Abstract
A new type of immunogenic molecule was engineered by replacing all three complementarity-determining-region (CDR) loops of the human immunoglobulin (Ig) heavy-chain variable (VH) domain with the Taenia crassiceps epitope PT1 (PPPVDYLYQT) and by displaying this construct on the surfaces of M13 bacteriophage. When BALB/c mice were immunized with such phage particles (PIgphage), a strong protection against challenge infection in very susceptible female hosts was obtained. When specifically stimulated, the in vivo-primed CD4+ and CD8+ T cells isolated from mice immunized with PT1, both as a free peptide and as the PIgphage construct, proliferated in vitro, indicating efficient epitope presentation by both major histocompatibility complex class II and class I molecules in the specifically antigen-pulsed macrophages used as antigen-presenting cells. These data demonstrate the immunogenic potential of recombinant phage particles displaying CDR epitope-grafted Ig VH domains and establish an alternative approach to the design of an effective subunit vaccine for prevention of cysticercosis. The key advantage of this type of immunogen is that no adjuvant is required for its application. The proposed strategy for immunogen construction is potentially suitable for use in any host-pathogen interaction.
Over the last few years, M13 and other filamentous phages have been used as expression vectors in which foreign gene products are fused to the phage coat proteins and are displayed on the surfaces of the phage particles. Phage-displayed peptide (9, 25) and antibody (Ab) (1, 36) libraries have been widely used in numerous studies. One of the important properties of phage particles is their high immunogenicity in different animal systems, and the use of genetically engineered filamentous phages as antigens for Ab production has been reported (14, 23). There is, however, a single study in which a recombinant phage displaying a disease-specific protective B-cell epitope was used as a vaccine to confer protection against human respiratory syncytial virus infection in mice (2). Also, the phage particles displaying recombinant anti-idiotypic Ab ScFv (single-chain fragment-variable) fragments expressed on the phage were used in maternal immunization, protecting neonatal mice against streptococcal infection (18).
Recently, Abs carrying antigenic peptides grafted into their complementarity-determining-region (CDR) loops at the immunoglobulin (Ig) heavy-chain variable (VH) region have been shown to be highly immunogenic and to serve as a very efficient vehicle to load the inserted epitopes onto major histocompatibility complex (MHC) molecules after processing by antigen-presenting cells (APC) (7, 37, 39, 41). Thus, it has been shown that a T-cell epitope of influenza virus nucleoprotein inserted into the CDR3 loop of the VH region of Ig was able to prime the virus-specific T cells in vivo (38). When influenza virus T- and B-cell epitopes were introduced into the CDR2 and CDR3 loops of the Ig VH domain, respectively, the DNA-immunized mice were protected against challenge with lethal doses of the virus (8).
So, taking advantage of the observations that Abs carrying T-cell epitopes inserted into CDR3 or CDR2 loops of the Ig VH domain and phages displaying a B-cell epitope or anti-idiotypic Ab ScFv fragment are strong immunogens, we have developed a new concept for immunogen construction, designing a human Ig VH domain grafted to a 10-amino-acid T-cell epitope, PT1, from the Taenia crassiceps antigen KETc7 (20) displayed on the M13 phage surface. The resulting PIgphage construct was used to immunize mice against experimental T. crassiceps cysticercosis, the simple disease model for testing candidate vaccine preparations against Taenia solium pig and human cysticercosis—a highly damaging and prevalent parasitic disease in the third world (20). To our knowledge, there is no report of the use of recombinant bacteriophages expressing any T-cell epitope alone or in the context of antigenized Abs or their fragments as immunogens. In our study, the mice immunized with the free synthetic T-cell epitope or with PIgphage developed a strong specific cellular immune response and resistance to challenge infection. The results point to this PT1 epitope as a promising vaccine candidate against cysticercosis and to the Ig VH-phage construct as an effective and inexpensive strategy for large-scale production of vaccines against various diseases.
MATERIALS AND METHODS
Immunogen construction.
A set of partially overlapping oligonucleotides collectively coding for the framework regions of the human Ig VH domain DP47 (OL.1, -3, -5, -6, and -8) (34) and the T. crassiceps T-cell epitope PT1 (PPPVDYLYQT) (OL.2, -4, and -7) was synthesized at Operon Technologies, Inc., Alameda, Calif. The oligonucleotides used were as follows: OL.1, GAGGTGCAGC TG T TGGAG TCTGGGGGAGGC T TGG TACAGCC TGGGGGG TCCCTGAGACTCTCCTGTGCA; OL.2 (PT1/H1), GCCTGGCGGACCCATGTCTGG TACAGATAATCAAC TGGCGG TGG TGCACAGGAGAG TC T; OL.3, TGGGTCCGCCAGGCTCCAGGGAAGGGGCTGGAGTGGGTCTCA; OL.4 (PT1/H2), GCCCTTCACGGAGTCTGTCTGGTACAGATAATCAACTGGCGGTGGTGGTGAGACCCACTCCA; OL.5, GACTCCGTGAAGGGCCGGTTCACCATCTCCAGAGACAAT TCCAAGAACACGC TG TATC TGCAAATGAAC; OL.6, ACGCTGTATCTGCAAATGAACAGCCTGAGAGCCGAGGACACGGCCGTATATTACTGTGCG; OL.7 (PT1/H3), GCCGTATATTACTGTGCGCCACCGCCAGTTGATTATCTGTACCAGACATGGGCCAGGGAACCCTGGTC; OL.8, TGGGGCCAGGGAACCCTGGTCACCGTCTCCTCA; 5′VR, GATGAATTCTGAGGTGCAGCTGTTGGAGTCTGG; and 3′VS, CTCGTCGACACGGTGACCAGGGTTCCCTGGCCC. Oligonucleotides 1 to 8 listed above (4 pmol each; the overlaps between the complementary oligonucleotides are 14 to 21 nucleotides) were combined and assembled in PCR (27) with Pfu DNA polymerase (Stratagene, La Jolla, Calif.) by cycling the reaction mixture (50 μl) 30 times (95°C for 2 min; 56°C for 2 min; 72°C for 1 min). An aliquot from this reaction (5 μl), containing a 350-bp DNA fragment coding for the Ig VH domain with the CDRs replaced by PT1 sequence, was amplified by PCR (50 μl) by cycling 30 times (94°C for 1 min; 65°C for 1 min; 72°C for 1 min) with the 5′VR and 3′VS primers (30 pmol each), which introduce EcoRI and SalI restriction sites at the 5′ and 3′ ends of the synthesized Ig VH domain, respectively. The assembly and amplification of PCR products were checked by agarose gel electrophoresis, and the DNA of the engineered VH domains, after purification from the gel with a Master Kit (Bio-Rad Laboratories, Hercules, Calif.), was cut with EcoRI and SalI (Stratagene) and purified again. Then, 1 μg of this DNA was ligated with 10 U of T4 DNA ligase (Amersham-Life Science, Cleveland, Ohio) to approximately 1 μg of EcoRI- and SalI-digested DNA of the pFACIB3 phagemid vector (kindly provided by J. Gavilondo) to fuse Ig VH in frame with M13 minor coat protein III (cpIII). The competent XL-1 Blue cells (Stratagene) were transformed with the ligation mixture, and the PIgphage phagemid clone obtained was rescued and amplified with helper phage VCS-M13 (Stratagene) as described previously (24). The correct PCR assembly and cloning were verified by dideoxy sequencing with [α-35S]dATP (Amersham) and the T7 Sequenase Quick-Denature plasmid sequencing kit (Amersham). Also, the same Ig VH domain gene with the CDRs replaced by PT1 sequence was cloned under the cytomegalovirus promoter into the eukaryotic expression vector pcDNA3 (Invitrogen) at EcoRI/SalI restriction sites, resulting in the pcDVH18 clone.
A nonrelated phage (NF) was previously isolated from a phage display heptapeptide library; it contains an autoimmune thrombocytopenic purpura-related epitope (ATSAIHG) displayed on the M13 phage surface (11).
Synthetic peptide.
To predict T-cell epitopes in T. crassiceps KETc7 protein (20), the method described by Margalit et al. (22) was used and the sequence PPPVDYLYQT with the highest amphipathic score was selected. The peptide PT1 (AAPPPVDYLYQTA) was prepared by stepwise solid-phase synthesis by the Nα-tert-BOC strategy, essentially as described previously (12). Purification was carried out by reverse-phase high-performance liquid chromatography on a Waters (Milford, Mass.) Delta Pak C18 column (7.8 by 150 mm) with a linear gradient (water-acetonitrile in 0.1% trifluoroacetic acid). The correct amino acid sequence was confirmed by protein sequencing on a pulsed liquid-phase protein sequencer (Applied Biosystems) at the Instituto Nacional de Cardiología by F. Masso. The molecular weight of the peptide was determined by fast-atom bombardment mass spectrometry on a JEOL JMS-SX102A mass spectrometer at the Instituto de Quimica, Universidad Nacional Autonoma de Mexico (Mexico D.F., Mexico) by L. Velasco.
Immunization and protection assays.
Four- to 6-week-old female BALB/c mice, originally purchased from Jackson Laboratories (Bar Harbor, Maine) and maintained at our animal facilities, were used. On days 0, 14, and 28, groups of 7 or 10 mice were immunized by intradermal (i.d.) inoculation and then boosted two times intraperitoneally (i.p.) with PIgphage (2 × 1010 phage), NF (2 × 1010 phage), Tris-buffered saline (TBS), or PT1 peptide (50 μg) in a 200-μl volume. Groups of six mice were immunized by single i.d. inoculations with PIgphage and NF. PT1 was administered in complete Freund’s adjuvant with two boosts in incomplete Freund’s adjuvant, and the phages were applied in TBS. Fourteen days after the last inoculation, the mice were challenged with T. crassiceps cysticerci, and 7 weeks later the individual parasite load in the peritoneal cavity of each mouse was counted as described previously (20). The data presented are representative of two experiments performed.
Separately, groups of five mice were immunized by single i.d. inoculations with PIgphage, NF, or PT1 peptide and with 100 μg of total antigen extract of T. crassiceps (TAg) (20) and used in lymphoproliferation assays. Also, sera from T. crassiceps-infected and noninfected mice were obtained and used in enzyme-linked immunosorbent assays (ELISAs).
Lymphoproliferation and cytokine assays.
Peritoneal macrophages (Mφ) were used as APC in all cell cultures, as previously reported (21). Briefly, Mφ were elicited in healthy female BALB/c mice by i.p. injection with 3 ml of 3% thioglycolate medium. Four days later, the mice were killed and Mφ were isolated by washing the peritoneal cavity with cold phosphate-buffered saline (PBS), following the procedure described elsewhere (19). The Mφ were scraped, adjusted to 106/ml, and seeded in the presence of PT1 (50 μg/ml), TAg (50 μg/ml), phage particles (2 × 109), pcDVH18 plasmid DNA (10 μg), or pcDNA3 plasmid DNA (10 μg). The plasmid DNAs were isolated with a Plasmid Midi kit (Qiagen Inc., Chatsworth, Calif.). After 3 h of incubation at 37°C in 5% CO2, the Mφ were scraped again and adjusted to 106/ml, and 100 μl of this suspension was seeded in flat-bottomed 96-well plates (Falcon, Oxnard, Calif.). The Mφ were cocultured with pools of CD4+ or CD8+ splenic cells (5 × 104) isolated from the mice (five mice per group) 10 days after the single inoculations with corresponding antigens, as described above. These cells were magnetically isolated with anti-CD4 and anti-CD8 monoclonal antibodies (PharMingen, San Diego, Calif.) bound to ferritin on a magnetic column (Miltenui Biotec, Bergisch Gladbach, Germany), as recommended by the manufacturer. Typically, the cell preparations were >90% pure as determined by staining and flow cytometric analysis. Plates were cultured in triplicate for 5 days, and 18 h before harvesting, 0.5 μCi of tritiated thymidine ([methyl-3H]TdR; specific activity, 247.9 GBq/mmol; NEN, Boston, Mass.) was added to the wells. The radioactivity in pelleted cells was measured with a Betaplate scintillation counter (Wallac). The results were expressed as total counts per minute. The levels of cytokines (gamma interferon [IFN-γ], interleukin-2 [IL-2], and IL-4) produced by T cells after 72 h of incubation with the corresponding antigen-pulsed Mφ were measured by sandwich ELISA with cytokine-specific monoclonal antibodies (PharMingen) according to the manufacturer’s instructions. The tests were done in duplicate. The data presented are representative of two experiments performed.
ELISA.
The levels of T. crassiceps- and phage-specific Abs in immune sera pooled from mice within each group were measured by ELISA essentially as described previously (11, 12). Briefly, flat-bottomed microtitration plates (96 well; Nunc, Roskilde, Denmark) were coated (in duplicate) with T. crassiceps TAg or PT1 peptide diluted in 0.2 M carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. Separate plates were coated with PIgphage or NF (109 phage/well) and incubated overnight at 4°C. After being washed with PBS, the plates were incubated with test sera diluted in PBS (1:20) containing 1% bovine serum albumin at 37°C for 1 h. Washing with PBS was repeated, and alkaline phosphatase-conjugated anti-mouse IgG (whole molecule) (Sigma, St. Louis, Mo.) was added. The subsequent reaction with p-nitrophenyl phosphate substrate (Sigma) in diethanolamine buffer (pH 9.8) was stopped by addition of 2 N NaOH. The absorbance was read at 405 nm with an automated ELISA reader. Another ELISA was performed with plates coated with murine sera diluted in carbonate-bicarbonate buffer. After overnight incubation at 4°C, the plates were washed with PBS and PIgphage or NF (109 phage/well) was added. The plates were incubated overnight at 4°C and washed with PBS, and rabbit anti-M13 serum (Stratagene) diluted in PBS (1:5,000) was added. After incubation at 37°C and washing with PBS, alkaline phosphatase-conjugated anti-rabbit IgG (whole molecule) was added, followed by reaction with p-nitrophenyl phosphate. The absorbance was read as described above.
Statistical analysis.
Parasite load data were analyzed for homoscedasticity by Levene’s test. Analysis of variance was then applied, considering all of the groups tested. Pairwise comparisons were made with Tukey’s post hoc test.
RESULTS
Engineering phage particles carrying PT1 grafted into the Ig VH domain.
First, the T-cell epitope PT1 (PPPVDYLYQT) was predicted (with GeneWorks from IntelliGenetics, Campbell, Calif.) by analyzing the amino acid sequence of the T. crassiceps proline-rich protective antigen KETc7 isolated from a cDNA expression library (20). The algorithm of Margalit et al. (22) was used, and the calculated amphipathic score was highest for the PT1 sequence.
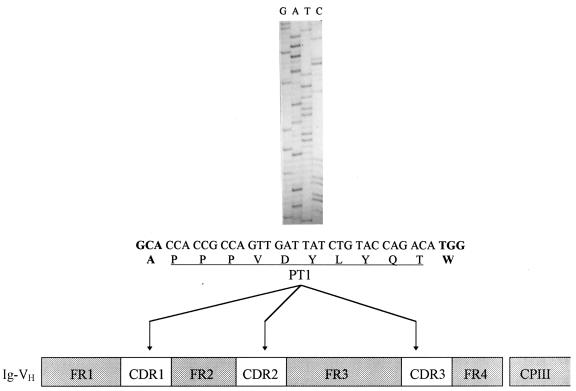
The general strategy of immunogen construction is summarized in Fig. 1. A set of synthetic overlapping oligonucleotides based on the DNA sequences of the framework regions of the human Ig germ line DP47 segment were used in PCR assembly to construct an entirely synthetic Ig VH domain carrying a 30-bp DNA fragment which codes for a PT1 epitope inserted in all three VH CDR loops. After PCR reamplification with 5′ and 3′ flanking primers carrying restriction sites, the antigenized VH segment was cloned into the EcoRI and SalI sites of the phagemid vector pFACIB3, resulting in the PIgphage clone, in which the VH domain was fused in frame to phage cpIII. The correct PCR assembly and cloning were verified by DNA sequencing. The nucleotide sequence of the CDR-grafted PT1 epitope and a diagram of the VH-PT1 chimeric polypeptide are shown in Fig. 1. Using the rescue procedure, the PIgphage clone was amplified and the phage particles were directly used in immunization trials. In parallel, the same Ig VH domain containing CDRs replaced by the PT1 epitope was cloned into the eukaryotic expression vector pcDNA3 (Invitrogen) by using EcoRI/SalI sites to obtain pcDVH18, which was used to pulse Mφ in a T-cell proliferation assay.
FIG. 1.
Schematic presentation of the engineered PIgphage immunogen. Oligonucleotides coding for the PT1 epitope (underlined) were inserted by PCR assembly into the CDRs of the Ig VH domain and cloned into the phagemid vector as a fusion with cpIII (see Materials and Methods). The correct PCR assembly was verified by DNA sequencing, and the sequence of the PT1 epitope, inserted into the CDR1 loop, is shown. Indicated in boldface letters are the alanine and tryptophan residues from the FR1 and FR2 regions, respectively. FR, immunoglobulin heavy-chain variable-domain framework region.
To evaluate the immunogenicity of the T. crassiceps epitope as a free peptide, the PT1 peptide was produced by solid-phase synthesis as reported previously (12).
Immunization and protection assays.
In order to analyze the protective potential of the PT1 epitope integrated in a PIgphage construct and as the free peptide, female BALB/c mice were immunized and tested in challenge experiments. Ten mice were immunized i.d. with 2 × 1010 PIgphage particles without adjuvant and boosted two times with the same immunogen by the i.p. route at 2-week intervals. The free peptide was injected i.d. in complete Freund’s adjuvant, and the animals were boosted two times with PT1 in incomplete Freund’s adjuvant. Mice from control groups were injected in the same way with NF and TBS alone. Two weeks after the last injection, the mice were challenged i.p. with T. crassiceps cysticerci, and 7 weeks later the level of protection was estimated by counting the individual parasite load in the peritoneal cavity of each mouse as described in detail elsewhere (20). Separately, mice were immunized with TAg, and T cells from these mice were used later in lymphoproliferation assays. These mice were not challenged with the parasite.
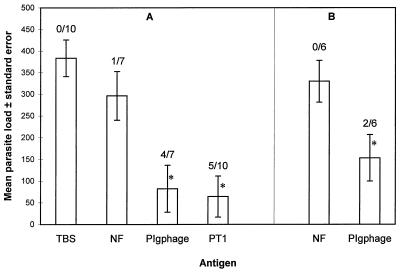
As shown in Fig. 2A, very similar protection effects were observed with the same epitope in both forms of presentation, as the free peptide and as PIgphage. Five of 10 and 4 of 7 mice immunized with PT1 peptide and the PIgphage construct, respectively, were completely protected against challenge, while in the rest of the immunized mice, with a single exception in both cases, a dramatic reduction in parasite load was obtained (80%) (Fig. 2A). Although few animals were tested, a single i.d. inoculation with PIgphage also induced strong protection against parasite challenge (Fig. 2B). In the mice immunized with the nonrelated phage NF, only a slight reduction in parasite load was observed compared to that in the TBS-immunized mice.
FIG. 2.
Protection against cysticercosis in immunized mice. The individual parasite loads were counted in mice immunized with corresponding antigen 7 weeks after challenge with T. crassiceps cysticerci. The number of completely protected mice of the total number of mice tested in each group is shown above each bar. (A) On days 0, 14, and 28, groups of mice were immunized by i.d. inoculation and boosted two times i.p. with PIgphage (2 × 1010 phage), nonrelated phage NF (2 × 1010 phage), and buffer solution (TBS) or free peptide (PT1) (50 μg) in a 200-μl volume. Fourteen days after the last inoculation, the mice were challenged with T. crassiceps cysticerci. (B) Mice were immunized by a single i.d. inoculation with PIgphage (2 × 1010 phage) and NF (2 × 1010 phage). Fourteen days later, the mice were challenged with T. crassiceps cysticerci. ∗, contrast with the negative controls (immunized with buffer and nonrelated phage) is statistically significant (P < 0.05).
Immune response to vaccination.
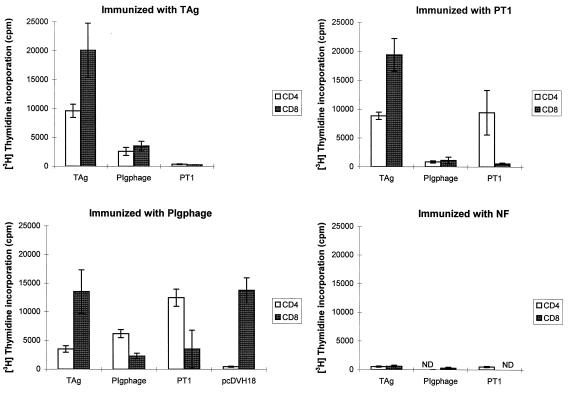
To study the type of protective immune response induced by the PT1 epitope in both forms of presentation and to find out whether immunization elicited a T-cell-proliferative response to this epitope, spleen lymphocytes and sera were obtained from the immunized mice (five mice in each group). CD4+ and CD8+ T cells were separated and tested in in vitro-proliferation assays to determine whether these in vivo-primed lymphocytes could be stimulated with the antigens. In general, proliferation was observed when CD4+ and CD8+ T cells from mice immunized with PT1 peptide, PIgphage, and TAg were stimulated in vitro with the same immunogens, with Mφ as the APC (Fig. 3). In contrast, no proliferation was observed in T cells isolated from mice immunized with NF phage (Fig. 3) or when T cells were cultured with NF-pulsed Mφ (data not shown). The levels of efficiency of the epitope presentation, however, differed for the three antigens. In almost all cases, the most efficient antigen was TAg, although for T cells from PIgphage-immunized mice, PIgphage and PT1 peptide were better stimulators for CD4+ cells, and CD8+ cells proliferated equally upon stimulation by TAg and pcDVH18 DNA. The nonresponsiveness of T cells from TAg-immunized mice to PT1 peptide stimulation possibly indicates the relative insignificance of the PT1-specific immune response when a complex antigen mixture is used as the immunogen. In contrast, the same cells respond positively to stimulation with the same epitope on a PIgphage carrier, indicating that the PT1 epitope as part of a PIgphage particle is more efficiently processed and presented to T cells by Mφ than as a free peptide. Similarly, PIgphage and PT1 peptide were stimulatory for the T cells from PIgphage-immunized mice but not for the T cells from PT1 peptide-primed mice stimulated with the same antigens, except for CD4+ cells stimulated with the peptide. Mφ were also pulsed with pcDVH18 plasmid DNA carrying the VH domain with CDRs grafted by the PT1 epitope to test the presentation of endogenously synthesized epitope. As expected, in this case the PT1 epitope was effectively presented by MHC class I molecules to CD8+ T cells but not to CD4+ T cells from PIgphage-immunized mice, as demonstrated in the T-cell proliferation assay (Fig. 3). The same T cells from PIgphage-immunized mice were cocultured with Mφ pulsed by pcDNA3 vector plasmid DNA as a control and did not proliferate (data not shown).
FIG. 3.
T-cell proliferation assay. The pools of CD4+ and CD8+ T cells (5 × 104) were isolated from the spleens of mice immunized with various antigens (five mice in each group) and were cultured for 5 days with intact Mφ pulsed in vitro with T. crassiceps TAg (50 μg/ml), recombinant PIgphage (2 × 109), synthetic PT1 peptide (50 μg/ml), and pcDVH18 plasmid DNA (10 μg). T cells not cultured with Mφ have shown a basal level of [3H]thymidine incorporation of <500 cpm. Each point represents the mean of determinations from triplicate wells ± standard deviation. ND, not determined.
Cytokine production was measured in supernatants of cultured T cells with different immunization protocols. In most cases, CD4+ and CD8+ T cells produced high levels of IFN-γ after stimulation by Mφ pulsed with TAg, PIgphage, and PT1 peptide (Table 1). Although the PIgphage- and PT1 peptide-stimulated CD8+ cells from PIgphage-immunized mice showed less proliferation than the CD4+ cells (Fig. 3), they produced more IFN-γ. The highest levels of IFN-γ were produced by PIgphage-stimulated CD4+ cells from TAg- and PT1-immunized mice and by PIgphage-stimulated CD8+ cells from TAg-immunized mice, while the lowest levels were detected in supernatants of NF-stimulated CD4+ cells. Surprisingly, low levels of IFN-γ were produced by PIgphage- and TAg-stimulated CD4+ cells from mice immunized with the respective antigens (Table 1). In the same supernatants, IL-4 was not detected and IL-2 was found only when CD8+ cells from PIgphage-immunized mice and CD4+ cells from PT1-immunized mice were cultured with PIgphage-pulsed Mφ (data not shown).
TABLE 1.
IFN-γ production by antigen-stimulated T cellsa
| Mice immunized with: | IFN-γ production (pg/ml) by:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4+ cells stimulated with:
|
CD8+ cells stimulated with:
|
|||||||
| PIgphage | TAg | PT1 | NF | PIgphage | TAg | PT1 | NF | |
| PIgphage | 411 | 2,504 | 1,130 | 474 | 1,362 | ND | 2,081 | 1,003 |
| TAg | 4,051 | 138 | 491 | 103 | 2,588 | 2,546 | 1,700 | <50 |
| PT1 | 3,011 | 2,948 | 72 | 116 | 85 | <50 | <50 | 138 |
| NF | <50 | <50 | <50 | <50 | ND | ND | ND | ND |
The pools of CD4+ and CD8+ cells (5 × 104) from mice (five mice in each group) immunized with corresponding antigen were cultured in the presence of Mφ stimulated with different antigens (see the legend to Fig. 2). Supernatants from duplicate cultures were harvested after 72 h and assayed for IFN-γ production. ND, not determined.
The levels of T. crassiceps-specific Abs in pooled sera from the immunized mice was measured by ELISA (12). As shown in Table 2, no positive signal was detected after incubation of the antigens containing the PT1 epitope (PT1, TAg, and PIgphage) with sera from PIgphage-immunized mice. Similar results were obtained with ELISA plates coated with the respective antisera and incubated in the presence of PIgphage (Table 2). In contrast, high levels of phage-specific antibodies were detected in sera of PIgphage and NF-immunized mice (Table 2), confirming the high immunogenicity of the phage itself. Interestingly, the same high levels of phage-specific Abs were detected in two different ELISA protocols: when plates were coated with PIgphage particles and incubated with the sera from PIgphage- and NF-immunized mice and when the plates were coated with the same sera and incubated in the presence of phages (Table 2).
TABLE 2.
Antibody response in immunized micea
| Plate coated with | Response when incubated with:
|
|||||
|---|---|---|---|---|---|---|
| Normal serum | Positive serum | Anti-PIgphage | Anti-NF | PIgphage | NF | |
| PT1 | 0.184 | 0.248 | 0.135 | 0.168 | ||
| TAg | 0.554 | 2.258 | 0.469 | 0.547 | ||
| PIgphage | 0.484 | 0.366 | 1.856 | 1.720 | ||
| Normal serum | 0.289 | 0.525 | ||||
| Anti-PIgphage | 1.624 | 1.627 | ||||
| Anti-TAg | 0.247 | 0.533 | ||||
| Anti-NF | 1.929 | 1.855 | ||||
The presence of specific antibodies was measured by ELISA as described in Materials and Methods. Sera from T. crassiceps-infected and noninfected mice were used as positive and negative controls, respectively. Phage particles (PIgphage and NF) were used at 2 × 109. Sera used were diluted 1:20. Anti-PIgphage, anti-TAg, and anti-NF are pooled sera from mice immunized with the corresponding antigen (five mice in each group).
DISCUSSION
The principal goal of the present research was to explore the immunogenic capacity of the Ig VH domain with CDRs replaced by a pathogen-specific epitope and expressed as a fusion product coupled to cpIII on the M13 phage surface. To achieve this aim, an entirely synthetic Ig VH segment was engineered by PCR assembly by simultaneously inserting a T-cell epitope, PT1 (PPPVDYLYQT), from T. crassiceps recombinant antigen KETc7 previously isolated from a cDNA expression library (20) into all of the CDRs of the VH domain (Fig. 1). In KETc7 proline-rich antigen (29% proline), prolines are tandemly repeated and are possibly involved in multiple epitopes. Generally, the proline-containing peptides are involved in many immunologically important phenomena and are commonly present in proteins at solvent-exposed sites, such as loops and turns (29), and VH loops seemed to be suitable sites for the introduction of peptide epitopes. According to our experimental design, we were expressing only the Ig VH domain, so altering the CDR structure by peptide insertion was not problematic, as it could be when expressing Fab fragments or the whole Ig molecule, where changes in CDRs can cause misfolding of Ig chains (37). As an example of a successful application of phages for vaccination purposes, the construction of recombinant anti-idiotypic Ab ScFv fragments expressed on the phage was reported and the phage particles displaying this Ab ScFv fragment were used in maternal immunization, leading to the protection of neonatal mice against streptococcal infection (18). In a recent elegant work, CDR-like loops in the Ig constant-region domains were replaced with antigenic peptides, and the ability of the mutant Ig to stimulate CD4+ T cells, by both the endogenous and the exogenous routes for class II presentation, was demonstrated (17). Those authors have identified another attractive site for the insertion of epitopes: the segments between β-strands of Ig C-region domains, although only in vitro efficiency of the epitope presentation by APC to peptide-specific T-helper 1 (Th1) cells has been shown.
Experimental murine T. crassiceps cysticercosis is a well-characterized model to study immunological (31), genetic (10), and gender-associated (30) factors of resistance and susceptibility to this parasite. This experimental system was also widely used for the evaluation of different vaccine preparations, such as recombinant antigens (20), synthetic peptides (33), and naked DNA (21). In order to test the immunogenic properties of the PT1 epitope, female BALB/c mice extremely susceptible to T. crassiceps infection were used in vaccination trials, and more than 50% of the mice immunized with PIgphage and free PT1 peptide were completely protected against pathogen challenge while in the rest of the immunized mice, the parasite load was dramatically reduced (Fig. 2A). Moreover, we have demonstrated that a single inoculation with PIgphage is able to confer protection against challenge (Fig. 2B). The results clearly indicate the importance of the PT1 epitope in immune protection against cysticercosis.
The detected immune response reflects an additional interesting phenomenon. We have shown that CD4+ and CD8+ T cells from PT1-, PIgphage-, and TAg-immunized mice proliferated in vitro upon stimulation by Mφ pulsed with TAg, PT1, and PIgphage (Fig. 3). These data indicate that the exogenously applied T-cell epitope in different molecular contexts (PT1 peptide and PIgphage) was effectively processed and presented in vivo to CD8+ and CD4+ T cells in the context of both MHC class I and class II molecules, respectively, and the same epitope was presented on Mφ to stimulate the T cells in vitro. Importantly, the T cells from TAg-immunized mice proliferated in the presence of PIgphage and TAg was stimulatory for T cells from PIgphage-immunized mice, suggesting that the PT1 epitope displayed on PIgphage contributes to the development of protective immunity to this pathogen. The positive response of T cells from peptide-immunized mice to TAg stimulation is an additional support for this suggestion.
The presentation of the PT1 epitope to CD8+ cells in the context of PIgphage is rather surprising, because peptides from exogenous sources are usually effectively presented on MHC class II but not class I molecules (39). Probably, the PT1 epitope attached to the phage particle accesses a classical or alternative MHC class I processing pathway to be delivered to CD8+ cells (4), acting like some bacterial toxins capable of delivering catalytic protein moieties to the cytosol of eukaryotic cells (13). Interestingly, PT1 peptide was presented by Mφ to both CD4+ and CD8+ cells from PIgphage-immunized mice in lymphoproliferation assays, indicating association of this 13-amino-acid peptide with both MHC class I and class II molecules, although the loading of class II molecules was much more efficient than that of class I molecules. In general, naturally occurring peptides eluted from MHC class I molecules are composed of 8 to 10 amino acid residues, and short peptide fragments (8 to 15 amino acids) derived from antigen are bound to both classes of MHC molecules (3). With respect to MHC class II-mediated peptide presentation to CD4+ cells, the efficient loading of identical viral peptides onto MHC class II molecules by antigenized Ig and influenza virus applied exogenously has already been demonstrated (7). As expected, in our experiment, when Mφ were pulsed with plasmid DNA (pcDVH18) carrying antigenized Ig VH domain to generate PT1 epitopes endogenously, only MHC class I molecules appeared to be charged with the PT1 epitope, resulting in proliferation of CD8+ but not CD4+ T cells isolated from the PIgphage-immunized mice (Fig. 3). Although the mechanism by which synthetic peptides induce cell-mediated immunity or stimulate T cells is not clear, possibly they can bind directly to MHC class II molecules (6) or to class I molecules by direct penetration into the cytoplasm (15).
Our data indicate that we have preferentially induced a type 1 (T1) immune response with production of IFN-γ by both CD4+ and CD8+ T cells and the absence of IL-4. The data presented in this study support the previous observations concerning the role of the immune response and cytokines in murine cysticercosis. Thus, it was shown that neonatal thymectomy of mice greatly increases susceptibility to T. crassiceps infection and that T-cell replacement restores it to normal levels (5), whereas the bulk of antiparasite Abs were not clearly related to protection and might even enhance parasite growth (16).
Importantly, Th1-type response has been shown to be protective in cysticercosis, while Th2-type response is permissive in this parasitic disease, and a progressive shift from Th1- to Th2-type response was observed during the experimental infection in mice (31, 35). By immunization with the PIgphage novel immunogen, we were able to strengthen this balance in favor of a Th1-type response. Moreover, in a recent work we have shown that treatment of mice with monoclonal anti-IFN-γ Ab resulted in a dramatic increase in susceptibility to pathogen challenge (32), while the mice receiving recombinant IFN-γ and IL-2 showed a low parasite load. In contrast, IL-10 induced a significant increase in parasite load (32). In the present study, no correlation was observed between the proliferative response and IFN-γ production. Similar results were reported in human (28) and murine (26) cells. No parasite- or PT1-specific antibodies were detected in the sera of PIgphage- and PT1-immunized mice, indicating that in our case the humoral immune response is not participating in immunoprotection against murine cysticercosis. Based on the presented data, we can conclude that cell-mediated T1 immune response is probably involved in the induction of resistance to the T. crassiceps challenge. At present, the exact mechanism and the role of components of immunity responsible for the protection obtained in this study are not clear. Obviously, more experiments are necessary to clarify the issues mentioned above, as well as to determine the MHC restriction element (H-2 haplotype) and the duration of the immune response, which were beyond the scope of this study.
In conclusion, in this study we demonstrated the engineering and use of a new kind of immunogen: a phage-displayed epitope grafted into Ig heavy-chain CDRs that seems to favor a T1 immune response. We showed that a CDR-grafted epitope is effectively processed and presented in vitro by APC to T cells and confers protection against pathogen challenge. Probably, using any other T-cell epitope, such an immunogen could serve as a universal vehicle to target specific cellular immune responses. Furthermore, the proposed approach for vaccine development has clear advantages over other systems described, since it is highly cost-effective and simple to manage. Importantly, no adjuvant is required for this type of immunogen application, and although there are no available data concerning the safety of the bacteriophage for use in humans, we hope that their use in veterinary medicine as a vaccine platform will be practical. Finally, although the proposed vaccine development strategy was successfully tested in a murine cysticercosis model, its application in other disease models, especially when the induction of cellular immune response is desirable, seems promising, considering that the engineering of Ig molecules grafted with biologically relevant peptides is already a well-established technique.
ACKNOWLEDGMENTS
We appreciate the help of J. C. Almagro in the design of oligonucleotides, the support in epitope prediction and helpful discussion of R. Saavedra, the critical reading of the manuscript by C. Larralde, L. Padilla, and E. Sciutto, and the technical help of A. Buendia and J. Aviles.
REFERENCES
- 1.Barbas C F, Hu D, Dunlop N, Sawyer L, Cababa D, Hendry R M, Nara P L, Burton D R. In vitro evolution of a neutralizing human antibody to human immunodeficiency virus type 1 to enhance affinity and broaden strain cross-reactivity. Proc Natl Acad Sci USA. 1994;91:3809–3813. doi: 10.1073/pnas.91.9.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastien N, Trudel M, Simard C. Protective immune responses induced by the immunization of mice with a recombinant bacteriophage displaying an epitope of the human respiratory syncytial virus. Virology. 1997;234:118–122. doi: 10.1006/viro.1997.8632. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M J. Lymphocyte activation in health and disease. Crit Rev Immunol. 1997;17:155–178. doi: 10.1615/critrevimmunol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 4.Billetta R, Filaci G, Zanetti M. Major histocompatibility complex class I-restricted presentation of influenza virus nucleoprotein peptide by B lymphoma cells harboring an antibody gene antigenized with the virus peptide. Eur J Immunol. 1995;25:776–783. doi: 10.1002/eji.1830250323. [DOI] [PubMed] [Google Scholar]
- 5.Bojalil R, Terrazas L I, Govezensky T, Sciutto E, Larralde C. Thymus-related cellular immune mechanisms in sex associated resistance to experimental murine cysticercosis (Taenia crassiceps) J Parasitol. 1993;79:384–389. [PubMed] [Google Scholar]
- 6.Bona C A, Casares S, Brumeanu T D. Towards development of T-cell vaccine. Immunol Today. 1998;19:126–132. doi: 10.1016/s0167-5699(97)01218-8. [DOI] [PubMed] [Google Scholar]
- 7.Brumeanu T D, Swiggard W J, Steinman R M, Bona C A, Zaghouani H. Efficient loading of identical viral peptide onto class II molecules by antigenized immunoglobulin and influenza virus. J Exp Med. 1993;178:1795–1799. doi: 10.1084/jem.178.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casares S, Brumeanu T D, Bot A, Bona C A. Protective immunity elicited by vaccination with DNA encoding for a B cell and a T cell epitope of the A/PR/8/34 influenza virus. Viral Immunol. 1997;10:129–136. doi: 10.1089/vim.1997.10.129. [DOI] [PubMed] [Google Scholar]
- 9.Cwirla S E, Peters E A, Barrett R, Dower W J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci USA. 1990;87:6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fragoso G, Lamoyi E, Mellor A, Lomeli C, Hernandez M, Sciutto E. Increased resistance to Taenia crassiceps murine cysticercosis in Qa-2 transgenic mice. Infect Immun. 1998;66:760–764. doi: 10.1128/iai.66.2.760-764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gevorkian G, Manoutcharian K, Almagro J C, Govezensky T, Dominguez V. Identification of autoimmune thrombocytopenic purpura-related epitopes using a phage-display peptide library. Clin Immunol Immunopathol. 1998;86:305–309. doi: 10.1006/clin.1997.4502. [DOI] [PubMed] [Google Scholar]
- 12.Gevorkian G, Manoutcharian K, Larralde C, Hernandez M, Almagro J C, Viveros M, Sotelo J, Garcia E, Sciutto E. Immunodominant synthetic peptides of Taenia crassiceps in murine and human cysticercosis. Immunol Lett. 1996;49:185–189. doi: 10.1016/0165-2478(96)02503-5. [DOI] [PubMed] [Google Scholar]
- 13.Goletz T J, Klimpel K R, Arora N, Leppla S H, Keith J M, Berzofsky J A. Targeting HIV proteins to the major histocompatibility complex class I processing pathway with a novel gp120-anthrax toxin fusion protein. Proc Natl Acad Sci USA. 1997;94:12059–12064. doi: 10.1073/pnas.94.22.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenwood J, Willis A E, Perham R N. Multiple display of foreign peptides on a filamentous bacteriophage. Peptides from Plasmodium falciparum circumsporozoite protein as antigens. J Mol Biol. 1991;220:821–827. doi: 10.1016/0022-2836(91)90354-9. [DOI] [PubMed] [Google Scholar]
- 15.Hsu S-C, Shaw D M, Steward M W. The induction of respiratory syncytial virus-specific cytotoxic T-cell responses following immunization with a synthetic peptide containing a fusion peptide linked to a cytotoxic T lymphocyte epitope. Immunology. 1995;85:347–350. [PMC free article] [PubMed] [Google Scholar]
- 16.Kunz J, Baumeister S, Dennis R, Kuytz B, Wiegandt H, Geyer E. Immunological recognition of larval Taenia crassiceps glycolipids by sera from parasite-infected mice. Parasitol Res. 1991;77:443–447. doi: 10.1007/BF00931642. [DOI] [PubMed] [Google Scholar]
- 17.Lunde E, Bogen B, Sandlie I. Immunoglobulin as a vehicle for foreign antigenic peptides immunogenic to T cells. Mol Immunol. 1997;34:1167–1176. doi: 10.1016/s0161-5890(97)00143-0. [DOI] [PubMed] [Google Scholar]
- 18.Magliani W, Polonelli L, Conti S, Salati A, Rocca P F, Cusumano V, Mancuso G, Teti G. Neonatal mouse immunity against B streptococcal infection by maternal vaccination with recombinant anti-idiotypes. Nat Med. 1998;4:705–709. doi: 10.1038/nm0698-705. [DOI] [PubMed] [Google Scholar]
- 19.Manickan E, Kanangat S, Rouse R J D, Yu Z, Rouse B T. Enhancement of immune response to naked DNA vaccine by immunization with transfected dendritic cells. J Leukoc Biol. 1997;16:125–132. doi: 10.1002/jlb.61.2.125. [DOI] [PubMed] [Google Scholar]
- 20.Manoutcharian K, Rosas G, Hernandez M, Fragoso G, Aluja A, Villalobos N, Rodarte L F, Sciutto E. Cysticercosis: identification and cloning of protective recombinant antigens. J Parasitol. 1996;82:250–254. [PubMed] [Google Scholar]
- 21.Manoutcharian K, Terrazas L I, Gevorkian G, Govezensky T. Protection against murine cysticercosis using cDNA expression library immunization. Immunol Lett. 1998;62:131–136. doi: 10.1016/s0165-2478(98)00039-x. [DOI] [PubMed] [Google Scholar]
- 22.Margalit H, Spouge J L, Cornette J L, Cease K B, Delisi C, Berzofsky J A. Prediction of immunological helper T cell antigenic sites from the primary sequence. J Immunol. 1987;138:2213–2229. [PubMed] [Google Scholar]
- 23.Meola A, Delmastro P, Monaci P, Luzzago A, Nicosia A, Felici F, Cortese R, Galfre G. Derivation of vaccines from mimotopes. Immunologic properties of human hepatitis B virus surface antigen mimotopes displayed on filamentous phage. J Immunol. 1995;154:3162–3172. [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Scott J K, Smith G P. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 26.Skinner M A, Yuan S, Prestidge R, Chuk D, Watson J D, Tan P L J. Immunization with heat-killed Mycobacterium vaccae stimulates CD8+ cytotoxic T cells specific for macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1997;65:4525–4530. doi: 10.1128/iai.65.11.4525-4530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stemmer W P C, Crameri A, Ha K D, Brennan T M, Heyneker H L. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene. 1995;164:49–53. doi: 10.1016/0378-1119(95)00511-4. [DOI] [PubMed] [Google Scholar]
- 28.Surcel H M, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G, Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–176. [PMC free article] [PubMed] [Google Scholar]
- 29.Tchernychev B, Cabilly S, Wilchek M. The epitopes for natural polyreactive antibodies are rich in proline. Proc Natl Acad Sci USA. 1997;94:6335–6339. doi: 10.1073/pnas.94.12.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terrazas L I, Bojalil R, Govezensky T, Larralde C. A role for 17-b-estradiol in immunoendocrine regulation of murine cysticercosis (Taenia crassiceps) J Parasitol. 1994;80:563–568. [PubMed] [Google Scholar]
- 31.Terrazas L I, Bojalil R, Govezensky T, Larralde C. Shift from an early protective TH1-type immune response to a late permissive TH2-type response in murine cysticercosis (Taenia crassiceps) J Parasitol. 1998;84:74–81. [PubMed] [Google Scholar]
- 32.Terrazas L I, Cruz M, Rodriguez-Sosa M, Bojalil R, Garcia-Tamayo F, Larralde C. Th1-type cytokines improve resistance to murine cysticercosis caused by Taenia crassiceps. Parasitol Res. 1999;85:135–141. doi: 10.1007/s004360050522. [DOI] [PubMed] [Google Scholar]
- 33.Toledo A, Larralde C, Fragoso G, Gevorkian G, Manoutcharian K, Hernandez M, Acero G, Rosas G, Lopez-Casillas F, Kubli C, Vazquez R, Terrazas L I, Sciutto E. Towards a Taenia solium cysticercosis vaccine: an epitope shared by Taenia crassiceps and Taenia solium protects mice against experimental cysticercosis. Infect Immun. 1999;67:1086–1098. doi: 10.1128/iai.67.5.2522-2530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlinson I M, Walter G, Marks J D, Liewelyn M B, Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992;227:776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- 35.Villa O F, Kuhn R E. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1996;112:561–570. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- 36.Winter G, Griffiths A D, Hawkins R E, Hoogenboom H R. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 37.Xiong S, Gerloni M, Zanetti M. Engineering vaccines with heterologous B and T cell epitopes using immunoglobulin genes. Nat Biotechnol. 1997;15:882–886. doi: 10.1038/nbt0997-882. [DOI] [PubMed] [Google Scholar]
- 38.Zaghouani H, Kuzo Y, Kuzo H, Mann N, Daian C, Bona C. Engineered immunoglobulin molecules as vehicles for T cell epitopes. Int Rev Immunol. 1993;10:265–278. doi: 10.3109/08830189309061701. [DOI] [PubMed] [Google Scholar]
- 39.Zaghouani H, Kuzu Y, Kuzu H, Brumeanu T D, Swiggard W J, Steinman R M, Bona C A. Contrasting efficacy of presentation by major histocompatibility complex class I and class II products when peptides are administered within a common protein carrier, self immunoglobulin. Eur J Immunol. 1993;23:2746–2750. doi: 10.1002/eji.1830231104. [DOI] [PubMed] [Google Scholar]
- 40.Zaghouani H, Steinman R, Nonacs R, Shah H, Gerhard W, Bona C. Presentation of a viral T cell epitope expressed in the CDR3 region of a self immunoglobulin molecule. Science. 1993;259:224–227. doi: 10.1126/science.7678469. [DOI] [PubMed] [Google Scholar]
- 41.Zanetti M, Rossi F, Lanza P, Filaci G, Lee R H, Billetta R. Theoretical and practical aspects of antigenized antibodies. Immunol Rev. 1992;130:125–150. doi: 10.1111/j.1600-065x.1992.tb01524.x. [DOI] [PubMed] [Google Scholar]