Abstract
We propose the beneficial effects of exercise are in part mediated through the prevention and elimination of senescent cells. Exercise counters multiple forms of age-related molecular damage that initiate the senescence program and activates immune cells responsible for senescent cell clearance. Preclinical and clinical evidence for exercise as a senescence-targeting therapy and areas needing further investigation are discussed.
Keywords: aging, geroscience, healthspan, senescence-associated secretory phenotype, senotherapeutics
INTRODUCTION
Aging has long been appreciated as the major risk factor for conditions and diseases affecting the cardiovascular, musculoskeletal, central nervous, immune, pulmonary, and other systems. However, until recently, targeting aging to thwart chronic disease, multimorbidity, or geriatric syndromes was viewed as science fiction. Fundamental advances in understanding the biology of aging and proof-of-concept studies in preclinical models have radically changed this perspective and provided a foundation for the new and rapidly expanding field of geroscience.
The geroscience hypothesis posits that interventions that target key hallmarks of aging potentially can prevent, delay, or even reverse age-related conditions as a group (1). If successful, the impact on human health would be transformative, in terms of both sociomedical costs and quality of life, through extending healthspan, the number of healthy and active years of life, and compressing the period of morbidity to the very end.
The promise of geroscience has resulted in extraordinary public and private investment into pharmacological approaches to counter the effects of aging. Although exhilarating, the profound effects of lifestyle factors on healthy aging should not be overlooked. Exercise, in particular, is a highly effective means to counter the onset and progression of the most prevalent and disabling aging-related conditions, including heart disease, diabetes, Alzheimer disease, osteoporosis, sarcopenia, cancer, and frailty. The effects of exercise on the fundamental mechanisms of aging warrant investigation and may yield new insights into behavioral strategies, pharmacological approaches, and combinations thereof to optimize health and function over the life course.
Over the past decade, mounting evidence highlights cellular senescence, a cell fate in response to diverse forms of molecular and cellular damage, as a driver of aging and aging-related conditions. Moreover, candidate drugs (senotherapeutics) that impact the abundance (senolytics) or behavior (senomorphics) of senescent cells have been shown to counter multiple conditions across multiple physiological systems in mouse models of aging and disease (2). Herein, we discuss the current evidence supporting that exercise, in part, acts as a senotherapeutic and discuss the future work necessary to gain a fundamental understanding for how exercise directly exerts senotherapeutic effects.
CELLULAR SENESCENCE
In a study of primary human fibroblasts published in 1961, Leonard Hayflick and Paul Moorhead (3) observed the transition of newly plated cells from an early growth phase to a prolonged period of rapid multiplication, which then was followed by a state characterized by the loss of mitotic activity, enlargement, the aggregation of debris, and “bizarre nuclear forms and sizes…reminiscent of irradiated cultures,” which could not be reversed. The authors referred to this cell fate of stable growth arrest as the phenomenon of replicative senescence and, notably, suggested it “may bear directly upon problems of aging.” Now 60 yr later, there is compelling evidence that this is indeed the case.
Causes of Cellular Senescence
Senescence is a consequence of multiple forms of molecular damage that increase with advancing age, exceed the cell’s capacity for repair, but are insufficient to trigger death by apoptosis. Through cell divisions, telomeres, which are short repeats of DNA sequences that cap the ends and protect chromosomes, progressively shorten. Ultimately, this triggers a DNA damage response (DDR) and activates key governors of the cell cycle and senescence program (thoughtfully reviewed in (4)). It also should be noted that telomere uncapping and DNA damage within telomeres can trigger senescence independent of changes in telomere length (5,6). As with replicative stress, the DDR is the lynchpin of other recognized inducers of cellular senescence, including reactive oxygen species (ROS), mitochondrial dysfunction, proteotoxic stress, and exogenous stressors such as irradiation and genotoxic drugs, which together are termed stress-induced premature senescence. In the context of cancer, overexpression of oncogenic Ras or loss of the tumor suppressor phosphatase and tensin homolog can also trigger the DDR as a consequence of DNA hyperreplication, referred to as mitotic stress (7). Inflammatory factors, including those produced by senescent cells (e.g., cytokines, transforming growth factor-β family ligands, and vascular endothelial growth factor), also have been implicated in the initiation and spread of senescence (8). Inflammation also is linked to DNA damage and subsequent activation of the cell cycle inhibitors. The functional links between DNA damage and cell cycle arrest and the distinguishing hallmarks of senescent cells are discussed.
Mediators and Markers of Cellular Senescence
DNA damage initiates cell cycle arrest through the DDR and downstream p53/p21 and p16 pathways (Fig. 1). Activation of cyclin-dependent kinase inhibitor (CDKI) P21Cip1 (P21) leads to inhibition of the cyclin E/CDK2 complex, whereas activation of the CDKI P16INK4a (P16) leads to inhibition of the cyclin D/CDK4/6 complex. These actions block cell cycle progression through preventing the phosphorylation and dissociation of retinoblastoma tumor suppressor protein RB, thereby inhibiting activation of E2F transcription factors and G1/S transition.
Figure 1.
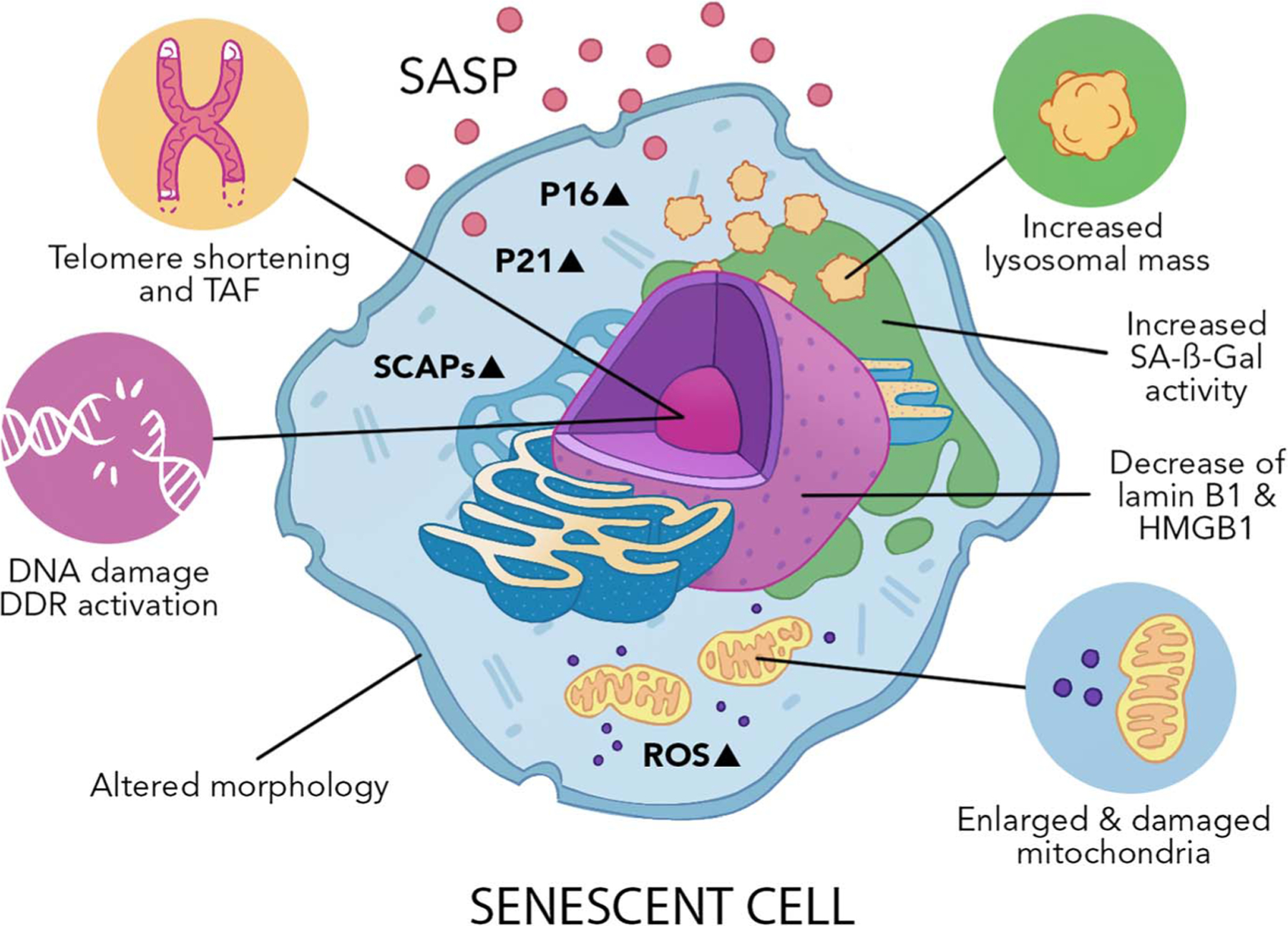
Hallmarks of senescent cells. Senescence is, fundamentally, a DNA damage response (DDR). DNA damage, telomere-associated DNA damage foci (TAF), and chromatin reorganization can be caused by several stressors, including mitochondrial dysfunction, excessive reactive oxygen species (ROS), inflammation, and exogenous triggers such as irradiation and genotoxic drugs. The DDR activates cyclin-dependent kinase inhibitors P21 and P16, ultimately leading to growth arrest. Established senescent cells will present an enlarged nucleus and aberrant cell morphology, accompanied by the loss of nuclear lamin B1 and HMGB1, high levels of lysosomal activity, and increased SA-β-gal activity. Senescent cells typically have a robust and heterogeneous bioactive secretome composed of proinflammatory cytokines and chemokines, growth factors, and matrix-modifying factors called the senescence-associated secretory phenotype (SASP). Another key feature of senescent cells is the upregulation of senescent cell antiapoptotic pathways (SCAPs), which confer their resistance to apoptosis.
As noted by Hayflick and Moorehead, growth arrest in the context of senescence is accompanied by significant morphological changes, including enlarged nuclei within cells of flattened, expanded, and granular appearance. These features of senescent cells can reflect alterations in chromatin, formation of senescence-associated heterochromatic foci, senescence-associated distension of satellites, telomere-associated DNA damage foci (TAF), compromised integrity of the nuclear envelope (e.g., loss of lamin B1 and high mobility group box protein 1 (HMGB1)), changes in structural proteins, and increased endoplasmic reticulum stress (9). Senescent cells also have altered lysosomal content and increased activity of senescence-associated beta-galactosidase (SA-β-gal). An additional feature of senescent cells is their robust and heterogeneous senescence-associated secretory phenotype (SASP), composed of a diverse collection of proinflammatory cytokines, chemokines, growth factors, matrix remodeling proteins, miRNAs, metabolites, and other bioactive molecules. The SASP is triggered by various regulatory pathways and transcription factors and exerts deleterious effects, locally and systemically. Key regulators of the SASP include the well-characterized nuclear factor–κB and C/EBP pathways, as well as the cGAS-STING pathway, which can be uniquely activated by DNA fragments released into the cytoplasm consequent to alterations in the nuclear envelope, as well as mitochondrial DNA (mtDNA) fragments leaked from dysfunctional mitochondria (10). Furthermore, senescent cells activate BCL2 family members and other senescent cell antiapoptotic pathways (SCAPs), contributing to their resistance to apoptosis (11).
Based on the dynamic nature and cell-to-cell differences in the senescence phenotype, it is critical to emphasize that there is not a stand-alone, specific marker that distinguishes a senescent cell from a healthy or acutely stressed cell. The potential for misinterpretation can stem from high basal expression of p16 in macrophages and the expression of CDKIs in response to cell proliferation and differentiation (e.g., in response to acute exercise), which occur in the absence of other core properties of the senescence program. The field largely has agreed that comprehensive analysis of senescence, whether in isolated cells or intact tissue, should include combinations of measures of cell cycle regulators, chromatin modifications, DNA and telomeric damage, SASP components, and SCAPs.
Biological Roles of Senescent Cells in Aging and Age-Related Diseases
Senescence plays a critical role in health throughout the life course, including development and parturition, and as an essential antitumor mechanism (9). Senescent cells can arise in younger tissues, but they are rare because of efficient removal by the immune system, as further detailed. With advancing age, senescent cells accumulate and are enriched at anatomical sites of disease, where they compromise the structure and function of multiple organs. For example, senescent cells promote ventricular hypertrophy and fibrosis in the heart, which cause myocardial dysfunction; drive atherosclerotic lesion formation and maturation in the vasculature; recruit, anchor, and amplify signals from proinflammatory macrophages in adipose tissue and exacerbate metabolic dysfunction; contribute to neurofibrillary tangle-associated pathology in the brain, characteristic of neurodegenerative diseases; increase bone resorption and reduce bone formation, characteristic of osteoporosis; and cause lung fibrosis and impair pulmonary function, characteristic of fibrotic pulmonary diseases (12).
Correspondingly, genetic and pharmacological clearance of senescent cells confers therapeutic benefits in models of myocardial infarction, atherosclerosis, diabetes, Alzheimer disease, osteoporosis, lung fibrosis, and a number of other age-related conditions (12). These exciting results in preclinical models of aging and disease have fueled a major interest in senotherapeutic interventions as a novel means to optimize late life health, in line with the geroscience hypothesis.
EFFECT OF EXERCISE ON MODULATORS OF CELLULAR SENESCENCE
In a compelling review, Booth et al. (13) underscore physical inactivity as a major cause of chronic diseases and a threat to human healthspan and showcase exercise as a profoundly effective means to prevent or delay the onset and progression of 35 conditions. Indeed, the mechanism through which exercise confers such remarkable benefits on health is multifactorial. Considering the therapeutic effects of both exercise and senotherapeutics on the pathogenesis of many aging-associated diseases and geriatric syndromes, it is plausible that exercise may act, in part, by preventing the accumulation and augmenting the clearance of senescent cells (Fig. 2). Potential mechanisms in support of this premise are discussed.
Figure 2.
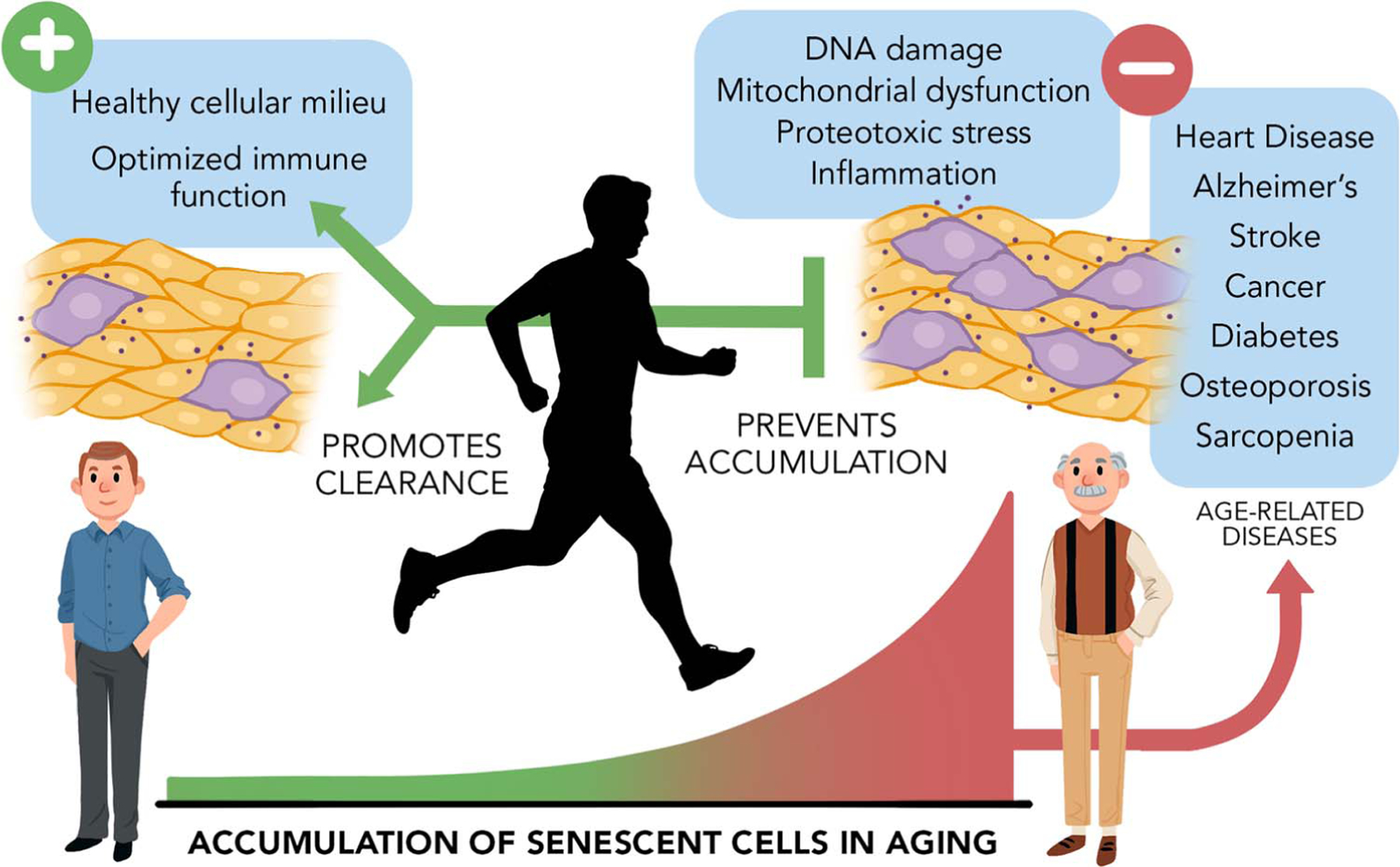
Exercise as a countermeasure to aging and chronic disease. Senescent cells accumulate with age and contribute to many age-associated diseases and geriatric syndromes. We propose exercise effectively delays the onset and progression of disease, in part, by preventing the accumulation of senescent cells. Mechanistically, exercise counters diverse forms of stimuli that cause senescence, including DNA damage, mitochondrial dysfunction, excessive reactive oxygen species (ROS), and inflammation. In parallel, exercise promotes a healthier cellular milieu that counters reinforcement of the senescence program and facilitates immune cell–mediated senescent cell clearance. The senotherapeutic effects of exercise contribute to its marked impact on healthspan.
DNA and Telomere Damage
DNA damage is a hallmark of aging and a primary trigger of the senescence program. Impressively, 8 wk of treadmill running attenuated the age-associated increase in DNA damage (8-OHdG content) within skeletal muscle of 30-month-old rats (14). This was attributed to enhanced DNA repair (8-OHdG excision assay) and resistance to oxidative stress. In humans, a cross-sectional study of approximately 100 healthy women and men aged 50–72 yr did not show an association between self-reported physical activity and DNA damage (measured via comet assay), but reported a positive association between both physical activity and high-intensity physical activity (metabolic equivalent task (MET) values ≥6) and DNA repair in lymphocytes (15). The combination of moderate- and high-intensity activity (activities with MET values ≥4) also was associated with a trend for 25% higher DNA repair.
In terms of telomere length, the effects of physical activity and structured exercise interventions have been inconsistent. Differences in study design, duration, cell/tissue types analyzed, and the methods used to measure telomere length prohibit consensus, as highlighted in a recent systematic review (16). In younger mice, neither 3 wk nor 6 months of voluntary wheel running affected telomere length within the aorta, although exercised mice had increased expression of telomere repeat-binding factor 1 (TRF1), TRF2, and Ku70 in the aorta, which has been shown to protect against telomere erosion and genome degradation (17). In addition, telomerase activity was higher in the aorta and mononuclear cells isolated from the blood, spleen, and bone marrow of mice exercised for 3 wk compared with sedentary controls. In this same study, both telomerase activity and TRF2 protein abundance were also significantly higher in young (early 20s) and middle-aged (early 50s) professional long-distance runners compared with their untrained peers. Collectively, these findings suggest exercise may prevent senescent cell accumulation by increasing the resilience of DNA and telomeres to damage-inducing stimuli and augmenting the capacity for DNA repair when damage occurs.
Mitochondria Dysfunction and ROS
Aging and physical inactivity independently and synergistically diminish mitochondrial content and oxidative capacity and exacerbate mitochondrial mutations and oxidative stress. Dysfunctional mitochondria and ROS have been strongly implicated in the induction and reinforcement of the senescence program (18). Exercise has powerful effects on mitochondrial abundance, quality, and function, as recently reviewed (19). Of note, peroxisome proliferator–activated receptor-gamma (PPAR) coactivator-1 (PGC1α)–mediated gene expression is a primary response to endurance exercise in mice and humans (20). PGC1α interacts with numerous transcription factors that control the expression of several bioenergetic pathways, including nuclear genes encoding mitochondrial proteins, like transcription factor A of the mitochondria (TFAM), which is essential for mitochondrial biogenesis (21). Moreover, exercise training restores age-associated deficits in autophagy (22), which translates into effective removal of damaged and dysfunctional organelles, including mitochondria. Mitochondrial DNA mutations also accumulate with age, secondary to increased ROS and continuous replication, which enables mutations that impair function to, potentially, expand clonally (23). Impressively, 30 wk of voluntary wheel running in mtDNA mutator mice (mice with defective mtDNA polymerase, PolgA) from 10 wk of age reduced mtDNA mutations and features of accelerated aging in skeletal muscle, heart, and brain (24). Finally, ROS is mainly generated during oxidative metabolism within mitochondria, and levels increase with mitochondrial dysfunction and advancing age. Exercise acutely activates ROS production, which is essential for force generation within skeletal muscle, signaling to distal tissues and activating antioxidants. This adaptation ensures homeostasis and prevention of excess oxidative stress (25). Indeed, just 2 wk of treadmill running has been shown to increase the abundance of antioxidants superoxide dismutase-1 and −2 and glutathione peroxidase and reduce ROS in the hippocampus of 12-month-old rats (26). Collectively, through the restoration of mitochondrial health and function and rebalancing ROS, exercise may prevent oxidative stress–mediated induction and reinforcement of the senescence program.
Inflammation and Secreted Factors
Advancing age is associated with chronic low-grade “sterile” inflammation characterized by elevated levels of proinflammatory markers in the absence of overt infection. Senescent cells are a plausible contributor to age-associated inflammation, as the SASP is a veritable source of proinflammatory cytokines and chemokines that recruit, anchor, and amplify the activities of immune cells (27). Furthermore, the SASP can mediate the spread of senescence both locally and systemically (28).
Several bioactive proteins and molecules, termed exerkines, are released into circulation after exercise and are thought to orchestrate systemic adaptations in several organs including bone, brain, and adipose tissue (29). Interestingly, although there is an acute and transient inflammatory response after exercise, exercise training suppresses sterile inflammation. For example, recognized proinflammatory SASP factors, interleukin-6 (IL-6), CCL2 (MCP1), and tumor necrosis factor α (TNF-α) are activated acutely after exercise (30), partially due to oxidative stress and the DDR, but basal levels are reduced after exercise intervention. A study from our group showed that voluntary wheel running for 4 months significantly reduced the expression of SASP factors including IL-6, CCL2, and PAI1 in adipose tissue of middle-aged high-fat diet (HFD)–fed mice (31). Another recent study from our laboratory showed that 3 months of progressive strength and endurance training in older adults (average 67-yr-old) significantly reduced expression of components of the cGAS-STING pathway (i.e., cGAS, IFNy, and TNFα) in circulating CD3+ T cells (32), known to induce premature senescence and reinforce the senescence program (33). These findings complement data from several cross-sectional studies in humans that show an inverse relation between markers of inflammation and physical activity levels and fitness (34).
In addition to modulating proinflammatory factors, it is possible that the transient rise in IL-6 in response to exercise stimulates the expression of anti-inflammatory mediators IL-1Ra and IL-10 and, in turn, downregulates the expression of TNF-α and IL-1β (35). Exercise also stimulates brain-derived neurotrophic factor (BDNF) activation and secretion from skeletal muscle (36). BDNF promotes DNA repair by activating the transcription factor cAMP responsive element binding protein 1 (CREB) and the base excision repair enzyme apurinic/apyrimidinic endonuclease 1 (APE1) (37). In this sense, exercise may function as a senomorphic, targeting the SASP and suppressing its deleterious effects. Furthermore, it is plausible that the acute cellular stress response activated in response to exercise (increased inflammatory markers, ROS, etc.) may have a hormetic effect and indeed make these cells more resilient to future senescence-inducing stimuli.
Immune Cells That Recognize and Eliminate Senescent Cells
Available evidence suggests that under physiological conditions, immune cells may target and clear senescent cells to maintain tissue homeostasis. The secretion of SASP factors is potentially involved in the recruitment of immune cells, although the mechanisms underlying the immunosurveillance of senescent cells are still not fully understood. Aging of the immune system may hamper the efficient removal of senescent cells and contribute to their progressive accumulation (38).
The acute response to exercise and the adaptations to long-term training can influence the immune cells recruited by skeletal muscle and mobilized in the peripheral blood. Exercise involving eccentric muscle contractions can induce cellular damage, which stimulates the recruitment of various immune cells to clear cellular debris and facilitate repair. This acute response is dominated at early stages by proinflammatory cells (including neutrophils and proinflammatory macrophages), whereas at later stages involves anti-inflammatory macrophages, CD8+, and regulatory T lymphocytes. Adaptions to exercise training can modulate this response (39). Twelve weeks of endurance exercise training composed of cycling 3 d·wk−1 at a target intensity corresponding to 65% V O2max increased M2 macrophage abundance in human skeletal muscle, suggesting that adaptive changes to training may reduce local inflammation (40). In peripheral blood, the concentrations of several immune cells can also be differentially modulated in response to acute and chronic exercise. For example, repeated bouts of “allout” ergometer rowing in competitive oarsmen increased the circulating concentrations of natural killer (NK) cells and subsets of lymphocytes, with higher levels after each bout. NK cell activity measured by an in vitro killing assay also increased during each row and was elevated even the day after the last bout (41). Moreover, a single bout of treadmill running resulted in an immediate but transient increase in circulating CD8+ T cells (42) and proinflammatory monocytes (43) in moderately trained male subjects in their mid-20s. Conversely, circulating proinflammatory monocytes were higher in physically inactive older adults than in age-matched physically active individuals, and 12 wk of combined endurance and resistance training lowered the percentage of these cells in habitually inactive older adults, supporting the hypothesis that regular exercise training can exert anti-inflammatory effects (44).
Favorable shifts in proinflammatory and anti-inflammatory immune cell populations in response to exercise and evidence that physical activity can ameliorate detrimental shifts in immune cell number, phenotype, and function with advancing age (45) support the premise that structured exercise and physical activity facilitate senescent cell clearance. Then again, conclusive evidence is needed still, and it must be noted that senescent cells use several strategies to evade the immune response. For example, senescent dermal fibroblasts can evade immune clearance by expressing the nonclassical major compatibility complex molecule HLA class I histocompatibility antigen, alpha chain E which interacts with the inhibitory receptor natural killer group 2 member A expressed by NK cells and highly differentiated CD8+ T cells (46). Similarly, senescent cells can evade immune clearance by shedding the NKG2D ligands, which are important for immune cell recognition, from their cell surface via matrix metalloproteinase–mediated cleavage (47). Advances in understanding the interplay between senescent and immune cells and the mechanisms of immune evasion have led to immune cell-based senotherapeutic approaches (38). Encouraging results were recently reported in experimental studies using antibody-dependent cell-mediated cytotoxicity assay targeting dipeptidyl peptidase 4 (DPP4), selectively expressed on the surface of senescent fibroblasts and conferred sensitivity to death by NK cells (48). Similarly, chimeric antigen receptor (CAR) T cells targeting the urokinase-type plasminogen activator receptor (uPAR), reported to be a specific cell surface marker in certain senescent cell populations, effectively ablated senescent cells in vitro and in murine models of lung adenocarcinomas and liver fibrosis (49). Given the effects of exercise on the immune system and conceptual and technical advances in cell-based therapies, additional research is warranted to better understand the interplay between exercise, immune cell adaptations, and senescent cell clearance across the lifespan.
THE IMPACT OF EXERCISE ON MARKERS OF CELLULAR SENESCENCE IN PRECLINICAL MODELS
There is a wealth of evidence supporting exercise as a strategy to prevent disease and extend healthspan. There is compelling, although modest, evidence that exercise counters inducers and activates clearance of senescent cells. Although these data collectively suggest that the therapeutic effects of exercise on health and function could be partly mediated through the prevention and elimination of senescent cells, to date, there is a limited body of preclinical work that supports this premise. We summarize these studies.
Cardiovascular System
Short-term (3 wk) voluntary wheel running started at 8 wk of age significantly increased myocardial telomerase activity and the protein expression of two telomere-stabilizing proteins, telomerase reverse transcriptase (TERT) and TRF2 in male mice compared with sedentary controls. Increased telomerase activity was associated with reduced myocardial levels of P16, P53, and cell-cycle-checkpoint kinase 2 (Chk2) (50). Similar effects were observed in the myocardium and aorta with longer-term (6 months) voluntary wheel running (17). When endothelial nitric oxide synthase (eNOS) knockout mice or TERT knockout mice (8-wk-old) were subjected to the same training stimulus, the positive effects of exercise were not present, suggesting that these enzymes are crucial mediators of myocardial and aortic adaptation. In a later study, the AMP-activated protein kinase (AMPK) was identified as another important mediator of the vascular protective effects of exercise. In relation to senescence, they found that the reduction in P16, P53, and Chk2 protein levels and the increase in TRF2 and TERT mRNA expression in the vasculature of young wild-type mice induced by an 8-wk voluntary wheel running exercise intervention were attenuated in α1AMPK knockout mice (51).
Adipose Tissue
Regarding adipose tissue, work from our group showed that 16 wk of voluntary wheel running started at 8 months of age in male mice significantly prevented HFD-induced increases in p21, p53, and SASP-factor expression and the percentage of SA-β-gal–positive cells in visceral adipose tissue. In addition, we found that when a 14-wk running wheel intervention was started in mice that already had been exposed to an HFD for 16 wk, it was able to attenuate the increase in p16, Pai1, and CD68 expression in visceral adipose tissue (31). Consistent with these findings, a 4-wk swimming intervention introduced after 5 wk of HFD reduced SA-β-gal activity in both subcutaneous (inguinal) and visceral (epididymal) white adipose tissue in 5-month-old mice (52). In addition, exercise reduced p21 expression in inguinal adipose tissue as well as p16 and p21 expression in epididymal fat–derived preadipocytes. Using immunofluorescence detection methods, exercise has been shown to reduce the frequency of P16+/PDGFRα+ and P21+/PDGFRα+ cells in both fat depots, phosphorylated-γH2A.X expression in inguinal fat, P16 and P21 in Mac3+ macrophages and P16 in CD3+ lymphocytes in the inguinal fat, and P21 in Mac3+ macrophages in the epididymal fat (52). A more recent study also showed a reduction in p16, p53, and Il6 expression in the visceral adipose tissue of young male mice after 20 wk of voluntary wheel running. These senescence markers were also lower in 4-wk-old mice that underwent 10 wk of wheel running followed by 10 wk of sedentary behavior, suggesting a legacy effect of exercise on the senescence program (53).
Liver
In relation to the liver, we reported that 16 wk of voluntary wheel running in male mice prevented an HFD-induced increase in p21 expression, but no changes in p16 or p53 expression were observed in response to either the diet or exercise (31). Recently, the influence of exercise on liver has been studied in 16-month male Nfkb1−/− mice, a model that develops inflammation-driven premature aging. The exercise intervention consisted of treadmill running for 30 min·d−1 three times a week for 3 months. They found that exercise reduced liver inflammation, oxidative damage, and cellular senescence; improved hepatic steatosis; and prevented tumor development. In relation to senescence markers, immunohistochemistry on liver sections showed a significant reduction of P21-positive hepatocytes in exercised mice compared with sedentary mice. Reduced P21 was associated with a reduction in TAF-positive hepatocytes and lower levels of 4HNE staining, which is a lipid peroxidation marker (54).
Brain
Only one study has examined the effects of endurance exercise on the levels of senescence markers in the brain. Young female mice were administered either normal chow or a high-fat/high-fructose diet for 12 wk. High-fat/high-fructose diet–fed mice were then randomized to no exercise or treadmill running (60 min·d−1, 5 d·wk−1) for 12 wk (55). The exercise intervention blunted diet-induced increases in P16, P21, and P53 protein, as well as SA-β-gal and lipofuscin in the hippocampus. In addition, exercise alleviated diet-induced neuroinflammation and oxidative stress in the hippocampus (55), consistent with prior reports (26).
Skeletal Muscle
To date, little is known about how exercise influences senescence markers within the skeletal muscle (56). Four weeks of treadmill exercise was found to reduce P21 protein level in the gastrocnemius muscle of 19-month-old mice compared with sedentary mice (57). In a recent study, an acute bout of 14 d of downhill running promoted a senescent phenotype in skeletal muscle fibroadipogenic progenitors from young wild-type mice but not from mice with chronic inflammatory myopathy (58). Induction of senescence with exercise and pharmacological activation of AMPK using 5-aminoimidazole-4-carboxamide ribonucleotide improved muscle function, cross-sectional area, and regeneration in the chronic inflammatory myopathy model. In future studies, it will be important to discern the impact of exercise on transient versus sustained rises in senescence markers because of their opposing implications.
Several other studies have investigated the impact of exercise on cellular senescence but relied on a single senescence marker (e.g., SA-β-gal or p16 expression), limiting the interpretation of these findings. A recent review on the senolytic effects of exercise summarized the evidence available with a metanalysis (59). They noted the high heterogeneity observed in animal studies and the need for additional investigations. Moreover, the current preclinical evidence that supports exercise as a strategy to prevent or clear senescent cells is derived from young and middle-aged animals. Whether exercise is an effective senotherapeutic in older animals needs further investigation.
THE EFFECTS OF EXERCISE ON MARKERS OF CELLULAR SENESCENCE IN HUMANS
Early work examining the relation between cellular senescence and exercise in humans primarily relied on measures of self-reported physical activity and their cross-sectional associations with senescence markers in circulating immune cells. A study of 170 healthy participants aged 18–80 yr found that those with more than 240 min a month of habitual physical activity had lower expression levels of P16 in circulating T cells than those who did not (60). A similar study in a subset (n = 136) of human samples from the same cohort corroborated these findings, showing relatively higher levels of habitual activity were associated with lower levels of P16 in circulating T cells (61). Among 63 healthy postmenopausal women, those who self-reported participation in vigorous physical activity had longer telomeres in circulating leukocytes than those who were sedentary (62). It seems the efficacy of exercise may be influenced by health status or specific disease states. In a recent study of 47 patients aged 56–81 yr with a high prevalence of frailty and comorbid conditions undergoing coronary artery bypass surgery, no association was found between levels of physical activity and P16 in circulating T cells (63). Notably, self-reported physical activity levels were relatively low in this cohort and, potentially, below a threshold required for therapeutic effect.
Similar cross-sectional analyses have been conducted in cohorts of highly active middle-aged to older adults. Healthy middle-aged marathon runners/triathletes have lower P53 and P16 in circulating leukocytes, greater telomerase activity and telomere length, and higher expression of telomere-stabilizing proteins compared with untrained controls (17). In line with these findings, exercise-trained older adults (~60 yr) were found to have reduced P53, P21, and P16 in endothelial cells compared with sedentary controls (64).
Studies investigating the direct effects of exercise interventions on senescent cell burden are lacking. To date, we are aware of only two such reports. Five months of resistance training in older (~73 yr) overweight/obese women reduced P16-expressing cells in thigh adipose tissue, and P16+ cell content was negatively associated with several parameters of physical function, including grip strength and 400-m walk time (65). Most recently, our group showed 12 wk of structured strength, and endurance training reduced the expression of key markers of the senescence program including P16, P21, cGAS, and TNFα in peripheral blood CD3+ T cells and lowered the circulating concentrations of several senescence-related proteins in older (~67 yr) adults (32). Furthermore, circulating levels of senescence-related proteins at baseline were predictive of changes in physical function in response to the exercise intervention.
Taken together, the findings presented above suggest higher levels of habitual physical activity and structured exercise are protective against age-related increases in senescence markers, particularly P16, in certain populations of circulating immune cells. High levels of physical activity may increase the resilience of DNA and telomeres to diverse forms of stress, including ROS, inflammation, and protein aggregates, and in turn reduce the induction of CDKIs and other governors of the senescence program. Exercise interventions seem to directly influence senescent cell burden, reducing P16-expressing cells and clinically relevant senescence markers. Interestingly, senescence markers are predictive of levels of physical function and even the responsiveness to intervention.
CHALLENGES AND FUTURE DIRECTIONS
As discussed in the previous paragraphs, the evidence currently available about the effects of exercise on cellular senescence, although promising, is still preliminary, and additional investigations are needed. Main goals and challenges to address in future studies should include the following:
The What: Defining senescence through a comprehensive and rigorous assessment of core properties in isolated cells and intact tissues by leveraging different and complementary methodological approaches. Advances in bulk, single-cell, and single-nuclei sequencing, and proteomics enable hypothesis- and discovery-driven approaches to define the presence and absence of core features of senescent cells (i.e., CDKIs, SASP-effector pathways, SASP components, prosurvival pathways) and discover novel features (e.g., unique cell membrane factors) (49) that may aid in their identification. These approaches can be complemented by imaging methods, such as digital spatial profiling and established histochemical approaches, to define location and other core properties, such as TAF, SA-β-gal activity, or loss of nuclear envelope proteins (e.g., lamin B1, HMGB1). A comprehensive and rigorous approach is needed to overcome the challenges posed by i) the absence of a specific, stand-alone marker of senescence, and ii) misinterpretation of acute responses to exercise (e.g., ROS, inflammation, CDKI expression in response to cell proliferation and differentiation) as indicators of cellular senescence. A systematic test of different markers has been proposed to comprehensively assess senescence burden both in vitro and in vivo (66). Similar standards should be applied to the assessment of senescence in the context of exercise.
The Where: Determining the cell types and tissues in which exercise counters senescent cell accumulation. It is remarkable to note that exercise benefits nearly all tissues. The promise of senotherapeutic drugs has accelerated efforts, including a National Institutes of Health Common Fund initiative, to define the cell types in mice and humans prone to senescence with chronological age. These studies can inform and be paralleled by investigations into the effects of exercise on modulators of senescence in different tissues and their resident cell populations. Skeletal muscle, a tissue that is composed of terminally differentiated multinucleated muscle fibers and several mitotically competent mononuclear cell populations, is highly responsive to exercise, understudied in the context of senescence, and serves as a noteworthy example (56). There is a parallel need for biomarkers of systemic senescent cell burden that are valid, easily accessible (e.g., blood based), and responsive to intervention (32,67,68). This task is challenged by the fact that many of the circulating biomarkers studied to date (i.e., circulating proteins, miRNAs, or mtDNA) are not unique to senescent cells.
The When: Examining when in the life course exercise effectively counters the accumulation of senescent cells. Early and mid-life behaviors (e.g., physical activity levels, nutrition) undoubtedly influence aging trajectory. The same can be said for exposures to damaging stimuli (e.g., chemotherapy, irradiation, infections). The extent to which exercise bolsters resistance to senescence-inducing stimuli and fosters the clearance of senescent cells in early, mid, and late life are worthy of investigation. In addition to studying the senotherapeutic effects of exercise in older adults, there is strong evidence for increased senescence and accelerated aging in childhood cancer survivors treated with chemotherapies (69). Whether exercise before exposure ( prehabilitation) or after recovery could counter the adverse effects of treatment is an opportunity worthy of further exploration. Of course, the challenge of when will require prospective long-term preclinical and clinical studies and assessment of the effects of exercise at different ages and conditions.
The How: Better understanding the mode(s), frequency, intensity, and duration of the exercise needed to counteract cellular senescence. As with pharmacotherapy, there are multiple forms of exercise (e.g., endurance, resistance, and combinations thereof ) that benefit different health parameters. The extent to which different forms of exercise and physical activity affect senescent cell abundance or behavior over the life course is unknown. This also holds true for the requisite frequency, intensity, and duration that is most effective, and underscores the need for further study. Moreover, rigorous studies are required to establish the senotherapeutic mechanisms of action of exercise. This will lead to a fundamental understanding for the distinct cellular processes and pathways being influenced by exercise in senescent cells that drive therapeutic benefit. Based on the current state of the literature, sex-specific differences in response to exercise, as it pertains to its effects as a senotherapeutic, are not apparent. However, this topic has not been thoroughly studied and warrants further investigation. Advances in accessible senescence markers, particularly in humans, will facilitate these efforts. Importantly, exercise can be tailored to all persons regardless of age and physical abilities.
CONCLUSION
Exercise is, arguably, the most effective means to extend human healthspan. New insights into the mechanisms through which exercise optimizes function and counters disease have the potential to guide public health initiatives to promote physical activity and, concurrently, reveal biology that may be targeted through pharmacological intervention. The literature summarized herein suggests exercise both counters diverse forms of molecular damage that cause cellular senescence and potentially promotes immune-mediated senescent cell clearance. This evidence supports the hypothesis that exercise prevents the age-associated accumulation of senescent cells. Although more comprehensive and confirmatory studies are needed, the senotherapeutic effects of exercise have been demonstrated in multiple tissues in both preclinical models and humans. We propose rigorous preclinical and human studies examining several senescence phenotypes in response to structured exercise interventions are needed to accurately promote exercise as an effective, safe, scalable, and readily implementable senotherapeutic.
Key Points.
Senescence plays an important role during embryonic development, parturition, and tumor suppression. However, it has become clear that the accumulation of senescent cells is a major driver of age-related chronic diseases and geriatric syndromes.
In preclinical models, targeted elimination of senescent cells by genetic and pharmacologic approaches restores tissue health and attenuates the progression of numerous age-related conditions.
High levels of physical activity and structured exercise profoundly delay the onset and progression of several chronic diseases and geriatric syndromes.
Exercise counters multiple forms of age-related molecular damage that can initiate the senescence program and potentially promotes senescent cell clearance by the immune system.
As summarized herein, the current evidence supports the premise that exercise is an effective countermeasure for the age-related accumulation of senescent cells.
Acknowledgments
X. Zhang, D.A. Englund, and Z. Aversa equally contributed to this work. We are grateful for the support of the National Institutes of Health, National Institute of Aging for grants P01 AG62413, R01 AG55529, and R01 AG53832 to N.K.L. and T32AG049672 to D.A.E., as well as the Glenn Foundation for Medical Research and the Pritzker Foundation. We also acknowledge the support of the Robert and Arlene Kogod Center on Aging Career Development Award to X.Z.
References
- 1.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell 2014; 159(4):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins PD, Jurk D, Khosla S, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu. Rev. Pharmacol. Toxicol 2021; 61: 779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res 1961; 25:585–621. [DOI] [PubMed] [Google Scholar]
- 4.Victorelli S, Passos JF. Telomeres and cell senescence—size matters not. EBioMedicine 2017; 21:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson R, Lagnado A, Maggiorani D, et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 2019; 38(5):e100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan RG, Ives SJ, Lesniewski LA, et al. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am. J. Physiol. Heart Circ. Physiol 2013; 305(2):H251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Micco R, Fumagalli M, Cicalese A, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006; 444(7119):638–42. [DOI] [PubMed] [Google Scholar]
- 8.Acosta JC, Banito A, Wuestefeld T, et al. A complex secretory program or- chestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol 2013; 15(8):978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol 2018; 28(6):436–53. [DOI] [PubMed] [Google Scholar]
- 10.Gluck S, Guey B, Gulen MF, et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol 2017; 19(9):1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015; 14(4):644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol 2020; 16(5):263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr. Physiol 2012; 2(2):1143–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radák Z, Naito H, Kaneko T, et al. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch 2002; 445(2):273–8. [DOI] [PubMed] [Google Scholar]
- 15.Cash SW, Beresford SA, Vaughan TL, et al. Recent physical activity in relation to DNA damage and repair using the comet assay. J. Phys. Act. Health 2014; 11(4):770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valente C, Andrade R, Alvarez L, Rebelo-Marques A, Stamatakis E, Espregueira-Mendes J. Effect of physical activity and exercise on telomere length: systematic review with meta-analysis. J. Am. Geriatr. Soc 2021; 69(11):3285–300. [DOI] [PubMed] [Google Scholar]
- 17.Werner C, Furster T, Widmann T, et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 2009; 120(24):2438–47. [DOI] [PubMed] [Google Scholar]
- 18.Martini H, Passos JF. Cellular senescence: all roads lead to mitochondria. FEBS J 2022. doi: 10.1111/febs.16361. [DOI] [PMC free article] [PubMed]
- 19.Oliveira AN, Richards BJ, Slavin M, Hood DA. Exercise is muscle mitochondrial medicine. Exerc. Sport Sci. Rev 2021; 49(2):67–76. [DOI] [PubMed] [Google Scholar]
- 20.Baar K, Wende AR, Jones TE, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 2002; 16(14):1879–86. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber SN, Emter R, Hock MB, et al. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)–induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. U. S. A 2004; 101(17):6472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp. Gerontol 2010; 45(2):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greaves LC, Nooteboom M, Elson JL, et al. Clonal expansion of early to mid-life mitochondrial DNA point mutations drives mitochondrial dysfunction during human ageing. PLoS Genet 2014; 10(9):e1004620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross JM, Coppotelli G, Branca RM, et al. Voluntary exercise normalizes the proteomic landscape in muscle and brain and improves the phenotype of progeroid mice. Aging Cell 2019; 18(6):e13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic. Biol. Med 2011; 51(5):942–50. [DOI] [PubMed] [Google Scholar]
- 26.Marosi K, Bori Z, Hart N, et al. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience 2012; 226:21–8. [DOI] [PubMed] [Google Scholar]
- 27.Camell CD, Yousefzadeh MJ, Zhu Y, et al. Senolytics reduce coronavirus-related mortality in old mice. Science 2021; 373(6552):eabe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med 2018; 24(8):1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catoire M, Kersten S. The search for exercise factors in humans. FASEB J 2015; 29(5):1615–28. [DOI] [PubMed] [Google Scholar]
- 30.Kinugawa T, Kato M, Ogino K, et al. Interleukin-6 and tumor necrosis factor-alpha levels increase in response to maximal exercise in patients with chronic heart failure. Int. J. Cardiol 2003; 87(1):83–90. [DOI] [PubMed] [Google Scholar]
- 31.Schafer MJ, White TA, Evans G, et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 2016; 65(6):1606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Englund DA, Sakamoto AE, Fritsche CM, et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 2021; 20:e13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med 2018; 215(5): 1287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ 2005; 172(9):1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J. Appl. Physiol 2007; 103(3):1093–8. [DOI] [PubMed] [Google Scholar]
- 36.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab 2014; 25(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med 2014; 16(1): 161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton DGA, Stolzing A. Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res. Rev 2018; 43:17–25. [DOI] [PubMed] [Google Scholar]
- 39.Peake JM, Neubauer O, Della Gatta PA, Nosaka K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol 2017; 122(3): 559–70. [DOI] [PubMed] [Google Scholar]
- 40.Walton RG, Kosmac K, Mula J, et al. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci. Rep 2019; 9(1):969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen HB, Secher NH, Christensen NJ, Pedersen BK. Lymphocytes and NK cell activity during repeated bouts of maximal exercise. Am. J. Physiol 1996; 271(1 Pt 2):R222–7. [DOI] [PubMed] [Google Scholar]
- 42.Simpson RJ, Cosgrove C, Chee MM, et al. Senescent phenotypes and telomere lengths of peripheral blood T-cells mobilized by acute exercise in humans. Exerc. Immunol. Rev 2010; 16:40–55. [PubMed] [Google Scholar]
- 43.Simpson RJ, McFarlin BK, McSporran C, Spielmann G, ó Hartaigh B, Guy K. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav. Immun 2009; 23(2):232–9. [DOI] [PubMed] [Google Scholar]
- 44.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J. Leukoc. Biol 2008; 84(5):1271–8. [DOI] [PubMed] [Google Scholar]
- 45.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol 2019; 19(9):563–72. [DOI] [PubMed] [Google Scholar]
- 46.Pereira BI, Devine OP, Vukmanovic-Stejic M, et al. Senescent cells evade immune clearance via HLA-E–mediated NK and CD8+ T cell inhibition. Nat. Commun 2019; 10:2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muñoz DP, Yannone SM, Daemen A, et al. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight 2019; 5(14):e124716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim KM, Noh JH, Bodogai M, et al. Identification of senescent cell surface targetable protein DPP4. Genes Dev 2017; 31(15):1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amor C, Feucht J, Leibold J, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020; 583(7814):127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J. Am. Coll. Cardiol 2008; 52(6):470–82. [DOI] [PubMed] [Google Scholar]
- 51.Kröller-Schön S, Jansen T, Hauptmann F, et al. α1AMP-activated protein kinase mediates vascular protective effects of exercise. Arterioscler. Thromb. Vasc. Biol 2012; 32(7):1632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pini M, Czibik G, Sawaki D, et al. Adipose tissue senescence is mediated by increased ATP content after a short-term high-fat diet exposure. Aging Cell 2021; 20(8):e13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimura M, Suzuki S, Moriya A, et al. The effects of continuous and withdrawal voluntary wheel running exercise on the expression of senescence-related genes in the visceral adipose tissue of young mice. Int. J. Mol. Sci 2020; 22(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bianchi A, Marchetti L, Hall Z, et al. Moderate exercise inhibits age-related inflammation, liver steatosis, senescence, and tumorigenesis. J. Immunol 2021; 206(4):904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang Y, Kwon I, Cosio-Lima L, Wirth C, Vinci DM, Lee Y. Endurance exercise prevents metabolic distress-induced senescence in the hippocampus. Med. Sci. Sports Exerc 2019; 51(10):2012–24. [DOI] [PubMed] [Google Scholar]
- 56.Englund DA, Zhang X, Aversa Z, LeBrasseur NK. Skeletal muscle aging, cellular senescence, and senotherapeutics: current knowledge and future directions. Mech. Ageing Dev 2021; 200:111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon KJ, Zhang D, Kim SJ, Lee MC, Moon HY. Exercise-induced AMPK activation is involved in delay of skeletal muscle senescence. Biochem. Biophys. Res. Commun 2019; 512(3):604–10. [DOI] [PubMed] [Google Scholar]
- 58.Saito Y, Chikenji TS, Matsumura T, Nakano M, Fujimiya M. Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors. Nat. Commun 2020; 11(1):889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen XK, Yi ZN, Wong GT, et al. Is exercise a senolytic medicine? A systematic review. Aging Cell 2021; 20(1):e13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 2009; 8(4):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song Z, von Figura G, Liu Y, et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell 2010; 9(4):607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. Plos One 2010; 5(5):e10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pustavoitau A, Barodka V, Sharpless NE, et al. Role of senescence marker p16 INK4a measured in peripheral blood T-lymphocytes in predicting length of hospital stay after coronary artery bypass surgery in older adults. Exp. Gerontol 2016; 74:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossman MJ, Kaplon RE, Hill SD, et al. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Heart Circ. Physiol 2017; 313(5):H890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Justice JN, Gregory H, Tchkonia T, et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci 2018; 73(7):939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohli J, Wang B, Brandenburg SM, et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat. Protoc 2021; 16(5):2471–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schafer MJ, Zhang X, Kumar A, et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 2020; 5(12):e133668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeBrasseur NK, de Cabo R, Fielding R, et al. Identifying biomarkers for biological age: Geroscience and the ICFSR Task Force. J. Frailty Aging 2021; 10(3):196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smitherman AB, Wood WA, Mitin N, et al. Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16INK4a and frailty. Cancer 2020; 126(22):4975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


