Abstract
The current investigation extended prior cross-sectional mapping of etiological factors, transdiagnostic effortful and affective traits, and ADHD symptoms to longitudinal pathways extending from two etiological domains: polygenic and prenatal risk. Hypotheses were (1) genetic risk for ADHD would be related to inattentive ADHD symptoms in adolescence and mediated by childhood effortful control; (2) prenatal smoking would be related to hyperactive-impulsive ADHD symptoms during childhood and mediated by childhood surgency; and (3) there would be age-related variation, such that mediation of genetic risk would be larger for older than younger ages, whereas mediation of prenatal risk would be larger in earlier childhood than at later ages. Participants were 849 children drawn from the Oregon ADHD-1000 Cohort, which used a case control sample and an accelerated longitudinal design to track development from childhood (at year 1 ages 7-13) through adolescence (at year 6 ages 13-19). Results showed the mediational pathway from prenatal smoking through surgency to hyperactivity-impulsivity at Year 1 was significant (indirect effect estimate = .053, p < .01). The mediational pathway from polygenic risk through effortful control to inattention at Year 6 was also significant (indirect effect estimate = .084, p < .01). Both results were independent of the association between inattention and hyperactivity-impulsivity and control for the alternative etiological input and held across parent- and teacher-report of ADHD symptoms. In line with dual pathway models of ADHD, early prenatal risk for hyperactivity-impulsivity appears to operate through surgency, while polygenic genetic risk for inattention appears mediated by effortful control.
Keywords: ADHD, effortful control, surgency, pathways, genetics, prenatal smoking
Attention-Deficit/Hyperactivity Disorder (ADHD) is a heterogeneous neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity-impulsivity, or both. Due to the fact that children with ADHD can look inattentive, hyperactive, or some combination of both, the DSM-versions have proposed subtypes, now called presentations (American Psychiatric Association, 2013). In fact, such patterns are consistent with current, best-fitting models of ADHD, specifically the bifactor model characterized by a shared “g,” or general, ADHD factor, and specific, “s,” inattention and hyperactivity-impulsivity factors (Goh et al., 2020; Martel et al., 2009; Toplak et al., 2009). However, the two ADHD symptom domains of inattention and hyperactivity-impulsivity are thought to have at least partially dissociable etiological inputs (Willcutt et al., 2012). For this reason, multiple pathway models of ADHD have been proposed (Nigg, 2004; Sonuga-Barke, 2002; 2005; 2010).
Although ADHD is not reducible to personality or temperament traits (Martel & Nigg, 2006; Nigg et al., 2002), ADHD has been associated with particular transdiagnostic trait-like markers including reduced executive function (defined as cognitive functions that support goal-oriented activity such as working memory, inhibition, and planning), low effortful control (defined as the ability to overcome an impulse to carry out a goal-oriented action, an early developing precursor to executive functioning and a component of it; Diamond, 2013; Nigg, 2017), high surgency (or approach), and high negative affectivity (Goth-Owens et al., 2010; Martel et al., 2009; Willcutt et al., 2005; early results reviewed in Nigg, 2006; also see Nigg et al., 2020 ). These map differentially on to diverse etiological pathways to ADHD.
Multiple pathways to ADHD generally coalesce around the idea that regulatory forms of control such as effortful control and executive function are more related to inattention, while more affective traits related to innate reactivity such as surgency and negative affectivity are more related to hyperactivity-impulsivity (Nigg, 2004; Sonuga-Barke, 2005). Particularly when partialing out the substantial shared variance between inattention and hyperactivity-impulsivity, empirical work generally supports somewhat differential associations between effortful control and inattention versus between surgency and hyperactivity-impulsivity (Martel et al., 2009). Likewise, in a cross sectional bifactor model, the ADHD “g” factor shows associations with both regulatory and reactive traits, whereas “s” inattention is more specifically related to regulatory traits such as effortful control, and “s” hyperactivity-impulsivity is related more to reactivity including reactive, or reflexive, control related to surgency, as well as extraversion (Martel et al., 2010). Yet, much of this work has only been conducted cross-sectionally, and more critical examination of dual pathways to these component symptom dimensions with partially distinct etiological and trait mediators requires longitudinal evaluation.
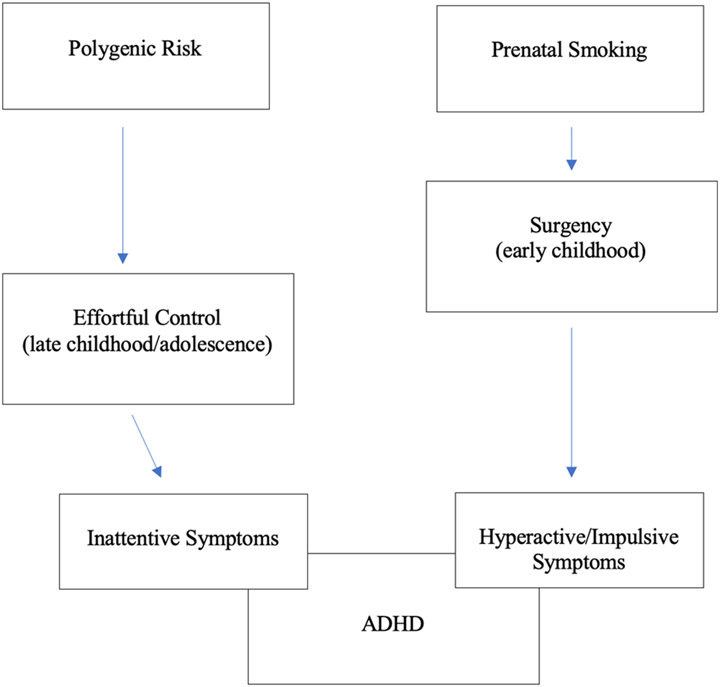
Cross-sectional work in different age ranges is consistent with the idea of developmental effects on etiological pathways to ADHD, although true longitudinal developmental pathways have not been tested. As shown in Figure 1, prenatal risk, particularly maternal substance abuse or smoking, seem to be related to hyperactivity-impulsivity (Roberts & Martel, 2014) as well as to surgency (Martel et al., 2012; 2014). We have proposed that this effect is particularly notable earlier in development such as during preschool and may fade out over time (Wagner et al., 2009). It may be mediated by maturation of subcortical and limbic neural circuits, potentially operating through effects on subcortical and limbic regions of the brain (Halperin & Schulz, 2006). That is, early prenatal insults may differentially slow down early development and increase reactivity and attendant activity levels. In contrast, the role of genetics on ADHD (as well as on related traits like effortful control) seem to become stronger as individuals age, begin elementary school, and go through adolescence, potentially operating through effects on the prefrontal cortex (Bouchard, 2013; Nigg et al., 2018; Rice et al., 2018). It may well be that innate predisposition leads to active and evocative gene-environment interactions manifested by effortful control and attention. Therefore, there may be some differences in the strength of these pathways over time, particularly between early childhood and adolescence.
Figure 1.
Conceptual Model
Again, largely cross-sectional work suggests that etiological factors such as genetics and exposure to prenatal risk factors show somewhat distinct associations with temperament/personality traits and ADHD symptoms in preliminary studies (Gizer & Waldman, 2009; Wiggs et al., 2016). For example, dopaminergic genetic alleles were related somewhat more clearly to inattentive symptoms (Gizer & Waldman, 2009), and explained cross-sectionally by trait conscientiousness (another measure of effortful forms of control at a trait level; Martel et al., 2010; 2011). Conversely, prenatal risk factors such as maternal smoking or substance use seem to increase risk for disruptive behaviors more generally (Buschgens et al., 2008; D’Onofrio et al., 2007; Skoglund et al., 2014), and--within ADHD--perhaps particularly hyperactivity-impulsivity (Martel & Roberts, 2014). Prenatal tobacco use may be a particularly useful marker of risk, given its known associations with increased risk for child ADHD (Langley et al., 2005; Linnet et al., 2003) as well as with more difficult child temperament (Huizink, 2012; Takegata et al., 2021) and impaired executive functioning (Desekin et al., 2015; Piper & Corbett, 2012). Further, etiological factors show differential associations with ADHD symptom domains mediated through different neurocognitive factors (Wiggs et al., 2016). In single gene studies, gene-by-environment interaction effects on ADHD, and particularly inattention, appeared mediated by more effortful forms of control such as trait conscientiousness, a personality trait related to the temperament trait of effortful control (Martel et al., 2011; 2012).
Altogether, cross-sectional studies provide support for at least somewhat differential pathways to inattention versus hyperactivity-impulsivity, in line with multiple pathways to ADHD. Namely, genetic risk may lead to worse cognitive and effortful control, which in turn differentially increases risk for inattentive ADHD symptoms. However, candidate gene studies are now known to have serious limitations, hence the need for follow up with a more robust approach using polygenic risk (Ronald, de Bode, & Polderman, 2021). Concomitantly, prenatal risk factors may be related less strongly to inattention and more generally to disruptive behavior, perhaps particularly hyperactive-impulsive ADHD symptoms. We hypothesize these effects are preferentially mediated by motivational and affective traits such as surgency. Yet, again, such claims have primarily relied upon cross-sectional work, and no known work to date has examined longitudinal pathways over an extended developmental period in childhood and adolescence.
Present Study
The goal of the current investigation was to extend prior work on cross-sectional associations of etiological factors, transdiagnostic effortful and affective traits, and ADHD symptoms to longitudinal pathway models related to ADHD. We primarily sought to determine whether at least somewhat distinct, dual pathways to ADHD inattention and hyperactivity-impulsivity hold longitudinally, the first test of its kind. Based on prior cross-sectional work, it was hypothesized that genetic risk would be related to inattentive ADHD symptoms and mediated by effortful control, particularly at older ages, while prenatal risk factors would be related to hyperactive-impulsive ADHD symptoms and mediated by surgency, particularly at younger ages. We further predicted that such pathways would be at least partially distinct, consistent with dual pathway models.
Methods
Participants were drawn from the Oregon ADHD-1000 Cohort, based on an accelerated longitudinal design to track ADHD development from childhood through adolescence. Details of recruitment, enrollment, and multi-method, multi-informant assessment procedures for ADHD diagnosis have been published in detail elsewhere (Musser et al., 2016; Karalunas et al., 2017); the supplement to Nigg et al. (2018) provides extensive further details of all methods and procedures. This background is summarized again below. Ethics approval was obtained from the Institutional Review Board at Oregon Health & Science University. A parent/legal guardian provided written informed consent and children provided written assent. 876 children were followed annually over 8 years. Data for the current study was drawn from 849 children from the baseline year (Year 1; aged 7-13 years) and 344 at a second time point (Year 6) six years later. Results were checked in a subset of the most prevalent ethnic group (northern European ancestry, n = 609). Family relatedness was handled as explained below (n = 656 were unrelated).
Participants and Sample Characterization
Recruitment.
Volunteers were recruited via mass mailings, using commercial mailing lists, to all families with children in the target age range within the geographic radius of 50 miles from a Northwest University in the United States. The mailing made clear that we were looking for children with possible or definite ADHD, as well as typically developing children with no history of learning or attention problems. In response to mailings, we received 2,144 inquiries. During an initial screening phone call, nearly half of the initial inquiries were excluded because of prescribed non-stimulant psychotropic medications, a history of non-febrile seizure, head injury with loss of consciousness > 60 seconds, autism spectrum disorder or intellectual disability, any other major medical conditions, history of psychosis, mania, or Tourette’s syndrome. Those who were excluded at this stage did not differ reliably from the final sample on sex ratio (p = .11) or non-white race (p = .22), but reported marginally lower family incomes (p = .06) and were slightly younger (p = .06).
Sample Characterization.
For those remaining (n = 1,449), an in-person “diagnostic” visit was then scheduled. Here, a parent completed the ADHD Rating Scale and a semi-structured clinical interview administered by a Master’s-level clinician, the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS; Puig-Antich & Ryan, 1986). Informants were asked to provide ratings of symptoms based on children’s behavior when they were not taking prescribed medications. Children completed a brief unstructured clinical interview with the same clinician. Interviewers and testers wrote detailed observational notes. Teachers were contacted and completed the ADHD Rating Scale (ADHD-RS). All clinical interviewers were trained to reliability of kappa >.80 for all diagnoses seen at ≥5% base rate in this sample on the KSADS to a master interviewer, and had videotapes viewed by a supervisor and reviewed periodically to prevent procedural drift. Psychometric testers were trained to an accuracy standard prior to beginning work and also had videotapes viewed periodically to prevent drift.
After the diagnostic team met (Step 2), 103 withdrew due to lack of further interest (e.g., only wanted the diagnostic screen), and 496 were ruled out for the following reasons: excess teacher-parent rating discrepancy (situational problems; 35%), subthreshold symptom count (not control or ADHD, 17%), psychosis, mania, current severe depressive episode, Tourette’s syndrome, or head injury (10%), autism (7%), other health condition (7%), ineligible medication (2%), IQ<80 (n = 1), or multiple rule outs. Among the eligible children with ADHD, 35% were prescribed stimulant medications and needed to complete a washout period, only slightly lower than rates in community surveys for pre-adolescent children.
Diagnostic assignment using a Best Estimate procedure.
All materials were scored and presented to a clinical diagnostic team comprising of a board certified child psychiatrist and a licensed child neuropsychologist. Team members independently formed a diagnostic opinion based on all available information. Their agreement rate for all diagnoses discussed in this paper was satisfactory (ADHD, kappa (k) = .88; ADHD subtype, k > .80, all other disorders with at least 5% base rate, k > .68). Disagreements were conferenced and consensus reached. Cases where consensus was not readily achieved for ADHD diagnosis were excluded.
Longitudinal Retention
Participants included 849 children in Year 1, from whom n = 610 were selected for the long term follow up study. They were followed annually for eight sequential years. With regard to retention, resource limitations mandated a planned missing design from among those youth. Three hundred forty-four children have been seen at Year 6 to date and that was the N for the Year 6 analyses. Those selected for follow up were chosen because their ADHD and non-ADHD status was clear and unambiguous. Those not followed included those with parent-teacher disagreement or sufficient comorbidity to reduce confidence that they were clear cases or non-cases by diagnostic team judgement.
Measures
ADHD symptoms.
The teacher-report version of the ADHD-Rating Scale for DSM-IV (ADHD-RS; DuPaul et al., 1998; Puig-Antich & Ryan, 1986) was used as the measure of ADHD symptomatology. Teachers responded to all 18 items on a 0 (rarely or never) to 3 (always or very often) scale (Year 1: α = .97; Year 5-8: α = .96). 0 and 1 responses were coded as 0, and 2 and 3 responses were coded as 1 to create sum scales on inattentive and hyperactive-impulsive symptom domains ranging from 0 to 18. Teacher response rate was 100% at Year 1 (required for inclusion) and >80% at Year 6. Informant demographic information was not available on teachers. For main analyses, clinician ratings of parent-report on the KSADS were utilized. Results were checked using teacher-report on the ADHD-RS with no change in main analyses.
Effortful and affective traits.
Effortful and affective traits were assessed by parent-report at each timepoint using the Temperament in Middle Childhood Questionnaire (TMCQ, Simonds & Rothbart, 2004). Based on the hypothesized pathways to ADHD of interest, Extraversion/Surgency, and Effortful Control were the focus in the current study (Rothbart, 1981). These scales had reliability of .64 or above at each time point. As a secondary check, analyses were rerun removing items that overlapped with ADHD symptoms which dropped reliability to .60 for both scales. Main result analyses did not change.
Prenatal maternal smoking.
At enrollment, each child’s primary caregiver (n = 625 biological mothers, n = 154 biological fathers, n = 34 non-biological parent respondents) completed a comprehensive developmental history questionnaire that is described in detail in Wiggs et al. (2016). Respondents were asked to report whether the mother smoked tobacco during her pregnancy with this child. Responses were coded into none (0) or any (1). Though more detailed information about frequency of prenatal tobacco use was collected as part of this measure, given relatively low rates of tobacco use in this sample (n = 83; 9.5%) the dichotomized version of this variable was used in analyses to optimize statistical power.
Genotyping and polygenic risk score.
DNA was extracted from saliva collected in OrageneR cups (DNA Genotek Inc., Kanata, ON, Canada) using manufacturer’s protocols in the OHSU Gene Profiling Shared Resource. All subjects were genotyped at the Stanley Center for Psychiatric Research (Broad Institute of MIT and Harvard, Cambridge, MA) using the PsychCHIP v1-1 (N = 603,132 single nucleotide polymorphisms (SNPs), developed by Illumina, Inc (San Diego, CA) in collaboration with the Psychiatric Genetics Consortium (PGC). Genotypes were generated from array intensity values using GenomeStudio (Illumina, Inc). Nongenotyped SNPs were imputed with IMPUTE2 (Howe, Donnelly, & Marchini, 2009) using the 1000 Genomes phase 3 reference panel. Additional details of the genotype data processing have been published previously (Nigg et al., 2018).
The ADHD polygenic risk score (PRS) was constructed using the PGC meta-analysis (Demontis, Walters, Martin, et, & al., 2019) as the discovery data set (19,099 ADHD cases and 34,194 controls, European-ancestry only). Higher scores indicate higher risk of diagnosis. Only SNPs with INFO score (imputation quality) ≥ 0.8 in both the PGC meta-analysis and our data were considered. SNPs were LD-clumped based on r2<0.1 using genotypes from the European-ancestry samples of the 1000 Genomes phase 3 data set, using PLINK v1.9 (https://www.cog-genomics.org/plink2). From this filtered (LD clumped) SNP list, SNPs with p ≤ 0.5 were selected for the polygenic score computation (N = 139,934). The PRS was calculated for all subjects in the Oregon ADHD-1000 data set by multiplying the number of risk alleles (0, 1, or 2) by the log(odds ratio) of that SNP in the discovery data set and averaging over all SNPs. The allelic scoring function (--score) in PLINK v1.9 was used for the PRS calculation. Relatedness was confirmed with the genotype data using the PC-Relate method (via the Bioconductor package GENESIS (Conomos et al., 2016) and was handled via the clustering algorithm in Mplus. We handled potential population stratification artifact by covarying the first 10 genomic principal components. We then checked results in the European-ancestry only subsample and in only unrelated European-ancestry subjects, with no change in the fundamental picture (see results below).
Statistical Analyses
First, correlational analyses were conducted between all risk factors (prenatal maternal smoking and polygenic risk), temperamental markers (surgency and effortful control), and ADHD symptoms (number of hyperactive/impulsive symptoms and number of inattentive symptoms). Next, variables were entered into differential regression analyses to assess the distinct concurrent value of risk factors and temperamental features in relation to ADHD inattentive versus hyperactive-impulsive ADHD symptoms.
Then, variables were entered into hierarchical regression analyses to assess the predictive value of risk factors and temperamental features in relation to ADHD symptoms. Bootstrapping mediation analyses estimating direct and indirect effects in Mplus were used. Models were developed using established path modeling logic (Kenny, 1979). In path diagram logic, models are designed to be consistent with causal theory (even though, in this case, observations are cross-sectional). A hypothesized causal path gets a one-directional arrow (and computes a regression coefficient). An unexamined association that exists between two constructs gets a curved dual-headed arrow (and computes a correlation).1 We developed the model based on the conceptual logic of how the variables relate. Namely, we tested whether effortful control accounted for the relationship between polygenic risk factors and inattentive symptoms, and whether surgency accounted for the relationship between prenatal risk factors and hyperactive/impulsive symptoms.
Secondary checks evaluating prediction of longitudinal change in symptoms over time were conducted, as well as specificity of analyses to etiological input and symptom domain.
Results
Correlational analyses.
As shown in Table 1, correlational analyses indicated that inattentive and hyperactive/impulsive ADHD symptoms at Year 1 and Year 6 were significantly associated with effortful control (r range −.44 to −.77, p < .01) and surgency (r range .33 to .59, p < .01) at Year 1, as well as with ADHD polygenic score (r range .18 to .24, p < .05) and prenatal maternal smoking (r range .16 to .18, p < .05). Effortful control was not significantly associated with prenatal maternal smoking (r = −.10), but otherwise, both traits were associated with both etiological factors (r range −.19 to .15, p < .01).
Table 1.
Correlations between temperament, ADHD symptoms, and risk factors at Years 1 and 6
| Factor M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
|
1 Year 1 Inattentive Symptoms 4.37 (3.52) |
1 | .69** | .73** | .50** | −.77** | .33** | .16** | .18** |
|
2 Year 1 Hyperactive Symptoms 3.19 (3.03) |
.69** | 1 | .59** | .65** | −.65** | .59** | .18** | .21** |
|
3 Year 6 Inattentive Symptoms 3.38 (3.42) |
.73** | .59** | 1 | .62** | −.62** | .33** | .17** | .24** |
|
4 Year 6 Hyperactive Symptoms 1.61 (2.30) |
.50** | .65** | .62** | 1 | −.44** | .44** | .16* | .20* |
|
5 Year 1 Effortful Control 3.22 (0.52) |
−.77** | −.65** | −.62** | −.44** | 1 | −.30** | −.10 | −.19** |
|
6 Year 1 Surgency 3.51 (0.55) |
.33** | .59** | .33** | .44** | −.30** | 1 | .15** | .12** |
|
7 Prenatal Maternal Smoking 84.7% no, 9.5% yes |
.16** | .18** | .17** | .16* | −.10 | .15** | 1 | 0.11 |
|
8 ADHD PRS Score 0.027 (0.00) |
.18** | .21** | .24** | .20* | −.19** | .12** | .11 | 1 |
Note. Participants were ages 7-13 years at Year 1 and 13-19 years at Year 6
p<.001
p<.05
Differential regression analyses.
Next, we tested whether these pathways were at least partially distinct to symptom domain by entering the two ADHD symptom domains as simultaneous, independent predictors in order to partial out their shared covariance. As shown in Table 2, shared variance between inattentive and hyperactive-impulsive ADHD symptoms at Year 6 were partialed out by entering them simultaneously in multivariate regression analyses as independent variables predicting the temperament traits of effortful control and surgency at Year 1 as dependent variables respectively. Effortful control was only significantly associated with inattentive ADHD symptoms (β = −.08, p < .01; not hyperactive-impulsive ADHD symptoms β = −.02, p = .081) at Year 6, and surgency was only significantly associated with hyperactive/impulsive ADHD symptoms (β = .09, p < .01; but not inattentive ADHD symptoms β = .01, p = .17) at Year 6.
Table 2.
Differential regression analyses predicting longitudinal ADHD symptoms as a function of temperament
| B | SE(B) | β | t | Sig. | |
|---|---|---|---|---|---|
| Effortful control → Year 6 Inattentive symptoms | −0.08 | 0.01 | −0.56 | −10.43 | p<.001 |
| Effortful control → Year 6 Hyperactive symptoms | −0.02 | 0.01 | −0.09 | −1.75 | p=.081 |
| Surgency → Year 6 Inattentive symptoms | 0.01 | 0.01 | 0.09 | 1.37 | p=0.17 |
| Surgency → Year 6 Hyperactive symptoms | 0.09 | 0.02 | 0.39 | 6.29 | p<.001 |
Note. Participants were 13-19 years of age at Year 6
Hierarchical regression analyses.
To provide a preliminary examination of mediation, hierarchical regression analyses were conducted in which etiological factors were entered at Step 1, and Year 1 traits were entered at Step 2; Year 1 or Year 6 parent- or teacher-report inattentive or hyperactive-impulsive ADHD symptoms were the dependent variables. Two theory-based models were tested: (1) effortful control as a mediator of the pathway between polygenic risk for ADHD and inattentive ADHD symptoms and (2) surgency as a mediator of the pathway between prenatal maternal smoking and hyperactive-impulsive ADHD symptoms. Pathways involving genetic risk were evaluated in a European-ancestry subsample and a European-ancestry (unrelated) subsample with similar results as the full sample which are the focus here.
As shown in Table 3, hierarchical regressions indicated in all instances that the significant association between etiological factors and ADHD symptoms became nonsignificant based on whether outcomes were assessed at Year 1 or 6 or whether assessed using parent-report or teacher-report (not shown). That is, the path between etiological factors and outcomes decreased significantly when traits were entered in Step 2 of the regression (β dropped from .24, p < .01 to .11, p > .01 for polygenic risk-inattention pathway; β dropped from .14, p < .05 to .09, p > .01 for prenatal maternal smoking-hyperactivity/impulsivity pathway).
Table 3.
Hierarchical regression analyses of predictors of concurrent and longitudinal ADHD symptoms
| DV | IV | B | SE(B) | β | R 2 | ΔR2 |
|---|---|---|---|---|---|---|
| Year 1 Inattentive Symptoms | Step 1: | |||||
| ADHD PRS score | 18482.06 | 3634.52 | .18** | .03 | ||
| Step 2: | .62 | .59 | ||||
| ADHD PRS score | 3457.10 | 2329.19 | .03 | |||
| Effortful control | −5.215 | .16 | −.78** | |||
| Year 1 Hyperactive Symptoms | Step 1: | |||||
| Prenatal maternal smoking | 1.72 | .35 | .17** | .03 | ||
| Step 2: | .35 | .32 | ||||
| Prenatal maternal smoking | .86 | .29 | .09* | |||
| Surgency | 3.14 | .16 | .57** | |||
| Year 6 Inattentive Symptoms | Step 1: | |||||
| ADHD PRS score | 24166.33 | 5631.38 | .24** | .06 | ||
| Step 2: | .40 | .34 | ||||
| ADHD PRS score | 11484.83 | 4612.91 | .11 | |||
| Effortful control | −4.10 | .32 | −.60** | |||
| Year 6 Hyperactive Symptoms | Step 1: | |||||
| Prenatal maternal smoking | .98 | .37 | .14* | .020 | ||
| Step 2: | ||||||
| Prenatal maternal smoking | .60 | .33 | .09 | .23 | .21 | |
| Surgency | 1.89 | .20 | .46** |
Note. Participants were ages 7-13 years at Year 1 and 13-19 years at Year 6
p<.001
p<.0
Mediation path analyses.
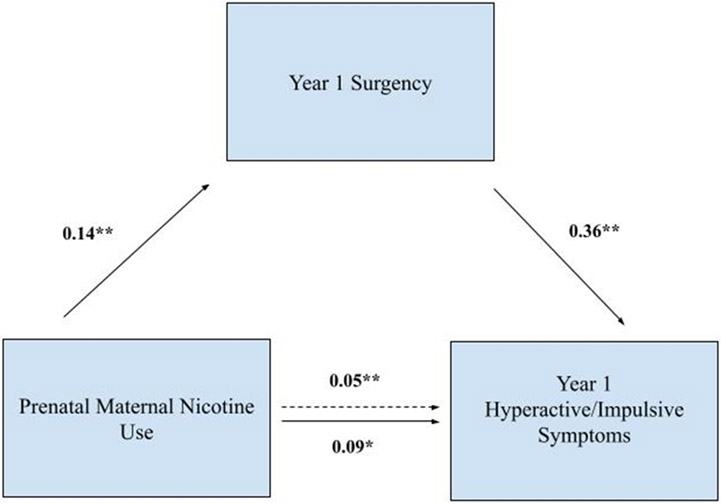
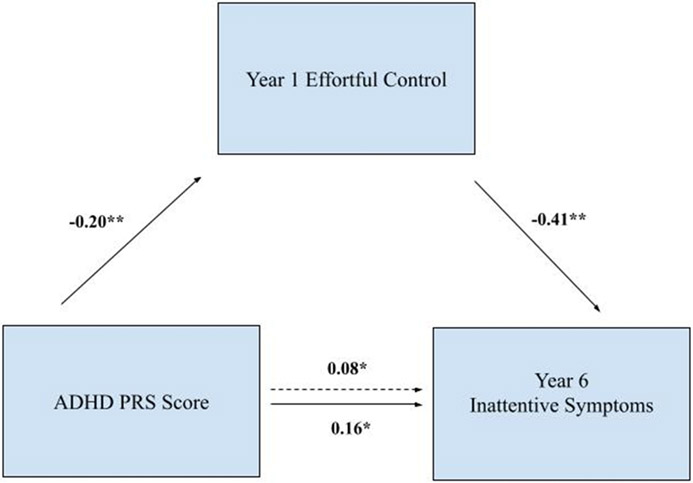
To more formally test mediation, shown in Table 4 and Figures 2 and 3, path analyses indicated that pathways were specific to symptom domain at the hypothesized developmental timepoint for etiological impacts. The mediational pathway from prenatal smoking through Time 1 surgency to Time 1 hyperactivity/impulsivity, survived control for inattention. The indirect effect was significant (indirect effect estimate = .05, p < .01). The mediation pathway from genetics through Time 1 effortful control to Time 6 inattention survived control for hyperactivity/impulsivity. The indirect effect was significant (indirect effect estimate = .08, p < .01). These path model indirect effects were not significant for the alternative symptom domains or for the alternative timepoints (after controlling for alternative symptom domain; all p > .05).
Table 4.
Standardized mediation path coefficients
| Standardized Parameter Estimate (SE) |
Est./SE | Sig. | |
|---|---|---|---|
| Model 1: Inattentive Symptoms at Year 6 | |||
| Path a: Effortful control → ADHD PRS score | −0.20 (0.06) | −3.62 | p<.01 |
| Path b: Effortful control | −0.41 (0.05) | −8.71 | p<.01 |
| Path c: ADHD PRS score total | 0.16 (0.05) | 3.38 | p<.05 |
| Path c’: ADHD PRS score indirect | 0.084 (0.03) | 3.30 | p<.05 |
| Model 2: Hyperactive Symptoms at Year 1 | |||
| Path a: Surgency → Prenatal maternal smoking | 0.14 (0.03) | 4.10 | p<.01 |
| Path b: Surgency | 0.36 (0.03) | 13.5 | p<.01 |
| Path c: Prenatal maternal smoking total | 0.091 (0.03) | 3.19 | p<.05 |
| Path c’: Prenatal maternal smoking indirect | 0.053 (0.01) | 3.90 | p<.001 |
Note. Participants were ages 7-13 years at Year 1 and 13-19 years at Year 6. Traits were measured at Year 1.
Figure 2. Prenatal maternal nicotine use mediation model.
Note. Participants were ages 7-13 years at Year 1 and 13-19 years at Year 6
Figure 3. Polygenic risk mediation model.
Note. Participants were ages 7-13 years at Year 1 and 13-19 years at Year 6
Additional specificity and longitudinal checks.
The alternative etiological domain could be covaried in mediation analyses with no change in mediation results. However, when both symptom domains AND both etiological inputs were included in the same model, then the model would not run, likely due to the high levels of collinearity, particularly for the ADHD symptom domains which were highly correlated with one another. Thus, although pathways were partially distinct, they are not fully distinct.
Longitudinal analyses were conducted in which Time 1 symptoms were entered to see if traits mediated associations between etiological factors and change in symptoms between Times 1 and 6. These checks indicated that there was no longer a significant association between etiological factors and Time 6 symptoms, after controlling for Time 1 symptoms, consistent with a pathway model in which etiological factors predict Time 1 and Time 6 symptoms, but not change in symptoms themselves. Mediation analyses did not differ when using teacher-report of ADHD symptoms.
Discussion
The current study extends prior cross-sectional work on temperament pathway models from etiology to inattention and hyperactivity/impulsivity to longitudinal data from childhood to adolescence. Consistent with dual pathway models to ADHD, effortful control and surgency exhibited differential associations with inattention and hyperactivity/impulsivity respectively over time. In particular, effortful control mediated associations between ADHD polygenic risk and inattentive symptoms at Year 6 (ages 13-19), while surgency mediated longitudinal associations between maternal prenatal smoking and hyperactivity/impulsivity at Year 1 (ages 7-13). Results held using teacher report of ADHD symptoms and when controlling for the alternative etiological domain or ADHD symptom domain. Importantly, the pathways appeared to be developmental. The prenatal pathway through surgency to hyperactivity/impulsivity held controlling for inattention at the first time point, while the genetic pathway through effortful control to inattention held controlling for hyperactivity/impulsivity only at the later timepoint.
In line with prior cross-sectional, correlational work (Martel & Nigg, 2006; Nigg, 2004), low effortful control and high surgency were associated with higher ADHD symptoms. Further, when accounting for shared overlap between inattention and hyperactivity-impulsivity, low effortful control was specifically and distinctly associated with inattention, while high surgency was specifically and distinctly associated with hyperactivity-impulsivity. Such findings are in line with dual pathway models of ADHD (Martel, Nigg, & von Eye, 2009; Nigg, 2004), and consistent with the idea that top-down control is more associated with inattention while surgency and other affective traits are associated with hyperactivity/impulsivity.
Traits mediated associations between known genetic and prenatal etiological factors and ADHD, findings which had shown promise in cross-sectional samples (Martel et al., 2009; 2010), but are replicated here for the first time using longitudinal data with evaluation of mediation effects across time. Such pathways are consistent with hypothesized specificity in dual pathway models of ADHD. Top-down regulation associated with ADHD inattention seems related etiologically to polygenic risk and may become more salient over time. Bottom-up reactivity related to hyperactivity/impulsivity seems to be more associated etiologically with maternal smoking in pregnancy. Its effect appears to fade over time more than the genetic risk effect (Wagner et al., 2009). Such results are consistent with partially distinct multiple pathways to ADHD (Nigg, 2004; Sonuga-Barke, 2005; 2010). Yet, although there was some specificity of effects given that the ADHD symptom domains could not be interchanged in the models, the pathways were not completely specific likely due to high correlations among the two ADHD symptom domains, consistent with recently supported bifactor models of ADHD (Martel & Nigg, 2010; Toplak et al., 2009). Yet partially distinct pathways to inattention versus hyperactivity-impulsivity are important to understand given heterogeneity in symptom presentations among children with ADHD.
Path analysis indicated that traits were an important part of mediational pathways between genetic and prenatal etiological factors and ADHD symptoms in childhood and adolescence. Importantly, these models did not predict change in ADHD over time, consistent with vulnerability or spectrum models of trait-psychopathology relations (vs. exacerbation; Tackett, 2006). Therefore, in these data, these traits seem to increase risk for initial severity of symptoms but not necessarily to impact the course of symptom change over time. These models were truly mediational with etiological factors predicting early symptoms and only temperamental traits (vs. etiological factors) predicting later symptoms, again consistent with pathway models from distal etiological through proximal vulnerability traits to psychopathology.
In addition, the specificity of mediational effects is consistent with some developmental specificity in pathways. The pathway from prenatal smoking through surgency to hyperactivity/impulsivity survived control of inattention only during childhood. This is in line with other findings from longitudinal work showing hyperactivity-impulsivity is salient earlier during development (vs. inattentive symptoms; Lahey et al., 2005), as well as the idea that early developmental trajectories of ADHD have primarily reactive, or affective, manifestation early during development (Sonuga-Barke, 2005; 2010). Further, early organizational hormonal effects may exert particularly salient effects on surgency and hyperactivity/impulsivity during early development (Arnold & Breedlove, 1985: Martel & Roberts, 2014). In contrast, analyses showing mediational pathways from genetic risk through effortful control to inattentive symptoms surviving control of hyperactivity-impulsivity later during development suggest genetic effects may be particularly salient on effortful control and inattention and may become increasingly prominent over time (Bergen, Gardner, & Kendler, 2007; Haworth et al., 2010). This is in line with prior work showing more thoughtful forms of regulation becoming particularly prominent during adolescence (Nigg, 2004; Sonuga-Barke, 2005; 2010).
Importantly, these relations held cross-sectionally and longitudinally between childhood and adolescence. Such findings suggest important implications for applied intervention and assessment work. Early assessment of reactivity such as surgency may be early markers for risk for ADHD which might be usefully targeted in preschool behavioral interventions targeted at parental management of traits such as impulsivity and outbursts. In contrast, later during development, interventions targeted toward more regulatory forms of control such as teaching of behavioral strategies like planning and organization may be more useful. An important future direction is to examine whether these longitudinal pathways hold at earlier timepoints, such as preschool.
In addition, these pathways were largely the same when clinician- and parent-report of ADHD symptoms were replaced with teacher-report symptoms, controlling for shared source variance and situational context. Therefore, these partially distinct temperament pathways of etiological effects on ADHD symptom domains seem consistent across settings and across different reporters of ADHD. We also accounted for item overlap between temperament traits and ADHD (De Pauw & Mervielde, 2010; Frick, 2004; Lemery et al., 2002), but examination of how these constructs relate to one another and their conceptual (but incomplete) relation is another important future direction. Candidate gene studies are now known to have serious limitations, hence the need for follow up with a more robust approach using polygenic risk, as was done here. For example, large genome-wide association studies (with samples sizes in the tens of thousands or larger) have consistently found that individual variants exert only very small effects on complex behavioral traits, like ADHD (Demontis, et al., 2019). In contrast, many candidate gene studies were done in much smaller samples, suggesting they were significantly underpowered to detect true effects and that the false-discovery rate in those studies is likely high. In fact, a number of recent large studies have failed to validate hypotheses from early candidate gene studies of behavioral traits (Border et al., 2019; Farrell et al., 2015). Therefore, attention to polygenic risk as done here was a study strength.
However, there are some study limitations. Notably, results will need to be replicated in other samples, across other developmental periods, particularly with more narrow age bands, and using other measures. Reliability of temperament scales was somewhat low, and reliance on parent-report (vs. other informants, observational measures) is a study limitation. Study sample retention was relatively low and is a study limitation. Longitudinal tests of more complex models of ADHD such as bifactor conceptualizations, is an important future direction. Finally, attention to comorbidity which also exhibits differential associations based on ADHD symptom domain, is an important future direction. In addition, the sample was largely Caucasian; therefore, replication in other groups will be important. Still, the current study remains an important extension of prior cross-sectional work.
Overall, the current study provides longitudinal evidence of partially distinct pathways to ADHD. Childhood effortful control specifically mediated longitudinal associations between polygenic risk and adolescent inattention, while childhood surgency specifically mediated longitudinal associations between prenatal maternal smoking and childhood hyperactivity-impulsivity. Such temperament markers and pathways were at least partially distinct in the expected developmental direction. These results support dual pathway models of ADHD and hold implication for identification of early markers and intervention targets for treatment of ADHD.
Acknowledgments:
Nigg and Mooney were supported by R37-59105. We are grateful for assistance with genotype preparation from the Integrated Genomics Laboratory at OHSU (Ms. Kristina Vartanian and Chris Harrington, Ph.D.). DNA extractions were performed by the OHSU Gene Profiling Shared Resource.
Footnotes
Statements and declarations: The authors have no competing interests.
In a path model, a hypothesized bidirectional effect gets two straight arrows, one pointing in each direction. However, models containing bidirectional effects can only be estimated given strong assumptions and other variables (or instruments) that cause each of the two variables involved in the bidirectional effect; therefore, we did not consider them viable here.
References
- American Psychiatric Association, A. P. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub.
- Arnold AP, & Breedlove SM (1985). Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Hormones and Behavior, 19(4), 469–498. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, & Kendler KS (2007). Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics, 10(3), 423–433. [DOI] [PubMed] [Google Scholar]
- Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, & Keller MC (2019). No support for historical candidate gene or candidate gene-by-interaction hypotheses for major depression across multiple large samples. American Journal of Psychiatry, 176(5): 376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TJ (2013). The Wilson effect: The increase in heritability of IQ with age. Twin Research and Human Genetics, 16(5), 923–930. [DOI] [PubMed] [Google Scholar]
- Buschgens C, Van Aken M, Swinkels S, Altink M, Fliers E, Rommelse N, … Buitelaar J (2008). Differential family and peer environmental factors are related to severity and comorbidity in children with ADHD. Journal of Neural Transmission, 115(2), 177–186. [DOI] [PubMed] [Google Scholar]
- Conomos MP, Reiner AP, Weir BS, & Thornton TA (2016). Model-free estimation of recent genetic relatedness. The American Journal of Human Genetics, 98(1), 127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Rathouz PJ, & Lahey BB (2007). Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Archives of General Psychiatry, 64(11), 1296–1304. [DOI] [PubMed] [Google Scholar]
- Daseking M, Petermann F, Tischler T, & Waldmann HC (2015). Smoking during pregnancy is a risk factor for executive function deficits in preschool-aged children. Geburtshilfe und Frauenheilkunde, 75(01), 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Baldursson G, Belliveau R, Bybjerg-Grauholm J, Bækvad-Hansen M, & Cerrato F (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics, 51(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pauw SS, & Mervielde I (2010). Temperament, personality and developmental psychopathology: A review based on the conceptual dimensions underlying childhood traits. Child Psychiatry & Human Development, 41(3), 313–329. [DOI] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64,135–68. doi: 10.1146/annurev-psych-113011-143750. Epub 2012 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, & Reid R (1998). ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation: Guilford Press. [Google Scholar]
- Farrell MS, Werge T, Sklar P, Owen MJ, Ophoff RA, O'Donovan MC, Corvin A, Cichon S, & Sullivan PF (2015). Evaluating historical candidate genes for schizophrenia. Molecular Psychiatry, 20(5):555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ (2004). Integrating research on temperament and childhood psychopathology: Its pitfalls and promise. Journal of Clinical Child and Adolescent Psychology, 33(1), 2–7. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, & Waldman ID (2009). Candidate gene studies of ADHD: A meta-analytic review. Human Genetics, 126(1), 51–90. [DOI] [PubMed] [Google Scholar]
- Goh PK, Lee CA, Bansal PS, Aguerrevere LE, Rucker AT, & Martel MM (2020). Interpretability and validity of a bifactor model of ADHD in young adults: Assessing the general “g” and specific IA and HI factors. Journal of Psychopathology and Behavioral Assessment, 42(2), 222–236. [Google Scholar]
- Halperin JM & Schulz KP (2006). Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin, 132(4), 560–81. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, … & Kovas Y (2010). The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry, 15(11), 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC (2012). Prenatal factors in temperament: The role of prenatal stress and substance use exposure. In Zentner M & Shiner RL (Eds.), Handbook of temperament (pp. 297–314). The Guilford Press. [Google Scholar]
- Howie BN, Donnelly P, & Marchini J (2009). A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genetics, 5(6), e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DA (1979). Correlation and Causality. New York: John Wiley & Sons. [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, & Willcutt E (2005). Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry, 62(8), 896–902. [DOI] [PubMed] [Google Scholar]
- Langley K, Rice F, van den Bree MB, & Thapar A (2005). Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatrica, 57(6), 359–371. [PubMed] [Google Scholar]
- Lemery KS, Essex MJ, & Smider NA (2002). Revealing the relation between temperament and behavior problem symptoms by eliminating measurement confounding: Expert ratings and factor analyses. Child Development, 73(3), 867–882. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, … & Jarvelin MR (2003). Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. American Journal of Psychiatry, 160(6), 1028–1040. [DOI] [PubMed] [Google Scholar]
- Martel MM, Eye A, & Nigg JT (2010). Revisiting the latent structure of ADHD: Is there a “g” factor? Journal of Child Psychology & Psychiatry, 51. doi: 10.1111/j.1469-7610.2010.02232.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Goth-Owens T, Martinez-Torteya C, & Nigg JT (2010). A person-centered personality approach to heterogeneity in attention-deficit/hyperactivity disorder (ADHD). Journal of Abnormal Psychology, 119(1), 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Gremillion ML, & Roberts B (2012). Temperament and common disruptive behavior problems in preschool. Personality and Individual Differences, 53(7), 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Gremillion ML, Roberts BA, Zastrow BL, & Tackett JL (2014). Longitudinal prediction of the one-year course of preschool ADHD symptoms: Implications for models of temperament–ADHD associations. Personality and Individual Differences, 64, 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, & Nigg JT (2006). Child ADHD and personality/temperament traits of reactive and effortful control, resiliency, and emotionality. Journal of Child Psychology and Psychiatry, 47(11), 1175–1183. [DOI] [PubMed] [Google Scholar]
- Martel MM, Nigg JT, & Von Eye A (2009). How do trait dimensions map onto ADHD symptom domains? Journal of Abnormal Child Psychology, 37(3), 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nikolas M, Jernigan K, Friderici K, & Nigg JT (2010). Personality mediation of genetic effects on attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology, 38(5), 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, & Roberts BA (2014). Prenatal testosterone increases sensitivity to prenatal stressors in males with disruptive behavior disorders. Neurotoxicology and Teratology, 44, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Roberts B, Gremillion M, Von Eye A, & Nigg JT (2011). External validation of bifactor model of ADHD: Explaining heterogeneity in psychiatric comorbidity, cognitive control, and personality trait profiles within DSM-IV ADHD. Journal of Abnormal Child Psychology, 39(8), 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Von Eye A, & Nigg J (2012). Developmental differences in structure of attention-deficit/hyperactivity disorder (ADHD) between childhood and adulthood. International Journal of Behavioral Development, 36(4), 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2006). Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, 47(3‐4), 395–422. [DOI] [PubMed] [Google Scholar]
- Nigg JT (2017). Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58(4), 361–383. doi: 10.1111/jcpp.12675. Epub 2016 Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, & Sachek J (2004). Evaluating the endophenotype model of ADHD neuropsychological deficit: Results for parents and siblings of children with ADHD combined and inattentive subtypes. Journal of Abnormal Psychology, 113(4), 614. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Goldsmith HH, & Sachek J (2004). Temperament and attention deficit hyperactivity disorder: The development of a multiple pathway model. Journal of Clinical Child and Adolescent Psychology, 33(1), 42–53. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Gustafsson HC, Karalunas SL, Ryabinin P, McWeeney SK, Faraone SV, … & Wilmot B (2018). Working memory and vigilance as multivariate endophenotypes related to common genetic risk for attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 57(3), 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, John OP, Blaskey LG, Huang-Pollock CL, Willcutt EG, Hinshaw SP, & Pennington B (2002). Big five dimensions and ADHD symptoms: links between personality traits and clinical symptoms. Journal of Personality and Social Psychology, 83(2), 451–469. doi: 10.1037/0022-3514.83.2.451. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Sibley MH, Thapar A, Karalunas SL (2020). Development of ADHD: Etiology, heterogeneity, and early life course. Annual Review of Developmental Psychology, 2(1), 559–583. doi: 10.1146/annurev-devpsych-060320-093413. Epub 2020 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, & Corbett SM (2012). Executive function profile in the offspring of women that smoked during pregnancy. Nicotine & Tobacco Research, 14(2), 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Antich J, & Ryan N (1986). Kiddie schedule for affective disorders and schizophrenia. Pittsburgh, PA: Western Psychiatric Institute. [Google Scholar]
- Rice ML, Zubrick SR, Taylor CL, Hoffman L, & Gayán J (2018). Longitudinal study of language and speech of twins at 4 and 6 years: twinning effects decrease, zygosity effects disappear, and heritability increases. Journal of Speech, Language, and Hearing Research, 61(1), 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald Angelica, de Bode N, & Polderman TJC (2021). Systematic review: How the attention-deficit/hyperactivity disorder polygenic risk score adds to our understanding of ADHD and associated traits. Journal of the American Academy of Child & Adolescent Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK (1981). Measurement of temperament in infancy. Child Development, 569–578. [Google Scholar]
- Skoglund C, Chen Q, D′ Onofrio BM, Lichtenstein P, & Larsson H (2014). Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. Journal of Child Psychology and Psychiatry, 55(1), 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ (2002). Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behavioural Brain Research, 130(1–2), 29–36. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ (2005). Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biological Psychiatry, 57(11), 1231–1238. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E, Bitsakou P, & Thompson M (2010). Beyond the dual pathway model: Evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 49(4), 345–355. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, & Sergeant J (2005). SPECIAL SECTION The neuroscience of ADHD: Multidisciplinary perspectives on a complex developmental disorder. Developmental Science, 8(2), 103–104. [DOI] [PubMed] [Google Scholar]
- Takegata M, Matsunaga A, Ohashi Y, Toizumi M, Yoshida LM, & Kitamura T (2021). Prenatal and ontrapartum factors associated with infant temperament: A systematic review. Frontiers in Psychiatry, 12, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL (2006). Evaluating models of the personality–psychopathology relationship in children and adolescents. Clinical Psychology Review, 26(5), 584–599. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Pitch A, Flora DB, Iwenofu L, Ghelani K, Jain U, & Tannock R (2009). The unity and diversity of inattention and hyperactivity/impulsivity in ADHD: Evidence for a general factor with separable dimensions. Journal of Abnormal Child Psychology, 37(8), 1137–1150. [DOI] [PubMed] [Google Scholar]
- Wagner AI, Schmidt NL, Lemery-Chalfant K, Leavitt LA, & Goldsmith HH (2009). The limited effects of obstetrical and neonatal complications on conduct and ADHD symptoms in middle childhood. Journal of Developmental and Behavioral Pediatrics: JDBP, 30(3), 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs K, Elmore AL, Nigg JT, & Nikolas MA (2016). Pre-and perinatal risk for attention-deficit hyperactivity disorder: Does neuropsychological weakness explain the link? Journal of Abnormal Child Psychology, 44(8), 1473–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of Attention-Deficit/Hyperactivity Disorder: A meta-analytic review. Biological Psychiatry, 57. doi: 10.1016/j.biopsych.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, … Lahey BB (2012). Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology, 121(4), 991. [DOI] [PMC free article] [PubMed] [Google Scholar]