Abstract
RNA is a polymer with pivotal functions in many biological processes. RNA structure determination is thus a vital step toward understanding its function. The secondary structure of RNA is stabilized by hydrogen bonds formed between nucleotide basepairs, and it defines the positions and shapes of functional stem-loops, internal loops, bulges, and other functional and structural elements. In this work, we present a methodology for studying large intact RNA biomolecules using homonuclear 15N solid-state NMR spectroscopy. We show that proton-driven spin-diffusion experiments with long mixing times, up to 16 s, improved by the incorporation of multiple rotor-synchronous 1H inversion pulses (termed radio-frequency dipolar recoupling pulses), reveal key hydrogen-bond contacts. In the full-length RNA isolated from MS2 phage, we observed strong and dominant contributions of guanine-cytosine Watson-Crick basepairs, and beyond these common interactions, we observe a significant contribution of the guanine-uracil wobble basepairs. Moreover, we can differentiate basepaired and non-basepaired nitrogen atoms. Using the improved technique facilitates characterization of hydrogen-bond types in intact large-scale RNA using solid-state NMR. It can be highly useful to guide secondary structure prediction techniques and possibly structure determination methods.
Why it matters
RNA is well known for its role as an information transfer molecule; it transfers the genetic code from the DNA to the ribosome, thus facilitating protein production. RNA also serves many other cellular functions, which are regulated by its structure. RNA structure is determined from the assembly of the different nucleotides to form hydrogen bonds—the close contacts between nucleotides governed by nitrogen and hydrogen atoms. Other nucleotides that do not participate in those bonds create small key functional structures such as hairpins and bulges. Here, we show how solid-state NMR can be utilized to detect hydrogen bonds in intact RNA molecules and to differentiate between nucleotides participating in hydrogen bonds and those that do not.
Introduction
RNA structure has long been a subject of intensive studies, mainly in view of the discovery that RNA is not only an information transfer molecule (messenger RNA) that transfers the DNA genetic code to the ribosome. Noncoding RNA molecules adopt a large variety of three-dimensional structures serving many cellular roles (1,2). Transfer RNA delivers amino acids to the ribosome that also contains ribosomal RNAs as part of its structure. Riboswitches regulate gene expression, ribozymes can catalyze various reactions similarly to enzymes, and additional roles of RNA exist and continue to be discovered, the most recent one being of RNA-glycan conjugates displayed on the cell surface (3).
Generally, most structural studies are performed on small synthetic RNA oligomers. For example, structures solved by NMR (4,5) and x-ray crystallography (6) reveal various secondary structure elements such as basepaired helices, stem-loops, bulges, and more. Structures of protein-RNA complexes determined using various techniques such as cryogenic electron microscopy (CryoEM) (7), x-ray (8), and solution NMR (9) are also prevalent. Solid-state NMR has also provided structures of isolated RNA molecules or in complex with proteins (10,11).
Structure determination of large RNA molecules (beyond 100–200 nucleotides) is challenging for solution NMR because of the high spectral overlap. For larger sizes, decreased relaxation times further complicate structure determination. Such limitations are partially solved by segmentally labeling the RNA to reduce the spectral congestion or by using custom synthesis of nucleotides with partial enrichment (12,13). RNA structures are also hard to obtain by crystallography techniques, and thus, advanced sequence-based algorithms are a key tool to predict their secondary structure. Such techniques are based on minimizing the free energy on the basis of the hydrogen-bond patterns (14,15). Other methods for assessing the structure of large RNA molecules utilize enzymatic digestion, radical labeling, and more (16).
Structures of nucleic acid oligomers and polymers are stabilized by interactions between nucleosides. The basepairs in polynucleic acids are stabilized by hydrogen bonds. Canonical basepairs in RNA are formed between the nucleobases adenine (A) and uracil (U) and cytosine (C) and guanine (G). The canonical and most common Watson-Crick (WC) basepairs are formed between the nucleobases and, in RNA, are referred to as the “WC edge” (17). This type of bond is illustrated in Fig. 1 A. However, there are other possible geometries for hydrogen bonds to form, namely the sugar edge and the Hoogsteen edge, as illustrated in Fig. 1 B. Two nucleotides can form hydrogen bonds involving each pair of the three edges. Together with cis- and trans-orientations of the glycoside bond, there can be 12 types of hydrogen bonds between nucleotides giving the RNA flexibility in tertiary structure formation. Therefore, detection of the interacting edge of the hydrogen bond is essential to understanding RNA structure.
Figure 1.

(A) Scheme of canonical WC hydrogen bonds between nucleotides. The dashed blue lines represent the hydrogen bonds. Biological magnetic resonance data bank (BMRB) (18) nomenclature is in red, and average 15N chemical shifts and standard deviations (in ppms) are in green. R, ribose ring. (B) A guanine base with its three edge types.
Hydrogen bonds can be distinguished and characterized by NMR (19,20). In particular, JNN scalar couplings between donor and acceptor nitrogen spins were observed using HNN-COSY solution NMR experiments and reported directly on hydrogen bonds in a 69-nucleotide-long RNA oligomer (21). In large RNA molecules, 15N spins across the hydrogen bond are coupled by dipolar interactions. These interactions are not averaged in precipitated or sedimented samples and are thus amenable to solid-state NMR studies. Solid-state NMR has been successfully used to study various types of biological systems including folded and unfolded proteins, protein-DNA complexes, protein-RNA complexes, intact viruses, and more (22, 23, 24). Recent progress in RNA characterization has been reviewed by Marchanka (25) and by Wang (26). Those mainly include synthetic RNA oligomers with or without bound proteins. For example, a 23-mer RNA fragment of human immunodeficiency virus RNA and a (CUG)97 oligomer were studied using 15N-15N correlations (27,28) and by 1H-detected 15N correlations at magic angle spinning (MAS) frequencies of 40 kHz (29). However, intact, nonrepetitive long RNA leads to high spectral congestion, which does not easily allow a resonance assignment for each nucleotide separately. Yet, the NMR correlations still hold information on the native hydrogen bonding patterns. For instance, if can be quantified, it can be used to determine the ratio between paired and non-paired bases.
RNA viruses are common in nature. The size of their genome can be a few thousands of bases, as in small spherical bacteriophages (30), or as large as 30 thousand bases, as in the case of severe acute respiratory syndrome coronavirus 2 (31). RNA viruses are fast mutating and, therefore, pose constant risk for global health (32), as encountered in this past year and a half. The packing, folding, and capsid binding properties of RNA within the context of intact viruses is therefore important to understand, and we constantly seek methods aimed to study intact viruses or isolated intact RNA.
MS2 bacteriophage infects Escherichia coli bacteria bearing positive F pili. It contains a 3569-nucleotide-long, single-stranded RNA encapsulated in a capsid made of 89 copies of coat protein dimers and a maturation protein. Despite being the first RNA molecule to be sequenced (33), initial structural studies focused on interactions of the capsid protein with small RNA epitopes (34, 35, 36). Only recently, the interaction of the full-length RNA with the MS2 capsid in a wild-type viral particle was studied in detail using CryoEM. Initially, a resolution of 8.7 Å (37) was obtained revealing a network of stem loop regions in the RNA. Later on, the resolution was significantly improved to 3.6 Å for the capsid and 6 Å for the RNA (38). This resolution was sufficient for tracing the secondary structure motifs of different segments in the RNA, revealing unprecedented details on its structure. In particular, the identity of the bases involved in basepair interactions could be determined for approximately two-thirds of the sequence.
Here, we show how 15N correlations in solid-state NMR can be used to study the intact isolated RNA extracted from the MS2 phage. We improved 15N-15N proton-driven spin-diffusion (PDSD) polarization transfer (39) with rotor-synchronous π-pulses applied to the 1H channel (as in radio-frequency driven recoupling (RFDR) (40)), termed here PDSD-RFDR. Consequently, we show that various kinds of hydrogen bonds found between bases can be detected and characterized using this technique. From the type of nitrogen atoms involved, we can also estimate the type and face of those hydrogen bonds. We then compare the patterns we observe in the isolated RNA with that of the enclosed RNA derived from CryoEM.
Materials and methods
Sample preparation
Wild-type MS2 bacteriophage was produced by infecting cultures of E. coli strain C3000 and purified using our lab protocols for phage preparation (41). The RNA was harvested from the phage using a method similar to the one described by Meir et al. (42). Phage were vortexed in TRIzol (Tri Reagent; Sigma-Aldrich, St. Louis, MO), precipitated in isopropanol, and followed by cold ethanol wash. The total yield of RNA from one litter bacterial culture was on average ∼24 mg. The RNA purity was confirmed using standard RNA agarose gel. The RNA precipitate was packed into a 4 mm ZrO2 MAS rotor using a centrifuge and then used for NMR experiments. More details on MS2 phage preparation and purifications as well as RNA isolation and integrity appear in the Supporting materials and methods.
NMR methods
NMR experiments were carried out on two Bruker AVANCE III spectrometers (Billerica, MA) operating at 9.4 and 14.1 T, both equipped with MAS 4 mm probes. Two-dimensional 15N-15N correlation experiments were collected using PDSD (39), with mixing times up to 16 s. Additional PDSD experiments were acquired by adding rotor-synchronized π-pulses (as in RFDR (40)) to the 1H channel during the mixing time (2 and 8 s). We term this experiment PDSD-RFDR. All experiments on the 9.4 T magnet were performed at a spinning speed of 8 kHz and a temperature set on the controller to −28°C. A complete list of experimental parameters appears in the Table S1. The chemical shifts of 15N were externally referenced to 15NH4Cl at 39.3 parts per million (ppm) (43).
Data analysis
NMR data were processed using TopSpin3.5 and NMRPipe (44). Analysis was performed using TopSpin3.5 and SPARKY version 3.134 (45).
Numerical simulation
The NMR simulation package SIMPSON (46) was used to verify the physical basis for the enhancement by RFDR but is not intended to reproduce experimental enhancements. A SIMPSON script was written to show that the application of π-pulses on protons enhances the magnetization transfer between two non-hydrogen 'X' spins in an X2H3 spin system (X ≡ 13C in this case and represents a low-γ spin 1/2). The script, the dipolar couplings used, and the magnetization build-up curves appear in the Supporting materials and methods.
Results and discussion
Identification of 15N resonances
In order to identify the different 15N spins and assign them to the different nucleobases appearing in Fig. 1, we performed several two-dimensional (2D) 15N-15N PDSD solid-state MAS NMR correlation experiments on MS2-RNA. A typical 2D spectrum, acquired with a mixing time of 16 s, is shown in Fig. 2 A. The off-diagonal correlation signals result from the dipolar coupling between 15N nuclei that are proximate in space, probably not beyond ∼4 Å. To obtain them with sufficient sensitivity, it was necessary to significantly increase the mixing time up to 16 s (exploiting the long T1 of the 15N spins) and simultaneously reduce the spinning speed to 8 kHz (thus reducing MAS averaging of the dipolar interaction) while maintaining the temperature controller on −28°C. Similar to our previous study on intact T7 bacteriophage double-stranded DNA (47), we see clear asymmetry in the spectrum resulting from the excitation difference between primary, secondary, and tertiary amines and between nucleobases having more or fewer protons.
Figure 2.
(A) A typical two-dimensional 15N-15N PDSD spectrum of the MS2 RNA. A, C, G, and U stand for the different nucleotides. Numbers identify the particular nitrogen in the nucleotide following BMRB nomenclature. Dashed lines mark the chemical shifts of G, and intersects between lines correspond to intranucleotide crosspeaks. The spectrum was acquired with a mixing time of 16 s and processed with an exponential line broadening of 100 Hz in both dimensions. 20 contours at multiples of 1.4 were generated with the lowest contour level set to a signal/noise ratio (SNR) of 5. (B) A theoretical plot of the most-probable positions of RNA intranucleotide signals. Colored squares, different for each nucleotide, are centered on the average value of a particular peak according to a set of 10 RNA oligomers, with their size corresponding to the standard deviations. Points mark the position of intranucleotide peaks observed in our spectra. The black ellipse represents an example of a hydrogen bond between G2 and C3 (see below). The dash line is as defined in (A). An equivalent plot that uses the average and standard deviation chemical shifts from the BMRB database is shown in Fig. S4.
The resonances can be assigned to one of the four nucleobases shown in Fig. 1 A but not to a specific nucleobase in the sequence (totaling 3569 bases). The assignment process relied on several strategies. Because biological magnetic resonance data bank (BMRB) averages for RNA oligomers have large standard deviations due to some errors in the database, initial data from 10 representative RNA oligomers (see Fig. S7; Table S4) were collected, and their average chemical shifts and standard deviations were extracted (for comparison with BMRB, see Fig. S6). Fig. 2 B shows blocks centered at positions corresponding to correlations between those average RNA nucleobase shifts (differentiated by color). Their size is given by the standard deviations of the shifts. Peaks from spectra similar to that in Fig. 2 A were then marked on the predicted positions and assigned to the corresponding nucleotides. Many of the signals are easily identified. For example, primary amines are unique, having shifts of 82 ppm (adenosine N6 or, in short, A6), 74 ppm (G2), and 97 ppm (C4). Additional unique individual shifts are C3 (197 ppm), A3 (214 ppm), and A1 (223 ppm). Other shifts have to be determined from two or three options (G1 and U1 at ∼146 ppm with C1 somewhat higher at 152; U3 and G3 at ∼160–162 ppm; G9 and A9 at ∼170 ppm; and A7 and G7 at ∼231 ppm), with U being the most difficult to identify unambiguously. To resolve these ambiguities, we followed correlations to resolved signals performing “side-chain walks” (48) along proximate nitrogen nuclei in the same base. For example, in the spectrum shown in Fig. 2 A, the linkage between different 15N signals of the G-base, marked by the dotted lines, can be clearly identified. Similarly, other nucleobases are assigned. Overall, we were able to identify all but one nitrogen shift, as shown in Table 1.
Table 1.
15N chemical shifts (in ppm) of full-length isolated MS2-RNA
| N1 | N2 | N3 | N4 | N6 | N7 | N9 | |
|---|---|---|---|---|---|---|---|
| A | 222.0 | – | 213.2 | – | 83.8 | 231.2 | |
| C | 151.4 | – | 196.8 | 99.0 | – | – | – |
| G | 147.5 | 75.1 | 162.7 | – | – | 234.4 | 170.3 |
| U | 150.5 | – | 159.1 | – | – | – | – |
The unassigned nucleus A9 is indicated by a gray cell. Dashes indicate nonexisting atoms. Chemical shifts have been deposited to the BMRB with accession number 50974.
Identification of hydrogen bonds
Once 15N shifts have been assigned, several internucleotide contacts could be identified. Those are attributed almost exclusively to basepairing interactions, which are characteristic of the helical double-strand-like secondary structure of the RNA (in MS2, 68% of the RNA that appears in the electron density maps is attributed to a basepairing arrangement). In Fig. 2 B (the schematics of the RNA spectrum), the position of a representative basepairing crosspeak is given by the ellipse, correlating G2 and C3 belonging to a G-C canonical WC basepair. Other contacts have been similarly identified.
Clear evidence for basepairing interactions is shown in the different 15N-15N spectra shown in Fig. 3. We can identify G-C basepairing via the contacts G1-C3 and G2-C3. We can also identify C4-G3 and C4-G1. Although the PDSD experiment is not sufficiently quantitative, the fact the G1-C3 is the strongest crosspeak suggests that these contacts are typical WC pairs. According to a CryoEM structure by Dai et al. (38), analysis of RNA secondary structure elements shows that ∼54% of the hydrogen bonds in the structure are formed between G and C. It coincides with the fact that most hydrogen-bond correlations found in our spectra were between these two nucleotides. Additionally, a WC G-C basepair involves five proton spins available to mediate magnetization transfer, whereas other possible basepairs have less. Moreover, all spectra show higher intensities for guanine 15N signals, followed by cytosine, and the weakest signals are of adenine. G and C also appear more frequently in the MS2 RNA. Another canonical basepair is expected between A and U and mainly A1/6 and U3 assuming that the WC face is the most common arrangement. However, we could not detect such contacts. Both diagonal signals of A6 and A1 are relatively weak. This is not surprising given the fact that for A, the closest proton polarization source to N1 is that on the carbon AC2, and only two additional protons are available (on the N6 amine) to polarize the entire nucleobase. We observe even weaker A1-A6 and A1-A3 crosspeaks at long mixing times. Thus, the expected crosspeaks of A1-U3 at 222.0–159.1 ppm or A6-U3 at 83.8–159.1 are probably too weak to detect at these experimental conditions (U contributes only a single proton to the hydrogen bond). Given the fact that A-U basepairs are stabilized by two hydrogen bonds (and three protons), whereas those of G-C are stabilized by three hydrogen bonds (and five protons), it is likely that the A-U basepair is also more mobile, further averaging the 15N-15N dipolar interaction. The weak signal on A6 in comparison with G2 and C4 (all primary amines) is another indication for enhanced dynamics in the adenine nucleobase.
Figure 3.
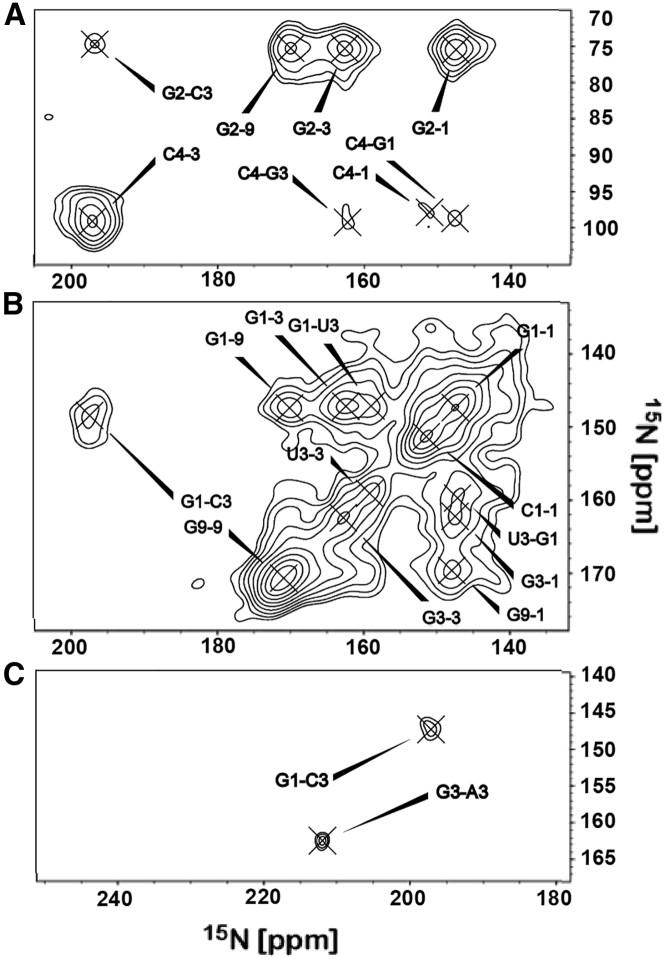
Examples of hydrogen bond and additional internucleotide crosspeaks from different 15N-15N spectra. The contour level was set such that the lowest signal has an SNR of 5. (A) PDSD-RFDR with a mixing time of 8 s. (B) PDSD with a mixing time of 16 s. This plot shows the G-U wobble basepair. (C) PDSD-RFDR, 2 s.
In addition to the G-C canonical basepair, RNA structure is also based on the formation of G-U wobble basepairs (49). Interestingly, we observe (Fig. 3 C) clear G1-U3 correlations between these two nucleotides, corresponding to the WC edge. Despite being energetically similar to the A-U basepair, the additional proton contributed by G and its much-preferred excitation efficiency provides sufficient polarization for this hydrogen bond to be detected. The assignment to U3 of the peak at 159.1 ppm is based on the absence of any other correlations to G, in which the G3 resonance at 162.3 is strongly correlated to G2 and G9. It is also supported by the correlation to U1 at 150.1 ppm, which we could detect using a PDSD spectrum taken at a higher field (Fig. S5). According to the CryoEM structure of MS2, ∼9% of the hydrogen bonds are formed between G and U.
Three additional uncommon contacts were observed in our spectra. Correlation peaks between G and A (G3-A1, G3-A3, and G3-A6) could only be found in the PDSD-RFDR experiments. Hydrogen bonds between G and A were reported for bacterial ribosomal RNA, in which A7 (part of the Hoogsteen edge) forms a bond with G1 (part of the WC edge) (50). However, in our case, we observe correlations to G3, which is part of the sugar edge of the nucleobase. Another possibility is that we are detecting weak stacking interactions that correspond to the positioning of the N3 atoms at distances smaller than 4 Å in space or even closer if they belong to a curved region. Indeed, in MS2 Protein Data Bank structures, a few such contacts could be detected. A weak (signal-to-noise ratio (SNR) of 5) A6-C4 contact is also detected (Fig. 3 A) in the PDSD-RFDR 8-s spectrum. The low intensity can indicate a low abundance hydrogen bond or, more likely, another stacking interaction.
Another significant observation would have been the identification of signals belonging to non-basepaired nucleobases. Moreover, if the ratio of basepaired to non-basepaired nucleotides could be determined quantitatively, the information could be input to structure prediction and calculation programs. According to the small set of RNA oligomers we summarized, shifts up to ∼3 ppm or more are expected for some 15N spins. Others are not significantly affected. Observation of such shifts requires that a pair of nitrogen spins will simultaneously be involved in a hydrogen bond and will be sufficiently shifted not to be obscured by the broadening of the signals resulting from averaging thousands of nucleotides. Fig. 4 shows such an example for the C nucleobase. Both C3 and C4 show broadening and shifting at the C3 diagonal signal around 197 ppm and at the C3-C4 crosspeak at 99 ppm. At the C3 position, where the signals are most intense (197 ppm), a basepairing crosspeak G1-C3 can also be detected (148 ppm). However, at the shoulders of the peaks, which have a higher chemical shift for C3 and a lower shift for C4, no basepairing crosspeak is detected. The reduced intensity of the shoulders and the absence of a crosspeak to G suggests that this signal belongs to non-basepaired C nucleotides, existing at smaller percentages in the RNA structure.
Figure 4.

A segment from a two-dimensional 15N-15N PDSD spectrum showing the distinction between basepaired (p) and non-basepaired (np) C-N3 and N4. The dashed line indicates the shift of a non-basepaired C-N3. The solid line corresponds to basepaired C-N3.
At this stage, the distinction of basepaired and non-basepaired nucleotides is qualitative because RNA dynamics and the PDSD transfer mechanism do not allow a more quantitative information to predict the ratio of non-basepaired and basepaired contributions. For example, our spectra required temperatures below −25°C, above which hardly any signals could be detected at all. It is therefore possible that more dynamic RNA parts are still missing in our spectrum (with probably greater contribution of nonstructured RNA regions) and that crosspeak signal intensities are affected by dynamics in addition to polarization transfer with varying efficiencies. Future studies could address such points as was suggested for 13C spin-diffusion correlation experiments in proteins (51).
Improving 15N polarization transfer with rotor-synchronous 1H π-pulses
The homonuclear interaction between 13C spins is significantly stronger than that of 15N spin pairs, and therefore, short mixing times (10–500 ms) are sufficient for magnetization transfer. Moreover, by constantly irradiating on the 1H spins at a field that resonates with the spinning frequency (γB1 = νR), known as DARR, dipolar-assisted rotational resonance (52), enhanced magnetization transfer is obtained. Yet, irradiating for seconds, as required for 15N recoupling, is impossible in practice because of hardware limitations, and therefore, using dipolar-assisted rotational resonance for 15N correlation experiments is not always feasible. Moreover, RNA is a dynamics biomolecule with a smaller density of proton spins as compared with proteins, further reducing the efficiency of dipolar recoupling. 15N correlation spectra in biological samples are therefore mostly obtained by the PDSD technique with the application of long mixing times (53,54) in the order of seconds or using direct polarization transfer via the application of rotor-synchronous 15N π-pulses (27). At high spinning speeds, the proton-assisted recoupling technique PAR has proved useful (29,55). PDSD is based on an indirect effect in which the 15N-15N magnetization transfer is achieved due to 1H-15N dipolar recoupling (resulting from incomplete MAS averaging of this interaction). The long mixing time required for 15N correlations always carry some signal decay due to relaxation, and as the mixing times in our studies was increased from 2 to 16 s, the SNR was reduced by up to sevenfold in some cases (see Table S2); however, as we demonstrated, new signals appeared.
Although the homonuclear 1H-1H dipolar interaction is theoretically not necessary for the PDSD effect to occur (56), clearly enhanced 1H-1H interaction can increase the efficiency of 1H-15N heteronuclear dipolar recoupling. We therefore applied synchronous π-pulses to the 1H channel after the RFDR scheme to directly recouple the 1H-1H homonuclear dipolar interaction. As demonstrated in Fig. S2, this approach is different from the original suggestion to recouple directly heteronuclear interaction by applying two pulses every rotor period (56) or directly recoupling the homonuclear 15N dipolar interaction using synchronous π-pulses (57). To demonstrate this new approach, four 15N-15N correlation experiments were conducted—two with a mixing time of 2 s and two with a mixing time of 8 s, each pair differing only by the application of the 1H-RFDR sequence. The overlay between the spectra is shown in Fig. 5. Although the average SNR at long mixing times is generally smaller, for most crosspeaks the SNR has consistently increased (average of ∼153% for the mixing time of 2 s and of ∼126% for 8 s) by the application of the π-pulses. Moreover, not only does the PDSD-RFDR spectrum contain more correlations, these correlations are mostly between two hydrogen-bonded nitrogen spins, thus providing essential additional information regarding the hydrogen-bond patterns in the RNA, enabling detection of the data described in previous sections. The actual SNR enhancement in both experiments are given in Tables S3a and S3b.
Figure 5.
A comparison between PDSD (black) and PDSD-RFDR (red) 15N-15N correlation spectra acquired with a similar mixing time of (A) 8 s and (B) 2 s. Crosspeaks that appear only in the PDSD-RFDR experiment are assigned. Enhancement is also apparent in many other signals, averaging ∼126% (8 s) and ∼153% (2 s) for the crosspeaks. The contour levels were chosen such that the lowest SNR was 5. The processing (100 Hz broadening, similar zero-filling, and similar acquisition times) was identical in all cases.
To verify that the application of π-pulses is the source of signal enhancement, we performed numerical simulations using the SIMPSON software (46). We calculated the polarization transfer between two nonhydrogen (X) spins in an X2H3 spin system in which all spins are coupled by hetero- and homonuclear interactions. We followed the evolution of a preliminary state in which the two X spins are oppositely polarized in two cases; one with the π-pulses (PDSD-RFDR) and another without. The numerical simulations in Fig. S3 show that polarization transfer is indeed enhanced with the π-pulses. These results qualitatively verify our experimental results.
Conclusions
The 1.1-MDa, full-length RNA isolated directly from the MS2 bacteriophage virus was studied using solid-state NMR. Using RFDR-enhanced PDSD 15N-15N correlation experiments, we could assign the 15N resonances to the four different nucleotides, and detect hydrogen bonds, both canonical as well as wobble basepairs. These hydrogen bonds stabilize the secondary structure of the viral RNA and reduce its overall dynamics. By recognizing the 15N shifts making up the hydrogen bonds, it is possible with such techniques to determine the face of the bond and the dominant pairs we detect have a WC face. Another observation is the ability to distinguish nitrogen spins involved in basepairing interactions from those in non-basepaired nucleobases.
Although we show how solid-state NMR can be useful to study such high-molecular-weight RNA biomolecules, detection of genomic 15N resonances in general, and more importantly hydrogen bonds, poses some challenges, some of which we have addressed here. We show that by recoupling the 1H-1H dipolar interaction using RFDR, signal enhancement is obtained without a need for seconds-long continuous irradiation that challenges the hardware. Consequently, the experimental time can be shortened significantly. It also allows the gain of information even when long mixing times are inapplicable due to short relaxation times. Moreover, using this technique, we have been able to detect new correlations not detected even at very long mixing times. Some of these correlations validate our assignment. Others are attributed to internucleotide contacts. Toward more quantitative estimation of hydrogen-bond patterns, it will be required to generate a more uniform and efficient 15N excitation and estimate and fit polarization transfer between the basepairs (58). The latter is required even if such hydrogen bonds are detected directly via proton detection (29).
The improved method we described for characterizing hydrogen bonds in intact RNA using solid-state NMR is applicable to RNA molecules extracted from natural sources, or even still embedded in their source organism, and can be utilized regardless of sequence length. Although not yet quantitative, it has the potential to guide RNA structure prediction protocols.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
We thank Prof. Uri Gophna from the Faculty of Life Sciences, Tel Aviv University, for supplying MS2 stocks and for fruitful discussions. We thank Meital Bachar and Roni Hassid from our group for the RNA agarose gel preparation.
This research was supported by the Israel Science Foundation grant #847/17.
Editor: Yuval Ebenstein.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.bpr.2021.100027.
Supporting material
References
- 1.Eddy S.R. Non-coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2001;2:919–929. doi: 10.1038/35103511. [DOI] [PubMed] [Google Scholar]
- 2.Ransohoff J.D., Wei Y., Khavari P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018;19:143–157. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn R.A., Pedram K., et al. Bertozzi C.R. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell. 2021;184:3109–3124.e22. doi: 10.1016/j.cell.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park C.-J., Bae S.H., et al. Choi B.S. Solution structure of the influenza A virus cRNA promoter: implications for differential recognition of viral promoter structures by RNA-dependent RNA polymerase. Nucleic Acids Res. 2003;31:2824–2832. doi: 10.1093/nar/gkg387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnwal R.P., Yang F., Varani G. Applications of NMR to structure determination of RNAs large and small. Arch. Biochem. Biophys. 2017;628:42–56. doi: 10.1016/j.abb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westhof E. Twenty years of RNA crystallography. RNA. 2015;21:486–487. doi: 10.1261/rna.049726.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugita Y., Matsunami H., et al. Wolf M. Cryo-EM structure of the Ebola virus nucleoprotein-RNA complex at 3.6 Å resolution. Nature. 2018;563:137–140. doi: 10.1038/s41586-018-0630-0. [DOI] [PubMed] [Google Scholar]
- 8.Ennifar E., Nikulin A., et al. Dumas P. The crystal structure of UUCG tetraloop. J. Mol. Biol. 2000;304:35–42. doi: 10.1006/jmbi.2000.4204. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Hartman E., et al. Feigon J. Structure of a yeast RNase III dsRBD complex with a noncanonical RNA substrate provides new insights into binding specificity of dsRBDs. Structure. 2011;19:999–1010. doi: 10.1016/j.str.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed M., Marchanka A., Carlomagno T. Structure of a protein-RNA complex by solid-state NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2020;59:6866–6873. doi: 10.1002/anie.201915465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchanka A., Simon B., et al. Carlomagno T. RNA structure determination by solid-state NMR spectroscopy. Nat. Commun. 2015;6:7024. doi: 10.1038/ncomms8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duss O., Maris C., et al. Allain F.H.-T. A fast, efficient and sequence-independent method for flexible multiple segmental isotope labeling of RNA using ribozyme and RNase H cleavage. Nucleic Acids Res. 2010;38:e188. doi: 10.1093/nar/gkq756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchanka A., Kreutz C., Carlomagno T. Isotope labeling for studying RNA by solid-state NMR spectroscopy. J. Biomol. NMR. 2018;71:151–164. doi: 10.1007/s10858-018-0180-7. [DOI] [PubMed] [Google Scholar]
- 14.Turner D.H., Sugimoto N., Freier S.M. RNA structure prediction. Annu. Rev. Biophys. Biophys. Chem. 1988;17:167–192. doi: 10.1146/annurev.bb.17.060188.001123. [DOI] [PubMed] [Google Scholar]
- 15.Reuter J.S., Mathews D.H. RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics. 2010;11:129. doi: 10.1186/1471-2105-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strobel E.J., Yu A.M., Lucks J.B. High-throughput determination of RNA structures. Nat. Rev. Genet. 2018;19:615–634. doi: 10.1038/s41576-018-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leontis N.B., Westhof E. Geometric nomenclature and classification of RNA base pairs. RNA. 2001;7:499–512. doi: 10.1017/s1355838201002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulrich E.L., Akutsu H., et al. Markley J.L. BioMagResBank. Nucleic Acids Res. 2008;36:D402–D408. doi: 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flinders J., Dieckmann T. NMR spectroscopy of ribonucleic acids. Prog. Nucl. Magn. Reson. Spectrosc. 2006;48:137–159. [Google Scholar]
- 20.Grzesiek S., Cordier F., et al. Barfield M. Insights into biomolecular hydrogen bonds from hydrogen bond scalar couplings. Prog. Nucl. Magn. Reson. Spectrosc. 2004;45:275–300. [Google Scholar]
- 21.Dingley A.J., Grzesiek S. Direct observation of hydrogen bonds in nucleic acid base pairs by internucleotide 2 JNN couplings. J. Am. Chem. Soc. 1998;120:8293–8297. [Google Scholar]
- 22.Ladizhansky V. Applications of solid-state NMR to membrane proteins. Biochim. Biophys. Acta. Proteins Proteomics. 2017;1865:1577–1586. doi: 10.1016/j.bbapap.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Lecoq L., Fogeron M.-L., et al. Böckmann A. Solid-state NMR for studying the structure and dynamics of viral assemblies. Viruses. 2020;12:1069. doi: 10.3390/v12101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habenstein B., Loquet A. Solid-state NMR: an emerging technique in structural biology of self-assemblies. Biophys. Chem. 2016;210:14–26. doi: 10.1016/j.bpc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Sreemantula A.K., Marchanka A. Solid-state NMR spectroscopy for characterization of RNA and RNP complexes. Biochem. Soc. Trans. 2020;48:1077–1087. doi: 10.1042/BST20191080. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y., Wang S. RNA characterization by solid-state NMR spectroscopy. Chemistry. 2018;24:8698–8707. doi: 10.1002/chem.201705583. [DOI] [PubMed] [Google Scholar]
- 27.Leppert J., Urbinati C.R., et al. Ramachandran R. Identification of NH...N hydrogen bonds by magic angle spinning solid state NMR in a double-stranded RNA associated with myotonic dystrophy. Nucleic Acids Res. 2004;32:1177–1183. doi: 10.1093/nar/gkh288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riedel K., Leppert J., et al. Ramachandran R. Characterisation of hydrogen bonding networks in RNAs via magic angle spinning solid state NMR spectroscopy. J. Biomol. NMR. 2005;31:331–336. doi: 10.1007/s10858-005-1614-6. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Xiang S., et al. Wang S. Proton-detected solid-state NMR detects the inter-nucleotide correlations and architecture of dimeric RNA in microcrystals. Chem. Commun. (Camb.) 2017;53:12886–12889. doi: 10.1039/c7cc07483b. [DOI] [PubMed] [Google Scholar]
- 30.Callanan J., Stockdale S.R., et al. Hill C. Expansion of known ssRNA phage genomes: from tens to over a thousand. Sci. Adv. 2020;6:eaay5981. doi: 10.1126/sciadv.aay5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu F., Zhao S., et al. Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrasco-Hernandez R., Jácome R., et al. Ponce de León S. Are RNA viruses candidate agents for the next global pandemic? A review. ILAR J. 2017;58:343–358. doi: 10.1093/ilar/ilx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiers W., Contreras R., et al. Ysebaert M. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976;260:500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama T., Hebert R.R., Hartman K.A. Ribonucleoprotein complexes formed between bacteriophage MS2 RNA and MS2 protein in vitro. J. Mol. Biol. 1967;25:455–463. doi: 10.1016/0022-2836(67)90198-2. [DOI] [PubMed] [Google Scholar]
- 35.Valegârd K., Murray J.B., et al. Liljas L. The three-dimensional structures of two complexes between recombinant MS2 capsids and RNA operator fragments reveal sequence-specific protein-RNA interactions. J. Mol. Biol. 1997;270:724–738. doi: 10.1006/jmbi.1997.1144. [DOI] [PubMed] [Google Scholar]
- 36.Helgstrand C., Grahn E., et al. Liljas L. Investigating the structural basis of purine specificity in the structures of MS2 coat protein RNA translational operator hairpins. Nucleic Acids Res. 2002;30:2678–2685. doi: 10.1093/nar/gkf371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koning R.I., Gomez-Blanco J., et al. Koster A.J. Asymmetric cryo-EM reconstruction of phage MS2 reveals genome structure in situ. Nat. Commun. 2016;7:12524. doi: 10.1038/ncomms12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai X., Li Z., et al. Sun R. In situ structures of the genome and genome-delivery apparatus in a single-stranded RNA virus. Nature. 2017;541:112–116. doi: 10.1038/nature20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szeverenyi N.M., Sullivan M.J., Maciel G.E. Observation of spin exchange by two-dimensional Fourier transform 13C cross polarization-magic-angle spinning. J. Magn. Reson. 1982;47:462–475. [Google Scholar]
- 40.Bennett A.E., Griffin R.G., et al. Vega S. Chemical shift correlation spectroscopy in rotating solids: radio frequency-driven dipolar recoupling and longitudinal exchange. J. Chem. Phys. 1992;96:8624–8627. [Google Scholar]
- 41.Morag O., Sgourakis N.G., et al. Goldbourt A. In: Protein NMR, Methods in Molecular Biology. Ghose R., editor. Humana Press; 2018. Filamentous bacteriophage viruses: preparation, magic-angle spinning solid-state NMR experiments, and structure determination; pp. 67–97. [DOI] [PubMed] [Google Scholar]
- 42.Meir M., Harel N., et al. Stern A. Competition between social cheater viruses is driven by mechanistically different cheating strategies. Sci. Adv. 2020;6:eabb7990. doi: 10.1126/sciadv.abb7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertani P., Raya J., Bechinger B. 15N chemical shift referencing in solid state NMR. Solid State Nucl. Magn. Reson. 2014;61–62:15–18. doi: 10.1016/j.ssnmr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Delaglio F., Grzesiek S., et al. Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 45.Lee W., Tonelli M., Markley J.L. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics. 2015;31:1325–1327. doi: 10.1093/bioinformatics/btu830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bak M., Rasmussen J.T., Nielsen N.C. SIMPSON: a general simulation program for solid-state NMR spectroscopy. J. Magn. Reson. 2000;147:296–330. doi: 10.1006/jmre.2000.2179. [DOI] [PubMed] [Google Scholar]
- 47.Abramov G., Goldbourt A. Nucleotide-type chemical shift assignment of the encapsulated 40 kbp dsDNA in intact bacteriophage T7 by MAS solid-state NMR. J. Biomol. NMR. 2014;59:219–230. doi: 10.1007/s10858-014-9840-4. [DOI] [PubMed] [Google Scholar]
- 48.Higman V.A. Solid-state MAS NMR resonance assignment methods for proteins. Prog. Nucl. Magn. Reson. Spectrosc. 2018;106-107:37–65. doi: 10.1016/j.pnmrs.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Varani G., McClain W.H. The G·U wobble base pair. A fundamental building block of RNA structure crucial to RNA function in diverse biological systems. EMBO Rep. 2000;1:18–23. doi: 10.1093/embo-reports/kvd001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Traub W., Sussman J.L. Adenine-guanine base pairing ribosomal RNA. Nucleic Acids Res. 1982;10:2701–2708. doi: 10.1093/nar/10.8.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duong N.T., Raran-Kurussi S., et al. Agarwal V. Quantitative 1H-1H distances in protonated solids by frequency-selective recoupling at fast magic angle spinning NMR. J. Phys. Chem. Lett. 2018;9:5948–5954. doi: 10.1021/acs.jpclett.8b02189. [DOI] [PubMed] [Google Scholar]
- 52.Takegoshi K., Nakamura S., Terao T. 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem. Phys. Lett. 2001;344:631–637. [Google Scholar]
- 53.Giraud N., Blackledge M., et al. Emsley L. The influence of nitrogen-15 proton-driven spin diffusion on the measurement of nitrogen-15 longitudinal relaxation times. J. Magn. Reson. 2007;184:51–61. doi: 10.1016/j.jmr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Traaseth N.J., Gopinath T., Veglia G. On the performance of spin diffusion NMR techniques in oriented solids: prospects for resonance assignments and distance measurements from separated local field experiments. J. Phys. Chem. B. 2010;114:13872–13880. doi: 10.1021/jp105718r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewandowski J.R., De Paëpe G., et al. Griffin R.G. (15)N-(15)N proton assisted recoupling in magic angle spinning NMR. J. Am. Chem. Soc. 2009;131:5769–5776. doi: 10.1021/ja806578y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takegoshi K., Nakamura S., Terao T. 13C–1H dipolar-driven 13C–13C recoupling without 13C rf irradiation in nuclear magnetic resonance of rotating solids. J. Chem. Phys. 2003;118:2325–2341. [Google Scholar]
- 57.Robyr P., Meier B.H., Ernst R.R. Radio-frequency-driven nuclear spin diffusion in solids. Chem. Phys. Lett. 1989;162:417–423. [Google Scholar]
- 58.Duong N.T., Raran-Kurussi S., et al. Agarwal V. Can proton-proton recoupling in fully protonated solids provide quantitative, selective and efficient polarization transfer? J. Magn. Reson. 2020;317:106777. doi: 10.1016/j.jmr.2020.106777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.