Abstract
Background
Venous thromboembolism (VTE), which comprises deep vein thrombosis (DVT) and pulmonary embolism (PE), is the leading cause of preventable death in hospitalised people and the third most common cause of mortality in surgical patients. People undergoing bariatric surgery have the additional risk factor of being overweight. Although VTE prophylaxis in surgical patients is well established, the best way to prevent VTE in those undergoing bariatric surgery is less clear.
Objectives
To evaluate the benefits and harms of pharmacological interventions (alone or in combination) on venous thromboembolism and other health outcomes in people undergoing bariatric surgery compared to the same pharmacological intervention administered at a different dose or frequency, the same pharmacological intervention or started at a different time point, another pharmacological intervention, no intervention or placebo.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 1 November 2021.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs in males and females of any age undergoing bariatric surgery comparing pharmacological interventions for VTE (alone or in combination) with the same pharmacological intervention administered at a different dose or frequency, the same pharmacological intervention started at a different time point, a different pharmacological intervention, no treatment or placebo.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. VTE and 2. major bleeding. Our secondary outcomes were 1. all‐cause mortality, 2. VTE‐related mortality, 3. PE, 4. DVT, 5. adverse effects and 6. quality of life. We used GRADE to assess certainty of evidence for each outcome.
Main results
We included seven RCTs with 1045 participants. Data for meta‐analysis were available from all participants.
Four RCTs (597 participants) compared higher‐dose heparin to standard‐dose heparin: one of these studies (139 participants) used unfractionated heparin (UFH) and the other three (458 participants) used low‐molecular‐weight heparin (LMWH). One study compared heparin versus pentasaccharide (198 participants), and one study compared starting heparin before versus after bariatric surgery (100 participants). One study (150 participants) compared combined mechanical and pharmacological (enoxaparin) prophylaxis versus mechanical prophylaxis alone. The duration of the interventions ranged from seven to 15 days, and follow‐up ranged from 10 to 180 days.
Higher‐dose heparin versus standard‐dose heparin
Compared to standard‐dose heparin, higher‐dose heparin may result in little or no difference in the risk of VTE (RR 0.55, 95% CI 0.05 to 5.99; 4 studies, 597 participants) or major bleeding (RR 1.19, 95% CI 0.48 to 2.96; I2 = 8%; 4 studies, 597 participants; low‐certainty) in people undergoing bariatric surgery. The evidence on all‐cause mortality, VTE‐related mortality, PE, DVT and adverse events (thrombocytopenia) is uncertain (effect not estimable or very low‐certainty evidence).
Heparin versus pentasaccharide
Heparin compared to a pentasaccharide after bariatric surgery may result in little or no difference in the risk of VTE (RR 0.83, 95% CI 0.19 to 3.61; 1 study, 175 participants) or DVT (RR 0.83, 95% CI 0.19 to 3.61; 1 study, 175 participants). The evidence on major bleeding, PE and mortality is uncertain (effect not estimable or very low‐certainty evidence).
Heparin started before versus after the surgical procedure
Starting prophylaxis with heparin 12 hours before surgery versus after surgery may result in little or no difference in the risk of VTE (RR 0.11, 95% CI 0.01 to 2.01; 1 study, 100 participants) or DVT (RR 0.11, 95% CI 0.01 to 2.01; 1 study, 100 participants). The evidence on major bleeding, all‐cause mortality and VTE‐related mortality is uncertain (effect not estimable or very low‐certainty evidence). We were unable to assess the effect of this intervention on PE or adverse effects, as the study did not measure these outcomes.
Combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone
Combining mechanical and pharmacological prophylaxis (started 12 hours before surgery) may reduce VTE events in people undergoing bariatric surgery compared to mechanical prophylaxis alone (RR 0.05, 95% CI 0.00 to 0.89; number needed to treat for an additional beneficial outcome (NNTB) = 9; 1 study, 150 participants; low‐certainty). We were unable to assess the effect of this intervention on major bleeding or morality (effect not estimable), or on PE or adverse events (not measured).
No studies measured quality of life.
Authors' conclusions
Higher‐dose heparin may make little or no difference to venous thromboembolism or major bleeding in people undergoing bariatric surgery when compared to standard‐dose heparin.
Heparin may make little or no difference to venous thromboembolism in people undergoing bariatric surgery when compared to pentasaccharide. There are inadequate data to draw conclusions about the effects of heparin compared to pentasaccharide on major bleeding.
Starting prophylaxis with heparin 12 hours before bariatric surgery may make little or no difference to venous thromboembolism in people undergoing bariatric surgery when compared to starting heparin after bariatric surgery. There are inadequate data to draw conclusions about the effects of heparin started before versus after surgery on major bleeding.
Combining mechanical and pharmacological prophylaxis (started 12 hours before surgery) may reduce VTE events in people undergoing bariatric surgery when compared to mechanical prophylaxis alone. No data are available relating to major bleeding.
The certainty of the evidence is limited by small sample sizes, few or no events, and risk of bias concerns. Future trials must be sufficiently large to enable analysis of relevant clinical outcomes, and should standardise the time of treatment and follow‐up. They should also address the effect of direct oral anticoagulants and antiplatelets, preferably grouping them according to the type of intervention.
Plain language summary
Can medicines prevent venous thromboembolism after weight‐loss surgery?
What is venous thromboembolism?
Venous thromboembolism (VTE) is a clinical condition that usually starts when a blood clot forms inside a vein. The condition includes both deep vein thrombosis (when the clot forms in a deep vein, usually in the legs) and pulmonary embolism (when the clot forms in or reaches a blood vessel in the lungs). Both situations can substantially reduce quality of life and can be life‐threatening. People with obesity or who undergo surgery are more likely to experience VTE. Therefore, people undergoing a bariatric surgical procedure (aimed at reducing bodyweight by restricting food intake or absorption) are at particularly high risk. However, it is unclear whether these people should receive the same intervention to prevent VTE as people with other clinical conditions.
How can venous thromboembolism be prevented?
VTE prophylaxis (preventive interventions) can be mechanical (e.g. elastic stockings or external compressive devices) or pharmacological (involving medicines that reduce blood clot formation, such as heparins, pentasaccharides or antiplatelet agents), or can combine both approaches.
What did we want to find out?
We wanted to assess whether any pharmacological intervention can prevent VTE in people undergoing bariatric surgery, and whether these interventions are safe.
What did we do?
We searched for studies that examined the effect of any medicine for preventing VTE in people undergoing bariatric surgery, compared with the same medicine at a different dose, or given more or less often, or started at a different time point; or compared with a different medicine; or compared with no treatment or placebo (dummy treatment). We also included combinations of interventions.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and size.
What did we find
We found seven studies involving 1045 people who underwent bariatric surgery. The studies provided information about four different comparisons:
1) heparin at a higher dose versus the same heparin at a standard dose; 2) heparin versus a pentasaccharide; 3) heparin started before versus after the surgical procedure; and 4) combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone.
Four studies provided information for the first comparison: one assessed unfractionated heparin (which is given by a medical professional and wears off quickly) and three assessed low‐molecular‐weight heparin (which patients can inject themselves and which lasts longer). There was one study for each of the other three comparisons.
Heparin at a higher dose compared to a standard dose may make little or no difference to the risk of VTE or major bleeding in people undergoing bariatric surgery. The evidence on death, pulmonary embolism, deep vein thrombosis and the side effect thrombocytopenia (low blood platelet count) is uncertain.
Heparin compared with pentasaccharide may make little or no difference to the risk of VTE or deep vein thrombosis in people undergoing bariatric surgery. The evidence on major bleeding, death, pulmonary embolism and side effects (thrombocytopenia, irregular heartbeat, rash and nausea and vomiting) is uncertain.
Heparin before compared with after surgery may make little or no difference to the risk of VTE or deep vein thrombosis in people undergoing bariatric surgery. The evidence on major bleeding and death is uncertain. The study did not measure pulmonary embolism or harmful side effects.
Mechanical prophylaxis plus pharmacological prophylaxis compared to mechanical prophylaxis alone may decrease the risk of VTE and deep vein thrombosis in people undergoing bariatric surgery. The evidence on major bleeding and death is uncertain. The study did not measure pulmonary embolism or harmful side effects.
No studies measured the effect of any intervention on quality of life.
Conclusion
Although there is some evidence on the effects of heparins, pentasaccharides and mechanical combined with pharmacological prophylaxis for preventing VTE in people undergoing bariatric surgery, we are still not sure which intervention works best.
What are the limitations of the evidence?
We have little or very little confidence in the evidence because the studies were of low quality. Many participants dropped out from one study, there were a low number of events overall, and most studies had few participants. Larger studies assessing important outcomes (e.g. VTE, major bleeding, death due to any cause, death due to VTE, pulmonary embolism, deep vein thrombosis, harmful side effects and quality of life) are needed to assess which medicines are more effective and safer and at which dose they should be used.
How up to date is this evidence?
This evidence is up‐to‐date to November 2021.
Summary of findings
Summary of findings 1. Higher‐dose heparin compared to standard‐dose heparin for preventing venous thromboembolism in people undergoing bariatric surgery.
| Higher‐dose heparin compared to standard‐dose heparin for preventing venous thromboembolism in people undergoing bariatric surgery | |||||
| Patient or population: people undergoing bariatric surgery Setting: hospital Intervention: higher‐dose heparin Comparison: standard‐dose heparin | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with standard‐dose heparin | Risk with higher‐dose heparin | ||||
| VTE Follow‐up: 10–90 days | 597 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | RR 0.55 (0.05 to 5.99) | Study population | |
| 7 per 1000 | 4 per 1000 (0 to 43) | ||||
| Major bleeding Follow‐up: 10–90 days | 597 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | RR 1.19 (0.48 to 2.96) | Study population | |
| 40 per 1000 | 47 per 1000 (19 to 118) | ||||
| All‐cause mortality Follow‐up: 10–90 days | 597 (4 RCTs) | ⊕⊕⊝⊝ Lowc | Not estimable | 4 studies reported no events. | |
| VTE‐related mortality Follow‐up: 10–90 days | 597 (4 RCTs) | ⊕⊕⊝⊝ Lowc | Not estimable | 4 studies reported no events. | |
| PE Follow‐up: up to 90 days | 310 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,d | RR 0.37 (0.02 to 8.92) | Study population | |
| 6 per 1000 | 2 per 1000 (0 to 55) | ||||
| DVT Follow‐up: 10–90 days | 597 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,d | RR 1.10 (0.07 to 17.40) | Study population | |
| 4 per 1000 | 4 per 1000 (0 to 63) | ||||
| Adverse events (thrombocytopenia) Follow‐up: up to 90 days | 310 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,d | RR 1.10 (0.07 to 17.40) | Study population | |
| 6 per 1000 | 7 per 1000 (0 to 108) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RCT: randomised controlled trial; RR: risk ratio; VTE: venous thromboembolism. | |||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of performance and detection bias. bDowngraded one level due to imprecision: few events and 95% CI consistent with possible benefit and possible harm. cDowngraded two levels due to imprecision: no events. dDowngraded two levels due to imprecision: very large CI of the absolute difference and few events.
Summary of findings 2. Heparin compared to pentasaccharide for preventing venous thromboembolism in people undergoing bariatric surgery.
| Heparin compared to pentasaccharide for preventing venous thromboembolism in people undergoing bariatric surgery | |||||
| Patient or population: people undergoing bariatric surgery Setting: hospital Intervention: heparin Comparison: pentasaccharide | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with pentasaccharide | Risk with heparin | ||||
| VTE Follow‐up: up to 14 days | 175 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR 0.83 (0.19 to 3.61) | Study population | |
| 43 per 1000 | 36 per 1000 (8 to 157) | ||||
| Major bleeding Follow‐up: up to 14 days | 198 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | RR 1.70 (0.42 to 6.92) | Study population | |
| 30 per 1000 | 51 per 1000 (13 to 208) | ||||
| All‐cause mortality Follow‐up: up to 14 days | 198 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | Not estimable | 1 study reported no events. | |
| VTE‐related mortality Follow‐up: up to 14 days | 198 (1 RCT) | ⊕⊝⊝⊝ Very lowa,d | Not estimable | 1 study reported no events. | |
| PE Follow‐up: up to 14 days | 198 (1 RCT) | ⊕⊝⊝⊝ Very lowa,d | Not estimable | 1 study reported no events. | |
| DVT Follow‐up: up to 14 days | 175 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR 0.83 (0.19 to 3.61) | Study population | |
| 43 per 1000 | 36 per 1000 (8 to 157) | ||||
| Adverse events (thrombocytopenia) Follow‐up: up to 14 days | 198 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | RR 0.34 (0.01 to 8.25) | Study population | |
| 10 per 1000 | 3 per 1000 (0 to 83) |
||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RCT: randomised controlled trial; RR: risk ratio; VTE: venous thromboembolism. | |||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of reporting and other bias. bDowngraded one level due to imprecision: few participants, and 95% CI consistent with possible benefit and possible harm. cDowngraded two levels due to imprecision: very large CI of the absolute difference and few events. dDowngraded two levels due to imprecision: no events.
Summary of findings 3. Heparin started before compared to after the surgical procedure for preventing venous thromboembolism in people undergoing bariatric surgery.
| Heparin started before versus after the surgical procedure for preventing venous thromboembolism in people undergoing bariatric surgery | |||||
| Patient or population: people undergoing bariatric surgery Setting: hospital Intervention: heparin started 12 hours before surgery Comparison: heparin started after surgery | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with heparin after surgery | Risk with heparin 12 h before surgery | ||||
| VTE Follow‐up: 15 days | 100 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR 0.11 (0.01 to 2.01) | Study population | |
| 80 per 1000 | 9 per 1000 (1 to 161) | ||||
| Major bleeding Follow‐up: 15 days | 100 (1 RCT) | ⊕⊝⊝⊝ Very lowa,d | RR 3.00 (0.13 to 71.92) | Study population | |
| 0 per 1000 | 0 per 1000 (0 to 0) |
||||
| All‐cause mortality Follow‐up: 15 days | 100 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | Not estimable | 1 study reported no events. | |
| VTE‐related mortality Follow‐up: 15 days | 100 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | Not estimable | 1 study reported no events. | |
| PE | Not reported | ||||
| DVT Follow‐up: 15 days | 100 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR 0.11 (0.01 to 2.01) | Study population | |
| 80 per 1000 | 9 per 1000 (1 to 161) | ||||
| Adverse events (thrombocytopenia) | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RCT: randomised controlled trial; RR: risk ratio; VTE: venous thromboembolism. | |||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level due to high risk of performance bias. bDowngraded one level due to imprecision: few participants, and 95% CI consistent with possible benefit and possible harm. cDowngraded two levels due to imprecision: no events. dDowngraded two levels due to imprecision: very large CI of the absolute difference and few events.
Summary of findings 4. Combined mechanical and pharmacological prophylaxis compared to mechanical prophylaxis alone for preventing venous thromboembolism in people undergoing bariatric surgery.
| Combined mechanical and pharmacological prophylaxis compared to mechanical prophylaxis alone for preventing venous thromboembolism in people undergoing bariatric surgery | |||||
| Patient or population: people undergoing bariatric surgery Setting: hospital Intervention: combined mechanical and pharmacological prophylaxis Comparison: mechanical prophylaxis alone | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with mechanical prophylaxis alone | Risk with combined mechanical and pharmacological prophylaxis | ||||
| VTE Follow‐up: 4 weeks | 150 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR 0.05 (0.00 to 0.89) | Study population | |
| 120 per 1000 | 6 per 1000 (0 to 107) | ||||
| Major bleeding Follow‐up: 4 weeks | 150 (1 RCT) | ⊕⊕⊝⊝ Lowb | Not estimable | 1 study reported no events. | |
|
All‐cause mortality Follow‐up: 4 weeks |
150 (1 RCT) | ⊕⊕⊝⊝ Lowb | Not estimable | 1 study reported no events. | |
|
VTE‐related mortality Follow‐up: 4 weeks |
150 (1 RCT) | ⊕⊕⊝⊝ Lowb | Not estimable | 1 study reported no events. | |
| PE | Not reported | ||||
|
DVT Follow‐up: 4 weeks |
150 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | RR 0.05 (0.00 to 0.89) | Study population | |
| 120 per 1000 | 6 per 1000 (0 to 107) | ||||
| Adverse events | Not reported | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; RCT: randomised controlled trial; RR: risk ratio; VTE: venous thromboembolism. | |||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aNumber needed to treat for an additional beneficial outcome (NNTB) = 9. bDowngraded two levels due to imprecision: few participants and few or no events.
Background
See Table 5 for a glossary of terms.
1. Glossary of terms.
| Term | Definition |
| Adjustable gastric banding | Surgical treatment for obesity where a silicone belt or band is placed around the upper portion of the stomach, shaping the stomach in an 'hourglass' form and restricting the flow of food |
| Anticoagulants | Drugs that suppress, delay or prevent blood clots |
| Antiplatelet agents | Drugs that prevent blood clots by inhibiting platelet function |
| Atherosclerosis | A disease characterised by a build‐up of abnormal fat, cholesterol and platelet deposits on the inner wall of the arteries |
| Biliopancreatic diversion with duodenal switch | Surgical treatment for obesity that involves reducing the size of the stomach by removing some of it, then bypassing most of the intestine |
| Body mass index (BMI) | Body mass divided by the square of the body height, universally expressed in units of kg/m2 |
| Bariatric surgery | Any type of surgery aimed at weight loss |
| Deep vein thrombosis (DVT) | Coagulation or clotting of the blood in a deep vein (i.e. far beneath the surface of the skin) |
| Duplex ultrasound | Non‐invasive evaluation of blood flow through the arteries and veins by ultrasound devices |
| Dyslipidaemia | Abnormal concentration of fats (lipids or lipoproteins) in the blood |
| Gastric imbrication | Also known as stomach folding; surgical procedure to reduce the stomach volume without resection |
| Heparin | A drug used to prevent blood clotting (anticoagulant, blood thinner) |
| Laparoscopic | A procedure performed via an endoscope inserted through an incision in the abdominal wall and used for viewing or performing minor surgery in the abdominal or pelvic cavities |
| Low‐molecular‐weight heparin | A drug used to prevent blood clotting (anticoagulant) |
| Obesity | Where the amount of body fat is beyond healthy conditions (BMI greater than 30 kg/m2) |
| Oedema | Excess watery fluid that collects in tissues of the body, causing swelling when fluid leaks out of the body's vessels |
| Overweight | Where the amount of body fat is over that of the average population, but less than unhealthy conditions (BMI between 25 kg/m2 and 30 kg/m2) |
| Placebo | Substance or treatment with no active effect, like a sugar pill |
| Pulmonary embolism (PE) | Blood clot in the lung or blood vessel leading to the lung. The clot originates in a vein (e.g. DVT) and travels to the lung. |
| Randomised clinical trial (RCT) | A study in which the participants are divided randomly into separate groups to compare different treatments |
| Roux‐en‐Y gastric bypass | Surgical treatment for obesity where the surgeon creates a small pouch at the top of the stomach, limiting the amount of food that can be eaten. The small intestine is also cut and connected to the new pouch. |
| Superficial thrombosis | Inflammatory thrombotic disorder in which a clot develops in a vein near the surface of the skin |
| Sleeve gastrectomy | Bariatric surgery in which most of the stomach is removed, leaving a small sleeve |
| Thrombosis | Local coagulation of blood (clot) in a part of the circulatory system |
| Unfractionated heparin (UFH) | A mixture of heparins obtained from animals that is used to prevent blood coagulation, to prevent and treat clotting disorders |
| Vascular | Relating to blood vessels (arteries and veins) |
| Vena cava | The largest human vein that returns blood to the heart after it has passed around the body |
| Venous | Relating to a vein |
| Venous thromboembolism (VTE) | A condition that involves a blood clot forming in a vein, and sometimes migrating to another location (e.g. the lung) |
| Virchow's Triad | 3 factors that contribute to thrombosis:
|
Description of the condition
Obesity is clinically defined as a body mass index (BMI) of 30 kg/m2 or greater; a BMI of 40 kg/m2 or greater is called severe obesity or morbid obesity (WHO 1998). The BMI is calculated by dividing the weight of an individual in kilograms by the square of their height in metres. Although there are other clinical definitions of obesity, BMI is the most widely used due to its ease of application in clinical practice (Table 6). The causes of obesity are multifactorial and include direct and indirect effects such as genetics, gene‐environment interactions and social determinants of health (e.g. area of residence, educational status and economic stability); however, the most important factor is an energy imbalance between physical activity and food intake (Arroyo‐Johnson 2016). Increased food industry productivity through technological development has led to a concomitant increase in the energy value of commonly consumed foods. At the same time, improvements in transport have resulted in a more sedentary lifestyle. These factors are often cited as the main reasons for the constant increase in BMI and obesity worldwide (Medina 2017). Since the mid‐1970s, the prevalence of obesity has tripled (from 3.2% in 1975 to 10.8% in 2014 in men, and from 6.4% in 1975 to 14.9% in 2014 in women); if this trend continues, it is estimated that by 2025 obesity will affect around 18% of men and 21% of women worldwide (NCD‐RisC 2016). In the USA, it is estimated that 69% of all adults are overweight and 35% are obese (NHLBI 2013).
2. Classification of adults according to BMI.
| Classification | BMI valuesa | |
| Underweight | < 18.5 | |
| Normal range | 18.5–24.99 | |
| Overweight | Preobese | 25.00–29.99 |
| Obese class I | 30.00–34.99 | |
| Obese class II | 35.00–39.99 | |
| Obese class Ill | ≥ 40 | |
BMI: body mass index.
aBody mass divided by the square of the body height, universally expressed in units of kg/m2.These BMI values are age‐ and sex‐independent.
Obesity and high BMI are associated with pathologies such as type 2 diabetes and dyslipidaemia, as well as various cancers and cardiovascular diseases (including hypertension, atherosclerosis, stroke, venous thromboembolism and coronary disease; Fresan 2019). One study that examined adherence to the 2015–2020 Dietary Guidelines for Americans in a Spanish population of almost 17,000 people found that high adherence scores were associated with reduced risk for all‐cause mortality, cardiovascular mortality and cancer mortality (Fresan 2019). These conditions lead to increased health costs, and it is estimated that obesity and high BMI were responsible for the 10% annual increase in health costs in the USA from 1995 to 2008. In 2006, people with obesity had a 42% greater annual per capita spending than those without obesity (Finkelstein 2009).
Since the 1990s, a number of surgical techniques (i.e. bariatric surgery) have been developed to treat obesity. Usually, they are indicated for people with severe obesity (BMI of 40 kg/m2 or greater, or 30 kg/m2 or greater with comorbidities), although some studies use 35 kg/m2 as the threshold (NHLBI 2013). Different studies have shown these procedures to be more effective than clinical treatments alone, in people eligible to undergo surgical treatment (Colquitt 2014; Reges 2018). The term 'bariatric' refers to treatments that aim to reduce weight and, therefore, treat obesity. Although 'bariatric' can refer to any treatment, either clinical or surgical, its use became popular after the 1960s, when the first surgical procedures for obesity came into practice (Google Ngram Viewer 2018). Bariatric surgery comprises surgical techniques that result in reduced alimentary intake or absorption (or both), through restriction of the stomach or digestive tract (or both). Some of the most common techniques include laparoscopic or open Roux‐en‐Y gastric bypass, laparoscopic adjustable gastric banding (although the use of this technique has decreased since the 2010s), laparoscopic sleeve gastrectomy, biliopancreatic diversion with duodenal switch, and laparoscopic gastric imbrication (Colquitt 2014; Puzziferri 2018).
Research has shown bariatric surgery to be safe, as overall mortality after the procedure is between 0.05% and 1.5% or 2% (Nguyen 2017). However, when surgical complications do occur, they can be life‐threatening. One such complication is venous thromboembolism (VTE; Colquitt 2014; Goldfeder 2006; Morino 2007).
VTE comprises two related diseases: deep vein thrombosis (DVT) and pulmonary embolism (PE). VTE has an estimated annual incidence of 100 to 200 persons per 100,000, depending on phenotype, age and sex (Heit 2015; Jacobs 2018). It is the third leading cause of cardiovascular death worldwide, and the leading cause of preventable death in hospitalised people (Goldfeder 2006). Usually, VTE begins as an episode of DVT (with or without symptoms) that may lead to complications. PE can occur as a complication of a DVT or as a primary event of VTE, and is associated with a high risk of death; while chronic complications related to DVT, including post‐thrombotic syndrome (PTS) or chronic pulmonary hypertension (CPH), can have a considerable impact on quality of life (QoL; Barnes 2015). The risk of VTE is higher in surgical patients, representing the third most common cause of mortality; and VTE incidence is further increased in people with obesity (Borch 2010). Consequently, VTE is of particular concern for people undergoing bariatric surgery; it is responsible for considerable morbidity and accounts for almost 50% of postoperative mortality in this population (Morino 2007).
The incidence of VTE in surgical patients varies according to the type of surgery and patient profile. Orthopaedic procedures have higher rates (40% to 60%) compared to other surgery (15% to 40%; Geerts 2004). Some other clinical conditions have increased the risk of VTE in hospitalised people. For example, 49% of hospitalised people who are severely ill with COVID‐19 will develop VTE, compared to 11.2% of all hospitalised people (COVIDSurg 2022; Flumignan 2021; Flumignan 2022a; Santos 2022). Additionally, in people with COVID‐19, 30‐day mortality is 7.4% in those without VTE compared with 40.8% in those with VTE (COVIDSurg 2022). Some guidelines recommend various agents for VTE prophylaxis (preventive treatment) in surgical patients and also recommend extended prophylaxis (30 days) in people undergoing orthopaedic surgery (Falck‐Ytter 2012).
The economic impact associated with VTE is significant and can be up to 1.5 times greater for surgical patients (Salous 2019). This difference in total cost occurs mainly in the first three months after confirmation of VTE (Cohoon 2015).
Description of the intervention
Interventions for the prevention of VTE all aim to affect one of the elements of Virchow's Triad (see Table 5). Although vena cava filters are not recommended for primary VTE prevention (Gould 2012), there are mechanical interventions (e.g. elastic stockings, pneumatic compression, early ambulation, vein recanalisation treatments) to reduce venous stasis (Broderick 2021; Flumignan 2015; Streiff 2016). Pharmacological interventions (anticoagulants or antiplatelet agents) focus on reducing the hypercoagulability factor (Ageno 2010; Flumignan 2021; Flumignan 2022a; Flumignan 2022b; Santos 2022). The decision of whether to use prophylaxis (mechanical or pharmacological, or both) depends on the risk stratification of each person according to criteria such as the Rogers or Caprini score (Gould 2012), or the National Institute for Health and Care Excellence (NICE) risk assessment tool for VTE (NICE 2018).
The latest guidelines from the American College of Chest Physicians recommend mechanical prophylaxis in all surgical patients (at least early ambulation), and pharmacological prophylaxis in those with a moderate or high risk of VTE and low or moderate risk of major bleeding (Douketis 2016). One Cochrane Review found that combining pharmacological prophylaxis with intermittent pneumatic compression resulted in a reduced incidence of VTE compared with intermittent pneumatic compression alone, but increased the incidence of major bleeding (Kakkos 2022). People undergoing bariatric surgery are considered to have at least moderate risk of a VTE event (Bartlett 2015), and should therefore receive pharmacological prophylaxis.
For the purpose of this review, we focused on pharmacological interventions for the prevention of VTE in people undergoing bariatric surgery.
How the intervention might work
Drugs available for the prevention of VTE are oral anticoagulants (OACs), heparins (either unfractionated or low‐molecular‐weight), direct oral anticoagulants (DOACs), pentasaccharides and antiplatelet agents (Flumignan 2021; Flumignan 2022a; Jacobs 2018; Kakkos 2021; Santos 2022; Stevens 2021).
Warfarin and coumarin agents are classified as OACs, and are also called antivitamin K agents because they act as competitive antagonists of vitamin K. They have been used in clinical practice since 1954, and are therefore the standard comparison agent for almost every trial that investigates VTE treatment (Smith 2018). Because OACS are associated with an increased risk of major bleeding and take longer to reach effective plasma levels, their use in primary prophylaxis is limited; these agents have more utility in the long‐term treatment and prevention of VTE recurrence (Stevens 2021).
Low‐molecular‐weight heparins (LMWHs) present a lower risk of bleeding and a higher effectiveness compared with unfractionated heparins (UFHs) in DVT treatment. However, in VTE prevention, this difference appears to be irrelevant (Gould 2012).
DOACs are the most recent class of anticoagulants to be released for medical use. They are direct inhibitors of factors IIa or Xa of the coagulation cascade and are similar in effectiveness and safety to LMWHs (Burnett 2016; Stevens 2021). The risk of bleeding in people with DVT is lower with DOACs compared with vitamin K antagonists (VKAs), and some randomised controlled trials (RCTs) have reported a reduced risk of recurrent VTE with DOACs (Stevens 2021). Nevertheless, DOACs are rarely used in studies of VTE prophylaxis in non‐orthopaedic surgery, and the ninth edition of the American College of Chest Physicians' evidence‐based clinical practice guidelines includes no recommendations for their use in VTE prophylaxis (Gould 2012).
Pentasaccharides are a relatively new class of anticoagulants and are indirect inhibitors of factor Xa. One Cochrane Review of pentasaccharides for the prevention of VTE showed increased effectiveness for prevention of total VTE, total DVT, PE and symptomatic VTE (Dong 2016). However, these anticoagulants did not affect mortality rate and increased the risk of major bleeding. Most of the included trials involved orthopaedic patients, so provided no evidence of the benefits and safety of pentasaccharides in other surgical patients, including those undergoing bariatric surgery (Dong 2016).
Although it is not common practice, the use of antiplatelets for the prevention of DVT might be considered in certain conditions, as they interfere with Virchow's Triad (see Table 5; Eikelboom 2012). Data regarding the effects of antiplatelets are lacking. While one Cochrane Review has evaluated the effects of antiplatelets for DVT treatment, there is no high‐quality evidence on the effectiveness of antiplatelets for VTE prevention (Flumignan 2022b).
Why it is important to do this review
Most strategies aimed at preventing postoperative complications, including VTE, are intended for use in general patients. While this approach can be efficient on a population‐wide basis, the risk‐to‐benefit ratio must be individualised. This is particularly important in populations with specific risk profiles (Pannucci 2017). Many people with obesity have some degree of non‐alcoholic liver disease, which may increase the risk of bleeding on some anticoagulants (Pillai 2009; Qamar 2018). In addition, bariatric surgery may cause changes to the anatomy of the gastrointestinal tract, bodyweight and composition of the adipose tissue, all of which can affect the absorption, distribution or elimination of orally administered drugs (Martin 2017).
Although some guidelines and expert consensus recognise the importance of VTE prevention for people undergoing bariatric surgery, the most widely used guidelines regarding antithrombotic therapy for VTE do not indicate any specific recommendations for this population (Burnett 2016; Kearon 2012; Stevens 2021). The 2018 NICE guideline for preventing VTE has a small section with recommendations regarding bariatric surgery patients, but it has limited information about drugs to be used and no information on dosage or time of administration (NICE 2018). While some RCTs have evaluated the use of pharmacological interventions in bariatric surgery, there are currently no high‐quality data for this group (Imberti 2014a; Shelkrot 2014; Steele 2015a). For instance, there are no recommendations on whether prevention should begin before or after surgery, whether the heparin dose must be adjusted for patient mass, or what the duration of pharmacological prophylaxis following a procedure should be (Aminian 2017; Bartlett 2015; Stroh 2016). We aimed to identify the best available evidence to answer these questions.
Objectives
To evaluate the benefits and harms of pharmacological interventions (alone or in combination) on venous thromboembolism and other health outcomes in people undergoing bariatric surgery compared to the same pharmacological intervention administered at a different dose or frequency, the same pharmacological intervention or started at a different time point, another pharmacological intervention, no intervention or placebo.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs and quasi‐RCTs that compared pharmacological interventions for preventing VTE in people undergoing bariatric surgery. Parallel (e.g. cluster or individual) and cross‐over designs were eligible for inclusion. We had planned to use data from the first phase of cross‐over studies to avoid the risk of carry‐over effects, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We included studies published as full‐text articles, those published as abstract only, and unpublished data.
Types of participants
We included males and females of all ages who underwent bariatric surgery, with no limitations regarding BMI or surgical technique. We excluded participants receiving treatment for current VTE episodes, and those treated for VTE within the previous 30 days. We considered the rationale for bariatric surgery (see Data extraction and management), but did not exclude participants based on this criterion.
If we had identified studies with mixed populations in which only a subset of the participants met our inclusion criteria, we would have attempted to obtain data for the subgroup of interest from the study authors. Whenever we were unable to obtain data on a subgroup of interest, we would have included all participants in our analysis, providing at least 50% of the study population fulfilled our inclusion criteria. We had planned to explore the effect of this decision in a sensitivity analysis. We would have excluded studies where less than 50% of the population met our inclusion criteria and data on the subgroup of interest were unavailable.
Types of interventions
We included studies comparing one pharmacological intervention (agent or drug) versus another pharmacological intervention, placebo or no treatment. Combinations of interventions were also eligible for inclusion, provided co‐treatments were balanced between the treatment and control arms. We also included studies that compared the same drug administered at different doses or frequencies, or started at different time points. We pooled studies that addressed the same comparisons.
We considered the following pharmacological interventions:
heparins, both UFHs and LMWHs;
VKAs:
DOACs, factor Xa inhibitors and direct thrombin inhibitors;
antiplatelet agents; and
pentasaccharides.
Types of outcome measures
All trials that met our inclusion criteria were eligible, regardless of whether they reported one or more of the outcomes listed below. Where a published report did not appear to report one of these outcomes, we accessed the trial protocol and contacted the trial authors to ascertain whether they had measured but not reported any of the outcomes. Relevant trials that measured these outcomes but did not report the data at all, or did not report them in a usable format, would be included in the review and narratively described. Because this was not a cost‐effectiveness review, we had planned to report direct costs data in the discussion section in a narrative form if such information was available.
We had planned to present the outcomes at two time points: at 90 days or less after the start of the intervention (early outcomes), and at more than 90 days after the start of the intervention (long‐term outcomes). Early outcomes were of primary interest; therefore, we produced summary of findings tables for this time point only. Long‐term outcomes would be reported at the longest possible time of follow‐up.
Primary outcomes
VTE (combined DVT or PE, symptomatic or asymptomatic, first episode or recurrent, fatal or non‐fatal). The diagnosis had to be confirmed by clinical examination and at least one additional objective diagnostic test. For DVT diagnosis from any site (e.g. lower limbs, abdominal) these tests included ultrasonography, angiography (e.g. computed tomography (CT), magnetic resonance imaging (MRI) or digital subtraction) and postmortem examination. For PE diagnosis, valid tests were angiography by any described method, ventilation‐perfusion scan or postmortem examination. If the participant had both DVT and PE events, we counted this as a single event of VTE for the analysis of this outcome.
Major bleeding, defined by a decrease in haemoglobin concentration of 2 g/dL or more, a retroperitoneal or intracranial bleed, a transfusion of two or more units of blood, or fatal haemorrhagic events, as per the International Society on Thrombosis and Haemostasis (Schulman 2010)
Secondary outcomes
All‐cause mortality
VTE‐related mortality
PE (fatal or non‐fatal), confirmed by angiography (e.g. CT, MRI or digital subtraction) or ventilation‐perfusion scan, or both
DVT (first episode or recurrent), confirmed by ultrasonography or angiography (e.g. CT, MRI or digital subtraction)
Adverse events. We considered all possible adverse events separately, as individual outcomes. These could be gastrointestinal adverse effects (e.g. nausea, vomiting, diarrhoea, abdominal pain), allergic reactions, renal failure, minor bleeding or thrombocytopenia.
QoL: participants' subjective perception of improvement (yes or no), as reported by the trial authors or using any validated scoring system such as the 36‐item Short Form Health Survey (SF‐36; Ware 1992)
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year or publication status restrictions:
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web; searched 1 November 2021);
Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 10) via the Cochrane Register of Studies Online (CRSO);
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE; 1946 to 1 November 2021);
Embase Ovid (1974 to 1 November 2021); and
CINAHL EBSCO (1982 to 1 November 2021).
We adapted the search strategy designed for MEDLINE to the other databases. Where appropriate, we combined search strategies with adaptations of the Cochrane highly sensitive search strategy for identifying RCTs and controlled clinical trials (as described in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions; Lefebvre 2021). Appendix 1 presents the search strategies for the major databases.
We searched the following trial registries on 1 November 2021:
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; www.who.int/clinical-trials-registry-platform); and
ClinicalTrials.gov (clinicaltrials.gov).
We designed and performed additional searches in Latin American and Caribbean Health Science Information database (LILACS) and Indice Bibliográfico Español de Ciencias de la Salud (IBECS) databases on 16 November 2021, both via Virtual Health Library (bvsalud.org/). We applied no filters and selected the RCTs and quasi‐RCTs manually in the LILACS and IBECS databases. For the search strategy, see Appendix 2.
Searching other resources
We checked the bibliographies of relevant studies for additional trials, and we also contacted the authors of the included trials for any possible unpublished data. In addition, we contacted field specialists and pharmaceutical companies to enquire about relevant ongoing or unpublished studies.
Data collection and analysis
Selection of studies
We merged the search results to remove duplicate records, then three review authors (FCFA, LCUN and RLGF) independently screened the titles and abstracts of the records using Covidence to determine which were potentially eligible. We resolved any disagreement by discussion within the review team (FCFA, JCCBS, LCUN and RLGF). Finally, we obtained the full‐text reports of all potentially eligible trials and assessed them for compliance with our eligibility criteria. We excluded trials that did not meet the eligibility criteria, documenting the reason for exclusion. We illustrated the study selection process in a PRISMA diagram (Liberati 2009).
Data extraction and management
Three review authors (FCFA, LCUN and RLGF) extracted data from the included studies and transferred them to an electronic data extraction form, which had been piloted by two review authors (FCFA and RLGF). Any inconsistencies were resolved by discussion within the review team. We extracted the following data:
publication details (e.g. year, country, authors) and study design;
population data (e.g. age, comorbidities, sex, BMI, bariatric surgery technique, rationale for bariatric surgery indication);
details of the intervention (e.g. manufacturer, dosage, additional procedures);
number of participants randomised into each treatment group, number of participants in each group who failed treatment, numbers of participants lost during follow‐up;
duration of follow‐up and cost of treatment; and
types and timing of measured outcomes.
One review author (FCFA) transferred data into the Review Manager 5 file (Review Manager 2020). We double‐checked correct data entry by comparing the data presented in the systematic review with the data extraction form. A second review author (RLGF) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Three review authors (FCFA, LCUN and RLGF) independently assessed all included studies for risk of bias using the Cochrane risk of bias tool (RoB 1), described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion within the review team.
We judged each study as being at high, low or unclear risk of bias for each of the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting; and
other potential threats to validity.
For cluster‐randomised trials, we had planned to consider particular biases as recommended in the Cochrane Handbook for Systematic Reviews of Interventions: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised trials (Higgins 2011). We reported the assessment judgements of each individual study in risk of bias tables (located in the Characteristics of included studies table). We contacted trial author to request missing information whenever we were unable to make judgements based on the published data.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Amaral 2020) and reported any deviations from it in the Differences between protocol and review section.
Measures of treatment effect
For dichotomous data, we presented the results using risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, we had planned to present the results as mean differences (MDs) with 95% CIs. Where studies did not use the same scales, we had planned to present the results as standardised mean differences (SMDs) with 95% CIs.
We had planned to narratively describe skewed data reported as medians and interquartile ranges. We calculated the number needed to treat (NNT) for the outcomes with direct implication for practice. We obtained the risk difference with Review Manager 5 software (Review Manager 2020). We expressed the NNT as the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), to indicate the direction of effect, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019a).
Unit of analysis issues
We considered each participant as the unit of analysis. Where included studies considered multiple interventions in the same group, we analysed only the partial data of interest.
Cross‐over trials
Had we identified any cross‐over RCTs, we would have used data from the first phase of the study only, to avoid the risk of carry‐over effects, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Cluster‐randomised trials
We had planned to include cluster‐randomised trials in the analyses along with individually randomised trials, adjusting their sample sizes according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). This would involve using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial or study of a similar population. If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. Had we identified both cluster‐randomised trials and individually randomised trials, we would have synthesised the relevant information. We would have combined the results from both types of trial if there was little heterogeneity between the study designs, and the effect of the intervention appeared unrelated to the choice of randomisation unit. We had also planned to perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
We contacted study authors or study sponsors to verify key study characteristics. We had planned to obtain missing numerical outcome data where possible (e.g. when we found only the abstract of a study), and use the calculator within Review Manager 5 to calculate missing standard deviations (SDs) using other data from the trial, such as CIs. Where this was not possible, and missing data were thought to introduce serious bias, we had planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis. For all outcomes, we followed intention‐to‐treat (ITT) principles to the greatest degree possible (i.e. we analysed participants in their randomised group regardless of what intervention they actually received). We used available case data for the denominator if ITT data were unavailable.
Assessment of heterogeneity
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between CIs. We used the I2 statistic to measure heterogeneity among the trials in each analysis, though we acknowledge that there is substantial uncertainty in the value of the I2 statisticwhen measuring heterogeneity among a small number of studies. Had we identified substantial heterogeneity, we would have reported it and explored possible causes though prespecified subgroup analyses. As strict thresholds for the interpretation of the I2 statistic are not recommended, we used the rough guide to the interpretation provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019), as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
When the I2 value lay in an area of overlap between two categories (e.g. between 50% and 60%), we considered differences in participants and interventions among the trials contributing data to the analysis (Deeks 2019).
Assessment of reporting biases
We performed literature searches in multiple sources to reduce the chance of reporting biases. We would have assessed the presence of publication bias and other reporting bias using funnel plots if we had identified sufficient studies (i.e. more than 10) for inclusion in the meta‐analysis (Sterne 2017). If asymmetry was present, we would have explored possible causes, including publication bias, poor methodological quality and true heterogeneity (Sterne 2017). We had planned to perform additional statistical analysis for continuous outcomes with intervention effects measured as MDs to assess reporting biases, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2017).
Data synthesis
We synthesised the data using Review Manager 5 (Review Manager 2020). We undertook meta‐analysis only where it was meaningful (i.e. where the treatments, participants and underlying clinical questions were similar enough for pooling to be appropriate). If we were confident that trials were estimating the same underlying treatment effect (i.e. that the population, interventions, comparators and outcome characteristics of the included studies were homogenous), we used a fixed‐effect meta‐analysis. If clinical heterogeneity had been sufficient to suggest differing underlying treatment effects between trials, or if we had identified at least substantial heterogeneity, we would have used a random‐effects meta‐analysis. Had there been substantial clinical, methodological or statistical heterogeneity across trials that precluded the pooling of data, we would have used a narrative approach to data synthesis (Deeks 2019).
We addressed all outcomes listed in Types of outcome measures in the Results section of the review under the heading Effects of interventions. In addition, for each comparison, we presented the key outcomes in a summary of findings table. We included the results of individual studies and any statistical summary of these in Data and analyses tables in the review.
Subgroup analysis and investigation of heterogeneity
With the available data, we could only perform subgroup analysis for one comparison (higher‐dose heparin versus standard‐dose heparin). However, we plan to perform the following additional subgroup analyses if sufficient data are available in future versions of this review.
-
Interventions:
different doses of drugs;
different combination of interventions; and
duration of prophylaxis (e.g. until 30 days after surgery or more).
-
Participant characteristics:
age (e.g. 15 years to 24 years, 25 years to 64 years and 65 years and over);
sex;
BMI;
race;
comorbidities; and
presence or absence of non‐alcoholic fatty liver disease (diagnosed either by ultrasonography, CT or biopsy).
-
Type of bariatric surgery:
gastric bypass, gastric sleeve, duodenal switch, gastric banding, gastric balloon, vagal blocking, aspiration therapy, etc.
Sensitivity analysis
We had planned to carry out the following sensitivity analyses, to test whether key methodological factors or decisions affected the main result, grouping by study design (individual, cross‐over or cluster‐randomised), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019):
exclusion of quasi‐RCTs to determine any impact on the overall results;
inclusion of studies with low risk of bias only (considering overall risk of bias of an included study as low if there was no high‐risk judgement in random sequence generation, allocation concealment, incomplete outcome data and selective reporting);
examination of fixed‐effect model and random‐effects model meta‐analyses, to explore the differences between the two estimates;
exclusion of trials with mixed populations where all participants were included (where at least 50% were of interest) to determine their impact on the primary analyses; and
exclusion of studies with missing data that were unobtainable, to determine their impact on the primary analyses.
We had also planned to carry out sensitivity analyses considering cross‐over and cluster‐RCTs. We had planned to investigate the effect of variation in the ICC, acknowledge heterogeneity in the randomisation unit, and perform a sensitivity analysis to investigate the effects of the randomisation unit. We would have presented these results and compared them with the overall findings.
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro GDT software (GRADEpro GDT) to prepare summary of findings tables with the key information on pharmacological interventions for preventing VTE in participants undergoing bariatric surgery (Schünemann 2019b). We created one table for each treatment comparison for the 'early' time point. We included the following outcomes in each table:
VTE;
major bleeding;
all‐cause mortality;
VTE‐related mortality;
PE;
DVT; and
adverse events (thrombocytopenia).
We used the GRADE approach to rate the certainty of the evidence for each outcome as high, moderate, low, or very low, based on the criteria of risk of bias, inconsistency, indirectness, imprecision, and publication bias (Atkins 2004; Schünemann 2019a). We based the summary of findings tables on methods described in Chapter 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions, and justified any departures from the standard methods (Atkins 2004; Schünemann 2019a; Schünemann 2019b).
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review, and we avoided making recommendations for practice. In the Implications for research section we suggested priorities for future research and outlined the remaining uncertainties in the area.
Results
Description of studies
Results of the search
We performed database searches in November 2021. After excluding duplicate records, we screened the titles and abstracts of 4982 unique records, excluding 4939 records and retrieving the full‐text articles of 43 potentially eligible records. After the full‐text assessment, we included seven studies (14 reports; Abdelsalam 2021; Ahmad 2021; Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steele 2015b; Steib 2016). We excluded nine studies (nine reports) with reasons (see Characteristics of excluded studies table). We considered 15 reports to be irrelevant. There are no studies awaiting classification. Five studies (five reports) are ongoing (see Characteristics of ongoing studies table). Figure 1 shows the flow of studies.
1.
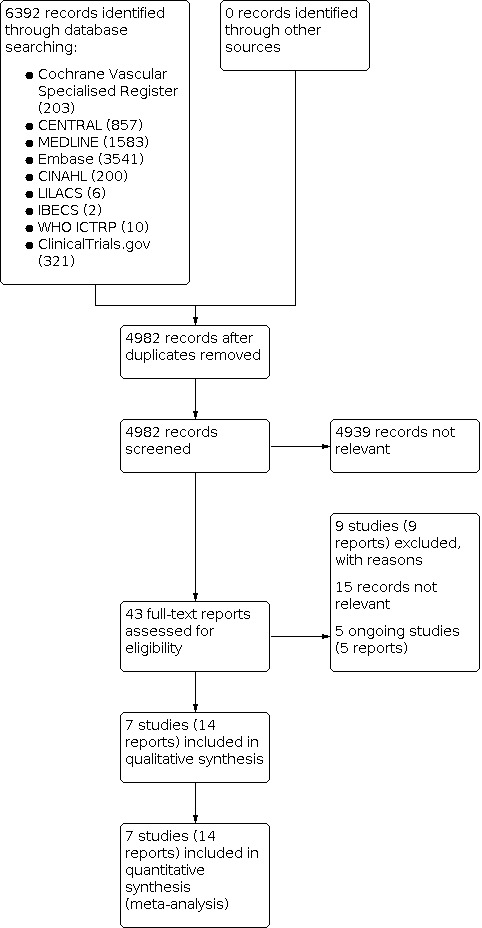
Study flow diagram.
Included studies
The seven included studies assessed 1045 participants who underwent bariatric surgery and received an intervention for preventing VTE. All studies analysed prophylactic anticoagulants. We extracted data for four comparisons:
higher‐dose heparin versus standard‐dose heparin (Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016);
heparin versus pentasaccharide (Steele 2015b);
heparin started before surgery versus heparin started after surgery (Abdelsalam 2021); and
combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone (Ahmad 2021).
For details of the included studies, see the Characteristics of included studies table.
Design
All seven included studies were classified as parallel RCTs, but three did not provide details on the method used for randomisation (Ebrahimifard 2012; Kalfarentzos 2001; Steib 2016). Six studies had two arms (Abdelsalam 2021; Ahmad 2021; Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steele 2015b) and one study had three arms (Steib 2016).
Although the nature of the intervention allowed for blinding of participants, personnel and outcome assessors, no studies were triple‐blind. Steele 2015b adequately described blinding of personnel and participants but did not describe blinding of outcome assessors. Ahmad 2021, Ebrahimifard 2012 and Kalfarentzos 2001 did not adequately describe the blinding methods, and two studies were unblinded (Imberti 2014b; Steib 2016). Abdelsalam 2021 reported that surgeons and ultrasonographers were blind to the study groups; however, the study authors did not describe any method of blinding participants (we considered participants unblinded for this review) or outcome assessors.
Settings
Five studies were performed in a single centre, in Egypt (Abdelsalam 2021; Ahmad 2021), Iran (Ebrahimifard 2012), Greece (Kalfarentzos 2001) and the USA (Steele 2015b). Two other studies were performed as single‐country, multicentre collaborations, in Italy (Imberti 2014b) and France (Steib 2016).
Participants
The studies randomised 1069 participants, of whom 1045 were effectively analysed. The reason for exclusion of participants after randomisation varied from withdrawn concealment, refusing surgery, inclusion criteria not met, or logistical problems (Imberti 2014b; Steib 2016). The mean age ranged from 33.7 (standard deviation (SD) 9.7) years to 41.8 (SD 9.4) years. Although all studies considered both sexes for enrolment, the number of female participants was considerably higher, with a total male to female ratio of 173:896.
Sample size
The sample size ranged from 60 participants in Kalfarentzos 2001 to 258 participants in Imberti 2014b. Only one study enroled fewer than 100 participants (Kalfarentzos 2001).
Funding
Abdelsalam 2021 declared that "there are no funds for this study as it was held in a university hospital". Ahmad 2021 declared that "the authors received no financial support for the research, authorship, and/or publication of this article". Ebrahimifard 2012 declared their study was funded by the Tehran University of Medical Sciences and Health Services. Imberti 2014b and Steele 2015b stated that they were funded by pharmaceutical companies (Alfa Wasserman for Imberti 2014b and GlaxoSmithKline for Steele 2015b), but that the companies did not interfere in the study design, results or reports. Steib 2016 reported that the study was partially funded by a pharmaceutical company (Sanofi‐Aventis) and partially funded by institutional grants, but that the funders did not have any role in their study design, conduction, analysis or report. Kalfarentzos 2001 did not report their funding source.
Conflicts of interest
The authors of Abdelsalam 2021, Ahmad 2021, Ebrahimifard 2012, Imberti 2014b, Steele 2015b and Steib 2016 declared no conflict of interest. Kalfarentzos 2001 made no statement about conflict of interest.
Interventions
Four studies compared different dose regimens of the same heparin. Ebrahimifard 2012 analysed UFH 5000 IU three times per day versus UFH 5000 IU twice per day. Imberti 2014b compared the LMWH parnaparin 6400 IU per day to parnaparin 4250 IU per day, started 12 hours preoperatively and administered for a mean period of 9 (SD 2) days. Kalfarentzos 2001 compared nadroparin (LMWH) 9500 IU per day to nadroparin 5700 IU per day, started preoperatively then administered once daily postoperatively until discharge. Steib 2016 compared enoxaparin (LMWH) at three different doses (4000 IU twice per day and once per day, and 6000 IU once per day).
Steele 2015b compared enoxaparin (LMWH) 40 mg twice a day during hospitalisation, starting on call to the operating room versus fondaparinux sodium (pentasaccharide) 5 mg once daily during hospitalisation, starting six hours following surgery.
Abdelsalam 2021 analysed the administration of enoxaparin (LMWH) 1 mg/kg/day (with a maximum dose of 120 mg per day), started 12 hours before surgery and administered at the same dose after surgery until the 15th postoperative day, versus the same drug at the same dose but administered only after surgery until the 15th postoperative day.
Ahmad 2021 compared enoxaparin (LMWH) 40 mg administered 12 hours before surgery and every 24 hours after surgery for two weeks, combined with mechanical interventions (elastic stocking and early ambulation) versus mechanical interventions alone (elastic stocking and early ambulation).
All participants in Ebrahimifard 2012, Steele 2015b, and Steib 2016 received mechanical interventions (e.g. compression stocking, early ambulation or sequential compression devices). Imberti 2014b reported that most (but not all) participants received at least one mechanical intervention. Kalfarentzos 2001 did not report on the use of mechanical interventions. Abdelsalam 2021 reported that "patients were encouraged to ambulate a few hours postoperatively and to continue mobility on a regular basis when discharged home", but provided no data on the effective adherence of participants to these recommendations. Abdelsalam 2021 did not report any other mechanical intervention.
No studies reported the level of experience of the person carrying out the procedure.
Outcomes
The seven studies measured similar outcomes, and we were able to extract data for all outcomes of this review except QoL. The most frequent outcome measures were VTE, major bleeding, all‐cause mortality and VTE‐related mortality. Studies assessed these outcomes at different time points, ranging from seven to 180 days after the start of the intervention. Four studies evaluated data up to 90 days after the start of the intervention (i.e. the cut‐off for our pre‐established early time point; Abdelsalam 2021; Ebrahimifard 2012; Imberti 2014b; Steib 2016). Steele 2015b reported data up to two weeks, without explaining why the follow‐up differed from that proposed in their protocol (two years). We tried to obtain clarification by email without success. Kalfarentzos 2001 provided data for long‐term follow‐up (up to 180 days after the intervention). Ahmad 2021 presented data for at least four weeks' follow‐up; participants who experienced a VTE event had their follow‐up increased to six months as they had to continue anticoagulation treatment.
Primary outcomes
All studies provided data on VTE and major bleeding.
Secondary outcomes
All studies reported all‐cause mortality, VTE‐related mortality and DVT until the end of the study period.
Only Imberti 2014b, Kalfarentzos 2001 and Steele 2015b provided data on PE and adverse events. Imberti 2014b and Kalfarentzos 2001 reported only thrombocytopenia adverse events, and Steele 2015b reported thrombocytopenia and other adverse events (atrial fibrillation, rash and nausea and vomiting). Abdelsalam 2021, Ebrahimifard 2012, Steib 2016 and Ahmad 2021 made no mention of adverse events or PE.
No studies provided data on QoL.
Excluded studies
We excluded eight studies due to ineligible study design (non‐randomised, cohort or retrospective studies; Birkmeyer 2012; Borkgren‐Okonek 2008; Goslan 2018; Kushnir 2019; Magee 2009; Raftopoulos 2008; Scholten 2002; Simone 2008), and one study due to wrong population (people undergoing plastic or reconstructive surgery; Pannucci 2021). See Characteristics of excluded studies table.
Ongoing studies
We identified five ongoing studies, evaluating at least one of the following interventions for preventing VTE in people undergoing bariatric surgery:
heparin (UFH or LMWH; Balibrea 2017; NCT01970202; NCT02128178; TCTR20201016001); or
DOACs, factor Xa inhibitors and direct thrombin inhibitors (Balibrea 2017; NCT03522259).
For our primary outcomes, Balibrea 2017, NCT02128178, and NCT03522259 plan to provide data on VTE, and Balibrea 2017 and NCT02128178 plan to provide data on major bleeding. For our secondary outcomes, only NCT03522259 plans to report all‐cause mortality, and only Balibrea 2017 plans to report adverse events. No ongoing studies plan to report VTE‐related mortality, DVT and PE (separately) or QoL. NCT01970202 plans to report laboratory outcomes but included none of the outcomes relevant to this review in their planning. TCTR20201016001 plans to report anti‐Xa levels but has made no mention of any other prespecified outcomes.
We tried to contact trial authors, and we searched by the trial registration number and title on all databases of interest, but we identified no additional data for any of these ongoing studies.
Risk of bias in included studies
We provided justifications for our risk of bias judgements in the Characteristics of included studies table. Figure 2 and Figure 3 summarise the review authors' judgements about each risk of bias item in graphical format.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We also judged the overall risk of bias as described in the Sensitivity analysis section. We considered Steele 2015b to have high overall risk of bias and all other studies to have low overall risk of bias (Abdelsalam 2021; Ahmad 2021; Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016).
Allocation
Random sequence generation
We considered four studies at low risk of bias related to random sequence generation (Abdelsalam 2021; Ahmad 2021; Imberti 2014b; Steele 2015b), and the remaining three at unclear risk as they did not describe the randomisation method (Ebrahimifard 2012; Kalfarentzos 2001; Steib 2016).
Allocation concealment
We judged Ahmad 2021 and Steele 2015b at low risk of bias related to allocation concealment, and the remaining five studies at unclear risk due to lack of information (Abdelsalam 2021; Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016).
Blinding
Blinding of participants and personnel
We considered only Steele 2015b at low risk of performance bias. Three studies provided no information about the blinding of participants, so we considered them at unclear risk (Ahmad 2021; Ebrahimifard 2012; Kalfarentzos 2001). We judged the remaining three studies at high risk of bias because they did not blind participants (Abdelsalam 2021; Imberti 2014b; Steib 2016).
Blinding of outcome assessment
We judged four studies at unclear risk of detection bias due to lack of information (Abdelsalam 2021; Ebrahimifard 2012; Kalfarentzos 2001; Steele 2015b), two studies at high risk because they did not blind outcome assessors (Imberti 2014b; Steib 2016), and only Ahmad 2021 at low risk.
Incomplete outcome data
We considered all studies at low risk of attrition bias (Abdelsalam 2021; Ahmad 2021; Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steele 2015b; Steib 2016).
Selective reporting
We considered six studies at low risk of reporting bias (Abdelsalam 2021; Ahmad 2021; Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016), and Steele 2015b at high risk because it excluded 21/198 (10.6%) participants from the assessment of VTE and DVT occurrence (in fact, two more participants were not included in the analysis of VTE and DVT, but Steele 2015b did not mention the reason for this loss of data).
Other potential sources of bias
We judged six studies at low risk of other bias (Abdelsalam 2021; Ahmad 2021; Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016), and Steele 2015b at high risk because the prespecified outcomes differed from those included in the final report.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Higher‐dose heparin versus standard‐dose heparin
Four studies compared higher‐dose to standard‐dose heparin in people undergoing bariatric surgery, reporting outcomes of interest for this review with a follow‐up of 90 days or less (Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016).
Primary outcomes
Venous thromboembolism
Only Imberti 2014b reported VTE events. The other three studies reported no events in either group (Ebrahimifard 2012; Kalfarentzos 2001; Steib 2016). Higher‐dose heparin may result in little or no difference in VTE compared to standard‐dose heparin in people undergoing bariatric surgery (RR 0.55, 95% CI 0.05 to 5.99; 4 studies, 597 participants; low‐certainty evidence; Analysis 1.1). We downgraded the certainty of the evidence by one level due to the high risk of performance and detection bias, and by one level due to imprecision (few events and CI consistent with possible benefit and possible harm). The test for subgroup differences and the sensitivity analysis was not applicable because only Imberti 2014b reported events.
1.1. Analysis.

Comparison 1: Higher‐dose heparin versus standard‐dose heparin, Outcome 1: Venous thromboembolism
Major bleeding
All four studies reported major bleeding with a follow‐up of 10 to 90 days (Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016). Higher‐dose heparin may result in little or no difference in major bleeding compared to standard‐dose heparin in people undergoing bariatric surgery (RR 1.19, 95% CI 0.48 to 2.96; I2 = 8%; 4 studies, 597 participants; low‐certainty evidence; Analysis 1.2). We downgraded the certainty of the evidence by one level due to the high risk of performance and detection bias, and one level due to imprecision (few events and CI consistent with possible benefit and possible harm). The test for subgroup differences suggested that the type of heparin had no modifying effect on major bleeding (I2 = 0%, Chi2 = 0.77, df = 1 (P = 0.38)). The sensitivity analysis was not applicable because we judged all studies as having low overall risk of bias.
1.2. Analysis.

Comparison 1: Higher‐dose heparin versus standard‐dose heparin, Outcome 2: Major bleeding
Secondary outcomes
All‐cause mortality
All four studies reported no deaths in either group during a follow‐up of 10 to 90 days (Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016). The effect of higher‐dose heparin on all‐cause mortality compared to standard‐dose heparins from 10 to 90 days was not estimable (no events in either group; 4 studies, 597 participants; low‐certainty evidence; Analysis 1.3). We downgraded the certainty of the evidence by two levels due to imprecision (no events). The test for subgroup differences and the sensitivity analysis were not applicable because there were no events.
1.3. Analysis.

Comparison 1: Higher‐dose heparin versus standard‐dose heparin, Outcome 3: All‐cause mortality
Venous thromboembolism‐related mortality
All four studies reported no deaths in either group during a follow‐up of 10 to 90 days (Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016). The effect of higher‐dose heparin on VTE‐related mortality compared to standard‐dose heparin from 10 to 90 days was not estimable (no events in either group; 4 studies, 597 participants; low‐certainty evidence; Analysis 1.4). We downgraded the certainty of the evidence by two levels due to imprecision (no events). The test for subgroup differences and the sensitivity analysis were not applicable because there were no events.
1.4. Analysis.

Comparison 1: Higher‐dose heparin versus standard‐dose heparin, Outcome 4: Venous thromboembolism‐related mortality
Pulmonary embolism
Imberti 2014b and Kalfarentzos 2001 assessed PE with a follow‐up of 90 days or less. Both studies used LMWH. Kalfarentzos 2001 reported no events in either group. Imberti 2014b reported one PE event in the standard‐dose heparin group. The evidence on PE is uncertain for people undergoing bariatric surgery (RR 0.37, 95% CI 0.02 to 8.92; 2 studies, 310 participants; very low‐certainty evidence; Analysis 1.5). We downgraded the certainty of the evidence by one level due to the high risk of performance and detection bias, and by two levels due to imprecision (very large CI of the absolute difference and few events). The test for subgroup differences and the sensitivity analysis were not applicable because only Imberti 2014b reported events.
1.5. Analysis.

Comparison 1: Higher‐dose heparin versus standard‐dose heparin, Outcome 5: Pulmonary embolism
Deep vein thrombosis
All four studies assessed DVT with a follow‐up of 10 to 90 days (Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016), but only Imberti 2014b reported events (one case of DVT in each group). The evidence on DVT is uncertain for people undergoing bariatric surgery (RR 1.10, 95% CI 0.07 to 17.40; 4 studies, 597 participants; very low‐certainty evidence; Analysis 1.6). We downgraded the certainty of the evidence by one level due to the high risk of performance and detection bias, and by two levels due to imprecision (very large CI of the absolute difference and few events). The test for subgroup differences and the sensitivity analysis were not applicable because only Imberti 2014b reported events.
1.6. Analysis.

Comparison 1: Higher‐dose heparin versus standard‐dose heparin, Outcome 6: Deep vein thrombosis
Adverse events
In this comparison, the only reported adverse event was thrombocytopenia. Imberti 2014b and Kalfarentzos 2001 assessed thrombocytopenia with a follow‐up of 90 days or less. Kalfarentzos 2001 reported no events in either group. Imberti 2014b reported one event in each group. Both studies used LMWH. The evidence on adverse events (thrombocytopenia) is uncertain for people undergoing bariatric surgery (RR 1.10, 95% CI 0.07 to 17.40; 2 studies, 310 participants; very low‐certainty evidence; Analysis 1.7). We downgraded the certainty of the evidence by one level due to the high risk of performance and detection bias, and by two levels due to imprecision (very large CI of the absolute difference and few events). The test for subgroup differences and the sensitivity analysis were not applicable because only Imberti 2014b reported events.
1.7. Analysis.

Comparison 1: Higher‐dose heparin versus standard‐dose heparin, Outcome 7: Adverse events (thrombocytopaenia)
Quality of life
There were no data for QoL.
Heparin versus pentasaccharide
Only Steele 2015b compared LMWH versus a pentasaccharide in people undergoing bariatric surgery, reporting outcomes of interest for this review with a follow‐up of up to 14 days. The test for subgroup differences and sensitivity analysis were not applicable for any outcome, as there was only one study in this comparison.
Primary outcomes
Venous thromboembolism
Heparin may result in little or no difference in risk of VTE compared to pentasaccharide in people undergoing bariatric surgery (RR 0.83, 95% CI 0.19 to 3.61; 175 participants; low‐certainty evidence; Analysis 2.1). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by one level due to imprecision (few participants, and 95% CI consistent with possible benefit and possible harm).
2.1. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 1: Venous thromboembolism
Major bleeding
The evidence on major bleeding is uncertain (RR 1.70, 95% CI 0.42 to 6.92; 198 participants; very low‐certainty evidence; Analysis 2.2). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by two levels due to imprecision (few events and very large CI of absolute difference).
2.2. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 2: Major bleeding
Secondary outcomes
All‐cause mortality
The effect of heparin compared to pentasaccharide up to 14 days on all‐cause mortality was not estimable (no events in either group; 198 participants; very low‐certainty evidence; Analysis 2.3). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by two levels due to imprecision (no events).
2.3. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 3: All‐cause mortality
Venous thromboembolism‐related mortality
The effect of heparin compared to pentasaccharide up to 14 days on VTE‐related mortality was not estimable (no events in either group; 198 participants; very low‐certainty evidence; Analysis 2.4). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by two levels due to imprecision (no events).
2.4. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 4: Venous thromboembolism‐related mortality
Pulmonary embolism
The effect of heparin compared to pentasaccharide up to 14 days on PE was not estimable (no events in either group; 198 participants; very low‐certainty evidence; Analysis 2.5). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by two levels due to imprecision (no events).
2.5. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 5: Pulmonary embolism
Deep vein thrombosis
Heparin may result in little or no difference in DVT compared to pentasaccharide in people undergoing bariatric surgery (RR 0.83, 95% CI 0.19 to 3.61; 175 participants; low‐certainty evidence; Analysis 2.6). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by one level due to imprecision (few participants and 95% CI consistent with possible benefit and possible harm).
2.6. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 6: Deep vein thrombosis
Adverse events
Steele 2015b reported the adverse events thrombocytopenia, atrial fibrillation, rash, and nausea and vomiting.
The evidence on adverse events (thrombocytopenia) is uncertain for people undergoing bariatric surgery (RR 0.34, 95% CI 0.01 to 8.25; 198 participants; very low‐certainty evidence; Analysis 2.7). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by two levels due to imprecision (few events and very large CI of absolute difference).
2.7. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 7: Adverse events (thrombocytopaenia)
The evidence on adverse events (atrial fibrillation) is uncertain for people undergoing bariatric surgery (RR 2.04, 95% CI 0.19 to 22.14; 198 participants; very low‐certainty evidence; Analysis 2.8). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by two levels due to imprecision (few events and very large CI of absolute difference).
2.8. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 8: Adverse events (atrial fibrillation)
The evidence on adverse events (rash) is uncertain for people undergoing bariatric surgery (RR 1.02, 95% CI 0.06 to 16.09; 198 participants; very low‐certainty evidence; Analysis 2.9). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by two levels due to imprecision (few events and very large CI of absolute difference).
2.9. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 9: Adverse events (rash)
The evidence on adverse events (nausea and vomiting) is uncertain for people undergoing bariatric surgery (RR 1.02, 95% CI 0.15 to 7.10; 198 participants; very low‐certainty evidence; Analysis 2.10). We downgraded the certainty of the evidence by one level due to the high risk of reporting and other bias, and by two levels due to imprecision (few events and very large CI of the absolute difference).
2.10. Analysis.

Comparison 2: Heparin versus pentasaccharide, Outcome 10: Adverse events (nausea and vomiting)
Quality of life
There were no data for QoL.
Heparin started before versus after the surgical procedure
Only Abdelsalam 2021 compared LMWH starting before versus after the bariatric surgery, reporting outcomes of interest for this review with a follow‐up of 15 days. The test for subgroup differences and sensitivity analysis were not applicable for any outcome, as there was only one study in this comparison.
Primary outcomes
Venous thromboembolism
Starting heparin before the surgical procedure may result in little or no difference in VTE compared to starting heparin after the procedure in people undergoing bariatric surgery (RR 0.11, 95% CI 0.01 to 2.01; 100 participants; low‐certainty evidence; Analysis 3.1). We downgraded the certainty of the evidence by one level due to the high risk of performance bias, and by one level due to imprecision (few participants and 95% CI consistent with possible benefit and possible harm).
3.1. Analysis.

Comparison 3: Heparin started before versus after the surgical procedure, Outcome 1: Venous thromboembolism
Major bleeding
The evidence on major bleeding is uncertain for people undergoing bariatric surgery (RR 3.00, 95% CI 0.13 to 71.92; 100 participants; very low‐certainty evidence; Analysis 3.2). We downgraded the certainty of the evidence by one level due to high performance bias, and by two levels due to imprecision (few events, few participants and very large CI).
3.2. Analysis.

Comparison 3: Heparin started before versus after the surgical procedure, Outcome 2: Major bleeding
Secondary outcomes
All‐cause mortality
The effect of starting heparin before versus after the surgical procedure on all‐cause mortality at 15 days was not estimable (no events in either group; 100 participants; very low‐certainty evidence; Analysis 3.3). We downgraded the certainty of the evidence by one level due to high performance bias, and by two levels due to imprecision (no events and few participants).
3.3. Analysis.

Comparison 3: Heparin started before versus after the surgical procedure, Outcome 3: All‐cause mortality
Venous thromboembolism‐related mortality
The effect of starting heparin before versus after the surgical procedure on VTE‐related mortality at 15 days was not estimable (no events in either group; 100 participants; very low‐certainty evidence; Analysis 3.4). We downgraded the certainty of the evidence by one level due to high performance bias, and by two levels due to imprecision (no events and few participants).
3.4. Analysis.

Comparison 3: Heparin started before versus after the surgical procedure, Outcome 4: Venous thromboembolism‐related mortality
Pulmonary embolism
There were no data for PE.
Deep vein thrombosis
Starting heparin before the surgical procedure may result in little or no difference in DVT compared with starting heparin after the procedure in people undergoing bariatric surgery (RR 0.11, 95% CI 0.01 to 2.01; 100 participants; low‐certainty evidence; Analysis 3.5). We downgraded the certainty of the evidence by one level due to high risk of performance bias, and by one level due to imprecision (few events and 95% CI consistent with possible benefit and possible harm).
3.5. Analysis.

Comparison 3: Heparin started before versus after the surgical procedure, Outcome 5: Deep vein thrombosis
Adverse events
There were no data for adverse events.
Quality of life
There were no data for QoL.
Combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone
Only Ahmad 2021 compared LMWH 40 mg started 12 hours before and administered every 24 hours after surgery, combined with elastic stockings and early ambulation, versus elastic stockings and early ambulation alone. The study reported outcomes of interest for this review with a follow‐up of four weeks. The test for subgroup differences and sensitivity analysis were not applicable for any outcome, as there was only one study in this comparison.
Primary outcomes
Venous thromboembolism
Combined mechanical and pharmacological prophylaxis may reduce the incidence of VTE compared to mechanical prophylaxis alone in people undergoing bariatric surgery within four weeks (RR 0.05, 95% CI 0.00 to 0.89; NNTB 9; 150 participants; low‐certainty evidence; Analysis 4.1). We downgraded the certainty of the evidence by two levels due to imprecision (few events and small sample size).
4.1. Analysis.

Comparison 4: Combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone, Outcome 1: Venous thromboembolism
Major bleeding
The effect of combined mechanical and pharmacological prophylaxis compared to mechanical prophylaxis alone within four weeks on major bleeding was not estimable (no events in either group; 150 participants; low‐certainty evidence; Analysis 4.2). We downgraded the certainty of the evidence by two levels due to imprecision (no events).
4.2. Analysis.

Comparison 4: Combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone, Outcome 2: Major bleeding
Secondary outcomes
All‐cause mortality
The effect of combined mechanical and pharmacological prophylaxis compared to mechanical prophylaxis alone within four weeks on all‐cause mortality was not estimable (no events in either group; 150 participants; low‐certainty evidence; Analysis 4.3). We downgraded the certainty of the evidence by two levels due to imprecision (no events).
4.3. Analysis.

Comparison 4: Combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone, Outcome 3: All‐cause mortality
Venous thromboembolism‐related mortality
The effect of combined mechanical and pharmacological prophylaxis compared to mechanical prophylaxis alone within four weeks on VTE‐related mortality was not estimable (no events in either group; 150 participants; low‐certainty evidence; Analysis 4.4). We downgraded the certainty of the evidence by two levels due to imprecision (no events).
4.4. Analysis.

Comparison 4: Combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone, Outcome 4: Venous thromboembolism‐related mortality
Pulmonary embolism
There were no data for PE.
Deep vein thrombosis
Combined mechanical and pharmacological prophylaxis may reduce the incidence of DVT compared to mechanical prophylaxis alone in people undergoing bariatric surgery (RR 0.05, 95% CI 0.00 to 0.89; 150 participants; low‐certainty evidence; Analysis 4.1). We downgraded the certainty of the evidence by two levels due to imprecision (few events and small sample size).
Adverse events
There were no data for adverse events.
Quality of life
There were no data for QoL.
Discussion
Summary of main results
We included seven RCTs with 1045 participants. The studies contributed to four comparisons in this review:
higher‐dose heparin versus standard‐dose heparin;
heparin versus a pentasaccharide;
heparin started before versus after the surgical procedure; and
combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone.
Six studies analysed only early time points, with follow‐up between seven and 90 days (which meets our protocol expectation). Only one study analysed participants 180 days after the intervention.
We found four RCTs (597 participants) that compared higher‐dose versus standard‐dose heparin. One of these studies (139 participants) used UFH and the remaining three (458 participants) used LMWH. Higher‐dose heparin may result in little or no difference in VTE and major bleeding compared to standard‐dose heparin in people undergoing bariatric surgery. The effect of higher‐dose heparin on all‐cause mortality and VTE‐related mortality compared to standard‐dose heparin was not estimable, as the studies reported no events. The evidence on PE, DVT and adverse events (thrombocytopenia) is uncertain.
One study with 198 participants compared heparin to a pentasaccharide. Heparin compared to pentasaccharide in people undergoing bariatric surgery may result in little or no difference in VTE or DVT. The effect on all‐cause and VTE‐related mortality was not estimable, as the study reported no events. The evidence on major bleeding, PE and adverse events (thrombocytopenia, atrial fibrillation, rash and nausea and vomiting) is uncertain. This study had 198 participants, but analysed only 175 of them for the thromboembolic outcomes (VTE, PE and DVT).
One study with 100 participants compared heparin started 12 hours before surgery versus after surgery. Starting heparin before surgery may result in little or no difference in VTE or DVT. The effect on all‐cause and VTE‐related mortality was not estimable, as the study reported no events. Although the study authors reported no PE events, they did not use diagnostic methods to ascertain asymptomatic or oligosymptomatic PE. Therefore, we considered that this study did not offer enough data to assess the evidence on the effect of the intervention on PE risk.
One study with 150 participants compared combined mechanical and pharmacological prophylaxis with mechanical prophylaxis alone in people undergoing bariatric surgery. Combined mechanical and pharmacological prophylaxis may reduce the risk of VTE and DVT. The effect on major bleeding, all‐cause mortality and VTE‐related mortality was not estimable, as the study reported no events in either group. Because the study authors did not attempt to ascertain asymptomatic or oligosymptomatic PE, we considered there were insufficient data to assess the effect of the intervention on PE.
There were no data to assess the effect of these interventions on QoL.
Overall completeness and applicability of evidence
Obesity is considered a major risk factor for VTE, and all people with obesity who undergo surgery would usually have VTE prophylaxis in the absence of major contraindications (Bartlett 2015). Although other pharmacological interventions are available in clinical practice, we found no studies using VKAs, DOACs or antiplatelet agents for preventing VTE in the bariatric surgery routine. In fact, it is unlikely that VKAs will ever be studied as a primary prophylactic intervention, as they are associated with higher risk of major bleeding and more complex anticoagulation control, as pointed out in the protocol of this review (Amaral 2020; Stevens 2021; Smith 2018). However, studies addressing the effects of DOACs or antiplatelet agents would have been of great value to this review, as these drugs are used in the prophylaxis of thromboembolism in other situations (Flumignan 2022b; Flumignan 2021; Flumignan 2022a; Santos 2022; Stevens 2021). We did find two records of ongoing studies addressing the effect of DOACs in the prophylaxis of VTE in bariatric surgery patients (Balibrea 2017; NCT03522259), but at the time of writing there is no estimate of when data may be available. Because we only included studies that addressed the effects of heparins (UFH and LMWH) and pentasaccharides, the evidence in this review is valid only for these interventions.
Although Ebrahimifard 2012 reported no DVT events, the participants only underwent duplex ultrasound at day 10 after surgery. Given that studies such as Froehling 2013 have reported a higher incidence of VTE within the first month of surgery, it is possible that Ebrahimifard 2012 underestimated the event occurrence. Nonetheless, Kalfarentzos 2001 and Steib 2016 also reported no DVT event, although their participants had duplex ultrasound at least 30 days after surgery. The other included studies were effective in diagnosing and reporting DVT. Only one study reported PE. This may be because it is easier to diagnose asymptomatic DVT (as most cases were) than asymptomatic PE. As a result, the incidence of VTE and of DVT were almost the same.
The included studies reported no deaths, which suggests a much larger sample is needed to address the effect of these interventions on mortality. No studies addressed QoL, and only three studies measured adverse events (Imberti 2014b; Kalfarentzos 2001; Steele 2015b).
Despite varying durations of follow‐up, the duration of the intervention itself was up to 15 days in all studies, with a minimum of seven days in Imberti 2014b. Therefore, there is a lack of evidence assessing the duration of anticoagulation in people undergoing bariatric surgery.
Because people undergoing bariatric surgery have increased weight, there is an argument for augmenting the heparin dose to reach an effective level, but because most trials have excluded people with severe obesity, there is little evidence to support this theory (Stevens 2021). In addition, some people with higher BMI may have non‐alcoholic fatty liver disease, which affects their bleeding risk on anticoagulants (Pillai 2009; Stevens 2021). There are insufficient data in the included studies to support any difference in VTE prevalence according to BMI of participants, surgery type or surgery access (open surgery or laparoscopic). Most studies reported that the participants had also received mechanical interventions (e.g. early ambulation, compression stockings); however, Kalfarentzos 2001 provided no information in this regard.
Quality of the evidence
We found four RCTs with data for one comparison (higher‐dose heparin compared to standard‐dose heparin) and one RCT with data each for other comparisons (heparin compared to pentasaccharide, heparin starting before versus after the surgical procedure, and combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone).
The overall risk of bias was low for the fourth comparison, which included only Ahmad 2021. In the first two comparisons, we considered the risk of bias related to blinding of participants, random sequence generation or allocation concealment to be unclear or high for most studies (Abdelsalam 2021; Ebrahimifard 2012; Imberti 2014b; Kalfarentzos 2001; Steib 2016). Risk of bias was high for selective reporting and other bias in the RCT included in the heparin versus pentasaccharide comparison, as 23 participants were lost from only one arm of the study (21 reported missing, two not reported missing but not included in the analysis), and the time point of outcome assessment in the published report differed from that prespecified in the protocol (Steele 2015b). See Characteristics of included studies for detailed information on the risk of bias assessment.
The certainty of the evidence for all outcomes was low to very low. We downgraded the certainty of the evidence due to risk of bias, particularly with regard to performance and detection bias in three RCTs, and also reporting and other bias in one RCT. We downgraded the certainty of the evidence due to study limitations (risk of bias) and imprecision (few events, few participants and large CI) the first three comparisons. For the fourth comparison, we downgraded the certainty of the evidence for imprecision (small sample size and few events). See Table 1, Table 2, Table 3 and Table 4.
Potential biases in the review process
With the aim of identifying all relevant randomised trials for this review, we performed a broad and sensitive search through the prespecified databases, with no limitations on language, date of publication or any other factor. Additionally, we searched through the references of the included studies, and through the references of other systematic reviews and guidelines (Abildgaard 2020; Agarwal 2010; Bartlett 2015; Becattini 2012; Brotman 2013; Ikesaka 2014; Rocha 2006; Venclauskas 2018). However, it is possible that we missed some trials, notably in the grey literature. We adhered to the eligibility criteria prespecified in the protocol to limit subjectivity (Amaral 2020). We tried to contact the study authors to obtain additional relevant data, but received no response in some cases. If additional data become available in the future, we will include them in future updates of this review. To reduce the potential bias of the review process, three review authors independently selected studies, extracted data and assessed risk of bias. Where possible, we performed additional analyses (subgroups and sensitivity analyses) as planned in our protocol, but our conclusions are based on our primary analysis (Amaral 2020).
Agreements and disagreements with other studies or reviews
There are other published systematic reviews on VTE prophylaxis for people undergoing bariatric surgery, but all have methodological differences compared with our review.
Ikesaka 2014 searched MEDLINE, Embase and Cochrane CENTRAL, and handsearched the references of the included studies and conference proceedings. Ikesaka 2014 considered all types of study design, included only studies reporting VTE or bleeding and imposed no restriction regarding language or year of publication. They reported the results of one of our included studies (Kalfarentzos 2001). They used the RoB 1 tool to assess the risk of bias in the RCT, but used an obsolete risk of bias tool (the Modified Newcastle‐Ottawa Scoring System) for non‐randomised studies, and did not assess the certainty of evidence. Their search strategy included only pharmacological terms for heparin and did not consider DOACs or VKAs. Ikesaka 2014 combined six studies (one RCT, one quasi‐RCT and four NRS) in meta‐analyses and concluded that "adjusting the dose of heparin products for thromboprophylaxis post‐bariatric surgery seems to be associated with a lower rate of in hospital VTE compared to a strategy of not adjusting the dose, although this did not reach statistical significance".
Becattini 2012 searched MEDLINE and Embase, and handsearched the references of the included studies and other reviews, applying no language or publication date restrictions. Becattini 2012 aimed to include RCTs and observational studies, but retrieved no RCTs. They included only studies with videolaparoscopic surgery, reporting VTE and bleeding events. They did not use a standardised tool to assess the risk of bias of the included studies, and they did not describe any tool for assessing the certainty of the evidence. They included 19 observational studies, (12 prospective cohort studies and seven retrospective cohort studies). Becattini 2012 concluded that "the incidence of postoperative VTE seems to be relatively low in this setting, and the benefit of weight adjusted heparin prophylaxis remains controversial".
In Rocha 2006, bariatric surgery patients were a subgroup of the target population. Rocha 2006 searched MEDLINE, LILACS and the Cochrane CENTRAL databases, but did not mention any strategy for searching the grey literature. Their search strategy appears limited, as they only used a few Medical Subject Headings (MeSH) terms for obesity and VTE, without using free terms or correlated terms, and without searching for the pharmacological interventions. They considered all types of study design, and included only studies reporting VTE or bleeding. They imposed no language restrictions, but limited the search to studies published from 1976. They included six studies, but only one RCT, which is one of our included studies (Kalfarentzos 2001). They did not mention any method for assessing risk of bias, but did assess the certainty of the evidence using the 2001 Oxford Centre for Evidence‐Based Medicine levels of evidence tool. Due to the limited number and quality of the included studies, Rocha 2006 were unable to reach a conclusion regarding the more effective and safe prophylactic regimen.
Agarwal 2010 performed an update to the Rocha 2006 review. They searched for studies from 2006 to 2009 in MEDLINE and Cochrane CENTRAL (without LILACS), and included the studies already retrieved in the Rocha 2006 search, as well as studies from the reference lists of other reviews and guidelines. They considered all types of study design and included only studies published in English that reported VTE or bleeding and mortality. They analysed 30 studies, but only one RCT (Kalfarentzos 2001), which is also in our review. The remaining 29 studies were non‐RCTs (N = 7) or uncontrolled studies (N = 22). They did not mention any tool for assessing risk of bias or certainty of the evidence. Agarwal 2010 concluded that "although patients undergoing bariatric surgery are at a major risk of VTE, the various thromboprophylactic agents have been poorly studied in this setting".
Bartlett 2015 searched MEDLINE, Embase, Cochrane CENTRAL and ClinicalTrials.gov, and handsearched the references of the included studies and other reviews, limiting the search to studies published in English. They considered RCTs and cohort studies that compared two or more groups and that reported VTE or bleeding and mortality. Their search strategy did not include terms describing the interventions. The review included 14 studies, with two RCTs (Imberti 2014b; Kalfarentzos 2001), both of which are in our review. Bartlett 2015 did not mention how they assessed risk of bias or certainty of the evidence. Bartlett 2015 concluded that "until more data are available, institutional quality improvement efforts should focus on ensuring consistent application of established methods".
Brotman 2013 searched MEDLINE, Embase, Scopus, CINAHL, International Pharmaceutical Abstracts, ClinicalTrials.gov and the Cochrane Library, and handsearched the references of the included studies and other reviews. They included 13 observational cohort studies. The outcomes of interest were VTE, bleeding events, all‐cause mortality and adverse drug reactions. Brotman 2013 assessed risk of bias with "10 items from the Downs and Black instrument" and the certainty of the evidence "by adapting a grading scheme recommended in the Methods Guide for Effectiveness and Comparative Effectiveness Reviews". Brotman 2013 did not find evidence to support the use of a higher dose of pharmacotherapy in people undergoing bariatric surgery.
Hussain 2018 is a systematic review of a variety of drugs in people with obesity; anticoagulants in bariatric surgery patients is a subgroup. Hussain 2018 searched MEDLINE, Embase, CINAHL and Cochrane databases with a comprehensive search strategy. They considered all types of study design, included only studies reporting VTE or bleeding and imposed no restrictions regarding language or year of publication. They identified seven studies that analysed the effect of anticoagulant prophylaxis in bariatric surgery patients, including three RCTs (Imberti 2014b; Kalfarentzos 2001; Steib 2016), all of which are also in our review. Hussain 2018 assessed risk of bias using the Meta‐Analysis of Statistics Assessment and Review Instrument (MAStARI) for the cohort (with control) and randomised studies, and assessed the certainty of the evidence using the National Health and Medical Research Council GRADE tool. Hussain 2018 concluded that "optimal dose adjustment for obese patients remained unclear".
Abildgaard 2020 is a systematic review on the prophylactic use of anticoagulants in obese clinical and surgical patients. Bariatric surgery patients constituted a subgroup of participants. Abildgaard 2020 searched MEDLINE and Embase, restricting the search to English‐language publications but applying no publication date limitation. They included RCTs and observational studies that reported clinical efficacy or biochemical efficacy (antifactor Xa levels) and bleeding. They identified 22 studies that addressed the same subject as this review, including two RCTs (Steele 2015b; Steib 2016), both of which are also in our review. Abildgaard 2020 concluded with some dosage recommendations, but did not describe any assessment of the certainty of the evidence or risk of bias.
Given the cited limitations of these reviews, our review appears to be more comprehensive, including more RCTs and more comparisons. However, due to the lack of high‐quality studies, the evidence remains of low or very low certainty.
Authors' conclusions
Implications for practice.
In people undergoing bariatric surgery, higher‐dose heparin may result in little or no difference in venous thromboembolism (VTE) and major bleeding at 10 to 90 days of follow‐up. The evidence on the effect of higher‐dose heparin compared to standard‐dose heparin on all‐cause mortality, VTE‐related mortality, pulmonary embolism (PE), deep vein thrombosis (DVT) and adverse events (thrombocytopenia) is uncertain (effect not estimable or very low‐certainty evidence).
Heparin compared with a pentasaccharide after bariatric surgery may result in little or no difference in the risk of VTE or DVT. The evidence on the effect of heparin compared to pentasaccharide on the other outcomes is uncertain (effect not estimable or very low‐certainty evidence).
Starting prophylaxis with heparin 12 hours before bariatric surgery compared with starting heparin after bariatric surgery may result in little or no difference in the risk of VTE or DVT. The evidence on the effect of this intervention on major bleeding, all‐cause mortality and VTE‐related mortality is uncertain (effect not estimable or very low‐certainty evidence). We were unable to assess the effect of this intervention on PE and adverse events.
Mechanical combined with pharmacological prophylaxis, compared to mechanical prophylaxis alone, starting 12 hours before bariatric surgery, may reduce the incidence of VTE (low‐certainty evidence). However, we could not assess the effect of this intervention on the incidence of major bleeding, PE, death or adverse events.
We were unable to assess the effect of any intervention on quality of life.
Implications for research.
We found limited evidence on the use of heparins, pentasaccharides and mechanical combined with pharmacological prophylaxis in people undergoing bariatric surgery. Given the low number of included studies and the low number of events in each study, there is a need for high‐quality randomised controlled trials (RCTs) with larger sample sizes. Future trials must be sufficiently large to assess all relevant clinical outcomes, particularly those that occur less frequently (e.g. PE, mortality and adverse events). With the aim of improving clinical practice, future trials should also assess the effect of the interventions on participants' quality of life. Moreover, there is a lack of evidence regarding other relevant interventions, such as direct oral anticoagulants and antiplatelet agents for people undergoing bariatric surgery.
Research is needed on the ideal duration of the intervention (i.e. the duration of prophylactic anticoagulation) based on VTE pathophysiology. In addition, new RCTs should follow up participants for at least 120 days. Although this time point is arbitrary, there is a need to assess the long‐term outcomes related to anticoagulation.
Evidence from the five ongoing studies we identified in our search may be more robust; we will add all relevant data to updates of this review. There is still a need for RCTs of high methodological quality, including adequate reporting of randomisation, allocation concealment and blinding.
The key outcomes to be measured are VTE and major bleeding. Other important outcomes are all‐cause mortality, VTE‐related mortality, PE, DVT, adverse events and quality of life.
History
Protocol first published: Issue 7, 2020
Notes
Parts of the Methods section of this review are based on a standard template established by Cochrane Vascular.
Acknowledgements
We wish to thank Cochrane Vascular, Cochrane Brazil and the Division of Vascular and Endovascular Surgery of Universidade Federal de São Paulo, Brazil, for their methodological support.
The review authors and the Cochrane Vascular editorial base are grateful to the following peer reviewers for their time and comments: Praveen Kumar M, Clinical Research Manager, Nference, India; Gianfranco Silecchia MD PhD, Professor of Surgery, Department of Medical‐Surgical Sciences and Translational Medicine, Faculty of Medicine and Psychology Sapienza, University of Rome, Italy.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW (date of most recent search 1 November 2021) | #1 venous thromboembolism OR VTE OR Pulmonary Embolism OR Thromboembolism OR Thrombosis OR PE AND INREGISTER #2 Bariatric OR Body Mass Index OR Body Weight OR Obesity OR Overweight OR obese AND INREGISTER #3 Antithrombins or Aspirin OR Coumarins OR Dabigatran OR Anticoagula* OR Heparin OR Hirudin Therapy OR Phenindione OR Platelet Aggregation Inhibitors OR Polysaccharides OR Rivaroxaban OR Warfarin OR acetylsalicylic acid OR antiplatelet OR DOAC OR LMWH AND INREGISTER #4 #1 AND #2 AND #3 |
Aug 2020: 188 Nov 2021: 15 |
| CENTRAL via CRSO (date of most recent search 1 November 2021) | #1 MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES 990 #2 MESH DESCRIPTOR Thromboembolism EXPLODE ALL TREES 2019 #3 MESH DESCRIPTOR Thrombosis EXPLODE ALL TREES 4672 #4 MESH DESCRIPTOR Venous Thromboembolism EXPLODE ALL TREES 618 #5 MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES 2637 #6 ((vein* or ven*) adj thromb*):TI,AB,KY 10705 #7 (blood adj3 clot*):TI,AB,KY 5054 #8 (deep vein thrombosis):TI,AB,KY 4574 #9 (lung adj3 clot*):TI,AB,KY 12 #10 (PE or DVT or VTE):TI,AB,KY 7680 #11 (peripheral vascular thrombosis):TI,AB,KY 0 #12 (post‐thrombotic syndrome):TI,AB,KY 214 #13 (pulmonary embolism):TI,AB,KY 3098 #14 (pulmonary adj3 clot*):TI,AB,KY 17 #15 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*):TI,AB,KY 31116 #16 thromboprophylaxis:TI,AB,KY 957 #17 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 39544 #18 MESH DESCRIPTOR Bariatric Surgery EXPLODE ALL TREES 968 #19 MESH DESCRIPTOR Body Mass Index EXPLODE ALL TREES 9957 #20 MESH DESCRIPTOR Body Weight EXPLODE ALL TREES 27370 #21 MESH DESCRIPTOR Obesity EXPLODE ALL TREES 13487 #22 MESH DESCRIPTOR Overweight EXPLODE ALL TREES 16016 #23 bariatric:TI,AB,KY 2407 #24 (biliopancreatic diversion):TI,AB,KY 76 #25 BMI:TI,AB,KY 33674 #26 (body mass index):TI,AB,KY 33813 #27 bodyweight:TI,AB,KY 1334 #28 (body weight):TI,AB,KY 44491 #29 (gastric band*):TI,AB,KY 358 #30 (gastric bypass):TI,AB,KY 1753 #31 (laparoscopic gastric imbrication):TI,AB,KY 2 #32 (laparoscopic sleeve gastrectomy):TI,AB,KY 537 #33 obese:TI,AB,KY 21148 #34 obesity:TI,AB,KY 34868 #35 (over weight):TI,AB,KY 123 #36 overweight:TI,AB,KY 15628 #37 #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 110411 #38 #17 AND #37 1997 #39 MESH DESCRIPTOR Antithrombins EXPLODE ALL TREES 1982 #40 MESH DESCRIPTOR Aspirin EXPLODE ALL TREES 5806 #41 MESH DESCRIPTOR Coumarins EXPLODE ALL TREES 2233 #42 MESH DESCRIPTOR Dabigatran EXPLODE ALL TREES 293 #43 MESH DESCRIPTOR Anticoagulants EXPLODE ALL TREES 10817 #44 MESH DESCRIPTOR Heparin EXPLODE ALL TREES 4695 #45 MESH DESCRIPTOR Hirudin Therapy EXPLODE ALL TREES 75 #46 MESH DESCRIPTOR Phenindione EXPLODE ALL TREES 30 #47 MESH DESCRIPTOR Platelet Aggregation Inhibitors EXPLODE ALL TREES 10996 #48 MESH DESCRIPTOR Polysaccharides EXPLODE ALL TREES 15741 #49 MESH DESCRIPTOR Rivaroxaban EXPLODE ALL TREES 495 #50 MESH DESCRIPTOR Warfarin EXPLODE ALL TREES 1661 #51 (Factor X* adj (antag* or inhib* or block*)):TI,AB,KY 985 #52 (2‐Propenoic acid, 3‐phenyl‐):TI,AB,KY 0 #53 (acetylsalicylic acid):TI,AB,KY 5097 #54 anticoagul*:TI,AB,KY 12645 #55 (antiplatelet agent*):TI,AB,KY 866 #56 (Antiplatelet Drug*):TI,AB,KY 529 #57 apixaban:TI,AB,KY 831 #58 aspirin:TI,AB,KY 13365 #59 betrixaban:TI,AB,KY 90 #60 (Blood Platelet Aggregation Inhibitor*):TI,AB,KY 65 #61 (Blood Platelet Antagonist*):TI,AB,KY 0 #62 (Blood Platelet Antiaggregant*):TI,AB,KY 0 #63 Clexane:TI,AB,KY 96 #64 Clivarin:TI,AB,KY 17 #65 Coumarin*:TI,AB,KY 317 #66 (CY 216):TI,AB,KY 36 #67 CY‐216:TI,AB,KY 36 #68 dabigatran:TI,AB,KY 966 #69 Dalteparin:TI,AB,KY 733 #70 danaproid:TI,AB,KY 2 #71 DOAC*:TI,AB,KY 230 #72 edoxaban:TI,AB,KY 517 #73 embolex:TI,AB,KY 17 #74 EMT‐966:TI,AB,KY 0 #75 EMT‐967:TI,AB,KY 0 #76 enoxaparin:TI,AB,KY 2146 #77 Etexilate:TI,AB,KY 307 #78 Exanta:TI,AB,KY 6 #79 Falithrom:TI,AB,KY 2 #80 fondaparinux:TI,AB,KY 409 #81 FR‐860:TI,AB,KY 5 #82 Fragmin:TI,AB,KY 218 #83 Fragmine:TI,AB,KY 4 #84 Fraxiparin:TI,AB,KY 30 #85 Fraxiparine:TI,AB,KY 76 #86 Heparin*:TI,AB,KY 11699 #87 hirudin*:TI,AB,KY 471 #88 idrabiotaparinux:TI,AB,KY 15 #89 idraparinux:TI,AB,KY 42 #90 Innohep:TI,AB,KY 33 #91 Kabi‐2165:TI,AB,KY 39 #92 Liquemine:TI,AB,KY 3 #93 LMWH:TI,AB,KY 1322 #94 lomoparan:TI,AB,KY 11 #95 Lovenox:TI,AB,KY 59 #96 (low molecular weight heparin):TI,AB,KY 3068 #97 Marcoumar:TI,AB,KY 13 #98 Marcumar:TI,AB,KY 17 #99 melagatran:TI,AB,KY 60 #100 nadroparin:TI,AB,KY 297 #101 (oral anticoagulants):TI,AB,KY 1270 #102 orgaran:TI,AB,KY 22 #103 parnaparin:TI,AB,KY 41 #104 Pentasaccharide*:TI,AB,KY 55 #105 phenindione:TI,AB,KY 36 #106 phenprocoumon:TI,AB,KY 247 #107 PK‐10,169:TI,AB,KY 0 #108 PK‐10169:TI,AB,KY 7 #109 (Platelet Antagonist*):TI,AB,KY 11 #110 (Platelet Antiaggregant*):TI,AB,KY 32 #111 (Platelet Inhibitor):TI,AB,KY 91 #112 Pradaxa:TI,AB,KY 44 #113 rivaroxaban:TI,AB,KY 1524 #114 Sinthrome:TI,AB,KY 0 #115 Tedelparin:TI,AB,KY 3 #116 thienopyridine:TI,AB,KY 383 #117 Tinzaparin:TI,AB,KY 225 #118 (vitamin k antagonist*):TI,AB,KY 914 #119 vorapaxar:TI,AB,KY 132 #120 warfarin:TI,AB,KY 4609 #121 Xarelto:TI,AB,KY 54 #122 ximelagatran:TI,AB,KY 173 #123 #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101 OR #102 OR #103 OR #104 OR #105 OR #106 OR #107 OR #108 OR #109 OR #110 OR #111 OR #112 OR #113 OR #114 OR #115 OR #116 OR #117 OR #118 OR #119 OR #120 OR #121 OR #122 54068 #124 #38 AND #123 709 |
Aug 2020: 709 Nov 2021: 148 |
| Clinicaltrials.gov (date of most recent search 1 November 2021 ) | Bariatric OR Body Mass Index OR Body Weight OR Obesity OR Overweight OR obese | venous thromboembolism OR VTE OR Pulmonary Embolism OR Thromboembolism OR Thrombosis | Antithrombins or Aspirin OR Coumarins OR Dabigatran OR Anticoagula* OR Heparin OR Hirudin Therapy OR Phenindione OR Platelet Aggregation Inhibitors OR Polysaccharides OR Rivaroxaban OR Warfarin OR acetylsalicylic acid OR antiplatelet OR DOAC OR LMWH | Aug 2020: 276 Nov 2021: 45 |
| ICTRP Search Portal (date of most recent search 1 November 2021 ) | Bariatric OR Body Mass Index OR Body Weight OR Obesity OR Overweight OR obese | venous thromboembolism OR VTE OR Pulmonary Embolism OR Thromboembolism OR Thrombosis | Antithrombins or Aspirin OR Coumarins OR Dabigatran OR Anticoagula* OR Heparin OR Hirudin Therapy OR Phenindione OR Platelet Aggregation Inhibitors OR Polysaccharides OR Rivaroxaban OR Warfarin OR acetylsalicylic acid OR antiplatelet OR DOAC OR LMWH | Aug 2020: N/A Nov 2021: 10 |
| MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) 1946 to present (date of most recent search 1 November 2021) | 1 exp Pulmonary Embolism/ 2 exp Thromboembolism/ 3 Thrombosis/ 4 exp Venous Thromboembolism/ 5 exp Venous Thrombosis/ 6 ((vein* or ven*) adj thromb*).ti,ab. 7 (blood adj3 clot*).ti,ab. 8 deep vein thrombosis.ti,ab. 9 (lung adj3 clot*).ti,ab. 10 (PE or DVT or VTE).ti,ab. 11 peripheral vascular thrombosis.ti,ab. 12 post‐thrombotic syndrome.ti,ab. 13 pulmonary embolism.ti,ab. 14 (pulmonary adj3 clot*).ti,ab. 15 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*).ti,ab. 16 thromboprophylaxis.ti,ab. 17 or/1‐16 18 exp Bariatric Surgery/ 19 Body Mass Index/ 20 exp Body Weight/ 21 exp Obesity/ 22 exp Overweight/ 23 bariatric.ti,ab. 24 biliopancreatic diversion.ti,ab. 25 BMI.ti,ab. 26 body mass index.ti,ab. 27 bodyweight.ti,ab. 28 body weight.ti,ab. 29 gastric band*.ti,ab. 30 gastric bypass.ti,ab. 31 laparoscopic gastric imbrication.ti,ab. 32 laparoscopic sleeve gastrectomy.ti,ab. 33 obese.ti,ab. 34 obesity.ti,ab. 35 over weight.ti,ab. 36 overweight.ti,ab. 37 or/18‐36 38 17 and 37 39 exp Antithrombins/ 40 exp Aspirin/ 41 exp Coumarins/ 42 Dabigatran/ 43 exp Anticoagulants/ 44 exp Heparin/ 45 Fondaparinux/ 46 Hirudin Therapy/ 47 Phenindione/ 48 Platelet Aggregation Inhibitors/ 49 exp Polysaccharides/ 50 Rivaroxaban/ 51 exp Warfarin/ 52 (Factor X* adj (antag* or inhib* or block*)).ti,ab. 53 2‐Propenoic acid, 3‐phenyl‐.ti,ab. 54 acetylsalicylic acid.ti,ab. 55 anticoagul*.ti,ab. 56 antiplatelet agent*.ti,ab. 57 Antiplatelet Drug*.ti,ab. 58 apixaban.ti,ab. 59 aspirin.ti,ab. 60 betrixaban.ti,ab. 61 Blood Platelet Aggregation Inhibitor*.ti,ab. 62 Blood Platelet Antagonist*.ti,ab. 63 Blood Platelet Antiaggregant*.ti,ab. 64 Clexane.ti,ab. 65 Clivarin.ti,ab. 66 Coumarin*.ti,ab. 67 CY 216.ti,ab. 68 CY‐216.ti,ab. 69 dabigatran.ti,ab. 70 Dalteparin.ti,ab. 71 danaproid.ti,ab. 72 DOAC*.ti,ab. 73 edoxaban.ti,ab. 74 embolex.ti,ab. 75 EMT‐966.ti,ab. 76 EMT‐967.ti,ab. 77 enoxaparin.ti,ab. 78 Etexilate.ti,ab. 79 Exanta.ti,ab. 80 Falithrom.ti,ab. 81 fondaparinux.ti,ab. 82 FR‐860.ti,ab. 83 Fragmin.ti,ab. 84 Fragmine.ti,ab. 85 Fraxiparin.ti,ab. 86 Fraxiparine.ti,ab. 87 Heparin*.ti,ab. 88 hirudin*.ti,ab. 89 idrabiotaparinux.ti,ab. 90 idraparinux.ti,ab. 91 Innohep.ti,ab. 92 Kabi‐2165.ti,ab. 93 Liquemine.ti,ab. 94 LMWH.ti,ab. 95 lomoparan.ti,ab. 96 Lovenox.ti,ab. 97 low molecular weight heparin.ti,ab. 98 Marcoumar.ti,ab. 99 Marcumar.ti,ab. 100 melagatran.ti,ab. 101 nadroparin.ti,ab. 102 oral anticoagulants.ti,ab. 103 orgaran.ti,ab. 104 parnaparin.ti,ab. 105 Pentasaccharide*.ti,ab. 106 phenindione.ti,ab. 107 phenprocoumon.ti,ab. 108 PK‐10,169.ti,ab. 109 PK‐10169.ti,ab. 110 Platelet Antagonist*.ti,ab. 111 Platelet Antiaggregant*.ti,ab. 112 Platelet Inhibitor.ti,ab. 113 Pradaxa.ti,ab. 114 rivaroxaban.ti,ab. 115 Sinthrome.ti,ab. 116 Tedelparin.ti,ab. 117 thienopyridine.ti,ab. 118 Tinzaparin.ti,ab. 119 vitamin k antagonist*.ti,ab. 120 vorapaxar.ti,ab. 121 warfarin.ti,ab. 122 Xarelto.ti,ab. 123 ximelagatran.ti,ab. 124 or/39‐123 125 38 and 124 126 randomized controlled trial.pt. 127 controlled clinical trial.pt. 128 randomized.ab. 129 placebo.ab. 130 drug therapy.fs. 131 randomly.ab. 132 trial.ab. 133 groups.ab. 134 or/126‐133 135 exp animals/ not humans.sh. 136 134 not 135 137 125 and 136 |
Aug 2020: 1334 Nov 2021: 249 |
| Embase (Ovid; date of most recent search 1 November 2021) | 1 exp lung embolism/ 2 exp thromboembolism/ 3 thrombosis/ 4 exp venous thromboembolism/ 5 exp vein thrombosis/ 6 ((vein* or ven*) adj thromb*).ti,ab. 7 (blood adj3 clot*).ti,ab. 8 deep vein thrombosis.ti,ab. 9 (lung adj3 clot*).ti,ab. 10 (PE or DVT or VTE).ti,ab. 11 peripheral vascular thrombosis.ti,ab. 12 post‐thrombotic syndrome.ti,ab. 13 pulmonary embolism.ti,ab. 14 (pulmonary adj3 clot*).ti,ab. 15 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol*).ti,ab. 16 thromboprophylaxis.ti,ab. 17 or/1‐16 18 exp bariatric surgery/ 19 body mass/ 20 body weight/ 21 exp obesity/ 22 bariatric.ti,ab. 23 biliopancreatic diversion.ti,ab. 24 BMI.ti,ab. 25 body mass index.ti,ab. 26 bodyweight.ti,ab. 27 body weight.ti,ab. 28 gastric band*.ti,ab. 29 gastric bypass.ti,ab. 30 laparoscopic gastric imbrication.ti,ab. 31 laparoscopic sleeve gastrectomy.ti,ab. 32 obese.ti,ab. 33 obesity.ti,ab. 34 over weight.ti,ab. 35 overweight.ti,ab. 36 or/18‐35 37 17 and 36 38 exp antithrombin/ 39 exp acetylsalicylic acid/ 40 exp coumarin derivative/ 41 exp dabigatran/ 42 exp anticoagulant agent/ 43 exp heparin/ 44 exp fondaparinux/ 45 exp anticoagulant therapy/ 46 exp phenindione/ 47 exp antithrombocytic agent/ 48 exp polysaccharide/ 49 exp rivaroxaban/ 50 exp warfarin/ 51 (Factor X* adj (antag* or inhib* or block*)).ti,ab. 52 2‐Propenoic acid, 3‐phenyl‐.ti,ab. 53 acetylsalicylic acid.ti,ab. 54 anticoagul*.ti,ab. 55 antiplatelet agent*.ti,ab. 56 Antiplatelet Drug*.ti,ab. 57 apixaban.ti,ab. 58 aspirin.ti,ab. 59 betrixaban.ti,ab. 60 Blood Platelet Aggregation Inhibitor*.ti,ab. 61 Blood Platelet Antagonist*.ti,ab. 62 Blood Platelet Antiaggregant*.ti,ab. 63 Clexane.ti,ab. 64 Clivarin.ti,ab. 65 Coumarin*.ti,ab. 66 CY 216.ti,ab. 67 CY‐216.ti,ab. 68 dabigatran.ti,ab. 69 Dalteparin.ti,ab. 70 danaproid.ti,ab. 71 DOAC*.ti,ab. 72 edoxaban.ti,ab. 73 embolex.ti,ab. 74 EMT‐966.ti,ab. 75 EMT‐967.ti,ab. 76 enoxaparin.ti,ab. 77 Etexilate.ti,ab. 78 Exanta.ti,ab. 79 Falithrom.ti,ab. 80 fondaparinux.ti,ab. 81 FR‐860.ti,ab. 82 Fragmin.ti,ab. 83 Fragmine.ti,ab. 84 Fraxiparin.ti,ab. 85 Fraxiparine.ti,ab. 86 Heparin*.ti,ab. 87 hirudin*.ti,ab. 88 idrabiotaparinux.ti,ab. 89 idraparinux.ti,ab. 90 Innohep.ti,ab. 91 Kabi‐2165.ti,ab. 92 Liquemine.ti,ab. 93 LMWH.ti,ab. 94 lomoparan.ti,ab. 95 Lovenox.ti,ab. 96 low molecular weight heparin.ti,ab. 97 Marcoumar.ti,ab. 98 Marcumar.ti,ab. 99 melagatran.ti,ab. 100 nadroparin.ti,ab. 101 oral anticoagulants.ti,ab. 102 orgaran.ti,ab. 103 parnaparin.ti,ab. 104 Pentasaccharide*.ti,ab. 105 phenindione.ti,ab. 106 phenprocoumon.ti,ab. 107 PK‐10,169.ti,ab. 108 PK‐10169.ti,ab. 109 Platelet Antagonist*.ti,ab. 110 Platelet Antiaggregant*.ti,ab. 111 Platelet Inhibitor.ti,ab. 112 Pradaxa.ti,ab. 113 rivaroxaban.ti,ab. 114 Sinthrome.ti,ab. 115 Tedelparin.ti,ab. 116 thienopyridine.ti,ab. 117 Tinzaparin.ti,ab. 118 vitamin k antagonist*.ti,ab. 119 vorapaxar.ti,ab. 120 warfarin.ti,ab. 121 Xarelto.ti,ab. 122 ximelagatran.ti,ab. 123 or/38‐122 124 37 and 123 125 randomized controlled trial/ 126 controlled clinical trial/ 127 random$.ti,ab. 128 randomization/ 129 intermethod comparison/ 130 placebo.ti,ab. 131 (compare or compared or comparison).ti. 132 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 133 (open adj label).ti,ab. 134 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 135 double blind procedure/ 136 parallel group$1.ti,ab. 137 (crossover or cross over).ti,ab. 138 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 139 (assigned or allocated).ti,ab. 140 (controlled adj7 (study or design or trial)).ti,ab. 141 (volunteer or volunteers).ti,ab. 142 trial.ti. 143 or/125‐142 144 124 and 143 |
Aug 2020: 2795 Nov 2021: 746 |
| CINAHL (EBSCO; date of most recent search 1 November 2021) | S134 S119 AND S133 S133 S120 OR S121 OR S122 OR S123 OR S124 OR S125 OR S126 OR S127 OR S128 OR S129 OR S130 OR S131 OR S132 S132 MH "Random Assignment" S131 MH "Triple‐Blind Studies" S130 MH "Double‐Blind Studies" S129 MH "Single‐Blind Studies" S128 MH "Crossover Design" S127 MH "Factorial Design" S126 MH "Placebos" S125 MH "Clinical Trials" S124 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S123 TX crossover OR "cross‐over" S122 AB placebo* S121 TX random* S120 TX "latin square" S119 S37 AND S118 S118 S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 OR S84 OR S85 OR S86 OR S87 OR S88 OR S89 OR S90 OR S91 OR S92 OR S93 OR S94 OR S95 OR S96 OR S97 OR S98 OR S99 OR S100 OR S101 OR S102 OR S103 OR S104 OR S105 OR S106 OR S107 OR S1 ... S117 TX ximelagatran S116 TX Xarelto S115 TX warfarin S114 TX vorapaxar S113 TX "vitamin k antagonist*" S112 TX Tinzaparin S111 TX thienopyridine S110 TX Tedelparin S109 TX Sinthrome S108 TX rivaroxaban S107 TX Pradaxa S106 TX "Platelet Inhibitor" S105 TX "Platelet Antiaggregant*" S104 TX "Platelet Antagonist*" S103 TX "PK‐10169" S102 TX "PK‐10,169" S101 TX phenprocoumon S100 TX phenindione S99 TX Pentasaccharide* S98 TX parnaparin S97 TX orgaran S96 TX "oral anticoagulants" S95 TX nadroparin S94 TX melagatran S93 TX Marcumar S92 TX Marcoumar S91 TX "low molecular weight heparin" S90 TX Lovenox S89 TX lomoparan S88 TX LMWH S87 TX Liquemine S86 TX "Kabi‐2165" S85 TX Innohep S84 TX idraparinux S83 TX idrabiotaparinux S82 TX hirudin* S81 TX Heparin* S80 TX Fraxiparin S79 TX Fragmine S78 TX Fragmin S77 TX "FR‐860" S76 TX fondaparinux S75 TX Falithrom S74 TX Exanta S73 TX Etexilate S72 TX enoxaparin S71 TX "EMT‐967" S70 TX "EMT‐966" S69 TX embolex S68 TX edoxaban S67 TX DOAC* S66 TX danaproid S65 TX Dalteparin S64 TX dabigatran S63 TX "CY‐216" S62 TX "CY 216" S61 TX Coumarin* S60 TX Clivarin S59 TX Clexane S58 TX "Blood Platelet Antiaggregant*" S57 TX "Blood Platelet Antagonist*" S56 TX "Blood Platelet Aggregation Inhibitor*" S55 TX betrixaban S54 TX aspirin S53 TX apixaban S52 TX "Antiplatelet Drug*" S51 TX "antiplatelet agent*" S50 TX anticoagul* S49 TX "acetylsalicylic acid" S48 TX "Propenoic acid, 3‐phenyl*" S47 TX Factor X* n2 (antag* or inhib* or block*) S46 (MH "Warfarin") S45 (MH "Rivaroxaban") S44 (MH "Polysaccharides+") S43 (MH "Platelet Aggregation Inhibitors+") S42 (MH "Fondaparinux Sodium") S41 (MH "Heparin+") S40 (MH "Anticoagulants+") S39 (MH "Dabigatran Etexilate") S38 (MH "Aspirin") S37 S17 AND S36 S36 S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 S35 TX bariatric S34 (MH "Obesity+") S33 (MH "Body Weight+") S32 (MH "Body Mass Index") S31 TX overweight S30 TX "over weight" S29 TX obesity S28 TX obese S27 TX "laparoscopic sleeve gastrectomy" S26 TX "laparoscopic gastric imbrication" S25 TX "gastric bypass" S24 TX "gastric band*" S23 TX "body weight" S22 TX bodyweight S21 TX "body mass index" S20 TX BMI S19 TX "biliopancreatic diversion" S18 (MH "Bariatric Surgery+") S17 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S16 TX thromboprophylaxis S15 TX thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* or microembol* S14 TX pulmonary n3 clot* S13 TX "pulmonary embolism" S12 TX "post‐thrombotic syndrome" S11 TX "peripheral vascular thrombosis" S10 TX PE or DVT or VTE S9 TX lung n3 clot* S8 TX "deep vein thrombosis" S7 TX blood n3 clot* S6 TX ((vein* or ven*) n2 thromb*) S5 (MH "Venous Thrombosis+") S4 (MH "Venous Thromboembolism") S3 (MH "Thrombosis") S2 (MH "Thromboembolism+") S1 (MH "Pulmonary Embolism") |
Aug 2020: 181 Nov 2021: 19 |
| TOTAL before deduplication |
Aug 2020: 5483 Nov 2021: 1232 |
|
| TOTAL after deduplication | Aug 2020: 4174 Nov 2021: 993 |
|
Appendix 2. LILACS/IBECS search strategy
| Source | Seach Strategy | Hits retrieved |
| LILACS/IBECS | (mh:"Cirurgia Bariátrica" OR (cirurgia bariátrica) OR (e02.570.500.062*) OR (e04.062*) OR mh:"Bariatria" OR (bariatria) OR (e02.570.500*)) AND (mh:heparina OR heparina OR alfa‐heparina OR (alfa heparina) OR (heparina alfa) OR (heparina‐alfa) OR (ácido heparínico) OR (d09.698.373.400*) OR mh:anticoagulantes OR (anticoagulantes) OR (agentes anticoagulantes) OR (d27.505.954.502.119*) OR mh:"Inibidores da Agregação de Plaquetas" OR (inibidores da agregação de plaquetas) OR (antiagregadores de plaquetas) OR (agentes antiplaquetas) OR (antagonistas de plaquetas) OR (antiagregantes de plaquetas) OR (inibidor da agregação de plaquetas) OR (d27.505.954.502.780*) OR fondaparinux OR dabigatrana OR rivaroxabana OR enoxaparina OR dalteparina OR edoxaban OR apixaban OR betrixaban) | Sep 2020: 6 Nov 2021: 2 |
| TOTAL before deduplication | Sep 2020: 6 Nov 2021: 2 |
|
| TOTAL after deduplication | Sep:2020: 6 Nov 2021: 0 |
|
Data and analyses
Comparison 1. Higher‐dose heparin versus standard‐dose heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Venous thromboembolism | 4 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.05, 5.99] |
| 1.2 Major bleeding | 4 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.48, 2.96] |
| 1.2.1 Unfractionated heparin | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 5.16] |
| 1.2.2 Low molecular weight heparin | 3 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.48, 5.14] |
| 1.3 All‐cause mortality | 4 | 597 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3.1 Unfractionated heparin | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.3.2 Low molecular weight heparin | 3 | 458 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4 Venous thromboembolism‐related mortality | 4 | 597 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4.1 Unfractionated heparin | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.4.2 Low molecular weight heparin | 3 | 458 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.5 Pulmonary embolism | 2 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 8.92] |
| 1.6 Deep vein thrombosis | 4 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 17.40] |
| 1.6.1 Unfractionated heparin | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6.2 Low molecular weight heparin | 3 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 17.40] |
| 1.7 Adverse events (thrombocytopaenia) | 2 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 17.40] |
Comparison 2. Heparin versus pentasaccharide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Venous thromboembolism | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.19, 3.61] |
| 2.2 Major bleeding | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.42, 6.92] |
| 2.3 All‐cause mortality | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4 Venous thromboembolism‐related mortality | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.5 Pulmonary embolism | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.6 Deep vein thrombosis | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.19, 3.61] |
| 2.7 Adverse events (thrombocytopaenia) | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.25] |
| 2.8 Adverse events (atrial fibrillation) | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.19, 22.14] |
| 2.9 Adverse events (rash) | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.09] |
| 2.10 Adverse events (nausea and vomiting) | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.15, 7.10] |
Comparison 3. Heparin started before versus after the surgical procedure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Venous thromboembolism | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.01] |
| 3.2 Major bleeding | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 71.92] |
| 3.3 All‐cause mortality | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.4 Venous thromboembolism‐related mortality | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5 Deep vein thrombosis | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.01] |
Comparison 4. Combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Venous thromboembolism | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.89] |
| 4.2 Major bleeding | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.3 All‐cause mortality | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.4 Venous thromboembolism‐related mortality | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5 Deep vein thrombosis | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.89] |
4.5. Analysis.

Comparison 4: Combined mechanical and pharmacological prophylaxis versus mechanical prophylaxis alone, Outcome 5: Deep vein thrombosis
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelsalam 2021.
| Study characteristics | ||
| Methods | Methods: single‐centre, prospective, randomised controlled, 2‐arm, parallel‐group, single‐blind (participants not blinded) Country: Egypt Duration: July 2018–January 2019 |
|
| Participants | Number randomised: 100 (50 in each arm) Number analysed: 100 (100%) Mean age: experimental group: 33.78 (SD 9.7) years; control group: 34.04 (SD 9.3) years Sex (male/female): 20/80 Comorbidities: not described Mean bodyweight: experimental group: 131.5 (SD 19.3) kg; control group: 127.0 (SD 17.4) kg Mean BMI: experimental group: 48.78 (SD 5.62) kg/m2; control group: 47.96 (SD 5.52) kg/m2 Bariatric surgery techniques
Rationale for bariatric surgery indication: BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with comorbidities Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: LMWH enoxaparin 1 mg/kg/day, maximum 120 mg/day, started 12 hours before surgery and continued for 1 to 15 days after surgery (manufacturer not reported) Control: LMWH enoxaparin 1 mg/kg/day, maximum 120 mg/day, 1 to 15 days after the surgery (manufacturer not reported) Level of experience of the person carrying out the procedure: not reported Concomitant interventions
Excluded medications: not reported |
|
| Outcomes | Primary outcomes (specified)
Primary outcomes (collected)
Secondary outcomes (specified)
Secondary outcomes (collected)
Time points reported: until 15 days after the procedure Cost of treatment: not reported |
|
| Funding source | Quote "There are no funds for this study as it was held in a university hospital." | |
| Declarations of interest of study authors | Quote "There were no conflict of interests for any of the authors participating in this work." | |
| Notes | Protocol not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned following simple randomisation procedures (computerized random numbers) to one of two treatment groups". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Only personnel were blinded. Participants were not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | No losses. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Ahmad 2021.
| Study characteristics | ||
| Methods | Methods: single‐centre, prospective, randomised controlled, 2‐arm, parallel‐group, single‐blind (participants not blinded) Country: Egypt Duration: June 2019–December 2019 |
|
| Participants | Number randomised: 150 (75 in each arm) Number analysed: 150 (100%) Mean age: experimental group: 41.8 (SD 9.4) years; control group: 37.6 (SD 8.7) years Sex (male/female): 15/135 Comorbidities
Mean bodyweight: not reported Mean BMI: experimental group 46.4 (SD 8.7) kg/m2; control group: 45.2 (SD 5.2) kg/m2 Bariatric surgery techniques
Rationale for bariatric surgery indication: morbid obesity with repeated failure of weight loss after multidisciplinary medical treatment Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: mechanical thromboprophylaxis (perioperative elastic stockings on both lower limbs and early ambulation) combined with chemical thromboprophylaxis (enoxaparin 40 mg SC 12 hours preoperatively and every 24 hours postoperatively for 2 weeks) Control: mechanical thromboprophylaxis alone (perioperative elastic stockings on both lower limbs and early ambulation) Level of experience of the person carrying out the procedure: experienced Concomitant interventions: not reported Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary outcomes (specified)
Secondary outcomes (collected)
Time points reported: until 4 weeks after the procedure Cost of treatment: not reported |
|
| Funding source | Quote: "The authors received no financial support for the research, authorship, and/or publication of this article." | |
| Declarations of interest of study authors | Quote: "The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article." | |
| Notes | Protocol available (TCTR20200127002) Participants who experienced a VTE‐event were followed up for 6 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An Excel sheet was used to create a randomization sequence with a 1:1 allocation using random block sizes of 2 and 4 by an independent doctor". |
| Allocation concealment (selection bias) | Low risk | Quote: "A researcher who was not included with the clinical trial determined the allocation of treatment by sequentially opening numbered, opaque, sealed envelopes". |
| Blinding of participants and personnel (performance bias) | Unclear risk | No method of blinding of participants or personnel was described. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The same person [the researcher that did the randomisation] was also responsible after the assignment to the interventions". |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "No patient was withdrawn from the study after randomisation in addition to no changes to methods and outcomes after the commencement of the trial". |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Ebrahimifard 2012.
| Study characteristics | ||
| Methods | Methods: single‐centre, prospective, randomised controlled, 2‐arm, parallel‐group, single‐blind (described only at protocol stage) Country: Iran Duration: October 2010–October 2012 (planned in the protocol but not reported) |
|
| Participants | Number randomised: 139; experimental group: 71; control group: 68 Number analysed: 139 (100%) Mean age: 36.7 (SD 9.5) years Sex (male/female): 13/126 Comorbidities: 2 participants with preoperative saphenofemoral junction reflux Mean bodyweight: 119.7 (SD 20.3) kg Mean BMI: 44.29 (SD 6.9) kg/m2 Bariatric surgery techniques
Rationale for bariatric surgery indication: BMI > 40 kg/m2, or BMI > 35 kg/m2 with comorbidities (DM, hypertension, sleep apnoea, osteoarthritis, other obesity‐induced diseases) Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: UFH 5000 IU tid (manufacturer not reported) Control: UFH 5000 IU bid (manufacturer not reported) Level of experience of the person carrying out the procedure: not reported Concomitant interventions
Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until 10 days after the procedure Cost of treatment: not reported |
|
| Funding source | Quote "This research has been supported by Tehran University of Medical Sciences and Health Services." | |
| Declarations of interest of study authors | Quote "The authors declare no financial disclosure." | |
| Notes | Protocol available (IRCT201008253384N3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Incomplete description. Quote: "Patients were randomly divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Although the trial authors mentioned blinding for outcome assessors in the protocol, there is no mention of blinding in the final report. |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Imberti 2014b.
| Study characteristics | ||
| Methods | Methods: multicentre, prospective, randomised controlled, 2‐arm, parallel‐group, open‐label Country: Italy Duration: April 2004–February 2012 |
|
| Participants | Number randomised: 258; experimental group: 119; control group: 131 Number analysed: 250 (97.0%). 8 participants excluded due to withdrawal of informed consent (n = 1), refusal to undergo surgery (n = 4) and ineligibility according to inclusion criteria (n = 3) Mean age: 40.9 (SD 9.7) years Sex (male/female): 51/199 Comorbidities
Mean bodyweight: not reported Mean BMI: experimental group: 44.2 (SD 5.4) kg/m2; control group: 44.6 (5.4) kg/m2 Bariatric surgery techniques
Rationale for bariatric surgery indication: BMI ≥ 36 kg/m2 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: LMWH parnaparin 6400 IU/day, starting 12 hours preoperatively for a period of 9 (SD 2) days (Alfa Wassermann, Bologna, Italy) Control: LMWH parnaparin 4250 IU/day, starting 12 hours preoperatively for a period of 9 (SD 2) days (Alfa Wassermann, Bologna, Italy) Level of experience of the person carrying out the procedure: not reported Concomitant interventions
Excluded medications: not reported |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until 3 months after the procedure Cost of treatment: not reported |
|
| Funding source | Alfa Wassermann, Italy supplied the drugs and insurance policy and for supported the study with an unrestricted educational grant. Quote: "The sponsor had no role in the design and conduct of the study, management, analysis, interpretation of the data and preparation, review or approval of the manuscript." |
|
| Declarations of interest of study authors | Quote: "Davide Imberti, Edoardo Baldini, Matteo Giorgi Pierfranceschi, Alberto Nicolini, Concetto Cartelli, Marco De Paoli, Marcello Boni, Esmeralda Filippucci, Stefano Cariani and Giorgio Bottani state that they have no conflict of interest to declare." | |
| Notes | Protocol not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A centralised block‐balanced randomisation plan was used, stratified by centre, sex and BMI". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "We therefore performed a pilot, randomised, controlled, open‐label study". |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "We therefore performed a pilot, randomised, controlled, open‐label study". |
| Incomplete outcome data (attrition bias) | Low risk | 8 (3.1%) participants were excluded after randomisation. There is no substantial difference between the number of participants in the groups (experimental 119 (47.6%) and control 131 (52.4%)). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Kalfarentzos 2001.
| Study characteristics | ||
| Methods | Methods: single‐centre, prospective, randomised controlled, 2‐arm, parallel‐group, open‐label Country: Greece Duration: March 1999–August 2000 |
|
| Participants | Number randomised: 60 (30 in each group) Number analysed: 60 (100%) Mean age: experimental group: 35.7 (SD 10.8) years; control group: 34 (SD 10) years Sex (male/female): 13/47 Comorbidities: no lower limb vein thrombosis Mean bodyweight: experimental group: 134.4 (SD 26.3) kg; control group: 131 (SD 24) kg Mean BMI: experimental group; 48.6 (SD 7.3) kg/m2; control group: 48.8 (SD 8) kg/m2 Bariatric surgery techniques
Rationale for bariatric surgery indication: BMI > 36 kg/m2 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: LMWH nadroparin 9500 IU/day, started preoperatively then given once daily postoperatively until discharge (Fraxiparine, Sanofi Winthrop, Paris) Control: LMWH nadroparin 5700 IU/day, started preoperatively then given once daily postoperatively until discharge (Fraxiparine, Sanofi Winthrop, Paris) Level of experience of the person carrying out the procedure: not reported Concomitant interventions: mechanical interventions not described Excluded medications: other drugs with effects on coagulation |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until 6 months after the procedure. Cost of treatment: not reported |
|
| Funding source | Not described | |
| Declarations of interest of study authors | Not described | |
| Notes | Protocol not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
Steele 2015b.
| Study characteristics | ||
| Methods | Methods: single‐centre, prospective, randomised controlled, 2‐arm, parallel‐group, double‐blind Country: USA Duration: July 2010–August 2013 | |
| Participants | Number randomised: 198; experimental group: 98, control group: 100 Number analysed: 198 (100%; 175 for two outcomes) Mean age: 41.1 (SD 9.6) years Sex (male/female): 32/166 Comorbidities
Mean bodyweight: not reported Mean BMI: experimental group 45.7 (SD 5.2) kg/m2; control group: 45.1 (SD 5.5) kg/m2 Bariatric surgery techniques
Rationale for bariatric surgery indication: BMI > 36 kg/m2 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: enoxaparin 40 mg bid during hospitalisation (mean 2.5 days), starting on call to the operating room Comparator: fondaparinux sodium 5 mg once daily during hospitalisation (mean 2.5 days), starting six hours after surgery Level of experience of the person carrying out the procedure: not reported Concomitant interventions
Excluded medications: aspirin, NSAIDs and other antiplatelet agents (during hospital stay) |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until 2 weeks after the procedure (protocol: 2 years) Cost of treatment: not reported |
|
| Funding source | Quote: "Study materials (drug) and/or additional financial support were provided by GlaxoSmithKline." Quote: "Financial supporters had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript." |
|
| Declarations of interest of study authors | Not described | |
| Notes | Protocol available (NCT00894283) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "consecutive patients were randomly assigned on the day of surgery in a 1:1 ratio to either enoxaparin or fondaparinux, using a computer‐generated randomization scheme (Microsoft Excel 2007 data analysis tool pack) with a block size of 4". |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was performed by the pharmacy and was concealed from patients and study personnel". |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Due to the different dosing schedules of enoxaparin and fondaparinux, placebo doses were prepared to maintain the blind. Active and placebo syringes were prepared by our inpatient pharmacy and were not identifiable by external appearance". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | 14 participants were not treated according to the protocol (7 in experimental group, 7 in control group; 7.1%), but the reasons were reported, and the losses were balanced between the groups. |
| Selective reporting (reporting bias) | High risk | Quote: "There were 21 patients (15 in the enoxaparin treatment arm and 6 in the fondaparinux treatment arm) who were not evaluated for asymptomatic DVT because of inability to tolerate the magnetic resonance venography or because they missed the 10 to 14 day follow‐up visit". Two further participants were not included in the analysis of VTE and DVT; the report did not mention the reason of this loss of data. |
| Other bias | High risk | The primary and secondary outcomes of interest prespecified in the protocol differ to those in the final report. The time point for outcome assessment was 2 years in the protocol and 2 weeks in the final report. |
Steib 2016.
| Study characteristics | ||
| Methods | Methods: multicentre, prospective, randomised controlled, 3‐arm, parallel‐group, double‐blind Country: France Duration: July 2010–August 2013 |
|
| Participants | Number randomised: 164; experimental group: 56; control group 1: 54; control group 2: 54 Number analysed: 148 (90.2%); experimental group: 53; control group 1: 47; control group 2: 48 Mean age: experimental group: 39.5 (SD 1.7) years; control group 1: 40 (SD 1.5) years; control group 2: 39 (SD 1.5) years Sex (male/female): 29/107 Comorbidities
Mean bodyweight: not reported Mean BMI: experimental group: 47 (SD 1) kg/m2; control group 1: 48 (SD 1) kg/m2; control group 2: 49 (SD 1) kg/m2 Bariatric surgery techniques
Rationale for bariatric surgery indication: BMI ≥ 40 kg/m2 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Experimental: enoxaparin 4000 IU bid Control 1: enoxaparin 6000 IU once daily Control 2: enoxaparin 4000 IU once daily Level of experience of the person carrying out the procedure: not reported Concomitant interventions
Excluded medications: NSAIDs |
|
| Outcomes | Primary (specified)
Primary (collected)
Secondary (specified)
Secondary (collected)
Time points reported: until 30 days after the procedure Cost of treatment: not reported |
|
| Funding source | Quote: "This work was sponsored two thirds by institutional grants (Program of Interregional Research) and one third by industrial grants (Sanofi‐Aventis). Funding was managed by the Research Department of Strasbourg University Hospital." Quote: "Financial supporters had no role in the design and conduct of the study, in the collection and analysis of the data, or in the preparation of the manuscript." |
|
| Declarations of interest of study authors | Quote: "The authors have no commercial associations that might be a conflict of interest in relation to this article." | |
| Notes | Protocol available (NCT00444652) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Incomplete information. Quote: "patients were randomly assigned to groups". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study (protocol information). |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study (protocol information). |
| Incomplete outcome data (attrition bias) | Low risk | 16 participants were not treated according to protocol (9%), but the reasons were reported, and the losses were balanced between the groups. Quote: "One patient refused to participate to the study before treatment, 15 patients were not treated for logistic reasons (4 cancellations of surgery, 6 absences of investigators, 4 preoperative deviations from protocol, and 1 gaseous embolism)". |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | We do not suspect any other bias related to this study. |
bid: twice daily; BMI: body mass index; DBP: diastolic blood pressure; DM: diabetes mellitus; DVT: deep vein thrombosis; GORD: gastro‐oesophageal reflux disease; HIT: heparin‐induced thrombocytopenia; IU: international unit; LMWH: low‐molecular‐weight heparin; LRYB: laparoscopic Roux‐en‐Y gastric bypass; MRV: magnetic resonance venography; NSAIDs: non‐steroidal anti‐inflammatory drugs; OCP: oral contraceptive pill; PE: pulmonary embolism; RYB: Roux‐en‐Y gastric bypass; SBP: systolic blood pressure; SC: subcutaneously; SD: standard deviation; tid: three times daily; UFH: unfractionated heparin; VKA: vitamin‐K antagonist; VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Birkmeyer 2012 | Inadequate study design (cohort) |
| Borkgren‐Okonek 2008 | Inadequate study design (non‐randomised) |
| Goslan 2018 | Inadequate study design (non‐randomised) |
| Kushnir 2019 | Inadequate study design (retrospective) |
| Magee 2009 | Inadequate study design (non‐randomised) |
| Pannucci 2021 | Wrong population (plastic or reconstructive surgery) |
| Raftopoulos 2008 | Inadequate study design (non‐randomised) |
| Scholten 2002 | Inadequate study design (non‐randomised) |
| Simone 2008 | Inadequate study design (non‐randomised) |
Characteristics of ongoing studies [ordered by study ID]
Balibrea 2017.
| Study name | Apixaban versus enoxaparin for postoperative thromboprophylaxis after sleeve gastrectomy, a proposal for randomized controlled trial |
| Methods | Multicentre, double‐blind (oral and subcutaneous placebo are considered), 2‐arm, non‐inferiority RCT |
| Participants | Morbid obese (BMI 40–50 kg/m2) people aged 18–65 years scheduled for laparoscopic sleeve gastrectomy Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: apixaban 2.5 mg PO bid during 14 days after sleeve gastrectomy. Control: enoxaparin 40 mg SC once daily during 14 days after sleeve gastrectomy. |
| Outcomes | Primary outcomes
Secondary outcomes:
|
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Register not reported |
NCT01970202.
| Study name | Anti Xa levels under two different regimens of enoxaparin VTE prophylaxis after sleeve gastrectomy for morbid obesity |
| Methods | Single‐centre, open‐label, 3‐arm, parallel‐group RCT |
| Participants | 55 estimated participants, aged ≥ 18 years, both sexes Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental 1: enoxaparin 60 mg SC, once daily for 3 days after surgery Experimental 2: enoxaparin 40 mg SC, once daily for 3 days after surgery Control: no treatment |
| Outcomes | Anti‐factor Xa plasma levels (within 3 days after surgery) |
| Starting date | November 2013 |
| Contact information | Guy Lahat, MD, 972527360237. guyla@tlvmc.gov.il |
| Notes | NCT01970202 |
NCT02128178.
| Study name | Laparoscopic bariatric surgery: two regimens of venous thromboprophylaxis: prospective randomized study |
| Methods | Single‐centre, double‐blind (participant and outcomes assessor), 2‐arm, parallel‐group RCT |
| Participants | 55 estimated participants, adults and children, both sexes Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: enoxaparin 40 mg 2 hours before surgery and continued daily for 10 days after Control: enoxaparin 40 mg 12 hours before surgery and continued daily for 10 days after |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | November 2013 |
| Contact information | Mohamed Abdellatif, Mansoura University, Egypt, surg_latif@hotmail.com |
| Notes | NCT02128178 |
NCT03522259.
| Study name | Rivaroxaban as thrombosis prophylaxis in bariatric surgery (BARIVA) |
| Methods | Single‐centre, open‐label, 2‐arm, parallel‐group RCT |
| Participants | People with scheduled elective bariatric surgery or redo surgery after bariatric interventions, both sexes, aged ≥ 18 years |
| Interventions | Experimental: rivaroxaban 10 mg started on the first postoperative day and continued for 28 days after surgery Control: rivaroxaban 10 mg started on the first postoperative day and continued for 7 days after surgery |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 19 July 2018 |
| Contact information | Guido Stirnimann, MD, +41 31 632 21 11, guido.stirnimann@insel.ch |
| Notes | NCT03522259 |
TCTR20201016001.
| Study name | A randomized controlled trial comparison of enoxaparin 40 mg versus 60 mg dosage for venous thromboembolism prophylaxis in bariatric surgery |
| Methods | Single‐centre, open‐label, 2‐arm, parallel‐group RCT |
| Participants | Target sample size: 56 participants Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: enoxaparin 60 mg SC 12 hours before surgery Comparator: enoxaparin 40 mg group SC 12 hours before surgery |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | 31 October 2020 |
| Contact information | Kritsada Kongsawat, kritsada.ko14@gmail.com |
| Notes | TCTR20201016001 |
bid: twice daily; BMI: body mass index; DM: diabetes mellitus; DVT: deep vein thrombosis; IVC: inferior vena cava; PE: pulmonary embolism; PO: administered orally; RCT: randomised controlled trial; SC: subcutaneously; VTE: venous thromboembolism.
Differences between protocol and review
Although we had planned to consider all reported adverse events, we did not list thrombocytopenia as a possible adverse event in our protocol. We have added thrombocytopenia to the review and reported this event in the summary of findings tables.
Since we considered the 'start of prophylaxis (e.g. before or after surgery)' in a different comparison in the review, we did not use this as a subgroup analysis as stated in our protocol (Amaral 2020).
Contributions of authors
FCFA: acquired trial reports; selected trials; extracted, analysed and interpreted data; and drafted the review. FCFA is the guarantor of the review. JCCBS: analysed and interpreted data; and drafted the review. LCUN: selected trials; extracted, analysed and interpreted data; and drafted the review. RLGF: selected trials; extracted, analysed and interpreted data; and drafted the review.
Sources of support
Internal sources
-
Division of Vascular and Endovascular Surgery, Universidade Federal de Sao Paulo, Brazil
Method support
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil
The protocol was financed in part by CAPES, finance code 001.
Declarations of interest
FCFA: none JCCBS: none LCUN: none RLGF: none
New
References
References to studies included in this review
Abdelsalam 2021 {published data only}
- Abdelsalam AM, ElAnsary AM, Salman MA, Nassef SA, Elfergany HM, Aisha HA. Adding a preoperative dose of LMWH may decrease VTE following bariatric surgery. World Journal of Surgery 2021;45(1):126-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ahmad 2021 {published data only}TCTR20200127002
- Ahmad KS, Zayed ME, Faheem MH, Essa MS. Incidence of silent deep venous thrombosis after laparoscopic bariatric surgery in patients who received combined mechanical and chemical thromboprophylaxis compared to patients who received mechanical thromboprophylaxis only. Surgical Innovation 2021;28(1):144-50. [DOI: 10.1177/1553350620965812] [DOI] [PubMed] [Google Scholar]
- TCTR20200127002. Incidence of silent DVT after laparoscopic bariatric surgery in patients who received combined mechanical and chemical thromboprophylaxis compared to patients who received mechanical thromboprophylaxis only. trialsearch.who.int/Trial2.aspx?TrialID=TCTR20200127002 (first received 27 January 2020).
Ebrahimifard 2012 {published data only}
- Ebrahimifard F, Pazouki A, Solaymani Dodaran M, Vaziri M. A comparison between two different prophylactic doses of unfractionated heparin for deep venous thrombosis prevention in laparoscopic bariatric surgery. Journal of Minimally Invasive Surgical Sciences 2012;1(2):58-61. [DOI: 10.5812/jmiss.4991] [DOI] [Google Scholar]
- Ebrahimifard F, Pazouki A, Solaymani Dodaran M, Vaziri M. A comparison between two different prophylactic doses of unfractionated heparin for deep venous thrombosis prevention in laparoscopic bariatric surgery. Obesity Surgery 2013;23(8):1167. [DOI: 10.1007/s11695-013-0986-z] [DOI] [Google Scholar]
- IRCT201008253384N3. Effect of different doses of heparin in prophylaxis of deep vein thrombosis following laparoscopic bariatric surgery; a randomized clinical trial. en.irct.ir/trial/3459 (first received 5 October 2010).
Imberti 2014b {published data only}
- Imberti D, Baldini E, Pierfranceschi MG, Nicolini A, Cartelli C, De Paoli M, et al. Prophylaxis of venous thromboembolism with low molecular weight heparin in bariatric surgery: a prospective, randomised pilot study evaluating two doses of parnaparin (BAFLUX Study). Obesity Surgery 2014;24(2):284-91. [DOI: 10.1007/s11695-013-1105-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imberti D, Legnani C, Baldini E, Cini M, Nicolini A, Guerra M, et al. Pharmacodynamics of low molecular weight heparin in patients undergoing bariatric surgery: a prospective, randomised study comparing two doses of parnaparin (BAFLUX study). Thrombosis Research 2009;124(6):667-71. [DOI: 10.1016/j.thromres.2009.04.021] [DOI] [PubMed] [Google Scholar]
Kalfarentzos 2001 {published data only}
- Kalfarentzos F, Stavropoulou F, Yarmenitis S, Kehagias I, Karamesini M, Dimitrakopoulos A, et al. Prophylaxis of venous thromboembolism using two different doses of low-molecular-weight heparin (nadroparin) in bariatric surgery: a prospective randomized trial. Obesity Surgery 2001;11(6):670-6. [DOI: 10.1381/09608920160558588] [DOI] [PubMed] [Google Scholar]
Steele 2015b {published data only}
- NCT00894283. Comparing enoxaparin to fondaparinux to prevent venous thromboembolism (VTE) in bariatric surgical patients. clinicaltrials.gov/ct2/show/NCT00894283 (first received 5 May 2009).
- Steele KE, Canner J, Prokopowicz G, Verde F, Beselman A, Wyse R, et al. The EFFORT trial: preoperative enoxaparin versus postoperative fondaparinux for thromboprophylaxis in bariatric surgical patients: a randomized double-blind pilot trial. Surgery for Obesity and Related Diseases 2015;11(3):672-83. [DOI: 10.1016/j.soard.2014.10.003] [DOI] [PubMed] [Google Scholar]
- Steele KE, Schweitzer MA. Prevalence of asymptomatic deep venous thrombosis among bariatric surgical patients receiving perioperative enoxaparin versus fondaparinux. Clinical and Translational Science 2013;6(2):117. [Google Scholar]
Steib 2016 {published data only}
- NCT00444652. Thromboprophylaxis and bariatric surgery. clinicaltrials.gov/ct2/show/NCT00444652 (first received 8 March 2007).
- Steib A, Degirmenci SE, Junke E, Asehnoune K, Figier M, Pericard C, et al. Once versus twice daily injection of enoxaparin for thromboprophylaxis in bariatric surgery: effects on antifactor Xa activity and procoagulant microparticles. A randomized controlled study. Surgery for Obesity and Related Diseases 2016;12(3):613-21. [DOI: 10.1016/j.soard.2015.08.505] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Birkmeyer 2012 {published data only}
- Birkmeyer NJ, Finks JF, Carlin AM, Chengelis DL, Krause KR, Hawasli AA, et al. Comparative effectiveness of unfractionated and low-molecular-weight heparin for prevention of venous thromboembolism following bariatric surgery. Archives of Surgery 2012;147(11):994-8. [DOI: 10.1001/archsurg.2012.2298] [DOI] [PubMed] [Google Scholar]
Borkgren‐Okonek 2008 {published data only}
- Borkgren-Okonek MJ, Hart RW, Pantano JE, Rantis PC, Guske PJ, Kane JM, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surgery for Obesity and Related Diseases 2008;4(5):625-31. [DOI: 10.1016/j.soard.2007.11.010] [DOI] [PubMed] [Google Scholar]
Goslan 2018 {published data only}
- Goslan CJ, Baretta GA, Souza HG, Orsi BZ, Zanoni EC, Lopes MA, et al. Deep venous thrombosis prevention in bariatric surgery: comparative study of different doses of low weight molecular heparin [Profilaxia da trombose venosa profunda em cirurgia bariátrica: estudo comparativo com doses diferentes de heparina de baixo peso molecular]. Jornal Vascular Brasileiro 2018;17(1):26-33. [DOI: 10.1590/1677-5449.008417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kushnir 2019 {published data only}
- Kushnir M, Gali R, Alexander M, Billett HH. Efficacy and safety of direct oral factor Xa inhibitors in patients after bariatric surgery. Blood 2019;134(Suppl 1):2439. [Google Scholar]
Magee 2009 {published data only}
- Magee C, Barry J, Ahmed J, Javed S, Macadam R, Kerrigan D. Comparison of dalteparin and enoxaparin in preventing venous thromboembolism following laparoscopic bariatric surgery. Obesity Surgery 2009;19:1000. [DOI: 10.1007/s11695-009-9904-9] [DOI] [Google Scholar]
Pannucci 2021 {published data only}
- Pannucci CJ, Fleming KI, Bertolaccini C, Agarwal J, Rockwell WB, Mendenhall SD, et al. Optimal dosing of prophylactic enoxaparin after surgical procedures: results of the double-blind, randomized, controlled fixed or variable enoxaparin (FIVE) trial. Plastic and Reconstructive Surgery 2021;147(4):947-58. [DOI] [PubMed] [Google Scholar]
Raftopoulos 2008 {published data only}
- Raftopoulos I, Martindale C, Cronin A, Steinberg J. The effect of extended post-discharge chemical thromboprophylaxis on venous thromboembolism rates after bariatric surgery: a prospective comparison trial. Surgical Endoscopy and Other Interventional Techniques 2008;22(11):2384-91. [DOI: 10.1007/s00464-008-0031-9] [DOI] [PubMed] [Google Scholar]
Scholten 2002 {published data only}
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obesity Surgery 2002;12(1):19-24. [DOI: 10.1381/096089202321144522] [DOI] [PubMed] [Google Scholar]
Simone 2008 {published data only}
- Simone EP, Madan AK, Tichansky DS, Kuhl DA, Lee MD. Comparison of two low-molecular-weight heparin dosing regimens for patients undergoing laparoscopic bariatric surgery. Surgical Endoscopy and Other Interventional Techniques 2008;22(11):2392-5. [DOI: 10.1007/s00464-008-9997-6] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Balibrea 2017 {published data only}
- Balibrea JM, Corcelles R, Moreno P, Mans E, Sabench F, Vilallonga R, et al. Apixaban versus enoxaparin for postoperative thromboprophylaxis after sleeve gastrectomy. A proposal for randomized controlled trial. Obesity Surgery 2017;27(1 Suppl 1):479-80. [DOI: 10.1007/s11695-017-2774-7] [DOI] [Google Scholar]
NCT01970202 {published data only}
- NCT01970202. Anti Xa levels under two different regimens of enoxaparin VTE prophylaxis after sleeve gastrectomy for morbid obesity. clinicaltrials.gov/ct2/show/NCT01970202 (first received 14 October 2013).
NCT02128178 {published data only}
- NCT02128178. Laparoscopic bariatric surgery: two regimens of venous thromboprophylaxis: prospective randomized study. clinicaltrials.gov/show/NCT02128178 (first received 22 April 2014).
NCT03522259 {published data only}
- NCT03522259. Rivaroxaban as thrombosis prophylaxis in bariatric surgery (BARIVA). clinicaltrials.gov/ct2/show/NCT03522259 (first received 30 April 2018).
TCTR20201016001 {published data only}
- TCTR20201016001. A randomized controlled trial comparison of enoxaparin 40 mg versus 60 mg dosage for venous thromboembolism prophylaxis in bariatric surgery. trialsearch.who.int/Trial2.aspx?TrialID=TCTR20201016001 (first received 16 October 2020).
Additional references
Abildgaard 2020
- Abildgaard A, Madsen SA, Hvas AM. Dosage of anticoagulants in obesity: recommendations based on a systematic review. Seminars in Thrombosis and Hemostasis 2020;46(8):932-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agarwal 2010
- Agarwal R, Hecht TE, Lazo MC, Umscheid CA. Venous thromboembolism prophylaxis for patients undergoing bariatric surgery: a systematic review. Surgery for Obesity and Related Diseases 2010;6(2):213-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ageno 2010
- Ageno W. Recent advances in the management of venous thromboembolism. Korean Journal of Hematology 2010;45(1):8-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aminian 2017
- Aminian A, Andalib A, Khorgami Z, Cetin D, Burguera B, Bartholomew J, et al. Who should get extended thromboprophylaxis after bariatric surgery?: a risk assessment tool to guide indications for post-discharge pharmacoprophylaxis. Annals of Surgery 2017;265(1):143-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arroyo‐Johnson 2016
- Arroyo-Johnson C, Mincey KD. Obesity epidemiology worldwide. Gastroenterology Clinics of North America 2016;45(4):571-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2015
- Barnes GD, Kanthi Y, Froehlich JB. Venous thromboembolism: predicting recurrence and the need for extended anticoagulation. Vascular Medicine 2015;20(2):143-52. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartlett 2015
- Bartlett MA, Mauck KF, Daniels PR. Prevention of venous thromboembolism in patients undergoing bariatric surgery. Vascular Health and Risk Management 2015;11:461-77. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Becattini 2012
- Becattini C, Agnelli G, Manina G, Noya G, Rondelli F. Venous thromboembolism after laparoscopic bariatric surgery for morbid obesity: clinical burden and prevention. Surgery for Obesity and Related diseases 2012;8(1):108-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Borch 2010
- Borch KH, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, et al. Anthropometric measures of obesity and risk of venous thromboembolism: the Tromso study. Arteriosclerosis, Thrombosis and Vascular Biology 2010;30(1):121-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Broderick 2021
- Broderick C, Watson L, Armon MP. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD002783. [DOI: 10.1002/14651858.CD002783.pub5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brotman 2013
- Brotman DJ, Shihab HM, Prakasa KR, Kebede S, Haut ER, Sharma R, et al. Pharmacologic and mechanical strategies for preventing venous thromboembolism after bariatric surgery: a systematic review and meta-analysis. JAMA Surgery 2013;148(7):675-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burnett 2016
- Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. Journal of Thrombosis and Thrombolysis 2016;41(1):206-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohoon 2015
- Cohoon KP, Leibson CL, Ransom JE, Ashrani AA, Petterson TM, Long KH, et al. Costs of venous thromboembolism associated with hospitalization for medical illness. American Journal of Managed Care 2015;21(4):e255-63. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Colquitt 2014
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database of Systematic Reviews 2014, Issue 8. Art. No: CD003641. [DOI: 10.1002/14651858.CD003641.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 5 April 2020. Available at covidence.org.
COVIDSurg 2022
- COVIDSurg Collaborative, GlobalSurg Collaborative. SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. Anaesthesia 2022;77(1):28-39. [DOI: 10.1111/anae.15563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Dong 2016
- Dong K, Song Y, Li X, Ding J, Gao Z, Lu D, et al. Pentasaccharides for the prevention of venous thromboembolism. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD005134. [DOI: 10.1002/14651858.CD005134.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Douketis 2016
- Douketis JD. The 2016 American College of Chest Physicians treatment guidelines for venous thromboembolism: a review and critical appraisal. Internal and Emergency Medicine 2016;11(8):1031-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eikelboom 2012
- Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e89S-e119S. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falck‐Ytter 2012
- Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e278S-325S. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkelstein 2009
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Affairs 2009;28(5):w822-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Flumignan 2015
- Flumignan RL, Flumignan CD, Baptista-Silva JC. Angioplasty for deep venous thrombosis. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD011468. [DOI: 10.1002/14651858.CD011468] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2021
- Flumignan RL, Tinôco JD, Pascoal PI, Areias LL, Cossi MS, Fernandes MI, et al. Prophylactic anticoagulants for people hospitalized with COVID-19: systematic review. British Journal of Surgery 2021;108(9):e299-300. [DOI: 10.1093/bjs/znab197] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2022a
- Flumignan RL, Civile VT, Tinôco JD, Pascoal PI, Areias LL, Matar CF, et al. Anticoagulants for people hospitalised with COVID-19. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD013739. [DOI: 10.1002/14651858.CD013739.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2022b
- Flumignan CD, Flumignan RL, Baptista-Silva JC. Antiplatelet agents for the treatment of deep venous thrombosis. Cochrane Database of Systematic Reviews 2022, Issue 9. Art. No: CD012369. [DOI: 10.1002/14651858.CD012369.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fresan 2019
- Fresan U, Sabate J, Martinez-Gonzalez MA, Segovia-Siapco G, la Fuente-Arrillaga C, Bes-Rastrollo M. Adherence to the 2015 dietary guidelines for Americans and mortality risk in a Mediterranean cohort: the SUN project. Preventive Medicine 2019;118:317-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Froehling 2013
- Froehling DA, Daniels PR, Mauck KF, Collazo-Clavell ML, Ashrani AA, Sarr MG, et al. Incidence of venous thromboembolism after bariatric surgery: a population-based cohort study. Obesity Surgery 2013;23(11):1874-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geerts 2004
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3 Suppl):338S-400S. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldfeder 2006
- Goldfeder LB, Ren CJ, Gill JR. Fatal complications of bariatric surgery. Obesity Surgery 2006;16(8):1050-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Google Ngram Viewer 2018
- Historic use of the word BARIATRIC, since 1800. Google Books Ngram Viewer (accessed 9 July 2020);Available from tinyurl.com/y7ech3cv.
Gould 2012
- Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in non-orthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e227S-77S. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 31 October 2022. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Heit 2015
- Heit JA. Epidemiology of venous thromboembolism. Nature Reviews Cardiology 2015;12(8):464-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Hussain 2018
- Hussain Z, Curtain C, Mirkazemi C, Zaidi ST. Peri-operative medication dosing in adult obese elective surgical patients: a systematic review of clinical studies. Clinical Drug Investigation 2018;38(8):673-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ikesaka 2014
- Ikesaka R, Delluc A, Le Gal G, Carrier M. Efficacy and safety of weight-adjusted heparin prophylaxis for the prevention of acute venous thromboembolism among obese patients undergoing bariatric surgery: a systematic review and meta-analysis. Thrombosis Research 2014;133(4):682-7. [DOI: 10.1016/j.thromres.2014.01.021] [DOI] [PubMed] [Google Scholar]
Imberti 2014a
- Imberti D, Baldini E, Pierfranceschi MG, Nicolini A, Cartelli C, De Paoli M, et al. Prophylaxis of venous thromboembolism with low molecular weight heparin in bariatric surgery: a prospective, randomised pilot study evaluating two doses of parnaparin (BAFLUX Study). Obesity Surgery 2014;24(2):284-91. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2018
- Jacobs B, Henke PK. Evidence-based therapies for pharmacologic prevention and treatment of acute deep vein thrombosis and pulmonary embolism. Surgical Clinics of North America 2018;98(2):239-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kakkos 2021
- Kakkos SK, Gohel M, Baekgaard N, Bauersachs R, Bellmunt-Montoya S, Black SA, et al. Editor's Choice – European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the management of venous thrombosis. European Journal of Vascular and Endovascular Surgery 2021;61(1):9-82. [PMID: 10.1016/j.ejvs.2020.09.023] [DOI] [PubMed] [Google Scholar]
Kakkos 2022
- Kakkos S, Kirkilesis G, Caprini JA, Geroulakos G, Nicolaides A, Stansby G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database of Systematic Reviews 2022, Issue 1. Art. No: CD005258. [DOI: 10.1002/14651858.CD005258.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearon 2012
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419S-96S. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2017
- Martin KA, Lee CR, Farrell TM, Moll S. Oral anticoagulant use after bariatric surgery: a literature review and clinical guidance. American Journal of Medicine 2017;130(5):517-24. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Medina 2017
- Medina C, Tolentino-Mayo L, Lopez-Ridaura R, Barquera S. Evidence of increasing sedentarism in Mexico City during the last decade: sitting time prevalence, trends, and associations with obesity and diabetes. PLOS One 2017;12(12):e0188518. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morino 2007
- Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, Scopinaro N. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Annals of Surgery 2007;246(6):1002-7; discussion 1007-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCD‐RisC 2016
- NCD Risk Factor Collaboration (NCD-RisC), Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387(10026):1377-96. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nguyen 2017
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nature Reviews. Gastroenterology and Hepatology 2017;14(3):160-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
NHLBI 2013
- American College of Cardiology/American Heart Association Task Force. Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014;22(Suppl 2):S41-410. [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism (NG89). Available from www.nice.org.uk/guidance/ng89 (accessed 5 April 2020).
Pannucci 2017
- Pannucci CJ, Swistun L, MacDonald JK, Henke PK, Brooke BS. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Annals of Surgery 2017;265(6):1094-103. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pillai 2009
- Pillai AA, Rinella ME. Non-alcoholic fatty liver disease: is bariatric surgery the answer? Clinics in Liver Disease 2009;13(4):689-710. [PMID: ] [DOI] [PubMed] [Google Scholar]
Puzziferri 2018
Qamar 2018
- Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. Journal of the American College of Cardiology 2018;71(19):2162-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Reges 2018
- Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer I, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA 2018;319(3):279-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, the Cochrane Collaboration, 2020.
Rocha 2006
- Rocha AT, Vasconcellos AG, da Luz Neto ER, Araújo DM, Alves ES, Lopes AA. Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obesity Surgery 2006;16(12):1645-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salous 2019
- Salous AK, Reyad A, Sweeney K, Mavanur A. A significant proportion of venous thromboembolism events in general surgical patients occurs after discharge: analysis of the ACS-NSQIP Essentials database. Perioperative Medicine 2019;8:18. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 2022
- Santos BC, Flumignan RL, Civile VT, Atallah AN, Nakano LC. Prophylactic anticoagulants for non-hospitalised people with COVID-19. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD015102. [DOI: 10.1002/14651858.CD015102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulman 2010
- Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. Journal of Thrombosis and Haemostasis 2010;8(1):202-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2019a
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Schünemann 2019b
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Shelkrot 2014
- Shelkrot M, Miraka J, Perez ME. Appropriate enoxaparin dose for venous thromboembolism prophylaxis in patients with extreme obesity. Hospital Pharmacy 2014;49(8):740-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2018
- Smith M, Wakam G, Wakefield T, Obi A. New trends in anticoagulation therapy. Surgical Clinics of North America 2018;98(2):219-38. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steele 2015a
- Steele KE, Canner J, Prokopowicz G, Verde F, Beselman A, Wyse R, et al. The EFFORT trial: preoperative enoxaparin versus postoperative fondaparinux for thromboprophylaxis in bariatric surgical patients: a randomized double-blind pilot trial. Surgery for Obesity and Related Diseases 2015;11(3):672-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Stevens 2021
- Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, et al. Antithrombotic Therapy for VTE Disease: second update of the CHEST guideline and expert panel report. Chest 2021;160(6):e545-608. [PMID: 10.1016/j.chest.2021.07.055] [DOI] [PubMed] [Google Scholar]
Streiff 2016
- Streiff MB, Agnelli G, Connors JM, Crowther M, Eichinger S, Lopes R, et al. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. Journal of Thrombosis and Thrombolysis 2016;41(1):32-67. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stroh 2016
- Stroh C, Michel N, Luderer D, Wolff S, Lange V, Köckerling F, et al. Risk of thrombosis and thromboembolic prophylaxis in obesity surgery: data analysis from the German Bariatric Surgery Registry. Obesity Surgery 2016;26(11):2562-71. [DOI] [PubMed] [Google Scholar]
Venclauskas 2018
- Venclauskas L, Maleckas A, Arcelus JI. European guidelines on perioperative venous thromboembolism prophylaxis: surgery in the obese patient. European Journal of Anaesthesiology 2018;35(2):147-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
WHO 1998
- World Health Organization. Obesity: preventing and managing the global epidemic. apps.who.int/iris/handle/10665/42330 (accessed 11 January 2019).
References to other published versions of this review
Amaral 2020
- Amaral FC, Baptista-Silva JC, Nakano LC, Flumignan RL. Pharmacological interventions for preventing venous thromboembolism in patients undergoing bariatric surgery. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013683. [DOI: 10.1002/14651858.CD013683] [DOI] [PMC free article] [PubMed] [Google Scholar]


