Abstract
The chances of ventilator-associated pneumonia (VAP) increases 6–20 folds when an endotracheal tube (ETT) is placed in a patient. VAP is one of the most common hospital-acquired infections and comprises 86% of the nosocomial pneumonia cases. This study introduces the idea of nitric oxide-releasing ETTs (NORel-ETTs) fabricated by the incorporation of the nitric oxide (NO) donor S-nitroso-N-acetylpenicillamine (SNAP) into commercially available ETTs via solvent swelling. The impregnation of SNAP provides NO release over a 7-day period without altering the mechanical properties of the ETT. The NORel-ETTs successfully reduced the bacterial infection from a commonly found pathogen in VAP, Pseudomonas aeruginosa, by 92.72 ± 0.97% when compared with the control ETTs. Overall, this study presents the incorporation of the active release of a bactericidal agent in ETTs as an efficient strategy to prevent the risk of VAP.
Keywords: antimicrobial, endotracheal tubes, nitric oxide, S-nitroso-N-acetylpenicillamine, ventilator-associated pneumonia
1 |. INTRODUCTION
Endotracheal intubation is a common hospital procedure implemented if the patient is not able to breathe oxygen on their own. In a normal, nonintubated respiratory tract, the region above the vocal cords is heavily colonized by bacteria due to the proximity of the oral and nasal cavity while the lower respiratory tract is sterile (Safdar, Crnich, & Maki, 2005). The major defense mechanisms in keeping the lower respiratory tract sterile include anatomic airway barriers, cough reflexes, mucus, and mucociliary clearance (Safdar et al., 2005). Mucociliary clearance clears the airways of its own secreted mucus, together with substances trapped in it, with coughing serving as a back-up system to mucociliary clearance (Robinson et al., 1996). The placement of an endotracheal tube (ETT) hinders the normal defense mechanisms especially the cough reflex, compromising the mucociliary clearance and providing a conduit for the microorganisms to invade the normally sterile lower respiratory tract (Efrati et al., 2010).
Ventilator-associated pneumonia (VAP) is pneumonia that occurs after patients have been intubated via an ETT and received mechanical ventilation (Koenig & Truwit, 2006). Endotracheal intubation increases the chances of VAP 6–20 folds (Raad et al., 2011), increasing by 3% per day during the first 5 days of ventilation, and by 2% during days 5 through 10 (May et al., 2014; Pneumatikos, Dragoumanis, & Bouros, 2009; Raad et al., 2011). Accounting for 86% of nosocomial pneumonia cases, VAP is one of the most common hospital-acquired infections (HAIs), increasing mortality rates, hospital stay, and hospital costs (Barnes, Feit, Grant, & Brisbois, 2019; Koenig & Truwit, 2006; May et al., 2014; Mietto, Pinciroli, Patel, & Berra, 2013). To contract VAP, microorganisms must reach the sterile lower respiratory tract and adhere to the mucosa to begin colonization (Safdar et al., 2005). The main perpetrators of VAP include Pseudomonas aeruginosa, Acinetobacter spp., and Stenotrophomonas maltophilia (Koenig & Truwit, 2006). Using the ETT as a vehicle, these microorganisms can gain access through a variety of mechanisms, mainly by the aspiration of microbe-laden secretions from the oropharynx directly, or from stomach reflux into the oropharynx (Efrati et al., 2010; Koenig & Truwit, 2006; Safdar et al., 2005). With an indwelling ETT, the likelihood of VAP development is increased due to the availability of a foreign surface, promoting microbial colonization and biofilm formation with the aforementioned mechanisms. Thus, microbes use ETTs as a vehicle for colonization, and patients with them are more susceptible to infections.
Due to the fact that VAP accounts for 60% of all deaths that occur from HAIs, (May et al., 2014) and considering the role ETTs play in increasing the chance for infection in the lower respiratory tract, researchers have strived to develop effective means to prevent VAP. ETTs are made from polyvinyl chloride (PVC), rubber, silicone, or metal (Feit, Chug, & Brisbois, 2019). Over the years, there have been designed changes to the ETT to improve the antimicrobial efforts without the need for additional antiseptics. These changes include adding a cuff to the end of the ETT for better sealing to prevent macroaspiration, or including a suctioning channel to remove subglottic secretion (Mietto et al., 2013). However, both of these additions have proven to be ineffective in preventing the spread of bacteria to the lower trachea and have the potential to damage the trachea (Inglis, Millar, Jones, & Robinson, 1989; Mietto et al., 2013). Additional prevention attempts have included various surface modifications to ETTs such as silver-coated (Barnes et al., 2019; Olson, Harmon, & Kollef, 2002) and gentamicin-containing hydrogel-coated ETTs (Barnes et al., 2019; Jones, McCoy, Andrews, McCrory, & Gorman, 2015). Currently, silver-coated ETTs are the only commercially approved antimicrobial ETTs in the United States. However, there are limiting factors to using silver. Silver-coated ETTs have been shown to be effective in in vivo studies and in clinical trials, but they were not shown to reduce secondary outcomes such as mortality rates, and length of intensive care unit stay (Kollef et al., 2008; Raad et al., 2011). It is the cost of silver that is a major drawback; however, as it is 10–20 folds more expensive compared with commercial ETTs (Kollef et al., 2008; de Lima, Seabra, & Duran, 2012; Zhang, Wang, Chen, & Chen, 2014). Furthermore, the risk with using antibiotics, either as a hydrogel antibiotic-releasing surface coating or a treatment to the infection, is the development of antibiotic-resistant bacteria. Despite these attempts to prevent bacterial adhesion and decrease the chance of VAP, these modifications have major limitations in being economically feasible and still provide the risk of infection. In addition, it has been previously reported that a major limiting factor in the use of surface-treated ETTs is that there is still an accumulation of secretions within the ETT lumen (Mietto et al., 2013). This accumulation covers the surface, blocking the specific antimicrobial treatment that is on the surface from reaching either the planktonic bacteria or biofilms present, all the while the risk of VAP persists.
An alternative approach to improving the antimicrobial activity of materials that have high infection rates, like ETTs, is to mimic one of the defense mechanisms of the human body. Nitric oxide (NO) is an endogenous gas that is released from the natural endothelium and is proven to be a strong antimicrobial agent (Brisbois et al., 2015; Brisbois, Major, Goudie, Bartlett et al., 2016; Sundaram, Pant, Goudie, Mani, & Handa, 2016). NO has a number of other biological function such as vasodilation, thrombosis, and aids in wound healing (Brisbois et al., 2015; Brisbois, Major, Goudie, Meyerhoff et al., 2016; Goudie, Brainard, Schmiedt, & Handa, 2017; Goudie et al., 2016). More specifically, NO that is released in the sinus cavities and by neutrophils and macrophages functions as an effective natural antiseptic agent (Brisbois et al., 2015; Degano, Genestal, Serrano, Rami, & Arnal, 2005). Endogenous NO upregulates ciliary motility, an important host defense mechanism, therefore, inducing alveolar macrophage activity (Ricciardolo, 2003). In addition, NO has the ability to act as a signaling molecule to reduce mucus secretions in the trachea (Ramnarine, Khawaja, Barnes, & Rogers, 1996). NO has been shown to have broad-spectrum antimicrobial properties, effectively killing both Gram-negative and Gram-positive bacteria (Brisbois et al., 2015; Brisbois, Major, Goudie, Bartlett et al., 2016; Regev-Shoshani, Ko, Miller, & Av-Gay, 2010; Sundaram et al., 2016). The development of NO-releasing materials, by incorporating donors such as S-nitrosothiols (RSNOs) into various polymers, has the potential to release NO exogenously mimicking the endogenous release. S-nitroso-N-acetylpenicillamine (SNAP) is a synthetic RSNO donor that has been extensively studied for its antimicrobial properties (Brisbois et al., 2015; Goudie et al., 2016; Ren et al., 2015). Exposure to heat, light, and catalysis (e.g., using metal ions like Cu+) are the main promoters of NO release from RSNOs (Brisbois, Major, Goudie, Bartlett et al., 2016; Goudie et al., 2016). Common methods of incorporating NO donors such as SNAP include swelling the polymer with a solvent swelling solution (Brisbois, Major, Goudie, Bartlett et al., 2016; Colletta et al., 2015), immobilization of the donor onto the polymer itself (Frost & Meyerhoff, 2005; Reynolds et al., 2006), or physically blending the NO donor with the polymer in solution (Brisbois et al., 2015; Brisbois, Handa, Major, Bartlett, & Meyerhoff, 2013). The efficacy of NO-releasing materials as a bactericidal agent has been demonstrated in numerous in vitro studies using materials incorporated with SNAP against common bacteria (Brisbois, Major, Goudie, Bartlett et al., 2016; Brisbois, Major, Goudie, Meyerhoff et al., 2016; Goudie, Pant, & Handa, 2017; Homeyer, Goudie, Singha, & Handa, 2019; Singha, Pant, Goudie, Workman, & Handa, 2017). Specifically, Feit et al. (2019) have shown that the incorporation of a NO-releasing donor into medical grade PVC, a common material used to produce ETTs, reduced viable bacteria colonization of Staphylococcus aureus and Escherichia coli over a 24-hr period. Therefore, incorporating NO donors into an ETT has the potential to decrease the build-up of the secretion on the ETT lumen and increase alveolar macrophage activity, but more important, it can simultaneously inhibit bacterial proliferation and decrease the risk of infection. Overall, the introduction of NO donor molecules into ETTs would be beneficial in inhibiting biofilm formation and contraction of VAP in intubated patients.
In this study, the development of nitric oxide-releasing ETTs (NORel-ETTs) is shown to significantly reduce bacteria cell proliferation, addressing the prevention of bacterial adhesion and VAP more effectively. Using a solvent swelling method, the NO donor, SNAP, was incorporated into the polymer of the ETT. The impregnation of SNAP demonstrated an effective NO release over a 7-day period while preserving the natural mechanical properties of commercial ETTs. The NORel-ETT was effective in significantly reducing the infection caused by P. aeruginosa, a pathogen that is frequently found to result in VAP over a 24-hr period. NORel-ETTs effectively provide the active release of a bactericidal agent commonly found in the natural body resulting in an effective method to decrease the risk of VAP, a common occurrence in hospitals that increases costs and mortality rates.
2 |. MATERIALS AND METHODS
2.1 |. Materials
N-acetyl-d-penicillamine, sodium chloride, potassium chloride, sodium phosphate dibasic, potassium phosphate monobasic, ethylenediaminetetraacetic acid (EDTA), tetrahydrofuran (THF), phosphate-buffered saline (PBS), trioctyl trimellitate (TOTM), and N,N-dimethylacetamide (DMAc) were purchased from Sigma-Aldrich (St. Louis, MO). Methanol, hydrochloric acid, and sulfuric acid were obtained from Thermo Fisher Scientific (Pittsburgh, PA). Acetone was purchased from VWR International (Radnor, PA). Uncuffed, PVC ETTs, 5 mm, were purchased from Dynarex Corporation (Orangeburg, NY). PBS, pH 7.4, containing 138 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate, 100 μM EDTA was used for all experiments. P. aeruginosa (ATCC 27853) was originally obtained from the American Type Culture Collection (Manassas, VA). Luria broth (LB) and Luria agar were bought from Thermo Fisher BioReagents (Fair Lawn, NJ). PBS was purchased from Sigma-Aldrich.
2.2 |. SNAP synthesis
The NO donor, SNAP, was synthesized using an amended version of a previously reported method (Chipinda & Simoyi, 2006). Concisely, an equimolar ratio of NAP and sodium nitrite was dissolved in a 1:1 mixture of water and methanol containing 2 M HCl and 2 M H2SO4. After stirring, the reaction vessel was cooled over 6 hr using an ice bath to form a crystal precipitate. Upon crystal formation, the crystals were collected via vacuum filtration, washed with deionized (DI) water, and allowed to dry under ambient conditions. The entirety of the synthesis and the crystals were protected from light at all times.
2.3 |. Preparation of SNAP-impregnated ETTs
NORel-ETTs were fabricated using a solvent swelling method. The SNAP swelling solution was prepared by dissolving SNAP in a mixture of acetone and THF, at a ratio of 95:5, respectively, by volume. Acetone successfully swells the ETT by a factor of two and the inclusion of THF in the swelling solution allows for a higher infiltration of SNAP into the polymer matrix. When used as a swelling agent for PVC, acetone enters the polymer matrix without destroying the crystallites that act as crosslinks within the polymer (Summers & Rabinovitch, 1981). Therefore, this system should allow the PVC to revert to its initial state after swelling. The ETT was cut into 2-cm segments for the swelling process to test various amounts of SNAP concentrations and plasticizers. Once the optimal amount was finalized, the ETT was cut into 8-cm segments for swelling. Various amounts of plasticizer concentrations were used to minimize any changes in the mechanical properties during the swelling process; TOTM was added at a concentration of 1%, 1.5%, and 2% per volume of solution. Due to the high molecular weight of TOTM, it is less extractable and noncytotoxic making it safe to use in biomedical applications (Courtney, Sundaram, Matata, & Gaylor, 1994; Lai et al., 2012). As later described, a concentration of 2% TOTM of solution proved to have no significant changes in the ultimate tensile strength (UTS) or Young’s modulus when compared with the commercial ETT and was added to the swelling solution for the fabrication of the NORel-ETTs used in the remainder of the methods to preserve the original plasticity of the material. Three concentrations of SNAP were initially used to determine the optimal concentration for the desired release kinetics without altering the properties of the material. The concentrations considered were 20, 40, and 60 mg/ml. For each solvent swelling solution variation, the commercial ETT was submerged in the swelling solution for 5 hr to allow for the proper infiltration of solution into the polymer. The samples were removed from solution and vacuum dried for 24 hr at 23°C to ensure the removal of the solvents. The samples were then placed in DI water and sonicated for 5 min in a Thermo Fisher Scientific 1.9 L sonicating bath to remove any residual SNAP crystals that may have adhered to the surface of the samples. Throughout the entire fabrication process, the samples were shielded from the light.
The surface properties were not observed for the prepared ETTs as previous studies report that no significant changes were observed to the surface characteristics when swelling PVC with SNAP (Feit et al., 2019). This is consistent with other reports that demonstrate swelling silicone with SNAP did not significantly alter the surface properties (Brisbois, Major, Goudie, Bartlett et al., 2016; Feit et al., 2019; Goudie et al., 2016). Therefore, we have substantial evidence that the impregnation of SNAP does not negatively affect the surface properties of PVC.
2.4 |. Tensile testing of the NORel-ETTs
Commercial ETTs were dissolved in THF then cast into films. The ETTs were cast to create testing samples that properly adhere to the IPC-TM-650 standards and for placement and measurements from the machine. These films were swelled with different variations of the swelling solution described above to observe the effect of plasticizer concentration on the mechanical properties when compared with the commercial ETT, as well as the addition of various swelling concentrations of SNAP. The variations are as follows: THF and acetone swelling solution without TOTM, with 1%, 1.5%, and 2% TOTM, and with SNAP concentrations of 20, 40, and 60 mg/ml. The ETT samples containing SNAP were swelled with 2% TOTM, later determined to be the optimal plasticizer concentration to add. The original ETT films and fabricated films were cut according to IPC-TM-650 standards with a 6:1 length/width ratio (n = 3; Sundaram et al., 2016). Tensile testing was performed by carefully securing the samples in place on the jaws of an Instron material testing machine. The samples were tested at a constant extension rate of crosshead speed of 2 mm/s at room temperature (23°C). The stress and strain relationship was derived from the test and the UTS was then determined for all samples and averaged for each variation.
2.5 |. Total SNAP-loaded percentage in fabricated NORel-ETTs
To determine the total amount of SNAP loaded in the ETT after swelling for each concentration, ultraviolet–visible (UV–Vis) spectroscopy was performed. The NORel-ETT was swelled and dried as described above, then massed before being dissolved in 2 ml of DMAc. UV absorbance values were recorded for each SNAP concentration at a wavelength of 340 nm (n = 3), corresponding to the local maxima of the SNAP molecule at 340 nm (Shishido, Seabra, Loh, & Ganzarolli de Oliveira, 2003). The absorbance value was compared with a previously created calibration curve, with consideration of the mass of the sample, to determine the percentage of SNAP present within each sample.
2.6 |. NO release measurements
The active release of NO from the fabricated NORel-ETT was measured using a Sievers chemiluminescence Nitric Oxide Analyzer (NOA) model 280i (Boulder, CO). The NORel-ETT was cut for a total surface area of 1 cm2 (n = 3). The samples were threaded with silk surgical suture to be suspended above 3 ml of PBS buffer with 100 mM EDTA in an amber glass vial at 37°C. The samples are suspended above the solution rather than submerged to create a humid environment mimicking the lower trachea and respiratory tract. Once the sample is placed in the vial, nitrogen is bubbled through the PBS buffer and the purged NO is continuously swept from the vial into the chemiluminescence detection chamber. The NO release measured from the NORel-ETT is normalized using the surface area and the flux unit (×10−10 mol · cm−2 · min−1). After each measurement, the samples were kept on the surgical suture and suspended in a vial above 5-ml PBS with EDTA in an incubator at 37°C to maintain physiological conditions.
2.7 |. Storage stability of NORel-ETT
To determine the ability of the NORel-ETT to be stored under various conditions, the sections of NORel-ETT tubing (40 mg/ml concentration) were stored under temperatures of 37°C, 23°C, 4°C, and −20°C with desiccant for 3 months. After being stored for 2 weeks, 1 month, and 3 months, segments (n = 3) of the samples were dissolved in DMAc, then using UV–Vis spectroscopy, the absorbance valve was recorded at 340 nm for each condition and compared to a predetermined calibration curve. The percent SNAP remaining was calculated and compared with the initial NORel-ETT. The NO release kinetics of the samples stored for 3 months at 23°C were measured and compared with newly made NORel-ETT samples with a SNAP concentration of 40 mg/ml. All samples were stored at the respective temperatures in the dark conditions.
2.8 |. Bacteria adhesion analysis: 24-hr test with P. aeruginosa
The fabricated NORel-ETTs were tested for bacterial adhesion inhibition by comparing them to the control tubes. The samples were 0.5 cm2 in surface area and data analysis was adjusted for this surface area. Inhibition of bacteria adhesion was studied using a modified American Society for Testing and Materials protocol (E2180) 24 hr of exposure to P. aeruginosa (Liu, Singha, Handa, & Locklin, 2017; Singha et al., 2017). To obtain the required pathogenic culture, a colony of P. aeruginosa was inoculated and grown overnight in LB media to a CFU/ml of 106–108. This overnight culture was washed twice in PBS by centrifuging at 2,500 rpm for 7.5 min. This centrifuge wash was done to get rid of nutrient waste and unused media from the overnight culture. The resulting bacteria pellet obtained from centrifugation was then resuspended with PBS to get an optical density-adjusted culture used for the bacteria adhesion study.
Once the suspension was ready, the samples were incubated with the bacteria culture in a shaker incubator (140 rpm, 37°C). After 24 hr of exposure to the bacteria, samples were gently rinsed with sterile PBS to get rid of any unattached bacteria and then homogenized to release all the attached bacteria into the sterile PBS. A consistent volume of sterile PBS was used for each sample. This process also helps in homogenously mixing any biofilm bacteria formed during the 24-hr study. The bacterial solutions attained were then serially diluted and plated. The plates were incubated in 37°C for 18 hr and “CFU of bacteria/cm2 of sample” measurements were then calculated from them using the following formula:
2.9 |. Statistical analysis
Data for the 24-hr bacteria adhesion analysis is expressed as a mean ± standard deviation. Data for all other experiments are expressed as mean ± standard error of the mean. The results between the data for the control ETT and NORel-ETT were analyzed by comparison of means using a two-tailed Student’s t test. Values of p < .05 were considered statistically significant for all tests.
3 |. RESULTS AND DISCUSSION
3.1 |. Total SNAP-loaded percentage
To determine the amount of SNAP loaded into the ETT for each concentration after swelling, the UV–Vis spectra were recorded and the amount of SNAP was calculated (Figure 1). The 60 mg/ml concentration had the highest percentage, 19.5 ± 0.39 wt%, as expected. The concentration of 20 mg/ml was the lowest with 7.97 ± 0.90 wt%, and the 40 mg/ml concentration ETT was 14.2 ± 0.82 wt% of SNAP. Wo et al. (2015) found the solubility of SNAP in CarboSil polymer to be ca. 3.4–4.0 wt%, with higher concentrations leading to crystal formation within the matrix of the polymer, which is why increasing the level of SNAP does not always result in an increase in the NO release. The formation of SNAP crystals within the polymer matrix leads to extended-release profiles as compared to typical burst release rates. However, an initial burst release is commonly seen in these materials, stemming from the portion of integrated SNAP that is soluble within the polymer matrix. Therefore, since the SNAP loading percentages for the NORel-ETT are higher than the solubility of SNAP in the polymer matrix, it is likely that there is SNAP crystal formation within the ETT polymer matrix. The formation of SNAP crystals is important for the NO release kinetics as it provides a longer release period and longer shelf life in its crystalline form (Goudie et al., 2016; Wo et al., 2015).
FIGURE 1.

Percent of SNAP loaded compared with a total mass of endotracheal tube after solvent swelling fabrication process for each concentration: 20, 40, and 60 mg/ml. The amount of SNAP loaded was measured using UV–Vis spectroscopy. NORel-ETT, nitric oxide-releasing endotracheal tube; SNAP, S-nitroso-N-acetylpenicillamine; UV–Vis, ultraviolet–visible
3.2 |. Mechanical testing analysis
The altering of polymers with additives can affect the mechanical properties of the base polymer, including the tensile strength. Due to ETTs being composed of PVC, plasticizers are often added to create the proper plasticity for the application of the material. PVC is characteristically a rigid and brittle polymer, thus the performance is modified by adding plasticizers to obtain the desired flexibility and durability for the particular application (Lai et al., 2012). TOTM is one of the main commercial plasticizers used in PVC products and due to its high molecular weight, it is less extractable and noncytotoxic making it safe to use in biomedical applications (Courtney et al., 1994; Lai et al., 2012). The swelling solution, therefore, included TOTM to preserve the plasticity of the original ETT since the TOTM diffuses out of the PVC during swelling. The mechanical strength of the ETTs was studied by measuring the UTS. The addition of plasticizer (TOTM) was seen to lower the UTS of the ETTs. The UTS of the ETTs approached the UTS of the commercial ETT as the amount of TOTM increased (Figure 2a). The commercial ETT (control) was found to have a UTS of 26.1 ± 1.5 MPa. The ETT swelled with the swelling solution with no plasticizer added had a UTS of 41.9 ± 3.6 MPa. Adding 1% of TOTM to solution decreased the UTS to 35.5 ± 1.2 MPa, while adding 1.5% TOTM had a UTS of 32.6 ± 0.96 MPa, and 2% had a UTS of 26.7 ± 1.2 MPa. The UTS was significantly higher for the ETT swelled with the swelling solution but no TOTM added compared with the control ETT (p = .014). The ETTs swelled with 1% and 1.5% TOTM were also significantly higher compared with the control, p = .0077 and p = .020, respectively. The ETTs with 2% TOTM added were not statistically significant from the control ETT. Therefore, the addition of 2% TOTM resembled the UTS of the control ETT and was chosen as the optimal percentage for the swelling solution used in the remainder of the studies.
FIGURE 2.
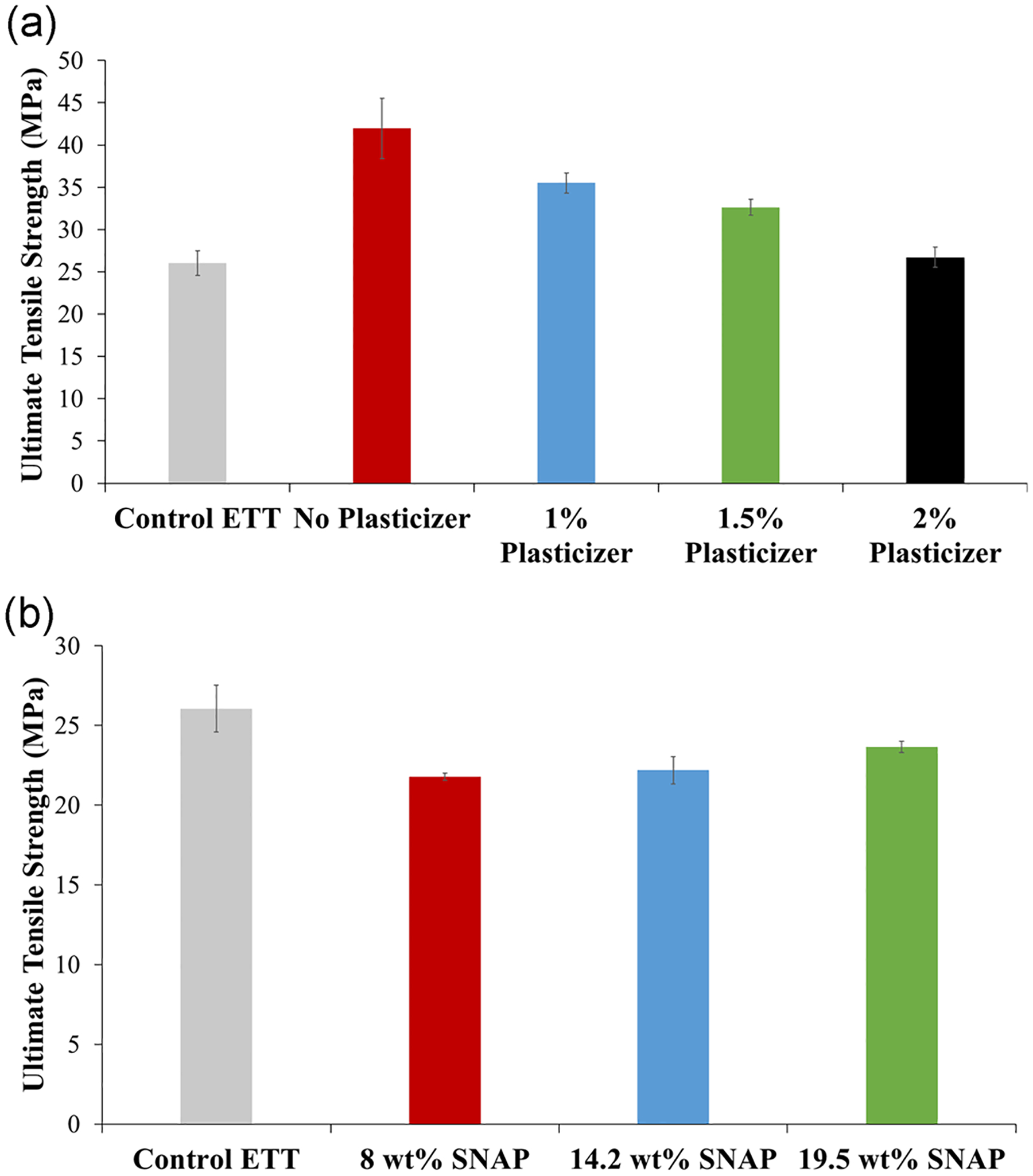
(a) Ultimate tensile strength of ETTs with varying amounts of plasticizer added to the swelling solution (0%, 1%, 1.5%, 2% added) at the breakpoint. (b) Ultimate tensile strength of ETTs with different concentrations of SNAP added to a swelling solution containing 2% TOTM in solution (20, 40, and 60 mg/ml added) at the breakpoint. Both measured by Instron tensile strength instrument. ETT, endotracheal tube; SNAP, S-nitroso-N-acetylpenicillamine; TOTM, trioctyl trimellitate
The addition of SNAP was seen to slightly increase the UTS as the amount of SNAP increased (Figure 2b). NORel-ETTs with 8 wt% SNAP had a UTS of 21.8 ± 0.24 MPa. The UTS of NORel-ETTs between 8 and 14.2 wt% was not found to be statistically significant (p = .67) with 14.2 wt% NORel-ETT having a UTS of 22.2 ± 0.84 MPa. As the SNAP concentration increased to 19.5 wt%, the UTS for 19.5 wt% NORel-ETT increased to 23.6 ± 0.35 MPa. The UTS of NORel-ETTs between 14.2 and 19.5 wt% was not statistically significant (p = .19); however, the UTS for NORel-ETTs between 8 and 19.5 wt% was statistically significant (p = .012). Each of the SNAP concentrations was not statistically significant when compared with the control ETT. Consequently, the addition of SNAP in either of these concentrations is not shown to statistically affect the tensile strength of the commercial ETT. In previous studies, the addition of SNAP to Elast-eon E2As above 10 wt% was seen to decrease the UTS drastically when compared to lower weight percentages of SNAP (Goudie et al., 2016; Sundaram et al., 2016). This discrepancy could be due to the material examined, Elast-eon E2As versus PVC, the crystal structure of SNAP within the polymer, or the addition of plasticizer during fabrication.
3.3 |. NO release measurements
NO release was measured for the NORel-ETTs using a Sievers chemiluminescence NOA (Figure 3). Since the risk of VAP is greatest during the first 5 days of endotracheal intubation, the NO release was measured over a 7-day period for each concentration. Release kinetics were examined as a function of SNAP swelling concentration. The 8 wt% NORel-ETTs initially released 4.04 ± 1.0 × 10−10 mol · cm−2 · min−1; however, only lasted until Day 3 with a release of 0.0300 ± 0.010 × 10−10 mol · cm−2 · min−1, running out before Day 7. The 14.2 wt% NORel-ETT lasted the entirety of the testing period with an initial release of 7.08 ± 2.3 × 10−10 mol · cm−2 · min−1 and a final release of 0.690 ± 0.19 × 10−10 mol · cm−2 · min−1. Finally, the 19.5 wt% NORel-ETT measured to have an initial release of 63.00 ± 8.3 × 10−10 mol · cm−2 · min−1 and a final release of 1.18 ± 0.20 × 10−10 mol · cm−2 · min−1. Higher concentrations of SNAP have been previously reported to have a 60-day release (Homeyer et al., 2019); however, due to the solubility of SNAP within the solvents used in this method, the maximum concentration is 60 mg/ml. The 8 wt % NORel-ETT did not have a NO release past a 3-day period, making this concentration of SNAP not ideal for in vivo use as it could potentially be ineffective at inhibiting bacteria proliferation and biofilm formation, therefore not helping to reduce the risk of VAP. While the 19.5 wt% NORel-ETT lasted the entirety of the testing period, this concentration is also not desirable due to the large initial burst release on Day 0. This burst release is typical of NO-releasing materials, an undesirable characteristic for medical devices as high NO fluxes have been shown to be cytotoxic (Joshi, Ponthier, & Lancaster, 1999; Lopez-Belmonte, Whittle, & Moncada, 1993). The 14.2 wt% NORel-ETT not only lasted the entire testing period but also had a relatively stable release, only gradually decreasing in NO release over the testing period. Considering that the first 5 days is crucial in preventing the bacteria colonization and biofilm formation that can lead to VAP (Raad et al., 2011), the 14.2 wt% NORel-ETT showed the most desirable release kinetics of the three concentrations tested, therefore, this concentration will be used in the storage stability study as well as the antimicrobial characterization of the material.
FIGURE 3.
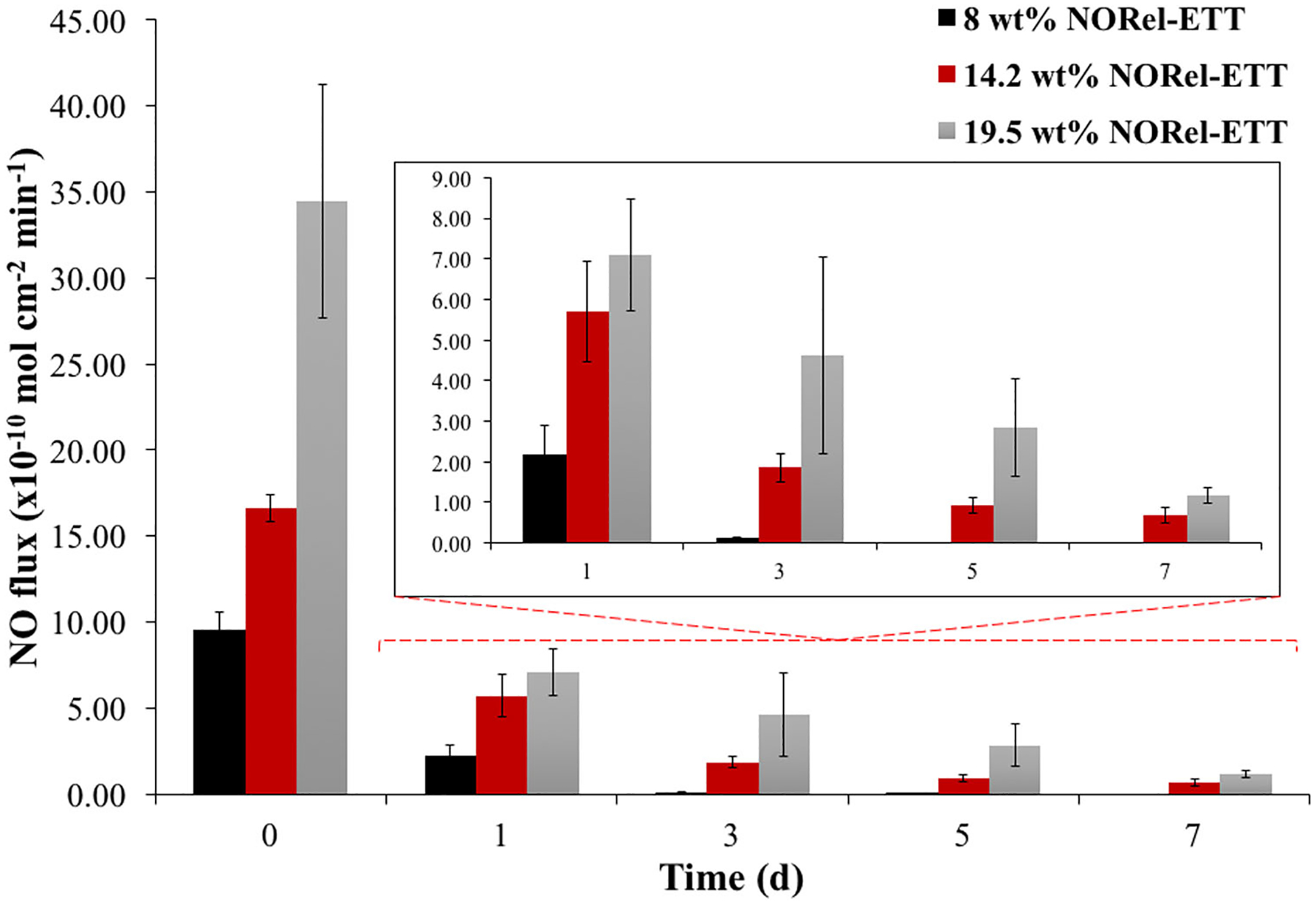
Average nitric oxide (NO) release measurements from freshly fabricated 8, 14.2, and 19.5 wt% NORel-ETT over 7-day period (n = 3). NO release measured from ETT samples suspended above PBS at 37°C using a Sievers Chemiluminescence Nitric Oxide Analyzer. ETT, endotracheal tube; NORel-ETT, nitric oxide-releasing endotracheal tube; PBS, phosphate-buffered saline
3.4 |. Storage stability of NORel-ETTs
Many NO donors, including SNAP, are sensitive to heat exposure, making their stable storage difficult. In order for NO-releasing materials to be feasible in the market, they need to be able to be stored without a large loss to the NO-releasing properties. Therefore, these materials need to be assessed as to their ability to be stored under various environments. NORel-ETTs with 14.2 wt% SNAP were stored at a variety of temperatures to observe the possibility of storage under various conditions (Figure 4). Room temperature (23°C) is the most realistic storage condition. Under this temperature, NORel-ETTs retained 91% SNAP after 1 month and 90.8% after 3 months. Naturally, the amount of SNAP remaining overtime decreased as the storage temperature increased, as seen when the NORel-ETTs were stored under 37°C. After 1 month only 69.2% SNAP remained and 61.6% after 3 months. The best storage conditions for the NORel-ETTs is at −20°C with 97.4% remaining after 1 month and 96.0% after 3 months. The NORel-ETTs were able to successfully retain the majority of the SNAP during the 3-month storage period under varying temperatures. It is important to have a high retention of SNAP throughout the storage period as well as a maintained release profile from the NORel-ETTs when they are ready to be used. The NO release from the NORel-ETTs with 14.2 wt% SNAP stored at 23°C was measured after 3 months of storage and compared with the release of freshly prepared NORel-ETTs (Figure 3). After 3 months of storage, the 14.2 wt% NORel-ETT initially released 16.3 ± 0.012 × 10−10 mol · cm−2 · min−1 with a final release after 7 days of 1.52 ± 0.65 × 10−10 mol · cm−2 · min−1. The release rates for the stored ETTs are very comparable to those of the freshly prepared samples. Typically, the higher release of NO on Day 0 is attributed to the soluble SNAP in the polymer, which is unstable and rapidly releases NO, and during storage, the soluble SNAP will decompose with the crystalline SNAP remaining (Goudie et al., 2016) However, after 3 months of storage, the NORel-ETT exhibited a high release on Day 0. This irregularity maybe because it takes more than 3 months for the soluble SNAP to completely degrade as previous storage stability studies on SNAP have been performed over 6 months (Goudie et al., 2016). Aside from storage at 37°C, at most 9.25% SNAP was lost during the 3-month storage period, observed by the UV–Vis results. The 7-day release after 3 months of storage confirmed the SNAP crystal formation in the polymer matrix during fabrication which is important for a controlled NO release and storing the NORel-ETTs.
FIGURE 4.
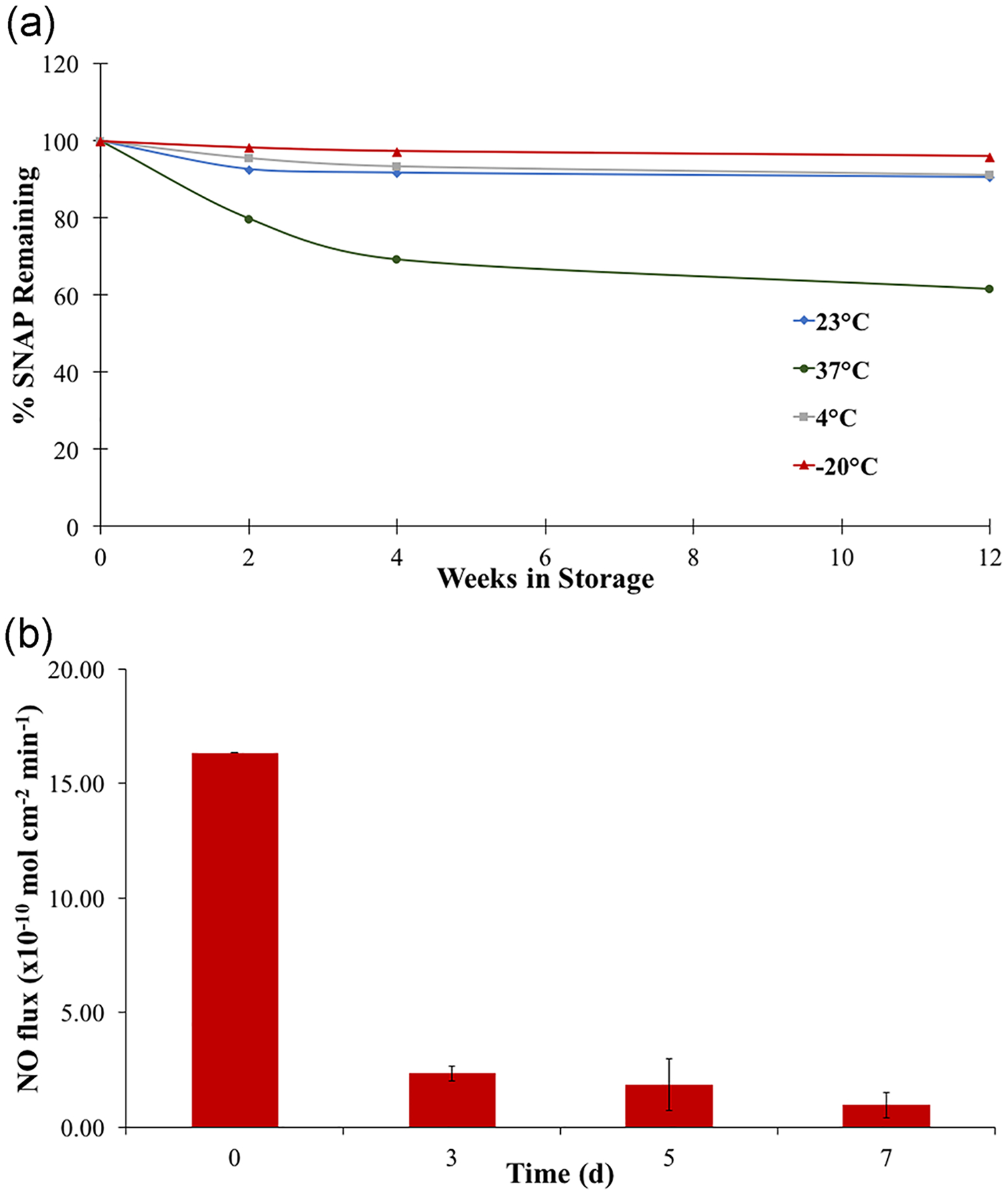
(a) Storage stability of SNAP in NORel-ETTs. The percent SNAP remaining was measured for NORel-ETTs stored under different storage conditions (−20°C, 4°C, 23°C, and 37°C). Error bars are excluded since they are on the order of data point size. (b) Average nitric oxide release measurements from 14.2 wt% NORel-ETT after 3-month storage at 23°C over the 7-day period (n = 3). NO, nitric oxide; NORel-ETT, nitric oxide-releasing endotracheal tube; SNAP, S-nitroso-N-acetylpenicillamine
3.5 |. Antimicrobial efficacy of NORel-ETTs
Despite prevention methods like silver ion-containing ETTs, improved antimicrobial therapy, and supportive care, VAP remains a common complication that has resulted in higher morbidity and mortality. Since VAP develops within 48 hr of infection, it is important to ward off and kill bacteria during the first few hours of intubation. The ETT provides a surface for bacteria to adhere to and develop into biofilms. Free-floating (or planktonic) bacteria can come across the ETT surface and attach within minutes. These free-floating bacteria are widely present in the microflora of the patient’s skin or respiratory tract and can easily find a way to the surface of the ETTs. The attached bacteria then produce slimy, extracellular polymeric substances (EPS) that cover the tubes and form the conditioning film for the stationary, attached bacteria. EPS production allows the emerging biofilm community to develop a complex, three-dimensional structure: A biofilm that protects the bacteria from antibiotics. Hence, biofilms have become a major hurdle for healthcare-associated infections and further validates why we need to develop more antimicrobial-infused materials to reduce the incidence of healthcare associate infections and therefore reduce healthcare costs significantly. In this study, the antimicrobial efficacy of the fabricated ETTs was examined against the common VAP pathogen P. aeruginosa in a 24-hr model. The total viable P. aeruginosa adhered to the ETT samples’ surface was determined after 24 hr at 37°C. Plate count for 24 hr P. aeruginosa showed that the viable bacteria attached on the surface of the NORel-ETT samples were 92.7 ± 0.97% less than on ETT controls (Figure 5; n = 4; p = .01). This corresponded to a ca. 1.5 log reduction in viable bacteria growth. This reduction is also comparable to the reduction of E. coli found in another study where silicone-modified antimicrobial polyethylene ETTs were tested (Jiang, Lv, Shen, & Wang, 2017), suggesting that the NO release does have significant antibacterial activity in P. aeruginosa. The reduction in P. aeruginosa attachment is also comparable with other NO-releasing, SNAP swollen materials (Goudie et al., 2017).
FIGURE 5.
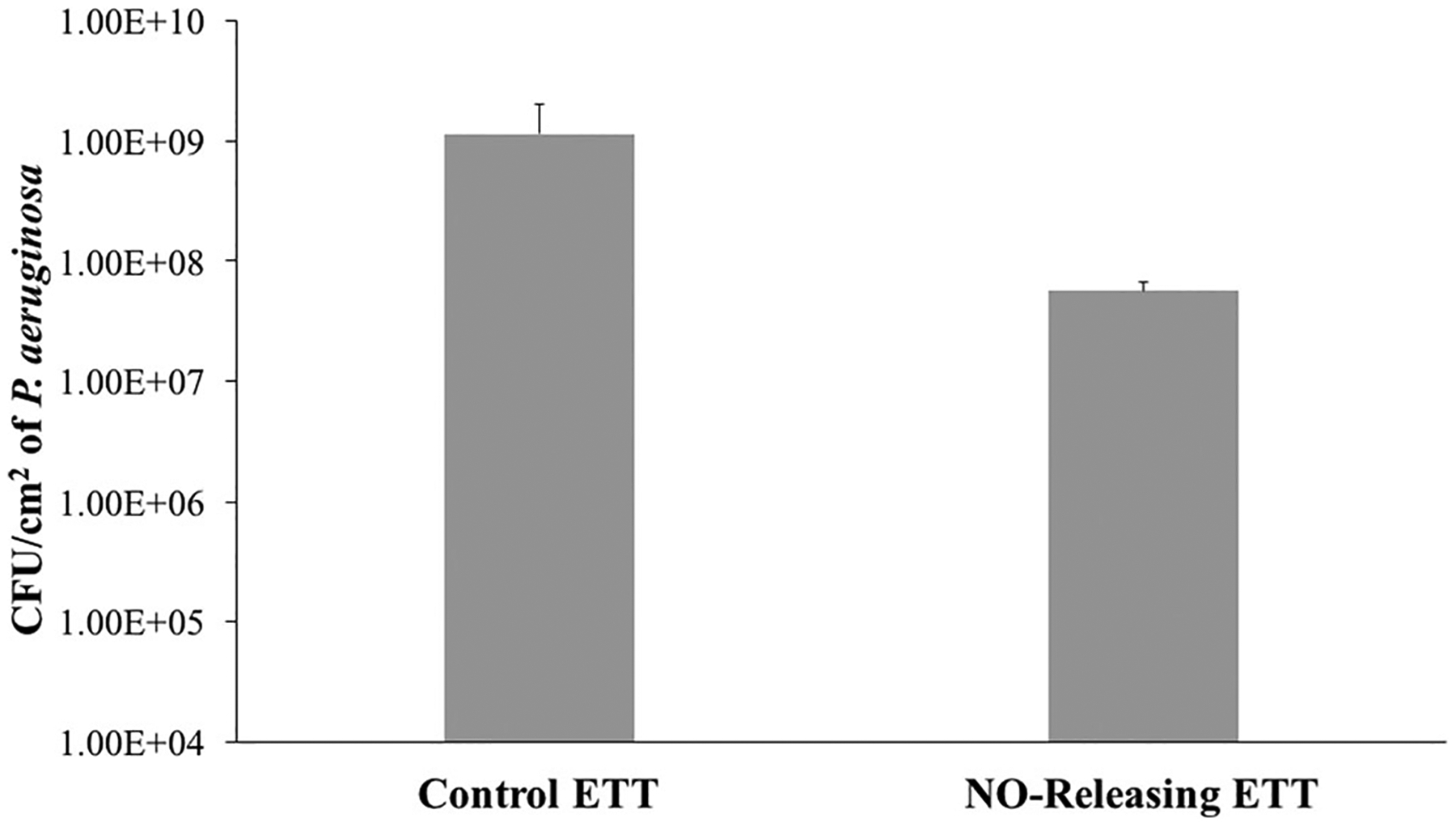
Pseudomonas aeruginosa bacteria adhesion per cm2 for control commercial endotracheal tube, and NORel-ETT over the 24-hr period (n = 3). Data represent mean ± SD. ETT, endotracheal tube; NORel-ETT, nitric oxide-releasing endotracheal tube; SD, standard deviation
Hence, from the biofilm adhesion study, it can be concluded that the NORel-ETT was able to significantly reduce the attachment of viable bacteria to its surface and thus prove to be a major development in the field of antimicrobial ETTs. However, despite the promising results, in the future in vivo studies need to be performed to demonstrate the long-term antimicrobial efficacy of these ETTs.
4 |. CONCLUSIONS
In this study, the incorporation of SNAP to create NORel-ETTs was investigated, including the effects of SNAP on the mechanical, physical, and bactericidal properties of ETTs. The storage stability of this material was also considered for clinical feasibility. The ETT was successfully loaded with SNAP using a solvent swelling method and the addition of SNAP did not alter the mechanical properties as the UTS was not significantly different from the original ETT. The NORel-ETTs successfully released NO over a 7-day period. Future long-term NO release studies need to be performed to determine the full potential of the release period for the NORel-ETTs. The present study also demonstrated the ability of NORel-ETTs to be stored for 3 months at room temperature while retaining 90.8 ± 1.2% of initial the SNAP concentration and at harsh conditions (37°C) retaining 61.6 ± 1.5%. The 3-month storage period did not alter the release kinetics of the NORel-ETTs as it was very similar to freshly made materials, demonstrating that these materials are feasible for use in clinical settings. Furthermore, the incorporation of an active release of NO into commercially available ETTs effectively reduced 92.7 ± 0.97% of viable bacteria attached to the surface of NORel-ETTs when compared with the control ETTs. Future long-term bacteria studies and in vivo studies need to be performed to validate the long-term antimicrobial efficacy of NORel-ETTs. As this study observed the effects of incorporating NO into an uncuffed ETT, future studies on the examination of NO in cuffed ETTs will be necessary to observe the possibility of VAP prevention in different types of ETTs. Overall, the impregnation of SNAP in ETTs to create NORel-ETTs serves as an inexpensive and effective approach to dramatically reduce the frequency of VAP.
ACKNOWLEDGMENT
The authors acknowledge the financial support of the National Institutes of Health (K25HL111213 and R01HL134899).
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Numbers: K25HL111213, R01HL134899
REFERENCES
- Barnes M, Feit C, Grant T-A, & Brisbois EJ (2019). Antimicrobial polymer modifications to reduce microbial bioburden on endotracheal tubes and ventilator-associated pneumonia. Acta Biomaterialia, 91, 220–234. [DOI] [PubMed] [Google Scholar]
- Brisbois EJ, Davis RP, Jones AM, Major TC, Bartlett RH, Meyerhoff ME, & Handa H (2015). Reduction in thrombosis and bacterial adhesion with 7 day implantation of S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As catheters in sheep. Journal of Materials Chemistry B, 3(8), 1639–1645. 10.1039/C4TB02036G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbois EJ, Handa H, Major TC, Bartlett RH, & Meyerhoff ME (2013). Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As polymer. Biomaterials, 34(28), 6957–6966. 10.1016/j.biomaterials.2013.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbois EJ, Major TC, Goudie MJ, Bartlett RH, Meyerhoff ME, & Handa H (2016). Improved hemocompatibility of silicone rubber extracorporeal tubing via solvent swelling-impregnation of S-nitroso-N-acetylpenicillamine (SNAP) and evaluation in rabbit thrombogenicity model. Acta Biomaterialia, 37, 111–119. 10.1016/j.actbio.2016.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbois EJ, Major TC, Goudie MJ, Meyerhoff ME, Bartlett RH, & Handa H (2016). Attenuation of thrombosis and bacterial infection using dual-function nitric oxide releasing central venous catheters in a 9day rabbit model. Acta Biomaterialia, 44, 304–312. 10.1016/j.actbio.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipinda I, & Simoyi RH (2006). Formation and stability of a nitric oxide donor: S-nitroso-N-acetylpenicillamine. Journal of Physical Chemistry B, 110(10), 5052–5661. 10.1021/jp0531107 [DOI] [PubMed] [Google Scholar]
- Colletta A, Wu J, Wo Y, Kappler M, Chen H, Xi C, & Meyerhoff ME (2015). S-Nitroso-N-acetylpenicillamine (SNAP) impregnated silicone foley catheters: A potential biomaterial/device to prevent catheter-associated urinary tract infections. ACS Biomaterials Science and Engineering, 1(6), 416–424. 10.1021/acsbiomaterials.5b00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney JM, Sundaram S, Matata BM, & Gaylor JD (1994). Biomaterials in cardiopulmonary bypass. Perfusion, 9(1), 3–10. 10.1177/026765919400900102 [DOI] [PubMed] [Google Scholar]
- Degano B, Genestal M, Serrano E, Rami J, & Arnal JF (2005). Effect of treatment on maxillary sinus and nasal nitric oxide concentrations in patients with nosocomial maxillary sinusitis. Chest, 128(3), 1699–1705. 10.1378/chest.128.3.1699 [DOI] [PubMed] [Google Scholar]
- Efrati S, Deutsch I, Antonelli M, Hockey PM, Rozenblum R, & Gurman GM (2010). Ventilator-associated pneumonia: Current status and future recommendations. Journal of Clinical Monitoring and Computing, 24(2), 161–168. 10.1007/s10877-010-9228-2 [DOI] [PubMed] [Google Scholar]
- Feit CG, Chug MK, & Brisbois EJ (2019). Development of S-nitroso-N-acetylpenicillamine impregnated medical grade polyvinyl chloride for antimicrobial medical device interfaces. ACS Applied Bio Materials, 2(10), 4335–4345. [DOI] [PubMed] [Google Scholar]
- Frost MC, & Meyerhoff ME (2005). Synthesis, characterization, and controlled nitric oxide release from S-nitrosothiol-derivatized fumed silica polymer filler particles. Journal of Biomedical Materials Research Part A, 72(4), 409–419. 10.1002/jbm.a.30275 [DOI] [PubMed] [Google Scholar]
- Goudie MJ, Brainard BM, Schmiedt CW, & Handa H (2017). Characterization and in vivo performance of nitric oxide-releasing extracorporeal circuits in a feline model of thrombogenicity. Journal of Biomedical Materials Research Part A, 105(2), 539–546. 10.1002/jbm.a.35932 [DOI] [PubMed] [Google Scholar]
- Goudie MJ, Brisbois EJ, Pant J, Thompson A, Potkay JA, & Handa H (2016). Characterization of an S-nitroso-N-acetylpenicillamine-based nitric oxide releasing polymer from a translational perspective. International Journal of Polymeric Materials, 65(15), 769–778. 10.1080/00914037.2016.1163570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie MJ, Pant J, & Handa H (2017). Liquid-infused nitric oxide-releasing (LINORel) silicone for decreased fouling, thrombosis, and infection of medical devices. Scientific Reports, 7(1):13623. 10.1038/s41598-017-14012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homeyer KH, Goudie MJ, Singha P, & Handa H (2019). Liquid-infused nitric-oxide-releasing silicone foley urinary catheters for prevention of catheter-associated urinary tract infections. ACS Biomaterials Science & Engineering, 5(4), 2021–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis T, Millar M, Jones J, & Robinson D (1989). Tracheal tube biofilm as a source of bacterial colonization of the lung. Journal of Clinical Microbiology, 27(9), 2014–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Lv B, Shen Q, & Wang X (2017). Preparation of silicon-modified antimicrobial polyethylene endotracheal tubes. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 105(1), 91–98. 10.1002/jbm.b.33530 [DOI] [PubMed] [Google Scholar]
- Jones DS, McCoy CP, Andrews GP, McCrory RM, & Gorman SP (2015). Hydrogel antimicrobial capture coatings for endotracheal tubes: A pharmaceutical strategy designed to prevent ventilator-associated pneumonia. Molecular Pharmaceutics, 12(8), 2928–2936. 10.1021/acs.molpharmaceut.5b00208 [DOI] [PubMed] [Google Scholar]
- Joshi MS, Ponthier JL, & Lancaster JR Jr. (1999). Cellular antioxidant and pro-oxidant actions of nitric oxide. Free Radical Biology and Medicine, 27(11–12), 1357–1366. [DOI] [PubMed] [Google Scholar]
- Koenig SM, & Truwit JD (2006). Ventilator-associated pneumonia: Diagnosis, treatment, and prevention. Clinical Microbiology Reviews, 19(4), 637–657. 10.1128/CMR.00051-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, … Schinner R NASCENT Investigation Group. (2008). Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: The NASCENT randomized trial. JAMA, 300(7), 805–813. 10.1001/jama.300.7.805 [DOI] [PubMed] [Google Scholar]
- Lai H, Wang Z, Wu P, Chaudhary BI, Sengupta SS, Cogen JM, & Li B (2012). Structure and diffusion behavior of trioctyl trimellitate (TOTM) in PVC film studied by ATR-IR spectroscopy. Industrial & Engineering Chemistry Research, 51(27), 9365–9375. [Google Scholar]
- de Lima R, Seabra AB, & Duran N (2012). Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. Journal of Applied Toxicology, 32(11), 867–879. 10.1002/jat.2780 [DOI] [PubMed] [Google Scholar]
- Liu Q, Singha P, Handa H, & Locklin J (2017). Covalent grafting of antifouling phosphorylcholine-based copolymers with antimicrobial nitric oxide releasing polymers to enhance infection-resistant properties of medical device coatings. Langmuir, 33(45), 13105–13113. 10.1021/acs.langmuir.7b02970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Belmonte J, Whittle BJ, & Moncada S (1993). The actions of nitric oxide donors in the prevention or induction of injury to the rat gastric mucosa. British Journal of Pharmacology, 108(1), 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R, Hoffman M, Sogo M, Parker A, O’Toole G, Brennan A, & Reddy S (2014). Micro-patterned surfaces reduce bacterial colonization and biofilm formation in vitro: Potential for enhancing endotracheal tube designs. Clinical and Translational Medicine, 3, 8. 10.1186/2001-1326-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietto C, Pinciroli R, Patel N, & Berra L (2013). Ventilator associated pneumonia: Evolving definitions and preventive strategies. Respiratory Care, 58(6), 990–1007. [DOI] [PubMed] [Google Scholar]
- Olson ME, Harmon BG, & Kollef MH (2002). Silver-coated endotracheal tubes associated with reduced bacterial burden in the lungs of mechanically ventilated dogs. Chest, 121(3), 863–870. [DOI] [PubMed] [Google Scholar]
- Pneumatikos IA, Dragoumanis CK, & Bouros DE (2009). Ventilator-associated pneumonia or endotracheal tube-associated pneumonia? An approach to the pathogenesis and preventive strategies emphasizing the importance of endotracheal tube. Anesthesiology, 110(3), 673–680. 10.1097/ALN.0b013e31819868e0 [DOI] [PubMed] [Google Scholar]
- Raad II, Mohamed JA, Reitzel RA, Jiang Y, Dvorak TL, Ghannoum MA, … Chaftari A-M (2011). The prevention of biofilm colonization by multidrug-resistant pathogens that cause ventilator-associated pneumonia with antimicrobial-coated endotracheal tubes. Biomaterials, 32(11), 2689–2694. [DOI] [PubMed] [Google Scholar]
- Ramnarine SI, Khawaja AM, Barnes PJ, & Rogers DF (1996). Nitric oxide inhibition of basal and neurogenic mucus secretion in ferret trachea in vitro. British Journal of Pharmacology, 118(4), 998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Shoshani G, Ko M, Miller C, & Av-Gay Y (2010). Slow release of nitric oxide from charged catheters and its effect on biofilm formation by Escherichia coli. Antimicrobial Agents and Chemotherapy, 54(1), 273–279. 10.1128/AAC.00511-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Colletta A, Koley D, Wu J, Xi C, Major TC, … Meyerhoff ME (2015). Thromboresistant/anti-biofilm catheters via electrochemically modulated nitric oxide release. Bioelectrochemistry, 104, 10–16. 10.1016/j.bioelechem.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MM, Hrabie JA, Oh BK, Politis JK, Citro ML, Keefer LK, & Meyerhoff ME (2006). Nitric oxide releasing polyurethanes with covalently linked diazeniumdiolated secondary amines. Biomacromolecules, 7(3), 987–94. 10.1021/bm060028o [DOI] [PubMed] [Google Scholar]
- Ricciardolo F (2003). Multiple roles of nitric oxide in the airways. Thorax, 58(2), 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Regnis JA, Bailey DL, King M, Bautovich GJ, & Bye PT (1996). Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine, 153(5), 1503–9. 10.1164/ajrccm.153.5.8630593 [DOI] [PubMed] [Google Scholar]
- Safdar N, Crnich CJ, & Maki DG (2005). The pathogenesis of ventilator-associated pneumonia: Its relevance to developing effective strategies for prevention. Respiratory Care, 50(6), 725–39. [PubMed] [Google Scholar]
- Shishido SM, Seabra AB, Loh W, & Ganzarolli de Oliveira M (2003). Thermal and photochemical nitric oxide release from S-nitrosothiols incorporated in Pluronic F127 gel: Potential uses for local and controlled nitric oxide release. Biomaterials, 24(20), 3543–3553. 10.1016/S0142-9612(03)00153-4 [DOI] [PubMed] [Google Scholar]
- Singha P, Pant J, Goudie MJ, Workman CD, & Handa H (2017). Enhanced antibacterial efficacy of nitric oxide releasing thermoplastic polyurethanes with antifouling hydrophilic topcoats. Biomaterials Science, 5(7), 1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers JW, & Rabinovitch EB (1981). Use of acetone in determining poly (vinyl chloride) processing morphology and product morphology. Journal of Macromolecular Science, Part B, 20(2), 219–233. [Google Scholar]
- Sundaram J, Pant J, Goudie MJ, Mani S, & Handa H (2016). Antimicrobial and physicochemical characterization of biodegradable, nitric oxide-releasing Nanocellulose–Chitosan packaging membranes. Journal of Agricultural and Food Chemistry, 64(25), 5260–5266. 10.1021/acs.jafc.6b01936 [DOI] [PubMed] [Google Scholar]
- Wo Y, Li Z, Brisbois EJ, Colletta A, Wu J, Major TC, … Meyerhoff ME (2015). Origin of long-term storage stability and nitric oxide release behavior of carbosil polymer doped with S-nitroso-N-acetyl-d-penicillamine. ACS Applied Materials & Interfaces, 7(40), 22218–22227. 10.1021/acsami.5b07501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wang L, Chen Q, & Chen C (2014). Cytotoxic potential of silver nanoparticles. Yonsei Medical Journal, 55(2), 283–291. 10.3349/ymj.2014.55.2.283 [DOI] [PMC free article] [PubMed] [Google Scholar]


