Abstract
Whether allelic variants of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) independently contribute to pulmonary outcome in CF patients has not been resolved. We used both cross-sectional and mixed-model longitudinal analyses of data from CF patients that were at least 12 years old to determine the influence on pulmonary function (percent predicted forced expiratory volume [FEV1]) of the CFTR gene genotype, gender, mucoid Pseudomonas aeruginosa (MPA) infection status, presence of total opsonic antibody to MPA, and, separately, the opsonic antibody activity specific to the mucoid exopolysaccharide (MEP) surface antigen. Two different factors were independently associated with the lack of MPA infection: a high level of MEP-specific opsonic activity (MSOA), implicating an immunologically based mechanism of resistance to infection, and a lack of any type of opsonic antibody to MPA, indicative of no significant exposure or infection. This latter phenotype was found in a subset of CF patients who carried at least one uncommon CFTR gene allele suggestive of a genetic basis for resistance to infection in this group of older CF patients. For CF patients in whom both CFTR gene alleles were identified by screening for the 12 most common variants (75% of alleles), cross-sectional analysis showed that MPA infection was best correlated with lower percent predicted FEV1, while genotype (two versus one ΔF508 CFTR gene allele) and a low level of MSOA were associated with increased risk of infection. A mixed-model analysis of longitudinal spirometric measurements that considered multiple risk factors to derive regression equations was used to determine which clinical parameters had the greatest effect on the annual rate of decline in percent predicted FEV1. This analysis showed that the CFTR gene genotype only modestly modified the constant (y intercept) of the derived equations, while gender and MPA infection status had the largest effects on annual rates of decline in percent predicted FEV1. These results indicate that the CFTR genotype is usually not a primary determinant of pulmonary function in most CF patients, but gender and MPA infection status are. Infection status is potentially influenced by both immunologic (a high level of MSOA) and genetic factors, such as carriage of a CFTR gene allele that leads to a diagnosis of CF but still confers resistance to infection that is comparable to that of the wild-type CFTR gene.
Cystic fibrosis (CF) occurs with mutation in the CF transmembrane conductance regulator (CFTR) gene. Sixty-six percent of the CFTR gene alleles contain a 3-bp deletion in the CFTR gene which results in the loss of a phenylalanine residue at position 508 (ΔF508 CFTR gene allele) (15). Therefore, about 44% of CF patients are homozygous for the ΔF508 CFTR gene allele. Survival in CF is limited by progressive obstructive pulmonary disease secondary to abnormal secretions and injury from chronic infection and inflammation. The predominant pathogen for CF patients is mucoid Pseudomonas aeruginosa (MPA), and infection with this pathogen is associated with more severe pulmonary disease (9, 16). While ΔF508 and other CFTR gene alleles (e.g., W1282X, and G551D) can be categorized as severe with respect to the level of exocrine pancreatic dysfunction (20), correlations of genotype with the severity of pulmonary disease have been less clear. Many analyses of cross-sectional data covarying measures of pulmonary function with the number of ΔF508 CFTR gene alleles failed to demonstrate a dose effect of this allele on pulmonary disease severity (1, 3, 8, 17, 22, 34). Other evidence, however, suggests that the CFTR gene genotype may influence pulmonary phenotype (4, 5, 14, 18, 23, 25, 35).
The observation that mild pulmonary disease can exist in some patients homozygous for the ΔF508 CFTR gene allele suggests that other genetic and environmental factors must modify the pulmonary phenotype in CF (2, 33). Given the impact of chronic MPA infection, factors that modify the time of onset or the persistence of infection may affect long-term outcome. One such factor may be a CF patient’s immunologic status. An immune response composed of opsonic antibodies specific to the mucoid exopolysaccharide (MEP) coat of MPA was associated with the absence of MPA infection in older CF patients, and these antibodies were protective against infection in animal models (30, 31). Tosi et al. (37) also found naturally occurring opsonic antibodies of undefined antigenic specificity present in CF patients prior to infection with a decline in opsonic activity following MPA infection. The effect of genotype on MPA infection or pulmonary status was not considered in these studies of CF patients.
The identification of factors that explain the observed variability in pulmonary outcome could be valuable both in furthering our understanding of CF pathophysiology and in accelerating evaluation of the efficacy of new therapies. Recently, Corey et al. (5) presented rates of decline in pulmonary function based on a mixed-model statistical approach for 363 CF patients analyzed according to age, sex, CFTR gene genotype, and pancreatic status. However, neither infection nor immunologic status to MPA was incorporated into their model. We therefore performed a multivariate assessment of known risk factors (5, 9), modeling age, MPA infection status, immune response to MPA, CFTR gene genotype, pancreatic supplement requirement, and gender to weigh and combine this information for a prediction of differences in pulmonary outcome. Assessment of longitudinal analysis, in addition to cross-sectional analysis, of pulmonary function measurements was chosen because of the failure of prior cross-sectional studies to detect an impact of genotype on pulmonary outcome in CF patients and because of the recently demonstrated value of longitudinal analysis (5).
(This work was presented in part at the Ninth Annual North American Cystic Fibrosis Conference, Dallas, Tex., October 1995.)
MATERIALS AND METHODS
Study population and sample collection.
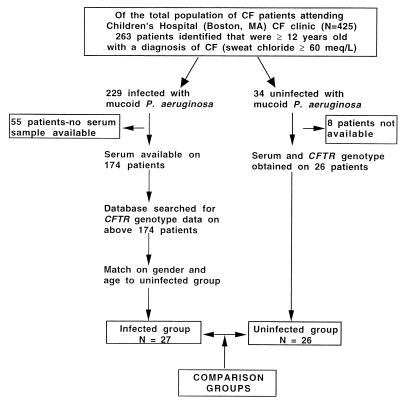
Of the 425 CF patients monitored at Children’s Hospital, Boston, Massachusetts, in 1993, 263 were at least 12 years of age, 87% of whom were infected with MPA. A matched-case control study was performed on CF patients (sweat chloride levels, >60 meq/liter) age 12 and over, with MPA infection as the exposure. Infected subjects grew MPA from two or more sputum cultures. Thirty-four of the 263 CF patients were uninfected subjects with sputum cultures that grew only normal bacterial throat flora, Staphylococcus aureus, Haemophilus influenzae, Klebsiella pneumoniae, or non-MPA. None had evidence of persistent MPA infection on routine biannual cultures. Patients colonized only with non-MPA maintain pulmonary function levels comparable to those of CF patients carrying only normal throat bacteria (9). Blood for antibody assessment and genotypes were obtained from 26 of the 34 eligible uninfected patients. Twenty-seven infected subjects with both genotype and serum available were matched on gender and age to the qualifying uninfected subjects (Fig. 1). The infected subjects were derived from the intersection of two subpopulations: (i) the 76% of clinic patients age 12 or older who had blood drawn for antibody assessment during an outpatient visit and (ii) a sample selected by random number generation who had participated in a genotyping study (32).
FIG. 1.
Schematic diagram of subject selection.
Genotype analysis.
Genomic DNA isolated from each subject was evaluated for the presence of any of twelve CFTR gene mutations (ΔF508, G551D, G542X, 621+1G→T, ΔI507, 1717-1 G→A, R117H, N1303K, W1282X, R560T, R553X, and 3849+10kb C→T) by one of three standard assays (10, 11, 32).
Antibody determinations.
As a measure of exposure to and/or infection with MPA, titers of total opsonic antibody to MPA were determined on blinded serum samples by using a well-established opsonophagocytic killing assay (30). Aliquots (100 μl) of heat-inactivated (56°C, 30 min) serum samples were diluted and mixed with equal volumes containing 2 × 106 CFU of MPA strain 2192, 2 × 106 peripheral blood leukocytes obtained from healthy donors and prepared by dextran sedimentation, and a 1:15 dilution of intact human serum as a complement source. Tubes were incubated at 37°C with end-over-end rotation for 90 min, after which the surviving CFU of MPA were counted. The overall opsonic antibody titer to MPA was the reciprocal of the highest serum dilution mediating killing of >50% of the CFU of MPA.
To determine the proportion of opsonic antibodies specific for the MEP antigen of MPA, serum samples were diluted to a point just prior to that at which the level of opsonic killing began to decline, and inhibition and adsorption studies with purified MEP antigen and MPA (MEP expressing) and non-MPA (non-MEP expressing) cells were conducted as described elsewhere (30). MEP-specific opsonic activity (MSOA) was calculated as the percentage of the opsonic killing activity specifically inhibited by purified MEP. In serum samples with a low level of MSOA, the majority (>50%) of the opsonic killing activity was removed by adsorption with a nonmucoid derivative of P. aeruginosa 2192.
Pulmonary function measurements.
Forced expiratory volume (FEV1) was determined by standard spirometry, and absolute volumes were converted to a percentage of the predicted volume expected for a healthy individual of the same age, gender, and height, on the basis of the regression equations developed by Knudson (19). For each subject, all percent predicted values, including those obtained prior to antibody testing, were included in the analysis. All values obtained subsequent to the initiation of DNase (Pulmozyme; Genentech, Inc., South San Francisco, Calif.) clinical trials were excluded. The time interval between evaluation points varied for each subject.
Statistical analysis.
Mean values for continuous variables were compared by the independent-sample t test. The analysis of proportional data was performed by using Fisher’s exact test (36). Logistic regression was used to estimate the probability of infection with MPA at a given level of MSOA, with the likelihood ratio chi-square test used to assess significance (12). All P values were two sided, with a P value of <0.05 considered statistically significant, unless otherwise stated.
Several methods were used to assess the pulmonary outcome of infected and uninfected subjects as reflected by percent predicted FEV1. In a cross-sectional analysis, the averages of the three most recent percent predicted FEV1 values for each subject were compared between infected and uninfected subjects by using an independent-sample t test. Because percent predicted FEV1 covaries with age, the most recent percent predicted FEV1 was regressed on the ages for infected and uninfected groups, and the differences in slopes and the intercepts of regression lines were compared, a method used in similar cross-sectional analyses of CF patients (1, 3, 17, 22). To derive formulas for predicting pulmonary function in CF patients at a given point in time by using the repeated measurements of pulmonary function generally available for most CF patients, the data from 1,680 determinations of percent predicted FEV1 were fitted to a mixed model incorporating the random patient effect by using the MIXED procedure in the SAS statistical package, version 6.12 (SAS Institute Inc., Cary, N.C.) (24). This model accounts for repeated measurements on individual subjects and unequal spacing between time points in order to capture age-related rates of change in pulmonary function (21). The percent predicted FEV1 measurements were analyzed in models designed to take into account each subject’s age, gender, MPA infection status, genotype (number of ΔF508 CFTR gene alleles), MPA antibody titer, MSOA level, sweat chloride level, and pancreatic supplement requirement. Univariate analysis did not support inclusion of sweat chloride levels or pancreatic supplement requirement in the final models. Several covariance structures were compared to rule out random patient effects, including compound symmetry, Toeplitz, autoregressiveness, and spatiality. The form of the covariance structure was determined according to the Akaike information criterion commonly used in longitudinal model fitting, with the compound symmetry function demonstrating superior model fit (13).
RESULTS
Study population characteristics.
CF patients 12 years old and older were chosen because this is the point at which 87% of patients in the overall clinic population had acquired MPA infection. Thus, it is likely that uninfected CF patients 12 years old and older represent those individuals whose lack of infection is not due to an environmental factor, such as not having been sufficiently exposed to MPA. After matching for age and gender, there were no significant differences in subject characteristics between MPA-infected and uninfected groups in terms of age at enrollment, sweat chloride level, or requirement for pancreatic supplementation (Table 1). The frequency of the ΔF508 CFTR gene allele was higher among infected subjects (P = 0.05, one-sided Fisher’s exact test) than among uninfected subjects. When infected and uninfected subjects were stratified by the count of ΔF508 CFTR gene alleles in a subject’s genotype, the distribution differed significantly, with more subjects in the MPA-infected group being homozygous for the ΔF508 CFTR gene allele (P = 0.03). The relative odds of MPA infection in subjects homozygous for the ΔF508 CFTR gene allele were 4.67 (95% confidence interval, 1.75 to 12.44; P = 0.01) compared to subjects heterozygous for this allele.
TABLE 1.
Study population characteristics and genotype distribution among MPA-infected and uninfected groups
| Characteristic | Infected group (n = 27) | Uninfected group (n = 26) | P value |
|---|---|---|---|
| Male/female ratio | 14/13 | 16/10 | 0.20 |
| Age at enrollment (yr) ± SD | 24.9 ± 8.2 | 21.3 ± 9.1 | 0.13 |
| Sweat chloride level (meq/liter) ± SD | 114.9 ± 15.8 | 111.0 ± 18.0 | 0.41 |
| No. requiring pancreatic supplementation | 27 | 23 | 0.11 |
| ΔF508 CFTR gene allele frequencya | 0.78 | 0.62 | 0.05 |
| No. homozygous for ΔF508 CFTR gene allele | 18 | 9 | 0.03 |
| No. heterozygous for ΔF508 CFTR gene allele | 6 | 14 | 0.02 |
| No. with neither allele identified | 3 | 3 | 1.00 |
| No. with overall opsonic antibody titer of ≥5 | 26 | 14 | <0.001 |
Distribution of non-ΔF508 CFTR gene alleles in the uninfected group: G542X, 3 alleles; G551D, 1 allele; W1282X, 1 allele; N1303K, 1 allele; and not identified, 14 alleles. Distribution in the infected group was as follows: G542X, 2 alleles; G551D, 2 alleles; 621+1G→T, 1 allele; and not identified, 7 alleles.
Characteristics of FEV1 determinations in infected and uninfected subjects.
The ages (years) at the first FEV1 determination were similar in the MPA-infected and uninfected subjects (mean ± standard deviation [SD], 12.2 ± 5.5 versus 11.4 ± 7.1, respectively [P = 0.66]), as were the durations (years) of follow-up in pulmonary function measurements (mean ± SD, 13.1 ± 4.5 versus 10.8 ± 4.5, respectively [P = 0.07]). The mean of the first percent predicted FEV1 was lower among infected subjects (mean ± SD, 80% ± 22% versus 95% ± 17% [P < 0.01]) than among uninfected subjects, likely reflecting the effect on pulmonary function of MPA infection prior to age 12 in infected subjects. To assess potential selection bias in the 76% of infected clinic patients 12 years old and older who provided blood samples, we compared the first percent predicted FEV1 used in the analysis from this group to data from the 1997 Cystic Fibrosis Foundation patient registry report and found that the mean ± SD of the percent predicted FEV1 in infected study subjects (80% ± 22%) was virtually the same as that in the national sample (age 13; mean ± SD, 79.1% ± 22.4% [n = 680]) (6). Follow-up for at least 6 years occurred in 25 infected (93%) and 22 uninfected (85%) subjects. MPA-infected subjects had over twice as many data points as uninfected subjects (mean number of FEV1 measurements per subject, 44 ± 27 versus 19 ± 13, respectively [P < 0.001]). Eighty-five percent of infected subjects had at least 16 FEV1 measurements, and 85% of uninfected subjects had at least 7 FEV1 measurements.
Antibody measurements and relationship to pulmonary function.
Forty of the 53 subjects had measurable serum opsonic activity that mediated killing of MPA without regard to the antigenic specificity of the antibodies, indicating exposure and/or infection with P. aeruginosa. However, when the opsonic antibodies were classified according to their specificity for the P. aeruginosa MEP antigen (i.e., MSOA), the mean (± standard error [SE]) MSOA level was dramatically lower in infected subjects than in uninfected subjects that were homozygous for the ΔF508 CFTR gene mutation (6.4% ± 2.5% [n = 18] versus 71.3% ± 6.8% [n = 9], respectively [P < 0.001]).
Among the group of 13 patients that totally lacked any type of serum opsonic killing activity against MPA, indicative of the lack of significant exposure or infection, only 1 patient was found to have evidence of MPA infection by culture, and this was the only patient in this group that was homozygous for the ΔF508 CFTR gene allele (Fisher’s exact test, P = 0.0002, compared with 27 of 40 ΔF508 CFTR gene homozygous subjects with opsonic antibody to MPA). All of the remaining 12 uninfected subjects lacking any opsonic antibody to MPA were compound heterozygotes for CFTR gene alleles. Of these 12, 9 had one ΔF508 CFTR gene allele, but the second allele was identified for only 2 of these 9 subjects (N1303K and G542X); the remaining 7 subjects carried a second CFTR gene allele that was not among the 12 most common ones we screened for. Two uninfected patients lacking any opsonic antibody were also compound heterozygotes with one of the two alleles not identified and the second allele being either G542X or G551D. The final uninfected patient lacking any antibody carried two unidentified CFTR gene alleles. Overall, 10 of 12 uninfected CF patients lacking any opsonic antibody had at least one CFTR gene allele that was not among the 75% screened for.
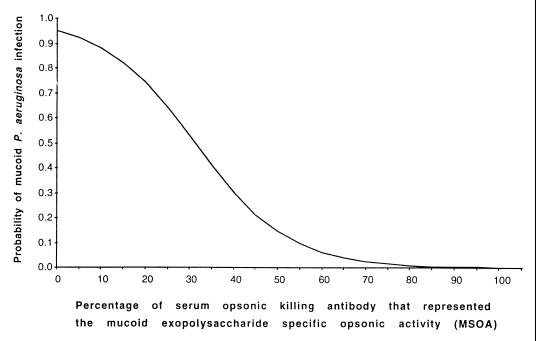
We next determined if the serum MSOA level could serve as a single explanatory variable for the likelihood of being infected with MPA. Using the MSOA level from all 40 subjects with serum opsonic killing antibodies against MPA, we derived a logistic regression model to estimate the probability of being infected with MPA by using the following formula: probability of infection = exp(7.6 − 0.2 M)/{1 + [exp(7.6 − 0.2 M)]}, where exp is the base of the natural logarithm and M is the level of MSOA, ranging from 0 to 100% (Fig. 2). This model revealed that if 35% of the serum opsonins are specific for MEP, there was an approximately 50% risk of infection. Higher levels of MSOA lead to lower risks of infection. There was a significant positive association (likelihood ratio test, 46.85; P < 0.0001) between MSOA and the probability of MPA infection.
FIG. 2.
Relationship between the percentage of serum opsonic antibodies to MPA that encompass the MSOA and the estimated probability of MPA infection.
Correlations of clinical parameters and pulmonary status determined by cross-sectional outcome analysis.
The ages (years) at which the most recent percent predicted FEV1 was obtained were comparable for MPA-infected and uninfected subjects (mean ± SD, 18.0 ± 8.0 versus 20.7 ± 6.8, respectively [P = 0.20, t test]), but the mean of the percent predicted FEV1 was lower among MPA-infected subjects than uninfected subjects (mean ± SE, 61% ± 5% versus 87% ± 5%, respectively [P < 0.0001, t test]). When the most recent percent predicted FEV1 for infected and uninfected subjects was regressed on age, analysis of covariance indicated that there was no difference in the estimated rate of decline of percent predicted FEV1 between these groups (P = 0.82), but there was a difference (Δ) between groups in the constants (y intercepts, Δ = 21.81; SE = 6.13; P < 0.001, t test).
The 40 subjects with any detectable opsonic antibody to MPA (titer, ≥5), regardless of its antigenic specificity, had a lower mean percent predicted FEV1 than the 13 subjects without any opsonic antibody (mean ± SD, 65% ± 25% versus 95% ± 20%, respectively [P < 0.0001, t test]). When subjects were compared by the number of ΔF508 CFTR gene alleles (0, 1, or 2), no differences were detected among these three groups with regard to the means of the three most recent percent predicted FEV1 (±SE) (no ΔF508 alleles, 82% ± 41% [n = 6]; one ΔF508 allele, 79% ± 29% [n = 20]; two ΔF508 alleles, 68% ± 21% [n = 27] [P = 0.30]). The percent predicted FEV1 for all males did not differ from that of all females. Thus, the cross-sectional analysis demonstrated an association of both genotype and MSOA with infection status and an effect of infection status on percent predicted FEV1 without revealing a direct association of genotype with percent predicted FEV1.
Correlations of clinical parameters and pulmonary status determined by longitudinal analysis.
We therefore sought to develop a model to estimate percent predicted FEV1 in CF patients by incorporating the factors shown to impact pulmonary function in the cross-sectional analysis, along with genotype and the immunologic status toward MPA that could also be expected to affect pulmonary function. In the statistical analysis with a mixed model, the main effects modeled were age, gender, infection status, number of ΔF508 CFTR gene alleles, pancreatic supplement requirement, and overall titer of opsonic antibody to MPA. The terms in the final mixed model were overall opsonic antibody titer (likelihood ratio test, 6.64; P < 0.01), age by infection status (likelihood ratio test, 31.13; P < 0.0001), age by gender (likelihood ratio test, 8.38; P < 0.01), and number of ΔF508 CFTR gene alleles by gender (likelihood ratio test, 27.17; P < 0.0001). From the mixed-model analysis, specific equations were generated for the estimation of percent predicted FEV1 in CF patients with evidence of exposure to P. aeruginosa as determined by the presence of an opsonic antibody of any specificity, classifying the patients by MPA infection status, gender, and genotype (Table 2).
TABLE 2.
Calculation of percent predicted FEV1 based on mixed-model equationa for CF patients with any antibody to P. aeruginosa
| Patient gender | Infection status | No. of ΔF508 CFTR gene alleles | Values for equation for percent predicted FEV1b [constant + (slope × age)]
|
|
|---|---|---|---|---|
| Constant | Slope (= annual rate of decline in FEV1) | |||
| Female | Uninfected | 0 | 104.31 | 1.40 |
| 1 | 115.03 | 1.40 | ||
| 2 | 114.13 | 1.40 | ||
| Infected | 0 | 104.74 | 2.21 | |
| 1 | 115.46 | 2.21 | ||
| 2 | 114.55 | 2.21 | ||
| Male | Uninfected | 0 | 109.98 | 0.90 |
| 1 | 98.27 | 0.90 | ||
| 2 | 101.65 | 0.90 | ||
| Infected | 0 | 110.41 | 1.71 | |
| 1 | 98.70 | 1.71 | ||
| 2 | 102.07 | 1.71 | ||
Overall mixed-model equation: FEV1 = 102.07 − 0.43(MPA uninfected) + 12.48 female) + 8.33(zero ΔF508 CFTR gene alleles) − 3.38(one ΔF508 CFTR gene allele) + 6.54(no MPA antibody) − 18.15(female with zero ΔF508 CFTR gene alleles) + 4.28(female with one ΔF508 CFTR gene allele) − 1.71(age) + 0.81(age)(MPA uninfected) − 0.50(age)(female). For variables other than age, each term in parentheses is replaced by a 1 if the patient meets that description or a 0 otherwise.
To calculate the percent predicted FEV1 for a CF patient, first categorize patients by gender, MPA infection status, and CFTR gene genotype. Next, use age in the following formula: percent predicted FEV1 = constant + (slope × age).
The importance of infection status can be readily seen in the equations in Table 2, wherein the slope of the regression line, which is the annual rate of decline in percent predicted FEV1, shows a slower rate of decline when comparing uninfected females with infected females (percent predicted annual decline in FEV1 of 1.40 versus 2.21, respectively) and uninfected males with infected males (percent predicted annual decline in FEV1 of 0.90 versus 1.71, respectively). Thus, for example, an infected female homozygous for the ΔF508 CFTR gene would be predicted to have a FEV1 of 47% at age 30.6, the median survival age for CF patients in the United States in 1997 (6), whereas an uninfected female of the same age and genotype would have a percent predicted FEV1 of 71%. The similar calculated percent predicted FEV1 for infected versus uninfected males homozygous for the ΔF508 CFTR gene would be 50% versus 74%, respectively.
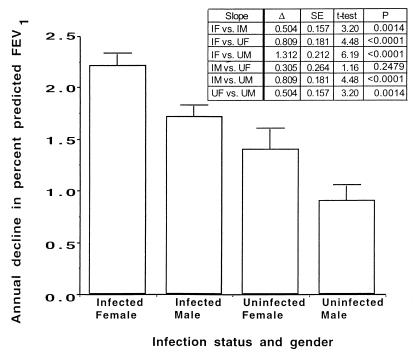
The differences between the pairs of slopes for infected and uninfected females and males were compared by t tests (Fig. 3). Infected females had a significantly more rapid annual rate of decline in percent predicted FEV1 (2.2%) than any of the other three groups (P ≤ 0.0014). The annual rate of decline of percent predicted FEV1 in infected males (1.7%) was not significantly different from that in uninfected females (1.4%; P = 0.25). The annual rate of decline of percent predicted FEV1 in uninfected males (0.9%) was significantly lower than that for the other three groups (P ≤ 0.0014). This analysis confirmed the importance of a subject’s gender and infection status in estimating percent predicted FEV1 in CF patients homozygous for the ΔF508 CFTR gene allele.
FIG. 3.
Comparison of the slopes for the annual rate of decline in percent predicted FEV1 among CF patients homozygous for the ΔF508 CFTR gene allele that had a titer of opsonic antibody to MPA of ≥5. Bars represent means of slope of annual rate of decline, and error bars indicate the standard errors. IF, infected female; IM, infected male; UF, uninfected female; UM, uninfected male; Δ, difference between means; SE, standard error of difference between means.
In contrast to the significant effect MPA infection status had on the annual rate of decline in percent predicted FEV1, differences among the groups due to the CFTR genotype, while statistically significant, were nonetheless of modest biologic and clinical importance. Because the number of subjects carrying no ΔF508 CFTR gene alleles was small (three males and three females, 10% of all CF patients studied), the model was used to compare male and female subjects with one versus two ΔF508 CFTR gene alleles (representing 70% of all individuals with CF). These comparisons yielded a likelihood ratio chi-square value of 6.65 (P < 0.01), indicating that the effect of the ΔF508 CFTR gene allele number is different in males and females. Although the results we obtained indicate that males with two ΔF508 CFTR gene alleles would have a higher percent predicted FEV1 than males with one ΔF508 CFTR gene allele, this difference is reflected only in the initial term (constant at age 12) of the regression equations comparing males with one or two ΔF508 CFTR gene alleles (Table 2). This difference in percent predicted FEV1 was no more than 3.4% between these two groups of males at any given age. As expected, females with two ΔF508 CFTR gene alleles had a lower percent predicted FEV1 compared with those with one allele, but again the difference was small (0.9% difference in FEV1). Overall, while the mixed-model analysis was sufficiently robust to determine that the impact of the CFTR gene genotype was statistically significant, the magnitude of the absolute difference at any age in percent predicted FEV1 based on genotype was quite small, ranging from 0.9 to 3.4%.
DISCUSSION
This study demonstrates that pulmonary function in CF, as reflected by percent predicted FEV1 measurements, can be modeled with an appropriate statistical analysis. Factors we identified as being statistically significant in the model included infection by MPA, the presence of a high MSOA, the subject’s gender, and the subject’s genotype. The first three factors have all been previously determined to be independent factors associated with pulmonary disease in CF (9, 30). However, the manner in which these independent factors interact to cause the loss in pulmonary function in CF, and the magnitude of the impact of these different factors, was revealed only by using the mixed-model approach. Importantly, our findings on the rate of decline in pulmonary function based on age and gender are very similar to those recently reported by Corey et al. (5), who also used a mixed-model analysis of multiple factors, but they did not include infection or immunologic status as main terms in their model. While the number of patients we studied (n = 53) was considerably fewer than the number (n = 363) studied by Corey et al. (5), the comparability of the results from the two mixed-model analyses indicates that the number of patients we studied, along with the large number of spirometric measurements (n = 1,680) used, was sufficient to model the effects of the CFTR gene genotype, gender, and immunologic status on rates of pulmonary decline in CF patients.
The mixed-model longitudinal analysis incorporates the effects of multiple characteristics and their combinations which influence the trajectory of change over time. The complexity of the factors that influence pulmonary function in CF, including interactions of infection and immune status and gender-specific effects of mutations in the CFTR gene, may have obscured previous attempts to use cross-sectional analysis to associate genotype, as an isolated factor, with pulmonary status in CF patients. Our results indicate that under these complex circumstances genotype by itself may be more of a determinant of susceptibility to infection, whereas infection has more of a direct effect on pulmonary status.
One example of this relationship was seen among nearly one-half of 26 older, uninfected subjects lacking any antibody to MPA. These subjects had a higher percent predicted FEV1 than those with any type of detectable opsonic antibody. Of the 13 subjects lacking any opsonic activity to MPA, only one was infected and this was the only patient in this group that was homozygous for the ΔF508 CFTR gene allele. Since all the serum samples were screened against a single MPA strain which produces both a MEP antigen and additional, conserved P. aeruginosa cell surface antigens (29, 30), it is possible that this single infected patient carried a rare strain lacking shared epitopes that are targets of an opsonic antibody. The other 12 patients lacking any opsonic antibody were compound heterozygotes, and 10 of these had at least one CFTR gene allele that was not among the 75% of the most common alleles we screened for. The lack of MPA infection in these 12 subjects may have resulted from CFTR gene alleles that cause altered chloride ion secretion properties, leading to elevated sweat chloride levels and a diagnosis of CF, that still confer natural resistance to MPA infection comparable to that of humans without CF, who rarely, if ever, have high levels of MSOA (30).
A potential mechanism to explain these findings has been proposed by Pier and colleagues, who demonstrated that the wild-type CFTR protein is a receptor for P. aeruginosa involved in airway epithelial cell internalization of this organism leading to bacterial clearance from the lung epithelium (27, 28). The absence of a CFTR in epithelial cells, as occurs with ΔF508 CFTR gene alleles and other alleles, has been proposed to prevent normal clearance of P. aeruginosa from the airway epithelium. Among the CF patients with no opsonic activity to MPA and no infection, most of whom carried at least one rare (<0.2% frequency) CFTR gene allele, it is possible that the rare allele encoded a protein with the wild-type property needed for P. aeruginosa clearance from the airway epithelium but diminished chloride ion conductance. Because the CFTR gene genotype is associated with both pulmonary function and infection status, it may have its greatest effect on pulmonary function indirectly by modifying infection status. The CF subjects lacking both MPA infection and a common CFTR gene allele likely maintain resistance to MPA infection that is comparable to that in individuals with wild-type CFTR gene alleles.
Our study reinforces results from previous reports demonstrating that lung infection with MPA is a key factor accelerating the decline in pulmonary function of CF patients (9, 14, 16). Better lung function is found among CF patients lacking MPA infection, and the presence of nonmucoid P. aeruginosa in the absence of MPA does not compromise lung function any differently in these patients than in those without any detectable P. aeruginosa in their sputum cultures (9, 26). High MSOA levels, an apparent specific immune resistance mechanism that has previously been related to lack of MPA infection in older, nongenotyped CF patients (30), was also shown in this study to independently modify the probability of MPA infection within a group of CF patients with a homogeneous CFTR gene genotype (ΔF508/ΔF508). Thus, host immune status is a determinant of the development of pulmonary pathology due to MPA infection and is unlikely to be affected by the CFTR gene locus.
Interestingly, when we grouped all patients with an opsonic antibody to MPA together, regardless of the antigenic specificity, the presence of an opsonic antibody to MEP (i.e., a high level of MSOA) was not found to be a main effect in the final statistical model. The finding that the presence of an opsonic antibody of any specificity to MPA was a factor to include in the final model likely reflects the association between this measurement and infection; almost all CF patients infected with MPA have a measurable opsonic antibody to this organism. The lack of an effect of a high level of MSOA in the final model was attributed to the small number of subjects with this phenotype. However, the fact that a high MSOA level independently predicted MPA infection and the infection had a major impact upon pulmonary status indicates that MSOA likely impacts the equations derived in the mixed model by its ability to categorize CF patients as either infected or uninfected with MPA.
The study was limited by several factors. We excluded patients under 12 years of age because the age-related rate of acquisition of MPA infection in CF patients indicates that some subjects under age 12 are too young to have been sufficiently exposed to become infected (6). Since 87% of our study population 12 years old and older was infected with MPA, this likely indicates an age where exposure or other age-related, nonimmunologic, and nongenetic factors impacting infection will be minimal. Indeed, as long as infection status is a major component for predicting pulmonary function in CF, it is unlikely that any model could be derived that would be applicable to patients under the specific age where nearly 90% of patients that will become infected have become infected. In addition, the frequency of severe non-ΔF508 CFTR gene mutations may vary geographically (7), indicating that differing results among genotype-phenotype studies (1, 3, 8, 14, 17, 22, 23, 34, 35) could be partially explained by an increased representation of unidentified mutations that more or less adversely impact the respiratory system. It is also clear that greater confidence in our results will follow once they are validated with a larger number of patients. However, the equations we derived for effects of gender and infection status on pulmonary status using 53 patients and 1,680 spirometric measurements were very similar to those of Corey et al. (5), who analyzed 363 patients and 9,362 pulmonary function tests. Overall, both studies showed the value of mixed-model analysis for predicting pulmonary outcomes in CF, and we have extended the work of Corey et al. (5) to include infection and immune status in the mixed-model equations for predicting pulmonary outcome.
ACKNOWLEDGMENTS
We are indebted to James H. Ware and Mary Ellen B. Wohl for their reviews of the manuscript and helpful discussions. We also thank Denise DesJardins and Gloria Meluleni for conducting the opsonophagocytosis assays.
This work was supported in part by NIH grants DK2273 (R.B.P.) and AI22806 (G.B.P.) and by a Trudeau Scholarship of the American Lung Association (R.B.P.).
REFERENCES
- 1.Borgo G, Gasparini P, Bonizzato A, Cabrini G, Mastella G, Pignatti P F. Cystic fibrosis: the ΔF508 mutation does not lead to an exceptionally severe phenotype. A cohort study. Eur J Pediatr. 1993;152:1006–1011. doi: 10.1007/BF01957227. [DOI] [PubMed] [Google Scholar]
- 2.Campbell P W, III, Parker R A, Roberts B T, Krishnamani M R, Phillips J A., III Association of poor clinical status and heavy exposure to tobacco smoke in patients with cystic fibrosis who are homozygous for the ΔF508 deletion. J Pediatr. 1992;120:261–264. doi: 10.1016/s0022-3476(05)80438-x. [DOI] [PubMed] [Google Scholar]
- 3.Campbell P W, III, Phillips III J A, Krishnamani M R, Maness K J, Hazinski T A. Cystic fibrosis: relationship between clinical status and ΔF508 deletion. J Pediatr. 1991;118:239–241. doi: 10.1016/s0022-3476(05)80490-1. [DOI] [PubMed] [Google Scholar]
- 4.Chillon M, Casals T, Mercier B, Bassas L, Lissens W, Silber S, Romey M C, Ruizromero J, Verlingue C, Claustres M, Nunes V, Ferec C, Estivill X. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332:1475–1480. doi: 10.1056/NEJM199506013322204. [DOI] [PubMed] [Google Scholar]
- 5.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation. Patient registry 1997 annual data report. Bethesda, Md: Cystic Fibrosis Foundation; 1998. [Google Scholar]
- 7.Cystic Fibrosis Genetic Analysis Consortium. Population variation of common cystic fibrosis mutations. Hum Mutat. 1994;4:167–177. doi: 10.1002/humu.1380040302. [DOI] [PubMed] [Google Scholar]
- 8.Cystic Fibrosis Genotype-Phenotype Consortium. Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med. 1993;329:1308–1313. doi: 10.1056/NEJM199310283291804. [DOI] [PubMed] [Google Scholar]
- 9.Demko C A, Byard P J, Davis P B. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48:1041–1049. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 10.Ferrie R M, Schwarz M J, Robertson N H, Vaudin S, Super M, Malone G, Little S. Development, multiplexing, and application of ARMS tests for common mutations in the CFTR gene. Am J Hum Genet. 1992;51:251–262. [PMC free article] [PubMed] [Google Scholar]
- 11.Highsmith W E, Burch L H, Zhou Z, Olsen J C, Boat T E, Spock A, Gorvoy J D, Quittel L, Friedman K J, Silverman L M, et al. A novel mutation in the cystic fibrosis gene in patients with pulmonary disease but normal sweat chloride concentrations. N Engl J Med. 1994;331:974–980. doi: 10.1056/NEJM199410133311503. [DOI] [PubMed] [Google Scholar]
- 12.Hosmer D W, Lemeshow S. Applied logistic regression. New York, N.Y: John Wiley; 1989. pp. 13–19. [Google Scholar]
- 13.Jennrich R I, Schluchter M D. Unbalanced repeated-measures models with structured covariance matrices. Biometrics. 1986;42:805–820. [PubMed] [Google Scholar]
- 14.Johansen H K, Nir M, Hoiby N, Koch C, Schwartz M. Severity of cystic fibrosis in patients homozygous and heterozygous for delta F508 mutation. Lancet. 1991;337:631–634. doi: 10.1016/0140-6736(91)92449-c. [DOI] [PubMed] [Google Scholar]
- 15.Kerem B S, Rommens J M, Buchanan J A, Markiewicz D, Cox T K, Chakravarti A, Buchwald M, Tsui L C. Identification of the cystic fibrosis gene—genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 16.Kerem E, Corey M, Gold R, Levison H. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr. 1990;116:714–719. doi: 10.1016/s0022-3476(05)82653-8. [DOI] [PubMed] [Google Scholar]
- 17.Kerem E, Corey M, Kerem B S, Rommens J, Markiewicz D, Levison H, Tsui L C, Durie P. The relation between genotype and phenotype in cystic fibrosis-analysis of the most common mutation. N Engl J Med. 1990;323:1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- 18.Khan T Z, Wagener J S, Bost T, Martinez J, Accurso F J, Riches D W H. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 19.Knudson R J, Lebowitz M D, Holberg C J, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 20.Kristidis P, Bozon D, Corey M, Markiewicz D, Rommens J, Tsui L C, Durie P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet. 1992;50:1178–1184. [PMC free article] [PubMed] [Google Scholar]
- 21.Laird N M, Ware J H. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Lester L A, Kraut J, Lloyd-Still J, Karrison T, Mott C, Billstrand C, Lemke A, Ober C. ΔF508 genotype does not predict disease severity in an ethnically diverse cystic fibrosis population. Pediatrics. 1994;93:114–118. [PubMed] [Google Scholar]
- 23.Liechti-Gallati S, Bonsall I, Malik N, Schneider V, Kraemer L G, Ruedeberg A, Moser H, Kraemer R. Genotype/phenotype association in cystic fibrosis: analyses of the ΔF508, R553X, and 3905insT mutations. Pediatr Res. 1992;32:175–178. doi: 10.1203/00006450-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Littell R C, Milliken G A, Stroup W W, Wolfinger R D. SAS system for mixed models. Cary, N.C: SAS Institute Inc.; 1996. [Google Scholar]
- 25.Mohon R T, Wagener J S, Abman S H, Seltzer W K, Accurso F J. Relationship of genotype to early pulmonary function in infants with cystic fibrosis identified through neonatal screening. J Pediatr. 1993;122:550–555. doi: 10.1016/s0022-3476(05)83534-6. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen S S, Kharazmi A, Espersen F, Hoiby N. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect Immun. 1990;58:3363–3368. doi: 10.1128/iai.58.10.3363-3368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pier G B, Grout M, Zaidi T S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pier G B, Matthews W J, Eardley D D. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J Infect Dis. 1983;147:494–503. doi: 10.1093/infdis/147.3.494. [DOI] [PubMed] [Google Scholar]
- 30.Pier G B, Saunders J M, Ames P, Edwards M S, Auerbach H, Goldfarb J, Speert D P, Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older, non-colonized cystic fibrosis patients. N Engl J Med. 1987;317:793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- 31.Pier G B, Small G J, Warren H B. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infection. Science. 1990;249:537–540. doi: 10.1126/science.2116663. [DOI] [PubMed] [Google Scholar]
- 32.Richards B, Skoletsky J, Shuber A P, Balfour R, Stern R C, Dorkin H L, Parad R B, Witt D, Klinger K W. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum Mol Genet. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- 33.Rosenstein B J. Genotype-phenotype correlations in cystic fibrosis. Lancet. 1994;343:746–747. doi: 10.1016/s0140-6736(94)91832-5. [DOI] [PubMed] [Google Scholar]
- 34.Santis G, Osborne L, Knight R A, Hodson M E. Independent genetic determinants of pancreatic and pulmonary status in cystic fibrosis. Lancet. 1990;336:1081–1084. doi: 10.1016/0140-6736(90)92566-z. [DOI] [PubMed] [Google Scholar]
- 35.Santis G, Osborne L, Knight R A, Hodson M E. Linked marker haplotypes and the delta F508 mutation in adults with mild pulmonary disease and cystic fibrosis. Lancet. 1990;335:1426–1429. doi: 10.1016/0140-6736(90)91448-j. [DOI] [PubMed] [Google Scholar]
- 36.Sokal R R, Rohlf F J. Biometry. 3rd ed. New York, N.Y: W. H. Freeman; 1995. pp. 730–734. [Google Scholar]
- 37.Tosi M F, Zakem-Cloud H, Demko C A, Schreiber J R, Stern R C, Konstan M W, Berger M. Cross-sectional and longitudinal studies of naturally occurring antibodies to Pseudomonas aeruginosa in cystic fibrosis indicate absence of antibody-mediated protection and decline in opsonic quality after infection. J Infect Dis. 1995;172:453–461. doi: 10.1093/infdis/172.2.453. [DOI] [PubMed] [Google Scholar]