Abstract
Burn patients are susceptible to infections due, in part, to immune dysfunction. Upregulation of programmed death‐1 (PD‐1) receptor on T cells and programmed cell death ligand‐1 (PD‐L1) on myeloid cells contribute to immune dysfunction in nonburn‐related sepsis. We hypothesized that PD‐1/PDL1 interactions contribute to immune dysfunction after burn injury. To determine the impact of burn injury and infection on PD‐L1, PD‐1 and costimulatory receptor expression by leukocytes and its relationship to T cell functions. The efficacy of anti‐PD‐L1 antibody was evaluated in a clinically relevant mouse model of burn injury and bacterial infection. Mice underwent 35% scald burn followed by Pseudomonas aeruginosa or Staphylococcus aureus infection on day 4 postburn. Anti‐PD‐L1 was administered on day 3 postburn. Numbers and phenotype of leukocytes, plasma cytokine concentrations, bacterial clearance, organ injury, and survival were assessed. Burn injury and infection with P. aeruginosa caused a significant upregulation of PD‐L1 on myeloid cells, along with a decrease in T cell numbers and function, significant multiorgan injury, and decreased survival. Treatment with anti‐PD‐L1 antibody improved bacterial clearance, reduced organ injury, and enhanced survival during Pseudomonas burn wound infection. Furthermore, anti‐PD‐L1 effectively protected against multiorgan injury, and improved bacterial clearance and survival following systemic S. aureus infection after burn injury. Blockade of PD‐1/PD‐L1 interactions might represent a viable treatment to improve outcomes among critically ill burn‐injured subjects and increased leukocyte PD‐L1 expression could serve as a valuable biomarker to select appropriate patients for such treatment.
Keywords: burn injury, checkpoint receptors, infection, myeloid cells, sepsis, T cells
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- CBC
complete blood count
- CLP
cecal ligation and puncture
- PD‐1
programmed death‐ 1 receptor
- PD‐L1
programmed death‐ligand 1
1. INTRODUCTION
Major injury, such as large burns, offers an advantageous environment for opportunistic pathogens to thrive. Infection is an important source of morbidity in burn patients, and is responsible for the majority of deaths in those who survive the initial burn injury.1, 2 Although there are many obvious risk factors for infection in burn patients, such as impaired skin defenses, long courses of immobility, and the use of life sustaining invasive devices like endotracheal tubes and central venous catheters, a developing concern is that development of immunosuppression amplifies the risk of infections in these patients.
Sepsis‐induced immunosuppression has been relatively well characterized in both animals and humans. Evidence indicates that inhibitory checkpoint receptors such as programmed death receptor‐1 (PD‐1) are increased on T cells of septic patients.3, 4, 5, 6 The corresponding inhibitory ligand for PD‐1, named as programmed death ligand‐1 (PD‐L1), has also been shown to be increased on monocytes, dendritic cells, and macrophages.5 PD‐1 is known to be normally upregulated on the surface of activated T lymphocytes to limit the magnitude of cellular activation.7 However, high antigen load and prolonged inflammation during sepsis induces sustained upregulation of PD‐1/PD‐L1 leading to impairment of normal innate and adaptive immune responses.8, 9 Interaction of PD‐1 with PD‐L1 can induce T cell exhaustion, which is characterized by loss of effector functions, decreased proliferation, and apoptotic cell death. Therefore, these immune checkpoint inhibitors are not only recognized as well‐defined biomarkers of sepsis‐induced immunosuppression, but also play a functional role in mediating immune dysfunction. The therapeutic efficacy of blocking antibodies against PD‐1 and PD‐L1 have been reported in recent nonburn‐related studies, which further supports the contention that upregulation of these inhibitory immune checkpoints contributes to immune dysfunction during sepsis.10, 11
Despite widespread use of antibiotics, infection remains the major cause of morbidity and mortality among burn patients. Therefore, there is a need to develop novel therapeutic strategies to treat burn‐associated infections and sepsis, which often leads to multiorgan failure and death.12, 13, 14 Novel therapies aimed at strengthening the immune response to infection provide a logical approach to tackle infections among critically ill burn patients. Preclinical studies from our laboratory, and others, show that burn injury combined with wound sepsis leads to impairment of both innate and adaptive immune system responses.15, 16, 17, 18, 19, 20, 21 Although previous studies have evaluated the use of anti‐PD‐L1 antibody in restoring immunological defects during sepsis,22, 23 none of the studies have evaluated the therapeutic potential of targeting PD‐1/PD‐L1 axis during burn injury combined with infection.
A major goal of this study was to study the therapeutic potential of anti‐PD‐L1 antibody to protect T cell function and improve survival in a clinically relevant mouse model of burn injury and infection. To our knowledge, our study is the first to report that treatment with anti‐PD‐L1 antibody improves bacterial clearance, maintains T cell numbers and function, and significantly improve survival during burn‐associated infection.
2. MATERIALS AND METHODS
2.1. Mouse model of burn injury and infection
All animal procedures were performed in accordance with the National Institutes of Health Guidelines and were approved by the Institutional Animal Care and Use Committee at Vanderbilt University Medical Center. Ten‐ to twelve‐week‐old male BALB/c mice were purchased from ENVIGO (Indianapolis, IN). A well‐established mouse model of full‐thickness cutaneous burn injury was used, as described in our previously published studies.15, 16, 17 For analgesia, buprenorphine (0.1 mg/kg, subcutaneously) was administered 30 min prior to burn injury. Mice were anesthetized using 2–3% isoflurane general anesthesia, then the dorsum was shaved and 1 mL normal saline was injected subcutaneously into the burn target area to prevent injury to the underlying tissues. Each mouse was placed supine and secured in a protective template with an opening corresponding to 30% of the total body surface area. The exposed skin was immersed in 97–98°C water for 10 s to induce a scald burn. Immediately following burn wound induction, intraperitoneal injection of 2 mL lactated Ringers solution was administered for fluid resuscitation. A second injection of buprenorphine (0.1 mg/kg, subcutaneously) was administered to all the mice at 8−12 h after the burn procedure and continued twice daily for 48 h after burn injury. Burned mice were housed individually in sterile cages and were provided sterile water and food. Sham mice underwent the same experimental procedure, but without the burn injury. Burned mice were monitored at least twice daily for any signs of morbidity such as decreased activity and response to stimuli combined with abnormal posturing, labored breathing, and a decrease in core body temperature. Additionally, the institutional veterinary staff performed daily monitoring.
The burn wound infection was induced by inoculating the wound with Pseudomonas aeruginosa obtained from American Type Culture and Collection (Manassas, VA; ATCC 19660). The culture was grown in tryptic soy broth and diluted in sterile saline solution prior to inoculation. On day 4 postburn, the wound surface was inoculated with P. aeruginosa using topical application of 1 × 106 CFU in 50 μL of sterile saline.
For S. aureus infection, 1 × 108 CFU of S. aureus in 200 μL of sterile saline was injected via intravenous route on day 4 postburn. S. aureus was obtained from American Type Culture and Collection (ATCC 25923).
2.2. Anti‐PD‐L1 antibody treatment protocol
Anti‐PD‐L1 antibody (50 μg in 200 μL sterile PBS for P. aeruginosa infection and 200 μg in 200 μL sterile PBS for S. aureus infection) was administered via the intraperitoneal route on day 3 after burn injury and 1 day prior to wound infection. Anti‐PD‐L1 antibody was purchased from BioXCell (catalog # BP0101; West Lebanon, NH). The anti‐PD‐L1 antibody dose was based on the previous studies that showed that a 50 μg dose of anti‐PD‐L1 provides protection in a cecal ligation and puncture (CLP) model of murine sepsis.22 The isotype IgG antibody (BioXCell; catalog # BP0090) was used as the control treatment (50 μg in 200 μL sterile PBS). Blood, spleen, and wound draining lymph nodes (inguinal, brachial, and axillary) were harvested on day 4 postburn (prior to infection and after one dose of anti‐PD‐L1) without infection, and on day 2 following P. aeruginosa wound infection to assess lymphocyte numbers and phenotype. For survival studies, mice were monitored for 7 days after wound infection. For S. aureus infection studies, blood, spleen, and lungs were harvested on day 3 after infection. The differential time point for blood and organ harvesting for P. aeruginosa versus S. aureus was based on the earliest time point we started to see morbidity and mortality among mice after infection.
2.3. Preparation of spleen and lymph node single cell suspensions
Single cell suspensions of splenocytes were prepared by gently pressing the spleen through 70 μm cell strainer as described previously.16 After centrifugation (300 × g for 10 min at 4°C), red blood cells were lysed using Red Blood Cell Lysis Buffer (5 mL for 10 min) (Sigma Life Sciences, St Louis, MO). Wound draining lymph nodes were dissociated by mincing and pressed through 70 μm cell strainer as described previously.16 The total cell counts for the spleen and pooled wound draining lymph nodes were measured using a TC20 cell counter (BioRad, Hercules, CA). Finally, splenocytes and lymph node cells were centrifuged (300 × g for 10 min at 4°C) and resuspended in PBS to achieve a concentration of 1 × 107 cells/mL.
2.4. Flow cytometry
Leukocytes isolated from spleen and lymph nodes were resuspended in cold PBS (1 × 107 cells/mL). The cells were then incubated with 1 μL/mL anti‐mouse CD16/32 (eBiosciences, San Diego, CA) for 5 min to block nonspecific Fc receptor‐mediated antibody binding. One million cells were then incubated with 0.5 μg of fluorochrome‐conjugated specific antibodies or isotype control antibodies (4°C, 30 min) in polystyrene tubes, followed by washing with 2 mL cold PBS and centrifugation (300 × g for 5 min). Finally, the cell pellet was resuspended in 200 μL cold PBS and flow cytometry was performed using BD Accuri C6 instrument (BD Biosciences, San Diego, CA). Data were analyzed using Accuri C6 software. The following fluorochrome‐conjugated antimouse antibodies (eBiosciences) were used: anti‐PD‐1‐FITC, anti‐F4/80‐FITC, anti‐CD‐28‐APC, anti‐Ly6C‐PerCPCy5.5, anti‐CD3‐FITC, anti‐CD8‐PE, anti‐CD8‐FITC, anti‐CD19‐PE, anti‐CD4‐PerCPCy5.5, anti‐CD4‐FITC, anti‐IFNγ‐PE, anti‐PD‐L1‐PE, anti‐CD11c‐FITC and respective isotype controls.
2.5. Measurement of bacterial counts
Blood, lung, spleen, and kidney tissues were harvested and processed as described previously.15 Serial dilutions of blood and tissue homogenates were grown on tryptic soy agar overnight and colony counts were performed to determine CFU of P. aeruginosa or S. aureus per milliliter of blood or per gram of tissue.
2.6. Measurement of organ injury markers and complete blood count
Under general isoflurane anesthesia, whole mouse blood was harvested by carotid artery laceration. Blood was collected in K3EDTA tubes (Greiner Bio‐Obe, Kremsmunster, Austria) and kept on ice until complete blood count (CBC) and differential leukocyte count analysis. CBC measurements were performed using a Forcyte veterinary hematology analyzer (Oxford Science, Oxford, CT). Remaining blood was centrifuged (1766 × g for 15 min at 4°C) to collect plasma for measurement of liver enzymes (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]); blood urea nitrogen (BUN), and cytokines. Organ injury markers were measured using Vet Axcel Chemistry Analyzer in the Translational Pathology Shared Resource facility at Vanderbilt University.
2.7. Plasma cytokine measurement
Plasma concentrations of IL‐6, IL‐10, IFN‐γ, KC, MIP‐2, TNF‐α, and IL‐17 were measured using a customized Bio‐Plex bead array and MAGPIX Multiplex Reader (Bio‐Rad Laboratories, Hercules, CA). Results were analyzed by Bio‐Plex Manager Software6.1, and graphs were prepared with GraphPad Prism Software 6.0 (GraphPad Software Inc., San Diego, CA).
2.8. Intracellular IFN‐γ measurement in CD4+ and CD8+ T cells
Intracellular IFN‐γ was measured by flow cytometry as described previously.15 Briefly, one million cells (both splenocytes and lymph nodes) were stimulated with PMA/Ionomycin (eBiosciences) for 5 h at 37°C in RPMI media containing 10% FCS, in a humidified incubator (5% CO2, 95% air). Protein transport inhibitors (Brefeldin A + Monesin) were added after 1 h of incubation with PMA/Ionomycin. At the end of 5 h stimulation period, the cells were harvested and labeled with fluorescence‐conjugated antibodies to CD4 and CD8. Cells were then fixed and permeabilized with 250 μL Cytofix/Cytoperm Plus (BD Biosciences) for 20 min at 4°C, followed by washing with BD Perm/Wash solution. Intracellular IFNγ production was detected using anti‐IFNγ‐PE antibody (clone XMG1.2; eBiosciences). Fluorochrome‐conjugated isotype‐specific IgGs served as controls. All samples were analyzed using an Accuri C6 flow cytometer (BD Biosciences). Data were analyzed using Accuri C6 software.
2.9. Survival study
Mice were closely monitored at least 3 times daily for 7 days after wound infection with P. aeruginosa or intravenous infection with S. aureus. Additionally, the institutional veterinary care technicians frequently monitored the health of mice on a daily basis. Mice were closely monitored for signs of morbidity including decreased activity and response to stimuli combined with abnormal posturing and labored breathing. Core body temperature was also measured using a lubricated rectal temperature probe and digital thermometer (Physitemp Instruments INC., Clifton, NJ), as an indicator of morbidity.12, 24 Mice displaying signs of morbidity and/or body temperature below 28°C were considered nonsurvivors and thus humanely euthanized under isoflurane anesthesia.15, 16
2.10. Statistical analysis
Graph preparations and data analyses were performed using Prism 6.0 (GraphPad Software Inc.). Data with 3 or more groups were analyzed using a one‐way ANOVA followed by the Tukey multicomparison posthoc test. Comparison between two groups was analyzed using Students t‐test. All data values are presented as mean ± SEM, except for bacterial counts, for which median values are designated. The median values derived from bacterial clearance studies were evaluated using the nonparametric Mann–Whitney U test. Survival curves were analyzed using a Mantel‐Cox log rank test. A value of P < 0.05 was considered statistically significant for all experiments.
3. RESULTS
3.1. Burn injury combined with wound infection with P. aeruginosa caused increased expression of PD‐L1 on splenic antigen presenting cells
Expression of PD‐L1 by myeloid cells and PD‐1 by lymphocytes in the spleens of burn injured mice was assessed at 4 days after burn injury and 2 days after burn wound infection with P. aeruginosa (postburn day 6) (Fig. 1A). Expression of PD‐L1 was significantly increased on CD11c+ dendritic cells (Fig. 1B) and F4/80+ macrophages (Fig. 1C) in the spleen at day 2 after burn wound infection, as compared with the noninfected sham and burn mice. PD‐L1 expression on Ly6C+ inflammatory monocytes and PD‐1 expression on CD4+ and CD8+ T cells was not significantly changed by burn and infection (Figs. 1D–1F). Burn injury alone did not affect either PD‐1 or PD‐L1 expression prior to infection.
Figure 1.
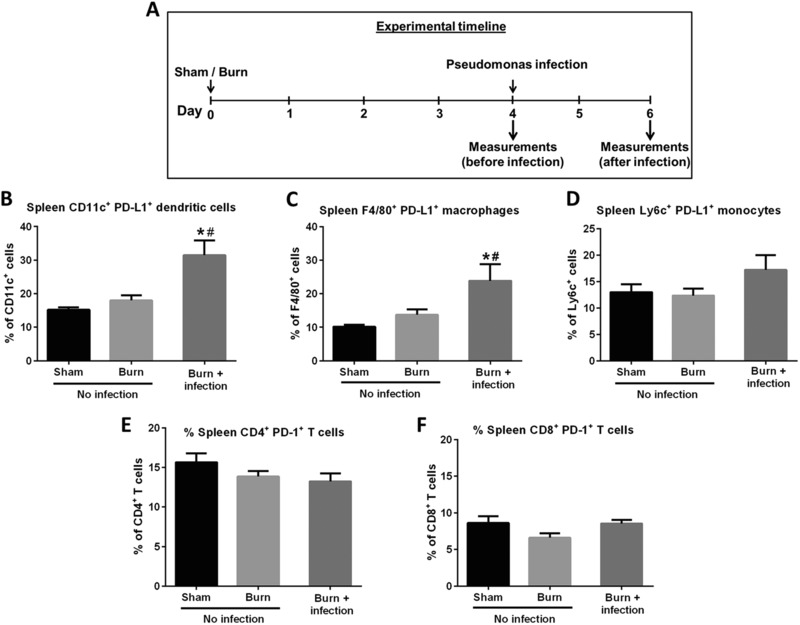
PD‐1 and PD‐L1 expression on leukocyte subsets in mice. PD‐1 and PD‐L1 expression by lymphocytes and antigen presenting cells in spleen and lymph nodes were measured using flow cytometry. [Sham = no burn and no infection; Burn = burn injury alone (no infection)]. Measurements were performed on day 4 after sham or burn procedures (before infection) or day 2 after wound infection. (A) The experimental timeline; (B, C, and D) the expression levels (%) of PD‐L1 on splenic CD11c+ dendritic cells, F4/80+ macrophages, and Ly6C+ inflammatory monocytes, respectively; (E and F) the expression levels (%) of PD‐1 on splenic CD4+ and CD8+ T cells, respectively. *Significantly (P < 0.05) different from sham group; and #significantly different from burn group (no infection)
3.2. Anti‐PD‐L1 antibody treatment improved systemic bacterial clearance, attenuated organ injury, decreased cytokine production, and improved survival during P. aeruginosa burn wound sepsis
Anti‐PD‐L1 antibody was administered on day 3 after burn injury (1 day before wound infection). Bacterial counts in blood and lung were measured 2 days after infection. Liver enzymes and BUN were measured prior to and 2 days after wound infection (Fig. 2A). Systemic dissemination of bacteria was evident in our model of burn wound infection as indicated by the high P. aeruginosa colony counts in the blood and lungs after wound infection (Figs. 2B and 2C). Treatment with anti‐PD‐L1 antibody significantly decreased the systemic bacterial colony counts compared with the mice treated with control IgG. Furthermore, wound infection led to a significant increase in BUN, serum ALT, and AST levels demonstrating organ injury (Figs. 2D–2F). Treatment with anti‐PD‐L1 antibody attenuated the wound infection‐induced increase in the levels of BUN, ALT, and AST.
Figure 2.
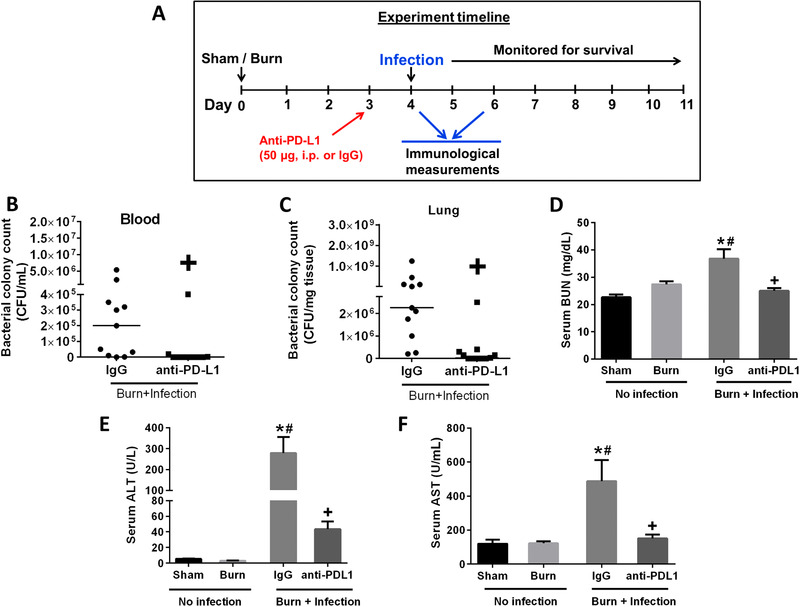
Treatment with anti‐PD‐L1 antibody decreases systemic bacterial burden and attenuates organ injury in mice after P. aeruginosa infection. (A) Time line depicting the burn injury and wound infection model along with anti‐PD‐L1 antibody treatment (50 μg, i.p., 1 day before wound infection). P. aeruginosa CFU in the blood (B) and the lungs (C) were measured at 2 days after infection. Organ injury markers were measured on day 4 after burn injury and day 2 postburn wound infection. (D) Serum blood urea nitrogen, BUN; (E) serum alanine aminotransferase, ALT; and (F) serum aspartate aminotransferase, AST. n = 8–10 per group. *Significantly (P < 0.05) different from sham group; #significantly different from burn group (no infection); and +significantly different from IgG group
As shown in Fig. 3, burn wound sepsis substantially increased the plasma concentrations of the cytokines IL‐6, IL‐10, KC, MIP‐2, and IL‐17. Treatment with anti‐PD‐L1 antibody significantly decreased the levels of all cytokines in mice with burn wound sepsis.
Figure 3.
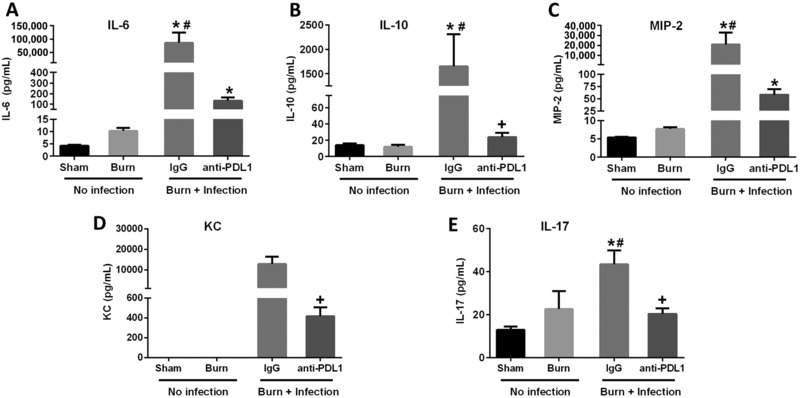
Effect of burn injury and wound infection with P. aeruginosa on plasma cytokine levels. Measurements were performed on day 4 (before wound infection) or 2 days postwound infection. Mice were treated with IgG or anti‐PD‐L1 on day 3 (1 day prior to infection). (A), (B), (C), (D), and (E) show the levels of IL‐6, IL‐10, MIP‐2, KC, and IL‐17 respectively. n = 8–10 per group. *Significantly (P < 0.05) different from sham group; #significantly different from burn group (no infection); and +significantly different from IgG group
Mice were monitored for 7 days after infection to analyze survival. As shown in Fig. 4, only 5% of mice with burn wound infection that were treated with control IgG survived over a 7 day period. Treatment with anti‐PD‐L1 antibody significantly improved the survival rate to greater than 70%.
Figure 4.
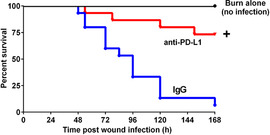
Assessment of survival in burn and P. aeruginosa infected mice treated with anti‐PD‐L1 antibody. Mice were treated with IgG or anti‐PD‐L1 at 3 days after burn injury (1 day prior to infection) and survival was assessed for 7 days. Groups included burn alone (no infection, black line); burned and IgG treated followed by wound infection (blue line); and burned and anti‐PD‐L1 antibody treated followed by wound infection (red line). n = 15 in each group. +Significantly (P < 0.05) different as compared with the IgG‐treated group
3.3. Anti‐PD‐L1 treatment attenuates multiorgan injury, improves bacterial clearance, and improves survival during systemic S. aureus infection after burn injury
In addition to P. aeruginosa infection, we also evaluated the effectiveness of anti‐PD‐L1 against a slowly progressive systemic S. aureus infection after burn injury. S. aureus was injected intravenously on day 4 after burn injury. Anti‐PD‐L1 treatment significantly attenuated the increase in liver (ALT and AST) and kidney (BUN) injury markers (Figs. 5A, 5B, and 5C), and significantly decreased the bacterial burden in major organs including lung and spleen (Figs. 5D and 5E). Importantly, treatment with anti‐PD‐L1 significantly improved the 7 day survival (>50%) as compared with the vehicle‐treated mice (<15%) (Fig. 5F).
Figure 5.
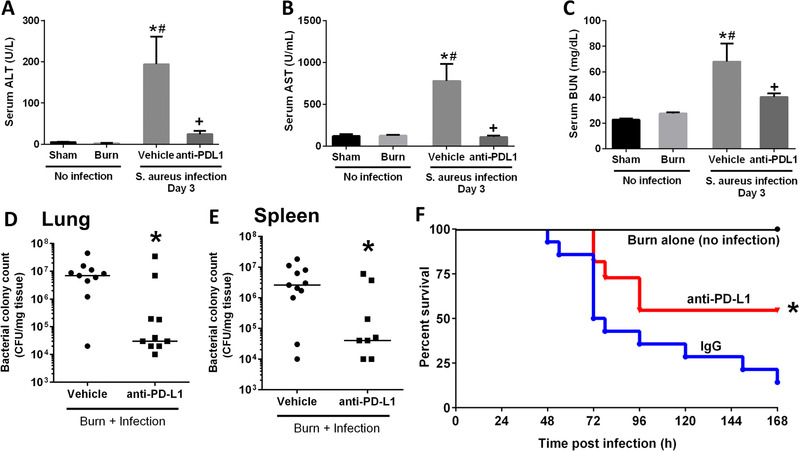
Anti‐PD‐L1 treatment protects against systemic S. aureus infection after burn injury. Anti‐PD‐L1 was administered 24 h before infection (on day 3 postburn). Mice were infected with S. aureus on day 4 after burn injury, and measurements performed on day 3 after infection. Organ injury markers were measured on day 4 after burn injury and day 3 postinfection. (A) Serum alanine aminotransferase, ALT; (B) serum aspartate aminotransferase, AST, and (C) serum blood urea nitrogen, BUN; S. aureus CFU in the lung (D) and the spleen (E) were measured at 3 days after infection. n = 8–10 per group. *Significantly (P < 0.05) different from sham group; #significantly different from burn group (no infection); and +significantly different from IgG group
3.4. Treatment with anti‐PD‐L1 antibody attenuated the decline in CD4+ and CD8+ lymphocyte counts in the spleen and wound draining lymph nodes after burn wound infection with P. aeruginosa
Burn injury alone (no infection) did not induce significant decreases in CD4+ and CD8+ T cell numbers in spleen or lymph nodes (Figs. 6A–6B and 6D–6E). In fact, both T cell subsets in burn draining lymph nodes were increased at 4 days after burn injury. In contrast, burn wound infection caused a significant decline in the CD4+ and CD8+ T cell counts in the spleen and burn wound draining lymph nodes on day 2 after infection (Figs. 6A–B and 6D–6E). Treatment with anti‐PD‐L1 antibody prevented the T cell loss in mice with burn wound infection. Splenic CD19+ B cell counts did not change after burn injury or wound infection (Fig. 6C). On the contrary, in the burn wound draining lymph nodes, wound infection caused a significant decline in B cell numbers, which was attenuated by anti‐PD‐L1 treatment (Fig. 6F). Similar to the increase in T cell numbers, burn injury alone also led to significant increase in the B cell numbers in the burn wound draining lymph nodes (Fig. 6F).
Figure 6.
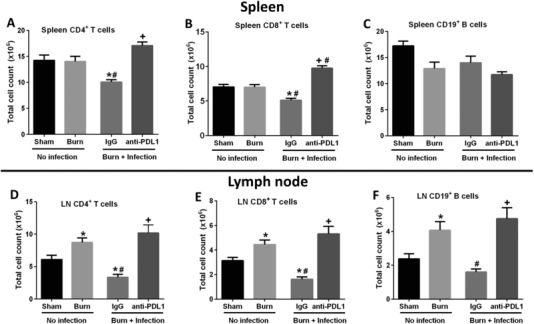
Measurement of CD4+, CD8+ T lymphocyte and CD19+ B lymphocyte counts in the spleen and burn wound draining lymph nodes. Upper panels represent cell counts in the spleen and lower panel represent cell counts in the burn wound draining lymph nodes. Flow cytometry was used to measure the individual CD4+ T (A, D), CD8+ T (B, E), and CD19+ B (C, F) cell counts. n = 10–15 per group. *Significantly (P < 0.05) different from sham group; #significantly different from burn group (no infection); and +significantly different from IgG group
3.5. Anti‐PD‐L1 antibody protected against the decline in CD8+ T cell IFN‐γ secretion in spleen and wound draining lymph nodes following burn wound sepsis induced by P. aeruginosa
Intracellular IFN‐γ production was measured by flow cytometry in ex vivo stimulated T cells as an index of function.25 Wound infection caused a significant decline in CD8+ T cell IFN‐γ production; in both the spleen and burn wound draining lymph nodes (Figs. 7B and 7D). The decline in CD8+ T cell IFN‐γ production was attenuated by treatment with anti‐PD‐L1 antibody. In fact, anti‐PDL1 treatment significantly augmented CD8+ T cell IFNγ production over sham control. Wound infection did not alter IFN‐γ production by CD4+ T cells in the spleen (Fig. 7A), but an increased CD4+ T cell IFN‐γ production was observed in the wound draining lymph nodes (Fig. 7C).
Figure 7.
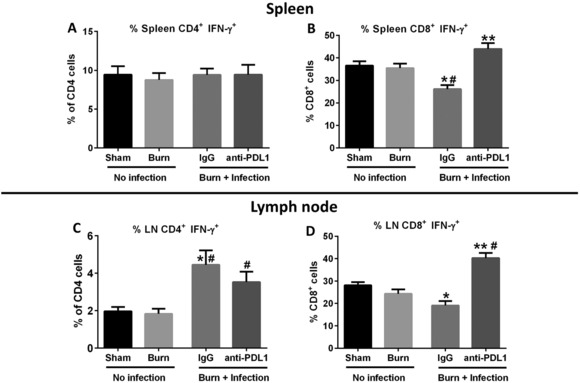
Effect of burn injury and P. aeruginosa wound infection on intracellular IFNγ production by CD4+ and CD8+ T cells. T cell function was assessed by measuring intracellular IFNγ production in ex vivo stimulated (PMA/ionomycin) T cells using flow cytometry. The percentage of intracellular IFNγ expressing CD4+ and CD8+ T cells in the spleen (A and B), and burn wound draining lymph nodes (C and D) was measured. n = 6–10 per group. *Significantly (P < 0.05) different from sham group; #significantly different from burn group (no infection); and +significantly different from IgG group
3.6. Burn wound sepsis caused significant decline in CD28 expression on CD4+ and CD8+ T cells, which was attenuated by anti‐PD‐L1 antibody treatment
Costimulation of T cells through the CD28 surface receptor is essential for effective T cell activation.26 Burn wound sepsis caused a significant decline in CD28 expression on both CD4+ and CD8+ T cells in the spleen, and on CD8+ T cells in the burn wound draining lymph nodes (Figs. 8A, 8B, and 8D). Treatment with anti‐PD‐L1 antibody significantly attenuated the decline in CD28 expression on CD4+ T cells in the spleen and CD8+ T cells in the wound draining lymph nodes. There was a trend toward increase in the CD28 expression on CD8+ T cells in the spleen after anti‐PD‐L1 treatment, but it was not statistically different as compared with the mice treated with control IgG antibody (Fig. 8B). Although, wound infection did not alter the CD28 expression on CD4+ T cells in the burn wound draining lymph nodes, treatment with anti‐PD‐L1 antibody significantly increased the CD28 expression on CD4+ T cells in the lymph nodes, above basal levels (Fig. 8C). Similar to the effect seen with percentage of CD28 expression, the mean fluorescence intensity of CD28 was also significantly decreased after burn wound sepsis on both CD4+ and CD8+ T cells in the spleen and lymph nodes, as compared with the sham group (data not shown). Although we observed a trend toward an increase in CD28 MFI on both CD4+ and CD8+ T cells with anti‐PD‐L1 treatment, the difference observed was not statistically significant (data not shown).
Figure 8.
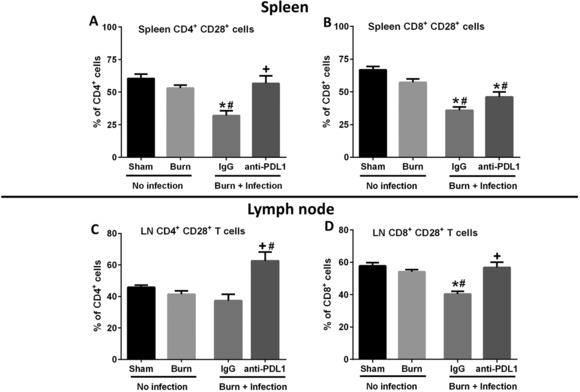
Analysis of CD28 expression on CD4+ and CD8+ T lymphocytes. Upper and lower panels show the percentage of CD28 expression on CD4+ and CD8+ T cells in the spleen and burn wound draining lymph nodes, respectively. (A and C) CD28 expression on CD4+ T cells, and (B and D) CD28 expression on CD8+ T cells. n = 10–15 per group. *Significantly (P < 0.05) different from sham group; #significantly different from burn group (no infection); and +significantly different from IgG group
4. DISCUSSION
Burn patients represent a critically ill patient population that suffers a high prevalence of infection and poor outcomes.1, 2 Results of this study show that increase in myeloid cell PD‐L1 expression in mice was associated with decreased numbers of T cells in spleen and wound draining lymph nodes and T cell dysfunction. Blockade of PD‐L1 with a neutralizing antibody reversed T cell dysfunction and improved the host response to burn injury and associated sepsis in mice, ultimately resulting in a large improvement in survival. This study extends our recent report, which showed that burn wound sepsis causes significant T cell dysfunction. At that time, the mechanisms underlying those alterations were unknown.15, 16 The present study provides evidence that PD‐1/PD‐L1 interactions contribute to immune dysfunction following major burn injury.
Prior studies among nonburned septic patients show that lymphopenia, upregulation of PD‐1 on T cells and PD‐L1 on monocytes correlate with increased susceptibility to secondary infections, viral reactivation, and increased mortality.3, 4, 9, 27, 28, 29 We observed a significant decline in lymphocyte numbers in mice with burn wound sepsis. Burn injury alone did not induce significant loss and dysfunction of T cells but required the presence of concomitant infection. Prior studies have shown that increased PD‐L1 expression is correlated with lymphopenia, and T cell apoptosis and dysfunction in septic patients.4, 10, 19, 30, 31 Treatment with anti‐PD‐L1 antibody significantly attenuated the decline in CD4+ and CD8+ T lymphocytes after burn wound sepsis in spleen and burn wound draining lymph nodes, as compared with the nonspecific IgG‐treated mice. Our findings are consistent with previous studies that show that blockade of PD‐1/PD‐L1 axis protects against T cell loss during sepsis (nonburn related), as a result of inhibition of T cell apoptosis.22, 30, 32
We hypothesized that increased PD‐L1 expression on antigen presenting cells might be a significant mechanism contributing to T cell dysfunction and increased susceptibility to infections following burn injury. Treatment with anti‐PD‐L1 antibody improved systemic bacterial clearance. These findings are consistent with previous studies that showed enhanced microbial clearance in primary and two‐hit models of CLP‐induced sepsis as a result of blockade of PD‐1/PD‐L1 interaction.22, 33, 34 Treatment with anti‐PD‐L1 antibody also protected against organ injury in our study as reflected by decreased liver and kidney injury. A study by Zhu et al.,35 showed increased PD‐L1 expression in the liver of septic mice (CLP) in association with significant liver injury. Treatment with anti‐PD‐L1 antibody decreased liver injury. The mechanisms by which anti‐PD‐L1 antibody reduced organ injury remain to be fully ascertained but might be mediated through the combination of antagonism of direct PD‐L1‐mediated organ injury and enhanced antimicrobial immunity.
We observed a significant decrease in plasma cytokine levels after wound infection in mice treated with anti‐PD‐L1 antibody, implying an overall decrease in systemic inflammation. We postulate that this could be a result of enhanced systemic bacterial clearance. With respect to IL‐6 levels, our findings are in contrast with some of the previous studies that show that blockade of PD‐1/PD‐L1 interaction augments IL‐6 production during sepsis,3, 22, 32 while the decline in IL‐10 observed in our study is consistent with previous reports.
IFN‐γ is one of the key cytokines produced by T cells and functions, in part, to amplify the antimicrobial functions of monocytes and macrophages.36 We observed a significant decline in IFN‐γ production by CD8+ T cells in the spleen and burn wound draining lymph nodes after burn wound sepsis, implying impaired T cell function. Treatment with anti‐PD‐L1 antibody attenuated the decline in IFN‐γ production by CD8+ T cells in mice with burn wound sepsis. This is a significant finding because restoring T cell IFN‐γ production has been shown to improve survival in animal models of sepsis.37 We also observed a significant decline in expression of costimulatory CD28 on CD4+ and CD8+ T lymphocytes during burn wound sepsis. This finding corroborates previous studies that demonstrate a sepsis‐induced decrease in CD28 expression on T cells in association with T cell dysfunction.9 PD‐L1 blockade significantly protected CD28 expression on CD4+ and CD8+ T cells during burn wound sepsis.
To broadly evaluate if the protective effects of anti‐PD‐L1 treatment extends to sepsis caused by Gram positive bacteria, we infected mice with S. aureus, a common burn‐associated pathogen. In contrast to burn wound inoculation with P. aeruginosa, S. aureus was injected intravenously to mimic bacteremia, systemic infection and sepsis. S. aureus typically causes wound colonization in burn patients but requires systemic dissemination in association with central line infections and other invasive procedures to cause systemic illness. Accordingly, previous studies have shown that topical S. aureus application to mouse skin only leads to a localized abscess lesion, which resolves over a week and intravenous inoculation is necessary to produce systemic S. aureus sepsis in mice.38 Systemic (intravenous) infection with S. aureus after burn injury caused significant kidney and liver injuries, lymphopenia among circulating immune cells, increased systemic bacterial load and decreased survival. Treatment with anti‐PD‐L1 attenuated organ injury, augmented bacterial clearance and improved survival. This shows that anti‐PD‐L1 treatment is effective in improving resistance against Gram‐negative and Gram‐positive pathogens bacteria that are commonly seen among burn patients.
A recent study by Patera et al.23 showed that sepsis caused a progressive decline in neutrophil and monocyte functions, which were restored by treatment with either anti‐PD‐L1 or anti‐PD‐1 antibody. Although, we did not evaluate innate immune cell functions in our current study, there remains a possibility of impaired innate immune cell function after burn wound sepsis. Furthermore, our current study demonstrates increased expression of PD‐L1 on antigen presenting cells after burn injury and infection. A study by Kim et al.39 showed that PD‐L1 ligation can back signal into the cell that expresses it. Although not yet investigated, there still remains a possibility that ligation of PD‐L1 by PD‐1 could reverse signal and inhibit the functions of dendritic cells, macrophages, and monocytes. Therefore, the beneficial effects of anti‐PD‐L1 antibody might result from reversal of both innate and adaptive immune cell dysfunction.
There are some limitations to our study. Only male mice were used in this investigation. Male mice were chosen due to the ease of performing intravenous injections. We also employed high mortality models and, in some cases, treatment approaches that are effective in high mortality sepsis have not been shown to be effective in low mortality models. Nevertheless, our models are clinically relevant and employ common burn‐associated pathogens that are introduced at the burn wound site or via systemic administration.
In conclusion, our study demonstrates that treatment with anti‐PD‐L1 reverses T cell dysfunction, improves bacterial clearance, reduces systemic inflammation, and attenuates organ injury in clinically relevant models of burn injury and infection. Survival benefit is an important effect of any therapeutic. Our studies demonstrate that treatment with a single dose of anti‐PD‐L1 antibody significantly increases survival during P. aeruginosa burn wound sepsis and systemic infection with S. aureus, as compared with the mice treated with nonspecific IgG antibody. This is a significant finding as this shows that anti‐PD‐L1 antibody could be used prophylactically to protect critically ill burn patients from life‐threatening infections. Moreover, we hypothesize that increased PD‐L1 expression could serve as useful biomarker to select patients that might benefit from anti‐PD‐L1 therapy.
AUTHORSHIP
N.K.P. and E.R.S. designed the experiments and wrote the manuscript. N.K.P. and E.R.S. analyzed the data and performed data interpretation. N.K.P., L.L., J.K.B., A.H., and Y.G. performed the experiments and critically revised the manuscript. All the authors have read and approved the manuscript.
ACKNOWLEDGMENTS
This work was supported by following grants: NIH RO1 GM66885 to E.R.S; American Heart Association grant 16POST29920007 to N.K.P.; and NIH RO1 GM121711 to J.K.B. The authors thank Dr. Kelli Boyd of the Translational Pathology Shared Resource at Vanderbilt University for assistance with running CBC analyses and serum ALT, AST and BUN measurements.
DISCLOSURES
All authors declare no conflicts of interest.
Patil NK, Luan L, Bohannon JK, Hernandez A, Guo Y, Sherwood ER. Anti‐PD‐L1 protects against infection with common bacterial pathogens after burn injury. J Leukoc Biol. 2018;103:23–33. 10.1002/jlb.10025
Summary sentence: Evaluation of immune checkpoint inhibitor, anti‐PD‐L1, in a murine model of burn wound infection induced sepsis.
REFERENCES
- 1. Mann E, Baun MM, Meininger JC, Wade CE. Comparison of mortality associated with sepsis in the burn, trauma, and general intensive care unit patient: a systematic review of the literature. Shock. 2012;37:4–16. [DOI] [PubMed] [Google Scholar]
- 2. Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care. 2009;13:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Li J, Lou J, et al. Upregulation of programmed death‐1 on T cells and programmed death ligand‐1 on monocytes in septic shock patients. Crit Care. 2011;15:R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guignant C, Lepape A, Huang X, et al. Programmed death‐1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15:R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boomer JS, Shuherk‐Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;16:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukocyte Biol. 2012;92:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patil NK, Bohannon JK, Sherwood ER. Immunotherapy: A promising approach to reverse sepsis‐induced immunosuppression. Pharmacol Res. 2016;111:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: is individualized immuno‐modulatory therapy the answer?. Virulence. 2014;5:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patil NK, Parajuli N, MacMillan‐Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria‐targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306:F734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Sims CR, Patil NK, Gokden N, Mayeux PR. Pharmacologic targeting of sphingosine‐1‐phosphate receptor 1 improves the renal microcirculation during sepsis in the mouse. J Pharmacol Exp Ther. 2015;352:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abraham E, Singer M. Mechanisms of sepsis‐induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. [DOI] [PubMed] [Google Scholar]
- 15. Patil NK, Bohannon JK, Luan L, et al. Flt3 ligand treatment attenuates T cell dysfunction and improves survival in a murine model of burn wound sepsis. Shock. 2017;47:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patil NK, Luan L, Bohannon JK, et al. IL‐15 Superagonist Expands mCD8+ T, NK and NKT Cells after Burn Injury but Fails to Improve Outcome during Burn Wound Infection. PloS One. 2016;11:e0148452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bohannon JK, Luan L, Hernandez A, et al. Role of G‐CSF in monophosphoryl lipid A‐mediated augmentation of neutrophil functions after burn injury. J Leukocyte Biol. 2016;99:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohannon J, Cui W, Sherwood E, Toliver‐Kinsky T. Dendritic cell modification of neutrophil responses to infection after burn injury. J Immunol. 2010;185:2847–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukuzuka K, 3rd Edwards CK, , Clare‐Salzler M, 3rd Copeland EM, , Moldawer LL, Mozingo DW. Glucocorticoid‐induced, caspase‐dependent organ apoptosis early after burn injury. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1005–18. [DOI] [PubMed] [Google Scholar]
- 20. O'Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper‐2 lymphocyte phenotype and diminished interleukin‐12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482–490. discussion 490–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen H, de Almeida PE, Kang KH, Yao P, Chan CW. Burn injury triggered dysfunction in dendritic cell response to TLR9 activation and resulted in skewed T cell functions. PloS One. 2012;7:e50238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Zhou Y, Lou J, et al. PD‐L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline Science: Defects in immune function in patients with sepsis are associated with PD‐1 or PD‐L1 expression and can be restored by antibodies targeting PD‐1 or PD‐L1. J Leukocyte Biol. 2016;100:1239–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warn PA, Brampton MW, Sharp A, et al. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim. 2003;37:126–131. [DOI] [PubMed] [Google Scholar]
- 25. Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507–1516. [DOI] [PubMed] [Google Scholar]
- 26. Chen L, Flies DB. Molecular mechanisms of T cell co‐stimulation and co‐inhibition. Nat Rev Immunol. 2013;13:227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Monaghan SF, Thakkar RK, Tran ML, et al. Programmed death 1 expression as a marker for immune and physiological dysfunction in the critically ill surgical patient. Shock. 2012;38:117–122. [DOI] [PubMed] [Google Scholar]
- 28. Monneret G, Venet F. A rapidly progressing lymphocyte exhaustion after severe sepsis. Crit Care. 2012;16:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomino A, Tsuda M, Aoki R, et al. Increased PD‐1 expression and altered T cell repertoire diversity predict mortality in patients with septic shock: a preliminary study. PLoS One. 2017;12:e0169653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang K, Svabek C, Vazquez‐Guillamet C, et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care. 2014;18:R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. [DOI] [PubMed] [Google Scholar]
- 32. Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti‐PD‐1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukocyte Biol. 2010;88:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang X, Venet F, Wang YL, et al. PD‐1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA. 2009;106:6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shindo Y, McDonough JS, Chang KC, Ramachandra M, Sasikumar PG, Hotchkiss RS. Anti‐PD‐L1 peptide improves survival in sepsis. J Surg Res. 2017;208:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu W, Bao R, Fan X, et al. PD‐L1 blockade attenuated sepsis‐induced liver injury in a mouse cecal ligation and puncture model. Mediat Inflamm 2013;2013:361501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murphey ED, Lin CY, McGuire RW, Toliver‐Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL‐12 and interferon‐gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21:415–425. [DOI] [PubMed] [Google Scholar]
- 37. Unsinger J, McGlynn M, Kasten KR, et al. IL‐7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184:3768–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim HK, Missiakas D, Schneewind O. Mouse models for infectious diseases caused by Staphylococcus aureus. J Immunol Methods. 2014;410:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim YS, Park GB, Lee HK, et al. Cross‐linking of B7‐H1 on EBV‐transformed B cells induces apoptosis through reactive oxygen species production, JNK signaling activation, and fasL expression. J Immunol. 2008;181:6158–6169. [DOI] [PubMed] [Google Scholar]


