Abstract
Kawasaki disease (KD) is an acute vasculitis of young children that can be complicated by coronary artery abnormalities. Recent findings suggest that a superantigen(s) may play an important role in stimulating the immune activation associated with the disease, although the origin of this superantigen(s) is unclear. Staphylococcus aureus, isolated from the rectum or pharynx of patients with KD, secretes toxic shock syndrome toxin 1 (TSST-1). The KD isolates express low levels of other exoproteins compared to isolates from patients with toxic shock syndrome (TSS). Thus, it was previously suggested that the KD isolates may be defective in the global regulatory locus agr (for accessory gene regulator), which positively regulates these factors (D. Y. M. Leung et al., Lancet 342:1385–1388, 1993). Here we describe another characteristic of KD isolates. When considered collectively, the KD isolates were found to express higher levels of staphylococcal protein A than the TSS isolates, another characteristic of an agr-defective phenotype. This correlated with a higher level of spa mRNA in these isolates. In contrast, the KD and TSS isolates expressed comparable levels of TSST-1, consistent with previous findings (D. Y. M. Leung et al., Lancet 342:1385–1388, 1993). Analysis of RNAIII transcript levels and nucleotide sequence analysis of the RNAIII-coding region suggested that the KD isolates are not defective in RNAIII, the effector molecule of the agr regulatory system. However, induction of RNAIII transcription in the KD isolates did not result in a dramatic decrease in the amount of spa mRNA, as has been reported for other strains (F. Vandenesch, J. Kornblum, and R. P. Novick, J. Bacteriol. 173:6313–6320, 1991).
Kawasaki disease (KD) is an acute illness of early childhood characterized by fever, induration and erythema of the hands and feet, inflammation of the mucous membranes, polymorphous skin rash, and cervical lymphadenopathy (23). As a complication of this multisystem vasculitis, coronary artery aneurysms or ectasias occur in approximately 25% of untreated patients (22). In the United States and Japan, KD is currently the leading cause of acquired heart disease in children. A number of observations suggest that immune mechanisms play an important role in the pathogenesis of the disease. In particular, acute KD is characterized by significant immune activation. Increased numbers of circulating activated T cells, B cells, and macrophages/monocytes lead to high levels of circulating cytokines and to the production of cytotoxic antibodies directed against vascular endothelial cell antigens (14, 30–32). These immunologic features, particularly the activation of T cells and macrophages, are characteristic of diseases which are caused by microbial superantigens (25). Indeed, the clinical symptoms and epidemiology of KD are highly suggestive of an infectious disease, overlapping with staphylococcal and streptococcal scarlet fevers and toxic shock syndromes (TSSs).
Recently, it was suggested that staphylococcal toxic shock syndrome toxin 1 (TSST-1) and streptococcal pyrogenic exotoxins might contribute to KD (34). In a blinded, controlled study, 13 of 16 KD patients were found to be infected with TSST-1-producing (n = 11) or streptococcal pyrogenic exotoxin C-producing (n = 2) bacteria, compared with only 1 of 15 febrile controls. We proposed that TSST-1-producing Staphylococcus aureus and streptococcal pyrogenic exotoxin C-producing streptococci could account for the selective expansion of Vβ2-expressing T cells in the blood and tissues of patients with acute KD, which has now been reported by three independent groups (1, 2, 11, 33, 50). In a follow-up multicenter study, it was found that TSST-1-producing S. aureus could be isolated from 31 of 32 patients with acute KD (36).
Staphylococcal protein A (SpA) is produced by over 95% of S. aureus strains and has the ability to bind to the Fc region of immunoglobulin G (IgG) in most mammalian species. This protein has been shown to inhibit opsonization and phagocytosis of staphylococci in vitro, a factor that may contribute to the virulence of this organism in vivo (15, 42, 43). SpA also exhibits diverse immunobiological properties, including an ability to activate B cells (B-cell superantigen) and complement (17, 26–28, 48). Interestingly, these laboratory parameters of immune activation have been reported during acute KD (24, 29). Thus, together with T-cell superantigens, such as TSST-1, SpA could account for at least some of the immune activation associated with this disease.
It was recently observed that S. aureus isolated from KD patients produced less lipase, hemolysin, and protease than isolates from TSS and other skin diseases. However, the KD isolates did not produce particularly low levels of TSST-1 (34). The expression of these exoproteins is controlled by global regulatory loci, including agr (for accessory gene regulator) (38, 44). This locus consists of two divergent transcripts, RNAII and RNAIII, driven by two distinct promoters, P2 and P3, respectively (40). RNAIII is the effector molecule of this regulatory system. During the postexponential and stationary phases of growth, this molecule upregulates the expression of various exoproteins and downregulates the expression of factors such as SpA, the fibronectin-binding proteins, and coagulase (20, 41). In bona fide agr-defective mutants, there is a lack of RNAIII expression, low expression levels of the positively regulated extracellular factors such as hemolysins, proteases, and TSST-1, and high expression levels of the negatively regulated factors, such as SpA. It has thus been speculated that the KD isolates may represent a new clone of TSST-1-secreting S. aureus which has a partially defective agr locus, or these isolates may be bona fide agr-defective mutants which have reverted in their ability to express TSST-1 (34).
In this study, we further characterized the KD isolates by examining the level of SpA expressed by these isolates compared to TSS isolates. We report that, when considered as a group, the KD isolates express significantly higher levels of cell wall-associated and extracellular SpA than TSS isolates, another characteristic of an agr-defective phenotype. However, consistent with previous observations, no difference was seen in the level of TSST-1 expressed by the two groups of isolates. We investigated the possibility that the KD isolates may be partially defective in their agr loci by analyzing spa and RNAIII transcription levels and by nucleotide sequence analysis of the RNAIII-coding region.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus strains Cowan I and Wood 46 were obtained from the American Type Culture Collection (Rockville, Md.). S. aureus strains 8325-4, RN4282, and RN4256 (RN4282 agrA::Tn551) were kindly provided by R. P. Novick (Public Health Research Institute, New York, N.Y.). TSST-1-expressing S. aureus isolates were obtained from either pharyngeal or rectal swabs of 21 patients with acute KD. Each of these patients fulfilled the diagnostic guidelines of the American Heart Association (3). TSST-1-expressing vaginal isolates of S. aureus were obtained from 12 patients with menstrual TSS. S. aureus strains were grown in tryptic soy broth (TSB). Escherichia coli JM101 (51) was used for plasmid DNA transformations and was grown in Luria-Bertani broth, containing 100 μg of ampicillin per ml when appropriate.
Analysis of cell wall-associated SpA expression by flow cytometry.
Overnight cultures were diluted 1:50 in 5 ml of fresh TSB and were grown with shaking at 37°C to early and late exponential phases (Fig. 1A, t1 and t2, respectively). All the isolates had similar growth rates and equivalent optical density at 600 nm (OD600) values at each 1-h time point. Cells were pelleted at 2,500 × g for 10 min at 4°C and then resuspended and blocked in staining solution (5% fetal calf serum, 0.1% intravenous immunoglobulin, 0.02% sodium azide in phosphate-buffered saline [PBS]) for 1 h at 4°C. Following the addition of 0.5 μg of biotinylated anti-SpA monoclonal antibody (MAb) (Sigma, St. Louis, Mo.), the cells were incubated for a further 30 min at 4°C. Cells were then washed twice in a wash solution (2% fetal calf serum, 0.02% sodium azide in PBS) and resuspended in staining solution. Phycoerythrin-conjugated streptavidin (Southern Biotechnology Associates, Inc., Birmingham, Ala.) was added, and the cells were incubated for 30 min at 4°C. Cells were then washed once in wash solution and twice in 0.02% sodium azide in PBS. Stained cells were fixed with 1% methanol-free formaldehyde in PBS (Polysciences, Inc., Warrington, Pa.) and analyzed with a Becton Dickinson FACScalibur, by using Cellquest software. Relative cell wall-associated SpA expression was measured by mean fluorescence intensity (MFI) with the anti-SpA MAb.
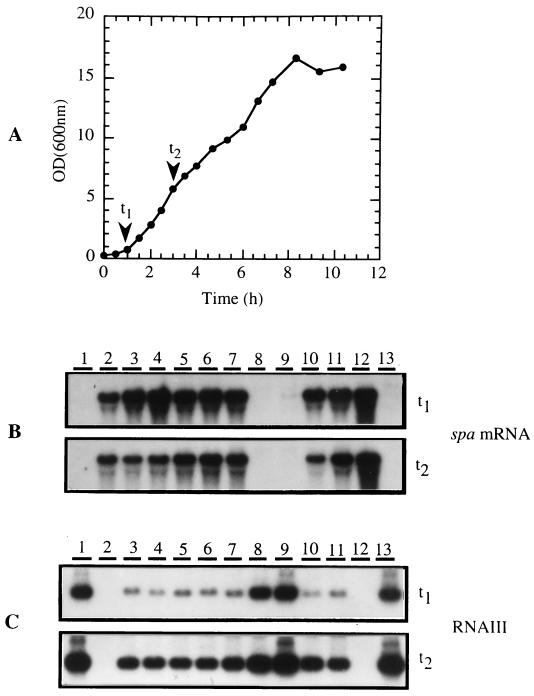
FIG. 1.
(A) Growth curve of S. aureus Cowan I. Growth curves obtained for the KD and TSS isolates were very similar. Time points (t1 and t2 represent early and late exponential phases, respectively) at which samples were taken for isolation of total RNA for Northern hybridization analysis are indicated by the arrows. Ten micrograms of total RNA was loaded in each well and probed for the (B) spa and (C) RNAIII transcripts, as described in the text. Samples were loaded as follows: lane 1, strain RN4282; lane 2, strain RN4256 (strain RN4282 agrA::Tn551); lanes 3 to 7, KD isolates (strains Yeh, KD1, KD6, KD-ATK, and Thomas, respectively); lanes 8 to 11, TSS isolates (strains MN8, FRI 1169, L. Park TN 1986, and K. Johnson MN 1985, respectively); lane 12, strain Cowan I; and lane 13, strain Wood 46. This analysis was performed in duplicate, with similar results.
Quantification of extracellular SpA expression by ELISA.
Extracellular SpA production was quantified by enzyme-linked immunosorbent assay (ELISA), as previously described (13). Overnight cultures were diluted 1:50 in 5 ml of fresh TSB and were grown with shaking at 37°C to early and late exponential phases (Fig. 1A, t1 and t2, respectively). All the isolates had similar growth rates and equivalent OD600 values at each time point. Cells were pelleted at 2,500 × g for 10 min at 4°C, and the supernatants were retained for analysis. Each well of a 96-well polystyrene plate (Corning, New York, N.Y.) was coated with 50 μl of a solution containing 10 μg of anti-SpA MAb (Sigma) per ml of 0.1 M NaHCO3 buffer (pH 8.2) for 18 h at 4°C. The wells were washed twice with PBS (pH 7.2) containing 0.05% Tween 20 (PBS/T) and then blocked with 200 μl of 1% bovine serum albumin (BSA) in PBS/T per well for 2 h at room temperature. After washing twice with PBS/T, 100 μl of a 1:10 dilution (in PBS/T containing 1% BSA) of each supernatant was added to the wells and incubated for 18 h at 4°C. The wells were washed four times with PBS/T, 100 μl of biotinylated anti-SpA MAb (Sigma) in PBS/T was added to each well, and the plate was incubated for 45 min at room temperature. After washing six times with PBS/T, 100 μl of avidin-peroxidase conjugate (Sigma) in PBS/T-BSA was added to each well, and the plate was incubated for 30 min at room temperature. Wells were washed eight times with PBS/T, 100 μl of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid] peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added to each well, and the plate was incubated at room temperature in the dark. After 9 min, the reaction was quenched by adding 100 μl of stop solution to each well. The OD405 was determined. A standard curve was prepared with known concentrations of a commercial preparation of SpA, purified from the cell wall of S. aureus Cowan I (Sigma).
Quantification of TSST-1 expression by ELISA.
Overnight cultures were diluted 1:50 in 5 ml of fresh TSB and were grown with shaking at 37°C to late exponential phase (Fig. 1A, t2). All the isolates had similar growth rates and equivalent OD600 values at each time point. Cells were pelleted at 2,500 × g for 10 min at 4°C. The supernatants were diluted 1:4 in PBS/T, normal rabbit serum was added to a final concentration of 1% (vol/vol), and the samples were incubated for 15 min at room temperature. Each well of a 96-well polystyrene plate (NUNC, Inc., Naperville, Ill.) was coated overnight with 100 μl of a solution containing 10 μg of anti-TSST-1 antibodies (Toxin Technology, Sarasota, Fla.) per ml of 0.1 M NaHCO3 buffer (pH 8.2) at 37°C. The plate was then washed twice with PBS/T and blocked with PBS/T for 30 min at room temperature. After washing twice with PBS/T, 100 μl of each diluted supernatant sample was added to the wells, and the plate was incubated for 2 h at 37°C. The plate was washed three times with PBS/T and then incubated with horseradish peroxidase-conjugated anti-TSST-1 antibodies (Toxin Technology) for 1 h at 37°C. Wells were washed 10 times with PBS/T, 100 μl of ABTS peroxidase (Sigma) was added to each well, and the plate was incubated at room temperature. After 15 min, the reaction was quenched by adding 50 μl of stop solution to each well. OD405 was determined. A standard curve was prepared with known concentrations of purified TSST-1 (Toxin Technology).
Isolation of RNA and Northern hybridization analysis.
Total cellular RNA was isolated from cultures grown to early and late exponential phases (Fig. 1A, t1 and t2, respectively) by using the RNeasy Mini kit (Qiagen Inc., Santa Clarita, Calif.). All the isolates had similar growth rates and equivalent OD600 values at each time point. Ten micrograms of each RNA sample (as determined by measuring the OD260) was resolved by 1.2% agarose-formaldehyde gel electrophoresis. RNA Millenium Markers (Ambion Inc., Austin, Tex.) were used as size standards. RNA was transferred onto a Hybond N+ membrane (Amersham Life Science, Inc., Arlington Heights, Ill.) and was hybridized with radioactively labeled DNA probes at 42°C, as described by Sambrook et al. (45). Probes were random primer labeled with [α-32P]dCTP to a specific activity of 1 × 108 to 5 × 108 cpm/μg by using the RadPrime DNA labeling system (Gibco BRL, Grand Island, N.Y.). Probe DNA for the spa and RNAIII transcripts was amplified by PCR from the chromosomes of strains Cowan I and 8325-4, respectively, as follows: spa, a 1.346-kb fragment (nucleotide [nt] 771 to 2117, according to Shuttleworth et al. [46]) by using the primers 5′-TTATATCTGGTGGCGTAACACCTGC-3′ and 5′-ATGCTTGAGCTTTGTTAGCATCTGC-3′, and RNAIII, a 945-bp fragment (nt 581 to 1526, according to Janzon et al. [21]) containing the entire RNAIII-coding sequence and the 5′ half of agrB by using the primers 5′-AAATACATAGCACTGAGTCCAAGG-3′ and 5′-TTTAATAAGTCGCACAGGAATGGG-3′. After hybridization, membranes were washed twice in a solution containing 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate (SDS) at 65°C and once in 0.1× SSC–0.1% SDS at 65°C, for 30 min each time. Membranes were exposed to Kodak X-Omat AR film at −80°C for various lengths of time.
Molecular cloning and sequencing.
An 862-bp fragment containing the entire RNAIII-coding region and approximately 160 bp of upstream sequence (nt 314 to 1175, according to Janzon et al. [21]) was amplified by PCR from the chromosomes of strain 8325-4, five KD isolates (Yeh, KD1, KD6, KD-ATK, and Thomas), and two TSS isolates (MN8 and FRI 1169) by using Pfu DNA polymerase (Stratagene, La Jolla, Calif.) and the primers 5′-ACCTTTTCCAACATTAGACTTATT-3′ and 5′-TTATTTCTCTTTTGAAGATACGTGG-3′. The products were cloned into the TA cloning vector pCR2.1 (Invitrogen, San Diego, Calif.). The constructs were purified with the QIAprep Spin Miniprep kit (Qiagen) and were sequenced in both directions with the Taq DyeDeoxy Terminator Cycle Sequencing kit (Perkin-Elmer Applied Biosystems, Foster City, Calif.), M13 reverse and T7 primers, and an automated DNA sequencer (model 377; Perkin-Elmer Applied Biosystems) at the University of Texas—Houston Medical School Molecular Genetics Core Facility. Nucleotide sequences were analyzed with SeqEd software (Perkin-Elmer Applied Biosystems).
RESULTS
Determination of cell wall-associated SpA expression levels.
The levels of cell wall-associated SpA produced by early- and late-exponential-phase (Fig. 1A, t1 and t2, respectively) cultures of the KD and TSS isolates were compared by flow cytometry, by using a biotinylated anti-SpA MAb and phycoerythrin-conjugated streptavidin. The MFI observed gives a relative measure of the cell wall-associated SpA expressed by each isolate (Fig. 2). Strains Cowan I and Wood 46 were included in this study as examples of strains which express high and very low levels of SpA, respectively. Strain RN4256 was also included as an example of an agr mutant that lacks RNAIII, a negative regulator of spa gene transcription. As anticipated, strains Cowan I and RN4256 produced significantly higher levels of cell wall-associated SpA than strain Wood 46 at both growth phases analyzed. When compared collectively, the KD isolates (n = 14) expressed significantly higher levels of cell wall-associated SpA than the TSS isolates (n = 11) at the early exponential growth phase (MFI of 950.04 ± 349.38 versus 95.98 ± 58.79; P < 0.05, Student’s t test). The same result was observed for late-exponential-phase cultures of the KD and TSS isolates (MFI of 1,597.07 ± 368.70 versus 228.99 ± 134.90; P < 0.05, Student’s t test).
FIG. 2.
Cell wall-associated SpA expression by KD and TSS isolates of S. aureus. Relative expression of SpA was determined using flow cytometry by measuring the MFI after incubation with biotinylated anti-SpA MAb and phycoerythrin-conjugated streptavidin, as described in the text. Each value represents the mean of duplicate analyses on the same isolate during the early and late exponential growth phases (t1 and t2 in Fig. 1A, respectively). Strains RN4256, Cowan I, and Wood 46 were included in the study as controls. KD isolates were found to express significantly (P < 0.05) more SpA on their cell surface than TSS isolates as calculated by Student’s t test.
Production of extracellular SpA.
The levels of extracellular SpA secreted by early- and late-exponential-phase cultures (Fig. 1A, t1 and t2, respectively) of the KD and TSS isolates were determined by ELISA (Fig. 3). Again, strains Cowan I, Wood 46, and RN4256 were included in the study as controls. When compared collectively, the KD isolates (n = 21) were found to secrete significantly higher levels of SpA than the TSS isolates (n = 12) at both growth phases analyzed (0.54 ± 0.10 versus 0.28 ± 0.22 μg/ml for early-exponential-phase cultures and 1.22 ± 0.42 versus 0.51 ± 0.35 μg/ml for late-exponential-phase cultures; P < 0.05, Student’s t test). However, when considered individually, a few TSS isolates were observed to express high levels of extracellular SpA comparable to the KD isolates.
FIG. 3.
Extracellular SpA secretion by KD and TSS isolates of S. aureus. The concentration of extracellular SpA in the supernatants of cultures grown to early and late exponential phases (t1 and t2 in Fig. 1A, respectively) was determined by ELISA with biotinylated anti-SpA MAb, as described in the text. Each value represents the mean of duplicate analyses of the same isolate. Strains RN4256, Cowan I, and Wood 46 were included in the study as controls. KD isolates were found to produce significantly (P < 0.05) more extracellular SpA than TSS isolates as calculated by Student’s t test.
Production of TSST-1.
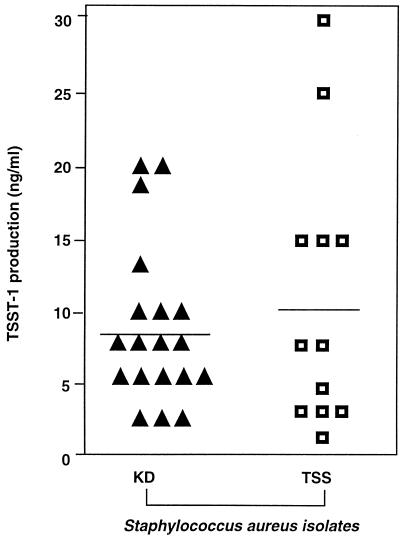
The amounts of TSST-1 secreted by late-exponential-phase cultures (Fig. 1A, t2) of the KD (n = 19) and TSS (n = 12) isolates were measured by ELISA (Fig. 4). The level of TSST-1 secretion was highly variable among individual isolates, ranging from 0.25 to 30.0 ng/ml. Furthermore, in contrast to cell wall-associated or extracellular SpA, there was no statistically significant difference in the levels of TSST-1 secreted by the KD or TSS groups of isolates at this growth phase (8.55 ± 5.41 and 10.00 ± 9.39 ng/ml, respectively).
FIG. 4.
Extracellular TSST-1 secretion by KD and TSS isolates of S. aureus. The concentration of TSST-1 in the supernatants of cultures grown to late exponential phase (t2 in Fig. 1A) was determined by ELISA with anti-TSST-1 MAb, as described in the text. Each value represents the mean of duplicate analyses of the same isolate. KD and TSS isolates secreted similar amounts of TSST-1.
Analysis of spa and RNAIII transcript levels.
To investigate the possibility that KD isolates may have a partially defective agr locus, the levels of spa and RNAIII transcripts in early- and late-exponential-phase cultures (Fig. 1A, t1 and t2, respectively) of the KD (n = 9) and TSS (n = 11) isolates were compared by Northern hybridization analysis. Strains Cowan I, Wood 46, and RN4282 and its isogenic, agr-negative mutant, RN4256, were included in the study as controls. The results obtained for some of the isolates analyzed are shown in Fig. 1B and 1C. High levels of spa mRNA (ca. 1.8 kb) were detected at both growth phases in all the KD isolates analyzed (Fig. 1B, lanes 3 to 7, and data not shown), strain RN4256 (Fig. 1B, lane 2), and strain Cowan I (Fig. 1B, lane 12). This is consistent with the finding that these strains express high levels of SpA. A similar result was observed for a few TSS isolates (Fig. 1B, lanes 10 and 11, and data not shown). These TSS isolates expressed high levels of SpA, comparable to the KD isolates. The spa mRNA was undetectable or barely detectable in the remaining TSS isolates analyzed (Fig. 1B, lanes 8 and 9, and data not shown), strain RN4282 (Fig. 1B, lane 1), and strain Wood 46 (Fig. 1B, lane 13). This is consistent with the observation that SpA is expressed at significantly lower levels by these strains.
The RNAIII transcript (ca. 510 bp) was detected in both early- and late-exponential-phase cultures of all the strains analyzed, except for strains RN4256 and Cowan I (Fig. 1C and data not shown). This is consistent with strain RN4256 being an agr-defective mutant. It also suggests that Cowan I has an uncharacterized agr defect, which may explain the high level of SpA expressed by this strain. The RNAIII transcript was more abundant in the late-exponential-phase cultures of all the isolates tested, consistent with previous reports on the regulation of the expression of this factor. However, significantly less RNAIII was detected in the early-exponential-phase cultures of all the KD isolates and a few of the TSS isolates than of strains RN4282, Wood 46, and the remaining TSS isolates. Furthermore, an inverse correlation was observed between the levels of the RNAIII and spa transcripts detected for each strain at this growth phase. This is consistent with RNAIII functioning as a negative regulator of spa gene transcription. Interestingly, despite the elevated levels of RNAIII in the late-exponential-phase cultures of the KD isolates and certain TSS isolates, the amount of spa mRNA did not decrease dramatically in these isolates.
DNA sequence analysis of the RNAIII-coding region.
Although all the KD isolates analyzed express RNAIII, it is possible that this transcript may not be functional in these strains. To address this question, the DNA sequence of a ca. 860-bp chromosomal fragment encompassing the RNAIII reading frame, the RNAIII P3 promoter, and the agr operon P2 promoter of five KD isolates and strains MN8 and FRI 1169 (TSS isolates) was determined. Each sequence was identical except that for strain FRI 1169 (data not shown). The sequences were compared to that published for strain 8325-4 (GenBank accession no. X17301), a well-characterized Agr+ strain. The FRI 1169 sequence was identical to this except for a single nucleotide substitution 2 nt upstream from the RNAIII transcriptional start site. The sequences of the other strains differed from that of strain 8325-4 in 10 nucleotide positions. However, all of these substitutions were outside the RNAIII-coding region and the −10 and −35 boxes of both the P2 and P3 promoters. There were also two insertions in the MN8/KD isolate sequence compared to strain 8325-4. A single nucleotide insertion which was not predicted to affect the RNAIII secondary structure was found at the 3′ end of the RNAIII-coding region (but outside the hld gene), and a 4-nt insertion was found just downstream from the RNAIII transcriptional termination site. Therefore, the sequence of the RNAIII molecule appears to be highly conserved in all the strains analyzed, with no apparent mutations that might affect the functionality or transcription level of the molecule.
DISCUSSION
It has been previously reported that TSST-1-secreting strains of S. aureus isolated from patients with KD exhibit an unusual phenotype which distinguishes them from TSS isolates, including the expression of lower levels of hemolyins, lipase, and protease (34). In this study, we describe another phenotype associated with KD isolates. When considered collectively, these isolates were found to express significantly more cell wall-associated and extracellular SpA than isolates from patients with TSS. This correlated with a higher level of spa mRNA expression in the KD isolates. Nonetheless, when considered individually, a few TSS isolates which expressed levels of spa mRNA and SpA comparable to the KD isolates were observed. Thus, high levels of SpA expression are not unique to the KD isolates but do appear to be a characteristic phenotype of these strains. In contrast, the KD and TSS isolates expressed comparable levels of TSST-1 in this study, consistent with previous findings (34).
It was recently suggested that the KD isolates might have a defective agr regulatory system that may be responsible for the usual phenotype associated with these strains (34). The high levels of SpA expressed by the KD isolates in this study are also typical of agr-defective mutants. Interestingly, the agr locus is genetically unstable in vitro (4, 37). Therefore, in this study, we chose to investigate the possibility that the KD isolates may have an agr defect. The agr locus consists of two divergent transcripts, RNAII, which contains agrB, -D, -C, and -A, and RNAIII, the effector molecule of the regulatory system (20, 41). The transcription of RNAIII is regulated by the agr system itself, and RNAIII is not transcribed in mutants that have a defect in any of the agr genes (40). In this study, all of the isolates analyzed expressed RNAIII, suggesting that all of the agr genes are functional. In addition, nucleotide sequence analysis of the RNAIII-coding regions of five representative KD isolates suggested that the RNAIII molecules were not mutated in these strains. Taken together, these results suggest that the KD isolates are not bona fide agr-defective mutants.
In this study, a correlation was observed between the levels of RNAIII and spa mRNA detected in each isolate in the early exponential phase (Fig. 1B and 1C, t1). In the TSS isolates which produced low levels of spa mRNA and SpA protein, high levels of RNAIII were detected (Fig. 1B and 1C, lanes 8 and 9, and data not shown). This suggests that the high levels of RNAIII in these isolates may be suppressing the transcription of the spa mRNA. In contrast, high levels of spa mRNA were detected in early-exponential-phase cultures of the KD isolates and in the few TSS isolates that expressed high levels of SpA (Fig. 1B and 1C, lanes 3 to 7, 10, and 11, and data not shown). Furthermore, despite an obvious increase in the amounts of RNAIII in the late-exponential-phase cultures of these isolates, the amount of spa mRNA detected did not decrease dramatically. This observation differs from the results of previous studies in which the spa mRNA was undetectable immediately following the induction of RNAIII (47). The reason for this discrepancy is unknown. How RNAIII suppresses spa gene transcription has not been determined. It is possible that RNAIII indirectly regulates this and other genes by its effect on an as-yet-unidentified regulatory gene(s). As the RNAIII expressed by the KD isolates appears to be intact (based on the DNA sequence of the RNAIII-coding region), it is possible that there may be a defect in one of these downstream regulatory genes such that spa mRNA transcription is not suppressed in these strains. Alternatively, there may be a mutation(s) in the spa promoter region that makes it less susceptible to the activity of these negative regulators.
It should also be pointed out that other trans-acting regulatory loci may be involved in determining the level at which the spa mRNA is transcribed or translated (7, 9, 16). In fact, it has recently been reported that the sar regulatory locus suppresses spa mRNA transcription by agr-dependent and agr-independent mechanisms (6). The SarA protein is an activator of RNAII and RNAIII transcription, binding to a nucleotide sequence between the P2 and P3 promoters of the agr locus (8, 10, 19). Thus, by optimizing the transcription of RNAIII, SarA indirectly suppresses spa mRNA transcription. How the sar locus suppresses spa mRNA transcription independently of agr has not yet been determined. Given that in sarA-defective mutants protease expression is increased and RNAIII transcription is greatly reduced or absent (5, 8, 10, 19), it seems unlikely that the KD isolates are defective in the sar regulatory locus.
It is clear from this and previous studies that the KD isolates have similar phenotypes (34). In addition, our preliminary observations suggest that these isolates may also be very similar at the genotypic level. We have noted that the chromosomal restriction enzyme digestion profiles of these isolates are very similar, if not identical. Furthermore, Southern hybridization and PCR analyses of the spa and coagulase loci of these strains suggest that they are highly conserved, encoding five N-terminal IgG-binding repeats and four C-terminal fibrinogen-binding repeats, respectively (unpublished data). In addition, the nucleotide sequence of a ca. 860-bp chromosomal region encompassing the RNAIII-coding region and flanking DNA was identical in the KD isolates analyzed. Indeed, it is tempting to speculate that the KD isolates may be clonal in origin, as is the case for TSS isolates (39). The KD isolates used in this study were collected from a variety of geographical locations within the United States. A multicenter epidemiological study of KD isolates using traditional typing methods, such as restriction fragment length polymorphism and multilocus enzyme electrophoresis analysis, or newer typing methods based on DNA sequence analysis is warranted to further investigate the clonality of these isolates.
It is a tantalizing possibility that the expression of high levels of extracellular SpA, secreted locally by S. aureus isolates colonizing the gastrointestinal tract of patients, may contribute to the symptoms of KD. It has been recently demonstrated that, in addition to exhibiting immunoregulatory activities, SpA has the ability to bind to von Willebrand factor, a protein that has a central role in hemostasis and thrombogenesis (18). This raises the exciting possibility that, in addition to inducing immune activation, SpA could perturb host hemostasis and increase the risk of vascular thrombosis, such as that associated with KD. Interestingly, it has been noted that coronary complications develop in some patients diagnosed with TSS (12, 49). Whether these patients are infected with S. aureus strains that secrete high levels of SpA remains to be determined. Significantly, such TSS isolates were observed in this study. However, additional bacterial and host factors are likely to be involved in determining whether a patient develops classical TSS or KD (35). Investigation of the potential role of SpA in the development of KD will require an animal model of this disease in which the contribution of this and other factors can be analyzed.
ACKNOWLEDGMENTS
This study was supported in part by Public Health Services Research grants HL36577, AR41256, and HL37260 and National Institutes of Health grant AI20624.
We thank Maureen Sandoval for her assistance in the preparation of the manuscript.
REFERENCES
- 1.Abe J, Kotzin B L, Jujo K, Melish M E, Glode M P, Kohsaka T, Leung D Y M. Selective expansion of T cells expressing T-cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–4070. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe J, Kotzin B L, Meissner C, Melish M E, Takahashi M, Fulton D, Romagne F, Malissen B, Leung D Y M. Characterization of T cell repertoire changes in acute Kawasaki disease. J Exp Med. 1993;177:791–796. doi: 10.1084/jem.177.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Heart Association Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease. Diagnostic guidelines for Kawasaki disease. JAMA. 1989;44:1218–1219. [PubMed] [Google Scholar]
- 4.Björklind A, Arvidson S. Mutants of Staphylococcus aureus affected in the regulation of exoprotein synthesis. FEMS Microbiol Lett. 1980;7:203–206. [Google Scholar]
- 5.Chan P F, Foster S J. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J Bacteriol. 1998;180:6232–6241. doi: 10.1128/jb.180.23.6232-6241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung A L, Eberhardt K, Heinrichs J H. Regulation of protein A synthesis by the sar and agr loci of Staphylococcus aureus. Infect Immun. 1997;65:2243–2249. doi: 10.1128/iai.65.6.2243-2249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A L, Projan S J. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994;176:4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A L, Wolz C, Yeaman M R, Bayer A S. Insertional inactivation of a chromosomal locus that modulates expression of potential virulence determinants in Staphylococcus aureus. J Bacteriol. 1995;177:3220–3226. doi: 10.1128/jb.177.11.3220-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien Y-T, Cheung A L. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J Biol Chem. 1998;273:2645–2652. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 11.Curtis N, Zheng R, Lamb J R, Levin M. Evidence for a superantigen mediated process in Kawasaki disease. Arch Dis Child. 1995;72:308–311. doi: 10.1136/adc.72.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies H D, Kirk V, Jadavji T, Kotzin B L. Simultaneous presentation of Kawasaki disease and toxic shock syndrome in an adolescent male. Pediatr Infect Dis J. 1996;15:1136–1138. doi: 10.1097/00006454-199612000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Ezepchuk Y V, Leung D Y M, Middleton M H, Bina P, Reiser R, Norris D A. Staphylococcal toxins and protein A differentially induce cytotoxicity and release of tumor necrosis factor-alpha from keratinocytes. J Investig Dermatol. 1996;107:603–609. doi: 10.1111/1523-1747.ep12583377. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, Kato H, Yabuta K. Peripheral blood monocyte/macrophage and serum tumor necrosis factor in Kawasaki disease. Clin Exp Immunol. 1988;48:247–251. doi: 10.1016/0090-1229(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 15.Gemmell C G, Tree R, Patel A, O’Reilly M, Foster T J. Susceptibility to opsonophagocytosis of protein A, α-haemolysin and β-toxin deficient mutants of S. aureus isolated by allele-replacement. Zentbl Bakteriol. 1991;21(Suppl.):273–277. [Google Scholar]
- 16.Giraudo A T, Raspanti C G, Calzolari A, Nagel R. Characterization of a Tn551-mutant of Staphylococcus aureus defective in the production of several exoproteins. Can J Microbiol. 1994;40:677–681. doi: 10.1139/m94-107. [DOI] [PubMed] [Google Scholar]
- 17.Gustafson G T, Stalenheim G, Forsgren A, Sjoquist J. “Protein A” from Staphylococcus aureus. IV. Production of anaphylaxis-like cutaneous and systemic reactions in non-immunized guinea pigs. J Immunol. 1968;100:530–534. [PubMed] [Google Scholar]
- 18.Hartleib J, Koehler N, Dickinson R, Chhatwal S, Sixma J J, Foster T, Peters G, Kehrel B, Herrmann M. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. 1998. Binding mechanisms of Staphylococcus aureus to von Willebrand factor: protein A revisited, abstr. B-80. [Google Scholar]
- 19.Heinrichs J H, Bayer M G, Cheung A L. Characterization of the sar locus and its interaction with agr in Staphylococcus aureus. J Bacteriol. 1996;178:418–423. doi: 10.1128/jb.178.2.418-423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janzon L, Arvidson S. The role of the δ-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990;9:1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janzon L, Lofdahl S, Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989;219:480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- 22.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children: clinical observations of 50 cases. Jpn J Allergol. 1967;16:178–222. [PubMed] [Google Scholar]
- 24.Kohsaka T, Abe J, Asahina T, Kobayashi N. Classical pathway complement activation in Kawasaki syndrome. J Allergy Clin Immunol. 1994;93:520–525. doi: 10.1016/0091-6749(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 25.Kotzin B L, Leung D Y M, Kappler J, Marrack P. Superantigens and human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski L M, Kunning S R, Zheng Y, Wheatley W L, Levinson A I. Staphylococcus aureus Cowan I-induced human immunoglobulin responses: preferential IgM rheumatoid factor production and VH3 mRNA expression by protein A binding cells. J Clin Immunol. 1995;15:145–151. doi: 10.1007/BF01543106. [DOI] [PubMed] [Google Scholar]
- 27.Kozlowski L M, Li W, Goldschmidt M, Levinson A I. In vivo inflammatory response to a prototypic B cell superantigen: elicitation of an Arthus reaction by staphylococcal protein A. J Immunol. 1998;160:5246–5252. [PubMed] [Google Scholar]
- 28.Kozlowski L M, Soulika A M, Silverman G J, Lambris J D, Levinson A I. Complement activation by a B cell superantigen. J Immunol. 1996;157:1200–1206. [PubMed] [Google Scholar]
- 29.Laxer R M, Schaffer F M, Myones B L, Yount W J, Rowe R D, Rubin L, Stein L D, Gelfand E W, Silverman E D. Lymphocyte abnormalities and complement activation in Kawasaki disease. Prog Clin Biol Res. 1987;250:175–184. [PubMed] [Google Scholar]
- 30.Leung D Y M, Chu E T, Wood N, Grady S, Meade R, Geha R S. Immunoregulatory T cell abnormalities in mucocutaneous lymph node syndrome. J Immunol. 1983;130:2002–2004. [PubMed] [Google Scholar]
- 31.Leung D Y M, Cotran R, Kurt-Jones E, Burns J, Newburger J, Pober J S. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989;ii:1298–1302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- 32.Leung D Y M, Geha R S, Newburger J W, Burns J C, Fiers W, Lapierre L A, Pober J S. Two monokines, interleukin 1 and tumor necrosis factor, render cultured vascular endothelial cells susceptible to lysis by antibodies circulating during Kawasaki syndrome. J Exp Med. 1986;164:1958–1972. doi: 10.1084/jem.164.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung D Y M, Giorno R C, Kazemi L V, Flynn P A, Busse J B. Evidence for superantigen involvement in cardiovascular injury due to Kawasaki syndrome. J Immunol. 1995;155:5018–5021. [PubMed] [Google Scholar]
- 34.Leung D Y M, Meissner H C, Fulton D R, Murray D L, Kotzin B L, Schlievert P M. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet. 1993;342:1385–1388. doi: 10.1016/0140-6736(93)92752-f. [DOI] [PubMed] [Google Scholar]
- 35.Leung D Y M, Schlievert P M, Meissner H C. The immunopathogenesis and management of Kawasaki syndrome. Arthritis Rheum. 1998;41:1538–1547. doi: 10.1002/1529-0131(199809)41:9<1538::AID-ART3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 36.Leung D Y M, Sullivan K E, Brown-Whitehorn T F, Fehringer A P, Allen S, Finkel T H, Washington R L, Schlievert P M. Association of toxic shock syndrome toxin- and exfoliative toxin-secreting Staphylococcus aureus with Kawasaki syndrome complicated by coronary artery disease. Pediatr Res. 1997;42:268–272. doi: 10.1203/00006450-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 37.McNamara P J, Iandolo J J. Genetic instability of the global regulator agr explains the phenotype of the xpr mutation in Staphylococcus aureus KSI9051. J Bacteriol. 1998;180:2609–2615. doi: 10.1128/jb.180.10.2609-2615.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morfeldt E, Janzon L, Arvidson S, Lofdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988;211:435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- 39.Musser J M, Schlievert P M, Chow A W, Ewan P, Kreiswirth B N, Rosdahl V T, Naidu A S, Witte W, Selander R K. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc Natl Acad Sci USA. 1990;87:225–229. doi: 10.1073/pnas.87.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novick R P, Projan S J, Kornblum J, Ross H F, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet. 1995;248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 41.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel A H, Nowlan P, Weavers E D, Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55:3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson P K, Verhoef J, Sabath L D, Quie P G. Effect of protein A on staphylococcal opsonization. Infect Immun. 1977;15:760–764. doi: 10.1128/iai.15.3.760-764.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Recsei P, Kreiswirth B, O’Reilly M, Schlievert P M, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Shuttleworth H L, Duggleby C J, Jones S A, Atkinson T, Minton N P. Nucleotide sequence analysis of the gene for protein A of S. aureus Cowan I (NCTC8530) and its enhanced expression in E. coli. Gene. 1987;58:283–295. doi: 10.1016/0378-1119(87)90383-0. [DOI] [PubMed] [Google Scholar]
- 47.Vandenesch F, Kornblum J, Novick R P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991;173:6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasquez K S, Pascual V, Lipsky P E. Staphylococcal protein A induces biased production of immunoglobulin by VH3 expressing human B lymphocytes. J Immunol. 1994;150:2974–2982. [PubMed] [Google Scholar]
- 49.Wiesenthal A M, Todd J K. Toxic shock syndrome in children aged 10 years or less. Pediatrics. 1984;74:112–117. [PubMed] [Google Scholar]
- 50.Yamashiro Y, Nagata S, Oguchi S, Shimizu I T. Selective increase of Vβ2+ T cells in the small intestinal mucosa in Kawasaki disease. Pediatr Res. 1996;39:264–266. doi: 10.1203/00006450-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]