Abstract
Both congestive heart failure (HF) and atrial fibrillation (AF) are important and increasingly common forms of cardiovascular disease in the 21st century. Heart failure is often complicated by AF, and AF can exacerbate and, in some cases, cause HF, also known as tachycardia-induced cardiomyopathy (TIC). Restoration and maintenance of sinus rhythm in the majority of AF patients with TIC can lead to an improvement in left ventricular function and dramatic symptomatic relief. This can be accomplished by surgical ablation; specifically, the Cox-Maze IV procedure (CMP IV), in those refractory to medical and catheter-based ablation, and those patients undergoing concomitant cardiac operation. However, many surgeons are reluctant to perform stand-alone or concomitant CMP IV in this high-risk cohort of patients. In this review, the over three decades of experience with surgical ablation will be reviewed along with the essential information that surgeons need to be aware of as they participate in the team-based care of patients with AF and HF.
Introduction
With the aging of the population, the incidence of both atrial fibrillation (AF) and heart failure (HF) continues to increase, with a spiraling cost to healthcare services globally [1, 2]. Atrial fibrillation and HF often coexist, promoting a vicious cycle that leads to worsening and often rapid deterioration in a patients’ condition. Atrial fibrillation is both a cause and consequence of HF, with complex interactions that can lead to impairment of systolic and diastolic function not present in sinus rhythm [2]. Atrial fibrillation is also associated with a three-fold increased risk of HF. The EuroHeart Failure Survey reported that 25% of HF patients with reduced ejection fraction (left ventricular ejection fraction (LVEF) less than 40%) had chronic AF [3], with many likely having a component of tachycardia-induced cardiomyopathy (TIC).
With the restoration of sinus rhythm and improved heart rate control, left ventricular function can be expected to normalize in patients with TIC. However, even in the absence of a rapid ventricular rate in AF, an irregular ventricular rhythm has also been shown to lead to a reduction in left ventricular function [4–7]. Conversely, the neurohormonal, and structural changes, including atrial and ventricular fibrosis observed in patients with HF, create a pro-arrhythmic environment that favors the initiation and maintenance of AF [8]. Canine studies by our group and others have shown that left atrial dilation leads to a significant shortening of the atrial effective refractory period, and slower and more inhomogeneous conduction [9, 10]. These changes have been implicated in the initiation of AF in humans and an increased AF inducibility in the canine model of mitral regurgitation [9, 11].
Medical Therapy and Catheter-Based Ablation
Regardless of which comes first, patients with concomitant AF and HF have a significantly worse prognosis [2, 5]. The goals of therapy are similar to other patients with AF to prevent stroke, control the ventricular rate and rhythm, reduce symptoms, and improve functional status and quality of life. Unfortunately, pharmacologic rate and rhythm control strategies, which come with serious side effects, have not led to improvements in left ventricular function, quality of life, or cardiovascular death [8, 12].
In contradistinction, multiple studies have shown catheter ablation to be superior to medical therapy with improved quality of life, exercise capacity, left ventricular function, and reduced AF burden [1, 13–16]. In 2018, Marrouche et al. reported a randomized controlled trial (CASTLE-AF; Catheter Ablation for Atrial Fibrillation with Heart Failure) comparing patients with AF and HF who underwent catheter-based ablation (n=179) to those with medical therapy alone (rate or rhythm control) (n=184). Catheter-based ablation for AF in this patient population was associated with a significantly lower mortality rate from any cause or hospitalization for worsening heart failure. Catheter ablation was associated with significant improvement in left ventricular function, exercise capacity, and a reduction in AF burden [15]. Patients with an ejection fraction of 35% or less were enrolled. In the ablation group, 63.1% of patients in comparison to 21.7% in the medical-therapy group (P<0.001) were in sinus rhythm at the 60-month follow-up visit. The adjudicated rate of recurrence of atrial fibrillation in the ablation group among those who had actually undergone ablation and who were followed for up to 60 months was 50.0% (75 of 151 patients), with an average of 1.3±0.5 ablation procedures per treated patient [15].
In agreement with this randomized study, a subgroup analysis of patients with heart failure from the largest randomized controlled trial to date, CABANA (Catheter Ablation Versus Antiarrhythmic Drug Therapy) was recently analyzed in a systemic review and showed that catheter ablation was associated with all-cause mortality benefit, improvement in ventricular function, and reduction in cardiovascular hospitalization and AF recurrence [17, 18].
Lifelong maintenance of rate and rhythm control is crucial in these patients as recurrence of the arrhythmia is associated with the rapid development of symptomatic heart failure and worsening of left ventricular systolic function [19]. For this reason, for patients who develop heart failure as a result of AF, both the 2013 ACCF/AHA guideline for the management of heart failure and the 2019 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation, recommend non-surgical rhythm control strategies [20, 21].
Despite improvements in technology and techniques, catheter-based ablation outcomes remain relatively poor, particularly in patients with chronic AF. In a study of 255 patients who underwent catheter ablation, of which 43% had paroxysmal AF, the AF-free survival after a single ablation procedure was 32% for the overall cohort at 10 years (39% for paroxysmal vs. 24% for non-paroxysmal AF, P=0.001). Allowing for multiple catheter ablations in those with failure improved the AF-free survival to 52% at 10 years (61% paroxysmal vs. 44% non-paroxysmal, P=0.002) [22]. Several other studies have reported early- and mid-term success rates after catheter ablations with equally disappointing results [23, 24].
Surgical Ablation
Owing to these disappointing results of catheter ablation, surgical ablation is generally the only treatment alternative to atrioventricular nodal ablation with permanent pacemaker implantation in symptomatic patients that are refractory to medical and catheter ablation therapy [25]. The most effective surgical technique for the management of AF has been the Cox-Maze procedure (CMP), introduced by James Cox and colleagues in 1987. The “cut-and-sew” version, or the Cox-Maze III, became the gold standard, as long-term follow-up had a greater than 90% freedom from symptomatic AF [26]. In this procedure, surgical incisions were made in both atria to create lines of conduction block to prevent AF. However, due to its technical complexity, few surgeons adopted the procedure. In 2002, a modified version, the Cox-Maze IV procedure (CMP IV), was introduced by our group utilizing bipolar radiofrequency and cryoablation devices to replace most of the atrial incisions [27]. The CMP IV has proven to be as effective as its predecessor at restoring sinus rhythm, while also reducing operative morbidity and mortality [28–30]. It is the only surgical operation to receive an FDA indication for the treatment of AF. Additionally, in contradistinction to catheter ablation and more limited surgical lesion sets, the CMP was found to be equally effective in patients with both paroxysmal and non-paroxysmal AF [29, 30].
The Society of Thoracic Surgeons (STS), The Heart Rhythm Society (HRS), European Heart Rhythm Association (EHRA), and the European Cardiac Arrhythmia Society (ECAS) have recommended surgical ablation as a primary stand-alone procedure to restore sinus rhythm for patients that are refractory to class I/III antiarrhythmic drugs and/or catheter-based therapy (Class IIA, Level B, non-randomized) [31, 32]. Nonetheless, there is currently no consensus regarding the referral for surgical ablation of patients diagnosed with heart failure and AF. This is due to the paucity of clinical series and randomized trials in patients with HF undergoing surgical ablation. However, it can be assumed that with the higher efficacy of the CMP, and its documented low morbidity and mortality, that the results of the large catheter ablation trials summarized above would be applicable to the surgical ablation population, and can be used to justify a more aggressive surgical approach.
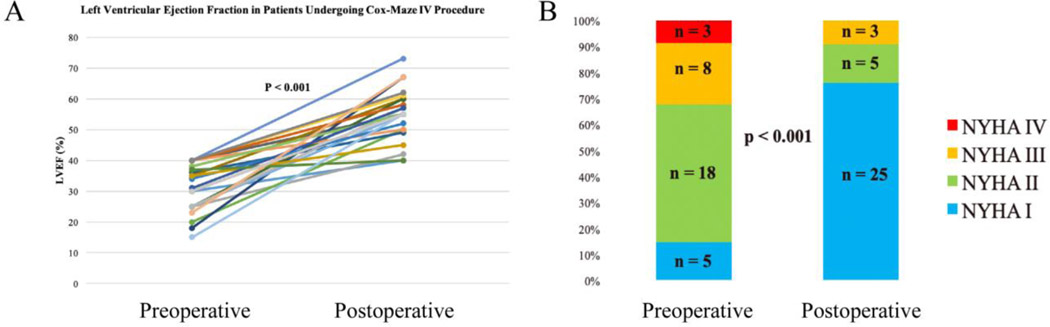
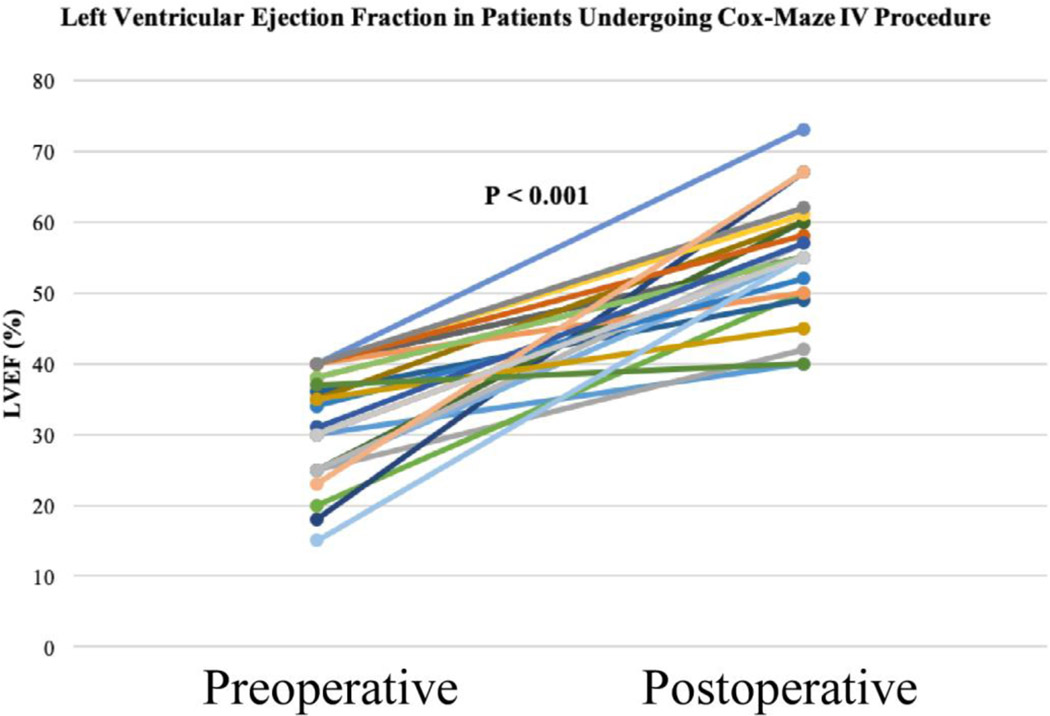
Several retrospective case series have shown improvement in left ventricular ejection fraction after the restoration of sinus rhythm with surgical ablation in the general population of patients with AF. These studies, however, had limited data regarding patients with severely depressed ejection and AF without structural or coronary heart disease [33–36]. More recently, our group reported a series of patients with severely depressed left ventricular function (left ventricular ejection fraction (LVEF) ≤40%) who underwent stand-alone CMP IV. In this patient cohort, 85% (29/34) of patients had clinical (NYHA class II or higher) heart failure symptoms. Restoration of sinus rhythm with the CMP IV was associated with significant improvement in LVEF (32±8% to 55±8%, P<0.001) (Figure 1-A). Additionally, patients’ NYHA functional classification improved significantly from baseline to final follow-up (2.3±0.8 to 1.3±0.6, P=0.02). At last follow-up, 91% of patients had NYHA class I/II symptoms (p<0.001) (Figure 1-B) [19]. The complications associated with surgical ablation in this patient population remained relatively low, as reported by our group and others [19, 33–36].
Figure 1.
Improvement in left ventricular function (A) in patients with a left ventricular ejection fraction of 40% or less at a median follow-up of 22 months after the stand-alone Cox-Maze IV procedure. Left ventricular function improved from 32 ± 8% to 55 ± 8% (95% confidence interval, 0.51 to 0.58; p < 0.001). Improvement in New York Heart Association Functional Classification (B) following the stand-alone Cox-Maze IV procedure. At late follow-up, 30 of 33 patients (91%) had NYHA I/II symptoms (p < 0.001). Reproduced with permission from Khiabani et al., The Annals of Thoracic Surgery [19].
In another similar study by Pozzoli et al., 39 highly symptomatic patients with reduced ejection fraction underwent stand-alone surgical ablation. At a mean follow-up of 29.4 ± 14.2 months, freedom from arrhythmias on or off antiarrhythmic drugs was 92 and 93% at 24 and 36 months, respectively. Ejection fraction normalized in all cases, from 51.3 ± 9% to 61.1 ± 3% (p < 0.001) overall, and from 37.0 ± 10% to 60.3 ± 3% (P < 0.001) when ≤ 45% preoperatively [36].
Patient Selection
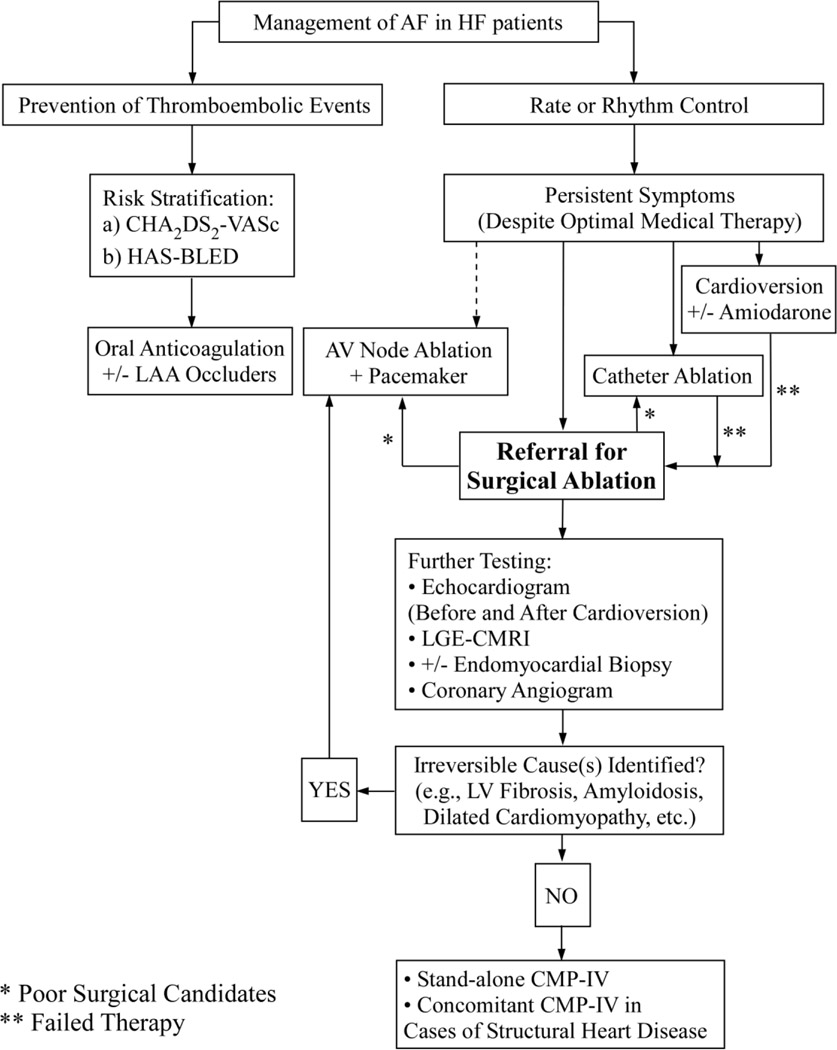
Patient selection can be challenging (Figure 2). Identifying a suitable patient who can benefit from surgical ablation is extremely important in this cohort of patients. It is important to distinguish patients with reversible heart failure secondary to atrial fibrillation with or without concomitant structural or coronary heart disease (e.g., tachycardia-induced cardiomyopathy), from those suffering from cardiomyopathies or other non-reversible forms caused of HF. At our center, all patients undergo an evaluation with echocardiography. Gadolinium-enhanced cardiac MRI is also performed to assess for other causes of cardiomyopathy, myocardial viability, and fibrosis. If there is any abnormality on cardiac MRI or a high index of suspicion, endomyocardial biopsies are performed [19]. As discussed previously, sustained tachycardia may result in both atrial and ventricular fibrosis, which can impact the degree of ventricular function improvement [1, 2, 16]. Recently, the CAMERA-MRI study randomized patients with persistent AF and idiopathic cardiomyopathy (LVEF≤45%) to medical treatment or catheter ablation. Randomization was done following cardiac MRI to assess the degree of left ventricular fibrosis by late gadolinium enhancement (LGE). Compared to the medical group, the catheter ablation group had superior rhythm control and left ventricular function improvement. However, the degree of ventricular fibrosis predicted the extent of LVEF recovery. On multivariate analysis, only the absence of LGE was found to predict LVEF normalization [16]. Thus, preoperative cardiac MRI should be strongly considered in all patients with a tentative diagnosis of tachycardia-induced cardiomyopathy to help gauge the degree of improvement from a patient may gain from surgical ablation.
Figure 2.
Management of patients with atrial fibrillation and heart failure at our institution. AV node, atrioventricular node; AF, atrial fibrillation; CMP-IV, Cox-Maze IV procedure; HF, heart failure; LAA, left atrial appendage; LGE-CMRI, late gadolinium enhancement cardiac MRI; LV, left ventricle.
Additionally, patients with dilated cardiomyopathy should be identified. Patients with idiopathic dilated cardiomyopathy tend to have larger left ventricular end-diastolic diameters than patients with TIC. Jeong et al. reported that LVEF ≤ 45% and LV end-diastolic dimension ≤ 61mm is predictive of TIC with a sensitivity of 100% and a specificity of 71%. Furthermore, in cases with LVEF < 30%, LV diastolic dimension ≤ 66mm is predictive of TIC with a sensitivity of 100% and a specificity of 83% [37]. Lastly, when in doubt, medical management should be initiated, and cardioversion should be considered. If the LVEF improved shortly after the restoration of sinus rhythm and when the rate is more adequately controlled, the diagnosis of TIC is more likely, and the patient is much more likely to benefit from surgical ablation [4, 34]. Patients with irreversible causes of heart failure (e.g., LV fibrosis, amyloidosis, ischemic and/or dilated cardiomyopathy, etc.) should not be offered stand-alone surgical ablation. Despite the restoration of sinus rhythm, left ventricular function often will not recover in these patients.
Summary and Conclusions
In summary, given the exceptional outcomes of the Cox-Maze procedure, it should always be considered as an option in treating patients with AF and HF. When performed correctly, in selected patients with HF, it provides reversal of AF-related cardiomyopathy with excellent outcomes, symptomatic relief, and negligible risk. Additionally, CMP IV is a better alternative to atrioventricular nodal ablation with permanent pacemaker implantation. The latter, while effective in controlling the ventricular rate, does not improve LVEF and exercise capacity as effectively as treatments that restore sinus rhythm [25]. Considering the excellent late outcomes of the CMP, with over 80% of patients free from recurrent AF at 5 years [38, 39], the positive results from the catheter ablation versus medical management trials in the HF population, such as CASTLE-AF, should be translatable to the surgical population. Moreover, given the other potential long-term benefits of CMP IV, including increased overall survival and stroke reduction, concomitant surgical ablation should be considered in this surgically high-risk population, despite the longer operative time [19, 30, 39].
While there have been only a few retrospective studies reporting the outcomes of surgical ablation for AF in patients with HF, it is our opinion that future guideline committees need to consider the addition of CMP IV to their recommendations in the management of patients with AF and HF who are undergoing cardiac surgery for another reason or as a stand-alone operation in carefully selected patients. While we need to create a better evidence base for surgical ablation in this population through the performance of prospective, multicenter clinical trials, there are ample published data establishing the important role of ablation in patients with AF and HF. Surgeons need to be aware that HF and the presence of tachycardia-induced cardiomyopathy is a strong indication for proceeding with AF ablation in properly selected patients.
Central Picture.
Improvement in left ventricular function following stand-alone CMP-IV. Reproduced with permission from Khiabani et al., The Annals of Thoracic Surgery [19].
Central Message.
Surgical ablation of AF can restore sinus rhythm in selected patients with reduced ejection fraction and is associated with significant improvement in left ventricular function and symptomatic relief.
Funding:
This work was supported by the National Institutes of Health R01-HL032257 to R.J.D., and R.B.S., T32-HL007776 to R.J.D., A.J.K., and Barnes-Jewish foundation.
Footnotes
Conflict of Interest Disclosure:
R.J.D. – Atricure, Inc: Speaker and receives research funding; LivaNova, Inc.: Speaker. Medtronic: Consultant; Edwards Lifesciences: Speaker. Other authors have nothing to disclose.
References:
- 1.Turagam MK, Garg J, Whang W, et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med. 2019;170(1):41–50. [DOI] [PubMed] [Google Scholar]
- 2.Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do?. Eur Heart J. 2015;36(46):3250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenzen MJ, Scholte op reimer WJ, Boersma E, et al. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25(14):1214–20. [DOI] [PubMed] [Google Scholar]
- 4.Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lakkireddy D, Olshansky B. Arrhythmia-Induced Cardiomyopathies: Mechanisms, Recognition, and Management. J Am Coll Cardiol. 2015;66(15):1714–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagens VE, Van veldhuisen DJ, Kamp O, et al. Effect of rate and rhythm control on left ventricular function and cardiac dimensions in patients with persistent atrial fibrillation: results from the RAte Control versus Electrical Cardioversion for Persistent Atrial Fibrillation (RACE) study. Heart Rhythm. 2005;2(1):19–24. [DOI] [PubMed] [Google Scholar]
- 6.Damiano RJ, Tripp HF, Asano T, Small KW, Jones RH, Lowe JE. Left ventricular dysfunction and dilatation resulting from chronic supraventricular tachycardia. J Thorac Cardiovasc Surg. 1987;94(1):135–43. [PubMed] [Google Scholar]
- 7.Daoud EG, Weiss R, Bahu M, et al. Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol. 1996;78(12):1433–6. [DOI] [PubMed] [Google Scholar]
- 8.Carlisle MA, Fudim M, Devore AD, Piccini JP. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019;7(6):447–456. [DOI] [PubMed] [Google Scholar]
- 9.Ruaengsri C, Schill MR, Khiabani AJ, et al. The hemodynamic and atrial electrophysiologic consequences of chronic left atrial volume overload in a controllable canine model. J Thorac Cardiovasc Surg. 2018;156(5):1871–1879.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solti F, Vecsey T, Kékesi V, Juhász-nagy A. The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc Res. 1989;23(10):882–6. [DOI] [PubMed] [Google Scholar]
- 11.Buxton AE, Waxman HL, Marchlinski FE, Josephson ME. Atrial conduction: effects of extrastimuli with and without atrial dysrhythmias. Am J Cardiol. 1984;54(7):755–61. [DOI] [PubMed] [Google Scholar]
- 12.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–77. [DOI] [PubMed] [Google Scholar]
- 13.Di biase L, Mohanty P, Mohanty S, et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation. 2016;133(17):1637–44. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald MR, Connelly DT, Hawkins NM, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97(9):740–7. [DOI] [PubMed] [Google Scholar]
- 15.Marrouche NF, Brachmann J, Andresen D, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med. 2018;378(5):417–427. [DOI] [PubMed] [Google Scholar]
- 16.Prabhu S, Taylor AJ, Costello BT, et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J Am Coll Cardiol. 2017;70(16):1949–1961. [DOI] [PubMed] [Google Scholar]
- 17.Packer DL, Mark DB, Robb RA, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321(13):1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asad ZUA, Yousif A, Khan MS, Al-khatib SM, Stavrakis S. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2019;12(9):e007414. [DOI] [PubMed] [Google Scholar]
- 19.Khiabani AJ, Adademir T, Schill MR, et al. Surgical Ablation of Atrial Fibrillation in Patients with Tachycardia-Induced Cardiomyopathy. Ann Thorac Surg. 2019;108(2):443–450. [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 21.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation. 2019; 140(2):e125–e151. [DOI] [PubMed] [Google Scholar]
- 22.Gaita F, Scaglione M, Battaglia A, et al. Very long-term outcome following transcatheter ablation of atrial fibrillation. Are results maintained after 10 years of follow up?. Europace. 2018;20(3):443–450. [DOI] [PubMed] [Google Scholar]
- 23.Gökoğlan Y, Mohanty S, Güneş MF, et al. Pulmonary Vein Antrum Isolation in Patients With Paroxysmal Atrial Fibrillation: More Than a Decade of Follow-Up. Circ Arrhythm Electrophysiol. 2016;9(5) [DOI] [PubMed] [Google Scholar]
- 24.Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2(2):e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee NA, Upadhyay GA, Ellenbogen KA, Mcalister FA, Choudhry NK, Singh JP. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis and systematic review. Circ Arrhythm Electrophysiol. 2012;5(1):68–76. [DOI] [PubMed] [Google Scholar]
- 26.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126(6):1822–8. [DOI] [PubMed] [Google Scholar]
- 27.Prasad SM, Maniar HS, Schuessler RB, Damiano RJ. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J Thorac Cardiovasc Surg. 2002;124(4):708–13. [DOI] [PubMed] [Google Scholar]
- 28.Lall SC, Melby SJ, Voeller RK, et al. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133(2):389–96. [DOI] [PubMed] [Google Scholar]
- 29.Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano RJ. The Cox-maze IV procedure in its second decade: still the gold standard?. Eur J Cardiothorac Surg. 2018;53(suppl_1):i19–i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weimar T, Schena S, Bailey MS, et al. The cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badhwar V, Rankin JS, Damiano RJ, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg. 2017;103(1):329–341. [DOI] [PubMed] [Google Scholar]
- 32.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ad N, Henry L, Hunt S. The impact of surgical ablation in patients with low ejection fraction, heart failure, and atrial fibrillation. Eur J Cardiothorac Surg. 2011;40(1):70–6. [DOI] [PubMed] [Google Scholar]
- 34.Stulak JM, Dearani JA, Daly RC, Zehr KJ, Sundt TM, Schaff HV. Left ventricular dysfunction in atrial fibrillation: restoration of sinus rhythm by the Cox-maze procedure significantly improves systolic function and functional status. Ann Thorac Surg. 2006;82(2):494–500. [DOI] [PubMed] [Google Scholar]
- 35.Pecha S, Ahmadzade T, Schäfer T, et al. Safety and feasibility of concomitant surgical ablation of atrial fibrillation in patients with severely reduced left ventricular ejection fraction. Eur J Cardiothorac Surg. 2014;46(1):67–71. [DOI] [PubMed] [Google Scholar]
- 36.Pozzoli A, Taramasso M, Coppola G, et al. Maze surgery normalizes left ventricular function in patients with persistent lone atrial fibrillation. Eur J Cardiothorac Surg. 2014;46(5):871–6. [DOI] [PubMed] [Google Scholar]
- 37.Jeong YH, Choi KJ, Song JM, et al. Diagnostic approach and treatment strategy in tachycardia-induced cardiomyopathy. Clin Cardiol. 2008;31(4):172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henn MC, Lancaster TS, Miller JR, et al. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2015;150(5):1168–76, 1178.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry L, Ad N. Performance of the Cox Maze procedure-a large surgical ablation center’s experience. Ann Cardiothorac Surg. 2014;3(1):62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]