Abstract
Although numerous prevention and intervention techniques have been developed to counteract catheter-associated urinary tract infections (CAUTIs), urinary catheters remain one of the most common sources of hospital-acquired infections. Nitric oxide (NO), a gaseous free radical responsible for regulating many physiological functions in the body, has gained immense popularity due to its potent, broad-spectrum antimicrobial activity, which is capable of combating medical device-associated infections. In this work, a straightforward solvent-swelling method was used to load the NO donor S-nitroso-N-acetyl-penicillamine (SNAP) into commercial latex catheters (SNAP-UCs) for the first time. The effects of swelling catheters with different concentrations of SNAP solutions (25–125 mg/mL SNAP in tetrahydrofuran (THF)) were studied by measuring the NO release kinetics, SNAP loading, and SNAP leaching. SNAP-UCs impregnated with a 50 mg/mL SNAP-THF solution were found to maximize the amount of SNAP loaded into the latex (0.115 ± 0.009 mg SNAP/mg catheter) and showed physiological levels of NO release (>2 × 10−10 mol min−1 cm−2) over 7 days and minimal SNAP leaching (<2%). SNAP-UCs showed impressive in vitro contact-based and diffusible antimicrobial efficacy against three CAUTI-associated pathogens, reducing the viability of adhered and planktonic Escherichia coli, Proteus mirabilis, and Staphylococcus aureus by ~98.0 to 99.1% (adhered) and 86.3–96.3% (planktonic) compared to control latex catheters. In vitro cytotoxicity against 3T3 mouse fibroblasts using a CCK-8 assay showed that SNAP-UCs were noncytotoxic (>90% viability). In summary, SNAP-UCs show stable, noncytotoxic NO release characteristics capable of potent, broad-spectrum antimicrobial activity, demonstrating great potential for reducing the devastating effects associated with CAUTIs.
Keywords: antimicrobial, nitric oxide, controlled release, medical device, urinary catheter
Graphical Abstract
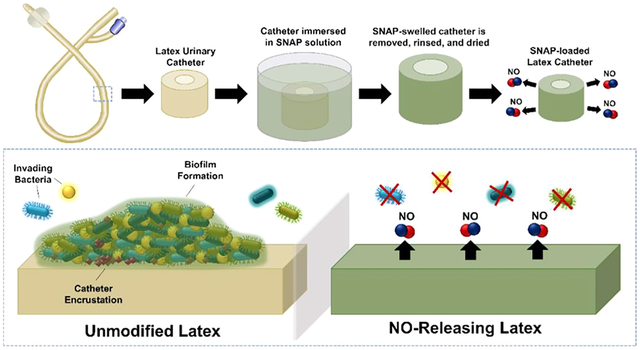
1. INTRODUCTION
Urinary tract infections are the most common type of nosocomial infection, composing approximately one-third of all infections that take place in acute-care hospitals.1 In a surveillance study examining nearly 200,000 intensive care unit (ICU) patients, the National Nosocomial Infections Surveillance System (NNIS) found that 95% of urinary tract infections (UTIs) were associated with urinary catheters.2 For catheterized patients, the risk of developing a UTI increases 3–7% per day, and for patients that require catheterization for ~7 days, the prevalence of catheter-associated urinary tract infections (CAUTIs) is approximately 25%, limiting long-term catheterization applications.3–5 The National Opinion Research Center at the University of Chicago (NORC) has estimated that the average additional cost related to CAUTIs is nearly $14,000 per case and approximated that such infections lead to 36 excess deaths per 1000 in-hospital CAUTI events.6 Hospital and healthcare prevention programs devised by the Centers for Disease Control and Prevention (CDC) have found some success in reducing the number of infections related to healthcare-acquired infections including CAUTIs in non-ICU settings, but the infections in ICU settings remained unchanged.7 Due to the devastating consequences associated with CAUTIs, once an infection is detected, a combination of antimicrobial therapies and catheter removal is often required.8 However, the emergence of antibiotic resistance coupled with the complicated, often polymicrobial composition of CAUTIs has made the eradication of biofilms and treatment of infection difficult.9 Microbial biofilms readily form on the surfaces of devices, significantly increasing antibiotic and host immune resistance.10 The shortage of novel antibiotics, inappropriate overuse, and decreased drug efficacy shortly after market introduction have led to a demand for new antimicrobial and infection mitigation strategies.
In addition to infection, indwelling urinary catheters are also plagued by encrustation. The presence of urease-producing bacteria such as Proteus mirabilis (P. mirabilis) catalyzes the hydrolysis of urea, generating ammonia and shifting the pH of the urine, causing magnesium and calcium phosphate to crystallize on the catheter surface.11 Catheter encrustation prevents urine drainage, potentially leading to pyelonephritis, septicemia, and endotoxic shock.12 Encrustation has been largely linked to CAUTI development, and therefore, methods to prevent the colonization of such bacteria are needed.13
Despite frequent complications, urinary catheters remain the most common indwelling medical device, used in approximately 25% of the hospitalized patients in the United States.14,15 Like the original foley catheter, the majority of urinary catheters manufactured currently are latex-based due to easy processing and low cost.14 However, latex-based urinary catheters have been associated with a number of complications, including increased risk of infection and catheter encrustation.16 In response, silicone-based catheters were introduced and were found to have some improvement in decreasing the risk of encrustation, infection, and urethritis.16,17 However, over half a century later, urinary catheters are still associated with high incidences of infection. Beginning at the turn of the century, several antimicrobial surface strategies for urinary catheters have been developed to reduce the frequency of CAUTIs.16,18 Silver-coated catheters, one of the only FDA-approved antimicrobial urinary catheters on the market, have become increasingly popular due to silver’s well-established antibacterial activity.19 However, clinical trials comparing silver-coated catheters with control catheters have generated inconsistent results.19 Antibiotic-eluting catheters have shown some improvements in antimicrobial efficacy but suffer from antibiotic resistance and quick depletion, depreciating the bactericidal effects for long-term indwelling applications.18,20 Recently, researchers have developed bio-inspired antimicrobial surface strategies to better combat hospital-acquired infections, and in that trend, nitric oxide (NO)-based polymer surfaces have shown great promise.
Nitric oxide is an endogenous free radical that is responsible for many vital functions in the body. In addition to its roles in the cardiovascular and nervous systems, NO is a potent antimicrobial utilized by the immune system through two dose-dependent means: (1) at low concentrations (<1 μM), NO supports the immune cell generation and activity; (2) at high concentrations (>1 μM), NO causes nitrosative and oxidative damage, resulting in protein alterations, DNA deamination and oxidative modifications, and lipid peroxidation.21–23 To combat device-associated infection, NO-releasing materials have been synthesized by incorporating NO donors including S-nitrosothiols (RSNOs) and N-diazeniumdiaolates through blending, covalent immobilization, or solvent-swelling impregnation methods.24 A unique feature of the solvent-swelling method is that it provides a straightforward means to load NO donors into already commercially available materials.25 S-Nitroso-N-acetyl-penicillamine (SNAP), a well-characterized, stable RSNO, has been successfully impregnated into different medical-grade polymers, including silicone,24–28 polyvinyl chloride,29,30 and CarboSil 2080A,31 but has yet to be impregnated or incorporated into latex materials.
In this study, commercial latex urinary catheters were loaded with the NO donor SNAP via a simple solvent-swelling impregnation method for the first time. The effect of different concentrations of SNAP swelling solutions (25–125 mg/mL) was studied by measuring the NO release kinetics, SNAP loading, and SNAP leaching. The sterilization stability of the materials was measured after ethylene oxide treatment and autoclaving. In addition, an in-depth antimicrobial study was performed through evaluating the diffusible and contact-based antimicrobial efficacies of the optimized materials in vitro against planktonic and adhered Escherichia coli (E. coli), Proteus mirabilis (P. mirabilis), and Staphylococcus aureus (S. aureus). The cytotoxicity of the materials was assessed using a 24 h CCK-8 assay against 3T3 mouse fibroblasts. The resulting NO-releasing latex catheter shows great promise in reducing the risk of infection associated with indwelling urinary catheters.
2. MATERIALS AND METHODS
2.1. Materials.
N-Acetyl-D-penicillamine (NAP), sodium nitrite (NaNO2), ethylenediaminetetraacetic acid (EDTA), tetrahydrofuran (THF), LB agar, CLED agar, and a Cell Counting Kit-8 (CCK-8) were purchased from Sigma Aldrich (St. Louis, MO). Phosphate buffered saline (PBS, pH 7.4) was prepared containing 138 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate, and 100 μM EDTA in deionized (DI) water. Hydrochloric acid, sulfuric acid, methanol, and LB broth were purchased from Thermo Fisher (Waltham, MA). 3T3 mouse fibroblast cells (ATCC 1658), S. aureus (ATCC 6538), E. coli (ATCC 25922), and P. mirabilis (ATCC 29906) were all obtained from American Type Culture Collection (Manassas, VA). Dulbecco’s Modified Eagle’s Medium (DMEM) and trypsin–EDTA were obtained from Corning (Corning, NY). Penicillin–Streptomycin (Pen-Strep) and fetal bovine serum (FBS) were purchased from Gibco Life Technologies (Grand Island, NY). Cross-sections of Bard latex catheters cut into 0.5 cm long segments were used.
2.2. SNAP Synthesis.
SNAP was prepared using a previously established protocol.32 Briefly, equimolar amounts of NAP (5 g) and NaNO2 (1 M in DI water) were completely dissolved in a methanol (100 mL) and DI water (30 mL) solution containing 20 mL of 12 M HCl and 5 mL of 18 M H2SO4. The solution was kept on an ice bath in the dark overnight to allow SNAP crystals to form. The precipitate was collected via vacuum filtration and dried for 24 h in a desiccator.
2.3. Preparation of SNAP-Impregnated Latex Urinary Catheters (SNAP-UCs).
SNAP-impregnated latex urinary catheters (SNAP-UCs) were prepared based on a modified solvent-swelling method previously established.24 Briefly, different concentrations of SNAP (25, 50, 100, and 125 mg/mL SNAP) were dissolved in THF. Latex urinary catheters were immersed in the SNAP-THF solutions and allowed to swell for 24 h in the dark under ambient conditions. The SNAP-swelled latex catheters were then removed and allowed to dry for 24 h to allow any excess THF to evaporate. After the THF evaporated, the catheters were quickly rinsed twice in methanol for 5 s to remove any additional SNAP crystals dried on the surface of the catheter and allowed to dry in the dark.
2.4. NO Release Analysis.
The NO release kinetics of the SNAP-UCs were measured using Sievers chemiluminescence nitric oxide analyzers (NOA, model 280i) over 7 days. The samples were immersed in an amber reaction vessel that contained 2 mL of PBS with EDTA and maintained at 37 °C using a water bath. Nitrogen bubbler and sweep gas purged NO released from the sample into the reaction cell of the NOA, where supplied ozone reacted with NO to produce excited-state nitrogen dioxide (NO2*), which emits a photon as it goes to the ground state. After accounting for the known calibration constant (mol PPB−1 s−1) and the surface area of the samples, the NO release rate (×10−10 mol cm−2 min−1) of each sample was calculated. The nitrogen supply gas was maintained at 200 mL/min. The samples remained immersed in a PBS solution throughout the duration of the study. Each day, the NO release was sampled for approximately 1 hour, which was averaged and represented in the graph as the NO flux for that day. Care was taken to ensure that measurements were made at day intervals (e.g., day 1 is the NO release at 24 h, day 3 is the NO release at 72 h, etc.). N = 4 samples were measured for each sample type.
2.5. SNAP Loading and Leaching Measurements.
SNAP loading and leaching were measured according to previously established methods using UV–vis spectrometry.24 First, total SNAP loading was measured by immersing the SNAP-UCs (n = 4) in 5 mL of THF for 24 h in the dark to extract any SNAP impregnated in the latex into solution. The optical densities (OD) of the THF samples were measured at 336 nm, which corresponds to the absorbance maxima of the S-NO group in SNAP.33,34 A calibration curve consisting of known SNAP concentrations dissolved in THF was used to calculate the total amount of SNAP loaded per mg of catheter.
SNAP leaching was similarly measured by immersing the SNAP-UCs in 1 mL of PBS with EDTA and periodically measuring the OD at 336 nm over a 24 h period using UV–vis. The samples were kept at 37 °C in between measurements. A calibration curve of known SNAP concentrations was used to measure the amount of SNAP leached per cm2 of the latex catheter.
2.6. Scanning Electron Microscopy (SEM).
SEM analysis was performed using a Thermo Fisher Scientific (FEI) Teneo at an accelerating voltage of 5.00 kV. Gold palladium was sputter coated on each sample at a thickness of 10 nm type using a Leica sputter coater prior to imaging.
2.7. Sterilization Stability Assessment.
To measure the sterilization stability of the SNAP-UCs, the prepared samples were sterilized using ethylene oxide and autoclaving methods. For ethylene oxide sterilization, samples enclosed in a sterilization pouch were exposed to ethylene oxide for 24 h in an Anprolene AN74i sterilizer. For autoclaving sterilization, samples enclosed in a sterilization pouch were autoclaved for 30 min at 121 °C at 15 psi. After sterilization, the samples were removed and added to 5 mL of THF each for 24 h to calculate the amount of SNAP remaining in the catheter (n = 4). The absorbance of the THF at 336 nm was measured to determine the total SNAP loaded and compared to the total SNAP loaded in unsterilized samples.
2.8. Antimicrobial Contact-Based and Diffusible Inhibitory Effects of SNAP-UCs.
To measure the diffusible and contact-based antimicrobial activity of the SNAP-UCs, the viability of both planktonic (in solution) and adhered bacteria was measured after 24 h of exposure to both control and test catheters (n = 4). First, each strain (E. coli, P. mirabilis, and S. aureus) was separately inoculated in LB broth at 37 °C until reaching ~108 colony forming units (CFUs) per mL validated by optical density measurements at 600 nm. The bacterial solution was then centrifuged at 2500×g for 7.5 min, resuspended in sterile PBS, and centrifuged again at 2500×g for 7.5 min. After again resuspending in sterile PBS, this bacterial solution was dispensed across a 24 well plate, each well containing a sample immersed in 1 mL of bacterial solution. The samples were exposed to bacteria for 24 h at 37 °C at 150 rpm. After incubation, two viability measurements were conducted to assess the antimicrobial efficacy of the samples. First, to assess the contact-based antibacterial activity of the samples, the viability of adhered bacteria was measured by gently rinsing each sample with sterile PBS and homogenizing for 60 s in sterile PBS to transfer any adhered bacteria from the catheters to the solution. Each solution was subsequently serially diluted and plated on LB agar (S. aureus and E. coli) or CLED agar (P. mirabilis). After 24 h incubation at 37 °C, the number of CFUs was counted. To assess the diffusible microbial inhibition of the materials, the viability of planktonic bacteria in the surrounding solution/environment was measured by directly taking the bacterial solution that the samples were incubated in, serially diluting, and plating. Similarly, after 24 h incubation at 37 °C, the number of CFUs was counted. Reduction in bacterial viability for both adhered and planktonic bacteria was calculated by the following equation (where ). Each experiment was duplicated to ensure reproducibility.
2.9. Cytotoxicity Assay.
The cytotoxicity of the SNAP-UCs toward 3T3 fibroblast cells (ATCC 1658) was tested using the cell counting kit-8 (CCK-8) assay according to the manufacturer’s protocol (Sigma Aldrich). All samples were sterilized by UV irradiation on both sides for 1 h. Catheter extracts were obtained by immersing the samples in DMEM (1 mg/mL) and incubating at 37 °C, for 24 h (n = 5). 3T3 fibroblast cells were cultured in 75 cm2 flasks containing DMEM supplemented by 10% FBS and 1% penicillin–streptomycin. After reaching a confluency of 80–90%, cells were harvested using 0.18% trypsin and a suspension was prepared with a density of 50,000 cells/mL. Then, 100 μL of the cell suspension was added to each well of a 96-well plate and incubated at 37 °C for 24 h. After the formation of a cell monolayer, the medium in each well was replaced with the obtained extracts and allowed to interact with cells for 24 h. Following exposure to the leachates, the viability of the cells was evaluated by adding 10 μL of CCK-8 dye to each well, incubating at 37 °C for 2 h, and measuring the absorbance at 450 nm. Wells containing cells exposed to DMEM with no extract were considered as control. The relative cell viability was calculated using the following equation:
2.10. Statistical Analysis.
All data measurements are reported in mean ± standard deviation. To measure significance of multiple groups, a one-way ANOVA with Tukey’s post hoc analysis was performed. p-values <0.05 were considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. Characterization of SNAP-UCs.
3.1.1. SNAP Loading Measurements.
Urinary catheters routinely fail due to infection, biofilm formation, and encrustation, potentially resulting in pyelonephritis, septicemia, endotoxic shock, and death.12 Capitalizing on the potent, broad-spectrum antimicrobial activity of NO, researchers have begun developing NO-releasing materials to extinguish the risk of infection associated with indwelling medical devices.23 One straightforward method of fabricating NO-releasing materials that has recently gained momentum is the impregnation of the NO donor SNAP via solvent swelling. However, although this method has been previously optimized to impregnate silicone or polyurethane-based polymers with SNAP,25 it has yet to be tested on latex-based materials. To optimize the SNAP swelling process, different concentrations of SNAP (25, 50, 100, and 125 mg/mL) were dissolved in THF and then utilized to swell and impregnate the latex catheter samples with SNAP. THF was used as the choice solvent due to its excellent SNAP solubility and ability to swell latex without dissolving the latex material. As shown in Figure 1A, the amount of SNAP loaded into the latex material maximized using a 50 mg/mL solution, resulting in 0.114 ± 0.009 mg SNAP/mg latex. Swelling catheters in 100 and 125 mg/mL SNAP-THF resulted in 0.109 ± 0.006 mg SNAP/mg latex and 0.115 ± 0.009 mg SNAP/mg latex, respectively. Therefore, swelling catheters in increased concentrations of SNAP beyond 50 mg/mL did not increase the amount of SNAP loaded in the latex (p > 0.05). However, swelling latex catheters in 25 mg/mL resulted in 0.043 ± 0.005 mg SNAP/mg latex, which is approximately half of the SNAP loaded compared to the 50 mg/mL SNAP-swelled catheters. In summary, the optimized swelling solution using SNAP and THF for latex materials was found to be the 50 mg/mL SNAP-THF solution likely due to the solubility limit of SNAP in the latex polymer matrix.
Figure 1.
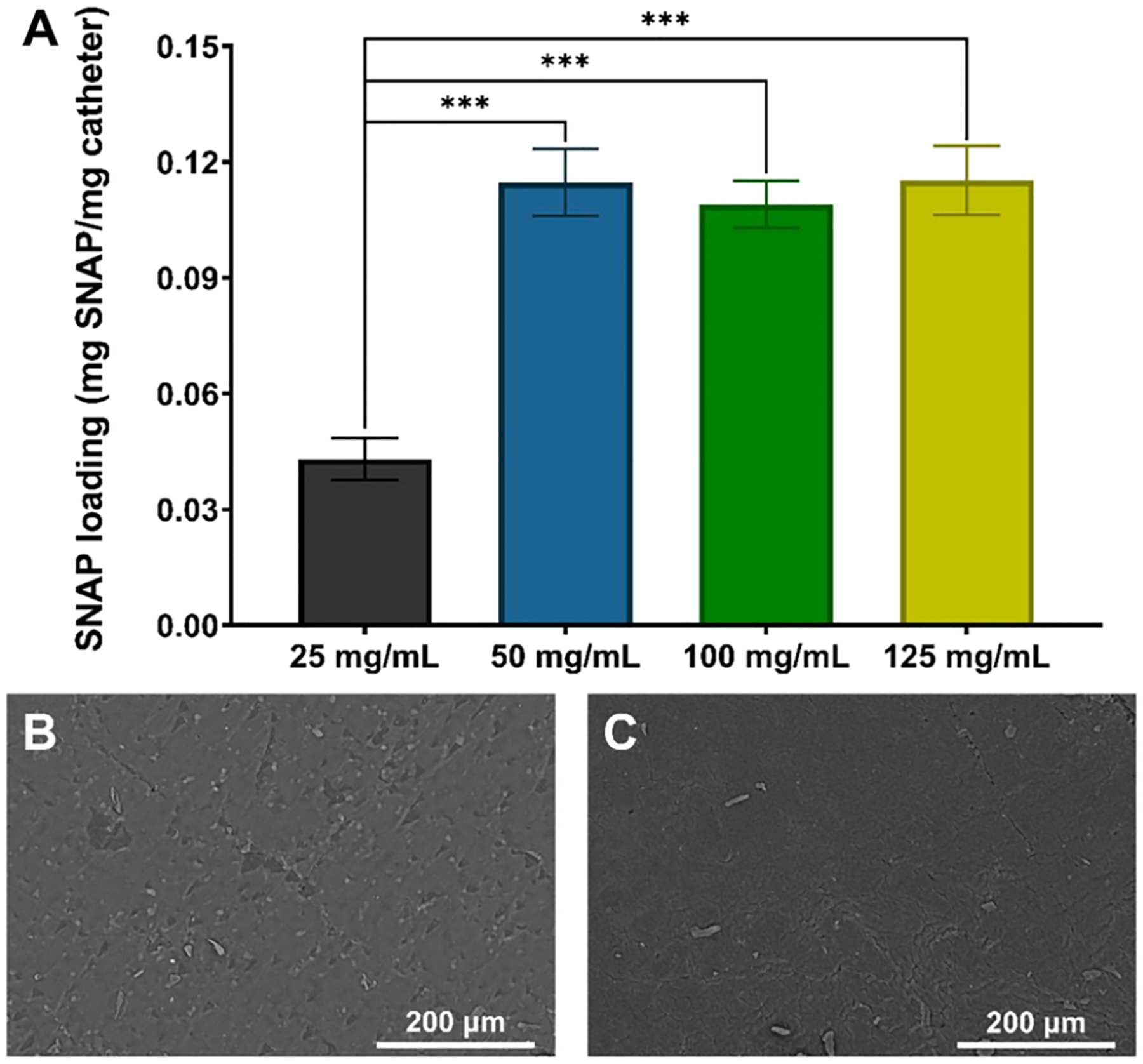
(A) Total SNAP loading (mg) per mg of latex after 24 h of swelling in 25, 50, 100, and 125 mg/mL SNAP-THF solutions. (*** represents p-values < 0.001). SEM images of (B) unmodified control latex catheters and (C) 50 mg/mL SNAP-UCs demonstrate no visible change in surface morphology after the swelling–deswelling process.
3.1.2. SEM Analysis of SNAP-UCs.
Significant changes in the surface morphology of medical devices can facilitate bacterial adhesion and colonization. Surface imperfections can increase the surface area or create crevices that bacteria can enter to evade the host environment.35 Therefore, when predicting the antimicrobial efficacy of a material, surface morphology is an important parameter to understand. To analyze the effect of the swelling and deswelling process on the latex catheters, SEM images were taken before (Figure 1B) and after (Figure 1C) swelling the catheters in a 50 mg/mL solution of THF. As shown, no change in surface morphology is visible between catheters with and without SNAP. Therefore, based on surface morphology alone, no disadvantage is expected from the SNAP-UCs.
3.1.3. NO Release Kinetics of SNAP-UCs.
To understand the effect of swelling latex catheters with different concentrations of SNAP (25–125 mg/mL), the NO release from the samples was measured over 7 days. As shown in Figure 2, catheters impregnated with 50, 100, and 125 mg/mL SNAP solutions exhibited NO release rates >2 × 10−10 cm−2 min−1 for at least 7 days. Interestingly, for the first 3 days, no significant difference was found between any of the sample types (p > 0.05). However, after day 3, latex catheters swelled with 25 mg/mL SNAP solutions did not exhibit any NO release, likely due to lower SNAP loading. Although materials with low levels of NO release (<0.1 × 10−10 cm−2 min−1) have shown some antimicrobial effects,36 previous studies evaluating elevated NO release patterns have shown substantially improved antimicrobial efficacy against both Gram-positive and Gram-negative pathogens compared to their low NO-releasing counterparts.32,37 When targeting invading microbes, immune cells (e.g., neutrophils) produce high concentrations of NO capable of DNA damage, lipid peroxidation, and disruption of protein function.23 Therefore, materials that release NO at high concentrations can disrupt bacterial viability subsequent colonization, reducing the risk of infection.
Figure 2.
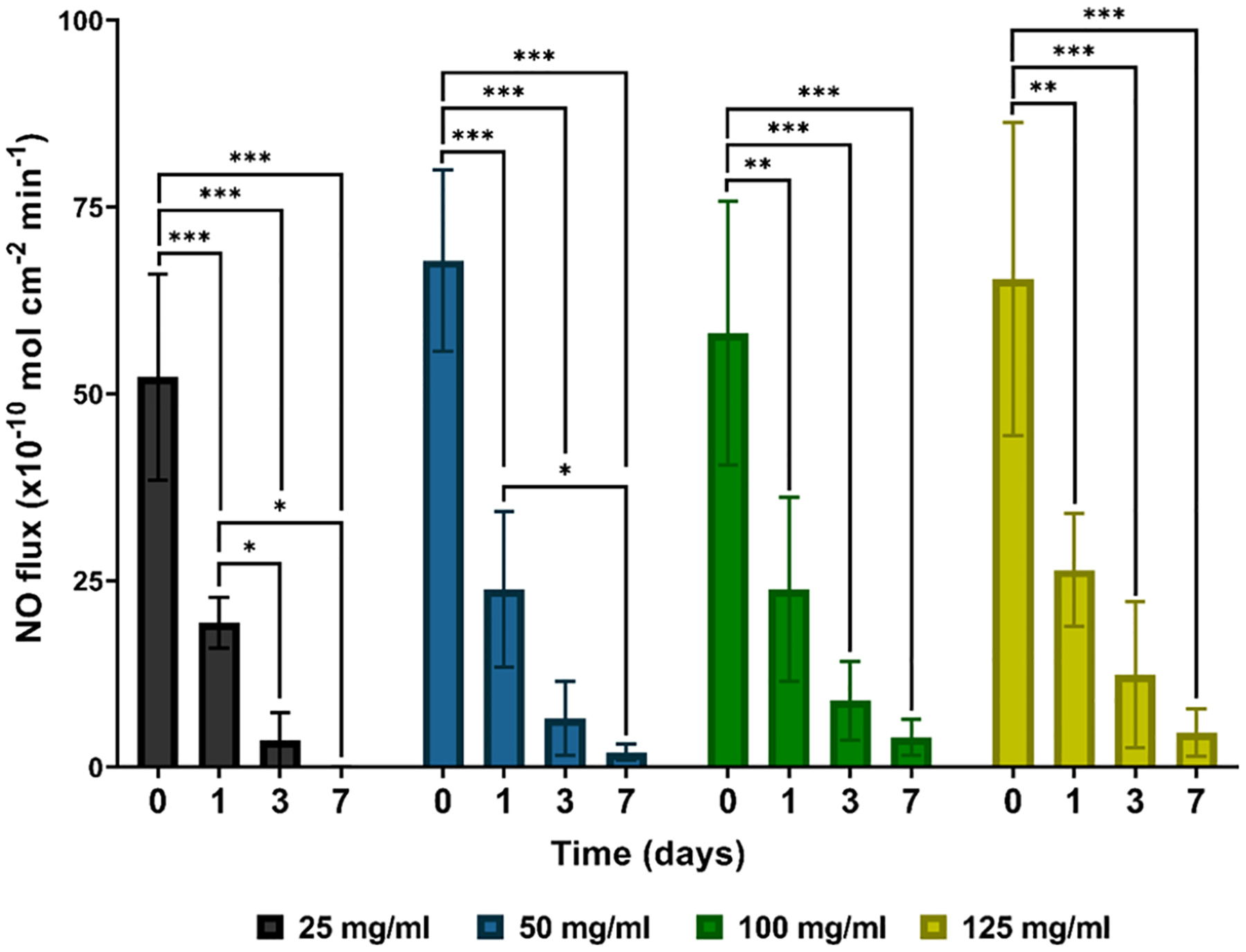
NO release measurements of 25, 50, 100, and 125 mg/mL SNAP-UCs over 7 days at 37 °C. The degree of statistical significance is indicated by * (p < 0.05), ** (p < 0.01), and *** (p < 0.001).
3.1.4. SNAP Leaching.
Leaching of NO donors from NO-releasing materials can diminish the NO reservoir stored in the material, significantly limiting the lifetime of the device. Moreover, the accumulation of NO donors as a product of leaching can lead to cytotoxic effects toward mammalian cells. Therefore, NO-releasing platforms that exhibit minimal NO donor leaching are necessary to ensure the longevity and cytocompatibility of indwelling medical devices. To examine the amount of SNAP leached from the SNAP-UCs, samples were incubated in PBS kept at 37 °C. The amount of SNAP leached was measured periodically over 24 h (Figure 3). No significant difference was found between any of the samples over the 24 h period regardless of the solvent-swelling system used to prepare the materials (25–125 mg/mL SNAP-THF) (p > 0.05). Irrespective of the sample type, <3% of the original SNAP loaded had leached from the sample after 24 h (25 mg/mL–2.3 ± 0.8%; 50 mg/mL–1.1 ± 0.4%; 100 mg/mL–1.3 ± 0.6%; and 125 mg/mL–0.8 ± 0.4%). Previous reports of SNAP-impregnated NO-releasing platforms exhibiting non-cytotoxic, extended NO release characteristics had similar low leaching profiles.24,25,27 Therefore, from the information gathered from the SNAP loading measurements (50 mg/mL SNAP-THF maxed SNAP loading), NO release studies (elevated NO release for 7 days), and SNAP leaching data (minimal SNAP leaching), all SNAP-UC catheters were prepared using a 50 mg/mL SNAP-THF solution for the duration of this study.
Figure 3.
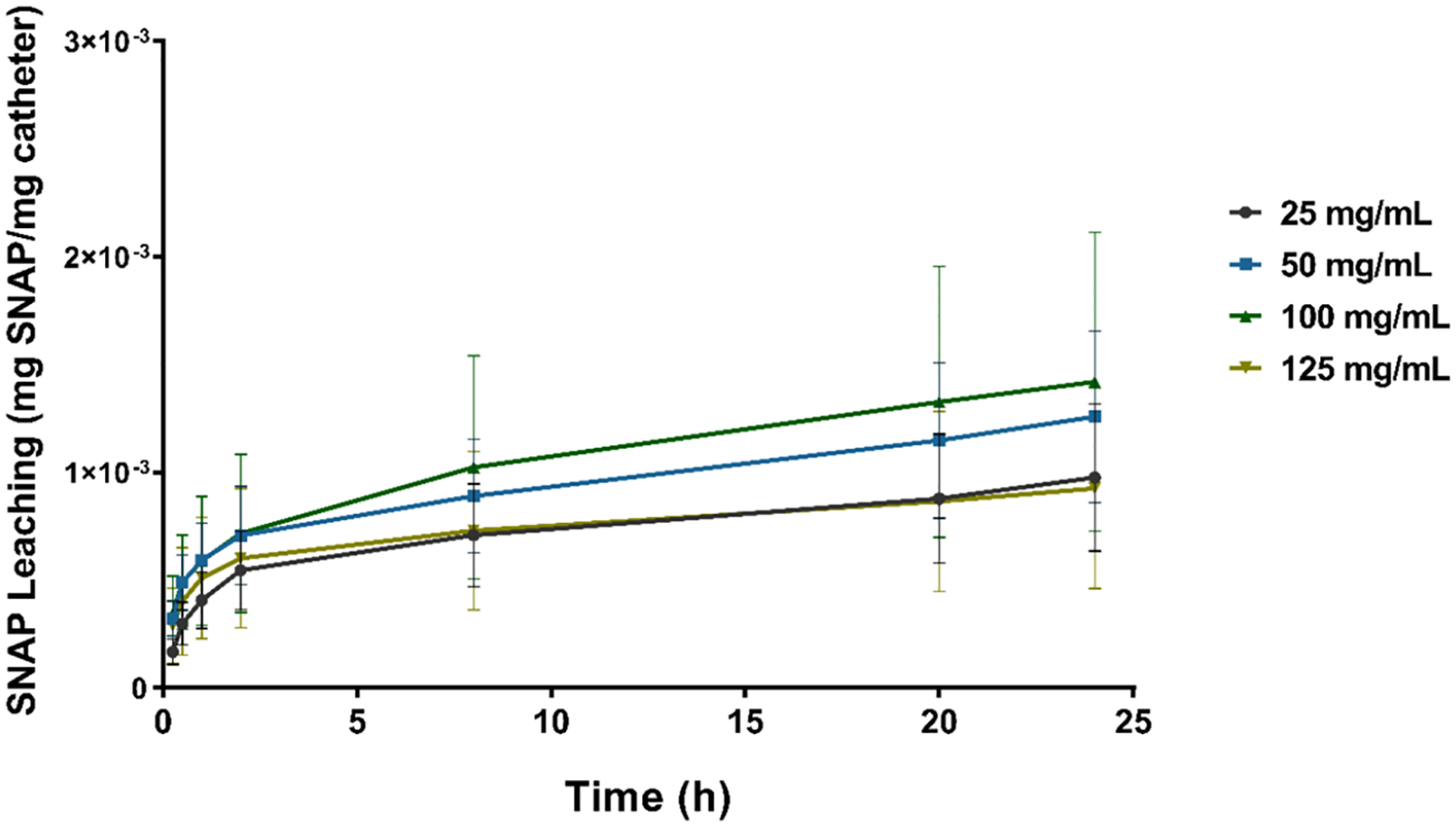
SNAP leaching measurements (mg SNAP per mg catheter) of 25, 50, 100, and 125 mg/mL SNAP-UCs using UV–vis spectroscopy. Samples were kept in PBS maintained at 37 °C over the 24 h period.
3.1.5. Sterilization Stability.
An important metric for ensuring the reproducibility of medical devices is evaluating the effect of sterilization techniques. To evaluate the stability of the SNAP-UCs, two common sterilization methods were executed: ethylene oxide (EO) sterilization (24 h) and autoclaving (30 min). Similar to the SNAP loading measurements, the total SNAP content (mg SNAP/mg catheter) was determined using UV–vis spectroscopy and compared to the SNAP content of nonsterilized SNAP-UCs (Figure 4). Comparable to previous studies34,38,39 that found that EO sterilization had minimal effect on NO-releasing materials, no statistical difference was found between the total SNAP content of EO-sterilized SNAP-UCs and the nonsterilized SNAP-UCs (102.8 ± 12.8% SNAP remaining, p > 0.05). However, after autoclaving, the SNAP content was significantly affected (2.5 ± 0.4%, p < 0.001 compared to both nonsterilized and EO-sterilized samples). This can be attributed to the rapid thermal decomposition of SNAP at elevated temperatures present during autoclaving. A previous study evaluating different sterilization techniques on SNAP-doped polymeric films also found that autoclaving rapidly diminished the amount of SNAP loaded.34 Therefore, EO sterilization was found to be an appropriate method of sterilizing SNAP-UCs.
Figure 4.
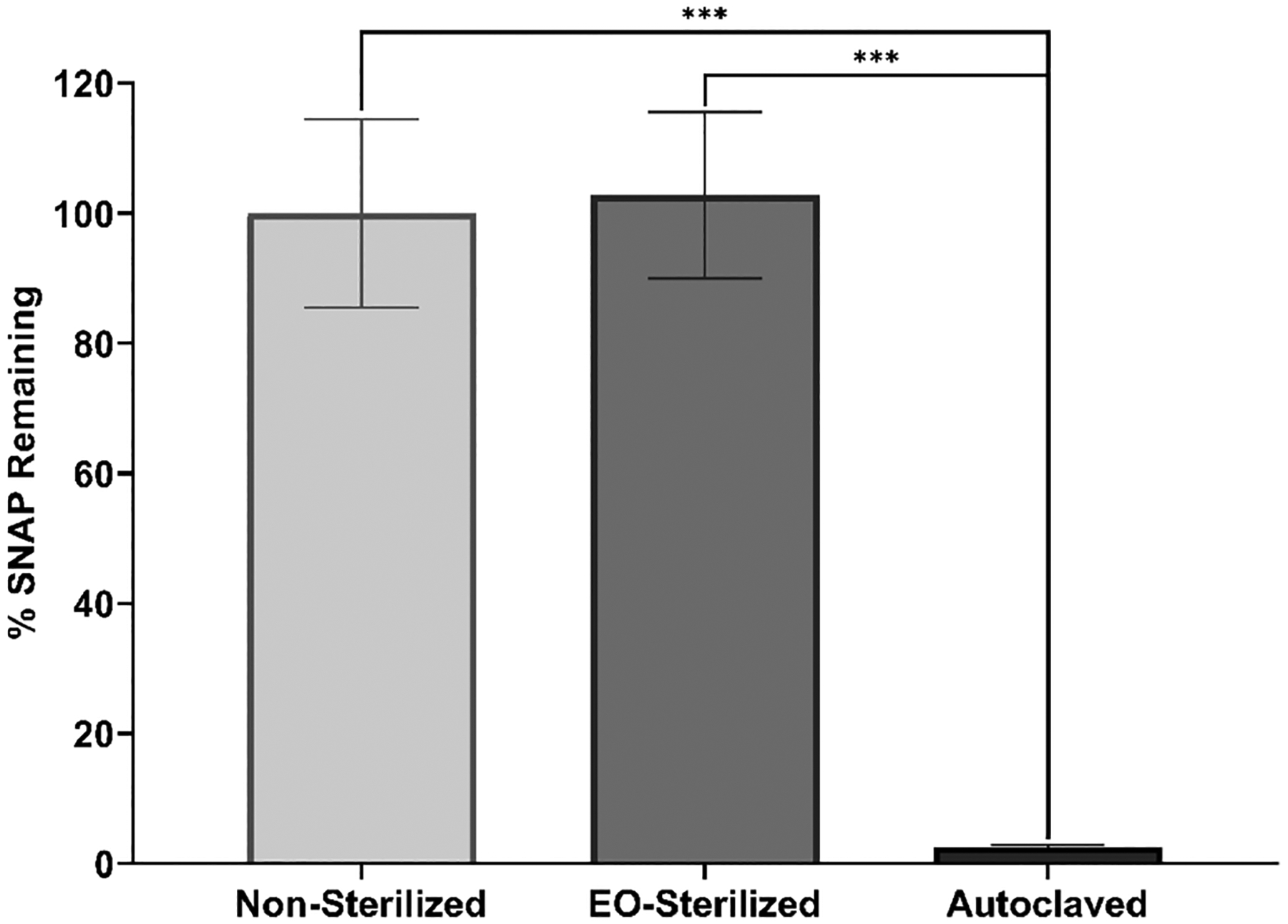
Sterilization stability (% SNAP remaining) after ethylene oxide (EO) sterilization (24 h) and autoclaving (30 min) compared to nonsterilized catheters. The degree of statistical significance is indicated by *** (p < 0.001).
3.2. Evaluation of Contact-Based and Diffusible Antibacterial Activity of SNAP-UCs.
Despite frequent intervention and improved healthcare habits, UTIs compose 40% of all nosocomial infections, and the majority of these infections are associated with catheter use.40 The majority of CAUTIs are thought to be derived from bacteria originating from the patient’s perineal flora or introduced from hospital personnel either extraluminally during catheter insertion or intraluminally through a break in the drainage line or contamination in the urine bag.41–43 Once exposed to the foreign surface, bacteria readily attach to the catheter and form protective exopolysaccharides, allowing for host defense evasion and biofilm development.42 To combat infection, antimicrobial surface modifications (e.g., silver-coated; antibiotic-eluting) have been added to commercial urinary catheters. However, the efficacy of silver-coated catheters both in vitro and clinically is mixed,20,44–47 and antibiotic resistance remains a serious concern of antibiotic-eluting medical devices.18 Therefore, the demand for a potent, broad-spectrum antimicrobial surface modification for indwelling urinary catheter use remains. To reduce bacterial colonization of both Gram-negative and Gram-positive pathogens, NO-releasing platforms have been synthesized and evaluated both in vitro and in vivo, showing significant promise.48–50 In this study, the NO donor SNAP was impregnated in latex-based commercial urinary catheters, a commonly used material for urinary catheters, through a straightforward solvent-swelling method to reduce the viability of invading pathogens. To evaluate the contact-based antimicrobial efficacy and diffusible inhibition of bacterial growth, the viability of E. coli, P. mirabilis, and S. aureus bacteria attached to the surface of the samples and in the surrounding solution was evaluated after 24 h of exposure (Figure 5).
Figure 5.
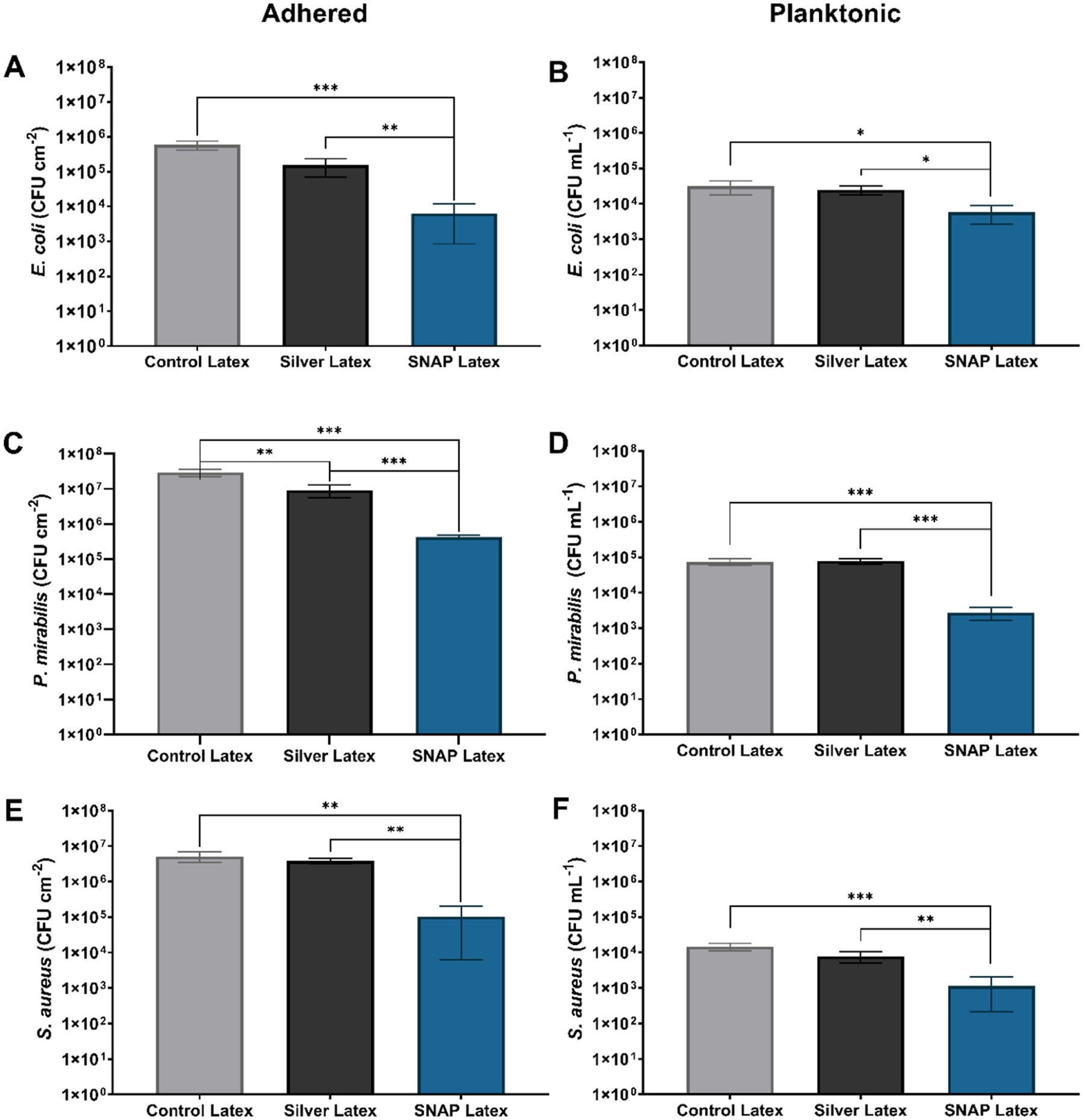
(A, C, E) Contact-based antimicrobial efficacy of SNAP-UCs compared to commercial latex and silver-coated latex urinary catheters. The viability of E. coli, P. mirabilis, and S. aureus attached to the surface was evaluated after 24 h of exposure. Similarly, the (B, D, F) diffusible inhibition of planktonic bacteria was evaluated by determining the number of viable bacteria in solution after 24 h of exposure. The degree of statistical significance is indicated by * (p < 0.05), ** (p < 0.01), and *** (p < 0.001).
E. coli is the most common pathogen associated with CAUTIs, isolated from half of hospital-acquired UTIs.40,42 After 24 h exposure to E. coli at 37 °C, SNAP-UCs were found to significantly reduce the viability of E. coli adhered to the catheter surface (98.9 ± 0.9% reduction, p < 0.001) and planktonic E. coli in the surrounding solution (81.6 ± 9.9% reduction, p < 0.05) compared to control latex catheters. Although silver-coated catheters, the current gold-standard for antimicrobial urinary catheters, reduced the viability of adhered E. coli 74.0 ± 14.0% compared to control latex catheters, the silver-coated catheters failed to significantly decrease the viability of E. coli in solution, reducing the viability by only 20.3 ± 22.8% (p > 0.05). Silver-coated catheters have largely been shown to be ineffective against Gram-negative bacilli including E. coli.51 In fact, previous studies comparing the diffusive inhibitory effects51,52 and contact-based antimicrobial effects20,52 of antimicrobial catheters toward adhered E. coli have found that silver-coated catheters failed to have any significant effect. However, NO-releasing platforms have shown substantial antimicrobial efficacy against Gram-negative bacteria commonly found with hospital-acquired infections including E. coli, Pseudomonas aeruginosa (P. aeruginosa), and Klebsiella pneumoniae.53,54 Moreover, compared to commercial silver-coated latex urinary catheters, SNAP-UCs reduced the viability of adhered E. coli by 96.9 ± 3.6% and the viability of planktonic E. coli by 76.9 ± 12.5%.
Particular uropathogens, including P. mirabilis, produce urease enzymes that hydrolyze urea, using it as a nitrogen source.42 Ammonia generated from this reaction shifts the pH of the urine, causing the accumulation of calcium and magnesium that result in crystalline biofilm formation, urethra and bladder trauma, urine retention, and septicemia.46 Proteus species also exhibit unique swarming behavior, allowing for the spread of infection through coordinated migration from the catheter surface to the urothelium in the bladder.55 Therefore, antimicrobial measures to combat P. mirabilis are imperative to prevent these events from occurring. SNAP-UCs significantly reduced the viability of adhered P. mirabilis by 99.1 ± 0.1% (p < 0.001) and the viability of planktonic P. mirabilis by 96.3 ± 1.4% (p < 0.001) compared to control latex catheters. However, similar to the antimicrobial trends shown against E. coli, although they are moderately able to reduce the viability of adhered P. mirabilis (82.3 ± 7.0% reduction, p < 0.01), silver-coated catheters failed to have a significant diffusible antimicrobial impact against P. mirabilis in the surrounding solution (p > 0.05). Morris and Stickler found that after exposing P. mirabilis-infected human urine to different commercial urinary catheters, silver-coated latex catheters occluded from encrustation the fastest, suggesting that silver coatings fail to prevent the colonization of P. mirabilis.46 Similarly, Morgan et al. found that indwelling silver-coated urinary catheters were unsuccessful in preventing encrustation and concluded that in order to prevent infection, catheters must contain antimicrobials that are able to diffuse out from the surface.56 In this study, SNAP-UCs resulted in significantly improved antimicrobial efficacy against P. mirabilis over silver-coated catheters. P. mirabilis are especially problematic for urinary infections due to the ability to catalyze the hydrolysis of urea, generating ammonia and shifting the pH of the urine, causing magnesium and calcium phosphate to crystallize on the catheter surface.11 Compared to the silver-coated catheters, SNAP-UCs reduced the viability of adhered P. mirabilis by 95.4 ± 0.6% (p < 0.01) and the viability of planktonic P. mirabilis in solution by 96.5 ± 1.4% (p < 0.001), showing great promise for eliminating P. mirabilis-related CAUTIs and subsequent encrustation complications.
An increasing number of Gram-positive cocci strains (S. aureus, Staphylococcus saprophyticus, Enterococcus spp., and Streptococcus spp.) have been detected in UTIs in recent years, identified in approximately 20% of these infections.57,58 Moreover, among patients with S. aureus bacteremia, patients with concomitant S. aureus bacteriuria have been linked to severe clinical outcomes including ICU admission and mortality compared to patients without bacteriuria.59,60 In this study, SNAP-UCs reduced the viability of adhered S. aureus by 98.0 ± 1.9% (p < 0.01) and the viability of planktonic S. aureus by 92.3 ± 6.3% (p < 0.001) compared to control latex catheters, as opposed to silver-coated catheters that were only able to reduce the viability of adhered S. aureus by only 16.8 ± 14.1% (p > 0.05) and the viability of planktonic S. aureus by 46.9 ± 19.2% (p > 0.05) compared to control latex catheters. However, SNAP-UCs performed significantly better than silver-coated catheters in reducing the viability of adhered S. aureus (97.3 ± 2.5% reduction, p < 0.01) and planktonic S. aureus in solution (85.5 ± 11.8% reduction, p < 0.01). To date, bacteria demonstrating resistance to exogenous sources of NO have yet to be demonstrated.23 Although S. aureus can evade host immune response through the induction of homolactic fermentation61 and increased expression of protective enzymes (e.g., flavohemoglobin) capable of detoxifying NO,62 the protective strategies are ineffective against potent, high levels of NO provided by NO donor drugs.23 Different NO-releasing platforms, including materials,63 nanoparticles,64,65 macro-molecular polymers,66 and ointments,67 have shown significant antimicrobial and antibiofilm efficacy against antibiotic-resistant strains of S. aureus, supporting the case for an NO-releasing urinary catheter.
A major advantage of NO-releasing surfaces is that NO exhibits multiple antimicrobial mechanisms against bacterial cells, which makes the development of resistance difficult (Figure 6). In fact, a previous study assessing bacterial resistance against exogenous NO through spontaneous and serial passage mutagenesis studies showed that no significant increase in MIC values was observed compared to parent S. aureus, MRSA, Staphylococcus epidermidis, E. coli, or P. aeruginosa.68 NO can easily diffuse across bacterial membranes, causing nitrosative and oxidative damage and resulting in protein alterations, DNA deamination and oxidative modifications, and lipid peroxidation.23 NO-releasing materials exhibiting extended NO release have still shown moderate reductions in decreased adhered bacterial viability when NO release rates are low (<0.1 × 10−10 mol cm−2 min−1) as a result of long incubation periods.36 Moreover, likely due to the high levels of NO release exhibited in the first 24 h, these SNAP-latex catheters were able to reduce not only the viability of bacteria adhered to the surface but also the viability of the pathogens in the solution. Therefore, NO-releasing materials are advantageous not only in their broad-spectrum antimicrobial capabilities but also from the potent, multimechanistic bactericidal nature NO, making the development of antimicrobial resistance less likely and creating a promising platform for preventing and combating CAUTIs.
Figure 6.
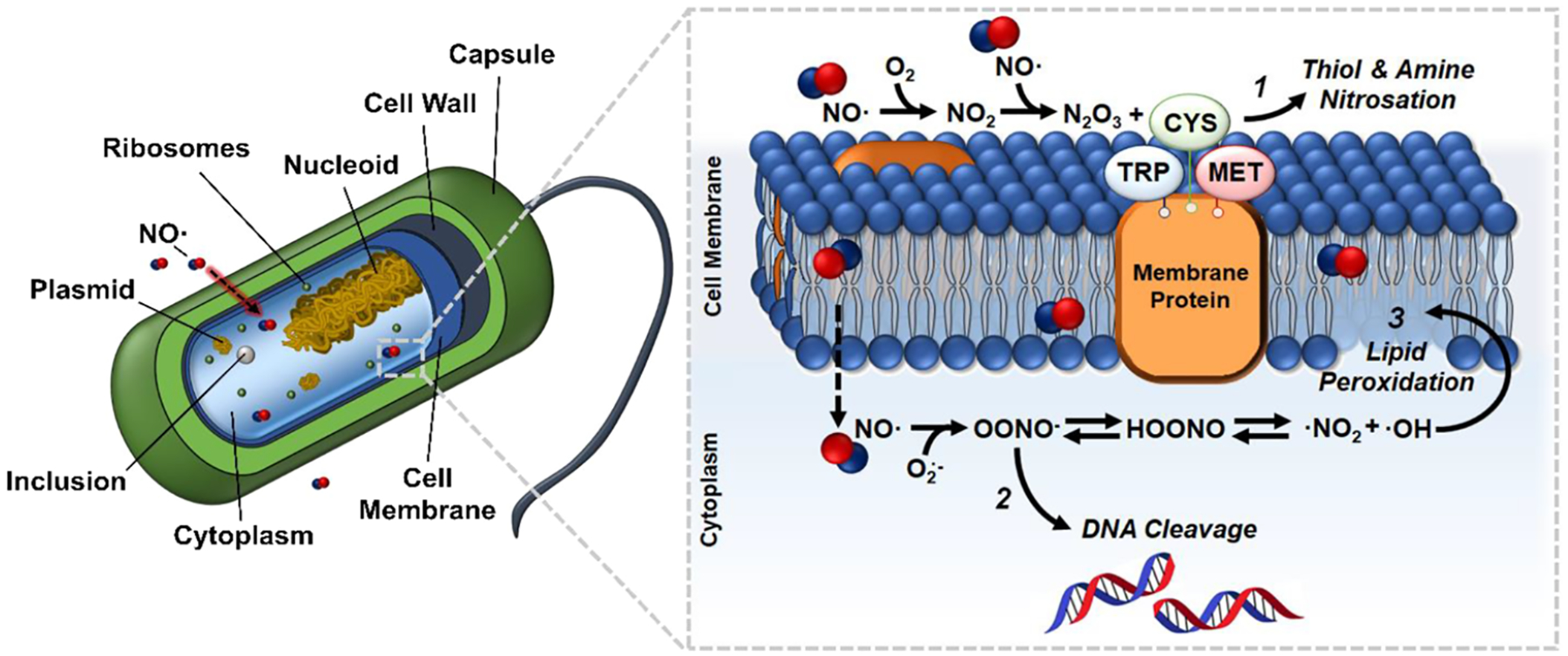
NO readily diffuses across the bacterial membrane, resulting in multiple antimicrobial reactions, including (1) thiol and amine nitrosation, (2) DNA cleavage, and (3) lipid peroxidation.
3.3. In Vitro Cytotoxicity of SNAP-UCs.
In addition to having strong antibacterial properties, urinary catheters must be biocompatible and not elicit any toxicity or adverse host response when used in the human body. Evaluation of the in vitro cytotoxicity is one of the most important initial steps toward the biocompatibility confirmation of the medical devices. In this study, 3T3 fibroblast cells were exposed to the leachate products of the tubing samples and a CCK-8 colorimetric assay was performed to investigate any possible cytotoxic responses. Many studies regarding the development of urinary catheters have used this cell line to evaluate the cytocompatibility of their materials.69,70 The intensity of the formazan dye produced during the CCK-8 assay by the activities of dehydrogenases in cells can be measured at 450 nm and is directly proportional to the numbers of living cells.
According to the obtained results (Figure 7), none of the samples were cytotoxic and they all maintained more than 90% cell viability. Although the viability of cells exposed to latex leachates was slightly lower compared to cells without leachate exposure (93.3 ± 1.8% relative cell viability, p = 0.0158), no significant difference was found between the viability of any SNAP-UCs and control cells (p > 0.05). This can be due to the regulating effect of NO on cell proliferation and protection, which has been demonstrated before in many studies.24,71 Latex urinary catheters have historically shown some degree of cytotoxicity both in vitro and in vivo.72–74 The results from this study show promising cytocompatibility from SNAP-UCs and should be further studied.
Figure 7.
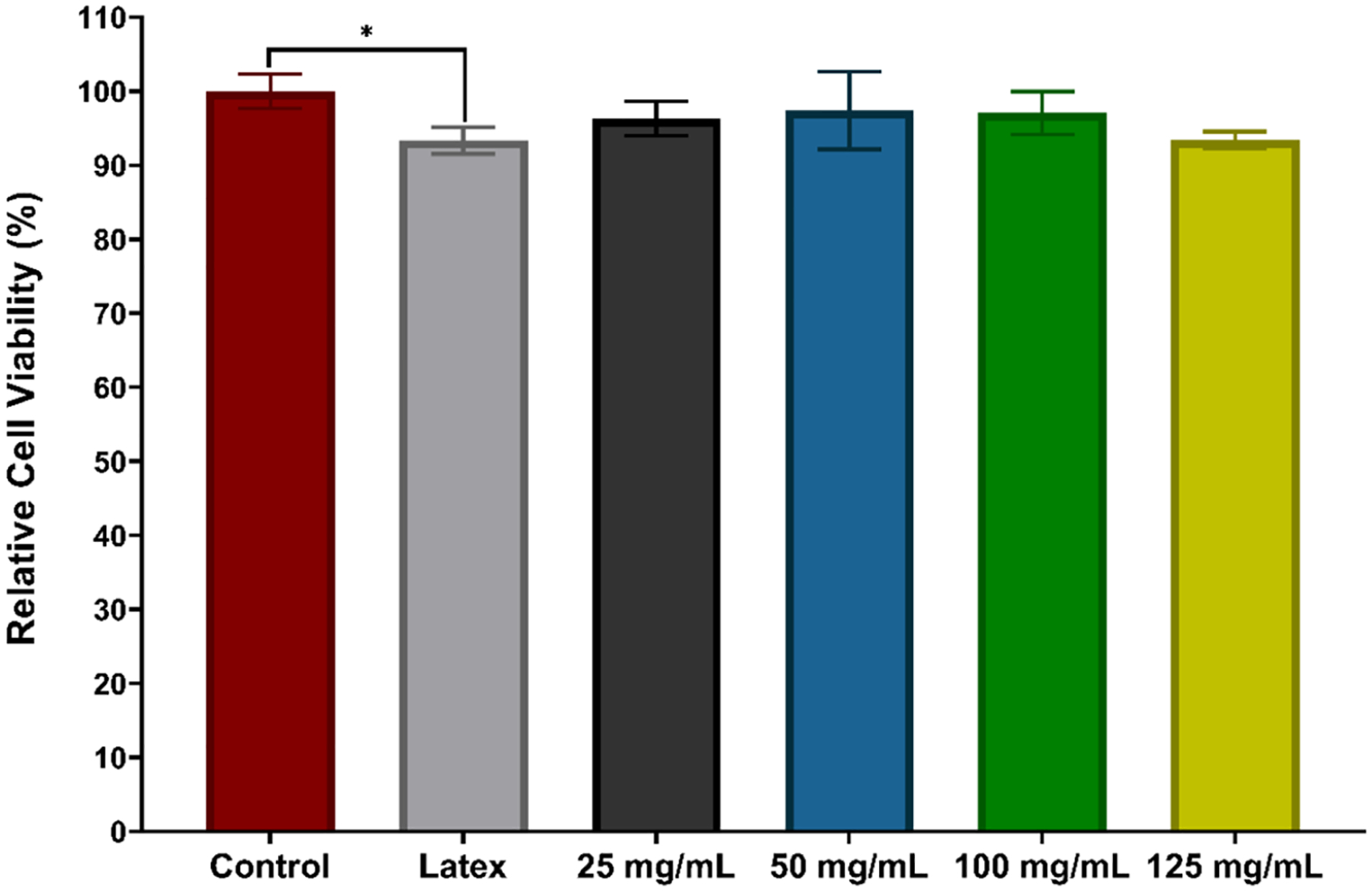
In vitro cytotoxicity measurements of samples against 3T3 mouse fibroblasts using a CCK-8 assay. The degree of statistical significance is indicated by * (p < 0.05).
4. CONCLUSIONS
Indwelling urinary catheter use is routinely complicated from bacterial colonization, resulting in infection and encrustation. In this work, the NO donor SNAP was loaded into commercial latex urinary catheters via a straightforward solvent-swelling technique to reduce the viability of adhered and planktonic bacteria. The solvent-swelling method was optimized by evaluating the SNAP loading, NO release, and SNAP leaching of latex catheters swelled with solutions containing different concentrations of SNAP. As a result, SNAP-UCs impregnated with a 50 mg/mL SNAP-THF solution maximized the amount of SNAP loaded into the material (0.115 ± 0.009 mg SNAP/mg catheter), exhibited high NO release (>2 × 10−10 mol min−1 cm−2) for 7 days, and had inconsequential SNAP leaching (<2%). Optimized SNAP-UCs demonstrated impressive contact-based and diffusible antimicrobial efficacy against three common CAUTI-associated pathogens, reducing the viability of adhered and planktonic E. coli, P. mirabilis, and S. aureus by 98.0–99.1% (adhered) and 86.3–96.3% (planktonic) compared to control latex catheters and outperforming commercial silver-coated catheters. After ethylene oxide sterilization, the SNAP loading was not significantly affected, showing great translational promise. Finally, the SNAP-UCs were found to be noncytotoxic (>90% cell viability retained) in vitro against 3T3 mouse fibroblasts using a CCK-8 assay. The resulting SNAP-UCs provide an exciting potent, broad-spectrum antimicrobial platform capable of reducing the risk of infections associated with urinary catheters and other latex-based medical devices.
ACKNOWLEDGMENTS
Funding for this work was supported by the National Institutes of Health, USA grant R01HL134899.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acsabm.1c01130
The authors declare no competing financial interest.
Contributor Information
Megan Douglass, School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
Sama Ghalei, School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
Elizabeth Brisbois, School of Chemical, Materials and Biomedical Engineering, College of Engineering, University of Georgia, Athens, Georgia 30602, United States.
Hitesh Handa, School of Chemical, Materials and Biomedical Engineering, College of Engineering and Pharmaceutical and Biomedical Sciences Department, College of Pharmacy, University of Georgia, Athens, Georgia 30602, United States.
REFERENCES
- (1).Klevens RM; Edwards JR; Richards CL Jr.; Horan TC; Gaynes RP; Pollock DA; Cardo DM Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Richards MJ; Edwards JR; Culver DH; Gaynes RP Nosocomial infections in medical intensive care units in the United States. Crit. Care Med 1999, 27, 887–892. [DOI] [PubMed] [Google Scholar]
- (3).Frontera JA; Wang E; Phillips M; Radford M; Sterling S; Delorenzo K; Saxena A; Yaghi S; Zhou T; Kahn DE; Lord AS; Weisstuch J Protocolized Urine Sampling is Associated with Reduced Catheter-Associated Urinary Tract Infections: A Pre- and Post-intervention Study. Clin. Infect. Dis 2020, 73, e2690–e2696. [DOI] [PubMed] [Google Scholar]
- (4).Lo E; Nicolle LE; Coffin SE; Gould C; Maragakis LL; Meddings J; Pegues DA; Pettis AM; Saint S; Yokoe DS Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect. Control. Hosp. Epidemiol 2014, 35, 464–479. [DOI] [PubMed] [Google Scholar]
- (5).Jaggi N; Sissodia P Multimodal supervision programme to reduce catheter associated urinary tract infections and its analysis to enable focus on labour and cost effective infection control measures in a tertiary care hospital in India. J. Clin. Diagn Res 2012, 6, 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Agency for Healthcare Research and Quality. Estimating the Additional Hospital Inpatient Cost and Mortality Associated With Selected Hospital-Acquired Conditions; AHRQ Publication, 2017; pp. 1–69. [Google Scholar]
- (7).Saint S; Greene MT; Krein SL; Rogers MA; Ratz D; Fowler KE; Edson BS; Watson SR; Meyer-Lucas B; Masuga M; Faulkner K; Gould CV; Battles J; Fakih MG A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. N. Engl. J. Med 2016, 374, 2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hooton TM; Bradley SF; Cardenas DD; Colgan R; Geerlings SE; Rice JC; Saint S; Schaeffer AJ; Tambayh PA; Tenke P; Nicolle LE; Infectious Diseases Society of America. Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis 2010, 50, 625–663. [DOI] [PubMed] [Google Scholar]
- (9).Azevedo AS; Almeida C; Melo LF; Azevedo NF Impact of polymicrobial biofilms in catheter-associated urinary tract infections. Crit. Rev. Microbiol 2017, 43, 423–439. [DOI] [PubMed] [Google Scholar]
- (10).Chen L; Wen YM The role of bacterial biofilm in persistent infections and control strategies. Int. J. Oral. Sci 2011, 3, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Dayyoub E; Frant M; Pinnapireddy SR; Liefeith K; Bakowsky U Antibacterial and anti-encrustation biodegradable polymer coating for urinary catheter. Int. J. Pharm 2017, 531, 205–214. [DOI] [PubMed] [Google Scholar]
- (12).Stickler DJ; Feneley RC The encrustation and blockage of long-term indwelling bladder catheters: a way forward in prevention and control. Spinal Cord 2010, 48, 784–790. [DOI] [PubMed] [Google Scholar]
- (13).Wilde MH; McMahon JM; Crean HF; Brasch J Exploring relationships of catheter-associated urinary tract infection and blockage in people with long-term indwelling urinary catheters. J. Clin. Nurs 2017, 26, 2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lawrence EL; Turner IG Materials for urinary catheters: a review of their history and development in the UK. Med. Eng. Phys 2005, 27, 443–453. [DOI] [PubMed] [Google Scholar]
- (15).Magill SS; Edwards JR; Bamberg W; Beldavs ZG; Dumyati G; Kainer MA; Lynfield R; Maloney M; McAllister-Hollod L; Nadle J; Ray SM; Thompson DL; Wilson LE; Fridkin SK; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med 2014, 370, 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Feneley RC; Hopley IB; Wells PN Urinary catheters: history, current status, adverse events and research agenda. J. Med. Eng. Technol 2015, 39, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Mangelson NL; Kado RT; Cockett AT Silicone rubber uses in the lower urinary tract. J. Urol 1968, 100, 573–577. [DOI] [PubMed] [Google Scholar]
- (18).Singha P; Locklin J; Handa H A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Saint S; Elmore JG; Sullivan SD; Emerson SS; Koepsell TD The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection: a meta-analysis. Am. J. Med 1998, 105, 236–241. [DOI] [PubMed] [Google Scholar]
- (20).Johnson JR; Johnston B; Kuskowski MA In vitro comparison of nitrofurazone- and silver alloy-coated foley catheters for contact-dependent and diffusible inhibition of urinary tract infection-associated microorganisms. Antimicrob. Agents Chemother 2012, 56, 4969–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Burney S; Caulfield JL; Niles JC; Wishnok JS; Tannenbaum SR The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res 1999, 424, 37–49. [DOI] [PubMed] [Google Scholar]
- (22).Carpenter AW; Schoenfisch MH Nitric oxide release: part II Therapeutic applications. Chem. Soc. Rev 2012, 41, 3742–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Schairer DO; Chouake JS; Nosanchuk JD; Friedman AJ The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Goudie MJ; Pant J; Handa H Liquid-infused nitric oxide-releasing (LINORel) silicone for decreased fouling, thrombosis, and infection of medical devices. Sci. Rep 2017, 7, 13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Brisbois EJ; Major TC; Goudie MJ; Bartlett RH; Meyerhoff ME; Handa H Improved hemocompatibility of silicone rubber extracorporeal tubing via solvent swelling-impregnation of S-nitroso-N-acetylpenicillamine (SNAP) and evaluation in rabbit thrombogenicity model. Acta Biomater. 2016, 37, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Colletta A; Wu J; Wo Y; Kappler M; Chen H; Xi C; Meyerhoff ME S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated Silicone Foley Catheters: A Potential Biomaterial/Device To Prevent Catheter-Associated Urinary Tract Infections. ACS Biomater. Sci. Eng 2015, 1, 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Homeyer KH; Goudie MJ; Singha P; Handa H Liquid-Infused Nitric-Oxide-Releasing Silicone Foley Urinary Catheters for Prevention of Catheter-Associated Urinary Tract Infections. ACS Biomater. Sci. Eng 2019, 5, 2021–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).McCabe MM; Hala P; Rojas-Pena A; Lautner-Csorba O; Major TC; Ren H; Bartlett RH; Brisbois EJ; Meyerhoff ME Enhancing analytical accuracy of intravascular electrochemical oxygen sensors via nitric oxide release using S-nitroso-N-acetyl-penicillamine (SNAP) impregnated catheter tubing. Talanta 2019, 205, No. 120077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Feit CG; Chug MK; Brisbois EJ Development of S-Nitroso-N-Acetylpenicillamine Impregnated Medical Grade Polyvinyl Chloride for Antimicrobial Medical Device Interfaces. ACS Appl. Bio Mater 2019, 2, 4335–4345. [DOI] [PubMed] [Google Scholar]
- (30).Homeyer KH; Singha P; Goudie MJ; Handa HS -Nitroso-N-acetylpenicillamine impregnated endotracheal tubes for prevention of ventilator-associated pneumonia. Biotechnol. Bioeng 2020, 117, 2237–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wo Y; Brisbois EJ; Wu J; Li Z; Major TC; Mohammed A; Wang X; Colletta A; Bull JL; Matzger AJ; Xi C; Bartlett RH; Meyerhoff ME Reduction of Thrombosis and Bacterial Infection via Controlled Nitric Oxide (NO) Release from S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated CarboSil Intra-vascular Catheters. ACS Biomater. Sci. Eng 2017, 3, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Mondal A; Douglass M; Hopkins SP; Singha P; Tran M; Handa H; Brisbois EJ Multifunctional S-Nitroso-N-acetylpenicill-amine-Incorporated Medical-Grade Polymer with Selenium Interface for Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 11, 34652–34662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Frost MC; Meyerhoff ME Controlled photoinitiated release of nitric oxide from polymer films containing S-nitroso-N-acetyl-DL-penicillamine derivatized fumed silica filler. J. Am. Chem. Soc 2004, 126, 1348–1349. [DOI] [PubMed] [Google Scholar]
- (34).Goudie MJ; Brisbois EJ; Pant J; Thompson A; Potkay JA; Handa H Characterization of an S-nitroso-N-acetylpenicillamine-based nitric oxide releasing polymer from a translational perspective. Int. J. Polym. Mater 2016, 65, 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Moriarty TF; Poulsson AHC; Rochford ETJ; Richards RG Bacterial Adhesion and Biomaterial Surfaces. In Comprehensive Biomaterials, Ducheyne P, Ed.; Elsevier: Oxford, 2011; pp. 75–100. [Google Scholar]
- (36).Hopkins SP; Pant J; Goudie MJ; Schmiedt C; Handa H Achieving Long-Term Biocompatible Silicone via Covalently Immobilized S-Nitroso-N-acetylpenicillamine (SNAP) That Exhibits 4 Months of Sustained Nitric Oxide Release. ACS Appl. Mater. Interfaces 2018, 10, 27316–27325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Douglass ME; Goudie MJ; Pant J; Singha P; Hopkins S; Devine R; Schmiedt CW; Handa H Catalyzed Nitric Oxide Release via Cu Nanoparticles Leads to an Increase in Antimicrobial Effects and Hemocompatibility for Short-Term Extracorporeal Circulation. ACS Appl. Bio Mater 2019, 2, 2539–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Brisbois EJ; Davis RP; Jones AM; Major TC; Bartlett RH; Meyerhoff ME; Handa H Reduction in Thrombosis and Bacterial Adhesion with 7 Day Implantation of S-Nitroso-N-acetylpenicillamine (SNAP)-Doped Elast-eon E2As Catheters in Sheep. J. Mater. Chem. B 2015, 3, 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Wo Y; Li Z; Brisbois EJ; Colletta A; Wu J; Major TC; Xi C; Bartlett RH; Matzger AJ; Meyerhoff ME Origin of Long-Term Storage Stability and Nitric Oxide Release Behavior of CarboSil Polymer Doped with S-Nitroso-N-acetyl-D-penicillamine. ACS Appl. Mater. Interfaces 2015, 7, 22218–22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Sabir N; Ikram A; Zaman G; Satti L; Gardezi A; Ahmed A; Ahmed P Bacterial biofilm-based catheter-associated urinary tract infections: Causative pathogens and antibiotic resistance. Am. J. Infect. Control 2017, 45, 1101–1105. [DOI] [PubMed] [Google Scholar]
- (41).Cortese YJ; Wagner VE; Tierney M; Devine D; Fogarty A Review of Catheter-Associated Urinary Tract Infections and In Vitro Urinary Tract Models. J. Healthc. Eng 2018, 2018, No. 2986742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Jacobsen SM; Stickler DJ; Mobley HL; Shirtliff ME Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev 2008, 21, 26–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Tambyah PA; Halvorson KT; Maki DG A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin. Proc 1999, 74, 131–136. [DOI] [PubMed] [Google Scholar]
- (44).Johnson JR; Kuskowski MA; Wilt TJ Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann. Intern. Med 2006, 144, 116–126. [DOI] [PubMed] [Google Scholar]
- (45).Kowalczuk D; Ginalska G; Piersiak T; Miazga-Karska M Prevention of biofilm formation on urinary catheters: comparison of the sparfloxacin-treated long-term antimicrobial catheters with silver-coated ones. J. Biomed. Mater. Res. B Appl. Biomater 2012, 100B, 1874–1882. [DOI] [PubMed] [Google Scholar]
- (46).Morris NS; Stickler DJ Encrustation of indwelling urethral catheters by Proteus mirabilis biofilms growing in human urine. J. Hosp. Infect 1998, 39, 227–234. [DOI] [PubMed] [Google Scholar]
- (47).Riley DK; Classen DC; Stevens LE; Burke JP A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection. Am. J. Med 1995, 98, 349–356. [DOI] [PubMed] [Google Scholar]
- (48).Brisbois EJ; Major TC; Goudie MJ; Meyerhoff ME; Bartlett RH; Handa H Attenuation of thrombosis and bacterial infection using dual function nitric oxide releasing central venous catheters in a 9day rabbit model. Acta Biomater. 2016, 44, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Nablo BJ; Prichard HL; Butler RD; Klitzman B; Schoenfisch MH Inhibition of implant-associated infections via nitric oxide release. Biomaterials 2005, 26, 6984–6990. [DOI] [PubMed] [Google Scholar]
- (50).Yang L; Jing L; Jiao Y; Wang L; Marchesan JT; Offenbacher S; Schoenfisch MH In Vivo Antibacterial Efficacy of Nitric Oxide-Releasing Hyperbranched Polymers against Porphyromonas gingivalis. Mol. Pharmaceutics 2019, 16, 4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Johnson JR; Delavari P; Azar M Activities of a nitrofurazone-containing urinary catheter and a silver hydrogel catheter against multidrug-resistant bacteria characteristic of catheter-associated urinary tract infection. Antimicrob. Agents Chemother 1999, 43, 2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Desai DG; Liao KS; Cevallos ME; Trautner BW Silver or nitrofurazone impregnation of urinary catheters has a minimal effect on uropathogen adherence. J. Urol 2010, 184, 2565–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Friedman A; Blecher K; Sanchez D; Tuckman-Vernon C; Gialanella P; Friedman JM; Martinez LR; Nosanchuk JD Susceptibility of Gram-positive and -negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence 2011, 2, 217–221. [DOI] [PubMed] [Google Scholar]
- (54).Hou Z; Wu Y; Xu C; Reghu S; Shang Z; Chen J; Pranantyo D; Marimuth K; De PP; Ng OT; Pethe K; Kang ET; Li P; Chan-Park MB Precisely Structured Nitric-Oxide-Releasing Copolymer Brush Defeats Broad-Spectrum Catheter-Associated Biofilm Infections In Vivo. ACS Cent. Sci 2020, 6, 2031–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Wasfi R; Hamed SM; Amer MA; Fahmy LI Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell Infect. Microbiol 2020, 10, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Morgan SD; Rigby D; Stickler DJ A study of the structure of the crystalline bacterial biofilms that can encrust and block silver Foley catheters. Urol. Res 2009, 37, 89–93. [DOI] [PubMed] [Google Scholar]
- (57).Gajdács M;Ábrók M; Lázár A; Burián K Increasing relevance of Gram-positive cocci in urinary tract infections: a 10-year analysis of their prevalence and resistance trends. Sci. Rep 2020, 10, 17658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Zarb P; Coignard B; Griskeviciene J; Muller A; Vankerckhoven V; Weist K; Goossens M; Vaerenberg S; Hopkins S; Catry B; Monnet D; Goossens H; Suetens C; National Contact Points for the ECD, C.; Hospital Contact Points for the ECD, C. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro. Surveill 2012, 17, 20316. [DOI] [PubMed] [Google Scholar]
- (59).Chihara S; Popovich KJ; Weinstein RA; Hota B Staphylococcus aureus bacteriuria as a prognosticator for outcome of Staphylococcus aureus bacteremia: a case-control study. BMC Infect. Dis 2010, 10, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Huggan PJ; Murdoch DR; Gallagher K; Chambers ST Concomitant Staphylococcus aureus bacteriuria is associated with poor clinical outcome in adults with S. aureus bacteraemia. J. Hosp. Infect 2008, 69, 345–349. [DOI] [PubMed] [Google Scholar]
- (61).Richardson AR; Libby SJ; Fang FC A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science 2008, 319, 1672–1676. [DOI] [PubMed] [Google Scholar]
- (62).Richardson AR; Dunman PM; Fang FC The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol. Microbiol 2006, 61, 927–939. [DOI] [PubMed] [Google Scholar]
- (63).Park J; Kim J; Singha K; Han DK; Park H; Kim WJ Nitric oxide integrated polyethylenimine-based tri-block copolymer for efficient antibacterial activity. Biomaterials 2013, 34, 8766–8775. [DOI] [PubMed] [Google Scholar]
- (64).Mihu MR; Cabral V; Pattabhi R; Tar MT; Davies KP; Friedman AJ; Martinez LR; Nosanchuk JD Sustained Nitric Oxide-Releasing Nanoparticles Interfere with Methicillin-Resistant Staphylococcus aureus Adhesion and Biofilm Formation in a Rat Central Venous Catheter Model. Antimicrob. Agents Chemother 2017, 61, e02020–e02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Nurhasni H; Cao J; Choi M; Kim I; Lee BL; Jung Y; Yoo JW Nitric oxide-releasing poly(lactic-co-glycolic acid)-polyethylenimine nanoparticles for prolonged nitric oxide release, antibacterial efficacy, and in vivo wound healing activity. Int. J. Nanomed 2015, 10, 3065–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Worley BV; Schilly KM; Schoenfisch MH Anti-Biofilm Efficacy of Dual-Action Nitric Oxide-Releasing Alkyl Chain Modified Poly(amidoamine) Dendrimers. Mol. Pharmaceutics 2015, 12, 1573–1583. [DOI] [PubMed] [Google Scholar]
- (67).Lee J; Hlaing SP; Cao J; Hasan N; Yoo J-W In vitro and in vivo evaluation of a novel nitric oxide-releasing ointment for the treatment of methicillin-resistant Staphylococcus aureus-infected wounds. J. Pharma. Investig 2020, 50, 505–512. [Google Scholar]
- (68).Privett BJ; Broadnax AD; Bauman SJ; Riccio DA; Schoenfisch MH Examination of bacterial resistance to exogenous nitric oxide. Nitric Oxide 2012, 26, 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Zhang S; Liang X; Gadd GM; Zhao Q Superhydrophobic Coatings for Urinary Catheters To Delay Bacterial Biofilm Formation and Catheter-Associated Urinary Tract Infection. ACS Appl. Bio Mater 2020, 3, 282–291. [DOI] [PubMed] [Google Scholar]
- (70).Zhang S; Wang L; Liang X; Vorstius J; Keatch R; Corner G; Nabi G; Davidson F; Gadd GM; Zhao Q Enhanced Antibacterial and Antiadhesive Activities of Silver-PTFE Nano-composite Coating for Urinary Catheters. ACS Biomater. Sci. Eng 2019, 5, 2804–2814. [DOI] [PubMed] [Google Scholar]
- (71).Champeau M; Póvoa V; Militão L; Cabrini FM; Picheth GF; Meneau F; Jara CP; de Araujo EP; de Oliveira MG Supramolecular poly(acrylic acid)/F127 hydrogel with hydration-controlled nitric oxide release for enhancing wound healing. Acta Biomater. 2018, 74, 312–325. [DOI] [PubMed] [Google Scholar]
- (72).Graham DT; Mark GE; Pomeroy AR; Macarthur EB In vivo validation of a cell culture test for biocompatibility testing of urinary catheters. J. Biomed. Mater. Res 1984, 18, 1125–1135. [DOI] [PubMed] [Google Scholar]
- (73).Pariente JL; Bordenave L; Jacob F; Bareille R; Baquey C; le Guillou M Cytotoxicity assessment of latex urinary catheters on cultured human urothelial cells. Eur. Urol 2000, 38, 640–643. [DOI] [PubMed] [Google Scholar]
- (74).Kowalczuk D; Ginalska G; Przekora A The cytotoxicity assessment of the novel latex urinary catheter with prolonged antimicrobial activity. J. Biomed. Mater. Res. A 2011, 98, 222–228. [DOI] [PubMed] [Google Scholar]


