Abstract
This review aimed to quantify the effect of therapeutic application of virtual reality (VR) on cognitive function in individuals with mild cognitive impairment (MCI). We searched for randomized controlled trials involving VR in the interventions provided to individuals with MCI. After searching four international electronic databases, we analyzed six studies involving 279 individuals with MCI. RevMan 5.4 was used for quality assessment and quantitative analysis. Therapeutic application of VR in individuals with MCI resulted in a significant improvement in cognitive function (mean difference = −1.46; 95% confidence interval: −2.53 to −0.39; heterogeneity: χ2 = 970.56, df = 18, I2 = 98%; and overall effect: Z = 2.67, p = 0.008). However, there was no significant improvement in the subcategories such as global cognition, working memory, executive function, memory function, and attention. In conclusion, feedback stimulation through VR has a potential value in improving cognitive function in individuals with MCI. However, on the basis of the results of the subcategories, a personalized VR program is required for the individual subcategories of cognitive function.
Keywords: virtual reality, mild cognitive impairment, cognitive function, rehabilitation
1. Introduction
Mild cognitive impairment (MCI) may be a precursor to dementia [1], a stage in which cognitive symptoms are not fully understood [2]. The prevalence of MCI in adults aged above 60 years ranges from 6.7% to 25.2% and varies according to age and educational level [3,4]. Divided attention, learning new information, verbal fluency, and reaction time tend to decline with normal aging [5]. However the diagnostic criteria for MCI also include changes in cognition, abnormal cognitive function in one or more areas, concerns about normal daily activities, and absence of dementia [6,7].
Early detection of MCI and appropriate interventions are very important since they can slow the progression to dementia or improve the symptoms [8]. The recommended non-pharmacological interventions for MCI include combined interventions with exercise and cognitive training [9,10]. Furthermore, studies using virtual reality (VR) for the prevention and treatment of MCI have been performed until relatively recently [11]. Exercise combined with VR showed significant improvement not only in physical function but also in cognitive function in normal elderly individuals, and there was a tendency to prefer this combination to general exercise [12,13].
With the development of VR technology, many studies have been conducted on MCI, and numerous systematic reviews have been published [14,15,16,17,18,19,20,21]. Systematic reviews have suggested that semi-immersive VR was more effective than immersive VR, and it showed significant improvement in global cognitive function and short-term memory. However, there was no significant improvement in other variables. Moreover, the effect size was not large, even for the variables with significant improvement. Therefore, we believed it necessary to classify and analyze the cognitive function in more detail to clarify controversial results.
Thus, we performed qualitative and quantitative analyses of the effect of VR on cognitive function in randomized controlled trials (RCTs) using the therapeutic application of VR for MCI.
2. Materials and Methods
2.1. Study Design
In this systematic review and meta-analysis, we aimed to perform qualitative and quantitative analyses based on studies involving therapeutic application of VR in individuals with MCI. A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (number: CRD42022360635).
2.2. Search Strategy and Selection of Studies
2.2.1. Inclusion Criteria
-
Participants
Participants were individuals with MCI alone.
-
Intervention
Interventions included VR alone or combined interventions.
-
Comparisons
Activities that did not involve an intervention or did not include VR were selected for comparisons.
-
Outcomes
To perform a meta-analysis, a comparative analysis was performed when there were three or more identical variables in the studies.
-
Types of studies
Among different study designs, only RCTs were selected.
2.2.2. Exclusion Criteria
Studies not published in English or studies not reporting the appropriate data were excluded. In addition, studies published before 2013 were excluded from the synthesis of relatively recent studies.
2.2.3. Strategy for Literature Search
We searched for studies published since 2013 wherein the study protocol was registered in PROSPERO until September 2022. The searched keywords were as follows: ‘mild cognitive impairment’ AND (‘virtual reality’ OR ‘rehabilitation’) AND (‘cognition’ OR ‘cognitive function’) AND ‘randomized controlled trial.’
The databases used for the search included the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Excerpta Medica Database (Embase), Medical Literature Analysis and Retrieval System Online (MEDLINE), and Physiotherapy Evidence Database (PEDro).
2.2.4. Study Selection and Data Extraction
Studies searched in the aforementioned electronic databases were exported to Microsoft Excel (Microsoft, Redmond, Washington, USA), and duplicate studies were excluded. According to the PRISMA guidelines, the full text of each study was checked after reviewing the title and abstract. Finally, studies were selected through consultation among researchers (H.K., J.J., and S.L.) with experience in meta-analyses.
2.2.5. Quality Assessment
Quality assessment was performed using the risk of bias (RoB) tool provided by RevMan 5.4 (The Cochrane Collaboration, Oxford, England). RoB is a tool consisting of seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each of the seven items was rated as high (−), low (+), or uncertain (?) by the researchers. If there was no agreement on the results, a consultation process was required.
2.3. Strategy for Data Synthesis
The included studies were synthesized and analyzed using RevMan 5.4. We performed a quantitative analysis using mean differences (MDs), considering RCTs with no homogeneity at baseline. For studies wherein the standard deviation was not reported in the values describing change from baseline, correlation coefficients were extracted and calculated from the results of the studies using the same variables. Therefore, data on outcome measures were extracted as MDs and presented as a random effects model considering the heterogeneity. In addition, the chi-squared and I2 tests provided in the software were used for heterogeneity.
An I2 value greater than 75% was considered to indicate high heterogeneity, and a value below 40% was considered to indicate low heterogeneity [22]. Publication bias in the studies was displayed using funnel plots [23].
3. Results
3.1. Literature Search and Characteristics of the Included Trials
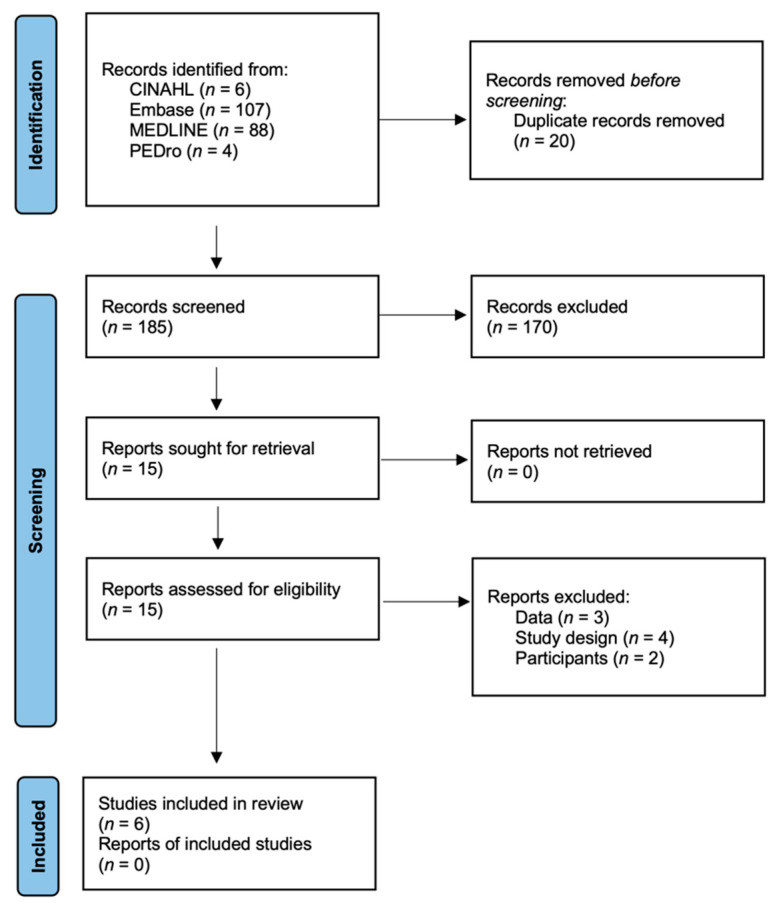
Altogether, 279 papers were identified using the four databases (Figure 1). Duplicate studies were classified using Excel, and 20 studies were excluded. Altogether, 170 studies were excluded for not conforming to the eligibility criteria. Following the review of full texts, three studies with inadequate data, four with inappropriate study designs, and two with an inadequate number of participants were excluded. Finally, six studies were selected in this systematic review and meta-analysis [24,25,26,27,28,29].
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram.
3.2. Assessment of Methodological Quality
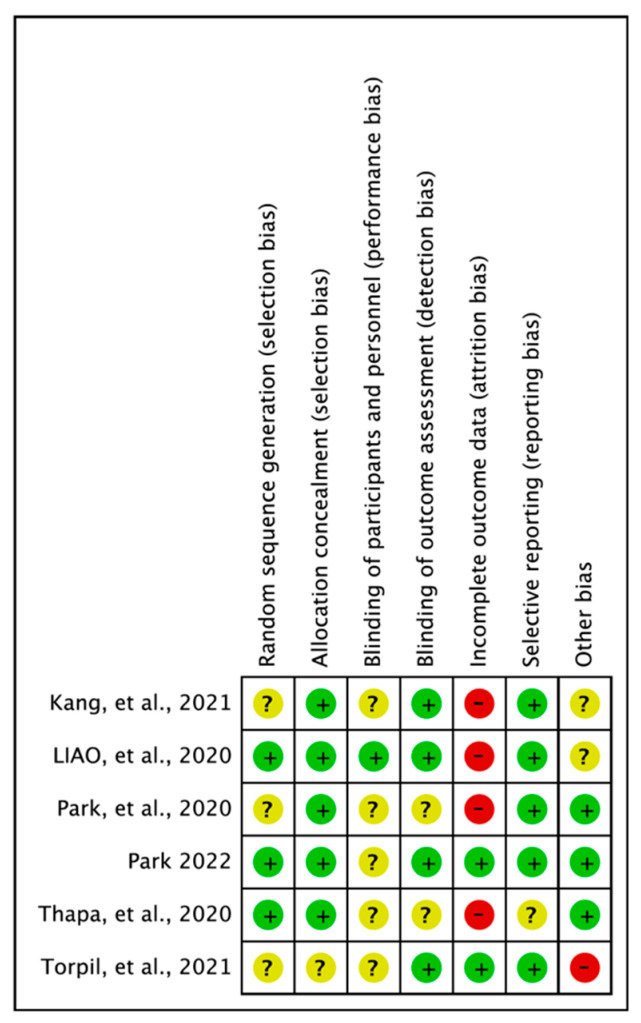
The results of quality assessment were as follows: random sequence generation (low: 3, uncertain: 3), allocation concealment (low: 5, uncertain: 1), blinding of participants and personnel (low: 1, uncertain: 5), blinding of outcome assessment (low: 1, uncertain: 5), incomplete outcome data (low: 2, high: 4), selective reporting (low: 5, uncertain: 1), and other biases (low: 3, uncertain: 2, high: 1). For other biases, items such as lack of sample size calculations, differences in baseline characteristics, and lack of study protocol registration were assessed as uncertain or high [30] (Figure 2).
Figure 2.
Risk of bias summary: review of authors’ judgments about each item for each included study. Kang et al., 2021 [24], Liao et al., 2020 [25], Park 2022 [26], Park et al., 2020 [27], Thapa et al., 2020 [28], Torpil et al., 2021 [29].
3.3. Virtual Reality for Individuals with Mild Cognitive Impairment
The six RCTs from this systematic review included 279 individuals with MCI. The interventions included VR without distinguishing between immersive and semi-immersive types. The treatment duration varied from 4 weeks to 3 months (Table 1). Cognitive function was classified into global cognition (Mini-Mental State Examination, Montreal Cognitive Assessment [31], and Loewenstein Occupational Therapy Cognitive Assessment-Geriatric [32]), working memory (Trail Making Test-part A [33,34] and digit span test [35]), executive function (Trail Making Test-part B [33,34], Digit Symbol Substitution Test, Weschsler Adult Intelligence Scale-revised Block Design Test [36], and Executive Interview 25 [37]), memory function (Seoul Verbal Learning Test [38] and California Verbal Learning Test [39]), and attention (Stroop test [40]) for outcome measurement (Table 1 and Table 2).
Table 1.
Classification of cognitive function.
| Cognition | Kang et al., 2021 [24] |
Liao et al., 2020 [25] |
Park 2022 [26] |
Park et al., 2020 [27] |
Thapa et al., 2020 [28] |
Torpil et al., 2021 [29] |
|---|---|---|---|---|---|---|
| Global cognition | MMSE | MoCA | K-MMSE | LOTCA-G | ||
| Working memory | TMT-A | Digit span | TMT-A | |||
| Executive function | TMT-B | EXIT-25 | WAIS-BDT | DSST, TMT-B | ||
| Memory function | SVLT | CVVLT | SVLT | LOTCA-G | ||
| Attention | Stroop test | Stroop test | LOTCA-G |
CVVLT, Chinese version of the California Verbal Learning Test; DSST, Digit Symbol Substitution Test; EXIT-25, Executive Interview 25; K-MMSE, Korean version of the Mini-Mental State Examination; LOTCA-G, Loewenstein Occupational Therapy Cognitive Assessment-Geriatric; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; SVLT, Seoul Verbal Learning Test; TMT-A, Trail Making Test-Part A; TMT-B, Trail Making Test-Part B; WAIS-BDT, Weschsler Adult Intelligence Scale-Revised Block Design Test.
Table 2.
Characteristics of the included trials.
| Study | Sample Size | Duration | Intervention | Authors’ Conclusion |
|---|---|---|---|---|
| Kang et al., 2021 [24] | EG = 23 CG = 18 |
4 weeks | EG = VR cognitive training twice a week, total eight sessions, fully immersive 3D setting CG = usual care |
Fully immersive VR cognitive training had positive effects on the visuospatial function, apathy, affect, and quality of life, and increased frontal-occipital functional connectivity in older individuals in a predementia state. |
| Liao et al., 2020 [25] | EG = 18 CG = 16 |
12 weeks | 60 min per session, three sessions per week, total of 36 sessions EG = VR-based PCT; take mass rapid transit, look for a store, kitchen chef, convenience-store clerk CG = PCT |
VR-based physical and cognitive training improved cognitive function. |
| Park, 2022 [26] | EG = 28 CG = 28 |
8 weeks | EG = VR-based spatial cognitive training; 24 sessions (45 min per session, 3 days per week), program in Unity game engine CG = no intervention |
VR-based spatial cognitive training might be clinically beneficial for improving spatial cognition and episodic memory in elderly individuals with MCI. |
| Park et al., 2020 [27] |
EG = 10 CG = 11 |
3 months | EG = Culture-based VR training; 24 sessions (30 min per day, 2 days per week), training with games (Crows and Seagulls, Janggu, Automated Teller Machine, Shopping in the Mart, Fireworks Party, Fruit Cocktail) CG = no intervention |
Culture-based VR training programs did not improve cognitive function. |
| Thapa et al., 2020 [28] | EG = 33 CG = 33 |
8 weeks | EG = VR; 100 min (three 20 min VR training sessions and three 10 min eye massage and stretching sessions), sessions held three times a week, VR training games (juice making, crow shooting, find the number of fireworks, memory object at the house) CG = HCE; 30–50 min per session, one session per week, total eight sessions |
VR-based training improved cognitive and physical function in patients with MCI when compared with controls. |
| Torpil et al., 2021 [29] | EG = 30 CG = 31 |
10–12 weeks | 45 min per session, two sessions per week, total 24 sessions EG = Cognitive rehabilitation plus VR; Microsoft Kinect for PC without immersion (Boxing Trainer, Jet Run, Superkick, Air Challenge) CG = cognitive rehabilitation |
Using VR applications in CR is recommended to improve cognitive function of older adults with MCI. |
CG, control group; CR, cognitive rehabilitation; EG, experimental group; HCE, home care education; MCI, mild cognitive impairment; PCT, physical and cognitive training; VR, virtual reality; 3D, three-dimensional.
3.4. Effectiveness of Virtual Reality in Treating Mild Cognitive Impairment
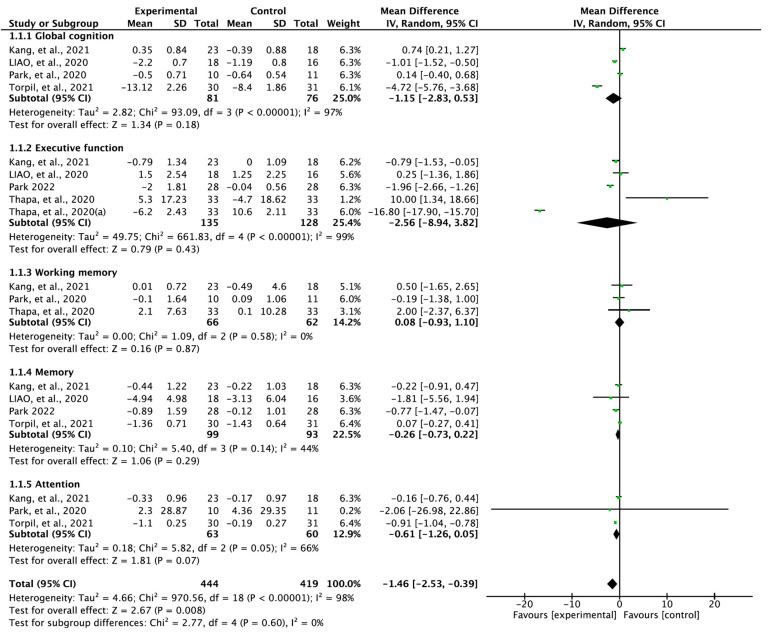
The studies showed a significant positive effect of therapeutically applied VR on the cognitive function of individuals with MCI (MD = −1.46; 95% confidence interval (CI): −2.53 to −0.39; heterogeneity: χ2 = 970.56, df = 18, I2 = 98%; overall effect: Z = 2.67, p = 0.008). Subcategories such as cognitive function (global cognition, working memory, executive function, memory function, and attention) were analyzed using subgroup analysis (Figure 3). There was no significant improvement in global cognition (MD = −1.15; 95% CI: −2.83 to 0.53), executive function (MD = −2.56; 95% CI: −8.94 to 3.82), working memory (MD = 0.08, 95% CI: −0.93 to 1.10), memory function (MD = −0.26, 95% CI: −0.73, 0.22), and attention (MD = −0.61, 95% CI: −1.26 to 0.05) when compared with the control group.
Figure 3.
Forest plot studying the effect of virtual reality on cognitive function. Thapa, et al. 2020 (a) Digit Symbol Substitution Test. Kang et al., 2021 [24], Liao et al., 2020 [25], Park 2022 [26], Park et al., 2020 [27], Thapa et al., 2020 [28], Torpil et al., 2021 [29].
3.5. Publication Bias
In this review, six studies were synthesized for meta-analysis according to eligibility criteria. The Cochrane Review [41] recommended that publication bias is not appropriate when fewer than 10 studies are synthesized, and thus it was not analyzed.
4. Discussion
In the present review, we performed qualitative and quantitative analyses by synthesizing RCTs that involved therapeutic application of VR in individuals with MCI. To the best of our knowledge, this is the first meta-analysis to classify the cognitive function and analyze the improvements in each subcategory.
Therapeutic use of VR had positive effects on cognitive function in individuals with MCI (MD = −1.46, 95% CI: −2.53 to −0.39; overall effect: Z = 2.67, p = 0.008). However, there was no significant improvement in the subcategories such as global cognition (MD = −1.15, 95% CI: −2.83 to 0.53), executive function (MD = −2.56, 95% CI: −8.94 to 3.82), working memory (MD = 0.08, 95% CI: −0.93 to 1.10), memory function (MD = −0.26, 95% CI: −0.73 to 0.22), and attention (MD = −0.61, 95% CI: −1.26 to 0.05) when compared with the control group. Our results differed from those reported in previous meta-analyses [14,15,16,17,18,19,20,21], which showed significant improvements in global cognition. A previous meta-analysis showed significant improvements in executive function [19,20,21] and memory function [15,19]. However, another meta-analysis reported no positive effects on memory function [17,20,21], execution function [17], and attention [17,21].
Some systematic reviews have reported results similar to those in the present review. However, the overall results in the present review were not consistent with those from previous reviews. This discrepancy might have been due to differences in methodological factors (determining the effect of VR alone through RCTs, the difference in search strategy, and lack of distinction between immersive and semi-immersive VR) and analyses (cognitive function was subdivided into categories, and each assessment tool was analyzed according to this classification). However, this does not imply that the results of the present review are absolute. The present review did not differentiate between immersive and semi-immersive VR images. According to a systematic review by Yu, Li and Lai [15], the semi-immersive and non-immersive types are more effective than the immersive type, since immersive technologies can be complex and difficult for individuals with MCI [42].
Although there was no significant improvement in the treatment effect of VR when compared with the control group, application of VR in the treatment environment might have a large potential impact in the future. VR elicits virtual sensations through the simulation of a virtual body [43], which can be provided with an immediate response to reduce compensatory movements by enhancing movement control as a feedback system [44]. Therefore, the provision of feedback should improve cognition and daily life functions by stimulating cognitive and motor domains [45]. Moreover, from a neuroscientific perspective, sharing the basic mechanism of the brain in VR should elicit physiological and psychological responses [46]. This involves observing the movement of the body in a virtual environment, which induces changes in muscle activity, heart rate, and stress [46].
Although the efficacy of VR-based cognitive training might decrease with age [47], it is suggested to be more effective when combined with physical training [48], since physical training increases brain-derived neurotrophic factor, which is concentrated in the hippocampus [49,50]. It has also led to activation of the frontal lobe in studies using magnetic resonance imaging [51]. Moreover, we found that combining VR-based training with physical training could be more effective [52] and could improve neuroplasticity in the ventral striatum by linking the motor and cognitive circuits [53]. Finally, from a functional point of view, the ability to switch between different tasks and to focus on tasks in a VR program that requires visual ability, attention [20], and real-time feedback stimulation should have a positive effect on individuals with MCI [48].
In the present systematic review and meta-analysis, therapeutic application of VR in individuals with MCI was more effective in improving cognitive function when compared with the control group. Despite the contradictory results, none of the subcategories of cognitive function showed significant improvement. However, the potential impact of immersive technology on enhancing the feedback systems and the neuroscientific mechanisms that can act as beneficial stimuli have identified therapeutic application of VR as an area that requires further study. This review has several limitations. Generalizability of a comprehensive review involving only six studies might be limited. We did not consider the different types of VR in the analysis. The intensity of interventions (duration and training protocol) was inconsistent in the present review. Finally, there was a significant improvement in cognitive function, but it was associated with a very high heterogeneity.
5. Conclusions
Therapeutic application of VR in individuals with MCI contributes to the improvement of cognitive function. However, its efficacy in some of the subcategories of cognitive function (global cognition, working memory, executive function, memory function, attention) is unclear. Further studies will require customized programs based on individual subcategories of cognitive function.
Author Contributions
Conceptualization, H.K.; methodology, H.K. and S.L.; software, H.K. and J.J.; formal analysis, H.K., J.J. and S.L.; investigation, H.K., J.J. and S.L.; resources, H.K., J.J. and S.L.; data curation, H.K.; writing—original draft preparation, H.K.; writing—review and editing, H.K., J.J. and S.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gauthier S., Reisberg B., Zaudig M., Petersen R.C., Ritchie K., Broich K., Belleville S., Brodaty H., Bennett D., Chertkow H. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 2.Grueso S., Raquel V.-S. Machine learning methods for predicting progression from mild cognitive impairment to Alzheimer’s disease dementia: A systematic review. Alzheimers Res. Ther. 2021;13:1–29. doi: 10.1186/s13195-021-00900-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen R.C., Lopez O., Armstrong M.J., Getchius T.S.D., Ganguli M., Gloss D., Gronseth G.S., Marson D., Pringsheim T., Day G.S. Practice guideline update summary: Mild cognitive impairment: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90:126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langa K.M., Levine D.A. The diagnosis and management of mild cognitive impairment: A clinical review. Jama. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halter J., Ouslander J., Tinetti M., Studenski S., High K., Asthana S. Hazzard’s Geriatric Medicine and Gerontology. McGraw-Hill Professional; New York, NY, USA: 2009. [Google Scholar]
- 6.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Nelson A.P., O’Connor M.G. Mild cognitive impairment: A neuropsychological perspective. CNS Spectr. 2008;13:56–64. doi: 10.1017/S1092852900016163. [DOI] [PubMed] [Google Scholar]
- 8.Wild K., Howieson D., Webbe F., Seelye A., Kaye J. Status of computerized cognitive testing in aging: A systematic review. Alzheimers Dement. 2008;4:428–437. doi: 10.1016/j.jalz.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daviglus M.L., Bell C.C., Berrettini W., Bowen P.E., Connolly E.S., Jr., Cox N.J., Dunbar-Jacob J.M., Granieri E.C., Hunt G., McGarry K. National Institutes of Health state-of-the-science conference statement: Preventing Alzheimer disease and cognitive decline. Ann. Intern. Med. 2010;153:176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 10.Park H., Park J.H., Na H.R., Hiroyuki S., Kim G.M., Jung M.K., Kim W.K., Park K.W. Combined intervention of physical activity, aerobic exercise, and cognitive exercise intervention to prevent cognitive decline for patients with mild cognitive impairment: A randomized controlled clinical study. J. Clin. Med. 2019;8:940. doi: 10.3390/jcm8070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zucchella C., Sinforiani E., Tamburin S., Federico A., Mantovani E., Bernini S., Casale R., Bartolo M. The multidisciplinary approach to Alzheimer’s disease and dementia. A narrative review of non-pharmacological treatment. Front. Neurol. 2018;9:1058. doi: 10.3389/fneur.2018.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Htut T.Z.C., Hiengkaew V., Jalayondeja C., Vongsirinavarat M. Effects of physical, virtual reality-based, and brain exercise on physical, cognition, and preference in older persons: A randomized controlled trial. Eur. Rev. Aging Phys. Act. 2018;15:1–12. doi: 10.1186/s11556-018-0199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Optale G., Urgesi C., Busato V., Marin S., Piron L., Priftis K., Gamberini L., Capodieci S., Bordin A. Controlling memory impairment in elderly adults using virtual reality memory training: A randomized controlled pilot study. Neurorehabil. Neural Repair. 2010;24:348–357. doi: 10.1177/1545968309353328. [DOI] [PubMed] [Google Scholar]
- 14.Kim O., Pang Y., Kim J.-H. The effectiveness of virtual reality for people with mild cognitive impairment or dementia: A meta-analysis. BMC Psychiatry. 2019;19:1–10. doi: 10.1186/s12888-019-2180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu D., Li X., Lai F.H.-Y. The effect of virtual reality on executive function in older adults with mild cognitive impairment: A systematic review and meta-analysis. Aging Ment. Health. 2022:1–11. doi: 10.1080/13607863.2022.2076202. [DOI] [PubMed] [Google Scholar]
- 16.Papaioannou T., Voinescu A., Petrini K., Fraser D.S. Efficacy and moderators of virtual reality for cognitive training in people with dementia and mild cognitive impairment: A systematic review and meta-analysis. J. Alzheimers Dis. 2022:1–30. doi: 10.3233/JAD-210672. [DOI] [PubMed] [Google Scholar]
- 17.Yan M., Zhao Y., Meng Q., Wang S., Ding Y., Liu Q., Yin H., Chen L. Effects of virtual reality combined cognitive and physical interventions on cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis. Aging Res. Rev. 2022:101708. doi: 10.1016/j.arr.2022.101708. [DOI] [PubMed] [Google Scholar]
- 18.Chao G., Chen L. Meta-analysis of virtual reality based on delaying mild cognitive impairment. J. Nerv. Ment. Dis. 2022;210:194–198. doi: 10.1097/NMD.0000000000001426. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S., Sui Y., Shen Y., Zhu Y., Ali N., Guo C., Wang T. Effects of virtual reality intervention on cognition and motor function in older adults with mild cognitive impairment or dementia: A systematic review and meta-analysis. Front. Aging Neurosci. 2021;13:586999. doi: 10.3389/fnagi.2021.586999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J., Ma Y., Ren Z. Rehabilitative effects of virtual reality technology for mild cognitive impairment: A systematic review with meta-analysis. Front. Psychol. 2020;11:1811. doi: 10.3389/fpsyg.2020.01811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong D., Chen L., Feng Y., Song R., Huang L., Liu J., Zhang L. Effects of virtual reality cognitive training in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry. 2021;36:1829–1847. doi: 10.1002/gps.5603. [DOI] [PubMed] [Google Scholar]
- 22.Deeks J.J., Higgins J.P.T., Altman D.G., Cochrane Statistical Methods Group . Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; London, UK: 2019. Analysing data and undertaking meta-analyses; pp. 241–284. [Google Scholar]
- 23.Duval S., Tweedie R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 24.Kang J.M., Kim N., Lee S.Y., Woo S.K., Park G., Yeon B.K., Park J.W., Youn J.-H., Ryu S.-H., Lee J.-Y. Effect of cognitive training in fully immersive virtual reality on visuospatial function and frontal-occipital functional connectivity in predementia: Randomized controlled trial. J. Med. Internet Res. 2021;23:e24526. doi: 10.2196/24526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Y.-Y., Tseng H.-Y., Lin Y.-J., Wang C.-J., Hsu W.-C. Using virtual reality-based training to improve cognitive function, instrumental activities of daily living and neural efficiency in older adults with mild cognitive impairment. Eur. J. Phys. Rehabil. Med. 2020;56:47–57. doi: 10.23736/S1973-9087.19.05899-4. [DOI] [PubMed] [Google Scholar]
- 26.Park J.-H. Effects of virtual reality-based spatial cognitive training on hippocampal function of older adults with mild cognitive impairment. Int. Psychogeriatr. 2022;34:157–163. doi: 10.1017/S1041610220001131. [DOI] [PubMed] [Google Scholar]
- 27.Park J.-H., Liao Y., Kim D.-R., Song S., Lim J.H., Park H., Lee Y., Park K.W. Feasibility and tolerability of a culture-based virtual reality (Vr) training program in patients with mild cognitive impairment: A randomized controlled pilot study. Int. J. Environ. Res. Public Health. 2020;17:3030. doi: 10.3390/ijerph17093030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thapa N., Park H.J., Yang J.-G., Son H., Jang M., Lee J., Kang S.W., Park K.W., Park H. The effect of a virtual reality-based intervention program on cognition in older adults with mild cognitive impairment: A randomized control trial. J. Clin. Med. 2020;9:1283. doi: 10.3390/jcm9051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torpil B., Şahin S., Pekçetin S., Uyanık M. The effectiveness of a virtual reality-based intervention on cognitive functions in older adults with mild cognitive impairment: A single-blind, randomized controlled trial. Games Health. 2021;10:109–114. doi: 10.1089/g4h.2020.0086. [DOI] [PubMed] [Google Scholar]
- 30.Babic A., Pijuk A., Brázdilová L., Georgieva Y., Pereira M.A.R., Pericic T.P., Puljak L. The judgement of biases included in the category “other bias” in cochrane systematic reviews of interventions: A systematic survey. BMC Med. Res. Methodol. 2019;19:1–10. doi: 10.1186/s12874-019-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The montreal cognitive assessment, Moca: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 32.Zwecker M., Levenkrohn S., Fleisig Y., Zeilig G., Ohry A., Adunsky A. Mini-mental state examination, cognitive fim instrument, and the loewenstein occupational therapy cognitive assessment: Relation to functional outcome of stroke patients. Arch. Phys. Med. Rehabil. 2002;83:342–345. doi: 10.1053/apmr.2002.29641. [DOI] [PubMed] [Google Scholar]
- 33.Tamura I., Kikuchi S., Otsuki M., Kitagawa M., Tashiro K. Deficits of working memory during mental calculation in patients with Parkinson’s disease. J. Neurol. Sci. 2003;209:19–23. doi: 10.1016/S0022-510X(02)00457-4. [DOI] [PubMed] [Google Scholar]
- 34.Crowe S.F. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts a and b of the trail making test. J. Clin. Psychol. 1998;54:585–591. doi: 10.1002/(SICI)1097-4679(199808)54:5<585::AID-JCLP4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Lynn R., Irwing P. Sex differences in mental arithmetic, digit span, and G defined as working memory capacity. Intelligence. 2008;36:226–235. doi: 10.1016/j.intell.2007.06.002. [DOI] [Google Scholar]
- 36.Jaeger J. Digit symbol substitution test: The case for sensitivity over specificity in neuropsychological testing. J. Clin. Psychopharmacol. 2018;38:513. doi: 10.1097/JCP.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho H.-T., Lin S.-I., Guo N.-W., Yang Y.-C., Lin M.-H., Wang C.-S. Executive function predict the quality of life and negative emotion in older adults with diabetes: A longitudinal study. Prim. Care Diabetes. 2022;16:537–542. doi: 10.1016/j.pcd.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Park K.W., Park H., Park J.-H., Sohn S.W. P4-112: Sensitive task measure of memory function on the story recall test in patients with mild cognitive impairment and mild Alzheimer’s disease. Alzheimers Dement. 2015;11:819. doi: 10.1016/j.jalz.2015.06.1818. [DOI] [Google Scholar]
- 39.Hermann B.P., Wyler A.R., Steenman H., Richey E.T. The interrelationship between language function and verbal learning/memory performance in patients with complex partial seizures. Cortex. 1988;24:245–253. doi: 10.1016/S0010-9452(88)80033-9. [DOI] [PubMed] [Google Scholar]
- 40.Dos Santos A., Carolina E., Capovilla A.G.S., Capovilla F.C. Computerized stroop test to assess selective attention in children with attention deficit hyperactivity disorder. Span. J. Psychol. 2007;10:33–40. doi: 10.1017/s1138741600006296. [DOI] [PubMed] [Google Scholar]
- 41.Page M.J., Higgins J.P.T., Sterne J.A.C. Assessing risk of bias due to missing results in a synthesis. Cochrane Handb. Syst. Rev. Interv. 2019:349–374. [Google Scholar]
- 42.Cherniack E.P. Not just fun and games: Applications of Virtual reality in the identification and rehabilitation of cognitive disorders of the elderly. Disabil. Rehabil. Assist. Technol. 2011;6:283–289. doi: 10.3109/17483107.2010.542570. [DOI] [PubMed] [Google Scholar]
- 43.Tieri G., Morone G., Paolucci S., Iosa M. Virtual reality in cognitive and motor rehabilitation: Facts, fiction and fallacies. Expert Rev. Med. Devices. 2018;15:107–117. doi: 10.1080/17434440.2018.1425613. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian S.K., Lourenço C.B., Chilingaryan G., Sveistrup H., Levin M.F. Arm motor recovery using a virtual reality intervention in chronic stroke: Randomized control trial. Neurorehabil. Neural. Repair. 2013;27:13–23. doi: 10.1177/1545968312449695. [DOI] [PubMed] [Google Scholar]
- 45.Coyle H., Traynor V., Solowij N. Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: Systematic review of the literature. Am. J. Geriatr. Psychiatry. 2015;23:335–359. doi: 10.1016/j.jagp.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Meehan M., Razzaque S., Insko B., Whitton M., Brooks F.P. Review of four studies on the use of physiological reaction as a measure of presence in stressfulvirtual environments. Appl. Psychophysiol. Biofeedback. 2005;30:239–258. doi: 10.1007/s10484-005-6381-3. [DOI] [PubMed] [Google Scholar]
- 47.Van Schaik P., Martyr A., Blackman T., Robinson J. Involving persons with dementia in the evaluation of outdoor environments. Cyberpsychol. Behav. 2008;11:415–424. doi: 10.1089/cpb.2007.0105. [DOI] [PubMed] [Google Scholar]
- 48.Liao Y.-Y., Chen I.-H., Lin Y.-J., Chen Y., Hsu W.-C. Effects of virtual reality-based physical and cognitive training on executive function and dual-task gait performance in older adults with mild cognitive impairment: A randomized control trial. Front. Aging Neurosci. 2019;11:162. doi: 10.3389/fnagi.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim H.-J., Lee D.J., Lee Y.S. The effect of aerobic exercise on brain-derived neurotrophic factor (Bdnf) in individuals with mild cognitive impairment: A systematic review and meta-analysis of a randomized controlled trials. Phys. Ther. Rehabil. Sci. 2022;11:304–310. doi: 10.14474/ptrs.2022.11.3.304. [DOI] [Google Scholar]
- 51.Colcombe S.J., Erickson K.I., Scalf P.E., Kim J.S., Prakash R., McAuley E., Elavsky S., Marquez D.X., Hu L., Kramer A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 52.Hötting K., Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013;37:2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt L., Lebreton M., Cléry-Melin M.-L., Daunizeau J., Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 2012;10:e1001266. doi: 10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.