Abstract
Multicellular organisms utilize a battery of extracellular and cellular mechanisms to defend against microbial infiltration. Among the armamentarium used by the small intestine to defend against microbial invasion are antimicrobial peptides called defensins. We previously have shown that gut barrier function is impaired following hemorrhagic shock, resulting in translocation of bacteria or endotoxin. Using a rat model, we examined the effect of hemorrhagic shock on α-defensin expression. We utilized the anchored reverse transcriptase PCR strategy to isolate a rat enteric defensin cDNA. The cDNA is 406 bases in length and encodes a putative prepro-enteric defensin that we have named rat defensin 5 (RD-5). RD-5 expression is restricted to the small intestine and is specifically localized by in situ hybridization to the Paneth cells. A 10-fold increase in its steady state levels was observed in the distal intestine immediately after the termination of shock. This is the first study to show that enteric defensins are inducible following injury. We suggest that enteric defensins may contribute to the complex and integrated barrier function of the intestinal mucosal surface.
The mucosal epithelium of the mammalian small intestine is a complex tissue composed of enterocytes, goblet cells, enteroendocrine cells, and Paneth cells. These cells arise from a common progenitor functionally anchored near the base of the small intestine and are perpetually renewed throughout life (12–14). Although the principal physiological function of this epithelial surface is nutrient absorption, it also forms a major barrier between the body and the environment of the lumen. Multiple defense mechanisms have been identified, which protect the villus epithelium from damage by prokaryotic organisms and viruses, thus averting potentially damaging inflammatory responses and the attachment of foreign microbes to its surface (9). These multiple defense mechanisms are the result of a combination of the anatomical design of the small intestine and the chemical armamentarium of the local and circulating cells. However, under adverse conditions such as hemorrhagic shock or injury, normal intestinal microbiota can cross this mucosal barrier and infect mesenteric lymph nodes and systemic organs in a process termed bacterial translocation (1, 4, 5).
Paneth cells have been suggested to be responsible for the defense of intestinal crypts from potential pathogens. They are located at the base of the crypts of Lieberkuhn throughout the small intestine and proximal colon, but they are most abundant in the ileum (16). These cells contain eosinophilic granules and possess ultrastructural features consistent with a secretory cell morphology (54). An array of known antimicrobial proteins, such as lysozyme (4, 39), tumor necrosis factor alpha (TNF-α) (19), α-1 antitrypsin (28), defensins/cryptdins (10, 18, 34, 36), and type II phospholipase A2 (15, 31, 42, 52), have been localized to these cells, suggesting that they may function to modulate the intestinal microbiota and the stem cell microenvironment, as well as contributing to the maintenance of mucosal barrier function.
α-Defensins are a family of small cationic antimicrobial and cytotoxic peptides that contain 29 to 35 amino acid residues, including 6 invariant cysteine residues whose intramolecular disulfide bonds cyclize and stabilize them in a complex folded triple-stranded β-sheet configuration (48, 49, 51). They possess a broad array of microbicidal activity in vitro against bacteria (12, 22, 40, 50), fungi (12), and enveloped viruses (23) and are highly abundant in neutrophils. Enteric defensins/cryptdins are homologs of myeloid defensins (18, 35, 38, 50). They contain eight conserved residues that include six cysteines whose disulfide bonding is characteristic of the family (22, 48). Enteric defensins also possess extended N termini compared to myeloid defensins (50). They are secreted and appear to function in the extracellular milieu rather than intracellularly (10, 46, 50). A second family of defensins, called β-defensins, differ from classical or α-defensins in their anatomic location and in some structural characteristics, notably the order in which their conserved cysteines are joined (8).
Here we describe the cloning of a rat enteric α-defensin cDNA whose expression is increased following hemorrhagic shock. We localized this defensin to the Paneth cells at the base of the crypts of Lieberkuhn. Our findings support the hypothesis that enteric defensins play a role in the defense of the intestinal mucosal epithelium and that the Paneth cell is a key cellular component that contributes to the barrier function of the intestinal mucosal surface.
MATERIALS AND METHODS
Animal preparation.
Animals were subjected to hemorrhagic shock as previously described (21). Briefly, male Sprague-Dawley rats were anesthetized with an intraperitoneal injection of 60 mg of ketamine mixed with 7.5 mg of xylazine/kg of body weight. A 1-cm incision was made, and the common femoral artery (proximal to the inferior epigastric) was catheterized. The catheter was connected to a swivel harness to allow the rat unrestrained activity. Animals were allowed to recover for 24 h. The following day animals were bled to a mean arterial pressure of 30 mm Hg. Shock was ended when 60% of the initial shed blood volume was returned. Animals were sacrificed either immediately or 2 or 24 h following termination of shock. At the time of sacrifice the small intestines were excised, rinsed with ice-cold normal saline, and cut into proximal, middle, and distal segments, corresponding to the duodenum, jejunum, and ileum, respectively. All tissue was flash frozen and stored at −80°C. (Animal procedures were conducted in accordance with National Institutes of Health guidelines and were approved by the New Jersey Medical School animal care committee.)
RNA analysis.
Total RNA was prepared from rat intestinal tissue by using the single-step guanidinium-acid phenol protocol of Chomczynski and Sacchi (3). Briefly, tissues were homogenized in denaturing solution containing 4 M guanidinium thiocyanate and 0.5% 2-mercaptoethanol. Homogenates were mixed with sodium acetate (pH 4), phenol, and chloroform-isoamyl alcohol and centrifuged, and the aqueous phase containing the RNA was saved. RNA was precipitated with isopropanol, washed with 70% ethanol, and dissolved in diethylpyrocarbonate-treated water. RNA concentrations were determined spectrophotometrically. Ethidium bromide staining was used initially to verify RNA integrity and uniform sample loading.
Anchored reverse transcriptase PCR analysis.
One microgram of total cellular RNA isolated from untreated rat small intestines was reverse transcribed by using the 3′ RACE (rapid amplification of cDNA ends) System (GIBCO/BRL) according to the manufacturer’s instructions. Part of the resulting cDNA was used as the template in PCR, using the universal adapter primer (5′CUACUACUACUAGGCCACGCGTCGACTAGTAC3′) as the anchor and the gene-specific primer MD-1s (5′CTCGCAGCCATGAAGAAACTAGTCCT3′). PCR cycling conditions consisted of 30 cycles of 1 min at 95°C, 1 min at 60°C, and 1 min at 72°C. The reaction mixtures were then incubated for an additional 7 min at 72°C before being placed at 4°C. PCR products were purified by glass milk adsorption (BIO101, La Jolla, Calif.), incubated in a standard fill-in reaction with T4 DNA polymerase (GIBCO/BRL), and subcloned into a BlueScript vector (Stratagene). Purified plasmid DNA was sequenced by using the dideoxy termination method (45) with Sequenase (U.S. Biochemical). Sequences were compared with the GenBank database (MacVector; IBI, New Haven, Conn.) for similarity to known sequences.
Slot blot and Northern analyses.
For slot blot analysis, RNA samples (5 μg) were mixed with denaturing solution (20× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]–formaldehyde) and heated for 15 min at 65°C, and each sample was applied to MagnaGraph nylon transfer membranes by using a VacuSlot-VS blotting apparatus (ABN; American Bionetics, Inc., Hayward, Calif.).
Filters were hybridized overnight at 55°C in 6× NET (1× NET is 0.15 M NaCl, 1 mM EDTA, and 15 mM Tris, pH 8.0)–5× Denhardt solution–0.1% sodium dodecyl sulfate (SDS)–250 μg of yeast tRNA per ml containing [32P]kinase-labeled oligonucleotide probe RCA4A (5′GTGTTTTTGGGGTAGGTTCAGCTTGGACCTGTAGGGCCAGCAGGACAAGGGCAG3′). Membranes were washed at room temperature in 6× NET–0.5% SDS, and the final high-stringency wash was in 6× NET–0.5% SDS for 10 min at the hybridization temperature. The filters were imaged with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). Membranes were stripped and rehybridized with an 18S ribosomal cDNA for normalization.
Total RNAs (5 μg) isolated from various organs were fractionated on an agarose-formaldehyde gel, transferred to nylon membranes, and hybridized in 5× SSC–5× Denhardt solution–0.1% SDS–100 μg of denatured salmon sperm DNA per ml at 65°C. Membranes were washed twice in 1× SSC–0.1% SDS at room temperature, and the final high-stringency wash was done in 0.5× SSC–0.1% SDS for 30 min at 65°C.
A 406-bp cDNA probe (16D11-1) generated by 3′ RACE from rat intestinal RNA and cloned in our laboratory was labeled with [α-32P]dCTP by using random-primed synthesis to a specific activity of >109 cpm/μg of DNA. A 4.3-kb 18S ribosomal cDNA was used as a loading control. Filters were imaged with a PhosphorImager.
In situ hybridization.
In situ hybridization was performed by the protocol of Young et al. (57). The oligonucleotide RCA4A probe was end labeled with 33P and added to hybridization buffer composed of 50% formamide, 4× SSC, 500 μg of sheared single-stranded DNA per ml, 250 μg of yeast tRNA per ml 1× Denhardt solution, and 10% dextran sulfate. Forty-five microliters of hybridization buffer containing 106 cpm was applied to the slides, to which coverslips were added and placed in humidified chambers for 18 h at 37°C. After hybridization, the slides were placed in 1× SSC to remove the coverslips and hybridization buffer. The highest-stringency wash was conducted at 55°C in 1× SSC for 1 h. Control slides were treated with RNase prior to hybridization. The slides were dipped in Kodak NTB 3 photographic emulsion, dried, and stored at 4°C. Following 4 days of exposure, the slides were developed with Kodak D-19 developer and stained with hematoxylin and eosin. Photographs were taken under bright-field optics.
Statistical methods.
Results are reported as means ± standard errors of the means. Data were analyzed by using the Student t test for comparison to control tissue. Significance is assigned for P < 0.05.
Nucleotide sequence accession number.
The nucleotide sequence of the RD-5 cDNA has been submitted to GenBank under accession no. AF115768.
RESULTS
Isolation of RD-5.
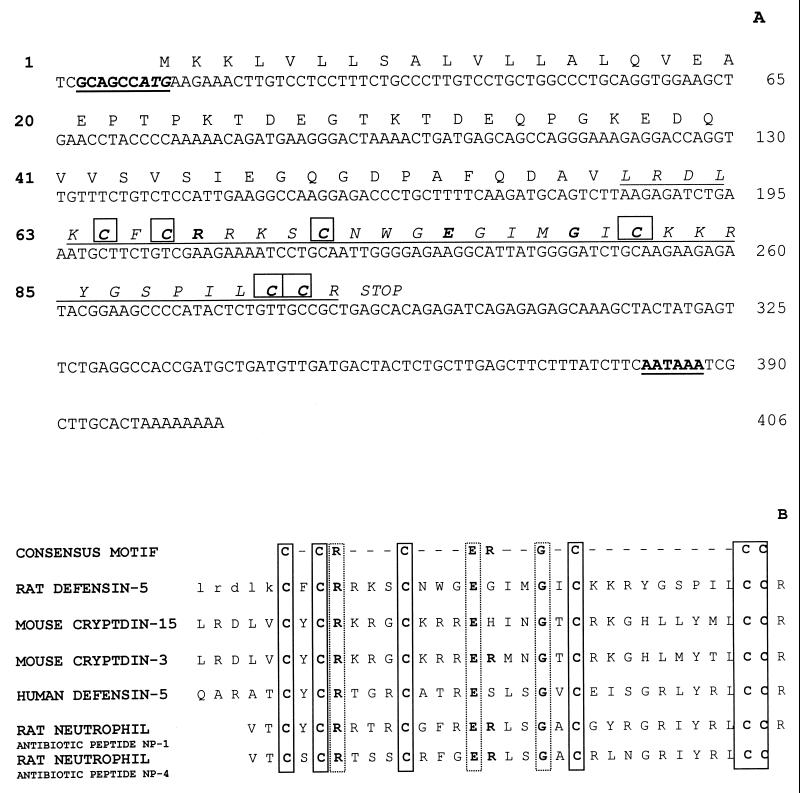
Previous studies by us and others have demonstrated that the immune response of the gut is suppressed following hemorrhagic shock, resulting in the translocation of bacteria or endotoxin (5, 6, 43). We therefore examined the effect of hemorrhagic shock on defensin expression in a rat model (47). We initially hybridized rat intestinal RNA to the mouse cryptdin 1 cDNA, kindly provided by Andre Ouellette (University of California, Irvine), and observed a signal corresponding to a size (0.75 kb) similar to that observed in the mouse (data not shown). To obtain a rat cDNA probe specific for the study of the induction of an enteric defensin gene(s) of the rat intestine during stress (hemorrhagic shock), we used an anchored reverse transcriptase PCR strategy to facilitate direct sequence analysis of rat intestinal RNA. Taking advantage of the high degree of sequence conservation in the 5′ regions of defensin mRNAs (18), we designed a gene-specific primer based on the mouse sequence capable of amplifying defensin-related cDNA. PCR products from these reactions were subcloned, and three independent clones were analyzed. All three clones were identical. As shown in Fig. 1A, the rat cDNA is composed of 406 nucleotides and possesses a typical polyadenylation recognition signal 11 nucleotides upstream of the poly(A) tail. The major open reading frame encodes a putative 93-amino-acid prepro-enteric defensin. A predicted 35-amino-acid mature peptide by comparison to other known enteric defensins is encoded starting at nucleotide 183. Comparison of the amino acid sequence of the predicted mature peptide (Fig. 1B) revealed greater identity and homology to mouse cryptdins than to rat neutrophil defensins, i.e., 48% identity and 65% homology to mouse cryptdin 15, 45% identity and 62% homology to mouse cryptdin 3, but only approximately 40% identity and 50% homology to rat neutrophil defensins 1 to 4 and to RIP-3, a rat neutrophil defensin isolated from the rat intestine (42). Comparison to the human defensin 5 (HD-5) precursor showed only 36% identity and 49% homology. The DNA corresponding to NH2 terminus of the predicted sequence contains the initiating methionine codon (CAGCCATGA) in an appropriate context for translation initiation (20). The putative prepropeptide has all of the structural features common to the numerous preprodefensins described to date (11, 18, 30, 34), and we call this sequence rat defensin 5 (RD-5). Database searches yielded no significant sequence similarity other than to known defensins.
FIG. 1.
(A) Nucleotide sequence of RD-5 cDNA and deduced sequence of the encoded immature protein. The consensus sequences for the start of translation and polyadenylation addition are in boldface and underlined. The putative methionine codon is in italics. The conserved cysteines characteristic of defensins are boxed. The predicted mature peptide of 35 amino acid residues is in italics and underlined. The nucleotide sequence is numbered on the right. The predicted amino acid sequence is numbered on the left. (B) Comparison of amino acid sequences of RD-5, mouse cryptdins 15 and 3, HD-5, rat neutrophil defensins 1 and 4, and the invariable consensus found in all α-defensins. The characteristic six conserved cysteines are indicated by solid boxes, while the three other invariant residues (arginine 6, glutamic acid 14, and glycine 18) are indicated by dashed boxes. The exact amino terminus of the putative mature RD-5 peptide cannot be predicted with certainty; therefore, several residues of the putative precursor peptide immediately proximal to the first cysteine are shown in lowercase.
Tissue distribution of expression.
The tissue distribution was determined by probing RNAs isolated from rat heart, liver, spleen, kidney, small intestine, large intestine, and colon with the RD-5 cDNA. A single transcript of approximately 700 bases was detected only in the small intestine, while the other tissues were negative (Fig. 2).
FIG. 2.
Tissue distribution of RD-5 expression. RNA samples (5 μg/lane) from the indicated adult rat tissues were electrophoresed, blotted, and hybridized with the RD-5 cDNA. A single band of approximately 700 nucleotides is observed only in the small intestine. Ethidium bromide staining of 18S rRNA demonstrating integrity and equality of loadings is shown in the bottom panel.
Localization of RD-5 mRNA.
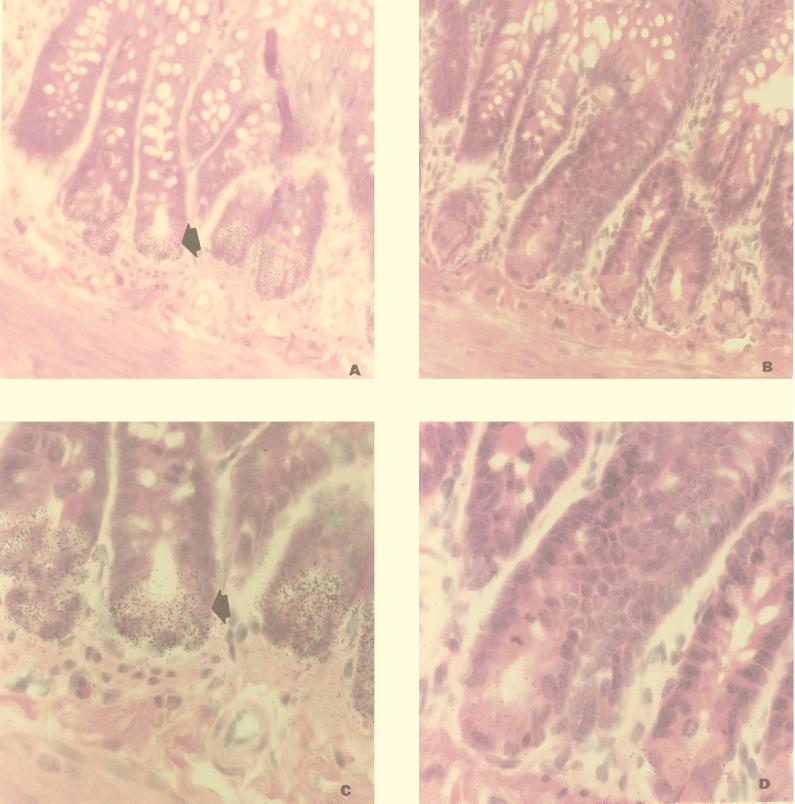
Cellular localization of the RD-5 message was determined by in situ hybridization. Tissue sections of adult rat small intestine were probed with the 33P-labeled antisense oligonucleotide RCA4A. A strong signal was observed with the antisense oligonucleotide probe in epithelial cells at the base of the crypts in sections of normal adult ileum (Fig. 3A and C). No signal was observed in parallel sections of tissue first treated with RNase prior to hybridization with RCA4A (Fig. 3B and D). In sections of ileum subjected to hemorrhagic shock, a strong signal was associated only with Paneth cells (data not shown). This indicates that the increased mRNA level is not due to other cell sources.
FIG. 3.
Detection of RD-5 mRNA in crypt cells of the adult rat small intestine by in situ hybridization. (A) Rat ileum hybridized with [33P]dATP-labeled RCA4A antisense oligonucleotide. Sections were counterstained with hematoxylin and eosin. The arrow indicates silver grains over a Paneth cell in the crypts. Magnification, ×200. (B) Serial section hybridized as for panel A except that the tissue was pretreated with RNase. (C and D) Selected regions shown in panels A and B, respectively. Magnification, ×400.
Expression of RD-5 following hemorrhagic shock.
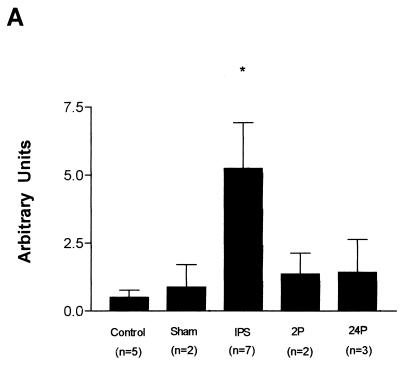
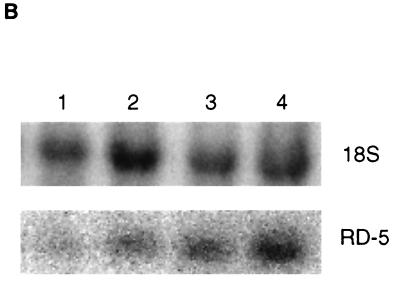
Total cellular RNA was prepared from the ilea of control and experimental animals. The controls consisted of untreated and sham-shocked rats. Sham-shocked animals were included to control for possible effects resulting from the surgical placement of the cannular. Shocked animals were bled to a mean arterial pressure of 30 mm Hg and maintained at this pressure until 60% of their shed blood was returned (approximately 2.5 h). Animals were sacrificed either immediately or 2 or 24 h after termination of shock. Each group included two to seven rats. Total RNA isolated from the ileum was analyzed by slot blot analysis, using the oligonucleotide probe RCA4A under high-stringency conditions. These conditions are designed to differentiate between highly homologous defensin family members (2). As seen in Fig. 4A, RD-5 mRNA steady-state levels in the ileum immediately after shock increase approximately 10-fold compared to those in control ileum. Steady-state levels by 24 h after shock appear to return to baseline levels.
FIG. 4.
(A) Effect of hemorrhagic shock on the level of RD-5 mRNA in the ileum of the rat small intestine. The histogram shows a quantitative representation of hybridization signals obtained from volume analysis of PhosphorImager data. Results are expressed in arbitrary units after normalization to the 18S rRNA signal. Abbreviations: IPS, immediately postshock; 2P, 2 h postshock; 24P, 24 h postshock. Error bars are standard errors of the means; the asterisk indicates statistical significance. (B) Representative Northern blot showing induction of RD-5. RNA samples (5 μg/lane) from control ileum (lanes 1 and 2) and ileum immediately after shock (lanes 3 and 4) were electrophoresed and blotted, and blots were hybridized with the RD-5 cDNA.
DISCUSSION
In the present study we describe RD-5, a new member of the rat α-defensin gene family. In situ hybridization experiments indicate that the RD-5 mRNA is localized to the Paneth cells, found at the base of the small intestinal crypts. The predicted peptide from the RD-5 cDNA sequence indicates that RD-5 exists as a possible 93-amino-acid precursor molecule with a structure similar to that reported for murine cryptdins/defensins. The deduced prepropeptide has a highly conserved sequence at the amino terminus, similar to those of previously described defensins (12, 18, 30, 49). By comparison to published defensin peptide sequences, a motif of six cysteines and three other invariant residues characteristic of mature defensin peptides is found at the carboxy-terminal end (Fig. 1B). (The exact amino terminus of the putative mature RD-5 peptide cannot be predicted with certainty.) The predicted primary structure of the prepropeptide derived from the RD-5 cDNA sequence shows significant similarity to those of previously described defensins (12, 18, 30, 49), supporting its potential role as an antimicrobial molecule in the rat intestine.
The RD-5 mRNA, like other α-defensin-encoding mRNAs, has a restricted tissue distribution. An abundant mRNA is detected only in the small intestine (Fig. 2). The observed expression of RD-5 mRNA in the control intestine suggests a baseline level of constitutive expression. As stated above, RD-5 is localized solely to the Paneth cells located at the base of the crypts of Lieberkuhn by in situ hybridization (Fig. 3).
The factor(s) that controls the tissue-specific and regulated expression of mammalian α-defensins is not known. We have previously shown that loss of intestinal barrier function is associated with our hemorrhagic shock model, as evidenced by translocation of bacteria or bacterial products such as endotoxin (43). Also associated with hemorrhagic shock are increased levels of proinflammatory mediators, in particular interleukin-6, interleukin-1, and TNF-α, which are known to have multiple cellular effects (7, 17, 55). Our results are the first to directly demonstrate that enteric defensins are inducible following injury. By comparison, Salzman and coworkers (44), using image analysis, have indirectly shown increased expression of human enteric defensins in necrotizing enterocolitis. These data, together with ours, suggest that enteric defensins can be induced by some exogenous signal, such as that provided by hemorrhage or necrotizing enterocolitis.
In contrast to shorter-lived myeloid cells, whose defensin expression is uninducible, Paneth cells are long-lived and metabolically active. The Paneth cells of germfree and conventionally reared mice and rats are reported to degranulate in response to oral administration of bacteria (46). However, the molecular signals that modulate Paneth cell responses to these stimuli are unknown (38). Hence, it is not unreasonable to assume that Paneth cells should be able to respond to these signals in an inducible manner as observed in our shock model. Paneth cells are found throughout the small intestine and proximal colon but are especially abundant in the region of the ileum (16). In fact, since the discovery that Paneth cells contain lysozyme and secrete it apically into the intestinal lumen, they have been implicated as effectors of mucosal barrier function (4, 39). More recently, a human α-defensin has been localized to their granules (41). These secretory cells also export numerous other host defense products, such as phospholipase A2 (15, 31, 42, 52), TNF-α (19), secretory immunoglobulin A (47), and matrilysin (37), implicating these cells in the mucosal defense against potential pathogens. Antimicrobial activity has been shown for murine cryptdins/defensins (10, 36) and a recombinant human defensin (rHD-5) (40). Hence, the continual release of these molecules by Paneth cells probably influences the crypt microenvironment (38).
The exact physiological role of enteric defensins is not entirely clear at this time. However, by analogy to neutrophils, the possibility that enteric defensins and these other numerous Paneth cell bioactive molecules can interact is a plausible hypothesis, since α-defensins and several other neutrophil granule components have been shown to synergize when combined (25, 26, 56).
A variety of nonmicrobicidal activities have also been ascribed to individual neutrophil α-defensins, leading to the speculation that Paneth cell defensins may also exhibit functions other than their antimicrobial roles. Neutrophil defensins have been reported to be chemotactic for monocytes (53), to be mitogenic (29), to modulate cell volume in intestinal enterocytes (27), to reduce monolayer integrity in cultured cells (32), and to be cytotoxic to mammalian cells at high concentrations (26, 33). In contrast, rHD-5 lacks mitogenic activity and shows limited and no cytotoxic activity against two human intestinal cell lines (40). However, two mouse cryptdins/defensins have been recently shown to induce epithelial cell chloride secretion by formation of an ion conductance channel (24), suggesting that these peptides may be multifunctional.
RD-5’s predicted structural similarities to other known defensins support its probable role as an antimicrobial molecule in the rat small intestine. However, due to the high degree of homology to mouse cryptdin 3, we speculate that RD-5 may also be multifunctional.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant HL53400 from the National Institutes of Health and by University of Medicine and Dentistry of New Jersey Foundation grant RA3619.
We thank Andre Ouellette for supplying the mouse cryptdin 1 cDNA probe and Michael Selsted and Charles Bevins for their helpful comments.
REFERENCES
- 1.Berg R D, Garllington A W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevins C L, Diamond G. Molecular biological strategies in the analysis of antibiotic peptide gene families. In: Shafer W, editor. Methods in molecular biology: current protocols in antimicrobial peptide research. Totowa, N.J: Humana Press; 1997. pp. 151–166. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–161. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Deckx R J, Vantrappen G R, Parein M M. Localization of lysozyme activity in Paneth cell granule fraction. Biochim Biophys Acta. 1967;139:204–207. doi: 10.1016/0005-2744(67)90136-2. [DOI] [PubMed] [Google Scholar]
- 5.Deitch E A, Kazuyoshi M, Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. J Trauma. 1985;25:385–392. doi: 10.1097/00005373-198505000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Deitch, E. A. Bacterial translocation of the gut flora. J. Trauma 30:S184–S189. [DOI] [PubMed]
- 7.Deitch E A, Xu D, Franko L, Ayala A, Chaudry I H. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–146. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Diamond G, Bevins C L. Molecule of the month. β-Defensins: Endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998;88:221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- 9.Duncan H E, Edberg S C. Host-microbe interaction in the gastrointestinal tract. Crit Rev Microbiol. 1995;21:85–100. doi: 10.3109/10408419509113535. [DOI] [PubMed] [Google Scholar]
- 10.Eisnhauer P B, Harwig S S S, Lehrer R I. Cryptdins: antimicrobial defensin of the murine small intestine. Infect Immun. 1992;60:3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz T, Rayner J R, Valore E V, Tumolo A, Talmadge K, Fuller F. The structure of the rabbit macrophage defensin genes and their organ-specific expression. J Immunol. 1989;143:1356–1358. [PubMed] [Google Scholar]
- 12.Ganz T, Lehrer R I. Defensins. Curr Opin Immunol. 1989;6:584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 13.Gordon J I. Intestinal epithelial cell differentiation: new insights form chimeric and transgenic mice. J Cell Biol. 1989;108:1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon J I, Schmidt G H, Roth K A. Studies of intestinal stem cells using normal, chimeric, and transgenic mice. FASEB J. 1992;6:3039–3050. doi: 10.1096/fasebj.6.12.1521737. [DOI] [PubMed] [Google Scholar]
- 15.Harwig S S L, Tan L, Qu X, Cho Y, Eisenhauer P B, Lehrer R I. Bactericidal properties of murine intestinal phospholipase A2. J Clin Investig. 1995;95:603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertzog A J. The Paneth cell. Am J Pathol. 1937;13:351–358. [PMC free article] [PubMed] [Google Scholar]
- 17.Hierholzer C, Kalff J C, Omeert L, Tsukada K, Loeffert J E, Loatkins S C, Billiar T R, Tweardy D J. Interleukin-6 production in hemorrhagic shock is accompanied by neutrophil recruitment and lung injury. Am J Physiol. 1998;275:L611–L621. doi: 10.1152/ajplung.1998.275.3.L611. [DOI] [PubMed] [Google Scholar]
- 18.Jones D E, Bevins C L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 19.Keshav S, Lawson L, Chung L P, Stein M, Perry V H, Gordon S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J Exp Med. 1990;171:327–332. doi: 10.1084/jem.171.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozrol J M, Rush B F, Smith S M, Machiedo G W. Occurrence of bacteria during and after hemorrhagic shock. J Trauma. 1988;28:10–15. [PubMed] [Google Scholar]
- 22.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 23.Lehrer R I, Daher K, Ganz T, Selsted M E. Direct inactivation of viruses by MCP-1 and MCP-2 natural peptide antibiotics from rabbit leukocytes. J Virol. 1985;54:467–472. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lencer W I, Cheung G, Strohmeier G R, Currie M G, Ouellette A J, Selsted M E, Madara J L. Induction of epithelial chloride secretion by channel-forming cryptdins 2 and 3. Proc Natl Acad Sci USA. 1997;94:8585–8589. doi: 10.1073/pnas.94.16.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy O, Ooi C E, Weiss J, Lehrer R I, Elsbach P. Individual and synergistic effects of rabbit granulocyte proteins on Escherichia coli. J Clin Investig. 1994;94:672–682. doi: 10.1172/JCI117384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtenstein A K, Ganz T, Selsted M E, Lehrer R I. Synergistic cytolysis mediated by hydrogen peroxide combined with peptide defensins. Cell Immunol. 1988;114:104–116. doi: 10.1016/0008-8749(88)90258-4. [DOI] [PubMed] [Google Scholar]
- 27.McLeod R J, Hamilton J R, Bateman A, Belcourt D, Hu J, Bennet H P J, Solomon S. Corticostatic peptides cause nifedipine-sensitive volume reduction in jejunal villus enterocytes. Proc Natl Acad Sci USA. 1991;88:552–556. doi: 10.1073/pnas.88.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molmenti E P, Perlmutter D H, Rubin D C. Cell-specific expression of α1-antitrypsin in human intestinal epithelium. J Clin Investig. 1993;92:2022–2034. doi: 10.1172/JCI116797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy C J, Foster B A, Mannis M J, Selsted M E, Reid T W. Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol. 1993;155:408–413. doi: 10.1002/jcp.1041550223. [DOI] [PubMed] [Google Scholar]
- 30.Nagaoka I, Someya A, Iwabuchi K, Yamashita T. Characterization of cDNA clones encoding guinea pig neutrophil cationic peptides. FEBS Lett. 1991;280:287–291. doi: 10.1016/0014-5793(91)80314-s. [DOI] [PubMed] [Google Scholar]
- 31.Nevalainen T J, Gronroos J M, Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Investig. 1995;72:201–208. [PubMed] [Google Scholar]
- 32.Nygaard S C, Ganz T, Peterson M W. Defensins reduce the barrier integrity of a cultured epithelial monolayer without cytotoxicity. Am J Respir Cell Mol Biol. 1993;8:193–200. doi: 10.1165/ajrcmb/8.2.193. [DOI] [PubMed] [Google Scholar]
- 33.Okrent D G, Lichtenstein A K, Ganz T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cell and endothelial cells. Am Rev Respir Dis. 1990;141:179–185. doi: 10.1164/ajrccm/141.1.179. [DOI] [PubMed] [Google Scholar]
- 34.Ouellette A J, Lualdi J C. A novel mouse gene family coding for cationic, cysteine-rich peptides. J Biol Chem. 1990;265:9831–9837. [PubMed] [Google Scholar]
- 35.Ouellette A J, Greco R M, James M, Frederick D, Naftilan J, Fallon J T. Developmental regulation of cryptdin a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989;108:1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouellette A J, Hsieh M M, Nosek M T, Cano-Gauci D F, Huttner K M, Buick R N, Selsted M E. Mouse Paneth cell defensins: primary structures and antimicrobial activities of numerous cryptdin isoforms. Infect Immun. 1994;62:5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouellette A J. Paneth cells and innate immunity in the crypt microenvironment. Gastroenterology. 1997;113:1779–1784. doi: 10.1053/gast.1997.v113.pm9352884. [DOI] [PubMed] [Google Scholar]
- 38.Ouellette A J, Selsted M E. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 39.Peeters T, Vantrappen G. The Paneth cell: a source of intestinal lysozyme. Gut. 1975;16:553–558. doi: 10.1136/gut.16.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter E M, van Dam E, Valore E V, Ganz T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun. 1997;65:2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter E M, Liu L, Oren A, Anton P A, Ganz T. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun. 1997;65:2389–2395. doi: 10.1128/iai.65.6.2389-2395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu X-D, Lloyd K C K, Walsh J H, Lehrer R I. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect Immun. 1996;64:5161–5165. doi: 10.1128/iai.64.12.5161-5165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rush B F, Sori A J, Murphy T F, Smith S, Flanagan J J, Machiedo G W. Endotoxemia and bacteremia during hemorrhagic shock: the link between trauma and sepsis? Ann Surg. 1988;207:549–554. doi: 10.1097/00000658-198805000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salzman N H, Polin R A, Harris M C, Ruchelli E, Hebra A, Zirin-Butler S, Jawad A, Porter E M, Bevins C L. Enteric defensin expression in necrotizing enterocolitis. Pediatr Res. 1998;44:20–26. doi: 10.1203/00006450-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satoh Y. Effect of live and heat-killed bacteria on the secretory activity of Paneth cells in germ-free mice. Cell Tissue Res. 1988;251:87–93. doi: 10.1007/BF00215451. [DOI] [PubMed] [Google Scholar]
- 47.Satoh Y, Ishikawa K, Tamaka H, Ono K. Immunohistochemical observations of immunoglobulin A in the Paneth cell of germ-free and formerly-germ-free rats. Histochemistry. 1986;85:197–201. doi: 10.1007/BF00494804. [DOI] [PubMed] [Google Scholar]
- 48.Selsted M E, Harwig S S L. Determination of the disulfide array in the human defensin HNP-2. A covalently cyclized peptide. J Biol Chem. 1989;264:4003–4007. [PubMed] [Google Scholar]
- 49.Selsted M E, Harwig S S L, Ganz T, Schilling J W, Lehrer R I. Primary structures of three human neutrophil defensin. J Clin Investig. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selsted M E, Miller S I, Henschen A H, Ouellette A J. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992;118:929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selsted M E, Brown D M, DeLange R J, Harwig S S, Lehrer R L. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985;260:4579–4584. [PubMed] [Google Scholar]
- 52.Senegas-Balas F, Balas D, Verger R, de Caro A, Figarello C, Ferrato F, Lechene P, Pertrand C, Ribet A. Immunohistochemical localization of intestinal phospholipase A2 in rat Paneth cell. Histochemistry. 1984;81:581–584. doi: 10.1007/BF00489538. [DOI] [PubMed] [Google Scholar]
- 53.Territo M C, Ganz T, Selsted M E, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Investig. 1989;89:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trier J S. Pattern of secretion by Paneth cells of the small intestine of mice. J Cell Biol. 1963;18:599–620. doi: 10.1083/jcb.18.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tulzo Y L, Shenkar R, Kaneko D, Moine P, Fantazzi G, Dinarello C A, Abraham E. Hemorrhage increases cytokine expression in lung mononucleated cell in mice. J Clin Investig. 1997;99:1516–1524. doi: 10.1172/JCI119314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wetering S, Mannesse-Lazeroms S P G, Dijkman J H, Hiemstra P S. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J Leukoc Biol. 1997;62:217–226. doi: 10.1002/jlb.62.2.217. [DOI] [PubMed] [Google Scholar]
- 57.Young W S, Mezey E, Siegel R E. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986;70:198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]