ABSTRACT
Probiotics are widely used to promote performance and improve gut health in weaning piglets. Therefore, the objective of this study was to investigate the effects of dietary supplementation with Bifidobacterium animalis subsp. lactis (B. animalis) JYBR-190 on the growth performance, intestine health, and gut microbiota of weaning piglets. The results showed that the dietary addition of B. animalis significantly improved growth performance and decreased diarrhea incidence. B. animalis increased villus height in the duodenum and elevated goblet cell numbers and amylase activity in the jejunum. Additionally, B. animalis supplementation markedly increased total antioxidant capacity in jejunal mucosa but declined the malondialdehyde content. B. animalis treatment did not affect the mRNA expressions associated with the intestinal barrier and inflammatory cytokine in various intestinal segments. Microbiota analysis indicated that a diet supplemented with B. animalis significantly increased the relative abundances of health-promoting bacteria in the lumen, such as Streptococcus, Erysipelotrichaceae, Coprococcus, and Oscillibacter. There was a trend for B. animalis fed piglets to have a higher relative abundance of B. animalis in ileal digesta. Moreover, B. animalis-treated pigs decreased the abundance of Helicobacter and Escherichia-Shigella in ileal mucosa-associated microbiota. In summary, this study showed that B. animalis supplementation stimulated growth performance, improved gut development, enriched beneficial bacteria abundances, and declined intestinal pathogens populations, while B. animalis had limited effects on the intestinal barrier and immune function.
IMPORTANCE In the modern swine industry, weaning is a critical period in the pig’s life cycle. Sudden dietary, social, and environmental changes can easily lead to gut microbiota dysbiosis, diarrhea, and a decrease in growth performance. To stabilize intestinal microbiota and promote animal growth, antibiotics were widely applied in swine diets during the past few decades. However, the side effects of antibiotics posed a great threat to public health and food safety. Therefore, it is urgent to find and develop antibiotic alternatives. The growing evidence suggested that probiotics can be preferable alternatives to antibiotics because they can modulate microbiota composition and resist pathogens colonization. In this study, our results indicated that dietary supplementation with Bifidobacterium animalis promoted growth in weaning piglets by improving gut development, increasing beneficial bacteria abundances, and declining pathogens populations.
KEYWORDS: Bifidobacterium animalis, gut microbiota, intestinal development, weaning piglets
INTRODUCTION
In the modern swine industry, weaning is a critical period in the pig’s life cycle. Piglets encounter multiple stressors during the postweaning period which cause disruptions of gut microbiota, intestinal inflammation, and diarrhea, consequently reducing their growth performance (1, 2). To stabilize intestinal microbiota and promote animal growth, antibiotics were widely applied in the swine diet in the past few decades (3, 4). However, the use of antibiotic growth promoters has been limited in pig production (3, 5). To maintain pig health during the postweaning period, it is urgent to find and develop antibiotic alternatives.
The mammalian gastrointestinal tract is inhabited by trillions of bacteria (6). A stable and well-balanced community of gut microbiota plays an important role in host health (2). Probiotics are living microorganisms when administered in adequate amounts, conferring a health benefit to the host (7). Supplementing probiotics in pig production can effectively modulate microbiota composition and inhibit pathogenic bacteria growth (8). In addition, probiotics can activate the immune system, resist pathogen colonization, and regulate intestinal barrier function (8, 9). Therefore, probiotics are considered desirable alternatives to benefit the weaning piglets (5, 10).
At present, the most frequently used commercial probiotics are Lactobacillus, Bifidobacterium, Streptococcus, and yeasts (10). A previous study has reported that Bifidobacterium enhanced gut health and immunity in weaning piglets (11). Meanwhile, the Bifidobacterium reduced the pathogen loads post-Salmonella challenge (12). Similarly, a recent report found that oral administration of Bifidobacterium animalis subsp. lactis (B. animalis) in germfree piglets can prevent Salmonella infection (13). Besides, B. animalis regulate inflammatory cytokines (14, 15), improve intestinal barrier function, and modulates gut microbiota (15). Moreover, Bifidobacterium species can improve their antioxidant capability (16, 17). However, the effects of B. animalis on the growth performance of weaning piglets have not been fully investigated. Therefore, we investigate whether B. animalis improve the growth performance of weaning piglets and then explore its underlying mechanisms.
RESULTS
Effects of Bifidobacterium animalis on growth performance of weaning piglets.
As shown in Table 1 to 3, dietary supplementation with B. animalis did not affect body weight (BW) on day 14 but significantly increased BW on day 28 (P < 0.05). No difference was found in average daily feed intake (ADFI) between the control group (Con) and the B. animalis group (Bif). Average daily gain (ADG) was increased in the group receiving the B. animalis-supplemented diet during days 15 to 28 and days 1 to 28 (P < 0.05). However, the feed conversion ratio (FCR) of piglets fed the B. animalis diet was decreased from day 15 to 28 and day 1 to 28 compared with piglets fed the control diet. Meanwhile, pigs fed the B. animalis diet markedly decreased the incidence of diarrhea (P < 0.05; Table 3).
TABLE 1.
Composition of the experimental diets (as-fed basis)
| Items | Phase 1, 1–14 day |
Phase2, 15–28 day |
||
|---|---|---|---|---|
| Con | Bif | Con | Bif | |
| Ingredient (%) | ||||
| Corn | 62.10 | 61.85 | 67.27 | 67.07 |
| Soybean meal 43% | 10.00 | 10.00 | 15.00 | 15.00 |
| Dehulled soybean meal 46% | 5.00 | 5.10 | 2.00 | 2.05 |
| Fish meal | 5.00 | 5.00 | 3.00 | 3.00 |
| Expanded soybean | 6.00 | 6.00 | 6.00 | 6.00 |
| Whey power | 8.00 | 8.00 | 3.00 | 3.00 |
| Soybean oil | 0.35 | 0.40 | 0.35 | 0.40 |
| CaHPO4 | 1.15 | 1.15 | 1.10 | 1.10 |
| Limestone | 0.70 | 0.70 | 0.60 | 0.60 |
| Salt | 0.20 | 0.20 | 0.20 | 0.20 |
| Primixa | 0.50 | 0.50 | 0.50 | 0.50 |
| L-Lys-HCl (78.8%) | 0.61 | 0.61 | 0.60 | 0.60 |
| DL-Met (98%) | 0.12 | 0.12 | 0.12 | 0.12 |
| L-Thr (98%) | 0.22 | 0.22 | 0.21 | 0.21 |
| L-Trp (98%) | 0.05 | 0.05 | 0.05 | 0.05 |
| Bifidobacterium animalisb | 0.00 | 0.10 | 0.00 | 0.10 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutrient level (%) | ||||
| Digestible energy (MJ/kg) | 14.44 | 14.44 | 14.48 | 14.48 |
| Crude protein (%) | 18.99 | 19.02 | 18.35 | 18.36 |
| Total Ca (%) | 0.88 | 0.88 | 0.72 | 0.72 |
| Total P (%) | 0.71 | 0.71 | 0.64 | 0.64 |
| Lys (%) | 1.35 | 1.35 | 1.28 | 1.28 |
| Met (%) | 0.40 | 0.40 | 0.38 | 0.38 |
| Thr (%) | 0.79 | 0.80 | 0.75 | 0.75 |
| Trp (%) | 0.23 | 0.23 | 0.23 | 0.23 |
The premix provided the following per kg of diets: VA 12,000 IU, VD3 2,500 IU, VE 30 IU, VK3 3 mg, VB5 10 mg, VB12 27.6 μg, niacin 30 mg, choline chloride 400 mg, Mn (as MnO) 40 mg, Fe (as FeSO4·H2O) 90 mg, Zn (as ZnO) 100 mg, Cu (as CuSO4·5H2O) 8.8 mg, I (as KI) 0.35 mg, Se (Na2SeO3) 0.3 mg.
Bifidobacterium animalis 1010 CFU per kg in the diet.
TABLE 2.
The primers used in this study
| Primera | Forward 5′ → 3′ | Reverse 5′ → 3′ |
|---|---|---|
| Total bacteria | ACTCCTACGGGAGGCAGCAG | ATTACCGCGGCTGCTGG |
| Bifidobacterium spp | CGCGTCCGGTGTGAAAG | CTTCCCGATATCTACACATTCCA |
| Lactobacillus spp | GAGGCAGCAGTAGGGAATCTTC | GGCCAGTTACTACCTCTATCCTTCTTC |
| E. coli | CATGCCGCGTGTATGAAGAA | CGGGTAACGTCAATGAGCAAA |
| Salmonella spp | CCTTTCTCCATCGTCCTGAA | TGGTGTTATCTGCCCGACCA |
| GAPDH | ACCCAGAAGACTGTGGATGG | AAGCAGGGATGATGTTCTGG |
| Mucin-1 | GTGCCGACGAAAGAACTG | TGCCAGGTTCGAGTAAGAG |
| Mucin-2 | CTGTGTGGGGCCTGACAA | AGTGCTTGCAGTCGAACTCA |
| ZO-1 | GCCATCCACTCCTGCCTAT | CGGGACCTGCTCATAACTTC |
| Claudin-1 | AAGGACAAAACCGTGTGGGA | CTCTCCCCACATTCGAGATGATT |
| Occludin | CAGCAGCAGTGGTAACTTGG | CAGCAGCAGTGGTAACTTGG |
| TNF-α | CCACGCTCTTCTGCCTACTGC | TCGGCTTTGACATTGGCTACAA |
| IL-1β | CCGCCAAGATATAACTGAC | GCAGCAACCATGTACCAA |
| IL-6 | AATGCTCTTCACCTCTCC | CACACTTCTCATACTTCTCAC |
| IL-8 | TGTCAATGGAAAAGAGGTCTGC | CTGCTGTTGTTGTTGCTTCTCA |
| IL-10 | GTCCGACTCAACGAAGAAGG | GCCAGGAAGATCAGGCAATA |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ZO-1, zonula occludens-1; TNF-α, tumor necrosis factor α; IL, interleukin.
TABLE 3.
The effects of Bifidobacterium animalis on the growth performance of weaning piglets
| Items | Cona | Bifa | SEMa | P value |
|---|---|---|---|---|
| BWa, kg | ||||
| Day 1 | 8.16 | 8.16 | 0.03 | 0.944 |
| Day 14 | 12.50 | 12.66 | 0.09 | 0.400 |
| Day 28 | 19.05 | 19.66 | 0.14 | 0.022 |
| ADGa, g | ||||
| Day 1–14 | 309.38 | 321.28 | 5.22 | 0.274 |
| Day 15–28 | 468.15 | 500.02 | 7.28 | 0.020 |
| Day 1–28 | 388.76 | 410.65 | 4.87 | 0.015 |
| ADFIa, g | ||||
| Day 1–14 | 449.97 | 443.93 | 6.65 | 0.671 |
| Day 15–28 | 777.78 | 785.91 | 7.92 | 0.631 |
| Day 1–28 | 613.88 | 614.92 | 6.76 | 0.943 |
| FCRa | ||||
| Day 1–14 | 1.45 | 1.38 | 0.02 | 0.066 |
| Day 15–28 | 1.66 | 1.57 | 0.02 | 0.034 |
| Day 1–28 | 1.58 | 1.50 | 0.02 | 0.008 |
| Diarrhea incidence, % | 12.20 | 6.85 | 0.018 | |
Con, Control group; Bif, Bifidobacterium animalis group; BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio; SEM, standard error of the means. n = 6 for each group.
Effects of Bifidobacterium animalis on immunological function and inflammatory cytokines of weaning piglets.
The concentrations of immunoglobulins (Ig) in serum, including IgA, IgG, and IgM, had no differences between the Con and Bif groups (Table 4). Similarly, there were no significant differences in serum of interleukin (IL)-1β, IL-6, and IL-10 between the two groups (Table 4).
TABLE 4.
The effects of Bifidobacterium animalis on immunological function and inflammatory cytokines of weaning piglets
| Itemsa | Cona | Bifa | SEMa | P value |
|---|---|---|---|---|
| IgA, mg/mL | 0.85 | 0.91 | 0.05 | 0.569 |
| IgG, mg/mL | 6.91 | 6.28 | 0.21 | 0.141 |
| IgM, mg/mL | 0.64 | 0.77 | 0.05 | 0.147 |
| IL-1β, pg/mL | 28.27 | 29.14 | 1.21 | 0.735 |
| IL-6, pg/mL | 87.40 | 83.61 | 3.18 | 0.573 |
| IL-10, pg/mL | 32.03 | 30.55 | 2.97 | 0.811 |
Con, Control group; Bif, Bifidobacterium animalis group; IgM, immunoglobulin M; IgG, immunoglobulin G; IgA, immunoglobulin A; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; SEM, standard error of the means. n = 7 for each group.
Effects of Bifidobacterium animalis on antioxidant indicators of weaning piglets.
The antioxidant indicators as presented in Table 5. The antioxidant indicators in serum, including catalase (CAT), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were not changed between the two groups. The addition of B. animalis to the diet did not influence the activities of CAT, T-SOD, and GSH-Px in the jejunum. However, B. animalis supplementation increased the activity of T-AOC (P < 0.05) and decreased the content of MDA in the jejunum (P < 0.05).
TABLE 5.
The effects of Bifidobacterium animalis on antioxidant indices of weaning piglets
| Itemsa | Cona | Bifa | SEMa | P value |
|---|---|---|---|---|
| Serum | ||||
| CAT, U/mL | 2.58 | 3.08 | 0.23 | 0.301 |
| T-SOD, U/mL | 146.98 | 148.80 | 1.28 | 0.500 |
| T-AOC, mmol/L | 0.31 | 0.27 | 0.01 | 0.085 |
| GSH-Px, U/mL | 317.68 | 306.41 | 9.76 | 0.584 |
| MDA, nmol/mL | 4.23 | 3.96 | 0.13 | 0.335 |
| Jejunum mucosa | ||||
| CAT, U/mg | 1.17 | 1.16 | 0.10 | 0.971 |
| T-SOD, U/mg | 76.81 | 78.29 | 3.12 | 0.827 |
| T-AOC, mmol/g | 0.50 | 0.98 | 0.10 | 0.003 |
| GSH-Px, U/mg | 218.84 | 172.89 | 14.42 | 0.114 |
| MDA, nmol/mg | 1.63 | 0.92 | 0.18 | 0.037 |
Con, Control group; Bif, Bifidobacterium animalis group; T-SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; T-AOC, total antioxidant capacity; MDA, malondialdehyde; SEM, standard error of the means. n = 7 for each group in serum, n = 5 for each group in the jejunum.
Effects of Bifidobacterium animalis intestinal morphology and barrier of weaning piglets.
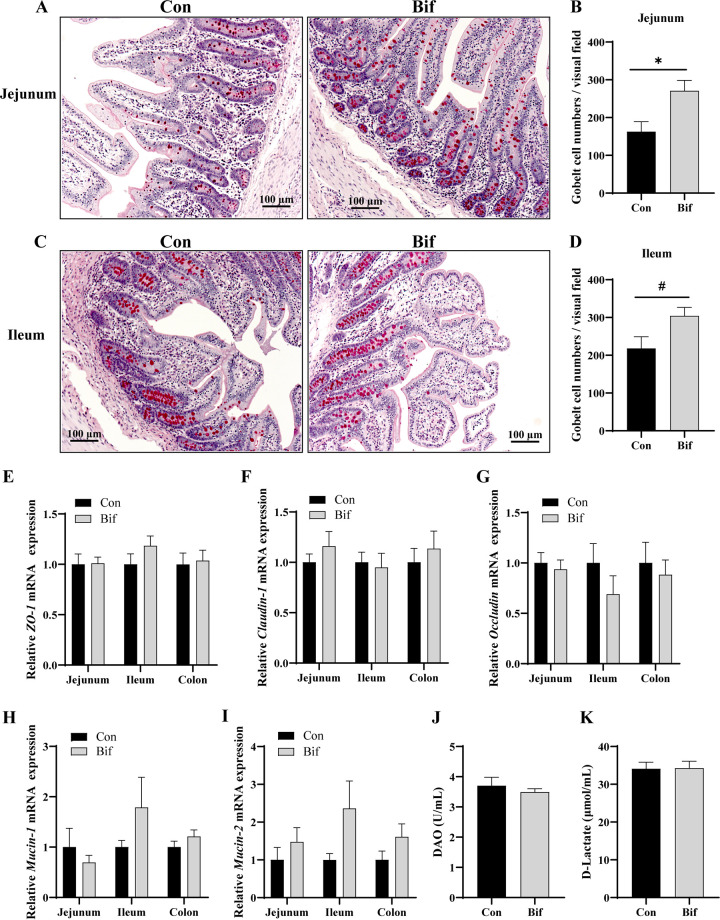
The intestinal morphology and barrier are shown in Table 6 and Fig. 1. There were no effects on villus height (VH), crypt depth (CD), and VH:CD ratio between the Con and Bif groups in the jejunum and ileum. The B. animalis treatment improved the VH and VH:CD ratio in the duodenum compared with the Con group (P < 0.05). In addition, supplementation of B. animalis to the weaning piglet's diet significantly increased the jejunal goblet cell numbers (P < 0.05) (Fig. 1A and B). The B. animalis supplementation tended to have higher goblet cell numbers in the ileum (P = 0.054, Fig. 1C and D).
TABLE 6.
The effects of Bifidobacterium animalis on intestinal morphology of weaning piglets
| Itemsa | Cona | Bifa | SEMa | P value |
|---|---|---|---|---|
| Duodenum | ||||
| VH, μm | 317 | 407 | 16.51 | <0.001 |
| CD, μm | 284 | 281 | 6.44 | 0.842 |
| VH:CD | 1.12 | 1.46 | 0.06 | <0.001 |
| Jejunum | ||||
| VH, μm | 414 | 418 | 6.15 | 0.732 |
| CD, μm | 282 | 297 | 12.92 | 0.592 |
| VH:CD | 1.47 | 1.45 | 0.07 | 0.873 |
| Ileum | ||||
| VH, μm | 289 | 312 | 6.90 | 0.085 |
| CD, μm | 198 | 189 | 6.66 | 0.504 |
| VH:CD | 1.47 | 1.66 | 0.06 | 0.091 |
Con, Control group; Bif, Bifidobacterium animalis group; VH, villus height; CD, crypt depth; SEM, standard error of the means. n = 5 for each group.
FIG 1.
The effects of Bifidobacterium animali on the intestinal barrier. Goblet cells were stained red color by Periodic acid-Schiff (PAS) in the jejunum (A) and ileum (C) of weaning piglets; The number of goblet cells of the visual field in the jejunum (B) and ileum (D); (E to I) the relative mRNA expression of ZO-1 (E), Claudin-1 (F), Occludin (G), Mucin-1 (H), and Mucin-2 (I) in intestinal mucosa; the concentrations of DAO (J) and d-Lactate (K) in serum. DAO, diamine oxidase; ZO-1, zonula occludens-1. SEM, standard error of the means. *, P < 0.05; #, significant trend. n = 7 for each group in serum samples; n = 5 for each group in the intestinal tissue samples.
No differences were observed for relative mRNA expression of tight junction molecules (including zonula occludens-1 [ZO-1], claudin-1, and occludin) in the jejunum, ileum, and colon between the two groups (Fig. 1E to G). Similarly, there were no differences between the B. animalis-supplemented and nonsupplemented groups for the intestinal mucus barrier proteins (mucin-1 and mucin-2) in the jejunum, ileum, and colon (Fig. 1H and I). d-lactate and diamine oxidase (DAO) reflect intestinal barrier integrity. The results showed that the B. animalis treatment also did not affect the contents of serum d-lactate and DAO (Fig. 1J and K).
Effects of Bifidobacterium animalis on jejunum mucosal enzyme activities.
In the jejunum, supplementation of B. animalis to the diet did not influence the activities of trypsin, pepsin, and lipase (Table 7). However, dietary supplementation with B. animalis increased the amylase activity (P < 0.05).
TABLE 7.
Effects of Bifidobacterium animalis on jejunum mucosal enzyme activities of weaning pigs
| Items | Cona | Bifa | SEMa | P value |
|---|---|---|---|---|
| Trypsin, U/mg protein | 86.63 | 79.5 | 5.20 | 0.526 |
| Pepsin, U/mg protein | 1.55 | 1.49 | 0.10 | 0.780 |
| Lipase, U/mg protein | 9.99 | 9.27 | 0.46 | 0.473 |
| Amylase, U/mg protein | 19.65 | 27.18 | 1.98 | 0.049 |
Con, Control group; Bif, Bifidobacterium animalis group; SEM, standard error of the means. n = 5 for each group.
Effects of Bifidobacterium animalis on intestinal inflammation.
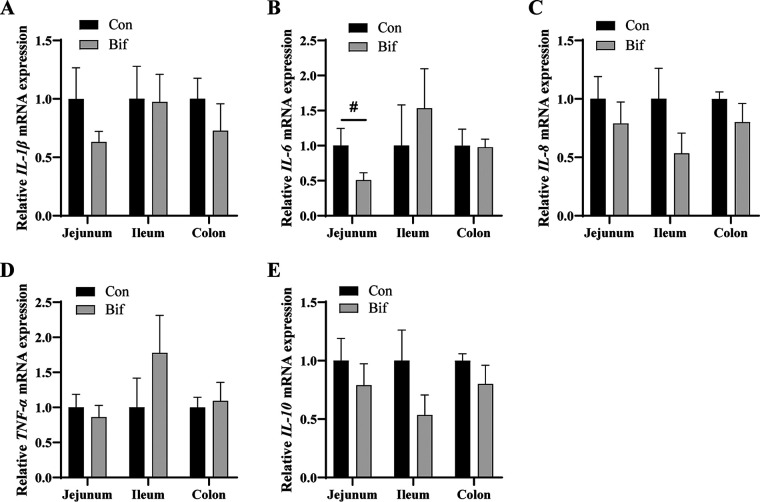
The inflammatory genes’ relative mRNA expression of different segments in the intestine are shown in Fig. 2. There were no remarkable effects on the relative mRNA expression of IL-1β, IL-6, IL-8, tumor necrosis factor α (TNF-α), and IL-10 in the intestine between the two groups (Fig. 2A to E). Compared with the Con group, B. animalis tended to decrease the relative mRNA expression of IL-6 in the jejunum (Fig. 2B, P = 0.10).
FIG 2.
The effects of Bifidobacterium animalis on intestinal inflammatory genes expression. The relative mRNA expression of IL-1β (A), IL-6 (B), IL-8 (C), TNF-α (D), and IL-10 (E). Con, Control group; Bif, Bifidobacterium animalis group; #, significant trend. SEM, standard error of the means. n = 5 for each group.
Effects of Bifidobacterium animalis on intestinal microbiota of weaning piglets.
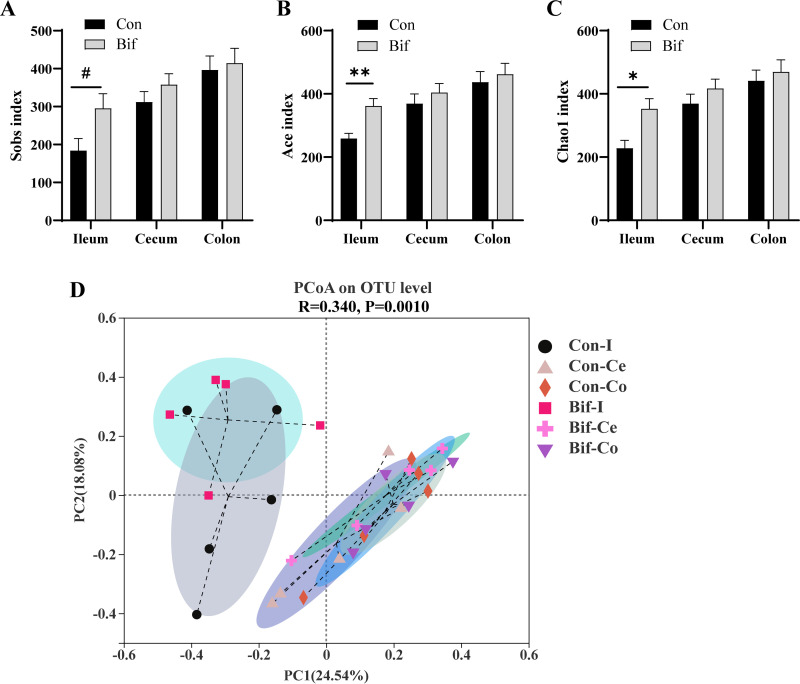
Next, the differences in microbiota composition in various segments of intestinal digesta samples were analyzed (Fig. S1A and B in Supplemental File 1). Alpha diversity (describing the diversity of populations within a sample) indices were calculated to assess the richness and diversity of bacterial communities. The richness of the bacterial community was evaluated by Ace, Chao1, and Sobs indices (Fig. 3A to C), and the diversity of the bacterial community was estimated by Shannon and Simpson indices (Fig. S2A and B in Supplemental File 1). Pigs in the Bif group had higher Ace and Chao1 indices in ileal digesta than pigs in the control group (P < 0.05, Fig. 3B and C). The Sobs index of ileum in the B. animalis-treated piglets displayed an increasing trend (P = 0.06, Fig. 3A). No effects were observed for alpha diversity indices in the hindgut between the two groups (Fig. 3A to C and Fig. S2A and B in Supplemental File 1). Beta diversity (describing the differences between populations among different samples) was assessed by principal component analysis (PCoA) plots based on Bray-Curtis distances. Beta diversity indicated no obvious separation in microbiota composition structure between the Bif and the Con groups in the same segment of the intestine (Fig. 3D, Fig. S2C to E in Supplemental File 1).
FIG 3.
The intestinal digesta microbiota diversities between the Bif and Con piglets. (A–C) Alpha diversity of Sobs (A), Ace (B), and (C) Chao1 indices; (D) principal coordinate analysis (PCoA) of ileal, cecal, and colonic digesta samples in the Bif and Con groups based on Bray-Curtis distances. Data are means ± SEM. *, P < 0.05; **, P < 0.01; #, significant trend P < 0.10. n = 5 for each group. Con, Control group; Bif, Bifidobacterium animalis group; Con-I, ileal digesta in control group; Con-Ce, cecal digesta in control group; Con-Co, colonic digesta in control group; Bif-I, ileal digesta in Bifidobacterium animalis group; Bif-Ce, cecal digesta in Bifidobacterium animalis group; Bif-Co, colonic digesta in Bifidobacterium animalis group.
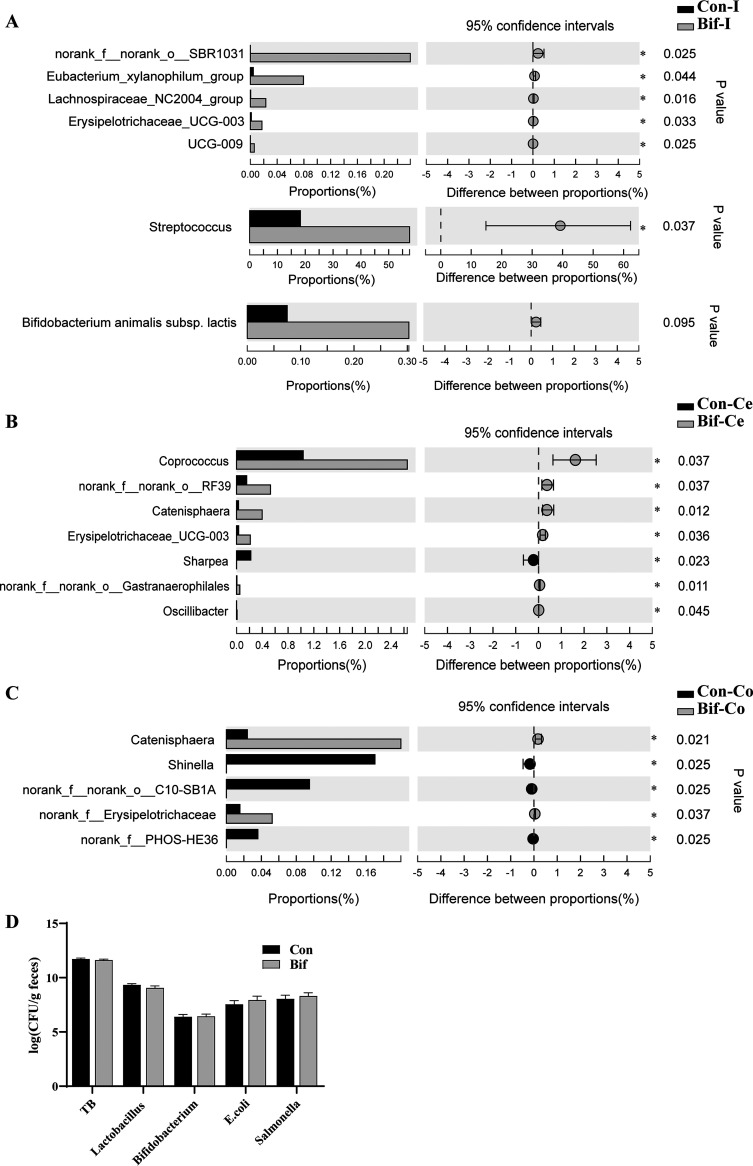
The relative abundances of genera Streptococcus and Erysipelotrichaceae_UCG-003 were increased in the ileal digesta of pigs fed the B. animalis diet compared with those fed the control diet (P < 0.05, Fig. 4A). In the cecal digesta, the relative abundances of Coprococcus, Erysipelotrichaceae_UCG-003, and Oscillibacter were elevated in pigs from the Bif group (P < 0.05, Fig. 4B). B. animalis supplementation promoted the relative abundances of Catenisphaera in the cecal and colonic digesta (P < 0.05, Fig. 4B and C). Importantly, the abundance of Bifidobacterium animalis subsp. lactis from ileal digesta in the Bif group showed an increasing trend compared to that in the Con group (P = 0.095, Fig. 4A). Next, real-time quantitative PCR was used to measure the absolute copy numbers of specific bacteria from the feces. The results revealed that there were no markable changes for the copies of total bacteria (TB), Lactobacillus, Bifidobacterium, Escherichia coli, and Salmonella from the feces between the two groups (Fig. 4D).
FIG 4.
The differential digesta bacteria between the Con and Bif group pigs. The differential bacteria from the ileal (A), cecal (B), and colonic (C) digesta samples between the Con and the Bif pigs (n = 5); (D) the absolute copy numbers of specific bacteria in the fecal samples (n = 8). Data are means ± SEM. *, P < 0.05. Con, Control group; Bif, Bifidobacterium animalis group; Con-I, ileal digesta samples in the control group; Con-Ce, cecal digesta samples in the control group; Con-Co, colonic digesta samples in the control group; Bif-I, ileal digesta samples in Bifidobacterium animalis group; Bif-Ce, cecal digesta samples in Bifidobacterium animalis group; Bif-Co, colonic digesta samples in Bifidobacterium animalis group. TB, total bacteria.
Effects of Bifidobacterium animalis on mucosal microbiota of weaning piglets.
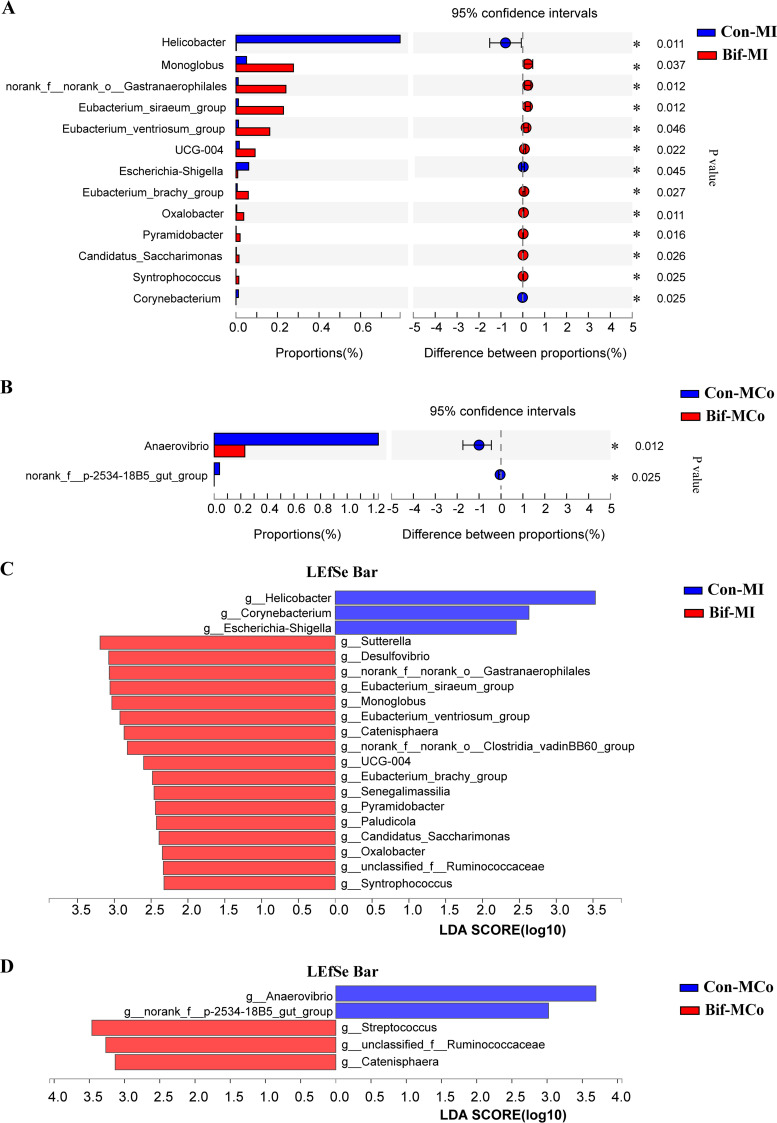
The mucosal microbiota was closely related to animal health. The mucosal microbiota in the ileum and colon were also analyzed (Fig. S1C and D in Supplemental File 1). The results showed that neither alpha diversity indices nor beta diversity had differences from ileal and colonic mucosa-associated microbiota between the Con and the Bif groups (Fig. S3 in Supplemental File 1). Differential bacteria analysis at the genus level found that the relative abundances of Helicobacter and Escherichia-Shigella were reduced in the ileal mucosa of pigs from the Bif group compared with those from the Con group (Fig. 5A, P < 0.05). Linear discriminant analysis (LDA) effect size (LEfSe) analysis was used to identify intergroup differential bacteria. Consistently, LEfSe analysis exhibited that the dietary addition of B. animalis also declined the Helicobacter and Escherichia-Shigella abundances of ileal mucosa-associated microbiota (Fig. 5C, LDA > 2). Additionally, Anaerovibrio was enriched in colonic mucosa samples of pigs from the Con group (Fig. 5B and D, P < 0.05)., whereas Streptococcus and Catenisphaera populations were enriched in colonic mucosa samples of pigs from the Bif group (Fig. 5D, LDA > 2).
FIG 5.
The differential mucosa bacteria between the Con and Bif group pigs. The differential bacteria from the ileal (A) and colonic (B) mucosa samples between the Con and the Bif pigs. Histograms of Linear discriminant analysis (LDA) score at the genus level in ileal (C), and colonic (D) mucosa samples between the Con and the Bif pigs. LDA score >2, n = 5 for each group. Con-MI, ileal mucosa samples in control group; Bif-MI, ileal mucosa samples in Bifidobacterium animalis group; Con-MCo, colonic mucosa samples in control group; Bif-MCo, colonic mucosa samples in Bifidobacterium animalis group. *, P < 0.05.
DISCUSSION
Probiotics have been extensively studied in animal production. Lactic acid bacteria, especially the Lactobacillus and Bifidobacterium species, exhibit various beneficial effects on hosts. Previous studies indicated that supplementation of Lactobacillus or Bifidobacterium improved the growth performance of weaning piglets and decreased diarrhea incidence by regulating the immune function, enhancing the epithelial barrier, increasing the antioxidant capability, and inhibiting the growth of pathogens (10, 18–20). In this study, we investigated the effects of B. animalis on the growth performance of weaning piglets. The results showed that dietary supplementation of B. animalis increased the final BW and ADG on day 15 to 28 and the whole experimental period of weaning pigs, but the FCR significantly decreased from day 15 to 28 and the whole period. Additionally, the pigs fed a diet with the addition of B. animalis reduced the incidence of diarrhea compared with the control group. The effects of Bifidobacterium animalis strains on growth performance in weaning pigs have a few reports. Modesto et al. (21) found that the ADG had a linear increase with increasing dietary levels of B. animalis strain Ra18 in weaning pigs for the Salmonella Typhimurium infection model. The diet supplementation of the probiotic mixture, containing Enterococcus faecium, B. animalis, and Lactobacillus salivarius, increased BW and body weight gain (BWG) on days 21, 28, 35, and 42 but markedly decreased the FCR at related time points in chicken for the Eimeria infection model (22). Broilers receiving in ovo inoculation with B. animalis improved BW and BWG and decreased the FCR on days 1 to 28, while no difference in ADFI was observed between the B. animalis and control groups (23). Similarly, a recent study found that rabbits received 1 × 10 9 CFU Bifidobacterium animalis subsp. lactis BB-12 (B. animalis BB12) for 30 days had remarkably increased final BW on day 60 in comparison with the control group (24). Meanwhile, B. animalis BB12 also declined the FCR in rabbits (24). Importantly, the amylase of jejunum in the Bif group was higher than in the control group. Numerous studies demonstrated that probiotics can improve the digestibility of dry matter, energy, crude protein, and crude fiber in weaning piglets (8, 10). Jin et al. (25) indicated that a lactobacillus mixture promotes amylase activities in broiler chickens. Thus, the improvement of dietary nutrient digestibility in pigs fed with probiotics may be explained by increased enzyme production and activity (8).
A mechanism of its health-promoting effects on animals is that probiotics could enhance immune function and suppress inflammatory reactions (10). Our results showed that B. animalis failed to affect the levels of immunoglobulin and inflammatory cytokines in serum or the relative inflammatory genes expression in the intestine, whereas the relative IL-6 mRNA expression in jejunum had a decreased tendency in B. animalis-treated pigs. Similarly, the serum contents of IgA, TNF-β, and IL-1β were not changed in B. animalis-treated broilers compared with the control group (26). In addition, our results were consistent with a recent report, which showed that there were no differences in serum levels of IL-8, TNF-α, and IL-10 or in different segments of the intestine (jejunum, ileum, and colon) between the germfree piglets and B. animalis BB12-treated germfree piglets (13).
The antioxidant enzymes, such as CAT, T-AOC, T-SOD, and GSH-Px play a pivotal role in eliminating oxidative damage and maintaining cell structure. The MDA is regarded as an important indicator of lipid peroxidation. Thus, these parameters are often used to reflect animal health and oxidative stress status. Under stress conditions, the activity of CAT, T-AOC, T-SOD, and GSH-Px was reduced, whereas the level of MDA increased (27). The growing evidence indicated that probiotics, especially lactic acid bacteria, possessed an antioxidant effect (19). Pigs fed a diet supplementation of Lactobacillus plantarum (L. plantarum) had increased contents of SOD and GSH-Px in serum, while the concentration of MDA was decreased (28). In the present study, no differences were observed in serum activities of antioxidant enzyme activities and level of MDA between the control and B. animalis pigs. However, B. animalis treatment significantly elevated the T-AOC activity and reduced the MDA level in the jejunum mucosa. In consistency with our findings, previous studies reported that B. animalis secretion of selenium-contained protein exhibited strong antioxidant activity in vivo (16, 29). Meanwhile, B. animalis culture supernatant or cells could significantly enhance the activities of antioxidant enzymes and reduce MDA levels in the d-galactose-induced-aging mice model (29). These results indicated that the beneficial effects of B. animalis on growth performance in weaning piglets might be associated with enhancing antioxidant capacity in the intestine.
Intestinal morphology indicators, such as VH, CD, and VH:CD ratio, are used to evaluate the intestinal mucosal function and are closely related to animal performance. In this study, our results found that B. animalis increased the VH and VH:CD ratio in the duodenum but did not differ in the jejunum and ileum. Barba-Vidal (12) also found that the VH and VH:CD ratio was not affected in B. animalis BPL6 combination with B. longum-treated weaning pigs without Salmonella infection, while B. animalis BPL6 significantly increased the VH:CD ratio on day 8 post Salmonella challenge (12). There was similar result indicated that B. animalis BB12 alleviated ileal epithelial damage post-Salmonella challenge (13).
The beneficial effect of probiotics on intestinal barrier function has also been suggested as a reason for their health-promoting effects on animals (9, 30). Intestinal epithelial tight junction proteins contribute to maintaining cell-cell interaction and determine the primarily paracellular permeability (31). To prevent pathogens infection, intestinal epithelia are covered with a thick and complex mucus layer, which is mainly composed of mucins secreted by goblet cells (32). Our results found that B. animalis did not influence the relative mRNA expression of tight junction molecules (ZO-1, claudin-1, and occludin) and mucin proteins. The serum DAO and d-lactate reflect intestinal permeability, and there were no differences between the Bif and Con groups as well. However, B. animalis-treated pigs markedly increased the number of goblet cells in the jejunum. A recent report demonstrated that the relative mRNA expression of claudin-1 and occludin were not affected in the ileum and colon of B. animalis BB12-treated germfree piglets, respectively, compared to those in the control group (13). Consistently, there was no difference in ileal goblet cells between Bifidobacterium boum RP36 or RP37 strain and control groups germfree piglets (13). It suggested that B. animalis may have a limited effect on intestinal barrier function for animals in normal conditions without pathogen infection. L. plantarum strain did not change the tight junction proteins expression without enterotoxigenic E. coli infection in the IPEC-J2 model, but L. plantarum improved the intestinal epithelial barrier under the E. coli infection (33). B. animalis BB12 treatment increased claudin-1 protein mRNA expression in the ileum of germfree piglets post Salmonella infection.
Gut microbiota is critical for improving growth performance and defending against pathogens' colonization (2, 34). Our results showed that the lumen of the ileal Ace index and Chao1 index remarkably increased in the Bif group pigs, indicating that dietary supplementation with B. animalis increased the alpha diversity in ileal microbiota. However, B. animalis did not change the bacterial alpha diversity in the hindgut. Notably, there were no differences in beta diversity in the ileum, cecum, and colon between the Bif and Con groups of pigs. Similarly, the alpha diversity indices and structure in ileal and colonic mucosa-associated microbiota were not affected in B. animalis-treated pigs compared with pigs in the control group. At the genus level, supplementation of B. animalis in diet promoted the relative abundances of the ileal lumen of Streptococcus and the cecal lumen of Coprococcus and Oscillibacter. Additionally, B. animalis supplementation increased the relative abundance of Catenisphaera in both lumens of the cecum and colon. Some Streptococcus spp belonging to lactic acid bacteria predominate in pigs as beneficial bacteria (20, 35). In the present study, unclassified Streptococcus may have a beneficial effect on weaning pigs. Similarly, the dietary addition of zinc oxide increased Streptococcus equinus and Streptococcus lutetiensis relative abundances (36). The Coprococcus and Oscillibacter were considered potentially beneficial bacteria or next-generation probiotics (37–40). Several studies found that Oscillibacter was associated with human health, especially inflammatory disease (41). Recent research reported that Oscillibacter showed a positive association in the high feed efficiency group of broiler chicken (42). In the current study, dietary supplements with B. animalis promoted Erysipelotrichaceae_UCG-003 populations in both lumens of the ileum and cecum, which may positively affect the growth performance of pigs. Polysaccharide utilization loci analysis indicated that Erysipelotrichaceae strains may have the ability to utilize plant polysaccharides (43). Concordantly, pigs fed a diet with high corn-based arabinoxylans increased Erysipelotrichaceae_UCG-002 abundance. Interestingly, the cecal relative abundance of the family Erysipelotrichaceae showed a negative correlation with FCR in broiler chickens (44). Besides, Catenisphaera, a member of the family Erysipelotrichaceae maybe also contribute to improving the growth performance of pigs (45). Importantly, dietary supplementation with B. animalis increased the abundance of B. animalis in the ileal digesta and decreased the abundance of potential pathogens Helicobacter and Escherichia-Shigella in the ileal mucosa. Collectively, these results indicated that B. animalis modulated the gut composition in different intestinal segments and enriched some beneficial bacteria, which consequently improved growth performance. Meanwhile, B. animalis decreased the potential pathogens populations in ileal mucosa.
In conclusion, this study showed that B. animalis increased growth performance, decreased diarrhea incidence, improved duodenal morphology, and enhanced antioxidant capacity in the jejunum of weaning piglets, but did not exert on the intestinal barrier and immune function significantly. Dietary supplementation with B. animalis increased the abundance of B. animalis in the ileum. However, B. animalis supplementation decreased the abundances of Helicobacter and Escherichia-Shigella might be a growth-promoting attribute. Thus, B. animalis can be considered a potential beneficial bacterium for pigs.
MATERIALS AND METHODS
Animals, diets, and experimental design.
A total of 96 (Duroc × Landrace × Large White) weaning piglets with average initial body weight (BW) of 8.16 ± 0.12 kg were randomly divided into 2 groups based on BW and sex. There were 6 replicate pens per treatment and 8 piglets per pen. The control group (Con) received a control diet without supplementation of probiotics. The Bifidobacterium animalis treated group (Bif) was fed a diet with 1010 CFU Bifidobacterium animalis subsp. lactis per kg. Bifidobacterium animalis subsp. lactis JYBR-190 was provided by ZhongKe-JiaYi Biological Engineering Co., Ltd. (Shandong, China). The experimental period included two feeding phases: phase 1, from day 1 to day 14 postweaning; phase 2, from day 15 to day 28 postweaning. The dietary compositions and nutrient concentrations are presented in Table 1. All diets without antibiotics were formulated to meet the nutrient requirements of NRC (2012) (46). Pigs were raised in an environmentally controlled room with slatted plastic flooring and a mechanical ventilation system. The environmental temperature was maintained at 24 to 28°C and relative humidity was controlled at 40% to 60%. Pigs were given ad libitum access to feed and water throughout the trial for 28 days.
Samples collection.
Individual pig BW was weighed on days 0, 14, and 28. Pen feed intake was also recorded on days 14 and 28 of the experiment. The average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated accordingly. The feces of pigs were scored daily for diarrhea according to the following criteria: 1, firm and well-form feces; 2, soft and form feces; 3, sloppy feces and mild diarrhea; and 4, pasty and liquid diarrhea. The incidence of diarrhea was calculated for the first 7 d after weaning. The incidence of diarrhea was calculated as follows: diarrhea incidence (%) = (total number of pigs per pen with diarrhea)/(number of pigs per pen × 7 d) × 100%.
On day 28, blood samples were collected from the jugular vein (at least one pig per pen) and serum was separated after centrifugation at 3,000 × g for 15 min at 4°C. Fresh fecal samples were collected into 2 mL sterile tubes on day 28 and stored at −80°C for further analysis.
On day 29, five pigs from each group were euthanized. The digesta samples from the ileum, cecum, and colon were immediately collected and placed in liquid nitrogen, and then stored at −80°C for further analysis. The different segments of intestinal samples were collected as described previously (47). Segments from the duodenum, jejunum, and ileum (approximately 3 cm long) were flushed with saline, fixed in 4% paraformaldehyde solution, and then embedded in paraffin for analysis of hematoxylin and eosin (HE) and Periodic acid-Schiff (PAS) staining. The mucosal tissues of the jejunum, ileum, and colon were scraped using the glass slide and stored at −80°C for determination of enzyme activities and related gene expression.
Biochemical analysis.
The contents of serum d-lactate, immunoglobulin A (IgA), IgG, IgM, interleukin-1β (IL-1β), IL-6, and IL-10 were detected by commercial ELISA kits (Jiancheng Co., Ltd., Nanjing, China) according to manufacturer’s instructions. The serum content of diamine oxidase (DAO) was measured using the biochemical kit following the manufacturer’s protocols (Solarbio Co., Ltd., Beijing China). The contents of total superoxide dismutase (T-SOD), catalase (CAT), total antioxidant capacity (T-AOC), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) capacity in serum were determined using commercial kits (Jiancheng Co., Ltd., Nanjing, China), respectively.
Jejunum mucosal tissues (approximately 100 mg) were weighed, homogenized in ice-cold 1 mL PBS (phosphate buffer solution), and centrifuged at 3,000 × g and 4°C for 10 min. The protein concentration of the jejunal supernatants was measured using the bicinchoninic acid (Thermo Fisher, USA) method according to the manufacturer's protocols. The jejunum mucosal antioxidant indices were determined as described above. The activities of trypsin, pepsin, lipase, and amylase were determined using commercial kits (Jiancheng Co., Ltd., Nanjing, China).
Morphological examination and measurement of intestinal goblet cell numbers.
The segments of the small intestine were sectioned (5 μm). The sections were stained with the HE method or PAS method. The images were captured under a light microscope (Carl Zeiss, Germany). The villus length (VH) and crypt depth (CD) was measured by random measurements of 10 VH and 10 CD per section. The intestinal goblet cell numbers in the field view were counted by a random selection of a field view in each section. The goblet cells are stained red color by PAS.
RNA extraction and quantification.
Total RNA was extracted from the tissue using TRIzol Reagent (Invitrogen, USA) according to the manufacturer’s protocol. 1.0 μg of RNA was used to synthesize the first-strand cDNA by using a reverse transcription kit (TaKaRa, Dalian, China). The synthesized cDNA was diluted (1:10, vol/vol) with ultrapure-water, and then stored at −20°C for future analysis. The quantitative real-time PCR was performed on Roche LightCycler 96 system (Roche, Sweden) with specific primers (Table 2). The method of 2−△△Ct was used to calculate the relative gene expression and glyceraldehyde-3-phosphate dehydrogenase (GADPH) was normalized as the internal control. All samples were measured in duplicate.
Microbiota analysis.
Total microbial DNA of the digesta and mucosa from the ileum, cecum, and colon was isolated using the QIAamp Fast DNA Stool Minikit (Qiagen, Germany) following the manufacturer’s protocol. The V3-V4 hypervariable regions of bacteria 16S rRNA genes were amplified with universal primers 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT). The purified PCR products were equimolarly combined and paired-end sequenced on the Illumina Miseq platform (Illumina, San Diego, CA, USA).
The raw reads were demultiplexed and quality filtered by QIIME (version 1.9) software with the following criteria (i) the lower-quality sequences with a length of <220 nt or >500 nt, an average quality score of <20, and sequences containing >3 nitrogenous bases were removed; (ii) only overlapping sequences longer than 10 bp and the reads without more than 2 nucleotide mismatches in the primer were assembled. The remaining sequences with 97% similarity were clustered into the same operational taxonomic units (OTUs) using UPARSE (version 7.1) and chimeric sequences were removed. The taxonomy of each OTU representative sequence was analyzed by Ribosomal Database Project (RDP) classifier against the SILVA 16S rRNA gene database using a confidence threshold of 70%. The analysis of the alpha diversity was calculated by the MOTHUR program. For the beta diversity, principal coordinate analysis (PCoA) was performed based on Bray-Curtis distances. The data were analyzed on the Majorbio I-Sanger cloud platform (https://cloud.majorbio.com/).
Quantification of specific bacteria.
Total bacterial DNA was separated from fecal samples as mentioned above. The specific primers of total bacteria, Lactobacillus, Bifidobacterium, Escherichia coli (E. coli), and Salmonella were presented in Table 2. Standard curves were obtained from constructing standard plasmids containing the 16S rRNA genes. The copies of target bacteria were calculated by corresponding standard curves as described previously (48).
Statistical analysis.
Data were performed using SAS 9.4 version (Cary, NC, United States). The data of the two groups were analyzed by unpaired Student's t test. The incidence of diarrhea was analyzed by χ2 tests. A Nonparametric Wilcoxon rank-sum test was performed to compare the microbiota difference between the Con and Bif piglets at different intestinal segments. P values were adjusted with a false discovery rate (FDR). The differential bacteria were identified by linear discriminant analysis (LDA) effect size (LEfSe) analysis. The level of statistical significance was P < 0.05, whereas 0.05 ≤ P ≤ 0.1 was considered a trend of significant difference. Data are presented as means ± standard error of the means (SEM).
Ethics statement.
This work was approved by the Care and Use of Experimental Animal Committee of China Agricultural University (CAU No. AW51211202-1-2).
Data availability.
All raw FASTQ reads for this study can be found in NCBI Sequence Read Archive (SRA) database PRJNA828137.
ACKNOWLEDGMENTS
The experiments were conducted at the FengNing Swine Research Unit of China Agricultural University (Academician Workstation in Chengdejiuyun Agricultural and Livestock Co., Ltd., Hebei, China).
This study was funded by grants from the National Natural Science Foundation of China (No. 32172750, 32125036, 31902170, and 31630074), the China Agricultural Research System (CARS-35), the National Key Research and Development Program of China (2021YFD1300201), and S&T Program of Hebei (199A7310H).
Jiaman Pang wrote the manuscript. Jiaman Pang analyzed the data. Jiaman Pang, Yisi Liu, and Luyuan Kang performed the experiments. Hao Ye, Junjun Wang, and Dandan Han revised the manuscript. Junjun Wang and Dandan Han designed the experiment. Junjun Wang, Dandan Han, and Jianjun Zang obtained financial support and oversaw this study.
All authors declare that there is no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Dandan Han, Email: handandan@cau.edu.cn.
Johanna Börkroth, University of Helsinki.
REFERENCES
- 1.Campbell JM, Crenshaw JD, Polo J. 2013. The biological stress of early weaned piglets. J Anim Sci Biotechnol 4:19–22. 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gresse R, Chaucheyras-Durand F, Fleury MA, Van de Wiele T, Forano E, Blanquet-Diot S. 2017. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol 25:851–873. 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Barton MD. 2014. Impact of antibiotic use in the swine industry. Curr Opin Microbiol 19:9–15. 10.1016/j.mib.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Cromwell GL. 2002. Why and how antibiotics are used in swine production. Anim Biotechnol 13:7–27. 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- 5.Thacker PA. 2013. Alternatives to antibiotics as growth promoters for use in swine production: a review. J Anim Sci Biotechnol 4:35. 10.1186/2049-1891-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308:1635–1638. 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. 2014. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 8.Liao SF, Nyachoti M. 2017. Using probiotics to improve swine gut health and nutrient utilization. Anim Nutr 3:331–343. 10.1016/j.aninu.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suez J, Zmora N, Segal E, Elinav E. 2019. The pros, cons, and many unknowns of probiotics. Nat Med 25:716–729. 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 10.Patil AK, Kumar S, Verma AK, Baghel RPS. 2015. Probiotics as feed additives in weaned pigs: a review. Livestock Res International 12:31–39. [Google Scholar]
- 11.Herfel TM, Jacobi SK, Lin X, Jouni ZE, Chichlowski M, Stahl CH, Odle J. 2013. Dietary supplementation of Bifidobacterium longum strain AH1206 increases its cecal abundance and elevates intestinal interleukin-10 expression in the neonatal piglet. Food Chem Toxicol 60:116–122. 10.1016/j.fct.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Barba-Vidal E, Castillejos L, Roll VFB, Cifuentes-Orjuela G, Moreno Muñoz JA, Martín-Orúe SM. 2017. The probiotic combination of Bifidobacterium longum subsp. infantis CECT 7210 and Bifidobacterium animalis subsp. lactis BPL6 reduces pathogen loads and improves gut health of weaned piglets orally challenged with Salmonella Typhimurium. Front Microbiol 8:1570. 10.3389/fmicb.2017.01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Splichalova A, Donovan SM, Tlaskalova-Hogenova H, Stranak Z, Splichalova Z, Splichal I. 2021. Monoassociation of preterm germ-free piglets with Bifidobacterium animalis subsp. lactis BB-12 and its impact on infection with Salmonella Typhimurium. Biomedicines 9:183. 10.3390/biomedicines9020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arenas-Padilla M, Duarte-Gutiérrez JL, Mata-Haro V. 2018. Bifidobacterium animalis ssp. lactis BB12 induces IL-10 through cell membrane-associated components via TLR2 in swine. J Appl Microbiol 125:1881–1889. 10.1111/jam.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N, Wang S, Xu B, Liu F, Huo G, Li B. 2021. Alleviation effects of Bifidobacterium animalis subsp. lactis XLTG11 on dextran sulfate sodium-induced colitis in mice. Microorganisms 9:2093. 10.3390/microorganisms9102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Q, Zhang B, Xu R, Wang Y, Ding X, Li P. 2010. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe 16:380–386. 10.1016/j.anaerobe.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Wang S, Zeng Z, Qin Y, Li P. 2019. The complete genome sequence of Bifidobacterium animalis subsp. lactis 01 and its integral components of antioxidant defense system. 3 Biotech 9:352. 10.1007/s13205-019-1890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barba-Vidal E, Martín-Orúe SM, Castillejos L. 2018. Review: are we using probiotics correctly in post-weaning piglets? Animal 12:2489–2498. 10.1017/S1751731118000873. [DOI] [PubMed] [Google Scholar]
- 19.Feng T, Wang J. 2020. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes 12:1801944. 10.1080/19490976.2020.1801944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Yao B, Gao H, Zang J, Tao S, Zhang S, Huang S, He B, Wang J. 2019. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs. BMC Vet Res 15:239. 10.1186/s12917-019-1991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modesto M, D'Aimmo MR, Stefanini I, Trevisi P, De Filippi S, Casini L, Mazzoni M, Bosi P, Biavati B. 2009. A novel strategy to select Bifidobacterium strains and prebiotics as natural growth promoters in newly weaned pigs. Livestock Science 122:248–258. 10.1016/j.livsci.2008.08.017. [DOI] [Google Scholar]
- 22.Giannenas I, Tsalie E, Triantafillou E, Hessenberger S, Teichmann K, Mohnl M, Tontis D. 2014. Assessment of probiotics supplementation via feed or water on the growth performance, intestinal morphology and microflora of chickens after experimental infection with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Avian Pathol 43:209–216. 10.1080/03079457.2014.899430. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Moneim AE, Elbaz AM, Khidr RE, Badri FB. 2020. Effect of in ovo inoculation of Bifidobacterium spp. on growth performance, thyroid activity, lleum histomorphometry, and microbial enumeration of broilers. Probiotics & Antimicro Prot 12:873–882. 10.1007/s12602-019-09613-x. [DOI] [PubMed] [Google Scholar]
- 24.Kadja L, Dib AL, Lakhdara N, Bouaziz A, Espigares E, Gagaoua M. 2021. Influence of three probiotics strains, Lactobacillus rhamnosus GG, Bifidobacterium animalis subsp. lactis BB-12 and Saccharomyces boulardii CNCM I-745 on the biochemical and haematological profiles and body weight of healthy rabbits. Biology (Basel) 10:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin LZ, Ho YW, Abdullah N, Jalaludin S. 2000. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult Sci 79:886–891. 10.1093/ps/79.6.886. [DOI] [PubMed] [Google Scholar]
- 26.Hernández-Granados MJ, Ortiz-Basurto RI, Jiménez-Fernández M, García-Munguía CA, Franco-Robles E. 2022. Dietary encapsulated Bifidobacterium animalis and Agave fructans improve growth performance, health parameters, and immune response in broiler chickens. Anim Biosci 35:587–595. 10.5713/ab.21.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Wang C, Huang K, Zhang M, Wang J, Pan X. 2020. Compound Lactobacillus sp. administration ameliorates stress and body growth through gut microbiota optimization on weaning piglets. Appl Microbiol Biotechnol 104:6749–6765. 10.1007/s00253-020-10727-4. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Ji HF, Wang SX, Zhang DY, Liu H, Shan DC, Wang YM. 2012. Lactobacillus plantarum ZLP001: in vitro assessment of antioxidant capacity and effect on growth performance and antioxidant status in weaning piglets. Asian Australas J Anim Sci 25:1153–1158. 10.5713/ajas.2012.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Q, Shang N, Li P. 2011. In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr Microbiol 62:1097–1103. 10.1007/s00284-010-9827-7. [DOI] [PubMed] [Google Scholar]
- 30.Natividad JM, Verdu EF. 2013. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res 69:42–51. 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Otani T, Furuse M. 2020. Tight junction structure and function revisited. Trends Cell Biol 30:805–817. 10.1016/j.tcb.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. 2008. Mucins in the mucosal barrier to infection. Mucosal Immunol 1:183–197. 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, Wang Y. 2018. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front Microbiol 9:1953. 10.3389/fmicb.2018.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ducarmon QR, Zwittink RD, Hornung BVH, van Schaik W, Young VB, Kuijper EJ. 2019. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol Mol Biol Rev 83:e00007. 10.1128/MMBR.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao S, Bai Y, Li T, Li N, Wang J. 2019. Original low birth weight deteriorates the hindgut epithelial barrier function in pigs at the growing stage. FASEB J 33:9897–9912. 10.1096/fj.201900204RR. [DOI] [PubMed] [Google Scholar]
- 36.Vahjen W, Pieper R, Zentek J. 2011. Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets. J Anim Sci 89:2430–2439. 10.2527/jas.2010-3270. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee A, Lordan C, Ross RP, Cotter PD. 2020. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 12:1802866. 10.1080/19490976.2020.1802866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. 2020. The controversial role of human gut Lachnospiraceae. Microorganisms 8:573. 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. 2015. Colonic bacterial composition in Parkinson's disease. Mov Disord 30:1351–1360. 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Li Y, Wen Z, Liu W, Meng L, Huang H. 2021. Oscillospira - a candidate for the next-generation probiotics. Gut Microbes 13:1987783. 10.1080/19490976.2021.1987783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konikoff T, Gophna U. 2016. Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol 24:523–524. 10.1016/j.tim.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Lv H, Song Y, Sun C, Zhang Z, Chen S. 2021. Community composition of cecal microbiota in commercial yellow broilers with high and low feed efficiencies. Poult Sci 100:100996. 10.1016/j.psj.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Liu M, Zhou M, Wu L, Yang H, Huang L, Chen C. 2021. Isolation and genomic characterization of five novel strains of Erysipelotrichaceae from commercial pigs. BMC Microbiol 21:125. 10.1186/s12866-021-02193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley D, Hughes RJ, Geier MS, Moore RJ. 2016. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Front Microbiol 7:187. 10.3389/fmicb.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanno M, Katayama T, Morita N, Tamaki H, Hanada S, Kamagata Y. 2015. Catenisphaera adipataccumulans gen. nov., sp. nov., a member of the family Erysipelotrichaceae isolated from an anaerobic digester. Int J Syst Evol Microbiol 65:805–810. 10.1099/ijs.0.000021. [DOI] [PubMed] [Google Scholar]
- 46.NRC. Nutrient requirements of swine [M]. National Academies Press, 2012. 11th rev. Ed Washington, DC. [Google Scholar]
- 47.Liu JB, Cao SC, Liu J, Xie YN, Zhang HF. 2018. Effect of probiotics and xylo-oligosaccharide supplementation on nutrient digestibility, intestinal health and noxious gas emission in weanling pigs. Asian-Australas J Anim Sci 31:1660–1669. 10.5713/ajas.17.0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N, Huang S, Jiang L, Dai Z, Li T, Han D, Wang J. 2019. Characterization of the early life microbiota development and predominant Lactobacillus species at distinct gut segments of low- and normal-birth-weight piglets. Front Microbiol 10:797. 10.3389/fmicb.2019.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aem.01296-22-s0001.pdf, PDF file, 0.5 MB (462.2KB, pdf)
Data Availability Statement
All raw FASTQ reads for this study can be found in NCBI Sequence Read Archive (SRA) database PRJNA828137.