Abstract
Thyroid cancer is the most common endocrine malignancy, and its incidence continues to rise. For clinicians with cancer patients, choosing and interpreting diagnostic laboratory studies has become increasingly important. Previously, changes in plasma free mitochondrial DNA levels have been found in colorectal, breast, lung, and urinary cancers, and have demonstrated diagnostic value. In this study, we investigated whether the occurrence and development of thyroid cancer might be predicted using mtDNA copy number (ND1), mtDNA integrity (ND4/ND1) and levels of cell-free nDNA (GAPDH). We analyzed ND1, ND4, and GAPDH levels in plasma and blood cells from 75 patients with thyroid cancer, 40 patients with nodular goiter, and 107 normal controls using real-time PCR. Although both the thyroid nodule and thyroid cancer patients had significantly increased ND1 levels, the ND4/ND1 ratio in the thyroid cancer group was higher than the thyroid nodule group (P < 0.05), and significantly higher than the normal control group (P < 0.01). Plasma levels of nuclear DNA (GAPDH) in the thyroid cancer group were also higher compared to normal (P < 0.05). These results indicate that increased intactness of plasma free mtDNA is associated with increased levels of plasma cell-free nDNA, and that the ND4/ ND1 ratio has the potential to be a new detection indicator in thyroid cancer. Furthermore, we classified thyroid cancer patients according to clinical data including age, tumor size, and metastasis. We found significantly higher levels of GAPDH in malignant tissues. Because ND4/ND1 correlated with plasma GAPDH in the plasma studies, this also suggests a potential relationship between ND4 intactness and thyroid tumor tissue size. Taken together, our findings suggest a tumor-specific process involving increased release of intact mtDNA, detectable in the plasma, which differentiates normal patients from patients with thyroid cancer.
Keywords: Cell free mtDNA, Thyroid cancer, mtDNA copy number, mtDNA integrity, ND4/ND1
1. Introduction
Thyroid cancer is the most common malignant endocrine tumor, comprising about 1% of all malignant tumors of the body. At the present, the key to successful treatment depends largely on early diagnosis of the disease. Thyroglobulin and calcitonin have been used as serum markers of thyroid malignancy, however both have demonstrated poor clinical sensitivity and specificity. The lack of effective serum markers has made screening and diagnosis of thyroid cancer difficult. Therefore, the investigation of new biomarkers for early screening and treatment of thyroid cancer is important.
Recent studies have found that cell free circulating DNA in the blood plays an important role in a wide variety of cancers. Circulating DNA was first reported by Mandel (Mandel and Metais, 1948), but it was a very long time before the potential of circulating DNA for diagnosis and prognosis in a variety of tumors was discovered (Anker et al., 2003; Bremnes et al., 2005; Fiegl et al., 2005; Leon et al., 1977). Compared with healthy patients, circulating DNA content has been shown to be significantly increased in the serum of patients with various malignant tumors (Ellinger et al., 2008; Seefeld et al., 2008). Of note, plasma has very recently been recognized as the specimen of choice over serum and whole blood for investigating levels of cell-free DNA (Lee et al., 2020).
At the present, it is believed that circulating DNA is mainly released through three ways: apoptosis (Giacona et al., 1998; Jahr et al., 2001; Thierry et al., 2010), cell necrosis (Jahr et al., 2001; Nawroz et al., 1996), and active release (Anker et al., 1975; Stroun and Anker, 1972). Through in vitro cell models, studies have found that apoptosis and active release are normally the two main mechanisms of circulating DNA release in healthy cells (Stroun et al., 2001). In cancer cells on the other hand, the two main release mechanisms are apoptosis and cell necrosis. Circulating DNA released by apoptosis can be identified specifically by ladder bands differing by 180 base pairs, while the circulating DNA released by necrosis mostly exists in the form of large fragments (Jahr et al., 2001).
Circulating tumor DNA includes nuclear DNA (nDNA) and mitochondrial DNA (mtDNA). Many studies have focused on nDNA, but the low copy number of nDNA in plasma has challenged the sensitivity of quantitative fluorescence detection methods for clinically relevant levels which can be as low as nanograms per microliter. MtDNA is widely recognized as a favorable option to nDNA for two reasons. First, a normal cell has hundreds to thousands of mitochondria, each containing two to ten copies of mtDNA. Therefore, due to its greater quantity, mtDNA is easier to detect than nDNA. Second, changes in circulating mtDNA structure and copy number are closely related to the occurrence and development of tumors; thus, changes or mutations in mtDNA could help distinguish tumor patients from non-tumor patients (Reznik et al., 2016). Detection and analysis of mtDNA might also provide important information for diagnosis and prognosis in cancer patients (Chen et al., 2016; Lin et al., 2016; Shen et al., 2016).
The mitochondrial electron transport chain is the main site for intracellular production of reactive oxygen species (ROS). Because mtDNA is located in close proximity to the electron transport chain, lacks histone protection, and possesses incomplete mutation repair mechanisms, mtDNA is highly susceptible to ROS-mediated gene mutations (Gao et al., 2010; Lezza et al., 1999). Mitochondrial respiratory chain complex I is the rate-limiting enzyme for electron transport and is the main site of ROS production; a change in complex I function is associated with the occurrence and development of disease due to mitochondrial dysfunction (Sharma et al., 2011; 2009). ND4 subunits are often missing from complex I and are a common indicator of mtDNA damage (Bua et al., 2006; Nishigaki et al., 2004). Referred to as the ‘common deletion,’ a 4977 bp deletion is associated with loss of all tRNA genes and 7 respiratory complex subunit genes, including ND4. (Yusoff et al., 2019) The 4977 bp common deletion is associated with oxidative stress in disease states such as cancer, and reflects a population of viable mitochondria but with poor mtDNA integrity (Yusoff et al., 2019). In contrast, the loss of ND1 subunits has a much more deleterious effect on complex I (Lim et al., 2016) and the mitochondria itself, making ND1 deletions rare in viable mitochondria. ND1 copy number is therefore a convenient marker for total mtDNA copy number, and the ND4/ND1 ratio can be used to evaluate the proportion of intact mtDNA, with a higher value indicating a lesser degree of ND4 gene deletion.
In this study, we used quantitative fluorescence PCR technology to analyze levels of ND1, ND4, and GAPDH (a surrogate marker of nDNA) in samples of both plasma and blood cells from 75 thyroid cancer patients, 40 patients with nodular goiter, and 107 normal patients. We also investigated the relationship between these markers and various clinical features of tumor severity. We constructed plasmids containing the entire gene for either ND1 or ND4, and created a standard curve using the actual ratio of ND4/ND1 plasmid concentration. We present that data here as well to demonstrate the feasibility of our methods.
2. Materials and methods
2.1. Patients and blood samples
Fresh blood samples were collected from a total of 75 pre-operative patients with thyroid cancer, 40 patients with nodular thyroid tumor, and 107 normal patients. The diagnosis was provided by the outpatient department of oncology and confirmed by final pathology in the First People’s Hospital of Wenzhou Medical University. Fresh blood was collected by EDTA-k2 vacuum anticoagulant tube, and blood cells were centrifuged and separated from the plasma within 4 h. Cell-free plasma samples and substratum blood cells were obtained by 1600g and 16000g two-step centrifugation. The collected samples were screened and classified according to the pathological diagnosis results provided by the pathology department, and the clinical data such as age, gender, lymph node metastasis, and cancer tissue size were collected.
2.2. Extraction of DNA from plasma and blood cells
We extracted the circulating DNA in the plasma with the commonly used commercial kit: MagMAX™ Cell-Free DNA Isolation Kit (MM; Applied Biosystems, Thermo Fisher Scientific, Foster City, CA, USA), and we performed DNA extraction using the kit instructions. The saturated NaCl method (Miller et al., 1988) was used to extract DNA from white blood cells.
2.3. Construction of plasmid standard and preparation of standard curve
The target fragment of the standard used in quantitative fluorescence PCR was from the peripheral blood genome of a local normal person in Wenzhou. The clinical data showed that the patient did not have hypertension, diabetes or other mitochondrial diseases.
Primer ND1 (F:TACTTCACAAAGCGCCTTCC; R:ATGAAGAATAGGG CGAAGGG),
ND4 (F: TATCACTCTCCTACTTACAG; R:AGAAGGTTATAATTCCT ACG),
GAPDH (F: TCAAAGAAGTGGGTTTATGGAGG; R: CTCAGCATCAT CATCAAACTCAAAGG) was synthesized by Dalian bao biological (dalian) co. LTD. The PCR products were sequenced by the sequencing department of Liuhe bgi technology co., LTD. Hangzhou branch. The sequencing results were compared with CodonCode Aligner 2.0.6, and the revised Cambridge Reference Sequence (rCRS) was obtained from the mitochondrial genome database (http://www.mitomap.org). The Assemble and Align to reference sequence functions in CodonCode Aligner software were used to compare the sequenced columns with the reference Cambridge sequence. Sequencing results showed that the mtDNA ND1 and ND4 of this normal specimen were the same as the Cambridge sequence, with no distinct polymorphism sites and ND4 region deletion, and the reference sequences of the nuclear genes GAPDH and GeneBank were the same, without mutation deletion. Sensory cells were prepared, and the target fragments were connected with the sensory cells and transformed to obtain the required colonies. The size of the target fragments was verified by PCR, and the bacteria were amplified by shaking. Plasmid extraction kit was used to extract plasmid, determine plasmid concentration, and calculate copy number, using the following parameters: the concentration of the specimen to be measured (ng/ul) = OD260 × 50; Molecular weight of the specimen = base number × 660; Copy number of specimen to be measured (copies/ul) = (sample molecular weight/sample concentration to be measured) × Avogadro’s number; Dilutions were performed at 109, 108, 107, 106, 105, 104, 103, 102 and 101 copies/ml and stored at −30°C. The standard curve was constructed, and the primer used for fluorescence quantification was designed within the extended segment of the primer. The primer of ND1 in the fluorescence quantification experiment (F: CCTCTCCACCCTTATCACAACAC;R: TCATATTATGGCC AAGGGTCAT) and probe of ND1 fragment (P: AGAACACCTCTGATTA CTC), primer of ND4 (F: TCCTCCTATCCCTCAACCCC;R: CACAATCTG ATGTTTTGGTTAAAC) and ND4 fragment probe (P: CATCATTACCGG GTTTTCCTCTTGTA), GAPDH primers (F: CCCCACACACATGCACTT ACC;R: CCTAGTCCCAGGGCTTTGATT) and GAPDH probes (P: TAGGA AGGACAGGCAAC). Plasmid standards with 5–6 gradients and negative control were performed in each experiment, and each gradient was repeated three times. The constructed standard curve had a good linear relationship (R2 above 0.996 ‘; amplification efficiency between 91% and 100%).
2.4. Quantitative fluorescence PCR
According to the above system, the 5′ ends of ND1, ND4 and GAPDH probes were labeled with 5-fam dye, the 3′ ends of ND1 and ND4 were labeled with ECLIPSE, and the 3′ ends of ND4 were labeled with TRAMA. PCR conditions were: pre-denaturation 20 s at 95°C, denaturation 5 s at 95°C, and extension 30 s at 58°C, run for a total of 40 cycles.
2.5. Verification of the degree of ND4 deletion
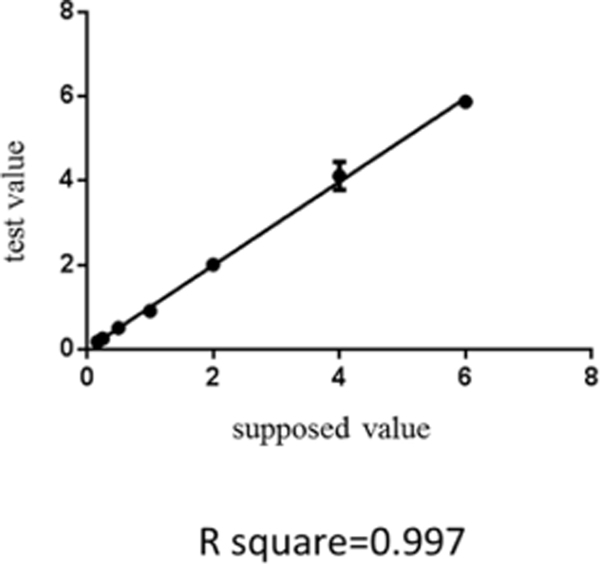
We designed an ND4 whole-gene plasmid and an ND1 whole-gene plasmid, which were synthesized by Beijing Qingke co., LTD. A plasmid extraction kit was used to extract the plasmid from culture. Determination of plasmid concentration was done by calculating the copy number. To prove feasibility of the detection method, serial dilutions were used to create samples with known proportions of ND4:ND1 = 6:1, 4:1, 2:1, 1:1, 1:2, 1:4, 1:6. The primers discussed above were used to detect copy number in samples of known ND4:ND1 in order to obtain a linear fitting curve, for which R2 = 0.997. The degree of ND4 deletion was calculated using the formula ND4/ND1. Note that a lower value indicates a greater degree of deletion.
2.6. Qualitative verification of ND4 deficiency
Blood samples of 7 thyroid cancer patients were selected from thyroid cancer samples for verification. The selected samples were the lowest in ND4 deletion level detection, representing a higher deletion level. The nested PCR method was used to design and amplify the external primers and internal primers of both ends of 4977 bp deletion regions by Primer Premier5.0. The external primers (F:AACCACAGTT TCATGCCCATC;R: TGTTAGTAAGGGTGGGGAAGC) and internal primers (F:ACCCTATTGCACCCCCTCTAC;R: CTTGTCAGGGAGGTAGCG ATG) Dalian bao biological (dalian) co. LTD.
2.7. Statistical analysis
SPSS16.0 software line chi-square test, independent sample t test, and other methods were used for statistical analysis of the obtained data, and GraphPad software was used for drawing. P values < 0.05 indicate statistically significant difference.
3. Results
3.1. Copy number detection of circulating nDNA (GAPDH) gene in plasma and blood cells
3.1.1. Copy number of GAPDH in plasma
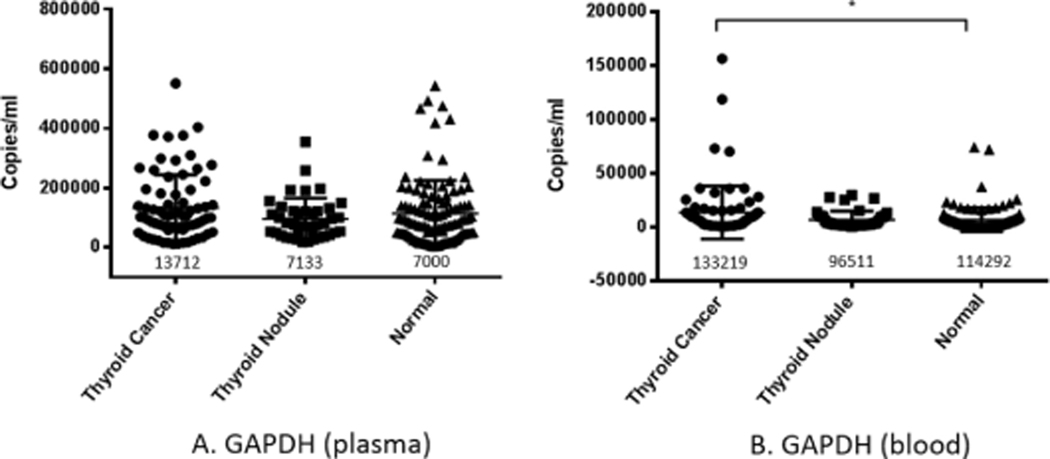
GAPDH is a cell housekeeping gene which has a stable expression pattern and other stable characteristics both in cancer and other diseases. We chose it to represent circulating nDNA. The copy number of GAPDH gene in thyroid cancer, nodular thyroid tumor, and normal human plasma was detected by quantitative fluorescence PCR. The results showed that circulating free DNA in the plasma of thyroid cancer patients was significantly higher than that of normal controls, while nodular thyroid tumors were not significantly different from the other two groups (Fig. 1A).
Fig. 1.
Copy number measurements of circulating nDNA (GAPDH) genes in plasma and blood cells. The copy number of nuclear DNA (measured via GAPDH) was detected in plasma (A) and blood (B) by quantitative PCR in thyroid cancer group, thyroid nodule group, and normal control group. In plasma, the GAPDH content of cancer group was significantly higher than normal group (P < 0.05). The number below the scatter in the figure is the mean value of each experimental group.
3.1.2. Copy number of GAPDH in blood cells
The number of copies of GAPDH in white blood cells represents the number of cells, and blood cells have been shown to actively release DNA. Therefore, we used quantitative fluorescence PCR to detect the copy number of GAPDH in the blood cells of the three groups to correct the circulating mtDNA, and the results showed that there was no significant difference in the copy number of GAPDH in the blood cells of the three groups (Fig. 1B).
3.2. Copy number detection of free mtDNA (ND1) gene in plasma and blood cells
3.2.1. Copy number of ND1 in plasma
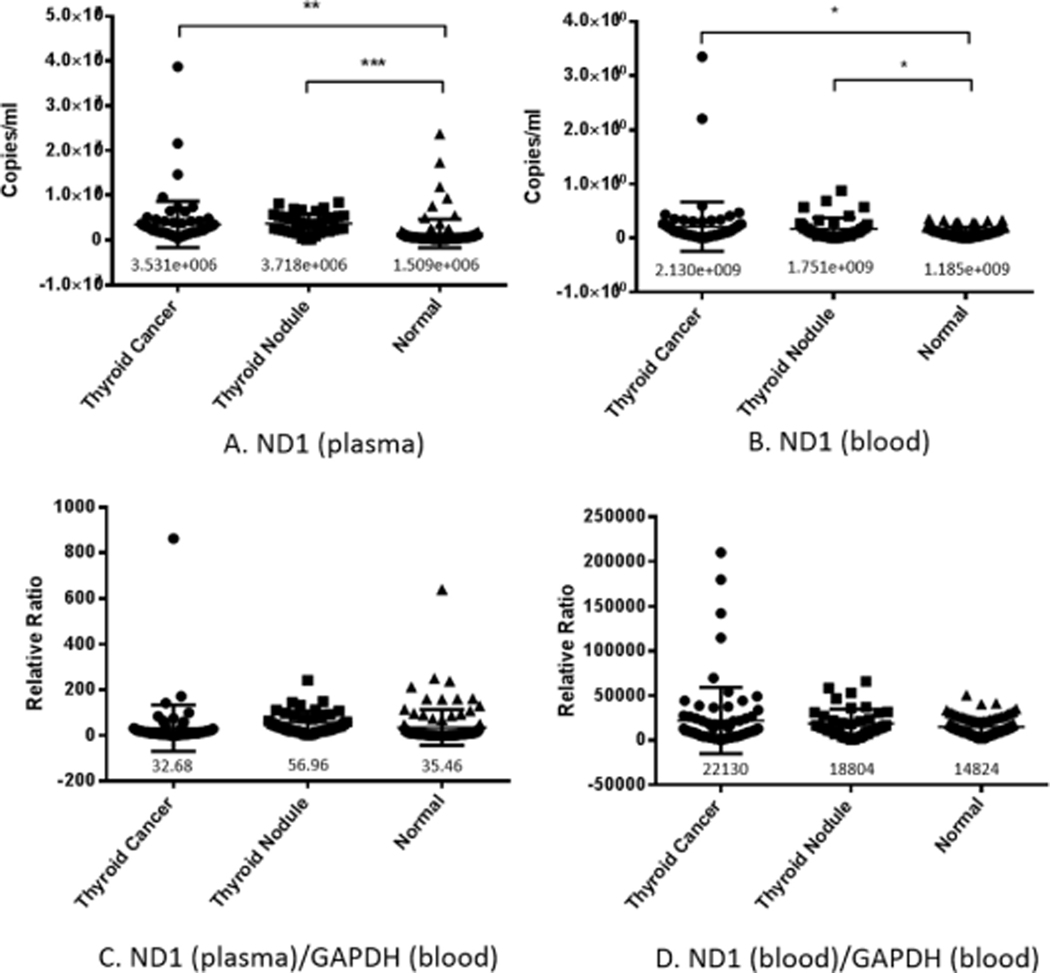
Free mtDNA was represented by ND1, and its copy number was detected by quantitative fluorescence PCR (Fig. 2A) and then normalized by GAPDH (Fig. 2C). MtDNA copy number in plasma was significantly higher in thyroid nodule and thyroid tumor patients compared to normal (Fig. 2A). There was no statistically significant difference in plasma mtDNA copy number between thyroid cancer, nodular thyroid tumor, and normal control group after normalization (Fig. 2C).
Fig. 2.
Copy number of cell-free ND1 DNA in plasma and blood cells. The copy number of ND1 in plasma (A) and blood cells (B), and copy number of ND1 normalized by nDNA (GAPDH) in plasma (C) and blood cells (D) of thyroid cancer and thyroid nodule patients were detected by quantitative fluorescence PCR. Total ND1 copy number was significantly higher in both blood and plasma in patients with thyroid nodule or thyroid tumor. No significant differences were found among the three groups after normalizing for nDNA. The number below the scatter in the figure is the mean value of each experimental group.
3.2.2. Copy number of ND1 in blood cells
We also measured the copy number of ND1 gene in blood cells to compare with that in plasma. MtDNA copy number in blood cells was significantly higher in thyroid nodule and thyroid tumor patients compared to normal (Fig. 2B), but showed no statistical difference after normalization for GAPDH (Fig. 2D).
3.3. Detection of ND4 copy number and mtDNA integrity (ND4/ND1) in plasma and blood cells
3.3.1. ND4 copy number and mtDNA integrity (ND4/ND1) in plasma
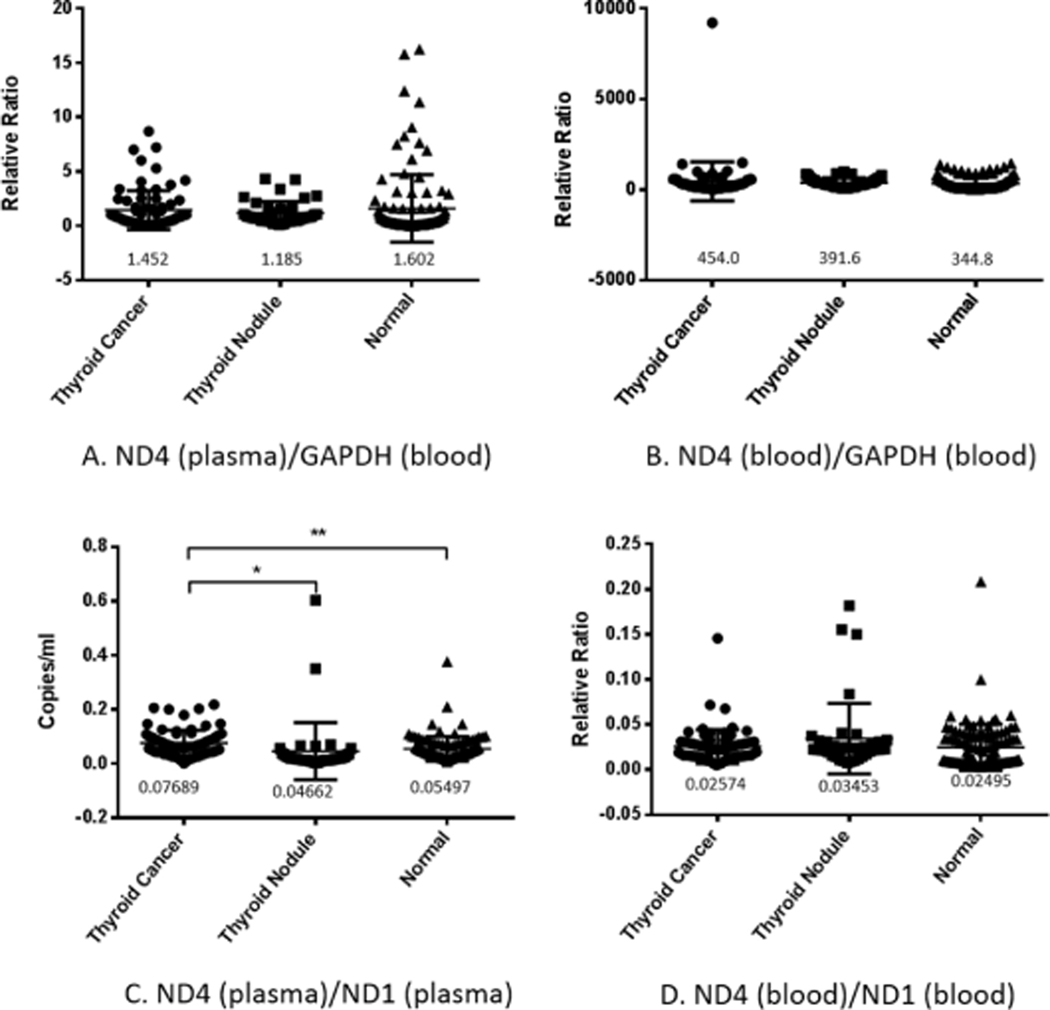
We detected the copy number of the target fragment ND4 in the plasma of the three groups by quantitative fluorescence PCR, and corrected it with blood cell GAPDH. The results showed no difference in the amount of ND4 in the three groups (Fig. 3A). Further examination of mtDNA integrity (ND4/ND1) revealed significant differences between the thyroid cancer group and the normal control group, and no significant differences between nodular thyroid tumors and the other two groups (Fig. 3C).
Fig. 3.
ND4 copy number and ND4 deletion level in plasma and blood cells. The copy number of ND4 in plasma (A) and blood cells (B) in normal patients, patients with thyroid cancer, or patients with thyroid nodules was detected by quantitative fluorescence PCR, and no significant difference was found among the three groups. ND4/ND1 ratio in plasma (C) and in blood cells (D) suggests that there was a greater proportion of intact mtDNA (lesser degree of ND4 deletion) in plasma from thyroid cancer patients compared to both normal patients (P < 0.1) and thyroid nodule patients (P < 0.5). The number below the scatter in the figure is the mean value of each experimental group.
3.3.2. ND4 copy number and mtDNA integrity (ND4/ND1) in blood cells
We detected the copy number of the target fragment ND4 in the blood cells of the three groups by quantitative fluorescence PCR, and corrected it with the blood cells GAPDH. The results showed no statistical difference in the copy number of ND4 among the three groups (Fig. 3B), and no difference in mtDNA integrity among the three groups (Fig. 3D).
3.4. Correlation of thyroid cancer samples according to age, tissue size, and metastasis
3.4.1. Relationship between GAPDH copy number in plasma and blood cells and clinical features of thyroid cancer
According to the clinical data, patients with thyroid cancer were classified according to age (≤45 years or>, 45 years), tissue size (≤1cm or > , 1 cm), whether or not they had metastasis (yes or no), and the expression of GAPDH was compared under different conditions. Patients with tumor tissues > 1 cm had higher plasma free DNA than those with tissues less than or equal to 1 cm, with significant statistical differences (Table 1A).Table 2.Table 3.
Table 1.
Relationship between GAPDH copy number in plasma and blood cells and clinical features of thyroid cancer. The relationship between GAPDH copy number and clinical data in plasma and blood leukocytes.
| Classification | Size | Age | Metastasis | |||
|---|---|---|---|---|---|---|
|
| ||||||
| < 1cm | > 1cm | < 45 | > 45 | Yes | No | |
|
| ||||||
| GAPDH (plasma) | 9546.685 | 19913.565 | 17870.78 | 9386.43 | 16567.54 | 11305.735 |
| P value | 0.0453 | 0.1460 | 0.3681 | |||
| GAPDH (blood) | 139932.95 | 178193.5 | 158761.8 | 147154.65 | 182202.2 | 131785.8 |
| P value | 0.4050 | 0.7917 | 0.1889 | |||
| GAPDH (plasma) /GAPDH (blood) | 0.148237 | 0.241589 | 0.157129 | 0.163816 | 0.161258 | 0.146558 |
| P value | 0.2515 | 0.9096 | 0.7951 | |||
P values were estimated by “unpaired t test”, the unit of copy number is “copies/ml”.
Thyroid cancer patients were classified by clinical features according to the following categories: (≤45 years old or> 45 years old), tissue size (≤1cm or 1 cm), and whether or not the cancer had metastasized (yes or no). Plasma GAPDH, our marker for cell-free nDNA, was significantly higher in patients with tumor size > 1 cm compared with patients whose tumors were ≤1 cm.
Table 2.
Relationship between ND1 copy number in plasma and blood cells and clinical features of thyroid cancer. The relationship between ND1 copy number and clinical data in plasma and blood leukocytes.
| Classification | Size | Age | Metastasis | |||
|---|---|---|---|---|---|---|
|
| ||||||
| < 1cm | > 1cm | < 45 | > 45 | Yes | No | |
|
| ||||||
| ND1 (plasma) | 3875189.0 | 2353950.5 | 3207535.5 | 3880968.0 | 2902514.0 | 3998332.0 |
| P value | 0.2972 | 0.5748 | 0.3653 | |||
| ND1 (blood) | 2.02E+09 | 2.57E+09 | 2.05E+09 | 2.21E+09 | 2.07E+09 | 2.18E+09 |
| P value | 0.6756 | 0.8770 | 0.9181 | |||
| ND1 (plasma) / GAPDH (blood) | 80.71175 | 24.17597 | 41.42541 | 44.81586 | 39.58636 | 84.52316 |
| P value | 0.3300 | 0.8106 | 0.3476 | |||
| ND1 (blood) / GAPDH (blood) | 23472.76 | 22814.06 | 17826.59 | 27085.53 | 21986.74 | 22237.34 |
| P value | 0.9514 | 0.2839 | 0.9772 | |||
P values were estimated by “unpaired t test”, the unit of copy number is “copies/ml”.
Thyroid cancer patients were classified by clinical features according to the following categories: (≤45 years old or> 45 years old), tissue size (≤1cm or 1 cm), and whether or not the cancer had metastasized (yes or no). No statistically significant differences were found when comparing ND1 copy number under different clinical conditions.
Table 3.
Relationship between ND4 deficiency in plasma and blood cells and clinical features of thyroid cancer. The Relationship between ND4 deletion level and clinical data in plasma and blood leukocytes.
| Classification | Size | Age | Metastasis | |||
|---|---|---|---|---|---|---|
|
| ||||||
| < 1cm | > 1cm | < 45 | > 45 | Yes | No | |
|
| ||||||
| ND4 (plasma) / ND1 (plasma) | 0.03087 | 0.023559 | 0.030895 | 0.027419 | 0.030404 | 0.028954 |
| P value | 0.1425 | 0.4175 | 0.7338 | |||
| ND4 (blood) / ND1 (blood) | 0.024367 | 0.030395 | 0.025674 | 0.026127 | 0.027571 | 0.02437 |
| P value | 0.2637 | 0.9180 | 0.4710 | |||
P values were estimated by “unpaired t test”.
Thyroid cancer patients were classified by clinical features according to the following categories: (≤45 years old or> 45 years old), tissue size (≤1cm or 1 cm), and whether or not the cancer had metastasized (yes or no). No statistically significant differences were found when comparing levels of ND4 deletion under different clinical conditions.
3.4.2. Relationship between ND1 copy number in plasma and blood cells and clinical features of thyroid cancer
As mentioned above, we compared ND1 gene expression in plasma and blood cells under different conditions. There was no significant difference (Table 1B).
3.4.3. Relationship between ND4 deficiency in plasma and blood cells and thyroid clinical features of thyroid cancer
As mentioned above, we compared ND4 deficiency levels in plasma and blood cells under different conditions. There was no statistical difference (Table 1C).
3.5. Verification of the feasibility of ND4 deletion level detection method
The plasmid concentration of the whole gene of ND4 and the whole gene of ND1 was used to calculate the copy number of the plasmid, and the ratio of ND4/ND1 was made linear. It was determined according to the method for the determination of ND4 deletion level above. After linear fitting, R2 = 0.997 (Fig. 4), proving that the detection method was feasible.
Fig. 4.
Verification of the feasibility of ND4 deletion detection method. According to the plasmid concentration, the copy number of ND1, ND4 and nd1–4 plasmids were calculated, and diluted in accordance with the hypothesis ratio. Quantitative fluorescence method was used to verify that the ratio was consistent with the supposed value.
3.6. Verification of 4977 bp deletion
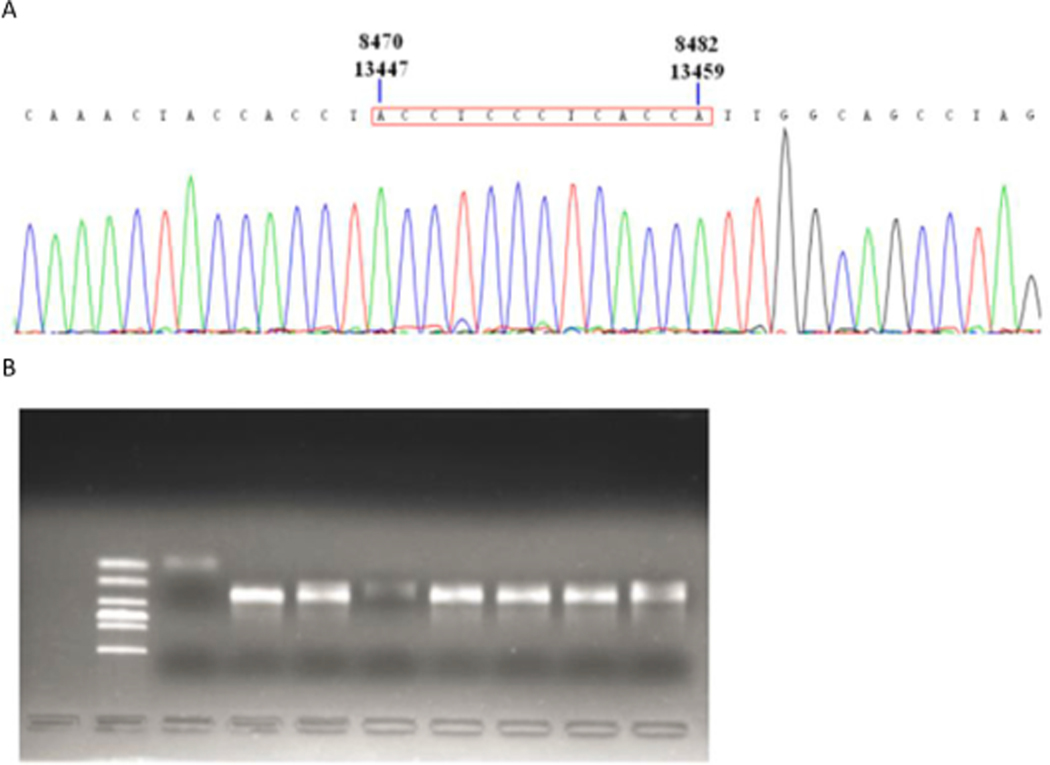
Electrophoresis and sequencing verification of 4977 bp missing nested PCR products (Fig. 5A and 5B).
Fig. 5.
Verification of 4977 bp deletion. In the 4977 bp deletion sequencing peak diagram, the red box shows that the repeated sequence at both ends of 4977 bp deletion is located in region 8470–8482 at one end and region 13447–13459 at the other end (A). The second well from the left is Marker DL2000, the third well is negative control, and the fourth well to the tenth well is 4977 bp missing band of 7 samples. The results above are the second amplification product of nested PCR (B).
4. Discussion
Early screening and timely treatment of cancer can significantly reduce mortality and prolong survival; hence many studies have focused on finding cancer-related biomarkers for early diagnosis. Circulating DNA in plasma is an attractive biomarker due to minimal invasiveness and ease of sampling. A large number of studies have found that changes in copy number and mutations of circulating DNA in the blood are closely related to the occurrence and development of tumors, suggesting that circulating DNA may become a new and potentially important indicator for cancer diagnosis, monitoring, and prognostic evaluation(Chen et al., 2016; Reznik et al., 2016; Shen et al., 2016; Stroun et al., 2001). Circulating DNA includes both mtDNA and nDNA, but most studies focus on either nDNA or mtDNA separately and do not investigate relationships between the two types of circulating DNA. Furthermore, circulating DNA is the result of a dynamic balance between free DNA in plasma and DNA on the surface of white blood cells; however, many studies focus only on the circulating DNA in plasma and ignore the DNA content on the surface of white blood cells. In this study, we addressed all of these sources of circulating DNA by analyzing both mitochondrial and nuclear genes in both blood cells and plasma. We evaluated the relationship between circulating mtDNA copy number, circulating mtDNA integrity, and circulating nDNA with the occurrence of thyroid cancer in hopes of finding a new tumor marker. We also analyzed the relationship of these markers with clinical features such as cancer tissue size, lymph node metastasis, and age.
Our experiments showed that the plasma circulating nDNA copy number was higher in the thyroid cancer group than in the normal control group. An increase in circulating nDNA copy number has also been found other cancers including colorectal (Agah et al., 2017), prostate (Ponti et al., 2019), and liver cancer (Oh et al., 2019). In malignant tumors, a unique microenvironment forms due to various factors like lack of oxygen, rapid growth, and relatively insufficient supply of energy and nutrients. This causes massive necrosis and apoptosis, which makes the cells release more circulating DNA into the plasma compared to normal cells. In addition, the tumor microenvironment induces aberrant cell signaling pathways. Phagocytes are recruited to the tumor, and after clearing some of the dead cells they are able to migrate back into the bloodstream, thus tumor DNA can be found within blood leukocytes as well. However, our results indicated that blood leukocytes are not a significant source of increased circulating mtDNA in thyroid cancer patients.
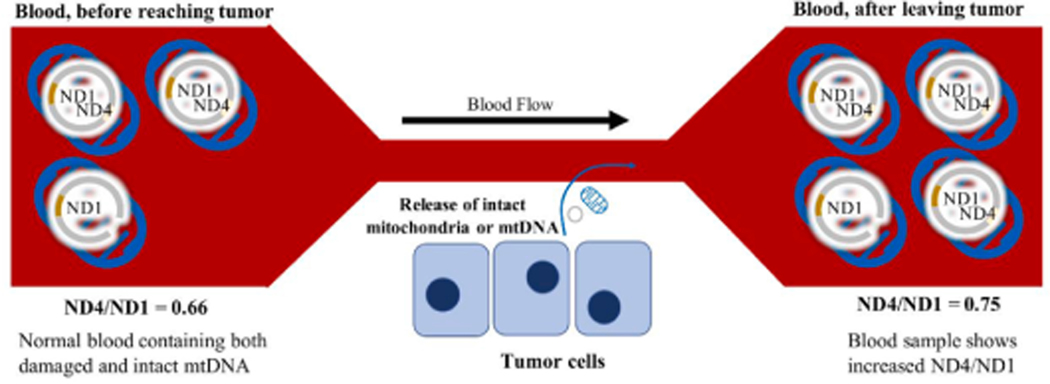
Aberrant signaling in cancer cells is also a means for promoting survival of the tumor cells, with such aberrancies often involving the mitochondrion and mitochondrial pathways (Lyu et al., 2019; Zhou et al., 2020). One example is the active secretion of mtDNA from tumor cells, which confers a survival advantage and enhances carcinogenesis by restoring functional mitochondrial genes to surrounding cells with respiratory gene deficiencies. This form of mtDNA transfer can take place by secretion of small exosomes containing mtDNA, as seen in breast cancer (Sansone et al., 2017), or by the secretion of whole mitochondria, as seen in melanoma (Dong et al., n.d.). Our results showed that both patients with a thyroid mass, either benign nodule or malignant tumor, had increased total mtDNA copy number (ND1) compared to normal. However, the patients with malignant tumors had significantly increased plasma ND4/ND1 ratio compared to patients with thyroid nodule and also control patients. Because ND4 deletion is a marker of mtDNA damage, the increased ND4/ND1 ratio together with increased ND1 suggests increased release of intact mtDNA in thyroid cancer patients. Our results are consistent with recent publications which describe the release of whole mitochondria or intact mtDNA genomes from cancer cells. For example, intact mitochondria are released from cancer cells undergoing TNF-mediated necroptosis (Maeda and Fadeel, 2014). Another groundbreaking study published this year showed that respiratory-competent mitochondria with intact mtDNA genomes are actively secreted by cancer cells, and separate experiments showed that respiratory-competent cell-free mitochondria can be found in patient plasma samples (Dache et al., 2020). Therefore, the increased intactness of mtDNA (measured by ND4/ND1 ratio) in patients with thyroid cancer could suggest a phenomenon involving release of free mitochondria, whose intact mtDNA can be detected in the plasma. Alternatively, the release of free mtDNA alone is also possible. Our findings in blood cells do not seem to support a mechanism of release involving cell death, however. Blood leukocytes would be expected to take up a significant amount of mtDNA due to phagocytosis of apoptotic or necrotic cell fragments. But in blood cell samples from thyroid cancer patients containing these leukocytes, we did not detect a significant increase in mtDNA copy number. A schematic diagram explaining the idea of how intact mtDNA release from thyroid tumor cells would increase the ND4/ND1 ratio is shown in Fig. 6.
Fig. 6.
Schematic diagram demonstrating how increased release of intact mitochondria by tumor cells would increase the ND4/ND1 ratio.
It is also worth noting here increased release of mtDNA in thyroid cancer patients could be related to aberrant mitophagy. Mitophagy is a key process used to repair mtDNA, and cancer cells commonly have alterations in mitophagy pathways (Panigrahi et al., 2019; Sharma et al., 2019; Song et al., 2020; Vara-Perez et al., 2019). Pathologic changes in mitophagy are linked with mtDNA release. For example, knockout of mitophagy receptor FUNDC1 in hepatocellular carcinoma cells resulted in the accumulation of damaged mitochondria within cells while simultaneously increasing release of mtDNA (Panigrahi et al., 2019). Transmitophagy is a process involving mitochondrial transfer between cells for mitochondrial degradation by the receiving cell (Bahr et al., 2020; Vara-Perez et al., 2019). However, these pathways of mitochondrial transfer are not necessarily limited to transporting damaged mitochondria; it appears that many cancers can hijack transmitophagy machinery to release intact mtDNA as a means of helping neighboring cells and promoting tumor growth (Vara-Perez et al., 2019). Future research in thyroid cancer cells should look for a link between mitophagy pathways and increased mtDNA release by thyroid cancer cells.
Finally, we also sought to determine if clinical severity of thyroid cancer was associated with differences in the same circulating DNA markers as the ones used for the blood samples. To do this, we investigated the relationships of cell-free nDNA (GAPDH), mtDNA copy number, and mtDNA integrity with tumor size, lymph node metastasis, and patient age. The only statistically significant result was a positive association between plasma GAPDH gene copy number and tumor size. Because results from the prior experiments in plasma showed that patients with thyroid cancer had both increased GAPDH and increased ND4/ND1 ratio, it is possible that a trial with a greater sample size might yield significant results for ND4/ND1 ratio as a predictor of clinical severity.
This study explored the possibilities for using circulating cell-free mtDNA from either blood or plasma as diagnostic markers for thyroid cancer. Our results indicate that increased mtDNA intactness in plasma, measured as an increased ND4/ND1 ratio, may have the greatest potential as a diagnostic marker. Future work should characterize the sensitivity, specificity, and receiver operating characteristic curves for ND4/ND1 ratio. Furthermore, additional studies must be performed to confirm the mechanism of intact mtDNA release from thyroid cancer cells.
Acknowledgement
This work was partly supported by the Zhejiang Provincial Key Laboratory of Medical Genetics at Wenzhou Medical University, and TB and YB are supported by William and Ella Owens Medical Research Foundation.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agah S, Akbari A, Talebi A, Masoudi M, Sarveazad A, Mirzaei A, Nazmi F, 2017. Quantification of plasma cell-free circulating DNA at different stages of colorectal cancer. Cancer Invest. 35, 625–632. 10.1080/07357907.2017.1408814. [DOI] [PubMed] [Google Scholar]
- Anker P, Mulcahy H, Stroun M, 2003. Circulating nucleic acids in plasma and serum as a noninvasive investigation for cancer: time for large-scale clinical studies? Int. J. Cancer 103, 149–152. 10.1002/ijc.10791. [DOI] [PubMed] [Google Scholar]
- Anker P, Stroun M, Maurice PA, 1975. Spontaneous release of DNA by human blood lymphocytes as shown in an in vitro system. Cancer Res. 35, 2375–2382. [PubMed] [Google Scholar]
- Bahr T, Welburn K, Donnelly J, Bai Y, 2020. Emerging model systems and treatment approaches for Leber’s hereditary optic neuropathy: Challenges and opportunities. Biochim. Biophys. Acta (BBA) – Molecular Basis of Disease 1866, 165743. doi: 10.1016/j.bbadis.2020.165743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremnes RM, Sirera R, Camps C, 2005. Circulating tumour-derived DNA and RNA markers in blood: a tool for early detection, diagnostics, and follow-up? Lung Cancer 49, 1–12. 10.1016/j.lungcan.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Bua E, Johnson J, Herbst A, Delong B, McKenzie D, Salamat S, Aiken JM, 2006. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am. J. Hum. Genet. 79, 469–480. 10.1086/507132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Wen S, Sun X, Fang Q, Huang L, Liu S, Li W, Qiu M, 2016. Elevated mitochondrial DNA copy number in peripheral blood and tissue predict the opposite outcome of cancer: a meta-analysis. Sci Rep 6, 37404. 10.1038/srep37404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dache ZAA, Otandault A, Tanos R, Pastor B, Meddeb R, Sanchez C, Arena G, Lasorsa L, Bennett A, Grange T, Messaoudi SE, Mazard T, Prevostel C, Thierry AR, 2020. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 34, 3616–3630. 10.1096/fj.201901917RR. [DOI] [PubMed] [Google Scholar]
- Dong L-F, Kovarova J, Bajzikova M, Bezawork-Geleta A, Svec D, Endaya B, Sachaphibulkij K, Coelho AR, Sebkova N, Ruzickova A, Tan AS, Kluckova K, Judasova K, Zamecnikova K, Rychtarcikova Z, Gopalan V, Andera L, Sobol M, Yan B, Pattnaik B, Bhatraju N, Truksa J, Stopka P, Hozak P, Lam AK, Sedlacek R, Oliveira PJ, Kubista M, Agrawal A, Dvorakova-Hortova K, Rohlena J, Berridge MV, Neuzil J, n.d. Horizontal transfer of whole mitochondria restores tumorigenic potential in mitochondrial DNA-deficient cancer cells. eLife 6. doi: 10.7554/eLife.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger J, Bastian PJ, Haan KI, Heukamp LC, Buettner R, Fimmers R, Mueller SC, von Ruecker A, 2008. Noncancerous PTGS2 DNA fragments of apoptotic origin in sera of prostate cancer patients qualify as diagnostic and prognostic indicators. Int. J. Cancer 122, 138–143. 10.1002/ijc.23057. [DOI] [PubMed] [Google Scholar]
- Fiegl H, Millinger S, Mueller-Holzner E, Marth C, Ensinger C, Berger A, Klocker H, Goebel G, Widschwendter M, 2005. Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 65, 1141–1145. 10.1158/0008-5472.CAN-04-2438. [DOI] [PubMed] [Google Scholar]
- Gao Y-J, He Y-J, Yang Z-L, Shao H-Y, Zuo Y, Bai Y, Chen H, Chen X-C, Qin F-X, Tan S, Wang J, Wang L, Zhang L, 2010. Increased integrity of circulating cell-free DNA in plasma of patients with acute leukemia. Clin. Chem. Lab. Med. 48, 1651–1656. 10.1515/CCLM.2010.311. [DOI] [PubMed] [Google Scholar]
- Giacona MB, Ruben GC, Iczkowski KA, Roos TB, Porter DM, Sorenson GD, 1998. Cell-free DNA in human blood plasma: length measurements in patients with pancreatic cancer and healthy controls. Pancreas 17, 89–97. 10.1097/00006676-199807000-00012. [DOI] [PubMed] [Google Scholar]
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R, 2001. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 61, 1659–1665. [PubMed] [Google Scholar]
- Lee J-S, Kim M, Seong M-W, Kim H-S, Lee YK, Kang HJ, 2020. Plasma vs. serum in circulating tumor DNA measurement: characterization by DNA fragment sizing and digital droplet polymerase chain reaction. Clin. Chem. Lab. Med. 58, 527–532. 10.1515/cclm-2019-0896. [DOI] [PubMed] [Google Scholar]
- Leon SA, Shapiro B, Sklaroff DM, Yaros MJ, 1977. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 37, 646–650. [PubMed] [Google Scholar]
- Lezza AM, Mecocci P, Cormio A, Beal MF, Cherubini A, Cantatore P, Senin U, Gadaleta MN, 1999. Mitochondrial DNA 4977 bp deletion and OH8dG levels correlate in the brain of aged subjects but not Alzheimer’s disease patients. FASEB J. 13, 1083–1088. 10.1096/fasebj.13.9.1083. [DOI] [PubMed] [Google Scholar]
- Lim SC, Hroudová J, Van Bergen NJ, Lopez Sanchez MIG, Trounce IA, McKenzie M, 2016. Loss of mitochondrial DNA–encoded protein ND1 results in disruption of complex I biogenesis during early stages of assembly. FASEB J. 30, 2236–2248. 10.1096/fj.201500137R. [DOI] [PubMed] [Google Scholar]
- Lin C-S, Lee H-T, Lee M-H, Pan S-C, Ke C-Y, Chiu A-W-H, Wei Y-H, 2016. Role of mitochondrial DNA copy number alteration in human renal cell carcinoma. Int. J. Mol. Sci. 17. 10.3390/ijms17060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu L, Wang Q, Song S, Li L, Zhou H, Li M, Jiang Z, Zhou C, Chen G, Lyu J, Bai Y, 2019. Oncocytic tumors are marked by enhanced mitochondrial content and mtDNA mutations of complex I in Chinese patients. Mitochondrion 45, 1–6. 10.1016/j.mito.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Maeda A, Fadeel B, 2014. Mitochondria released by cells undergoing TNF-α-induced necroptosis act as danger signals. Cell Death Dis. 5, e1312. 10.1038/cddis.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel P, Metais P, 1948. Les Acides Nucléiques Du Plasma Sanguin Chez l’Homme. C. R. Seances Soc. Biol. Fil. 142, 241–243. [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF, 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucl. Acids Res 16, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroz H, Koch W, Anker P, Stroun M, Sidransky D, 1996. Microsatellite alterations in serum DNA of head and neck cancer patients. Nature Med. 2, 1035–1037. 10.1038/nm0996-1035. [DOI] [PubMed] [Google Scholar]
- Nishigaki Y, Marti R, Hirano M, 2004. ND5 is a hot-spot for multiple atypical mitochondrial DNA deletions in mitochondrial neurogastrointestinal encephalomyopathy. Hum. Mol. Genet. 13, 91–101. 10.1093/hmg/ddh010. [DOI] [PubMed] [Google Scholar]
- Oh CR, Kong S-Y, Im H-S, Kim HJ, Kim MK, Yoon K-A, Cho E-H, Jang J-H, Lee J, Kang J, Park SR, Ryoo B-Y, 2019. Genome-wide copy number alteration and VEGFA amplification of circulating cell-free DNA as a biomarker in advanced hepatocellular carcinoma patients treated with Sorafenib. BMC Cancer 19, 292. 10.1186/s12885-019-5483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi DP, Praharaj PP, Bhol CS, Mahapatra KK, Patra S, Behera BP, Mishra SR, Bhutia SK, 2019. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin. Cancer Biol. 10.1016/j.semcancer.2019.07.015. [DOI] [PubMed] [Google Scholar]
- Ponti G, Maccaferri M, Manfredini M, Micali S, Torricelli F, Milandri R, Del Prete C, Ciarrocchi A, Ruini C, Benassi L, Bettelli S, Kaleci S, Ozben T, Tomasi A, 2019. Quick assessment of cell-free DNA in seminal fluid and fragment size for early non-invasive prostate cancer diagnosis. Clin. Chim. Acta 497, 76–80. 10.1016/j.cca.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Reznik E, Miller ML, Şenbabaoğlu Y, Riaz N, Sarungbam J, Tickoo SK, AlAhmadie HA, Lee W, Seshan VE, Hakimi AA, Sander C, 2016. Mitochondrial DNA copy number variation across human cancers. eLife 5, e10769. 10.7554/eLife.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, Galkin A, Thakur BK, Soplop N, Uryu K, Hoshino A, Norton L, Bonafé M, Cricca M, Gasparre G, Lyden D, Bromberg J, 2017. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. U.S.A. 114, E9066–E9075. 10.1073/pnas.1704862114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeld M, El Tarhouny S, Fan AXC, Hahn S, Holzgreve W, Zhong XY, 2008. Parallel assessment of circulatory cell-free DNA by PCR and nucleosomes by ELISA in breast tumors. Int. J. Biol. Markers 23, 69–73. 10.5301/jbm.2008.3658. [DOI] [PubMed] [Google Scholar]
- Sharma LK, Fang H, Liu J, Vartak R, Deng J, Bai Y, 2011. Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Hum. Mol. Genet. 20, 4605–4616. 10.1093/hmg/ddr395 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma LK, Lu J, Bai Y, 2009. Mitochondrial respiratory complex I: structure, function and implication in human diseases. Curr Med Chem 16, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma LK, Tiwari M, Rai NK, Bai Y, 2019. Mitophagy activation repairs Leber’s hereditary optic neuropathy-associated mitochondrial dysfunction and improves cell survival. Hum. Mol. Genet. 28, 422–433. 10.1093/hmg/ddy354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Song R, Lu Z, Zhao H, 2016. Mitochondrial DNA copy number in whole blood and glioma risk: a case control study. Mol. Carcinog. 55, 2089–2094. 10.1002/mc.22453. [DOI] [PubMed] [Google Scholar]
- Song S, Jiang Z, Spezia-Lindner DE, Liang T, Xu C, Wang H, Tian Y, Bai Y, 2020. BHRF1 enhances EBV mediated nasopharyngeal carcinoma tumorigenesis through modulating mitophagy associated with mitochondrial membrane permeabilization transition. Cells 9, 1158. 10.3390/cells9051158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroun M, Anker P, 1972. Nucleic acids spontaneously released by living frog auricles. Biochem. J. 128, 100P–101P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P, 2001. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta 313, 139–142. 10.1016/s0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- Thierry AR, Mouliere F, Gongora C, Ollier J, Robert B, Ychou M, Del Rio M, Molina F, 2010. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucl. Acids Res. 38, 6159–6175. 10.1093/nar/gkq421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara-Perez M, Felipe-Abrio B, Agostinis P, 2019. Mitophagy in cancer: a tale of adaptation. Cells 8. 10.3390/cells8050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusoff AAM, Abdullah WSW, Khair SZNM, Radzak SMA, 2019. A comprehensive overview of mitochondrial DNA 4977-bp deletion in cancer studies. Oncol Rev 13. 10.4081/oncol.2019.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Lyu L-H, Miao H-K, Bahr T, Zhang Q-Y, Liang T, Zhou H-B, Chen G-R, Bai Y, 2020. Redox regulation by SOD2 modulates colorectal cancer tumorigenesis through AMPK-mediated energy metabolism. Mol. Carcinog. 59, 545–556. 10.1002/mc.23178. [DOI] [PubMed] [Google Scholar]