Abstract
Objective
Enhanced recovery after surgery (ERAS) with a pre-emptive pain management program has been shown to decrease opioid prescriptions after thoracic surgery. We sought to determine which patient or procedural factors were associated with the need for prescription opioid medications after thoracic surgical procedures.
Methods
We performed a retrospective analysis of a postoperative pain survey at the time of follow-up in combination with procedural and patient characteristic data. We then performed univariate and multivariate logistic regression to determine factors associated with prescription opioids use.
Results
Two hundred twenty-eight patients completed questionnaires at a median of 37 days after surgery. Most patients received minimally invasive surgery (n = 213, 93%) with the 2 most common types of operations being foregut (n = 92, 40%) and pulmonary resection (n = 80, 35%). Thirty-nine percent of patients (n = 89) were taking chronic pain medications preoperatively, with 15% on chronic opioids medication (n = 33). After surgery, 166 patients (72%) did not take opioids at home. Multivariate analysis showed any chronic opioid medications before surgery (odds ratio, 28.8; 95% confidence interval, 9.13-90.8, P < .001) were associated with opioid use postoperatively. In contrast, increase in age was associated with a decrease in opioid use (odds ratio, 0.96; 95% confidence interval, 0.93-0.99, P = .01).
Conclusions
ERAS with pre-emptive pain management was associated with patients avoiding opioid prescriptions during recovery. The patient factor of preoperative opioid pain medication(s) and younger age is a significant factor for the patient needing opioids at home after surgery instead of procedural factors. Patient characteristics should be considered when tailoring the patient's pain management after thoracic surgical procedures.
Key Words: enhanced recovery after surgery, minimally invasive thoracic surgery, postoperative pain control, opioid medication
Abbreviations and Acronyms: ASA, American Society of Anesthesiologists; CI, confidence interval; ERAS, Enhanced Recovery After Surgery; IQR, interquartile range; OR, odds ratio
Graphical abstract

Opioid use before surgery and younger age were associated with opioid use after surgery.
Central Message.
Taking pain medication before a thoracic operation is the main risk factor for requiring opioid medication after surgery.
Perspective.
An ERAS program with an emphasis on pre-emptive pain control was associated with most of the patients recovering from the thoracic operation without a need for opioid prescriptions. The main risk factor requiring opioid pain medication after surgery is chronic pain medication before surgery.
See Commentaries on pages 187 and 189.
Enhanced Recovery After Surgery (ERAS) with pre-emptive pain management, opioid-sparing protocols has been shown to decrease postoperative opioid requirements in thoracic surgery.1, 2, 3 Moreover, enhanced recovery programs in thoracic surgery have been shown to reduce the length of stay, total fluid balance, and mean inflation-adjusted hospital costs.4 Consequently, most institutions have implemented these protocols to curb the opioid epidemic and decrease hospital costs.5 Still, some patients require opioid medications in the perioperative period for adequate pain management following thoracic surgical procedures. In the era of ERAS, it is unclear which patient or procedural factors are associated with being able to manage without opioid pain medications at home after thoracic surgery. Moreover, studies have shown that patient-derived outcomes data can provide a unique insight into patient pain medication use and overall pain control.6,7 Using patient-derived outcomes, we sought to determine which factors were associated with the need for prescription opioid medications after thoracic surgical procedures.
Methods
The institutional review board at Houston Methodist Research Institute approved the study (Pro00013680), and patients consented. We performed a retrospective cohort analysis of patients who underwent thoracic surgery procedures from 2018 to 2019 who completed our postoperative pain survey. Before the study period, we had implemented an ERAS program (Table 1)1,8 with pre-emptive pain control (Table 2).2 To summarize, all study patients went through the ERAS program that emphasized improvements in the preoperative, operative, and postoperative phases of care (Table 1). One of the most important aspects of the operative phase of care was performing minimally invasive surgery and thoracic nerve block with liposomal bupivacaine (Video 1) for chest cases and injection of liposomal bupivacaine around transversus abdominis plane in abdominal cases. The pre-emptive pain control program emphasizes the use of nonopioid scheduled pain mediation as the primary mode of pain control at home, along with patient education about the program (Table 2). Patients were discharged by a physician assistant or resident. Most patients were given pain medication based on the standard protocol (Table 2). If the patients' pain was not well controlled at the time of discharge on a standard protocol, patients were prescribed opioids. For patients with chest surgery, pain assessment was made after the removal of the chest tube. The evaluation was made based on the probability that the standard pain medication protocol would be successful in managing pain. We set the patient expectation by explaining to the patient that the goal is to manage the pain instead of having no pain. If the standard nonopioid pain medication is providing excellent pain relief, there is a high likelihood that the patient's pain will be successfully managed without opioid pain medication, and they will be discharged home on standard medication. If the standard nonopioid pain medications do not provide any relief, the patient will be discharged home on opioids. If patients called within 5 days of surgery due to pain, the provider who discharged the patient received the call to manage patient's pain. This improved the ability of the provider to assess and predict the pain medication need of the patient. We obtained institutional data collected for the Society of Thoracic Surgeons database, as well as data from electronic health records on patient and procedural characteristics. We recorded patient features such as demographics, comorbidities, surgical history, social history, and American Society of Anesthesiologists (ASA) classification. Surgical factors were categorized as anatomy, open versus minimally invasive, type of minimally invasive procedure (robot-assisted, video-assisted thoracoscopic surgery, or laparoscopic), surgery through the abdominal or thoracic cavity, time in the operating room, and primary surgeon who operated. The postoperative pain survey was given at the time of follow-up visit in the office. The survey inquired which, if any, pain medications patients took before surgery and after surgery, as well as the pain level (1-10 scale) at the time of follow-up (3-8 weeks after surgery) (Figure 1).
Table 1.
Enhanced recovery after surgery protocol with pre-emptive pain control
| Preoperative | Intraoperative | Postoperative | |
|---|---|---|---|
| Patient | Walk 1 mile a day Practice incentive spirometer Quit smoking 4 wk before surgery Stop drinking alcohol 2 wk before surgery Evaluation of steroids and blood thinners |
Ambulate 3 × a day Sit in a chair for total of 6 h Incentive spirometer 10 × every hour Take around-the-clock pain medication |
|
| Nurse | Give pain medication Inject enoxaparin |
Give pain medication Inject enoxaparin |
|
| Surgeon | Minimally invasive surgery Inject liposomal bupivacaine |
||
| Anesthesiologist | Total intravenous anesthetic Less CVL, Foley, and a-line IV antibiotics before incision Maintain normothermia Control postoperative nausea and vomiting Maintain euvolemia Give pain medication |
CVL, Central venous line; a-line, arterial line; IV, intravenous.
Table 2.
Standard and breakthrough pain medication for pre-emptive pain control
| Preoperative | Intraoperative | Postoperative inpatient | Postoperative outpatient | |
|---|---|---|---|---|
| Standard |
Tramadol Gabapentin |
Acetaminophen Ketorolac∗ |
Acetaminophen Ketorolac∗ Gabapentin Methocarbamol Lidoderm patch Tramadol as needed |
Acetaminophen 1 g PO 3 × a day for 5 d Naproxen 220 mg PO 2 × a day for 3 d∗ Gabapentin 300 mg PO 3 × a day for 7 d† Methocarbamol as needed† Lidoderm patch as needed† |
| Breakthrough |
|
|
Dilaudid IV × 2 Dilaudid PCA Convert to PO opioid |
Tramadol Acetaminophen with codeine #3 Acetaminophen with hydrocodone |
PO, Per os; IV, intravenous; PCA, patient-controlled analgesia.
Ketorolac and Naproxen was given for patients <75 years old with Cr < 1 and approval by surgeon.
For chest surgery.
Figure 1.
Postoperative pain questionnaire asking about specific pain medication patient took after surgery and before surgery as well as patient's pain at the time of the survey.
Statistical Analysis
Multiple logistic regression modeling (with the clustered variance estimator option for surgeon level) was performed to determine statistical association with the opioid prescription at discharge after thoracic surgery. Variables for multiple logistic regression models were selected based on the clinical significance and also by the Stata's Lasso technique with the cross-validation selection option.9,10 To summarize, all variables used in the univariable analysis were assessed by the Lasso. The program suggested models that included the variables with the greatest probability of being a risk factor. The likelihood ratio test further reduced model subsets. During the modeling process, the potential risk factors were discussed with the senior clinicians who have extensive clinical experience in the field to ensure the biological plausibility of the selected covariates. Logistic regression analysis was also repeated in the cohort of patients who were opioid-naive to identify additional factors associated with an opioid prescription at discharge after thoracic surgery. Missing data were assessed for missing completely at random using the Little's χ2 test.11 All the analyses were performed on Stata, version 16.1 (StataCorp LLC, College Station, Tex).9
Results
Two hundred twenty-eight patients met the inclusion and exclusion criteria and all patients consented to be part of the study. The median age of the cohort was 63 years old; patients were mostly female (58%), and white (90%, Table 3). The 3 most common comorbidities were hypertension (56%), coronary artery disease (17%), and diabetes (13%). There were 94 patients (41.2%) who were current or former smokers, 17 patients (8%) had a clinical diagnosis of opioid dependence, and 19 patients (8%) had a diagnosis of alcohol dependence (Table 3). In the survey, there were 33 patients (15%) who were taking opioids chronically before surgery, and there were 89 patients (39%) who were taking either opioids or nonopioid pain medication to treat chronic pain. There were 63 patients (29%) who took opioids after surgery (Table 4). The 2 most common operations for the patients in the cohort were foregut (n = 92, 40%) and pulmonary resection (n = 80, 35%, Table 4). Most patients underwent minimally invasive surgery (n = 212, 93%) with the 2 most common minimally invasive approaches being robot-assisted thoracoscopic surgery (n = 93, 41%) and robot-assisted laparoscopic surgery (n = 94, 41%). Most patients underwent a transthoracic surgical procedure (n = 131, 58%) with median time for the operation of 2.9 hours. Most of the cases were performed by one surgeon (n = 145, 83%). The median time between surgery and the questionnaire was 37 days. There was no significant difference in the time between surgery and opioid users versus nonusers filling out the survey (P = .14, Table 4). The median postoperative length of stay of the entire cohort was 1 day (interquartile range [IQR], 1, 3) (Table E1).
Table E1.
Postoperative parameters∗
| Total |
Complete data |
Missing data |
P values∗ | |
|---|---|---|---|---|
| (N = 228) | (n = 168) | (n = 60) | ||
| Opioid after surgery | 63 (27.6) | 46 (27.4) | 17 (28.3) | .89 |
| Any pain medication after surgery | 215 (94.3) | 160 (95.2) | 55 (91.7) | .31 |
| Tylenol after surgery | 167 (73.2) | 128 (76.2) | 39 (65.0) | .09 |
| Aleve after surgery | 55 (24.1) | 40 (23.8) | 15 (25.0) | .85 |
| Motrin after surgery | 35 (15.4) | 24 (14.3) | 11 (18.3) | .46 |
| Neurontin after surgery | 96 (42.1) | 67 (39.9) | 29 (48.3) | .25 |
| Flexeril after surgery | 2 (0.9) | 1 (0.6) | 1 (1.7) | .44 |
| Celebrex after surgery | 1 (0.4) | 0 (0.0) | 1 (1.7) | .09 |
| Lidoderm patch after surgery | 2 (0.9) | 2 (1.2) | 0 (0.0) | .40 |
| Lyrica after surgery | 1 (0.4) | 1 (0.6) | 0 (0.0) | .55 |
| Ultram after surgery | 30 (13.2) | 26 (15.5) | 4 (6.7) | .08 |
| Tylenol #3 after surgery | 10 (4.4) | 7 (4.2) | 3 (5.0) | .79 |
| Percocet after surgery | 3 (1.3) | 2 (1.2) | 1 (1.7) | .78 |
| Norco after surgery | 28 (12.3) | 17 (10.1) | 11 (18.3) | .10 |
| Morphine after surgery | 3 (1.3) | 1 (0.6) | 2 (3.3) | .11 |
| Hydrocodone after surgery | 1 (0.4) | 0 (0.0) | 1 (1.7) | .09 |
| Fentanyl after surgery | 2 (0.9) | 1 (0.6) | 1 (1.7) | .44 |
| Oxycodone after surgery | 1 (0.4) | 1 (0.6) | 0 (0.0) | .55 |
| Hydromorphone after surgery | 1 (0.4) | 1 (0.6) | 0 (0.0) | .55 |
| Number of different types of medications after surgery | 2.0 (1.0, 3.0) | 2.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | .90 |
| Narcotics after surgery | 63 (27.6) | 46 (27.4) | 17 (28.3) | .89 |
| Pain level at time of follow-up | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 3.0) | .06 |
| Time in OR, h, median (IQR) | 2.9 (1.9, 3.9) | 3.1 (2.3, 4.1) | 1.9 (1.2, 3.0) | <.001 |
| Postoperative length of stay, d, median (IQR) | 1.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.5) | .02 |
OR, Operating room; IQR, interquartile range. Values are in frequency and % unless otherwise specified. Values in bold are statistical significance at P values < .05.
Comparison between patients having complete data versus patients having missing data, using the χ2 or Fisher exact tests for categorical variables and Wilcoxon rank-sum for continuous variables.
Table 3.
Patient characteristics and need for opioid prescription after surgery
| Total |
Opioids after surgery |
P value | ||
|---|---|---|---|---|
| No |
Yes |
|||
| (N = 228) | (n = 165) | (n = 63) | ||
| Age at surgery, y, median (IQR) | 63.0 (54.0, 72.0) | 65.0 (54.0, 73.0) | 60.0 (53.0, 70.0) | .09 |
| Female | 132 (57.9) | 98 (59.4) | 34 (54.0) | .46 |
| White | 151 (89.9) | 113 (92.6) | 38 (82.6) | .08 |
| Height, cm, median (IQR) | 170.0 (163.0, 178.0) | 169.0 (163.0, 178.0) | 170.0 (164.0, 178.0) | .49 |
| Weight, kg, median (IQR) | 81.6 (66.5, 94.0) | 80.0 (65.6, 94.3) | 84.1 (69.4, 93.8) | .31 |
| Body mass index, median (IQR) | 27.6 (23.6, 31.5) | 27.4 (23.5, 31.5) | 28.0 (24.8, 31.4) | .79 |
| Comorbidities | ||||
| Hypertension | 127 (55.7) | 91 (55.2) | 36 (57.1) | .79 |
| Congestive heart failure | 6 (2.6) | 4 (2.4) | 2 (3.2) | .67 |
| Coronary artery disease | 39 (17.1) | 26 (15.8) | 13 (20.6) | .38 |
| Myocardial infarction | 16 (7.0) | 8 (4.8) | 8 (12.7) | .046 |
| Atrial fibrillation | 9 (3.9) | 7 (4.2) | 2 (3.2) | 1.00 |
| Valvular heart disease | 6 (2.6) | 4 (2.4) | 2 (3.2) | .67 |
| Interstitial lung disease | 9 (3.9) | 8 (4.8) | 1 (1.6) | .45 |
| Major vascular disease | 15 (6.6) | 11 (6.7) | 4 (6.3) | .93 |
| DVT/PE | 10 (4.4) | 7 (4.2) | 3 (4.8) | .86 |
| Cerebrovascular history | 16 (7.0) | 9 (5.5) | 7 (11.1) | .13 |
| Diabetes | 29 (12.7) | 22 (13.3) | 7 (11.1) | .65 |
| Liver dysfunction | 4 (1.8) | 1 (0.6) | 3 (4.8) | .07 |
| On dialysis | 2 (0.9) | 1 (0.6) | 1 (1.6) | .48 |
| Coexisting cancer | 21 (9.2) | 12 (7.3) | 9 (14.3) | .10 |
| Preoperative history of home oxygen | 6 (2.6) | 3 (1.8) | 3 (4.8) | .35 |
| Dementia or neurocognitive dysfunction | 3 (1.3) | 2 (1.2) | 1 (1.6) | 1.00 |
| Major psychiatric disorder | 64 (28.1) | 46 (27.9) | 18 (28.6) | .92 |
| Previous cardiothoracic surgery | 15 (6.6) | 10 (6.1) | 5 (7.9) | .61 |
| Reoperation | 34 (14.9) | 26 (15.8) | 8 (12.7) | .56 |
| Preoperative chemotherapy | 13 (5.7) | 10 (6.1) | 3 (4.8) | 1.00 |
| Preoperative thoracic radiation therapy | 5 (2.2) | 5 (3.0) | 0 (0.0) | .33 |
| Smoking | 94 (41.2) | 56 (33.9) | 38 (60.3) | <.001 |
| Opioid dependency | 17 (7.5) | 3 (1.8) | 14 (22.2) | <.001 |
| Alcohol abuse | 19 (8.3) | 10 (6.1) | 9 (14.3) | .04 |
| Opioids before surgery | 33 (14.5) | 5 (3.0) | 28 (44.4) | <.001 |
| Any pain medication before surgery | 89 (39.0) | 46 (27.9) | 43 (68.3) | <.001 |
| ASA classification | .10 | |||
| 2 | 65 (28.5) | 53 (32.1) | 12 (19.0) | |
| 3 | 122 (53.5) | 86 (52.1) | 36 (57.1) | |
| 4 | 41 (18.0) | 26 (15.8) | 15 (23.8) | |
Values in bold are statistical significance at P values < .05. IQR, Interquartile range; DVT, deep vein thrombosis; PE, pulmonary emboli; ASA, American Society of Anesthesiologists.
Table 4.
Procedural characteristics and opioid prescription after surgery
| Total |
Opioids after surgery |
P value | ||
|---|---|---|---|---|
| No |
Yes |
|||
| (N = 228) | (n = 165) | (n = 63) | ||
| Inpatient | 97 (42.5) | 64 (38.8) | 33 (52.4) | .06 |
| Anatomy | .001 | |||
| Lungs | 80 (35.1) | 56 (33.9) | 24 (38.1) | |
| Esophagus | 17 (7.5) | 14 (8.5) | 3 (4.8) | |
| Chest wall | 8 (3.5) | 2 (1.2) | 6 (9.5) | |
| Pleura | 12 (5.3) | 7 (4.2) | 5 (7.9) | |
| Mediastinum | 15 (6.6) | 9 (5.5) | 6 (9.5) | |
| Diaphragm | 4 (1.8) | 1 (0.6) | 3 (4.8) | |
| Foregut | 92 (40.4) | 76 (46.1) | 16 (25.4) | |
| Approach | .04 | |||
| Minimally invasive | 212 (93.0) | 157 (95.2) | 55 (87.3) | |
| Open | 16 (7.0) | 8 (4.8) | 8 (12.7) | |
| Procedure type | .004 | |||
| Open | 16 (7.0) | 8 (4.8) | 8 (12.7) | |
| Robot-assisted thoracoscopic | 93 (40.8) | 60 (36.4) | 33 (52.4) | |
| Robot-assisted laparoscopic | 94 (41.2) | 78 (47.3) | 16 (25.4) | |
| VATS | 24 (10.5) | 19 (11.5) | 5 (7.9) | |
| Laparoscopic | 1 (0.4) | 0 (0.0) | 1 (1.6) | |
| Thoracic versus abdominal cavity | .003 | |||
| Abdominal cavity | 97 (42.5) | 80 (48.5) | 17 (27.0) | |
| Thoracic cavity | 131 (57.5) | 85 (51.5) | 46 (73.0) | |
| Time in OR, h, median (IQR) | 2.9 (1.9, 3.9) | 2.7 (1.8, 3.8) | 3.3 (2.4, 4.5) | .01 |
| Surgeon | .02 | |||
| Surgeon 1 | 145 (83.3) | 111 (88.1) | 34 (70.8) | |
| Surgeon 2 | 22 (12.6) | 12 (9.5) | 10 (20.8) | |
| Surgeon 3 | 7 (4.0) | 3 (2.4) | 4 (8.3) | |
| Time between surgery and survey, d, median (IQR) | 37.0 (34.0, 40.0) | 39.0 (35.0, 40.0) | 36.0 (33.0, 40.0) | .14 |
Values are in frequency and % unless otherwise specified. Foregut–hiatal hernia repair with Nissen, Toupet, or LINX and Heller myotomy with Dor fundoplication. Values in bold are statistical significance at P values < .05. VATS, Video-assisted thoracoscopic surgery; OR, operating room; IQR, interquartile range.
Multiple logistic regression analysis of patient and procedural factors associated with requiring home opioid medication demonstrated that opioid use before surgery (odds ratio [OR], 28.80; 95% confidence interval [CI], 9.13-90.80, P < .001) was associated with postoperative home opioid use. In contrast, increase in age was associated with a decrease in home opioid use (OR, 0.96; 95% CI, 0.93-0.99, P = .01) (Table 5). Since opioid use before surgery is such a strong independent factor associated with home opioid use after surgery, we analyzed the opioid-naïve patients before surgery to determine additional factors associated with home opioid use in this subset of patients. Multiple logistic regression in opioid-naïve individuals (n = 195) showed that tobacco use (OR, 2.51; 95% CI, 1.09-5.78, P = .03), increased ASA classification (ASA 3: OR, 4.09; 95% CI, 1.15-14.53, P = .03; ASA 4: OR, 5.25; 95% CI, 1.23-22.45, P = .03), and any pain medication usage before surgery (OR, 2.78; 95% CI, 1.18-6.53, P = .02) were all significantly associated with need for opioid pain management at home after surgery. Moreover, increased age (OR, 0.95; 95% CI, 0.92-0.98, P = .001) was associated with a decrease in home opioid use (Table 5). Except for the body mass index, which had 23% of missing data, all the evaluated variables have complete data. Little's χ2 test for missing completely at random had a nonsignificant P value (.72), which suggests that the missing values could be completely random and do not influence the outcome. Of all the variables used in the analysis, only race and body mass index had missing data (26.3% and 23.7%, respectively, Table E2). The descriptive sensitivity analysis indicated that except for time in operation room and postoperative length of stay, the 2 groups of patients had similar level of postoperative outcomes, and specifically no difference in the primary outcome was found (opioids after surgery, P = .89, Table 5).
Table E2.
Missingness of variables used in the analysis
| Variable | Total | Missing no. | Percent missing |
|---|---|---|---|
| Age at surgery, y, median (IQR) | 228 | 0 | 0.0 |
| Sex | 228 | 0 | 0.0 |
| Race | 228 | 60 | 26.3 |
| Ethnicity | 228 | 0 | 0.0 |
| Inpatient | 228 | 0 | 0.0 |
| Primary payor cat4 | 228 | 0 | 0.0 |
| Body mass index, median (IQR) | 228 | 54 | 23.7 |
| Hypertension | 228 | 0 | 0.0 |
| Congestive heart failure | 228 | 0 | 0.0 |
| Coronary artery disease | 228 | 0 | 0.0 |
| Myocardial Infarction preop | 228 | 0 | 0.0 |
| Afib per EKG within last year | 228 | 0 | 0.0 |
| Valvular heart disease | 228 | 0 | 0.0 |
| Pulmonary hypertension | 228 | 0 | 0.0 |
| Interstitial fibrosis or interstitial lung disease | 228 | 0 | 0.0 |
| Major vascular disease | 228 | 0 | 0.0 |
| Deep vein thrombosis/pulmonary embolism | 228 | 0 | 0.0 |
| Cerebrovascular history | 228 | 0 | 0.0 |
| Diabetes | 228 | 0 | 0.0 |
| Liver dysfunction | 228 | 0 | 0.0 |
| On dialysis | 228 | 0 | 0.0 |
| Coexisting cancer | 228 | 0 | 0.0 |
| Preoperative chemotherapy or immunotherapy | 228 | 0 | 0.0 |
| Preoperative thoracic radiation therapy | 228 | 0 | 0.0 |
| Previous cardiothoracic surgery | 228 | 0 | 0.0 |
| Preoperative history of home O2 | 228 | 0 | 0.0 |
| Smoking | 228 | 0 | 0.0 |
| Narcotic dependency | 228 | 0 | 0.0 |
| Alcohol abuse | 228 | 0 | 0.0 |
| Dementia or neurocognitive dysfunction | 228 | 0 | 0.0 |
| Major psychiatric disorder | 228 | 0 | 0.0 |
| ECOG score | 228 | 0 | 0.0 |
| Reoperation | 228 | 0 | 0.0 |
| Surgical approach conversion | 228 | 0 | 0.0 |
| Intraoperative packed RBCs | 228 | 0 | 0.0 |
| ASA classification | 228 | 0 | 0.0 |
| Time between surgery and survey, d | 228 | 0 | 0.0 |
| Surgery type | 228 | 0 | 0.0 |
| Surgical type | 228 | 0 | 0.0 |
| Open | 228 | 0 | 0.0 |
| Minimally invasive type | 228 | 0 | 0.0 |
| Thoracic versus abdominal cavity | 228 | 0 | 0.0 |
| Time in OR, h | 228 | 0 | 0.0 |
| Any pain medication before surgery | 228 | 0 | 0.0 |
| Any non-narcotic pain medication before surgery | 228 | 0 | 0.0 |
| Narcotics before surgery | 228 | 0 | 0.0 |
| Tylenol before surgery | 228 | 0 | 0.0 |
| Aleve before surgery | 228 | 0 | 0.0 |
| Motrin before surgery | 228 | 0 | 0.0 |
| Neurontin before surgery | 228 | 0 | 0.0 |
| Ultram before surgery | 228 | 0 | 0.0 |
| T#3 before surgery | 228 | 0 | 0.0 |
| Percocet before surgery | 228 | 0 | 0.0 |
| Norco before surgery | 228 | 0 | 0.0 |
| Fentanyl before surgery | 228 | 0 | 0.0 |
| Oxycodone before surgery | 228 | 0 | 0.0 |
| Morphine before surgery | 228 | 0 | 0.0 |
| Number of different pain medications before surgery | 228 | 0 | 0.0 |
| Number of different pain medications before surgery | 228 | 0 | 0.0 |
IQR, Interquartile range; Afib, atrial fibrillation; EKG, electrocardiogram; ECOG, Eastern Cooperative Oncology Group; RBC, red blood cell; ASA, American Society of Anesthesiologists; OR, operating room.
Table 5.
Univariable and multivariable logistic regression of characteristics associated with opioid prescription (n = 228)
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Age at surgery, y, median (IQR) | 0.98 (0.97-1.00) | .12 | 0.96 (0.93-0.99) | .01 |
| Smoking | 2.96 (1.63-5.38) | <.001 | 2.16 (0.99-4.72) | .052 |
| Alcohol abuse | 2.58 (1.00-6.70) | .051 | 2.75 (0.86-8.75) | .09 |
| ASA classification | ||||
| 2 | (reference) | (reference) | ||
| 3 | 1.85 (0.88-3.87) | .10 | 2.43 (0.85-6.92) | .10 |
| 4 | 2.55 (1.04-6.22) | .04 | 2.84 (0.82-9.89) | .10 |
| Opioids before surgery | 25.60 (9.24-70.95) | <.001 | 28.80 (9.13-90.80) | <.001 |
| Surgical approach | ||||
| Minimally invasive | (reference) | (reference) | ||
| Open | 2.85 (1.02-7.97) | .045 | 2.57 (0.67-9.87) | .17 |
| Cavity | (reference) | |||
| Abdominal cavity | 2.55 (1.35-4.80) | .004 | (reference) | |
| Thoracic cavity | 1.15 (0.98-1.35) | .09 | 2.17 (0.91-5.18) | .08 |
| Time in operating room, h | 0.98 (0.97-1.00) | .12 | 1.18 (0.97-1.45) | .10 |
Values in bold are statistical significance at P values < .05. OR, Odds ratio; CI, confidence interval; IQR, interquartile range; ASA, American Society of Anesthesiologists.
Analysis of patients who took opioid medication(s) at home showed that most of the patients who took opioid medications at home required 2 different pain medications (47.6%) to manage pain. In contrast, patients who did not take opioid medications required 1 pain medication (38.8%, P < .001, Figure 2). The most common nonopioid pain medication in the opioid group was gabapentin (55.6%), and the most common opioid medication was tramadol (47.6%) and acetaminophen with hydrocodone (47.6%). In contrast, the most common medication to treat pain after surgery in a group of patients who did not use opioid medication was acetaminophen (82%) (Figure 3). The overall median pain score for the group at follow-up was 0 (IQR, 0.0-2.0). Between patients who had opioids at home versus no opioids, patients who were on opioids at home after surgery had a significantly greater median pain score of 2 (IQR, 0.0, 4.0, pain scale 1-10) compared with the patients who were taking no opioids at home after surgery, with a median pain score of 0 (IQR, 0, 1; P < .001) at follow-up. The use of different pain medications after a specific type of surgery is presented in the Tables 6 and E3.
Table E3.
Different pain medications used after surgery, stratified by type of surgery
| Type of surgery |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total |
Lungs |
Esophagus |
Chest wall |
Pleura |
Mediastinum |
Diaphragm |
Foregut |
P value | |
| N = 228 | N = 80 | N = 17 | N = 8 | N = 12 | N = 15 | N = 4 | N = 92 | ||
| No pain medication | 13 (5.7) | 4 (5.0) | 4 (23.5) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 4 (4.3) | .06 |
| Tylenol | 167 (73.2) | 57 (71.3) | 12 (70.6) | 4 (50.0) | 5 (41.7) | 11 (73.3) | 1 (25.0) | 77 (83.7) | .01 |
| Aleve | 55 (24.1) | 22 (27.5) | 1 (5.9) | 1 (12.5) | 2 (16.7) | 4 (26.7) | 0 (0.0) | 25 (27.2) | .39 |
| Motrin | 35 (15.4) | 13 (16.3) | 4 (23.5) | 1 (12.5) | 2 (16.7) | 1 (6.7) | 2 (50.0) | 12 (13.0) | .43 |
| Neurontin | 96 (42.1) | 57 (71.3) | 3 (17.6) | 5 (62.5) | 8 (66.7) | 9 (60.0) | 1 (25.0) | 13 (14.1) | <.001 |
| Flexeril | 2 (0.9) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) | .03 |
| Celebrex | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001 |
| Lidoderm patch | 2 (0.9) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1.00 |
| Lyrica | 1 (0.4) | 0 (0.0) | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .052 |
| Ultram | 30 (13.2) | 15 (18.8) | 0 (0.0) | 1 (12.5) | 2 (16.7) | 2 (13.3) | 2 (50.0) | 8 (8.7) | .08 |
| T#3 | 10 (4.4) | 1 (1.3) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 4 (26.7) | 2 (50.0) | 2 (2.2) | <.001 |
| Percocet | 3 (1.3) | 1 (1.3) | 0 (0.0) | 2 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001 |
| Norco | 28 (12.3) | 11 (13.8) | 3 (17.6) | 4 (50.0) | 2 (16.7) | 1 (6.7) | 0 (0.0) | 7 (7.6) | .03 |
| Morphine | 3 (1.3) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (1.1) | .03 |
| Hydrocodone | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001 |
| Fentanyl | 2 (0.9) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .17 |
| Oxycodone | 1 (0.4) | 1 (1.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .93 |
| Hydromorphone | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) | .96 |
| Number of different types of medications | <.001 | ||||||||
| 0 | 12 (5.3) | 4 (5.0) | 4 (23.5) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 3 (3.3) | |
| 1 | 69 (30.3) | 13 (16.3) | 5 (29.4) | 2 (25.0) | 4 (33.3) | 3 (20.0) | 1 (25.0) | 41 (44.6) | |
| 2 | 87 (38.2) | 29 (36.3) | 6 (35.3) | 3 (37.5) | 3 (25.0) | 7 (46.7) | 2 (50.0) | 37 (40.2) | |
| 3 | 49 (21.5) | 28 (35.0) | 1 (5.9) | 0 (0.0) | 3 (25.0) | 5 (33.3) | 1 (25.0) | 11 (12.0) | |
| 4 | 8 (3.5) | 5 (6.3) | 1 (5.9) | 1 (12.5) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 5 | 2 (0.9) | 1 (1.3) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 6 | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Narcotics | .001 | ||||||||
| No | 165 (72.4) | 56 (70.0) | 14 (82.4) | 2 (25.0) | 7 (58.3) | 9 (60.0) | 1 (25.0) | 76 (82.6) | |
| Yes | 63 (27.6) | 24 (30.0) | 3 (17.6) | 6 (75.0) | 5 (41.7) | 6 (40.0) | 3 (75.0) | 16 (17.4) | |
Values are in number and %.
Figure 2.
The number of different types of pain medications patients took after thoracic surgery. The most predominant proportion (48%) of the patients who took opioids as part of postoperative pain medication (Opioids) took 2 different types of pain medications. In contrast, patients who did not take opioid pain medication after surgery (No opioids) took 1 pain medication (39%, P < .001).
Figure 3.
Type of pain medication after surgery. The most common nonopioid pain medication in the opioid group was gabapentin (56%), and the most common opioid medication was tramadol (48%) and acetaminophen with hydrocodone (48%). The most common pain medication in patients who did not take opioid pain medication (No opioids) was acetaminophen (82%).
Table 6.
Multivariable logistic regression of characteristics associated with opioid prescription in patients naïve with both opioids and nonopioids before surgery (n = 139)
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| Age at surgery, y | 0.95 (0.91-0.99) | .02 |
| Smoking | 4.42 (1.45-13.50) | .01 |
| ASA classification | ||
| 2 | (reference) | |
| 3 | 3.65 (0.74-18.10) | .11 |
| 4 | 3.97 (0.71-22.13) | .12 |
| Cavity | ||
| Abdominal cavity | (reference) | |
| Thoracic cavity | 3.86 (0.89-16.76) | .07 |
Area under receiver operating characteristic curve = 0.78. Values in bold are statistical significance at P values < .05. OR, Odds ratio; CI, confidence interval; ASA, American Society of Anesthesiologists.
Discussion
Our previous studies showed that an ERAS program with an emphasis on pre-emptive pain control in thoracic surgery shows the ability to manage a patient's pain after a thoracic surgical procedure at home without opioids.2 A majority of patients in our cohort did not require opioid pain medications at home after surgery, and at follow-up, patients had excellent pain control. Our study shows that in the era before ERAS, thoracic surgeons often prescribed more opioids than patients needed to control their pain adequately. Studies looking at opioid prescriptions and usage after surgery before the ERAS era show that 7%-14% of patients fill opioid prescriptions without taking any pills dispensed, and up to 21% of the patients do not fill their opioid prescriptions at all.12,13 Moreover, Bartels and colleagues14 found 45% of patients after thoracic surgery took no or very few (5 or less) prescribed opioid pills. Thus, the ERAS program, especially with the emphasis of pre-emptive pain control, can have a significant impact reduction of opioid use after surgery. However, in our study, there was still a group of patients who required opioid pain medication at home.
In our cohort, the 2 most significant factors that contributed to the requirement of opioid pain medication after surgery were the use of opioid medications before surgery and younger age. The prevalence of opioid use before thoracic surgery was in line with observational study at a tertiary care academic medical center that included 34,186 patients.15 Their research showed that overall, about 23% of patients were taking preoperative opioids before all surgical procedures, with 65% in the orthopedic, 55% in the neurosurgical spine, and 15% in the thoracic surgery group. However, the use of opioids before surgery is not a factor that researchers often look at when they perform analysis of risk factors associated with the need for opioids after surgery. The population-based study of opioid prescriptions across health systems in Michigan in 2392 patients did not show that opioid use before surgery or any pain medication before surgery for chronic pain conditions.16 The main reason for lack of association between the pain medication before surgery and opioid pain medication after surgery in their study is that they did not use opioid or pain medication before surgery as a factor in their analysis. When this factor is used in the report, the use of opioids before surgery is associated with the need for opioid use after surgery.17,18 Moreover, using chronic pain as a patient factor shows an increase in risk for prolonged postoperative opioid use.19 Since our study shows a strong association between the pain medication before surgery and the need for opioids at home after surgery, it is essential to consider these factors in tailoring the patient's pain regimen after surgery. More likely than not, this group of patients will quickly fail the nonopioid pre-emptive pain regimen to manage pain after surgery. Although age was not significantly different in the univariable analysis between groups of no-home opioids and home opioids, this difference is borderline, with a median of 65.0 (IQR, 54.0, 73.0) versus 60.0 (IQR, 53.0, 70.0), respectively, P = .09. After we adjusted for the effect of other covariates in the multivariable analysis, the association between home opioids and age became significant.
In the cohort of patients who were opioid-naïve before surgery, we discovered that any pain medication before surgery, history of tobacco use, greater ASA classification, and younger age were associated with the need for opioid pain medication at home after thoracic surgery. The history of tobacco use is an independent factor for opioid use in other studies.16,20 A retrospective analysis of national insurance claims data of 36,177 patients after surgery has shown that smoking is an independent factor associated with persistent opioid use.20 The association between smoking and opioid use is likely due to those patients with a history of smoking may have a different perception of pain after surgery. Retrospective analysis of patient-reported outcomes evaluating pain after major surgery showed that current smokers have high pain intensity after surgery as well as a greater need for opioid pain medication after surgery.21 In addition, younger age16,19,22,23 as well as a high ASA score16 has been identified as patient factors for the need for an opioid medication after surgical procedures in other studies. It is unknown why these factors are associated with more pain after surgery.
In our study, there was no procedural factor that contributed to the need for opioids after surgery. This points to the fact that patient characteristics and there use of pain before surgery play a more critical factor in the need for opioids at home after surgery compared with the procedural factors. This is in contrast to other studies that show that procedure is a factor in the risk of opioid use after major surgery.16,24,25 For example, Clarke and colleagues25 performed a retrospective analysis of 39,140 patients who underwent major elective surgery at acute care hospitals found that patients who had the thoracic procedure had significantly prolonged opioid use compared with patients undergoing open radical prostatectomies. One major limitation of such analysis is that there are different groups of surgeons who are proscribing opioids after prostatectomy compared with thoracic procedures. Moreover, each group might have a different protocol in terms of evaluating pain and prescribing opioids. Thus, the major flaw of the study that looks at a large health care system is that it does not control for the prescriber. In contrast, in our cohort, all patients were treated in a similar fashion using a standardized regimen for the treatment of pain. This is one of the reasons for the lack of association of procedure with opioid medication in our study in contrast to the other studies. Another possible reason for the muting effect of the procedural factor may be that most of the patients underwent a minimally invasive approach. Overall, patients have lower pain levels after minimally invasive surgery, leading to less need for opioid medication at the time of discharge.
The pain survey was a very effective patient-reported outcome measure at the time of follow up that provided a quick and more accurate snapshot of the patient's pain medication requirement after surgery, their pain level at the time of the visit and the pain medications patients took before surgery. The survey was a better measure of the medications that patients took compared with the data on the prescribed medication. The analysis of the pain medication that the patient took showed that not all patients received the recommended regimen of pain medication at home. All patients who were going home without opioids were instructed to take acetaminophen; however, only 82% of the patients took the medication. This shows that the medications that were given to patients at discharge are not necessarily what the patient will take at home. Thus, this patient-reported outcome provided a more accurate assessment of the patient's pain level and pain medication needs after surgery.
There were several additional limitations to our study. Our study is a retrospective analysis of data that was collected in a single institution with most of the operations performed by a single surgeon. However, the data were collected from the patient's report of their perception of pain and use of pain medication before and after surgery at the time of follow-up visit, providing an accurate assessment of pain and the pain medication that was taken after the surgery. Moreover, having a single-institution analysis allowed for an outcome of a mature ERAS program with an emphasis on pre-emptive pain control. Moreover, one of the limitations was a wide range of dates of follow-up between 3 and 8 weeks. However, there was no significant difference in the follow-up time between the patients who required opioids after surgery and who did not require opioids after surgery. Furthermore, since the survey asked for all pain medications patients took after surgery, the range of the dates had a limited impact on the conclusion of the study. Another limitation of the study is that most of the patients underwent a minimally invasive approach; thus, the conclusions may not apply to a program that performs most of the operations in an open manner. The final limitation is that we may not have included all factors that could contribute to pain after surgery. Although we had a comprehensive list of patient and procedural factors, we may have missed other factors that could have contributed to the need for an opioid medication after surgery. Factors such as major complications after surgery were not studied in the analysis since the current cohort was unable to answer the question about the relationship between complications and opioid use.
Conclusions
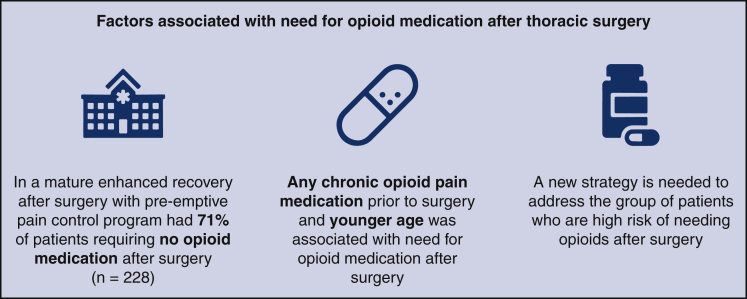
In a mature ERAS program, with an emphasis on pre-emptive pain control in the thoracic surgery program, a majority of patients were able to be managed without opioid medications at home. Also, procedural factors did not have an impact on the need for opioid medication at home; instead, patient factors contributed to the need for opioid medication after surgery. The most substantial factors that are associated with home opioid medicines after surgery are patients taking an opioid medication before surgery. Thus, in this group of patients, prescribers should have a lower threshold to add opioid pain medication after surgery. In patients who are opioid-naïve before surgery, the use of nonopioid pain medication before surgery, younger age, current or former smokers, and patients with greater ASA scores should be factors to be mindful while managing their postoperative pain. Thus, knowing these personal characteristics provides a way to personalize home pain medication regimen after surgery (Figure 4).
Figure 4.
In a mature thoracic program with enhanced recovery after surgery with pre-emptive pain control (n = 228), the majority of patients did not require opioid medication after surgery (71%). Any chronic opioid pain medication before surgery and younger age were associated with the need for opioid pain medication after surgery. A different strategy is needed to address this group of patients to maintain pain control after surgery.
Conflict of Interest Statement
Dr Kim has taught courses for Intuitive Surgical, Veran, and Medtronic. Dr Chan has taught courses for Veran. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
We thank Elaine Jordan, Libra Broussard, and Jeanette Corvera for their help with the pain questionnaire.
Appendix
Tables E1 to E3:
Supplementary Data
Port placement and liposomal bupivacaine nerve block. Video demonstrates the typical port placement and liposomal bupivacaine nerve block during a robot-assisted lobectomy. Video available at: https://www.jtcvs.org/article/S2666-2736(20)30174-1/fulltext.
References
- 1.Kim M.P., Chan E.Y., Meisenbach L.M., Dumitru R., Brown J.K., Masud F.N. Enhanced recovery after thoracic surgery reduces discharge on highly dependent narcotics. J Thorac Dis. 2018;10:984–990. doi: 10.21037/jtd.2018.01.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim M.P., Godoy C., Nguyen D.T., Meisenbach L.M., Chihara R., Chan E.Y., et al. Preemptive pain-management program is associated with reduction of opioid prescriptions after benign minimally invasive foregut surgery. J Thorac Cardiovasc Surg. 2020;159:734–744.e4. doi: 10.1016/j.jtcvs.2019.06.056. [DOI] [PubMed] [Google Scholar]
- 3.Thompson C., French D.G., Costache I. Pain management within an enhanced recovery program after thoracic surgery. J Thorac Dis. 2018;10(Suppl 32):S3773–S3780. doi: 10.21037/jtd.2018.09.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin L.W., Sarosiek B.M., Harrison M.A., Hedrick T., Isbell J.M., Krupnick A.S., et al. Implementing a thoracic enhanced recovery program: lessons learned in the first year. Ann Thorac Surg. 2018;105:1597–1604. doi: 10.1016/j.athoracsur.2018.01.080. [DOI] [PubMed] [Google Scholar]
- 5.Batchelor T.J.P., Rasburn N.J., Abdelnour-Berchtold E., Brunelli A., Cerfolio R.J., Gonzalez M., et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS(R)) Society and the European Society of Thoracic Surgeons (ESTS) Eur J Cardiothorac Surg. 2019;55:91–115. doi: 10.1093/ejcts/ezy301. [DOI] [PubMed] [Google Scholar]
- 6.Medbery R.L., Fernandez F.G., Khullar O.V. ERAS and patient reported outcomes in thoracic surgery: a review of current data. J Thorac Dis. 2019;11(Suppl 7):S976–S986. doi: 10.21037/jtd.2019.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes M.M., Lewith G., Newell D., Field J., Bishop F.L. The impact of patient-reported outcome measures in clinical practice for pain: a systematic review. Qual Life Res. 2017;26:245–257. doi: 10.1007/s11136-016-1449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J.K., Singh K., Dumitru R., Chan E., Kim M.P. The benefits of enhanced recovery after surgery programs and their application in cardiothoracic surgery. Methodist Debakey Cardiovasc J. 2018;14:77–88. doi: 10.14797/mdcj-14-2-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stata Base Reference Manual Release 16: A Stata Press publication. StataCorp; College Station, TX: 2019. [Google Scholar]
- 10.Hastie T., Tibshirani R., Wainwright M. 1st ed. CRC Press; Boca Raton, FL: 2015. Statistical Learning with Sparsity: The Lasso and Generalizations. [Google Scholar]
- 11.Little R.J.A. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1202. [Google Scholar]
- 12.Holst K.A., Thiels C.A., Ubl D.S., Blackmon S.H., Cassivi S.D., Nichols F.C., III, et al. Postoperative opioid consumption in thoracic surgery patients: how much is actually used? Ann Thorac Surg. 2020;109:1033–1039. doi: 10.1016/j.athoracsur.2019.08.115. [DOI] [PubMed] [Google Scholar]
- 13.Bicket M.C., Long J.J., Pronovost P.J., Alexander G.C., Wu C.L. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152:1066–1071. doi: 10.1001/jamasurg.2017.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartels K., Mayes L.M., Dingmann C., Bullard K.J., Hopfer C.J., Binswanger I.A. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One. 2016;11:e0147972. doi: 10.1371/journal.pone.0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilliard P.E., Waljee J., Moser S., Metz L., Mathis M., Goesling J., et al. Prevalence of preoperative opioid use and characteristics associated with opioid use among patients presenting for surgery. JAMA Surg. 2018;153:929–937. doi: 10.1001/jamasurg.2018.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard R., Fry B., Gunaseelan V., Lee J., Waljee J., Brummett C., et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surg. 2019;154:e184234. doi: 10.1001/jamasurg.2018.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirji S.A., Landino S., Cote C., Lee J., Orhurhu V., Shah R.M., et al. Chronic opioid use after coronary bypass surgery. J Card Surg. 2019;34:67–73. doi: 10.1111/jocs.13981. [DOI] [PubMed] [Google Scholar]
- 18.Harbaugh C.M., Lee J.S., Hu H.M., McCabe S.E., Voepel-Lewis T., Englesbe M.J., et al. Persistent opioid use among pediatric patients after surgery. Pediatrics. 2018;141:e20172439. doi: 10.1542/peds.2017-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stafford C., Francone T., Roberts P.L., Ricciardi R. What factors are associated with increased risk for prolonged postoperative opioid usage after colorectal surgery? Surg Endosc. 2018;32:3557–3561. doi: 10.1007/s00464-018-6078-3. [DOI] [PubMed] [Google Scholar]
- 20.Brummett C.M., Waljee J.F., Goesling J., Moser S., Lin P., Englesbe M.J., et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montbriand J.J., Weinrib A.Z., Azam M.A., Ladak S.S.J., Shah B.R., Jiang J., et al. Smoking, pain intensity, and opioid consumption 1-3 months after major surgery: a retrospective study in a hospital-based transitional pain service. Nicotine Tob Res. 2018;20:1144–1151. doi: 10.1093/ntr/ntx094. [DOI] [PubMed] [Google Scholar]
- 22.Behman R., Cleary S., McHardy P., Kiss A., Sawyer J., Ladak S.S.J., et al. Predictors of post-operative pain and opioid consumption in patients undergoing liver surgery. World J Surg. 2019;43:2579–2586. doi: 10.1007/s00268-019-05050-7. [DOI] [PubMed] [Google Scholar]
- 23.Namba R.S., Singh A., Paxton E.W., Inacio M.C.S. Patient factors associated with prolonged postoperative opioid use after total knee arthroplasty. J Arthroplasty. 2018;33:2449–2454. doi: 10.1016/j.arth.2018.03.068. [DOI] [PubMed] [Google Scholar]
- 24.Sun E.C., Darnall B.D., Baker L.C., Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176:1286–1293. doi: 10.1001/jamainternmed.2016.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke H., Soneji N., Ko D.T., Yun L., Wijeysundera D.N. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Port placement and liposomal bupivacaine nerve block. Video demonstrates the typical port placement and liposomal bupivacaine nerve block during a robot-assisted lobectomy. Video available at: https://www.jtcvs.org/article/S2666-2736(20)30174-1/fulltext.