ABSTRACT
Background and Aims:
Many pain syndromes such as chronic phantom limb pain (PLP) and stump pain (SP), involving nociceptive and neuropathic pain, develop after amputation. Recent literature suggests that the use of regional blocks reduces repeated stimulation of transected nerve roots and thus prevents central sensitisation. This randomised, double-blind study was conducted to evaluate the effect of pre-emptive ultrasound-guided single-shot lateral sciatic nerve block on the occurrence of chronic pain at six months after traumatic below-knee amputation.
Methods:
Thirty patients undergoing traumatic lower limb amputation under general anaesthesia were randomised into two groups: Group B received sciatic nerve block pre-emptively using ultrasound with 20 ml of 0.75% ropivacaine, whereas group C received 20 ml of normal saline. Follow-up of patients was done till six months post-amputation. The primary objective was to assess the occurrence of chronic pain at six months. Pain at 15 days and one month after surgery, post-operative morphine consumption and post-operative nausea and vomiting (PONV) were the secondary outcomes assessed.
Results:
The occurrence of PLP at six months was comparable in the two groups, group B (46.7%) and C (66.7%). None of the patients developed SP at six months. Median intensities of phantom pain were 1.0 (range, 1–2.0) versus 1.0 (range, 1–2.0) (P = 0.36), and median intensities of SP 2 (range, 2–3.0) versus 3 (range, 2–3.0) (P = 0.39) at 1 month.
Conclusion:
Pre-emptive sciatic nerve block did not decrease the occurrence or severity of chronic pain after traumatic below-knee amputation.
Keywords: Amputation, chronic pain, nerve block, phantom limb, pre-emptive, sciatic nerve, stump pain, ultrasound-guided
INTRODUCTION
Phantom limb pain (PLP) is a painful sensation of the absent limb, whereas stump pain (SP) represents pain in the residual limb. On an average, 60–80% of patients continue to have PLP in the first-year post-amputation.[1,2]
Chronic pain syndromes develop as a result of interaction between the peripheral, central and sympathetic nervous systems. Any transection at the level of peripheral nerves leads to the afferent nociceptive barrage, augmented excitation of dorsal horn cells in the spinal cord and reduction of inhibitory impulses leading to persistent post-surgical chronic pain.[3,4]
In the past, several modalities have been tried to prevent the phenomenon with variable results. Literature suggests that the use of central and peripheral nerve blocks in the prevention of central sensitisation shows promising results in decreasing chronic pain via a reduction in repeated stimulation of transected nerve roots.[5,6] Previous studies using peripheral nerve blocks and perineural infiltration, respectively, demonstrated a decrease in acute pain in the post-operative period.[7,8]
We hypothesised that the use of ultrasound-guided single-shot lateral sciatic nerve block pre-emptively would reduce the occurrence of chronic pain at six months after traumatic below-knee amputation. The primary outcome included the frequency and severity of chronic pain at six months. Pain at 15 days and one-month post-surgery, post-operative morphine consumption and occurrence of nausea and vomiting were the secondary outcomes recorded.
METHODS
After institutional ethics committee approval (NK/3055/MD/797) and written informed consent, this study was conducted over one year at the trauma operation theatre complex in a tertiary care hospital. The study was registered prospectively in the Clinical Trials Registry of India and followed all the principles of the Declaration of Helsinki. All trauma patients with American Society of Anesthesiologists physical status I and II in the age group of 18–60 years with isolated lower limb surgery requiring below-knee amputation were assessed for eligibility. Patients with crush injury, mangled extremity, revision of traumatic amputation, infected open wound and open fracture with distal neurovascular deficit were included. Patients with bilateral amputation, peripheral limb ischaemia, malignancy of foot, diabetic foot, pregnancy, coagulation disorder and head injury were excluded.
The patients were explained to report the intensity of pain on the numeric rating scale (NRS) (0–10), and also the use of patient-controlled analgesia (PCA) pump before surgery. They were allocated to one of the two groups; group B (block group) and group C (control group), using computer-generated random number tables, and numbers kept in opaque sealed envelopes. Group B received ultrasound-guided sciatic nerve block with 20 ml of 0.75% ropivacaine, and group C received ultrasound-guided sciatic nerve block with 20 ml of normal saline. An anaesthetist not involved in the perioperative management of the patient prepared the drug. Another blinded investigator did intraoperative management, block placement, data recording and subsequent follow-up in the post-anaesthesia care unit (PACU) and pain clinic.
All patients were anaesthetised using a standard general anaesthesia protocol followed by an ultrasound-guided lateral sciatic nerve block.
Block Procedure: The operative leg was placed in the lateral position along the long axis of the patient’s foot at an angle of 90° to the operating table for ultrasound-guided (Sonosite Inc., Bothell, USA) block placement. All blocks were given by an anaesthetist with a minimum experience of two years. A high frequency (8–12 MHz) linear array probe was placed transversely at the level of the popliteal crease, across the popliteal fossa. The femur was identified by a hyperechoic stripe and dense shadowing of a posterior segment; the pulsating popliteal artery and vein were sighted superficial and medial to the femur. Tibial and common peroneal nerves were traced proximally till they formed the sciatic nerve. A 22-gauge echogenic needle (Pajunk sonoplex cannula, Geisingen, Germany) was inserted in an in-plane approach and advanced till the deep border of the sciatic nerve, and 20 ml of either drug or normal saline was injected into the perineural sheath of the nerve.[9]
In the case of mean arterial pressure (MAP) >120% of baseline, an injection of fentanyl 1–2 μg/kg was given intravenously. Any episode of hypotension (MAP <80%) was managed with boluses of Ringer’s lactate solution.
In the PACU, all patients received intravenous PCA [pump (PCA plus; Abbott, Chicago, USA), injection morphine with bolus 1 mg and lockout interval 5 min] along with injection acetaminophen 1 g 8 hourly for 48 h. Patients were instructed to press the PCA button every time they had pain. Post-operatively, NRS score, heart rate, non-invasive blood pressure and pulse oximetry were recorded in the immediate post-operative period and at 30 min, 1, 2, 4, 8, 12, 24 and 48 h. Post-operative nausea and vomiting (PONV) was assessed at arrival, 6 h and 24 h, using a four-point scale: 0 = none, 1 = mild, 2 = moderate, 3 = severe nausea.[10] Total morphine consumption and the number of times PCA was activated in 48 h were recorded.
Patients were followed up and interviewed in the pain clinic on post-operative day 15, one month and six months. All patients received gabapentin in the post-operative period as per institutional protocol. SP was well-defined as pain localised to the region of the stump. Phantom pain was described as pain experienced in the missing part of the limb. Stump and phantom pain were described using the McGill Pain Questionnaire. At three interviews performed in the pain clinic, if phantom pain and SP were present, its frequency (constant, daily or daily with intervals), number and duration of phantom pain attacks on days with pain and intensity of the pain (mild, moderate, severe) were recorded.[11]
To detect a reduction in the incidence of phantom pain from 80% to 30%, with 80% power and an alpha error of 0.05, 14 patients were required in each group.[12] A total of 50 patients were included considering possible dropouts. Demographic data analyses were done using either the Student’s t-test or the Chi-square test. The results were presented as median with interquartile range, or mean (standard deviation). Categorical/qualitative variables were presented as frequency/percentage. For continuous normally distributed data, parametric t-test or Chi-square test was used, and for continuous skewed data, non-parametric, Mann–Whitney test and Fisher’s exact test were used. For intragroup analysis over time, a two-way analysis of variance was performed using repeated measures analysis of variance (ANOVA). Pearson correlation coefficient was used for the correlation between pre-surgery NRS and chronic pain score. A P value of less than 0.05 was considered to indicate statistical significance. Analysis of data of this study was done using Statistical Package for the Social Sciences [Chicago, IL and Microsoft Excel (Microsoft company) 2007] version 22.0.
RESULTS
One hundred and six patients were assessed for eligibility; 56 patients were excluded, for not fulfilling the inclusion criteria. Finally, 30 patients were randomly assigned to block group (n = 15) and control group (n = 15) during the enrolment period [Figure 1]. Demographic variables were comparable between the two groups [Table 1].
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram
Table 1.
Demographic data of two groups
| Group B (n=15) | Group C (n=15) | P | |
|---|---|---|---|
| Age (years) | 31.87 (11.10) | 36.87 (14.69) | 0.301 |
| Gender (M:F) | 15:0 | 14:1 | |
| Mean NRS score before surgery | 6.07 (1.03) | 5.80 (1.20) | 0.513 |
| Duration of surgery (minutes) | 150.00 (22.03) | 150.67 (25.76) | 0.939 |
Data expressed as mean (standard deviation); M:F (male: female); NRS (numerical rating scale)
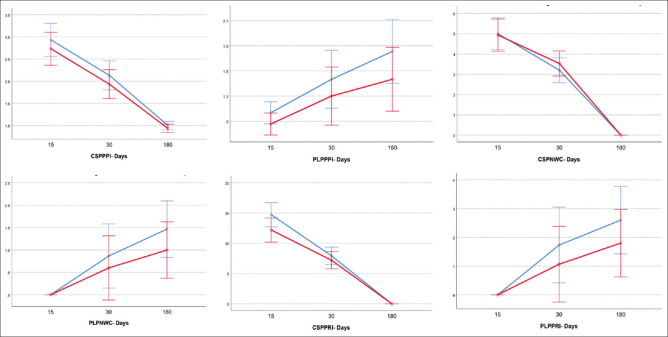
PLP at six months was seen in 46.7% in Group B (7/15) versus 66.7% in Group C (10/15) [risk difference of 20%, 95% confidence interval (CI) 14.74%, 54.74%]. None of the patients developed chronic stump pain (CSP) at six months post-surgery. Median intensities of phantom pain were 1.0 (1–2.0) versus 2 (1–2.0) (P = 0.246) in group B and C, respectively. Description of pain using McGill Pain Questionnaire revealed median pain rating index (PRI) of 4 (3-4) versus 3 (3-4) (P = 0.238), median number of new chosen words (NCW) of 2 (2-2) versus 2 (2-2) (P = 0.213), median present pain intensity (PPI) of 2 (2-2) versus 2 (2-2) (P = 0.383) and the median number of pain attacks per day was 1 (1-1.5) versus 1 (1-2) (P = 0.339) in group B and C, respectively [Figure 2].
Figure 2.
Trend of pain indices between groups over time. Error bars show a 95% confidence interval at each recorded time point. CSPPPI - chronic stump pain present pain intensity; PLPPPI - phantom limb pain present pain intensity; CSPNWC - chronic stump pain new chosen words; PLPNWC - phantom limb pain new chosen words; CSPPRI - chronic stump pain pain rating index; PLPPRI - phantom limb pain pain rating index
Three patients (20%) in Group B and 5 (33.3%) patients in group C developed PLP at 1-month post-surgery with a risk difference of 13% (95% CI (-17.95%–44.62%)). All the patients had stump pain at 1 month post-surgery (100%).
Median intensities of phantom pain were 1.0 (1–2.0) versus 1.0 (1–2.0) (P = 0.289), and median intensities of SP were 2 (2–3.0) versus 3 (2–3.0) P = 0.262, respectively, in group B and C [Figure 2].
McGill Pain Questionnaire for PLP at one month revealed median PRI of 4 (4-8) versus 6 (4-6) (P = 0.408), median NCW of 2 (2-5) vs 3 (3-4) (P = 0.408), median PPI of 2 (2-3) versus 2 (2-2.5) (P = 0.453) and the median number of pain attacks per day was 1.5 (1-1.5) versus 1.5 (1-2) (P = 0.455) in group B and C, respectively.
McGill Pain Questionnaire for SP at one month showed that median PRI [8 (5-8) versus 10 (6-10) (P = 0.185)], median NCW [3 (3-4) versus 3 (3-4) (P = 0.679)] and median PPI [2 (2-2) versus 2 (2-2) (P = 0.471)] were similar among the two groups. There was a statistically significant difference in median pain frequency at 1-month post-surgery between the two groups [1.5 (1-2) versus 2 (2-3) (P value = 0.021)].
None of the patients had PLP at the end of 15 days. The occurrence of SP at 15 days was 100% in both groups.
The median PPI of SP was 3.0 (range, 2-3) in group B versus 3 (range, 3-3) in group C (P = 0.309) and the median number of NCW was 5 (4-5) versus 5 (4-6) (P = 0.547), in group B and C, respectively, and was comparable in the two groups. There was a statistically significant difference between median PRI [12 (10-14) versus 15 (14-18) (P = 0.007)] and the median number of pain attacks per day [2.5 (2-3.5) versus 5 (3-5) (P = 0.011)] between the two groups [Figure 2].
The median number of times PCA was activated was statistically significant between the two groups (3 (2-6) group B versus 14 (11-15) group C (P = 0.000)). Total morphine consumption was more in group C, 13.4 mg (3.35) versus 5 mg (4) as compared to group B (P = 0.000).
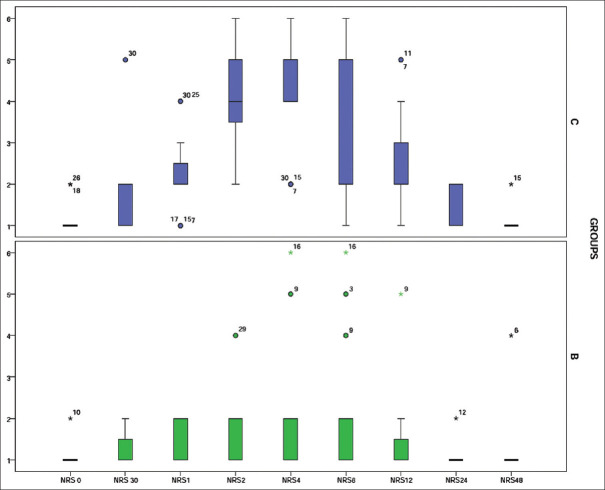
Baseline NRS scores were comparable between the groups, whereas median NRS was significantly increased in group C at 1, 2, 4, 12 and 24 h post-operatively [Figure 3]. Pre-operative NRS pain scores showed a weak negative correlation with PLP intensity in the preceding week of the interview at six months (Pearson’s correlation coefficient r = -0.19).
Figure 3.
Box and whiskers plot showing post-operative median NRS score. Horizontal line between the boxes indicates median, boundaries of the box indicate 25th and 75th quartiles,  indicates outliers and
indicates outliers and  indicates extremes
indicates extremes
Median PONV score at arrival in PACU and at 6 h post-operatively was statistically significant between two groups [0 (0-0) and 2 (0-2) at 0 min and 0 (0-1) and 1 (0-2), respectively, in group B and C]. None of the patients developed severe PONV during the entire observation period. Eleven patients (73.3%) in group C, and three patients (20%) in group B had sleep disturbance in the first 24 h post-operatively; 14 patients had increased post-operative NRS values.
DISCUSSION
This study was conducted to evaluate the effect of pre-emptive ultrasound-guided single-shot lateral sciatic nerve block on the occurrence of chronic pain at six months in trauma patients undergoing below-knee amputation. A 20% reduction was found in the occurrence of PLP at the end of six months in patients who pre-emptively received the sciatic nerve block. None of the patients developed CSP at six months. The use of sciatic nerve block was not associated with any difference in frequency and severity of PLP and SP at 15 days and one-month post-surgery.
Amputations result in both nociceptive and neuropathic symptoms with a high risk of developing chronic pain. As a result, the perioperative period has been an obvious target for varied interventions including physical, behavioural and pharmacological interventions (beta-blockers, antidepressants, anticonvulsants, selective serotonin reuptake inhibitors, ketamine), and percutaneous peripheral nerve stimulation, peripheral nerve blocks (popliteal, sciatic nerve blocks) and radiofrequency ablation to prevent the occurrence of chronic pain but have provided variable results,[13,14,15,16,17,18,19,20] aiming to reduce or prevent the phenomenon.[21] Both central and peripheral nerve blocks have been used; however, the short- and long-term outcomes remain debatable.[22,23,24]
A previous prospective study in 80 patients undergoing lower limb amputation, reported the effect of single-shot infiltration of bupivacaine and clonidine in the sciatic and posterior tibial nerve at the time of nerve exposure. The authors reported a decrease in acute post-operative pain but failed to demonstrate a decrease in CSP and PLP. However, as the block was performed after the surgical incision, pain signals may have already reached the central nervous system (CNS) resulting in sensitisation.[25] In the current study, the block was performed pre-emptively using ultrasound guidance, decreasing the probability of block failure. Also, in the current study, the reduction in morphine consumption in the block group at the end of 24 h was similar to that of Reuben et al.[8]
In a prospective pilot study, 0.25% bupivacaine was injected via a perineural catheter either in the sciatic and posterior tibial nerve (above and below-knee amputation, respectively) in 11 patients undergoing lower limb amputation.[5] None of the patients developed PLP. Another study in patients with peripheral vascular disease compared perineural local anaesthetic (LA) infusion in the sciatic nerve and posterior tibial nerve (above and below-knee amputation, respectively) with normal saline infiltration in patients undergoing amputation.[7] The authors reported a reduction in acute pain and morphine consumption in the treatment group but failed to demonstrate any difference in CSP and PLP between the groups, or in subjective pain at 3 and 6 months. Elizaga et al.[6] did a retrospective study comparing perineural bupivacaine and systemic analgesia in patients who underwent amputation and reported no effect in the occurrence of PLP.
The current study did not find a statistically significant difference in the occurrence of PLP with the use of pre-emptive analgesia, possibly due to the complex and poorly understood pathophysiology of PLP. None of the patients developed CSP at six months; however, the occurrence of SP was 100% at the end of one month. Also, the number of pain attacks per day was significantly lower in the block group, probably due to the use of nerve block, which led to decreased formation of a neuroma and hence decreased the pain.[26]
The study results also show a negative correlation between pre-operative NRS value and chronic pain; hence, pre-amputation pain did not seem to be a risk factor for pain after surgery. As pre-operative pain in trauma victims is not of long-standing duration, CNS plasticity may not have developed in these patients.[27] This is in agreement with Nikolajsen et al.,[2] who reported a total absence of pain in patients with severe pre-amputation pain.
The lateral sciatic nerve block was used in this study as most of the patients had significant acute pain, heavy bandages/external fixators on, and hence it was not possible to make the patients prone. Though it takes a longer time for onset, the quality of the block is similar to the posterior approach.[28] Ultrasound imaging provides direct imaging of the peripheral nerves, needle tip and distribution of LA.[29] Direct visualisation of LA spread minimises the chances of inadvertent intravascular injection.
The study has a few limitations. Only trauma patients undergoing lower limb amputation were included and hence the results cannot be generalised to the larger population. Secondly, the phantom limb sensation was not recorded. Thirdly, the perineural catheter was not used. Lastly, the follow-up period was only six months post-surgery, and the post-operative oral medications for chronic pain management were not compared. The study did not demonstrate the difference in pain indices over time between the groups. This was an exploratory outcome and the trial was probably underpowered to delineate these differences across time.
CONCLUSION
Pre-emptive administration of USG-guided sciatic nerve block is a safe and effective technique for decreasing acute post-operative pain, but it does not alter the occurrence of PLP. Further studies comparing pre-emptive single shot peripheral nerve block with a perineural catheter are required to see if insertion of the catheter and prolonged treatment confers any long-term advantage of decreasing the occurrence of PLP.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nikolajsen L, Ilkjaer S, Kroner K, Christensen JH, Jensen TS. The influence of preamputation pain on postamputation stump and phantom pain. Pain. 1997;72:393–405. doi: 10.1016/s0304-3959(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 2.Nikolajsen L, Finnerup NB, Kramp S, Vimtrup AS, Keller J, Jensen TS. A randomised study of the effects of gabapentin on postamputation pain. Anesthesiology. 2006;105:1008–15. doi: 10.1097/00000542-200611000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Nystrom B, Hagbarth KE. Microelectrode recordings from transected nerves in amputees with phantom limb pain. Neurosci Lett. 1981;27:211–6. doi: 10.1016/0304-3940(81)90270-6. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra PU, Geertzen JH, Stewart R, van der Schans CP. Phantom pain and risk factors:A multivariate analysis. J Pain Symptom Manage. 2002;24:578–85. doi: 10.1016/s0885-3924(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Fisher A, Meller Y. Continuous post-operative regional analgesia by nerve sheath block for amputation surgery—A pilot study. Anesth Analg. 1991;72:300–3. doi: 10.1213/00000539-199103000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Elizaga AM, Smith DG, Sharar SR, Edwards WT, Hansen ST., Jr Continuous regional analgesia by intraneural block:Effect on post-operative opioid requirements and phantom limb pain following amputation. J Rehabil Res Dev. 1994;31:179–87. [PubMed] [Google Scholar]
- 7.Pinzur MS, Garla PGN, Pluth T, Vrbos L. Continuous post-operative infusion of a regional anesthetic after an amputation of the lower extremity. J Bone Joint Surg. 1996;78:1501–5. doi: 10.2106/00004623-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Reuben SS, Raghunathan K, Roissing S. Evaluating the analgesic effect of the perioperative perineural infiltration of bupivacaine and clonidine at the site of injury following lower extremity amputation. Acute Pain. 2006;8:117–23. [Google Scholar]
- 9.Berde CB, Strichartz GR. Local Anesthetics. In: Miller RD, editor. Miller's Anesthesia. 8th ed. Philadelphia PA: Elseviers Saunders; 2014. pp. 1028–55. [Google Scholar]
- 10.Boogaerts JG, Vanacker E, Seidel L, Albert A, Bardiau FM. Assessment of post-operative nausea using a visual analogue scale. Acta Anaesthesiol Scand. 2000;44:470–4. doi: 10.1034/j.1399-6576.2000.440420.x. [DOI] [PubMed] [Google Scholar]
- 11.Melzack R. The McGill pain questionnaire:Major properties and scoring methods. Pain. 1975;1:277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 12.Ephraim PL, Wegener ST, MacKenzie EL, Dillingham TR, Pezzin L. Phantom pain, residual limb pain, and back pain in amputees:Results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–9. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 13.Glimore C, Iifeld B, Rosenow J, Li S, Desai M, Hunter C, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic post amputation pain:A multicenter, randomised, placebo- controlled trial. Reg Anesth Pain Med. 2019;44:637–45. doi: 10.1136/rapm-2018-100109. [DOI] [PubMed] [Google Scholar]
- 14.Chan BL, Witt R, Charrow AP, Magee A, Howard R, Pasquina PF, et al. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357:2206–7. doi: 10.1056/NEJMc071927. [DOI] [PubMed] [Google Scholar]
- 15.Ezzo J, Berman B, Hadhazy VA, Jadad AR, Lao L, Singh BB. Is acupuncture effective for the treatment of chronic pain? A systematic review. Pain. 2000;86:217–25. doi: 10.1016/S0304-3959(99)00304-8. [DOI] [PubMed] [Google Scholar]
- 16.De Caridi G, Massara M, Serra R, Risitano C, Giardina M, Acri IE, et al. Spinal cord stimulation therapy for the treatment of concomitant phantom limb pain and critical limb ischemia. Ann Vasc Surg. 2016;32:131.e11–4. doi: 10.1016/j.avsg.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Smith DG, Ehde DM, Hanley MA, Campbell KM, Jensen MP, Hoffman AJ, et al. Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. J Rehabil Res Dev. 2005;42:645–54. doi: 10.1682/jrrd.2005.05.0082. [DOI] [PubMed] [Google Scholar]
- 18.Wiech K, Kiefer RT, Topfner S, Preissl H, Braun C, Unertl K, et al. A placebo-controlled randomised crossover trial of the N-methyl-D-aspartic acid receptor antagonist, memantine, in patients with chronic phantom limb pain. Anesth Analg. 2004;98:408–13. doi: 10.1213/01.ANE.0000096002.53818.BD. [DOI] [PubMed] [Google Scholar]
- 19.Arjun BK, Prijith RS, Sreeraghu GM, Narendrababu MC. Ultrasound-guided popliteal sciatic and adductor canal block for below-knee surgeries in high-risk patients. Indian J Anaesth. 2019;63:635–9. doi: 10.4103/ija.IJA_296_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gowler VS, Goswami S. Pulsed radiofrequency ablation of stellate ganglion for chronic facial pain. Indian J Anaesth. 2020;64:1091–2. doi: 10.4103/ija.IJA_908_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halligan PW. Phantom limbs:The body in mind. Cogn Neuropsychiatry. 2002;7:251–69. doi: 10.1080/13546800244000111. [DOI] [PubMed] [Google Scholar]
- 22.Karanikolas M, Aretha D, Tsolakis I, Monantera G, Kiekkas P, Papadoulas S, et al. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency:A prospective, randomised, clinical trial. Anesthesiology. 2011;114:1144–54. doi: 10.1097/ALN.0b013e31820fc7d2. [DOI] [PubMed] [Google Scholar]
- 23.Nikolajsen L, Ilkjaer S, Christensen JH, Kroner K, Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet. 1997;350:1353–7. doi: 10.1016/S0140-6736(97)06315-0. [DOI] [PubMed] [Google Scholar]
- 24.Lambert AW, Dashfield AK, Cosgrove C, Wilkins DC, Walker AJ, Ashley S. Randomised prospective study comparing pre-operative epidural and intraoperative perineural analgesia for the prevention of post-operative stump and phantom limb pain following major amputation. Reg Anesth Pain Med. 2001;26:316–21. doi: 10.1053/rapm.2001.23934. [DOI] [PubMed] [Google Scholar]
- 25.Latremoliere A, Woolf CJ. Central sensitization:A generator of pain hypersensitivity by cental neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flor H, Nikolajsen L, Jensen TS. Phantom limb pain:A case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–81. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- 27.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain:Specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:49–64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadzic A, Vloka JD. A comparison of the posterior versus lateral approaches to the block of the sciatic nerve in the popliteal fossa. Anesthesiology. 1998;88:1480–6. doi: 10.1097/00000542-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Gray AT. Ultrasound-guided regional anesthesia:Current state of the art. Anesthesiology. 2006;104:368–73. doi: 10.1097/00000542-200602000-00024. [DOI] [PubMed] [Google Scholar]