Abstract
Objective
To examine the effect on adherence to disease modifying anti-rheumatic drugs (DMARDs) in participants with rheumatoid arthritis (RA) of a serious game that targeted implicit attitudes toward medication.
Methods
A multicentre randomised controlled trial (RCT) was performed with adults with RA that used DMARDs and possessed a smartphone/tablet. Control and intervention groups received care as usual. The intervention group played the serious game at will during 3 months. Game play data and online questionnaires Compliance Questionnaire on Rheumatology (CQR), Beliefs about Medicine Questionnaire (BMQ), Health Assessment Questionnaire (HAQ) and Rheumatoid Arthritis Disease Activity Index (RADAI) were collected. Primary outcome was DMARD implementation adherence operationalised as the difference in proportion of non-adherent participants (<80% taking adherence) between intervention and control group after 3 months using a Chi-squared test. Two sample t-tests and Wilcoxon rank-sum test were performed to test for differences on secondary outcomes.
Results
Of the 110 intervention participants that started the study, 87 participants (79%) installed the game and had a median playtime of 9.7 hours at 3 months. Overall, 186 participants completed the study. Adherence in intervention group (63%) and control group (54%) did not differ significantly (p=0.13) at 3 months. Neither were there differences oberved in CQR continuous score, beliefs about medication (BMQ) or clinical outcomes (HAQ and RADAI).
Conclusion
A serious game aimed at reinterpreting attitudes toward medication failed to show an effect on adherence to DMARDs or clinical outcomes in patients with RA. The game was played frequently indicating that it can be an effective channel for reaching patients.
Trial registration number
NL7217.
Keywords: Rheumatoid Arthritis, Antirheumatic Agents, Health services research
WHAT IS ALREADY KNOWN ON THIS TOPIC
In half of the patients with rheumatoid arthritis (RA), explicit attitudes towards methotrexate are positive whereas implicit attitudes are negative and this latter is a possible target for improving medication adherence.
Serious games that target implicit attitudes are effective in health behaviour change but have not been applied to improving medication adherence.
WHAT THIS STUDY ADDS
Our serious game that targeted implicit medication attitudes did not improve disease modifying anti-rheumatic drug adherence in patients with RA.
Patients engaged frequently with our serious game during 3 months.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
A serious game can be an effective channel in reaching patients with RA over time.
Introduction
Disease-modifying anti-rheumatic drugs (DMARDs) are effective in reducing disease activity and radiological progression and in increasing daily functioning in patients with rheumatoid arthritis (RA).1 2 These benefits can only be achieved when patients adhere to the agreed pharmacotherapeutic regimen.3 However around one third of patients with RA fail to correctly implement DMARD therapy in their daily routines, leading to suboptimal treatment effectiveness.4–6 As a result, there is a need for adherence improving interventions.
To date, interventions that aim to improve implementation adherence appear only partly effective.7–10 Part of this ineffectiveness might be caused by interventions insufficiently targeting implicit behavioural factors of non-adherence. Behavioural intentions such as taking medication are the net result of a person’s explicit (conscious) and implicit (unconscious) attitudes and these attitudes do not necessarily align.11 Explicitly a person might say that medication alleviates symptoms whereas implicitly the same person regards medication as unnatural.11 12 Habitual behaviour, like medication taking behaviour, happens mainly on an unconscious level where implicit attitudes dominate.13 An effective strategy to improve medication adherence might thus be to target implicit attitudes.14
Implicit attitudes are readjusted by training the brain to interpret a stimulus differently and consequently change non-conscious processes.15 This could, for instance, be done by performing behavioural tasks that lead to attending to a neutral or positive stimulus when confronted with a cue, which, in our case, would be medication.16 Such training needs rigorous and repetitive performing of behavioural tasks to change non-conscious processes and eventually behaviour. eHealth can be a suitable mode of delivery for repetitive practicing as it is easily accessible and allows patients to perform tasks at a convenient place and time. Repetitive practicing requires ongoing engagement with the intervention that is best achieved when participants are intrinsically motivated. Intrinsic motivation can be elicited by serious games: games that intend to entertain and to achieve at least one additional goal simultaneously such as learning or health.17 Serious games have been shown to positively influence eating behaviour by targeting implicit attitudes in children.18 No games have, as yet, been developed to counter suboptimal long-term medication adherence by targeting implicit attitudes in adults.
We developed a serious puzzle game aimed at improving medication adherence by targeting implicit attitudes toward medication in patients with RA.19 The serious game was built as an application on smartphone or tablet and contains four puzzle types: crossword, sudoku, word search and tangram. When opening the game or a puzzle, players had to perform behavioural tasks that aimed at reinterpreting their attitudes toward medication. The Gaming for Adherence to Medication using Ehealth in Rheumatoid arthritis (GAMER) trial aims to assess the effectiveness of this serious game on the implementation adherence of DMARDs compared with usual care alone.
Methods
Trial design and setting
This is a multi-centre randomised assessor-blinded controlled trial with a follow-up of 3 months. The trial has been registered in the Dutch trial register (https://www.trialregister.nl/trial/7217) and reporting adheres to both the CONSORT-EHEALTH and EMERGE guideline (see online supplemental material S1 for checklists). Ethical approval was asked for and waived by the local Medical Research Ethics Committee of the Radboud university medical centre (METC Oost Nederland, protocol number 2018-4648) and the trial complies with the Helsinki declaration. Two patient research partners were involved in the design phase of the study and another two patient research partners discussed the results and its implications with one of the researchers (BP). The GAMER trial was conducted in the outpatient rheumatology clinics of six hospitals in The Netherlands between August 2019 and April 2021.
rmdopen-2022-002616supp001.pdf (260.2KB, pdf)
Recruitment and eligibility criteria
The hospital information system provided a list of eligible participants who were randomly selected by using a random number generator and were sent an information letter with informed consent form and a reminder after no response by 3 weeks. For participants, the goal of the study was framed as assessing the effect of playing a puzzle game on the experience of RA disease burden. Medication adherence was not mentioned to prevent participants from modifying their adherence behaviour.
Inclusion criteria were: clinical diagnosis of RA, current DMARD use (no adherence criteria), self-management of medication (no support of caregiver, home care or use of a multi-dose drug dispensing system), possession of a smartphone or tablet running on iOS/Android software and a valid email address. Participants were excluded if they were not proficient in the Dutch language or participated in another trial. After providing written informed consent, participants were telephoned by the research team to check if they were compliant with eligibility criteria.
Randomisation and blinding
Participants were allocated to the intervention or control group on a 1:1 ratio. Randomisation was concealed before allocation and performed by CastorEDC, stratified by hospital and variable block randomisation with block sizes of two, four and six. Due to the design of the trial, blinding of participants and researchers was not possible although the assessor was blinded. Caregivers were not informed of study allocation.
Study arms
Control group
The control group received care as usual only. This consisted of regular consultations with the rheumatologist and is detailed in the treating guideline of the Dutch Rheumatology Association.20 Implementation adherence is subject of the consultation only if problems arise or if there are reasons to believe there is non-adherence. Control group participants were offered access to the intervention when they finished the final questionnaire at 3 months.
Intervention group
Intervention participants also received care as usual. Next to this, they received email instructions to download and install the serious game free of charge using their research code and were reminded to do so twice. Participants were told to play the intervention at will. If participants allowed app notifications they received a daily ‘come-and-play’ reminder.
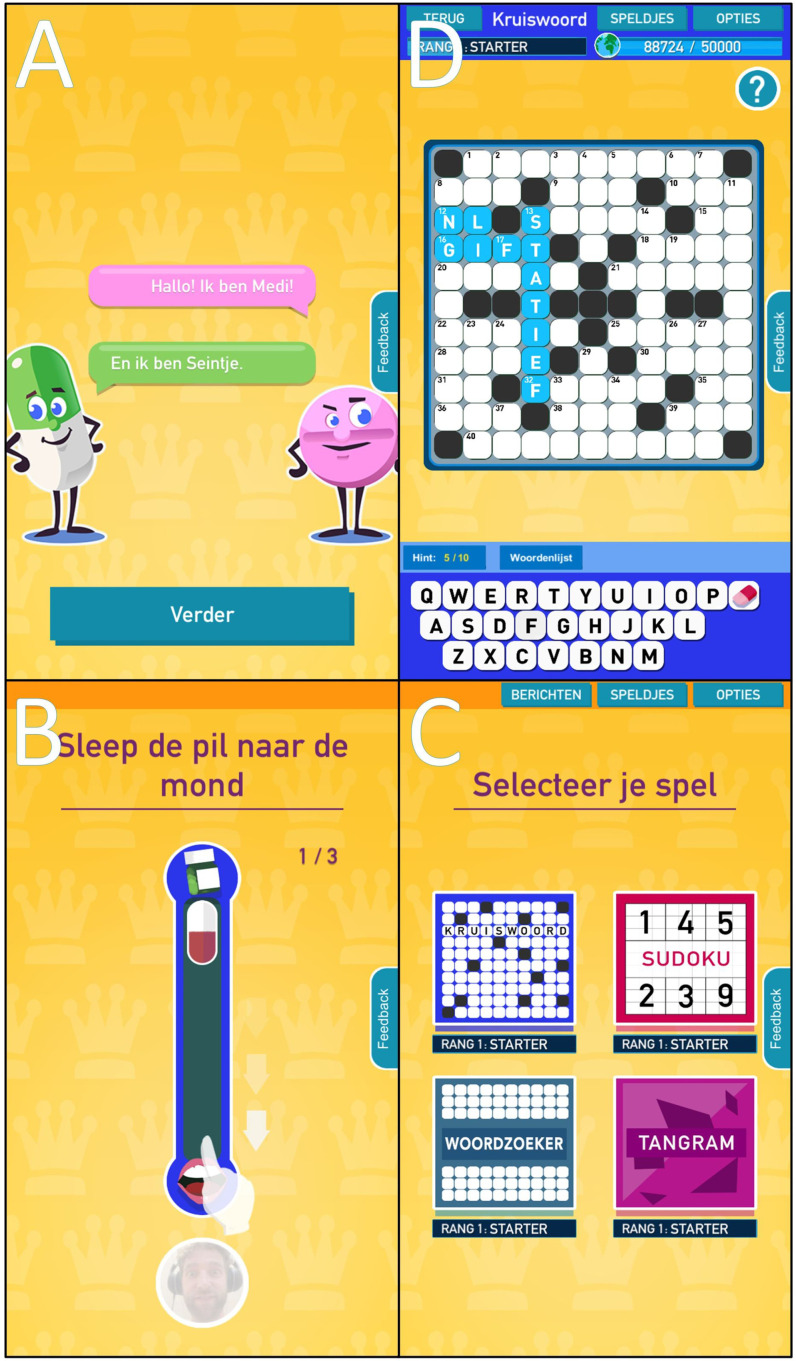
The development and participant pilot-testing of the serious game was guided by the Intervention Mapping framework and published elsewhere.19 Game Solutions Lab developed the game in co-creation with the Sint Maartenskliniek and AbbVie. In short, the storyboard of the serious game consisted of two ‘hosts’: a cartoon tablet and capsule (see figure 1A). They gave puzzle instructions, encouragements and daily ‘come-and-play’ notifications if these were allowed in the game’s settings. The ‘game’ part contained four puzzle types (see figure 1C): crossword (see figure 1D), sudoku, word search and tangram. Each puzzle type had varying difficulty levels and at least 50 puzzles to play. The ‘serious’ part consisted of behavioural tasks that players had to perform to open the game or a puzzle (see figure 1B). The behavioural tasks aimed to target implicit attitudes toward medication and were gamified behaviour change techniques based on attention bias modification, evaluative conditioning and goal priming.
Figure 1.
Serious game screenshots. Four screenshots of the studied Dutch serious game ‘Medi & Seintje’. Stills (A), (B), (C) and (D) are explained in the text.
Technical issues were resolved during the trial but content and functionality of the app remained unaltered. During the trial, one technical error occurred where the app failed to communicate with the server. Forty-one participants were possibly hindered by this error and informed by email how to resolve the issue.
Data collection
Participants received a study code and all data were logged using electronic data management software CastorEDC (ISO 27001 and ISO 9001 compliant). CastorEDC was also used to send questionnaires through email. Medication adherence and beliefs about medication questionnaires were collected at baseline, 1 and 3 months. In addition, clinical patient-reported outcomes were collected at 3 months, intervention play data at 1 and 3 months and demographic data and gaming experience at baseline.
When the study commenced on August 2019, participants were telephoned to make a start-of-study appointment in the pharmacy to allow for a pill/syringe count. Due to the COVID-19 regulations effective from March 2020 (leading to pharmacies delivering medication), pill/syringe count was abandoned and the study became fully digital.
Measurement instruments
Medication adherence
Primary outcome was DMARD implementation adherence at 3 months, assessed as the difference in proportion of non-adherent participants (<80% taking adherence3) between intervention (serious game and usual care) and control group (usual care) using the discriminant function of the Compliance Questionnaire on Rheumatology (CQR, 19 Likert-scaled items, item scores ranging from 1 to 421). The negative formulated items were recoded after which the critical cut-off score of −0.5849 was calculated to discriminate between adherent (≥80%) and non-adherent (<80%) as described by de Klerk et al.22 The discriminant function is able to detect whether a patient is adherent with a sensitivity of 62% and a specificity of 95% as validated using an electronic medication monitoring device over a period of 6 months.22 Because it was uncertain if participants would engage with the game for 3 months, the effect of the intervention on medication adherence was also assessed at 1 month using the CQR. Additionally, we report on the continuous CQR score, which was calculated by transforming sum scores to a scale between 0 and 100.21
Medication adherence was also assessed using pill/syringe count. Participants were supplied with a set and surplus amount of one of their DMARDs at study start and asked to commit to using this stock only during the study. At the end of the study, participants brought the remainder to the pharmacy and the pharmacy technician counted the medication in the presence of the participant. This outcome was abandoned in March 2020 when Dutch COVID-19 regulations took effect.
Beliefs about medication
The Beliefs about Medicine Questionnaire Specific (BMQ-Specific, 10 Likert-scale items, item scores ranging from 1 to 523 24) that assesses both beliefs about the necessity of medication and concerns about medication was also completed at 1 and 3 months. The sum scale score for necessity beliefs was subtracted from the sum scale score for concern beliefs to yield the necessity–concerns differential (NCD) score (range: −20 to 20). A positive NCD score indicates that necessity beliefs dominate concern beliefs.
Clinical outcomes
To assess the effect of the intervention on clinical outcomes, the Rheumatoid Arthritis Disease Activity Index (RADAI, 5 items) and the Health Assessment Questionnaire (HAQ, 20 items with five dimensions) were collected at 3 months. The patient-reported disease activity using RADAI correlated with physician’s assessment and swollen joint count (Spearman’s ρ=0.54, p<0.01 for both) and changes in the RADAI correlate strongly (r2=0.70, p<0.0001) with changes in the Disease Activity Score-28 (the golden standard for clinical disease activity in RA). As such, the RADAI is deemed a highly reliable and valid self-administered measure of disease activity. All five items are transformed into a 0–10 scale and averaged to provide a single 0–10 index of patient-assessed disease activity where a higher score indicates higher disease activity. The HAQ provides a single index value for health status with good reliability (α=0.88). This disability index (HAQ-DI) is determined by the highest subcategory score for each category unless aids or devices were used. Participants were included in this calculation only if at least six of the eight categories were completed. The HAQ-DI (range: 0–3) is the average of these eight category scores with higher scores indicating more disability (category 0–1: mild to moderate disability, 1–2: moderate to severe disability, 2–3: severe to very severe disability).
Intervention use
Intervention use was determined by extracting the following statistics from Google Firebase: total play time, number of sessions, average session time, number of completed behavioural tasks and the time span in which activity was observed. Additionally, acceptability of playing the serious game was assessed according to the Technology Acceptance Model.25 Methods and results are available as online supplemental material S2.
Sample size
Previous studies in the Sint Maartenskliniek demonstrated that 35% of patients with RA that use DMARDs are non-adherent.26 27 A slight Hawthorne effect was expected for all participants due to actively measuring adherence, meaning that non-adherence was expected to decrease to 30% of the population irrespective of randomisation. With an assumed intervention effect of 50% on non-adherent participants (without effecting adherent participants allowing for one-sided testing), the hypothesis was that 15% of the intervention group would be non-adherent compared with 30% in the control group at 3 months. A target sample size of 110 participants per arm was computed to provide 80% power to detect a single-sided 15% difference in adherence after 3 months with 15% loss to follow-up.
Statistical analysis
Descriptive statistics were performed to describe patient and disease characteristics.
Primary endpoint of the study, adherence at 3 months using the discriminant function of the CQR, was assessed with a Chi-square test to test for difference in proportions between study groups. Two sample t-tests and Wilcoxon rank-sum test were performed to test for differences between study groups for normally distributed and non-normally distributed variables, respectively. Primary analyses were performed according to the intention-to-treat principle (ITT). Secondary analyses included a per-protocol analysis where all intervention participants who played the game for more than 1 hour during the study period were considered adherent to the protocol. Exposure-response analyses were also performed: total play time was plotted against the continuous outcomes (CQR, BMQ NCD, RADAI and HAQ) to determine regression coefficient. In addition, playtime was plotted for both adherent and non-adherent intervention participants, based on the CQR, to determine whether there was a difference in average playtime between both groups.
P values <0.05 were considered statistically significant. Statistical analyses were performed using Stata V.13.1.
Results
Participants
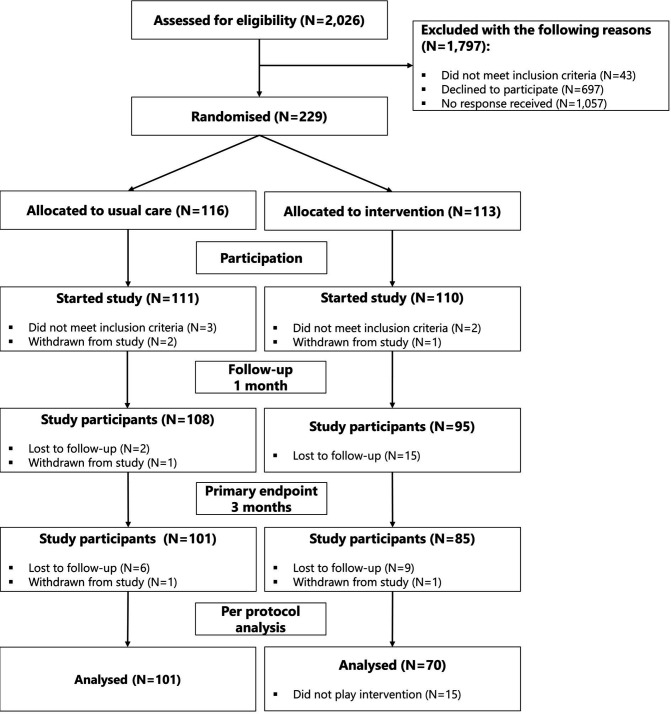
A total of 2026 eligible participants were invited for participation, which led to 111 participants starting the study in the control group and 110 participants in the intervention group (see figure 2). Apart from more men being lost to follow-up in the intervention group, there were no differences between study population and dropouts (data not shown). As 15 participants did not play the intervention for >1 hour, they were excluded per-protocol leaving 70 participants for analysis.
Figure 2.
GAMER study participant flow. GAMER, Gaming for Adherence to Medication using Ehealth in Rheumatoid arthritis; N, number.
Participant’s mean age was 61 years (SD 12) with the majority being women (73%) and living together (81%) (see table 1). Participants had RA for a median duration of 10 years and 67% were rheumatoid factor/anti-citrullinated protein antibody positive. At baseline, 38 participants in the control group (35%) and 43 participants in the intervention group (39%) were non-adherent.
Table 1.
Baseline characteristics of participants in control and intervention group
| Control (N=111)* |
Intervention (N=110)* |
|
| Age in years (mean, SD) | 61±12 | 61±12 |
| Female (N, %) | 78 (70) | 84 (76) |
| Living situation (N, %) | ||
| Alone | 22 (20) | 20 (18) |
| With partner and/or children | 88 (80) | 90 (82) |
| Educational level (N, %) | ||
| Low | 11 (10) | 20 (18) |
| Middle | 52 (47) | 45 (41) |
| High | 46 (41) | 45 (41) |
| Frequency of playing games (N, %) | ||
| Never to once a month | 31 (28) | 31 (28) |
| Once to multiple times a week | 41 (37) | 36 (33) |
| Once to multiple times a day | 38 (34) | 43 (39) |
| Disease duration in years (median, IQR) | 10 (4–17) | 9 (4 – 15) |
| RF/ACPA positive (N, %) | 77 (69) | 70 (64) |
| Current DMARD use (N, %) | ||
| 1 | 52 (47) | 62 (56) |
| 2 | 50 (45) | 39 (35) |
| ≥3 | 9 (8) | 9 (8) |
| Non-adherent according to CQR (N, %) | 38 (35) | 43 (39) |
| BMQ-Specific NCD score (mean, SD) | 5.8±4.3 | 5.0±5.1 |
*Some categories do not add up to 100% due to missing data.
BMQ, Beliefs about Medication Questionnaire; CQR, Compliance Questionnaire on Rheumatology; DMARD, disease-modifying antirheumatic drugs; IQR, inter quartile range; N, number; RF/ACPA, rheumatoid factor/anti-citrullinated protein antibody.
Primary outcome
At 3 months, 63% of the intervention participants were adherent compared with 54% of the control group (see figure 3). This difference was not statistically significant (p=0.13). Even though the difference in percentage of adherent participants was slightly larger at 1 month (64% vs 53%; p=0.06), the difference remained statistically non-significant.
Figure 3.
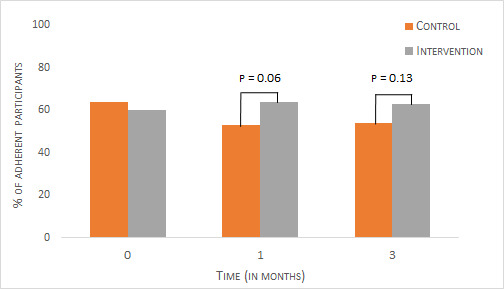
Medication adherence rates for control and intervention groups over time. Proportion of adherent participants as determined by the Compliance Questionnaire on Rheumatology (CQR) at baseline, 1 month and 3 months for control and intervention groups.
Secondary outcomes
The serious game did not show an effect on secondary medication outcomes at 3 months (see table 2). Medication adherence as measured using the objective pill count was higher in the total population (mean adherence around 95%) when compared with the proportion of adherent participants according to the subjective CQR self-report. Self-reported medication outcomes at 1 month were comparable (data not shown). The serious game intervention failed to show an effect on self-reported clinical outcomes as well (see table 2).
Table 2.
Study outcomes at endpoint (3 months)
| Control group (N=101) |
Intervention group (N=85) | Group difference (95% CI) | |
| Primary outcome | |||
| Adherent (N, %) | 55 (54) | 52 (63) | −8 (−22 to 6) |
| Secondary medication outcomes | |||
| CQR continuous (mean, SD) | 75±12 | 73±11 | 2.2 (−1.1 to 5.5) |
| Pill count in percentage† (mean, SD) | 95±16 | 97±8 | −2.3 (−9.7 to 5.1) |
| BMQ-Specific NCD score (mean, SD) | 4.8±4.2 | 5.3±4.7 | −0.5 (−1.8 to 0.8) |
| Secondary clinical outcomes | |||
| RADAI score (median, IQR) | 2.5 (1.2–4.0) | 2.5 (1.5–4.2) | 0.0 (−0.8 to 0.8) |
| HAQ score (median, IQR) | 0.8 (0.3–1.4) | 0.6 (0.3–1.4) | −0.1 (−0.5 to 0.2) |
*Percentage of the total number of participants excluding missing data.
†N=21 for the control group and N=24 for the intervention group.
BMQ NCD, Beliefs about Medication Questionnaire necessity-concerns differential; CQR, Compliance Questionnaire on Rheumatology; HAQ, Health Assessment Questionnaire; IQR, interquartile range; n, number; RADAI, Rheumatoid Arthritis Disease Activity Index.
Serious game play data
Of the 110 intervention participants that started the study, 87 participants (79%) installed the game. These participants had a median playtime of 6.2 hours at 1 month and 9.7 hours at 3 months (see table 3). Average session time was approximately 25 min throughout the study and the median number of sessions increased from 16 at 1 month to 36 at 3 months. During play, participants completed a median of 20 behavioural tasks at 1 month and 46 at 3 months. Seventy-five per cent (64) of the participants that installed the game was active for at least 30 days out of 90. Due to a communication error with Google Firebase, there were no user data between 6 January 2020 and 24 February 2020. As a result, the data of seven participants were incomplete.
Table 3.
Serious game play data at 1 and 3 months
| Actual use | 1 month (N=86)* |
3 months (N=78)† |
| Play time in hours (median, IQR) | 6.2 (2.3–11.9) | 9.7 (3.3–24.3) |
| Number of sessions (median, IQR) | 17(6–36) | 36 (11–78) |
| Session time in minutes (mean, SD) | 25±15 | 23±15 |
| Total number of behavioural tasks (median, IQR) | 20 (7–50) | 46 (13–115) |
| Active time span (maximum of 90 days) (median, IQR) | 79 (30–90)‡ | |
* Data of one player is missing at 1 month due to a communication error with Google Firebase.
†N=78 because eight participants played the serious game but did not respond to the study questionnaires at month 3. N=70 for the per protocol analysis on the primary outcome (see figure 2).
‡N = 86
IQR, interquartile range; n, number.
Per-protocol and exposure–response analyses
Per-protocol analyses did not differ from the ITT analyses on primary and secondary outcomes. No exposure–response effect was found on any of the outcomes (results not shown).
Discussion
This multicentre randomised controlled trial evaluated the effect of a serious game at improving implementation adherence of DMARDs. It showed that the serious game was frequently played but did not lead to improved medication adherence or clinical outcomes at 3 months.
Comparison with similar interventions is difficult because there have been limited studies on serious games aimed at enhancing medication adherence. In addition, there is great heterogeneity in intervention approach, study design and medication adherence assessment. Previous studies mainly describe development and testing of serious games that either gamify adherence behaviour by rewarding medication intake or indirectly promote medication adherence through education.28 29 Both effect on medication adherence and medication knowledge is modest and inconsistent.28 29 Apart from serious games, evidence on other interactive eHealth interventions for improving medication adherence is more abundant. A recent systematic review showed interactive eHealth interventions can be effective in improving medication adherence especially when channelled through Short Messaging Service, Interactive Voice Response, calls or mobile apps.30 This illustrates eHealth can be a suitable channel for improving medication adherence but application of serious games needs further development.
Two aspects of the intervention will be discussed that could possibly relate to the lack of effect: behavioural task effectiveness and the absence of integration in care. First, targeting implicit attitudes using eHealth has shown to be effective in changing health behaviour16 18 but has not been applied to medication taking behaviour. Several reasons can be given for this: (i) the behavioural tasks are not effective in changing implicit medication attitudes, (ii) changing implicit medication attitudes does not automatically lead to improved medication adherence, (iii) patients with longstanding RA are less susceptive to changing implicit medication attitudes (median disease duration was 10 years in our study) and (iv) there was insufficient or infrequent exposure to the behavioural tasks as exposure has shown to be a significant moderator of behaviour change technique effectiveness.16 Second, the serious game was not integrated in the RA care pathway and operated independently of the care context. Research showed that combining the eHealth intervention with healthcare professional interaction increases the chances of intervention effectiveness.31 32 The GAMER study refrained from integrating the serious game in the care pathway because it was expected to be at the expense of feasibility.
Besides the beforementioned intervention restraints, study methodology may also explain the negative outcomes of our trial. Medication adherence is difficult to determine, and it is, therefore, advised to combine subjective and objective measures.33 Although full comparison of both measures in our study was not possible due to missing pill count data, CQR discriminant function and pill count (with a cut-off at 80%) aligned in only 50% of the cases (data not shown). The self-reported CQR is easier to collect but might underestimate true adherence. In addition, the study population could have been ill-matched with the intervention’s target population because a large proportion of participants were adherent and/or had no negative implicit attitudes about DMARDs. Adherence was no criteria for inclusion in order to reflect clinical practice and measuring implicit attitude using implicit association tests was deemed too high a participant burden. As a result, the intervention target (ie, implicit attitudes) was not assessed as a study outcome, which is a flaw of this study.
The intervention was channelled as a serious mobile game because the smartphone is omnipresent in patient’s everyday life. As a result, game retention was high (median voluntary playtime of 9.7 hours at 3 months) and comparable to serious games where participants were encouraged to play.34 35 This channel, therefore, appears to be effective in reaching the patient but our serious game only reached part of the population with a response rate of 11% for the GAMER study. Of note, participants were only invited by a posted information letter with a reminder letter if they had not responded within 4 weeks. Our experience is that such low-intensity recruitment strategy generally leads to a participation rate of 20–30%.36
To increase the chances of intervention effectiveness, future endeavours should explore integration of the serious game in the care pathway. Additionally, the behavioural tasks should be further investigated to determine the most effective behavioural tasks and corresponding dose intensity. When investigating the effects of the adjustments, the trial design should fit the rapidly evolving nature of eHealth to prevent the intervention from being static over longer periods of time, for example using a trial within cohorts design where a cohort is continuously measured and for each design cycle, a new random participant sample is offered the intervention and outcomes compared between the sample and the cohort.37
In conclusion, our serious game aimed at encouraging a positive attitude towards DMARDs failed to show an effect on adherence to DMARDs or clinical outcomes in patients with RA. The serious game was played frequently indicating that it can be an effective channel for reaching patients.
Acknowledgments
Game Solutions Lab developed the game in co-creation with the Sint Maartenskliniek and AbbVie Inc. We thank all patients who participated in our study. We also thank Willemijn van Sterkenburg for her help in conducting the trial and Katrina Go for her help in the data analysis.
Footnotes
Contributors: Substantial contributions to study conception and design: BPHP, CLB, RT, SvD, BVdB; substantial contributions to data acquisition: BPHP, FG, RCFH, HEV, HAWvO, LIvdV; substantial contributions to analysis and interpretation of the data: BPHP, CLB, RT, SvD, BVdB; drafting the article or revising it critically for important intellectual content: BPHP, CLB, FG, RCFH, HEV, HAWvO, LIvdV, RT, SvD, BVdB; final approval of the version of the article to be published: BPHP, CLB, FG, RCFH, HEV, HAWvO, LIvdV, RT, SvD, BVdB. Guarantor: BPHP.
Funding: This work was supported by AbbVie Inc. AbbVie Inc. did not have any influence on the conduct, results or interpretation of findings of this study.
Competing interests: BPHP, CLB, FG, RCFH, HAWvO, LIvdV, RT and SvD have no conflicts of interest to report. HEV reports grants and/or personal fees from AbbVie, Amgen, AstraZeneca, BMS, Celgene, Celltrion, Galapagos, Gilead, GSK, Janssen-Cilag, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi-Genzyme, UCB all outside the submitted work. JEV reports speakers’ fees from Eli Lilly and Galapagos all outside the submitted work. BVdB reports grants and/or personal fees from UCB, Pfizer, Sanofi-Aventis, Galapagos, Amgen en Eli Lilly all outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but METC Oost Nederland, protocol number 2018-4648 exempted this study Participants gave informed consent to participate in the study before taking part.
References
- 1. Pasma A, Schenk CV, Timman R, et al. Non-adherence to disease-modifying antirheumatic drugs is associated with higher disease activity in early arthritis patients in the first year of the disease. Arthritis Res Ther 2015;17:1–10. 10.1186/s13075-015-0801-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 3. Waimann CA, Marengo MF, de Achaval S, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum 2013;65:1421–9. 10.1002/art.37917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murage MJ, Tongbram V, Feldman SR, et al. Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence 2018;12:1483–503. 10.2147/PPA.S167508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheiman-Elazary A, Duan L, Shourt C, et al. The rate of adherence to antiarthritis medications and associated factors among patients with rheumatoid arthritis: a systematic literature review and metaanalysis. J Rheumatol 2016;43:512–23. 10.3899/jrheum.141371 [DOI] [PubMed] [Google Scholar]
- 6. van den Bemt BJF, Zwikker HE, van den Ende CHM. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol 2012;8:337–51. 10.1586/eci.12.23 [DOI] [PubMed] [Google Scholar]
- 7. Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med 2017;99:269–76. 10.1016/j.ypmed.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilhelmsen NC, Eriksson T. Medication adherence interventions and outcomes: an overview of systematic reviews. Eur J Hosp Pharm 2019;26:187–92. 10.1136/ejhpharm-2018-001725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kini V, Ho PM. Interventions to improve medication adherence: a review. JAMA 2018;320:2461–73. 10.1001/jama.2018.19271 [DOI] [PubMed] [Google Scholar]
- 10. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014:CD000011. 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson TD, Lindsey S, Schooler TY. A model of dual attitudes. Psychol Rev 2000;107:101–26. 10.1037/0033-295X.107.1.101 [DOI] [PubMed] [Google Scholar]
- 12. St Quinton T, Brunton JA. Implicit processes, self-regulation, and interventions for behavior change. Front Psychol 2017;8:1–7. 10.3389/fpsyg.2017.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friese M, Wänke M, Plessner H. Implicit consumer preferences and their influence on product choice. Psychol. Mark. 2006;23:727–40. 10.1002/mar.20126 [DOI] [Google Scholar]
- 14. Linn AJ, Vandeberg L, Wennekers AM, et al. Disentangling rheumatoid arthritis patients’ implicit and explicit attitudes toward methotrexate. Front Pharmacol 2016;7:1–7. 10.3389/fphar.2016.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheeran P, Gollwitzer PM, Bargh JA. Nonconscious processes and health. Health Psychol 2013;32:460–73. 10.1037/a0029203 [DOI] [PubMed] [Google Scholar]
- 16. Jones EB, Sharpe L. Cognitive bias modification: a review of meta-analyses. J Affect Disord 2017;223:175–83. 10.1016/j.jad.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 17. Dörner R, Göbel S, Effelsberg W. Serious Games. In: Technol Rev. Cham: Springer International Publishing, 2016. [Google Scholar]
- 18. Alblas EE, Folkvord F, Anschütz DJ, et al. A health game targeting children's implicit attitudes and snack choices. Games Health J 2020;9:425–35. 10.1089/g4h.2019.0103 [DOI] [PubMed] [Google Scholar]
- 19. Pouls BP, Bekker CL, van Dulmen S, et al. A serious puzzle game to enhance adherence to antirheumatic drugs in patients with rheumatoid arthritis: systematic development using intervention mapping. JMIR Serious Games 2022;10:e31570. 10.2196/31570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dutch Rheumatology Association . Treatment of rheumatoid arthritis 2019.
- 21. de Klerk E, van der Heijde D, van der Tempel H, et al. Development of a questionnaire to investigate patient compliance with antirheumatic drug therapy. J Rheumatol 1999;26:2635–41. [PubMed] [Google Scholar]
- 22. de Klerk E, van der Heijde D, Landewé R, et al. The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: a validation study. J Rheumatol 2003;30:2469–75. [PubMed] [Google Scholar]
- 23. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1–24. 10.1080/08870449908407311 [DOI] [Google Scholar]
- 24. Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity-Concerns framework. J Psychosom Res 2008;64:41–6. 10.1016/j.jpsychores.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 25. Davis FD. A technology acceptance model for empirically testing new end-user information systems : theory and results. Massachusetts Institute of Technology, 1985. [Google Scholar]
- 26. van HM, den ECHMvan, Dulmen S. Electronic monitoring feedbackfor improving medication adherence and clinical outcomes in patients with early rheumatoid arthritis: a randomised clinical trial;15:1107–9. 10.2147/PPA.S297170.eCollection2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zwikker HE, van den Ende CH, van Lankveld WG, et al. Effectiveness of a group-based intervention to change medication beliefs and improve medication adherence in patients with rheumatoid arthritis: a randomized controlled trial. Patient Educ Couns 2014;94:356–61. 10.1016/j.pec.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 28. Tran S, Smith L, El-Den S, et al. The use of Gamification and incentives in mobile health Apps to improve medication adherence: Scoping review. JMIR Mhealth Uhealth 2022;10:e30671. 10.2196/30671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abraham O, LeMay S, Bittner S, et al. Investigating serious games that incorporate medication use for patients: systematic literature review. JMIR Serious Games 2020;8:e16096. 10.2196/16096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pouls BPH, Van den Bemt BJF, Vriezekolk J. Effectiveness of eHealth interventions on medication adherence in adults with chronic medication: a systematic review 2019;23:e18901. 10.2196/18901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zuidema RM, Van Dulmen S, Nijhuis-van der Sanden MWG, et al. qLessons learned from patients with access to an online self-management enhancing program for RA patients: qualitative analysis of interviews alongside a randomized clinical trial. Patient Educ Couns 2019;102:1170–7. 10.1016/j.pec.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 32. Hong M, Esse T, Vadhariya A, et al. Evaluating success factors of a medication adherence Tracker pilot program in improving Part D medication adherence metrics in a Medicare advantage plan: importance of provider engagement. J Manag Care Spec Pharm 2020;26:662–7. 10.18553/jmcp.2020.26.5.662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bekker CL, Aslani P, Chen TF. The use of medication adherence guidelines in medication taking behaviour research. Res Social Adm Pharm 2022;18:2325–30. 10.1016/j.sapharm.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 34. Kim HJ, Kim SM, Shin H, et al. A mobile game for patients with breast cancer for chemotherapy self-management and quality-of-life improvement: randomized controlled trial. J Med Internet Res 2018;20:e273. 10.2196/jmir.9559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. LeGrand S, Muessig KE, McNulty T, et al. Epic allies: development of a gaming APP to improve antiretroviral therapy adherence among young HIV-positive men who have sex with men. JMIR Serious Games 2016;4:e6. 10.2196/games.5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sinclair M, O'Toole J, Malawaraarachchi M, et al. Comparison of response rates and cost-effectiveness for a community-based survey: postal, Internet and telephone modes with generic or personalised recruitment approaches. BMC Med Res Methodol 2012;12:132. 10.1186/1471-2288-12-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Relton C, Torgerson D, O'Cathain A, et al. Rethinking pragmatic randomised controlled trials: introducing the "cohort multiple randomised controlled trial" design. BMJ 2010;340:c1066. 10.1136/bmj.c1066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002616supp001.pdf (260.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data underlying this article will be shared on reasonable request to the corresponding author.