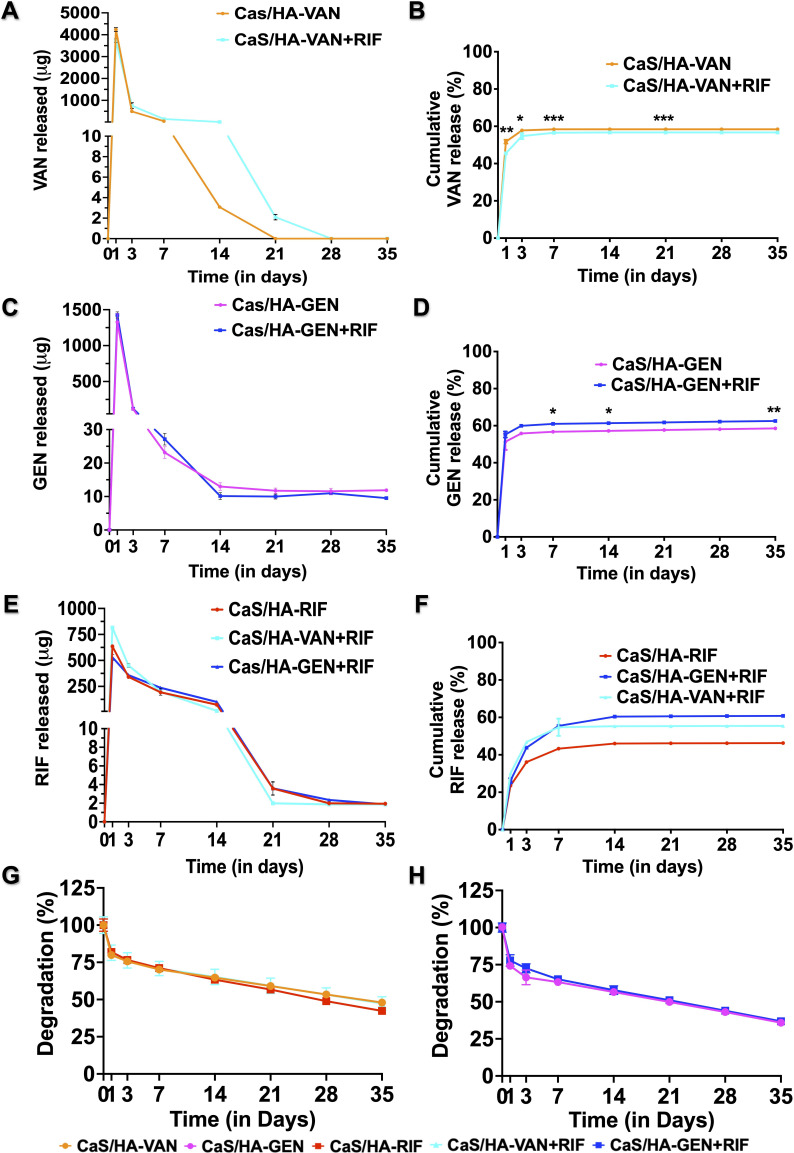
Antibiotic release profile and material degradation
In the tested CaS/HA pellets of both VAN or a combination of VAN + RIF, at days 1, 3, 7, and 21 there was a significant difference in the amount of VAN released per day. The overall cumulative release profile of VAN remained almost unchanged (58% and 57%, respectively) (Figures 2a and 2b). A similar cumulative release trend was observed with GEN as well (CaS/HA-GEN, 59% and CaS/HA-GEN + RIF, 63%) (Figures 2c and 2d). From CaS/HA-VAN and CaS/HA-VAN + RIF, VAN concentrations above the MIC levels of S. aureus were obtained until day 14 (3.08 µg/ml) and day 21 (2.1 µg/ml), respectively. Until study endpoint at day 35, CaS/HA-GEN and CaS/HA- GEN + RIF pellets eluted GEN of concentrations, well above the MIC levels of S. aureus; 11.8 µg/ml and 9.5 µg/ml, respectively. For RIF, an increased cumulative release profile was obtained when it was combined with CaS/HA-VAN (56%) or GEN (61%), compared to CaS/HA-RIF alone (46%) (Figures 2e and 2f). On day 35, concentrations of RIF from CaS/HA-RIF (1.94 µg/ml), CaS/HA-VAN + RIF (1.87 µg/ml), and CaS/HA-GEN + RIF (1.87 µg/ml) were all found to be above the MIC levels of S. aureus.
Fig. 2.
In vitro antibiotic release profile and material degradation. a) to f) Release per day and cumulative release profile of vancomycin (VAN), gentimicin (GEN), and rifampicin (RIF) from calcium sulphate/hydroxyapatite (CaS/HA)-VAN/GEN with or without RIF at days 1, 3, 7, 14, 21, 28, and 35. g) and h) In vitro material degradation profile of tested CaS/HA-antibiotic composites in phosphate-buffered saline. An independent-samples t-test was used to compare the in vitro material degradation profile and cumulative release profile CaS/HA-VAN/GEN alone or in combination with RIF. For comparison of cumulative release profile of RIF, one-way analysis of variance with Dunn’s multiple comparison test was used. *p < 0.05, **p < 0.01, ***p < 0.001.
During the first week, almost 30% of CaS/HA-VAN, CaS/HA-VAN + RIF, and CaS/HA-RIF pellets degraded, whereas it was approximately 36% for CaS/HA-GEN and CaS/HA-GEN + RIF pellets (Figures 2g and 2h). Until day 35, no statistically significant difference in the material degradation was observed for any of the CaS/HA-antibiotic combinations.
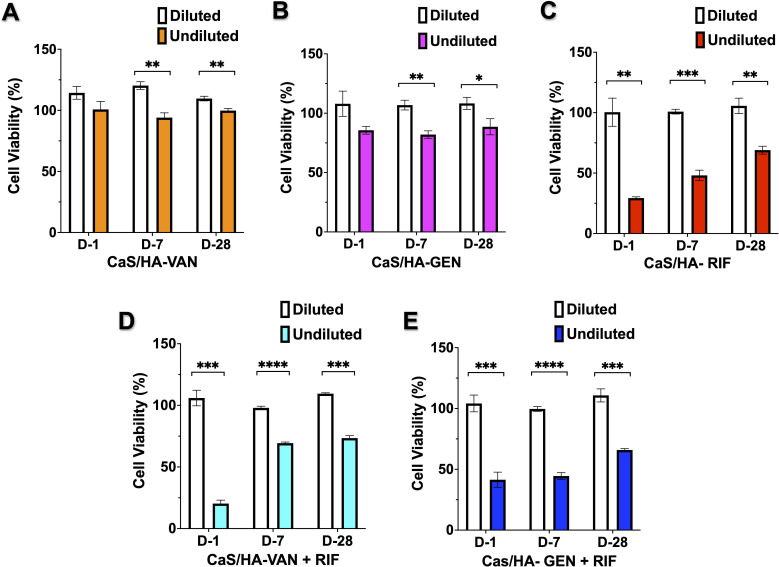
Cell viability via MTT assay
MTT assay of both CaS/HA-VAN/GEN undiluted fractions from all tested timepoints exhibited no significant toxic effects on the MC3T3-e1 cell lines (Figures 3a and 3b). Undiluted fractions from CaS/HA-VAN/GEN + RIF or CaS/HA-RIF did not achieve ≥ 80% cell viability (Figures 3c to 3e). However, with 100% cell viability, all diluted fractions of CaS/HA-antibiotic combinations showed a significant difference in the cell viability with that of undiluted fractions.
Fig. 3.
Cell viability via MTT assay. a) to e) Evaluation of in vitro cytotoxicity on MC3T3-E1 cell lines by MTT assay of both diluted and undiluted antibiotic fractions from day 1, 7, and 28. An independent-samples t-test was used to compare the difference in cytotoxicity between diluted and undiluted antibiotic fractions. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. CaS/HA, calcium sulphate/hydroxyapatite; GEN, gentamicin; RIF, rifampicin; VAN, vancomycin.
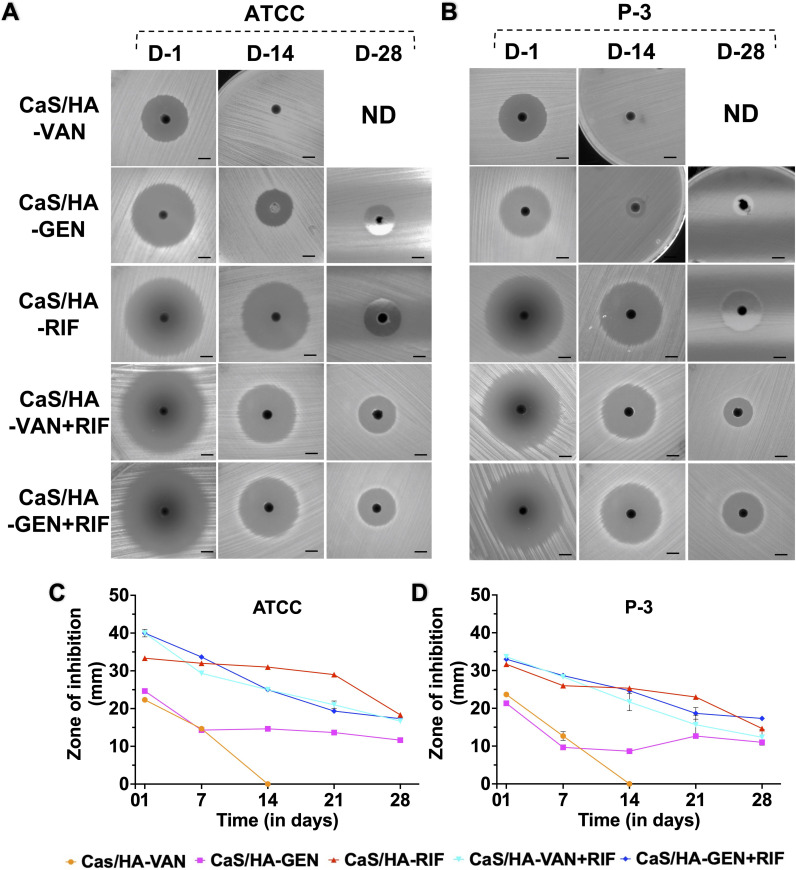
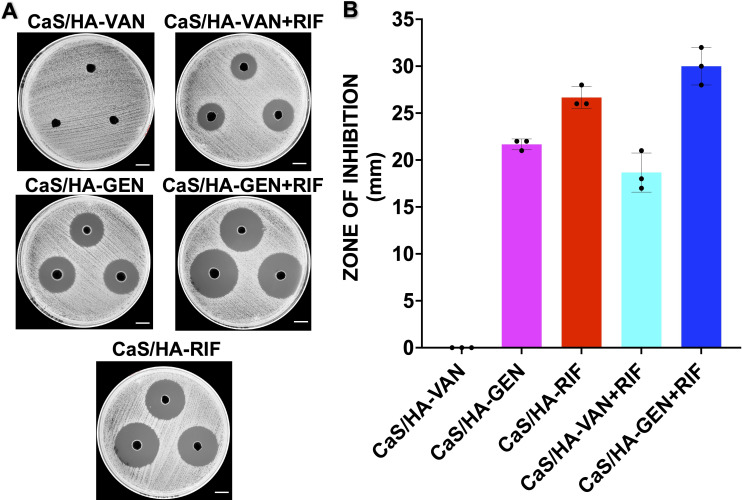
Efficacy on planktonic bacteria by continuous Kirby-Bauer disk diffusion assay
There was a general trend that ZOI of all different composite pellets decreased over time (Figures 4a to 4d; Supplementary Figure a). Out of all the tested CaS/HA pellets, antibacterial effect of CaS/HA-VAN against both S. aureus strains declined sharply and showed no effect by day 12 (Supplementary Figure a). CaS/HA-GEN pellets exhibited a strong antibacterial effect until day 15 and day 3 against S. aureus ATCC 25923 and clinical strain P-3, respectively (Supplementary Figure a). Thereafter it maintained a low but almost steady antibacterial effect until day 28. Against S. aureus ATCC 25923, both CaS/HA-VAN/GEN + RIF combinations showed a strong antibacterial effect until day 20 but in case of clinical strain P-3, it was day 16 for CaS/HA-VAN + RIF and day 20 for CaS/HA-GEN + RIF. Compared to pellets of all combinations, the antibacterial effect persisted for a longer time for CaS/HA-RIF pellets against both S. aureus ATCC 25923 (until day 26) and clinical strain P-3 (until day 25) (Supplementary Figure a). Overall, against both tested bacterial strains, CaS/HA-VAN/GEN + RIF combinations were found to be significantly more effective than CaS/HA-VAN/GEN alone composites (Supplementary Tables ii and iii).
Fig. 4.
Testing of antibacterial efficacy of calcium sulphate/hydroxyapatite (CaS/HA)-antibiotic pellets on planktonic bacteria by continuous Kirby-Bauer disk diffusion assay. a) and b) Mueller–Hinton agar plates inoculated with Staphylococcus aureus ATCC 25923 and clinical strain P3 showing zone of inhibition (ZOI) obtained on days 1, 14, and 28 (scale bar = 1.6 cm). c) and d) Graph data showing the ZOI (in mm) of CaS/HA-antibiotic pellets against c) S. aureus ATCC 25923 and d) S. aureus clinical strain P3. For comparison of difference in ZOI between different groups, one-way analysis of variance with Tukey’s multiple comparison test was used. Against both S. aureus ATCC 25923 and clinical strain P3, CaS/HA-vancomycin (VAN)/ gentamicin (GEN) + rifampicin (RIF) combinations were found to be significantly more effective than CaS/HA-VAN/GEN alone composites. Detailed statistical findings are provided in Supplementary Tables ii and iii. ND, not done.
Antibacterial effect of pellets after day 35
Except for CaS/HA-VAN, all other tested CaS/HA-antibiotics groups showed strong antibacterial effects at day 35 (Figures 5a and 5b). By exhibiting larger ZOI of all groups, CaS/HA-GEN + RIF appears to have the strongest antibacterial effects.
Fig. 5.
Antibacterial effect of pellets after day 35. a) Mueller-Hinton agar plates inoculated with Staphylococcus aureus ATCC 25923 showing zone of inhibition (scale bar = 2.6 cm). b) Graph data showing the antibacterial effect of the pellets placed in phosphate-buffered saline for 35 days. CaS/HA, calcium sulphate/hydroxyapatite; GEN, gentamicin; RIF, rifampicin; VAN, vancomycin.
Efficacy on preformed biofilms
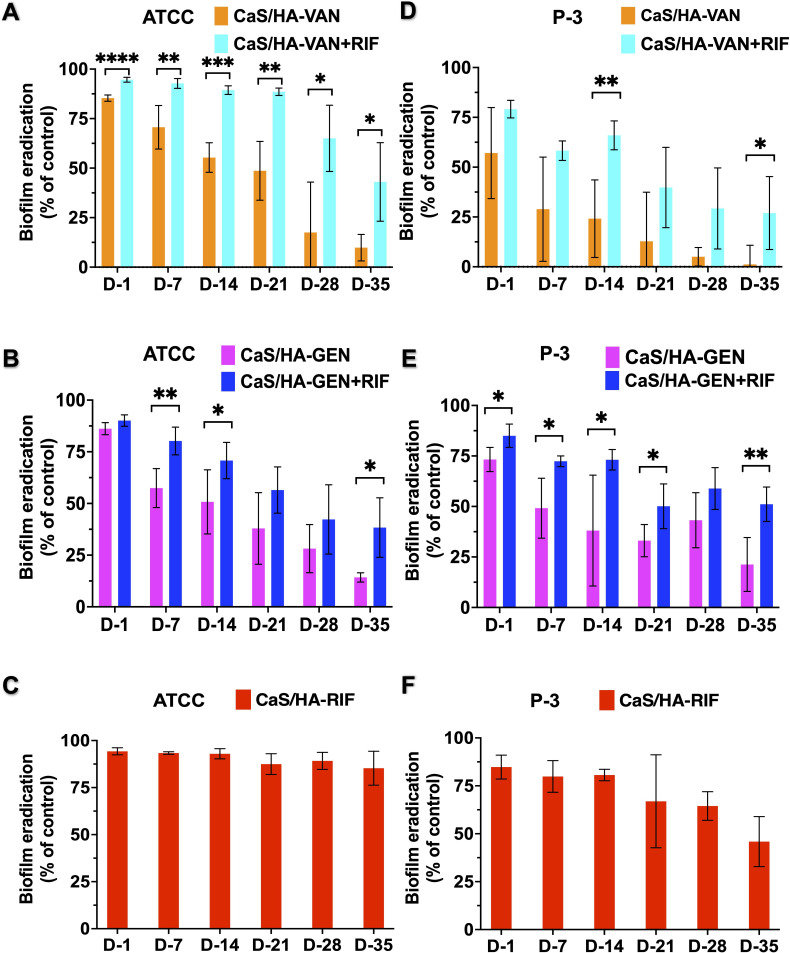
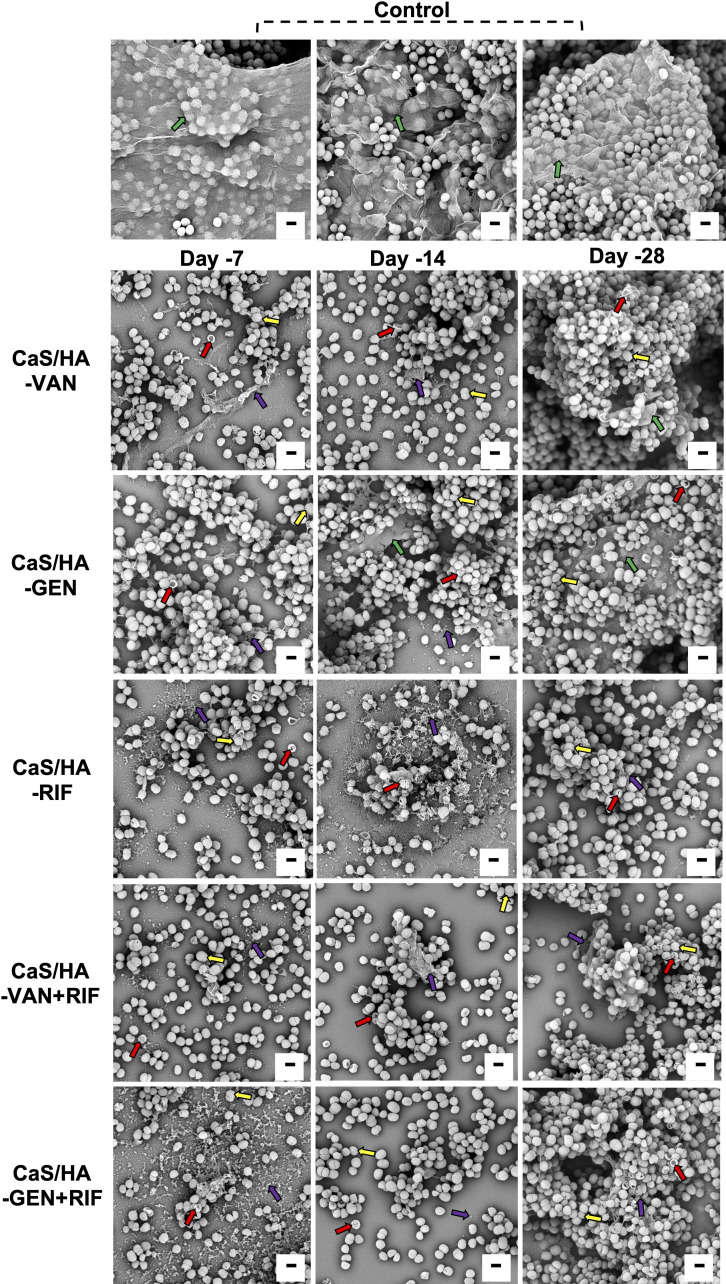
Overall, there was an excellent biofilm destruction trend observed with CaS/HA-VAN/GEN + RIF combinations compared with CaS/HA-VAN/GEN alone (Figures 6a to 6f). In comparison to CaS/HA-VAN, all tested fractions from CaS/HA-VAN + RIF showed significant destruction of S. aureus ATCC biofilms, but against clinical strain P-3 biofilms only day 14 and day 35 achieved significant difference. Almost all tested fractions of CaS/HA-GEN with RIF showed significant biofilm destruction against clinical strain P-3 biofilms. Compared to CaS/HA-VAN/GEN alone, CaS/HA-RIF fractions exhibited strong antibiofilm properties against both S. aureus ATCC and P-3 biofilms (Figure 6). SEM analysis of the preformed biofilm destruction also showed a comparable trend with that of crystal violet staining results (Figure 7).
Fig. 6.
Efficacy of antibiotic fractions from days 1, 7, 14, 21, 28, and 35 on preformed biofilms. a) to f) Graphical representation of the Staphylococcus aureus ATCC 25923 and clinical strain P3 biofilm destruction obtained by crystal violet staining. An independent-samples t-test was used to compare the differences in biofilm destruction between calcium sulphate hydroxyapatite (CaS/HA)-vancomycin (VAN)/gentamicin (GEN) with or without RIF. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. RIF, rifampicin.
Fig. 7.
Scanning electron microscopy images of Staphylococcus aureus ATCC 25923 biofilm destruction. For all tested CaS/HA-antibiotic groups, biofilm destruction and antibacterial effects were evident on day 7 and day 14. At day 28, number of planktonic bacteria were increased and biofilm were visible. Green arrow indicates biofilm while violet arrow indicates destructed biofilm. Red arrow indicates dead bacteria. Yellow arrow indicates membrane disruption and extravasation of cytoplasmic components of bacteria. Scale bar = 1 μm. CaS/HA, calcium sulphate/hydroxyapatite; GEN, gentamicin; RIF, rifampicin; VAN, vancomycin.
Testing for development of bacterial resistance to VAN, GEN, and RIF
Following continuous treatment of S. aureus ATCC 25923 and clinical strain P-3 biofilms with CaS/HA-VAN/GEN/RIF alone antibiotic fractions, the number of viable colony-forming units (CFUs) obtained on antibiotic-free TSA ranged from 0 to 6.5 × 107 CFU and 1.4 to 3.1 × 108 CFU, respectively (Table I). However with complete destruction of biofilm-embedded bacteria, no viable CFUs were obtained for CaS/HA-VAN/GEN + RIF combinations, which were clearly more potent in biofilm eradication than CaS/HA-VAN/GEN/RIF alone groups.
Table I.
Viable colony-forming units after continuous treatment with antibiotic fractions, and development of rifampicin-resistant CFUs.
| Antibiotic fractions from CaS/HA-antibiotics pellets | Bacteria tested, and total CFUs before treatment | Mean remaining viable CFUs (range) | Mean rifampicin-resistant CFUs (range) |
|---|---|---|---|
| CaS/HA-VAN CaS/HA-GEN CaS/HA-RIF CaS/HA-VAN+RIF CaS/HA-GEN+RIF |
S. aureus
ATCC 25923, 6.5 × 107 |
5.9 × 107 (5.6 × 107 to 6.5 × 107) 2.4 × 107 (1.4 × 107 to 3.7 × 107) 1.1 × 107 (0 to 4.5 × 107) 0 0 |
0 0 1.8 × 105 (0 to 7.5 × 105) 0 0 |
| CaS/HA-VAN CaS/HA-GEN CaS/HA-RIF CaS/HA-VAN+RIF CaS/HA-GEN+RIF |
S. aureus
clinical strain P-3, 2.8 × 108 |
1.73 × 108 (1.4 × 108 to 1.92 × 108) 2.54 × 108 (2.2 × 108 to 3.1 × 108) 1.05 × 108 (2.5 × 102 to 2.8 × 108) 0 0 |
0 0 9.2 × 107 (0 to 1.9 × 108) 0 0 |
CaS/HA, calcium sulphate/hydroxyapatite; CFU, colony-forming unit; GEN, gentamicin; RIF, rifampicin; VAN, vancomycin.
S. aureus ATCC and clinical strain P-3 biofilms that had been subjected to CaS/HA-RIF alone developed resistance to RIF, and were grown on plates with 32 mg/l of RIF indicating high level of resistance. This was further confirmed using MALDI-TOF. However, none of the tested biofilms exposed to RIF in combination with VAN/GEN developed resistance to RIF. Also, no resistance to VAN or GEN was noted for any of the CaS/HA-antibiotic combinations. Bacteria in control wells were sensitive to all tested antibiotics.
Discussion
Herein, we show that RIF could be combined with, and delivered locally via, the clinically approved biphasic ceramic carrier CaS/HA containing GEN or VAN. There are a few published studies that have reported carriers capable of RIF delivery for the treatment of bone infections. 18,22-24 In an in vitro study, Ahola et al 23 reported the potential of a β-tri-calcium phosphate-based material to deliver RIF or ciprofloxacin, but no combination studies were performed. In two separate studies, Qayoom et al 18,25 reported on a biphasic ceramic material as a carrier for RIF. In their first in vitro study, via local delivery, RIF and isoniazid showed potential for the treatment of bone tuberculosis. In a subsequent paper, the carrier delivered RIF alone for in vivo osteomyelitis treatment without focusing on the risk of resistance development with RIF monotherapy. Recently, a silorane-based biomaterial made up of two silicon-containing oxiranes combined with SiO3 fillers and Lamoreaux’s catalyst initiators was able to deliver RIF locally but lacked the biodegradable properties of the CaS/HA material. 22 In comparison to the materials used in these studies, the CaS/HA material used in the present study is a clinically proven and approved material, which potentially makes it easy for future clinical studies and clinical translation of CaS/HA as a carrier of RIF. One of the main indications for local delivery with an antibiotic containing CaS/HA is cavity filling of eradicated osteomyelitis. There may be a potential risk of abrasive HA wear in component-retaining treatment of prosthetic joint infections.
Using our experimental setup, combinations of CaS/HA-GEN/VAN + RIF were injectable at the manufacturer’s recommended starting injection time of three or four minutes. However, compared to the CaS/HA-GEN/VAN composites, a significant reduction in injectability was noticed at later timepoints with the addition of RIF. With the use of a commercial mixing system, which is recommended by the manufacturer for mixing CaS/HA materials, we believe that it would be easier to mix and deliver these composites. However, during surgery clinicians should aim to start the injection immediately after mixing rather than waiting for the recommended injection time, which needs to be revised for the new combinations of antibiotics containing RIF. Although the possibility of increasing the volume of mixing solutions to be used for combinations of RIF was not in the scope of the present study, future studies are planned to explore this possibility as well.
Adding RIF substantially prolongs the setting time of PMMA to more than 30 minutes, preventing it from being used clinically as a RIF carrier. 10 To circumvent this, Sanz-Ruiz et al 26 used microencapsulation of RIF to introduce it in PMMA, while Cyphert et al 27 added RIF to insoluble cyclodextrin. However, none of these methods of RIF incorporation in PMMA are in clinical use. In the present study, all tested combinations of RIF showed a recommended setting time of 15 minutes when combined with either GEN or VAN containing CaS/HA material.
The elution patterns of all tested antibiotics from CaS/HA had an initial burst release, which is congruent with previous reports on CaS/HA-VAN or GEN composites. 28,29 Since the MBEC levels of many antibiotics are reported to be 1,000-fold higher than the MIC levels, high levels of antibiotics delivered by burst release are indeed necessary to penetrate the biofilms and achieve bactericidal activity. 30,31 In agreement with the previous reports, following the initial high concentration, both GEN and RIF, either alone or in combination, maintained a sustained low concentration, still well above the MIC levels of most common pathogens such as staphylococci. 18,29 Similarly, as reported by Stravinskas et al, 28 from the CaS/HA-VAN + RIF composites, VAN with concentrations above the MIC levels was eluted for three weeks but from CaS/HA-VAN it was only eluted for two weeks. This could be attributable to the differences in the experimental setup with previous in vitro and clinical studies. Although the mixing of RIF did not affect the cumulative release profile of both VAN and GEN, it significantly changed the release profile of RIF itself. Since both GEN and VAN have no accretion to particulate HA, their fast elution from the CaS-based material might have influenced the RIF as well. 28,29
Most of the current guidelines for the treatment of bone- and implant-associated infections recommend the use of longer duration of systemic treatment of RIF in combination, one to two weeks post-surgery. 32,33 However, its timing of introduction in treatment, length of use, dose, and efficacy, especially in acute Staphylococcus or Cutibacterium infections, is repeatedly debated. 34 A recent multicentre observational study reported the efficacy of RIF in acute staphylococcal PJIs treated with debridement, and found no association with the dose of RIF but its early start within the first five days after debridement was associated with treatment failure. 9 The explanation pointed to a possible drug-drug interaction when the bacteria are still in planktonic phase, but the authors fell short of any definitive conclusions. In contrast, Becker et al 35 reported no association on the timing of RIF introduction in treatment, but rather a minimum RIF treatment duration of 14 days or more is recommended. In a cohort of acute staphylococcal PJIs treated with debridement, implant retention, and a short course of RIF which the authors started intraoperatively, Scheper et al 36 reported good clinical outcomes without any resistance development to RIF. So the available literature is inconclusive on the immediate starting of RIF following surgery due to issues with development of resistance. Moreover, it is well known that long-term systemic or oral treatment of RIF is associated with systemic toxicities and often patients find it difficult to comply with the treatment regimen. In such cases, local application of RIF via a controlled delivery system could definitely circumvent such problems. Since there is no current recommendation on the duration of local antibiotic delivery, and recommended systemic treatment duration of bone infections is six to 12 weeks, we believe that the RIF release profile observed in the current study may be valid.
Due to the low dose of RIF added to the material, the possible systemic effects should be less than those of systemic administration of the same drug. We used a concentration (300 mg) that corresponds to half of one clinically used dose (600 mg). Based on the in vitro data, it would not be possible for us to comment on the potential development of resistance, particularly in the gut microbiome, but in an in vivo model Kim et al 37 reported no RIF resistance development following treatment with rifaximin, a drug belonging to the same class. We hope that future in vivo data will give us more detailed information on low level of RIF in systemic circulation, and how it may affect the patient’s normal bacterial flora.
Although local delivery via carriers results in high initial level of antibiotics in bone infections, it should not negatively affect either osteoblasts or their ability to induce bone formation. Previous studies using different antibiotic classes reported their toxic effects in high concentrations in vitro on osteoblast cells. 38-41 Isefuku et al 40 reported that RIF at concentrations of 3 μg/ml can decrease the proliferation of osteoblast-like cells in vitro. However, using a 3D-printed calcium phosphate scaffold containing RIF (~33 μg/scaffold) and sitafloxacin in a murine model of femoral implant-associated osteomyelitis, Trombetta et al 42 conversely reported increase in bone formation at three and ten weeks. 42 In an in vivo osteomyelitis rat model, Qayoom et al 25 implanted RIF 3 mg/animal locally with nano-HA, which corresponds to clinically used RIF dose, 600 mg. The locally applied nano-HA-RIF combination completely eradicated the infection and increased bone formation, and the defect was completely healed. In a recent study, single and multiple doses of RIF < 16 μg/ml had no impairment on osteoblast proliferation or metabolism, but results differed when RIF concentrations were ≥ 50 μg/ml. 43 Interestingly, when the same authors monitored the effect of multiple doses of RIF 8 μg/ml between S. aureus-infected osteoblasts and uninfected control groups during 21 days of osteogenesis, a lower amount of mineralized extracellular organic matrix was observed in S. aureus-infected osteoblasts on day 7 but this significantly increased on day 21. These mentioned reports indicate that even at minimal concentrations RIF is toxic to normal uninfected osteoblast cells, but in bone infections where bacteria such as S. aureus are hiding within the osteoblasts, minimal to high RIF concentrations seem to improve the osteoblasts’ viability in the long term. In the present study, in vitro RIF elution shows concentrations > 16 μg/ml for at least two weeks, which clearly had an effect on the osteoblasts. However, we would also like to highlight the fact that bone is a tightly organized matrix of collagen and HA with embedded osteocytes, osteoblasts, and osteoclasts, therefore perfusion of drugs within this tight matrix is somewhat difficult, which should potentially help in alleviating the cytotoxic effect of antibiotics on bone and its cells. Previously, our group has shown that the carrier CaS/HA also plays a role in reducing the toxic effects of the drugs which arise when they are administered alone in the targeted site, 15 but conclusions on the actual biocompatibility and osteogenic properties of CaS/HA-GEN/VAN + RIF combinations could only be drawn from an experimental in vivo study designed to answer those particular questions.
While testing the antimicrobial efficacy, we used entire antibiotic-loaded biomaterial pellets for disk diffusion assay. In a previous study, Wahl et al 44 raised the concerns of this method over variation of antibiotic quantity in each pellet and its contact with agar surface. During the entire study period, we did not observe any notable variations in ZOI within each group, which means that the concentration of antibiotics present in each pellet was nearly equal. Although the contact surface of the pellet with the agar is not standardized and varies over time, it could be managed to be within an acceptable limit by standardizing the placement of CaS/HA pellets on the agar. The antibacterial effect of CaS/HA-VAN pellets decreased sharply by day 12 but correlated well with the VAN elution profile at day 14, when the VAN concentrations were below the MIC. Also, this validates our results obtained from the antibiotic release experiment. A moderate antibacterial effect of GEN was seen until day 28, and a strong antibacterial effect of RIF was seen for almost four weeks. These results were also in line with the release profile of both GEN and RIF. Using CaS beads, Aiken et al 24 reported a similar trend of comparable release profile and antimicrobial efficacy obtained with modified Kirby-Bauer assay of RIF. Cas/HA-RIF exhibited strong antibacterial effects for four weeks, but in the case of CaS/HA-VAN/GEN + RIF this was three weeks. As mentioned previously, the release trend of RIF when combined with VAN/GEN might be the reason. However, as shown repeatedly in previous studies, to prevent the development of RIF resistant bacterial populations, combinations of RIF with other antibiotics exert better antibiotic effects on biofilms than non-RIF combinations. 31,45 Moreover, in clinical conditions the initial burst release of antibiotics could potentially kill the majority of bacteria, and thereafter the residual bacterial populations could be eradicated by the sustained MIC levels released from the carriers.
Although the antibacterial effects of the CaS/HA-antibiotic pellets were tested by continuous disk diffusion assay, we also evaluated the antibacterial effects of the pellets used for the antibiotic elution assay, since they had been placed in PBS which was replenished at predetermined timepoints, thereby closely mimicking in vivo conditions. No extended antibacterial effect exhibited by CaS/HA-VAN was expected since its elusion profile showed no VAN release by day 21. In parallel to the consistent ZOI observed during continuous disk diffusion assay, CaS/HA-GEN showed antibacterial effects at day 35. This persistent antibacterial effect of CaS/HA-GEN points to the possibly limited interaction between GEN and HA particles or the slower resorption of CaS. However, this needs to be validated in future studies. The strong antibacterial effects exhibited by CaS/HA-VAN/GEN + RIF combinations after 35 days confirm RIF accretion to microparticulate HA. 46 This implies that these composites have the potential to elute antibiotics that could exert an antibacterial effect for at least a month even under actual in vivo conditions through either passive diffusion via material fluid interaction, material degradation, or osteoclast scavenging of antibiotic-bound HA particles.
Although biofilm-embedded bacteria are characterized by their resistance to antibacterial agents, our results showed that most of the tested antibiotic fractions of CaS/HA-VAN/GEN + RIF combinations showed excellent antibiofilm effects against 48-hour-old biofilms. It is important to note the significant difference in biofilm destruction effects between fractions of CaS/HA-VAN/GEN with or without RIF, within two weeks and at day 35. This highlights the potential of the initial high concentrations of combinations of the selected antibiotics to eradicate the biofilm. Using a porcine model of osteomyelitis, Blirup-Plum et al 47 reported that following limited or extended debridement, CaS/HA-GEN alone cannot eradicate infection without the addition of systemic antibiotics. Drawing similarity from their findings, our results show that even after continuous treatment of biofilms, CaS/HA-VAN/GEN fractions could not effectively eradicate established biofilms. Further, a recent study by Goetz et al 48 showed the significant effect of RIF combinations in reducing the bacterial counts on bone and implants compared with antibiotic monotherapy, and highlights the crucial therapeutic role of RIF combinations in bone infections.
Various studies have reported that RIF monotherapy leads to rapid development of resistance. 49,50 In agreement, we also noted the emergence of RIF resistance following continuous treatment of all tested S. aureus strains with CaS/HA-RIF fractions. Interestingly, when RIF was combined with VAN or GEN, resistance to RIF was not observed. This is not in line with a previous study by Holmberg et al, 50 where low rates of resistance to RIF were noted even when combined with other antibiotics. As observed, the delivery mode with an initial high concentration of antibiotics, and their excellent antibacterial and antibiofilm properties, could be the possible reasons for not developing RIF resistance. In agreement with a study by Bidossi et al, 51 no resistance to VAN or GEN was detected in the present study. In another study, Butini et al 52 reported the emergence of resistance to GEN following exposure to sub-MIC concentrations of GEN eluted from beads of CaS/HA-GEN. By employing the continuous exposure of sub-MIC concentrations of GEN, their experimental methodology differed from the present study where we exposed fractions from different timepoints including the initial high concentration of GEN from day 1 to above MIC at day 35.
This study has a few limitations. First, the in vitro findings need to be validated in relevant animal models. However, being a proof-of-concept study, we believe that our findings are valuable and aid the ongoing in vivo experiments. Second, although the use of current release profile setup using PBS is a well-established system to study the release of various biomolecules added to different biomaterial carriers, we acknowledge that an elution test using plasma or serum as exchange fluid could have added values to our observations. 29,44 For biofilm eradication we used a 48-hour-old immature biofilm rather than a 14-day-old biofilm, which is the reported time for biofilm maturation post-infection. 53 However, in a recent study Okae et al 54 reported that the MBEC of GEN and cefazolin obtained on 72-hour-old biofilms was similar to or even twice that of 14-day-old biofilms. VAN also showed a similar trend when tested using a methicillin-resistant S. aureus strain. 54 Taking these findings into consideration, we believe that the preliminary data obtained using a 48-hour-old biofilm provide useful insights about the biofilm destructive action of RIF combinations eluted via CaS/HA carrier. Finally, an analysis of the mechanical properties of CaS/HA alone and in combination with antibiotics was lacking in this study. However, these osteoconductive biomaterials are used as bone void fillers, and in weightbearing applications need additional mechanical support.
In conclusion, in this study we demonstrated a biphasic ceramic platform for the local and sustained delivery of RIF along with GEN or VAN. With a favourable injectability and sustained release of antibiotics, this bioresorbable carrier could avoid the off-target toxic effects of systemically administered RIF, and has the potential to be used as a novel treatment regime for bone infections. Compared to the VAN or GEN alone composites, RIF and VAN or GEN combinations did yield convincing antibacterial and antibiofilm effects without the development of resistance to RIF. Since CaS/HA is a clinically approved biomaterial for bone applications, translation of this as an off-the-shelf RIF carrier would be easier. However, for further validation of our in vitro findings, in vivo models are necessary and currently ongoing.
Author contributions
S. Sebastian: Investigation, Methodology, Data curation, Formal analysis, Funding acquisition, Writing – original draft.
F. Tandberg: Investigation, Data curation, Writing – review & editing.
Y. Liu: Investigation, Methodology, Data curation, Writing – review & editing.
D. B. Raina: Conceptualization, Project administration, Supervision, Resources, Validation, Writing – review & editing.
M. Tägil: Supervision, Resources, Validation, Writing – review & editing.
M. Collin: Supervision, Resources, Validation, Writing – review & editing.
L. Lidgren: Conceptualization, Supervision, Funding acquisition, Resources, Validation, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: funding from the Royal Fysiographic Society of Lund (grant number 142843-2020) and Landshövding Per Westlings Minnesfond (grant number RMv 2021-0022).
ICMJE COI statement
Y. Liu, D. B. Raina, M. Tägil, and L. Lidgren are stockholders in Moroxite AB, Sweden.
L. Lidgren is a board member of Bonesupport AB, Sweden and Orthocell, Australia.
All other authors have nothing to declare.
Data sharing
Data are available upon reasonable request to the corresponding author.
Acknowledgements
The authors would like to thank Anna Stefansdottir and Linnéa Polland for providing the clinical strains of Staphylococcus aureus. The authors deeply acknowledge the excellent technical assistance provided by Maria Baumgarten in performing the biofilm scanning electron microscopy imaging and its analysis. Ann-Charlotte Strömdahl, Ganna Petruk, Ariane Neumann, and Anna Dahlman are acknowledged for all their help during the bacteriology laboratory work. Xiaoya Li is greatly acknowledged for her help during the Fourier transform infrared spectroscopy experiment.
Open access funding
The authors report that they received open access funding for their manuscript from Lund University.
Follow the authors @lunduniversity
Supplementary material
Details of the following: materials used in the study, amount and concentration of materials and antibiotics used for pellet preparation, scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy protocol for analysis of calcium sulphate/hydroxyapatite composites, testing of injectability and setting time, degradation assay, MTT assay, efficacy on planktonic bacteria by continuous Kirby-Bauer disk diffusion assay, antibacterial effects of the pellets after day-35, efficacy on preformed biofilms, biofilm SEM protocol and viable bacteria after continuous treatment with antibiotic fractions, and development of resistance to vancomycin, gentamicin, and rifampicin. Figure showing the continuous Kirby-Bauer disk diffusion assay of pellets, and tables showing their detailed statistical findings.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Sujeesh Sebastian, Email: sujeeshsebastian@gmail.com.
Felix Tandberg, Email: felix.tandberg.291@student.lu.se.
Yang Liu, Email: liu.yang@med.lu.se.
Deepak B. Raina, Email: deepak.raina@med.lu.se.
Magnus Tägil, Email: magnus.tagil@med.lu.se.
Mattias Collin, Email: mattias.collin@med.lu.se.
Lars Lidgren, Email: lars.lidgren@med.lu.se.
References
- 1. Gogia JS, Meehan JP, Di Cesare PE, Jamali AA. Local antibiotic therapy in osteomyelitis. Semin Plast Surg. 2009;23(2):100–107. 10.1055/s-0029-1214162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gimza BD, Cassat JE. Mechanisms of antibiotic failure during Staphylococcus aureus osteomyelitis. Front Immunol. 2021;12:638085. 10.3389/fimmu.2021.638085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu K, Song L, Kang HP, Kwon H-K, Back J, Lee FY. Recalcitrant methicillin-resistant Staphylococcus aureus infection of bone cells: Intracellular penetration and control strategies. Bone Joint Res. 2020;9(2):49–59. 10.1302/2046-3758.92.BJR-2019-0131.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moojen DJF, Hentenaar B, Charles Vogely H, Verbout AJ, Castelein RM, Dhert WJA. In vitro release of antibiotics from commercial PMMA beads and articulating hip spacers. J Arthroplasty. 2008;23(8):1152–1156. 10.1016/j.arth.2007.08.020 [DOI] [PubMed] [Google Scholar]
- 5. McNally MA, Ferguson JY, Lau ACK, et al. . Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. Bone Joint J. 2016;98-B(9):1289–1296. 10.1302/0301-620X.98B9.38057 [DOI] [PubMed] [Google Scholar]
- 6. Logoluso N, Drago L, Gallazzi E, George DA, Morelli I, Romanò CL. Calcium-based, antibiotic-loaded bone substitute as an implant coating: A pilot clinical study. J Bone Jt Infect. 2016;1:59–64. 10.7150/jbji.17586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colding-Rasmussen T, Horstmann P, Petersen MM, Hettwer W. Antibiotic elution characteristics and pharmacokinetics of gentamicin and vancomycin from a mineral antibiotic carrier: An in vivo evaluation of 32 clinical cases. J Bone Jt Infect. 2018;3(4):234–240. 10.7150/jbji.26301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279(19):1537–1541. 10.1001/jama.279.19.1537 [DOI] [PubMed] [Google Scholar]
- 9. Beldman M, Löwik C, Soriano A, et al. . If, when, and how to use rifampin in acute staphylococcal periprosthetic joint infections, a multicentre observational study. Clin Infect Dis. 2021;73(9):1634–1641. 10.1093/cid/ciab426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Funk GA, Menuey EM, Cole KA, Schuman TP, Kilway KV, McIff TE. Radical scavenging of poly(methyl methacrylate) bone cement by rifampin and clinically relevant properties of the rifampin-loaded cement. Bone Joint Res. 2019;8(2):81–89. 10.1302/2046-3758.82.BJR-2018-0170.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gálvez-López R, Peña-Monje A, Antelo-Lorenzo R, et al. . Elution kinetics, antimicrobial activity, and mechanical properties of 11 different antibiotic loaded acrylic bone cement. Diagn Microbiol Infect Dis. 2014;78(1):70–74. 10.1016/j.diagmicrobio.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 12. Raina DB, Isaksson H, Hettwer W, Kumar A, Lidgren L, Tägil M. A biphasic calcium sulphate/hydroxyapatite carrier containing bone morphogenic protein-2 and zoledronic acid generates bone. Sci Rep. 2016;6(1):26033. 10.1038/srep26033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horstmann PF, Raina DB, Isaksson H, et al. . Composite biomaterial as a carrier for bone-active substances for metaphyseal tibial bone defect reconstruction in rats. Tissue Eng Part A. 2017;23(23–24):1403–1412. 10.1089/ten.TEA.2017.0040 [DOI] [PubMed] [Google Scholar]
- 14. Raina DB, Qayoom I, Larsson D, et al. . Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery. Biomaterials. 2019;188:38–49. 10.1016/j.biomaterials.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Raina DB, Sebastian S, et al. . Sustained and controlled delivery of doxorubicin from an in-situ setting biphasic hydroxyapatite carrier for local treatment of a highly proliferative human osteosarcoma. Acta Biomater. 2021;131:555–571. 10.1016/j.actbio.2021.07.016 [DOI] [PubMed] [Google Scholar]
- 16. Stravinskas M, Tarasevicius S, Laukaitis S, Nilsson M, Raina DB, Lidgren L. A ceramic bone substitute containing gentamicin gives good outcome in trochanteric hip fractures treated with dynamic hip screw and in revision of total hip arthroplasty: A case series. BMC Musculoskelet Disord. 2018;19(1):438. 10.1186/s12891-018-2360-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sebastian S, Liu Y, Sezgin EA, et al. . Injectability of a ceramic bone substitute mixed with rifampicin for local delivery. Orthopaedic Proceedings. 2020;102-B(SUPP_11). [Google Scholar]
- 18. Qayoom I, Verma R, Murugan PA, et al. . A biphasic nanohydroxyapatite/calcium sulphate carrier containing Rifampicin and Isoniazid for local delivery gives sustained and effective antibiotic release and prevents biofilm formation. Sci Rep. 2020;10(1):14128. 10.1038/s41598-020-70726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kadurugamuwa JL, Sin LV, Yu J, Francis KP, Purchio TF, Contag PR. Noninvasive optical imaging method to evaluate postantibiotic effects on biofilm infection in vivo. Antimicrob Agents Chemother. 2004;48(6):2283–2287. 10.1128/AAC.48.6.2283-2287.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. No authors listed . M100: Performance Standards for Antimicrobial Susceptability Testing. Clinical and Laboratory Standards Institute (CLSI), USA, 2021. [Google Scholar]
- 21. Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37(6):1771–1776. 10.1128/JCM.37.6.1771-1776.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Funk GA, Menuey EM, Ensminger WP, Kilway KV, McIff TE. Elution of rifampin and vancomycin from a weight-bearing silorane-based bone cement. Bone Joint Res. 2021;10(4):277–284. 10.1302/2046-3758.104.BJR-2020-0430.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahola N, Veiranto M, Männistö N, et al. . Processing and sustained in vitro release of rifampicin containing composites to enhance the treatment of osteomyelitis. Biomatter. 2012;2(4):213–225. 10.4161/biom.22793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aiken SS, Cooper JJ, Florance H, Robinson MT, Michell S. Local release of antibiotics for surgical site infection management using high-purity calcium sulfate: an in vitro elution study. Surg Infect (Larchmt). 2015;16(1):54–61. 10.1089/sur.2013.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qayoom I, Teotia AK, Panjla A, Verma S, Kumar A. Local and sustained delivery of rifampicin from a bioactive ceramic carrier treats bone infection in rat tibia. ACS Infect Dis. 2020;6(11):2938–2949. 10.1021/acsinfecdis.0c00369 [DOI] [PubMed] [Google Scholar]
- 26. Sanz-Ruiz P, Carbó-Laso E, Del Real-Romero JC, et al. . Microencapsulation of rifampicin: A technique to preserve the mechanical properties of bone cement. J Orthop Res. 2018;36(1):459–466. 10.1002/jor.23614 [DOI] [PubMed] [Google Scholar]
- 27. Cyphert EL, Learn GD, Hurley SK, Lu C-Y, von Recum HA. An additive to PMMA bone cement enables postimplantation drug refilling, broadens range of compatible antibiotics, and prolongs antimicrobial therapy. Adv Healthc Mater. 2018;7(21):21. 10.1002/adhm.201800812 [DOI] [PubMed] [Google Scholar]
- 28. Stravinskas M, Nilsson M, Vitkauskiene A, Tarasevicius S, Lidgren L. Vancomycin elution from a biphasic ceramic bone substitute. Bone Joint Res. 2019;8(2):49–54. 10.1302/2046-3758.82.BJR-2018-0174.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stravinskas M, Horstmann P, Ferguson J, et al. . Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: In vitro and clinical release studies. Bone Joint Res. 2016;5(9):427–435. 10.1302/2046-3758.59.BJR-2016-0108.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LaPlante KL, Mermel LA. In vitro activities of telavancin and vancomycin against biofilm-producing Staphylococcus aureus, S. epidermidis, and Enterococcus faecalis strains. Antimicrob Agents Chemother. 2009;53(7):3166–3169. 10.1128/AAC.01642-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jørgensen NP, Skovdal SM, Meyer RL, Dagnæs-Hansen F, Fuursted K, Petersen E. Rifampicin-containing combinations are superior to combinations of vancomycin, linezolid and daptomycin against Staphylococcus aureus biofilm infection in vivo and in vitro. Pathog Dis. 2016;74(4):ftw019. 10.1093/femspd/ftw019 [DOI] [PubMed] [Google Scholar]
- 32. Osmon DR, Berbari EF, Berendt AR, et al. . Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–e25. 10.1093/cid/cis803 [DOI] [PubMed] [Google Scholar]
- 33. Depypere M, Kuehl R, Metsemakers W-J, et al. . Recommendations for systemic antimicrobial therapy in fracture-related infection: A consensus from an international expert group. J Orthop Trauma. 2020;34(1):30–41. 10.1097/BOT.0000000000001626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Renz N, Trampuz A, Zimmerli W. Controversy about the role of rifampin in biofilm infections: Is it justified? Antibiotics (Basel). 2021;10(2):165. 10.3390/antibiotics10020165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Becker A, Kreitmann L, Triffaut-Fillit C, et al. . Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France. J Bone Jt Infect. 2020;5(1):28–34. 10.7150/jbji.40333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scheper H, van Hooven D, van de Sande M, et al. . Outcome of acute staphylococcal prosthetic joint infection treated with debridement, implant retention and antimicrobial treatment with short duration of rifampicin. J Infect. 2018;76(5):498–500. 10.1016/j.jinf.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 37. Kim M-S, Morales W, Hani AA, et al. . The effect of rifaximin on gut flora and Staphylococcus resistance. Dig Dis Sci. 2013;58(6):1676–1682. 10.1007/s10620-013-2675-0 [DOI] [PubMed] [Google Scholar]
- 38. Miclau T, Edin ML, Lester GE, Lindsey RW, Dahners LE. Bone toxicity of locally applied aminoglycosides. J Orthop Trauma. 1995;9(5):401–406. 10.1097/00005131-199505000-00007 [DOI] [PubMed] [Google Scholar]
- 39. Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 2011;29(7):1070–1074. 10.1002/jor.21343 [DOI] [PubMed] [Google Scholar]
- 40. Isefuku S, Joyner CJ, Simpson AH. Toxic effect of rifampicin on human osteoblast-like cells. J Orthop Res. 2001;19(5):950–954. 10.1016/S0736-0266(01)00022-5 [DOI] [PubMed] [Google Scholar]
- 41. Wiesli MG, Kaiser J-P, Gautier E, et al. . Influence of ceftriaxone on human bone cell viability and in vitro mineralization potential is concentration- and time-dependent. Bone Joint Res. 2021;10(3):218–225. 10.1302/2046-3758.103.BJR-2020-0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trombetta RP, Ninomiya MJ, El-Atawneh IM, et al. . Calcium phosphate spacers for the local delivery of sitafloxacin and rifampin to treat orthopedic infections: Efficacy and proof of concept in a mouse model of single-stage revision of device-associated osteomyelitis. Pharmaceutics. 2019;11(2):E94. 10.3390/pharmaceutics11020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alagboso FI, Mannala GK, Walter N, et al. . Rifampicin restores extracellular organic matrix formation and mineralization of osteoblasts after intracellular Staphylococcus aureus infection. Bone Joint Res. 2022;11(5):327–341. 10.1302/2046-3758.115.BJR-2021-0395.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wahl P, Rönn K, Bohner M, et al. . In vitro study of new combinations for local antibiotic therapy with calcium sulphate - Near constant release of ceftriaxone offers new treatment options. J Bone Jt Infect. 2018;3(4):212–221. 10.7150/jbji.26218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cirioni O, Mocchegiani F, Ghiselli R, et al. . Daptomycin and rifampin alone and in combination prevent vascular graft biofilm formation and emergence of antibiotic resistance in a subcutaneous rat pouch model of staphylococcal infection. Eur J Vasc Endovasc Surg. 2010;40(6):817–822. 10.1016/j.ejvs.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 46. Raina DB, Liu Y, Jacobson OLP, Tanner KE, Tägil M, Lidgren L. Bone mineral as a drug-seeking moiety and a waste dump. Bone Joint Res. 2020;9(10):709–718. 10.1302/2046-3758.910.BJR-2020-0097.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blirup-Plum SA, Bjarnsholt T, Jensen HE, et al. . Pathological and microbiological impact of a gentamicin-loaded biocomposite following limited or extensive debridement in a porcine model of osteomyelitis. Bone Joint Res. 2020;9(7):394–401. 10.1302/2046-3758.97.BJR-2020-0007.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goetz J, Keyssner V, Hanses F, et al. . Animal experimental investigation on the efficacy of antibiotic therapy with linezolid, vancomycin, cotrimoxazole, and rifampin in treatment of periprosthetic knee joint infections by MRSA. Bone Joint Res. 2022;11(3):143–151. 10.1302/2046-3758.113.BJR-2021-0268.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chaisson RE. Treatment of chronic infections with rifamycins: is resistance likely to follow? Antimicrob Agents Chemother. 2003;47(10):3037–3039. 10.1128/AAC.47.10.3037-3039.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holmberg A, Mörgelin M, Rasmussen M. Effectiveness of ciprofloxacin or linezolid in combination with rifampicin against Enterococcus faecalis in biofilms. J Antimicrob Chemother. 2012;67(2):433–439. 10.1093/jac/dkr477 [DOI] [PubMed] [Google Scholar]
- 51. Bidossi A, Bottagisio M, Logoluso N, De Vecchi E. In vitro evaluation of gentamicin or vancomycin containing bone graft substitute in the prevention of orthopedic implant-related infections. Int J Mol Sci. 2020;21(23):23. 10.3390/ijms21239250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Butini ME, Cabric S, Trampuz A, Di Luca M. In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf B Biointerfaces. 2018;161:252–260. 10.1016/j.colsurfb.2017.10.050 [DOI] [PubMed] [Google Scholar]
- 53. Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, et al. . Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J Orthop Res. 2015;33(9):1311–1319. 10.1002/jor.22907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Okae Y, Nishitani K, Sakamoto A, et al. . Estimation of minimum biofilm eradication concentration (MBEC) on in vivo biofilm on orthopedic implants in a rodent femoral infection model. Front Cell Infect Microbiol. 2022;12:896978. 10.3389/fcimb.2022.896978 [DOI] [PMC free article] [PubMed] [Google Scholar]