Abstract
Radiotherapy is indispensable in clinical cancer treatment, but because both tumor and normal tissues have similar sensitivity to X-rays, their clinical curative effect is intrinsically limited. Advanced nanomaterials and nanotechnologies have been developed for radiotherapy sensitization, typically employing high atomic number (high-Z) materials to enhance the energy deposition of X-rays in tumor tissues, but the efficiency is largely limited by the toxicity of heavy metals. A new and promising approach for radiosensitization is catalytic radiosensitization, which takes advantage of the catalytic activity of nanomaterials triggered by radiation. The efficiency of catalytic radiosensitization can be greatly enhanced by electron modulation and energy conversion of nanocatalysts upon X-ray irradiation, further enhancing the clinical curative effect. In this review, we highlight the challenges and opportunities in cancer radiosensitization, discuss novel approaches to catalytic radiosensitization, and finally describe the development of catalytic radiosensitization based on an in-depth understanding of radio-nano interactions and catalysis–biological interactions.
Keywords: Catalytic radiosensitization, Electron modulation, Energy conversion, Radiotherapy, Nanomaterials
Introduction
Radiotherapy (RT) plays an irreplaceable role in the current treatment of most solid malignancies [1]. Generally, its therapeutic effect is positively correlated with the radiation dose, but side effects can also be aggravated. As a local treatment method, RT seriously threatens the vital organs near the tumor sites with exposure to X-ray radiation, which limits the maximum tolerated dose of the human body. In addition, some malignancies are not as sensitive to RT as other normal tissues, which also contributes to low RT efficiency [2]. Thus, radiosensitization is urgently needed to improve the efficacy of RT [3–6].
Various strategies have been developed to enhance the radiosensitivity of tumor cells [7,8]. Depending on the interaction process and mechanism, they can be generally divided into physical enhancement, chemical enhancement and biological enhancement [9,10]. Catalytic radiosensitization is an emerging chemical enhancement strategy that has shown great potential in cancer RT. Unlike traditional physical enhancement, which only focuses on the physical process of X-ray absorption and energy deposition by high atomic number (high-Z) nanoparticles [11,12], catalytic radiosensitization focuses on catalytic reactions and their chemical products [13]. Here, catalytic radiosensitization is defined as the design and construction of nanocatalysts to purposefully initiate or enhance specific chemical reactions that are induced by X-ray radiation to generate active species or molecules with therapeutic functions, thereby improving the efficacy of RT. The concept of catalytic radiosensitization has not only created a new space for further enhancement of RT but also made more opportunities for bioapplication of various nanocatalysts.
Benefiting from the development of heterogeneous catalysis by nanomaterials, a considerable number of nanomaterials can serve as catalytic sensitizers, such as heterostructures [13], semiconductors [14], defective materials [15] and metal organic frameworks (MOFs) [16,17]. Their physical and chemical properties, such as size, morphology, structure, crystal face, chemical state, and surface ligands, can greatly influence catalytic activities under X-ray irradiation [18,19]. In addition to the catalytic reactions directly induced by X-ray radiation on nanocatalysts, secondary electrons or fluorescence photons emitted from high-Z materials under X-rays can also serve as an excitation source to enhance the catalytic activity of nanocatalysts [20]. With this in mind, more catalytic materials, not limited to high-Z materials, such as inorganic semiconductors and organic photosensitizers, etc., can be designed for catalytic radiosensitization as long as their catalytic activity can be excited by low-energy UV–Vis photons or electrons. Therefore, the design of catalytic materials or platforms is particularly important in catalytic radiosensitization.
In recent years, a series of nanomaterials and platforms have been ingeniously designed to induce catalytic reactions or enhance the kinetic rate of catalytic reactions under X-ray irradiation. However, a systematic classification and summary of these systems is still lacking. Herein, we attempt to present the progress of this emerging field from the perspective of materials science. From the perspective of the mechanism, we divide catalytic radiosensitization into two categories, electron modulation and X-ray energy conversion (Fig. 1), which are discussed in detail in this review. First, we present a brief overview of the background of the field, including the fundamentals of the physical interaction between X-rays and nanoparticles, as well as the catalytic potential of nanoparticles under X-ray irradiation. Then, the progress of research in catalytic radiosensitization is discussed. Finally, as a nascent area, prospects for development in the future and key issues in catalytic radiosensitization are considered.
FIGURE 1.
Overview of catalytic radiosensitization based on electron modulation and energy conversion.
Physical interactions between nanomaterials and X-rays
Catalytic radiosensitization of nanomaterials cannot occur independently but depends on the process and products of the physical interaction of materials under X-rays. Therefore, it is necessary to introduce the physical interaction between X-rays and nanomaterials first.
A series of physical reactions, including the photoelectric effect, Auger effect, and Compton scattering, can occur upon incident X-ray irradiation on nanoparticles, resulting in electrons, secondary X-rays, and fluorescence emission [21] (Fig. 2). According to the energy and effective distance, the freed electrons can be divided into low-energy and high-energy electrons. Low-energy electrons function primarily within 10 nm from the particles, while high-energy electrons can move farther [22–24]. Photoelectrons produced by X-ray irradiation of heavy atoms typically carry kinetic energies in the keV range and can deposit their energies over distances from few micrometers to millimeters from the heavy atom from which they were freed, depending on the energies of the incident X-rays, the types of nanoparticles and the surroundings [25]. Considering that the probability of photoelectrons generation upon the interaction of elements with X-rays is directly proportional to the atomic number but inversely proportional to the energies of X-rays, high-Z nanoparticles such as Au (Z = 79) can be used to increase the number of photoelectrons generated in biological tissue upon X-ray irradiation (Zaverage = 7.4) [12,26]. If the photoelectron is freed from a lower atomic orbital, an electron in a higher orbital can drop to the newly vacant spot, referred to as a “hole”. However, it needs to release excess energy, which is the energy difference between the higher and lower orbitals. The excess energy may simply result in fluorescent photon emission, or it may ionize an electron in a higher orbital, which is referred to as an Auger electron. This process can propagate to higher orbitals that have lower ionization energies, such as M, N, and valence shells. Since Auger electrons have relatively low energies, they can only travel short distances, within tens of nanometers, and therefore deposit energies only near the surface of nanoparticles [27,28]. Compton electrons are produced by Compton scattering, which describes the basic interaction of a single photon and a free or weakly bound electron. The process is inelastic and energetic X-rays lose a portion of their incident energy to the electron [29]. Fluorescence emission can occur from scintillation nanoparticles under X-rays. The orbital electrons of the atoms in the scintillator obtain energy greater than their forbidden band gap energy from X-rays and are excited to gain transit to the conduction band. After a series of physical processes, it returns to the ground state and emits fluorescence light with a short decay time (approximately 10 ns) or longer phosphorescence light depending on the mechanism of de-excitation. Since the probability of X-ray absorption is proportional to the electron density, high-Z nanoparticles have essential in physical enhancement [30–32]. A series of high-Z nanoparticles have been used as RT sensitizers, including noble metals (Au, Ag, Pt, etc.), rare earth elements (Gd, Ho, Tm, etc.), and other heavy elements (Hf, Ta, W, Bi, etc.) [33–36].
FIGURE 2.

Physical interaction of X-rays with high-Z nanoparticles.
However, physical enhancement alone can hardly achieve a desired therapeutic effect due to the relatively low conversion rate from physical enhancement products to therapeutically active species such as hydroxyl radicals (▪OH) and singlet oxygen (1O2). Because the chemical reaction energy barrier for free radical generation can be greatly reduced by catalysts, one of the advantages of catalytic radiosensitization over physical enhancement is that it can greatly improve the yield of free radicals in RT [13]. The reactivity of catalysts can be regulated by the electronic state and local electron density of the materials, so electrons emission during the physical enhancement process is expected to increase the electrons density of catalysts, thereby improving their catalytic activities when irradiated by X-rays. Fluorescence emission in the process of physical enhancement can also be used to excite photosensitizers or semiconductor catalysts for photocatalytic reactions. Therefore, physical enhancement provides crucial support for catalytic reactions in two ways: electron regulation in nanocatalysts and energy conversion from energic X-rays to low-energy fluorescence photons.
Catalytic effect in X-ray-nanoparticle interactions
The first clue to reveal the effect of a catalytic reaction in RT enhancement was reported by Guo et al. in 2007 when they investigated DNA damage with 3 nm Au nanoparticles [22]. The result demonstrated that the detected enhancement was 5 times higher than the theoretical physical enhancement, indicating that there were other processes contributing to the enhancement. In 2011, Misawa et al. attributed the increase generation of superoxide radicals under X-rays to the catalytic effect of Au nanoparticles [37]. In 2012, the chemical enhancement concept was proposed by Guo et al., who discovered a significantly increased yield of ▪OH in the presence of Au nanoparticles at only ~10 ppm, which is too low a content to produce measurable physical enhancement [19]. The enhancement disappeared completely when the surface of Au nanoparticles was covered by SiO2, implying the surface catalysis of Au nanoparticles under X-ray irradiation. The concept of catalytic radiosensitization was formally introduced for the first time in 2020 by Bu et al. [13]. In this study, Janus-like Au–Fe2C nanoheterostructures were designed and synthesized, aiming to increase the Au surface charge densities, further enhancing their catalytic activity of producing ▪OH under X-ray irradiation. The Au–Fe2C heterostructures yielded 5 times more ▪OH than pure Au nanoparticles at the same concentration under 6 MeV X-rays. The concept of catalytic radiosensitization provides a direction and basis for the rational design of catalytic sensitizers.
It is well known that one of the catalytic mechanisms in chemical reactions is to facilitate electron transfer [38,39]. Therefore, the electronic state and surface charge densities of catalysts are closely related to their catalytic activities. In particular, increasing the catalyst’s local electron density is an important method to enhance catalytic activity. Electrons that are ionized during RT provide a sufficient electron source for the electronic regulation of the catalyst. For instance, a low-state catalytic reaction center such as Fe(II) can be recovered by absorbing low-energy electrons, leading to continuous catalytic activity in Fenton or Fenton-like reactions [20]. In addition, through heterostructure design, the local charge densities can be controlled in a planned way to enhance their catalytic activities in reactions such as water radiolysis [13,15], which also plays important role in catalytic radiosensitization. As an example, nanoheterostructures combining two materials that have different Fermi levels and work functions can lead to directional advancement of electrons from low-Fermi level materials to high-Fermi level materials [13]. In addition, vacancy defects or heteroatom doping can also tune the local electronic structures of catalysts [15].
Catalytic reactions excited by external fields have the advantages in controllability of space and time, which are important for controlling tumor therapies such as photodynamic therapy and ultrasonic piezodynamic therapy [40,41]. However, very few catalysts can match the energy levels of high-energy X-rays in RT, so energy conversion is essential [42]. High-energy X-rays can be converted into low-energy UV–Vis photons by functional materials such as scintillation crystals, which in turn stimulate the photocatalytic reaction of photosensitizers or semiconductors to generate ROS [43,44]. In the following section, the application and contribution of electron modulation and energy conversion strategies in tumor catalytic radiosensitization are discussed with specific cases.
Catalytic radiosensitization
Catalytic radiosensitization based on electron modulation
According to the electron theory of catalysis on semiconductors [45], catalysis can be described as a process of electron transfer between reactants and the surface of a catalyst. Free electrons in the conduction band or holes in the valence band of semiconductors can be used as catalysts that are responsible for this transfer task. Electron theory also clarifies the relationship between the band structure of materials with electrical properties, the nature of adsorption bonds, and catalytic activity, which affects the reaction activity and selectivity by adjusting the Fermi level [46] on the surface or changing the density of electron–hole pairs. In recent years, fierce sparks have continued to collide between catalysis and biomedicine [47–49]. Meanwhile, utilizing nanomaterials for RT sensitization has attracted increasing attention [50–52], and the synthesis methods or performance control strategies have also been developed rapidly. Previous strategies have mainly focused on the adjustment of morphology and geometric structure characteristics [53,54], but unfortunately, these strategies might not meet the needs for the efficient production of reactive species for radiosensitization. By constructing abundant active sites and adjusting the electronic structure through surface design [55,56], the efficiency of a catalytic reaction can be greatly improved, bringing us closer to the perfect integration of catalysis and tumor radiosensitization. In this section, we discuss research progress in the electron regulation of catalytic radiosensitization in detail, including defect construction, heterostructure design and valence transition (Table 1).
TABLE 1.
A summary of nanocatalysts based on electron modulation for catalytic radiosensitization.
| Material types | Nanocatalysts | Mechanism | Products | Ref. |
|---|---|---|---|---|
| Defective materials | BiOCl nanocrystals | Bi vacancies to construct confined H2O | ▪OH | [15] |
| BiO2−x nanosheets | Oxygen vacancies enhance electrons transfer | [69] | ||
| Heterostructure | Au–Fe2C heterostructure | Local enrichment of electrons promotes H2O dissociation | ▪OH | [13] |
| Au–Bi2S3 heterostructure | Fermi level formation enables efficient charge separation | ▪OH | [81] | |
| BiOI@Bi2S3 | Interfacial interactions separate electron-hole pairs | [82] | ||
| WO2.9–WSe2–PEG | [83] | |||
| BiNPs/graphene oxide | Establishment of intrinsic electric field | ▪OH | [88] | |
| Valance transition materials | SnS2@Fe3O4 NPs | Electron transfer enhanced Fe3+/Fe2+ cycling | ▪OH | [99] |
| Cu2(OH)PO4 nanocrystals | X-ray-enhanced Cu2+/Cu+ cycling | ▪OH | [101] | |
| 125I–TiO2 nanoparticles | Reducing Ti4+ to Ti3+ creates active centers | ▪OH | [102] | |
| Antimonene nanoparticles | Reducing Sb5+ to Sb3+ forms toxic Sb2O3 | Sb2O3 | [103] |
Defect construction
During catalytic reactions, not only does a catalyst experience adsorption and desorption of various species on its surface, it also experiences electron transfer and thus a change in the local electronic structure [57,58]. The electronic structure of a catalyst’s surface has a great influence on its catalytic performance. To this end, various surface modifications have been exploited to regulate the electronic structure of the surface of given catalysts, in the hope of improving their catalytic activity. Among them, defect engineering is a significantly effective strategy [59]. It can accurately control the surface composition on the atomic scale, thus building a local microenvironment for catalysts and adjusting the electronic structure of nanomaterials [60,61]. Due to the change in electronic structure or local density, the activation energy required for catalytic reactions is critically reduced, thereby promoting catalytic activities. Improving catalytic performance inevitably leads to a better radiosensitization effect.
RT, as a conventional method for clinical tumor treatment, utilizes high-energy X-ray radiation to decompose H2O molecules to produce cytotoxic ▪OH [62]. However, owing to the low linear energy transfer density of X-rays, the interaction between H2O molecules and X-rays is weak. Additionally, the energy required to break down H2O molecules is relatively high, which leads to unsatisfactory ▪OH yields. Therefore, reducing the bond energy of the O–H bond in H2O molecules contributes to the catalysis of H2O molecules to produce toxic ▪OH [63,64], and then realize desirable catalytic radiosensitization efficiency. Fortunately, the rapid development of nanomaterials has brought new opportunities for activating H2O molecules. Nanocatalysts can adsorb H2O molecules and distort their structure through surface defect sites (such as vacancies, valence–variable atoms, steps and impurity atoms), thereby favoring the dissociation of H2O molecules [65–67]. Accordingly, Bu et al. synthesized BiOCl nanocrystals with Bi vacancies (BvNCs) to con-fine H2O molecules in situ and further employed them as radiosensitizers to realize efficient catalysis of H2O molecules [15] (Fig. 3a). Under X-ray irradiation, confined H2O molecules with higher activity were more easily ionized and catalyzed into free radicals than unconfined H2O molecules. Based on density functional theory (DFT), the O–H bond lengths of H2O molecules confined by VBi in BvNCs were 0.983 Å on one end and 0.909 Å on the other. Meanwhile, the bond lengths of H2O molecules without VBi were 0.978 Å and 0.977 Å (Fig. 3b), which indicated the existence of confined H2O molecules on the surface of BvNCS. In addition, the dissociation energy of BvNCs surface H2O molecules (2.68 eV) was also lower than that of BNCs (3.11 eV), which showed that VBi-constructed confined H2O molecules had twisted structures and reduced dissociation energies (Fig. 3c). Taken altogether, these evidences suggest that the catalytic reaction of H2O molecules becomes easy in the presence of Bi vacancies. What’s more, Fig. 3d indicated that BvNCs could induce the lowest colony formation rate. The strategy of using defects to construct confined H2O molecules to improve catalytic radiosensitization greatly expands the biomedical applications of nanocatalysts.
FIGURE 3.

(a) Schematic of the mechanism of defect construction mediated catalytic radiosensitization. (b) Simulated O–H bond length of H2O on the surface of perfect (001) facet (left) and in the presence of VBi (right). (c) Dissociation energy of H2O on perfect (001) facet (left) and in the presence of VBi (right). (d) Cell survival assay by using colony formation evaluation. Reproduced with permission [15]. Copyright 2021, Elsevier Ltd.
In addition to modifying H2O to make ROS production energetically easier, efficient regulation of electron–hole pairs in nanomaterials are also beneficial to the generation of ROS. For example, the formation of oxygen vacancies usually introduces intermediate energy levels [68]. Under the action of X-rays, electrons are first be transferred to these intermediate energy levels, after which they can be transferred to either the conduction band or oxygen, thereby generating more ROS. ROS produced in this way may enhance the sensitization of catalytic reaction-mediated RT. For this, Zhao et al. designed and synthesized an ultrathin defect-abundant bismuth oxide nanosheet (BiO2−x) [69], in which the oxygen vacancies could act as traps, allowing electrons to be easily transferred to O2 and formed . Moreover, oxygen vacancies endowed BiO2−x with catalase activity, which could catalyze H2O2 into O2 in the tumor microenvironment to relieve tumor hypoxia and increase the efficiency of RT. To summarize, the effective transfer of electrons from the surface of nanomaterials exposed to incident X-ray irradiation can be regulated by oxygen vacancies, thereby enhancing catalytic efficiency and contributing to efficient catalytic radiosensitization.
Heterostructure design
By matching the special band structure of noble metals and other semiconductor nanomaterials, heterostructures, such as Janus-like nanoparticles [70], satellite-like nanoparticles [71] and dumbbell-like nanoparticles [72], can be designed to induce electron transfer and rearrangement across the interface [73,74]. They effectively adjust the interfacial electronic structure and improve the efficiency of electron transfer and carrier separation [75,76]. Many studies have shown that heterostructures exhibit enhanced physical and chemical properties, which display significant potential in catalytic radiosensitization [77].
Regarding catalytic radiosensitization, the design of catalysts is the key to improving the efficacy of RT. The structural design of heterostructures can be used to efficiently manipulate the local charge density on the surface and interface, endowing materials with highly catalytically active sites [78]. Au nanoparticles are commonly used as RT sensitizers. To obtain Au nanoparticles with a high local charge density on their surface, which is favorable for increasing the conversion rate of activated H2O to ▪OH, Bu et al. selected a Janus-like Au–Fe2C nanocatalyst to investigate its catalytic radiosensitization effect [13] (Fig. 4a). As shown in Fig. 4b, Au–Fe2C heterostructure was composed of Au (approximately 9 nm) and Fe2C moiety (approximately 13 nm). The lattice spacings of 2.35 Å and 2.12 Å also corresponded to the plane of Au (1 1 1) and Fe2C (−1 0 1) respectively (Fig. 4c). Besides, theoretical simulations demonstrated that the difference in the work functions and Fermi levels of Au and Fe2C resulted in the local enrichment of electrons on the hetero-interface and Au surface (Fig. 4d, e). Consequently, this massive enrichment of electrons enhanced the production of H2O+ and H2O*. Then, H2O+ and H2O* could be effectively converted into ▪OH with X-ray irradiation. Increasing the charge density on the surface of Au through heterostructure construction indeed improved the catalytic conversion of H2O under X-rays, which was beneficial to the development of catalytic radiosensitization.
FIGURE 4.

(a) Schematic of AFCNPs heterostructure induced catalytic radiosensitization under X-ray irradiation. (b) TEM images of AFCNPs. (c) HRTEM images of the AFCNPs. Ground-state DFT method for calculating the work functions of (d) Au (1 1 1) and (e) Fe2C (−1 0 1) planes. Reproduced with permission [13]. Copyright 2021, Elsevier Ltd. (f) Schematic of X-ray-induced catalytic progress based on the Schottkytype heterostructures Au–Bi2S3 for enhancing free radical generation. (g) Cytotoxicity assay of heterostructures Au–Bi2S3 under X-rays with different treatments in HeLa cells. Reproduced with permission [81]. Copyright 2019, American Chemical Society.
Similar to photocatalysis, X-ray-induced electron–hole pairs may also be generated within a few femtoseconds. The reaction time between reactants and carriers varies from nanoseconds to microseconds, while the carriers recombine in picoseconds to nanoseconds. Therefore, improving the separation efficiency of carriers is extremely important to improve the catalytic reaction efficiency. Effective separation and transmission of electrons and holes can be achieved through electronic structure control. However, many semiconductors have narrow bandgaps, which results in easier recombination of electron–hole pairs and greatly reduces the generation efficiency of free radicals [79,80]. In view of this, Zhao et al. developed a Schottky-type heterostructure of Au–Bi2S3 to realize efficient carrier separation [81]. In principle, the Schottky barrier created between Au and Bi2S3 could trap low-energy electrons generated by X-rays. Then, electrons would transfer from Bi2S3 to Au until the Fermi levels of Au and Bi2S3 reached equilibrium, thus forming a new Fermi level and achieving effective charge separation (Fig. 4f). Due to the matched energy level, electrons transferred to Au were captured by H2O2 and then catalyzed into highly toxic ▪OH. Under X-ray irradiation, Au–Bi2S3 resulted in an obvious decline of cell viability to 8.68% on HeLa cells (Fig. 4g). The generation of ROS in this process could be completely oxygen-independent, indicating significant potential for heterostructure nanomaterials to shine in the treatment of hypoxic tumors.
In addition to establishing new Fermi levels, heterostructures can also trigger electron transfer through interfacial interactions. Under X-ray irradiation, the heterostructure formed by two semiconductors are both excited to generate corresponding electrons and holes. Based on the close interfacial interactions, the changes in flow direction of electrons and holes not only increase the concentration of electrons and holes but also achieve high separation efficiency of reductive electrons and oxidative holes. Therefore, efficient separation of electron–hole pairs greatly improve the reaction efficiency of catalyzing H2O and O2 to ROS, which naturally promotes catalytic radiosensitization.
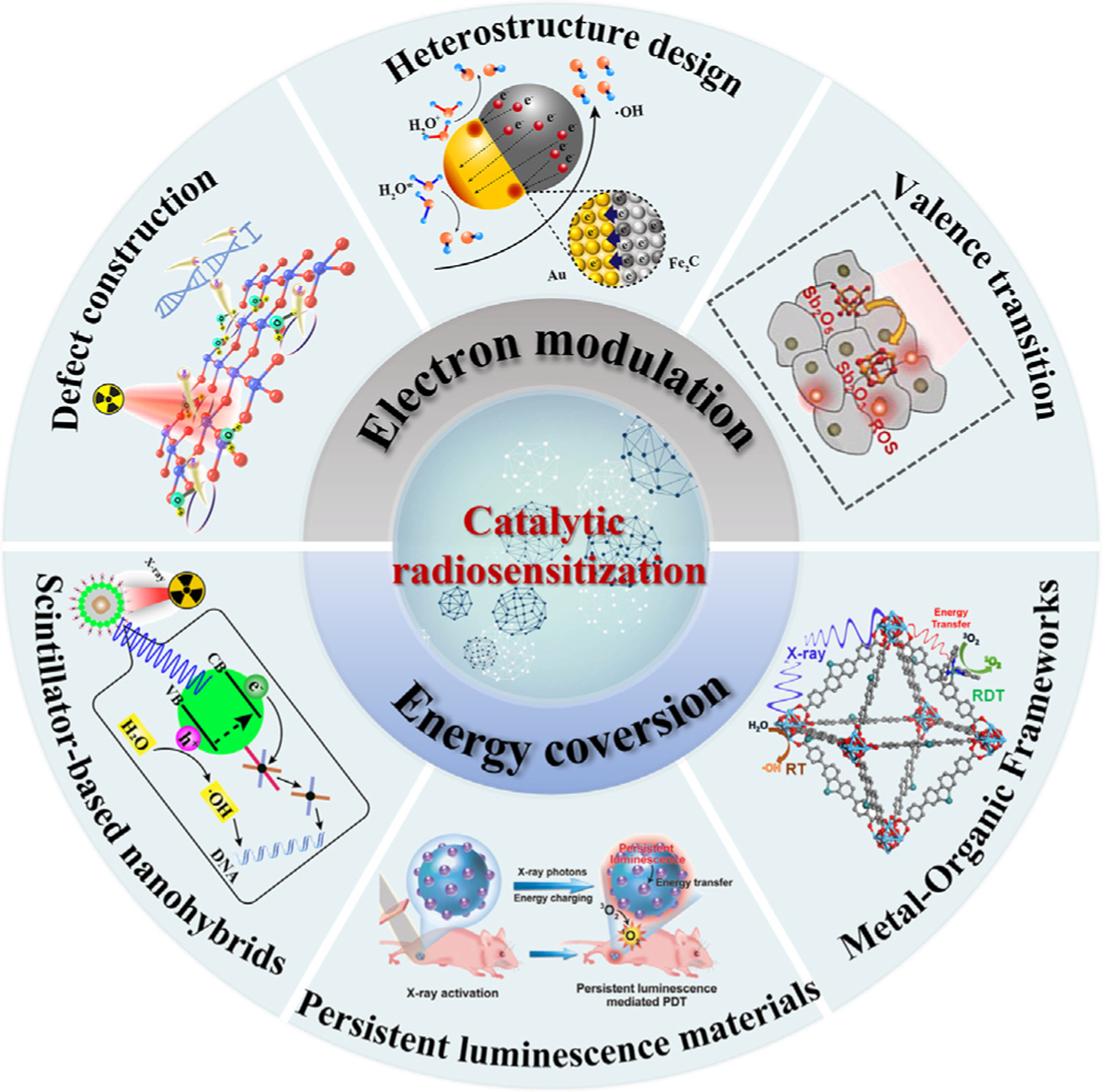
Zhao et al. developed a new type of nanodiagnostic material based on a semiconductor heterostructure (BiOI@Bi2S3) to obtain a higher separation efficiency of electron–hole pairs [82]. In particular, due to the interfacial interaction of the heterostructures, X-ray-induced electrons were easily transferred from the lowest conduction band of Bi2S3 to the lowest conduction band of BiOI, while the holes at the valence band top of the Bi2S3 moved in the opposite direction (Fig. 5a). Therefore, the electrons and holes were separated effectively to catalyze H2O and O2 into highly reactive ▪OH and . As shown in Fig. 5b, with X-ray irradiation, the fluorescence intensity of BiOI@Bi2S3 heterojunction was obviously increased compared to other groups, indicating the efficient ROS generation ability of BiOI@Bi2S3 heterostructure. Consistent with the above assay results, under X-ray irradiation, DNA double-strand damage was remarkably enhanced by BiOI@Bi2S3 (Fig. 5c), suggesting its strong radiotherapy enhancement effect. Similarly, Zhao et al. further designed and synthesized WO2.9-WSe2-PEG nanoparticles with a semiconductor heterojunction structure to achieve enhanced antitumor therapy [83]. The interfacial interaction between WO2.9 and WSe2 made it easy for electrons to transfer from the lowest conduction band of WO2.9 to WSe2. The holes left on the highest valence band of WO2.9 run in the opposite direction, resulting in effective separation of electron–hole pairs. In contrast to Bi2S3, due to the matching redox potential of WSe2 and H2O2, the electrons in WSe2 could immediately react with the overexpressed H2O2 in the tumor to generate ▪OH, thus realizing a highly effective catalytic reaction induced by X-rays.
FIGURE 5.
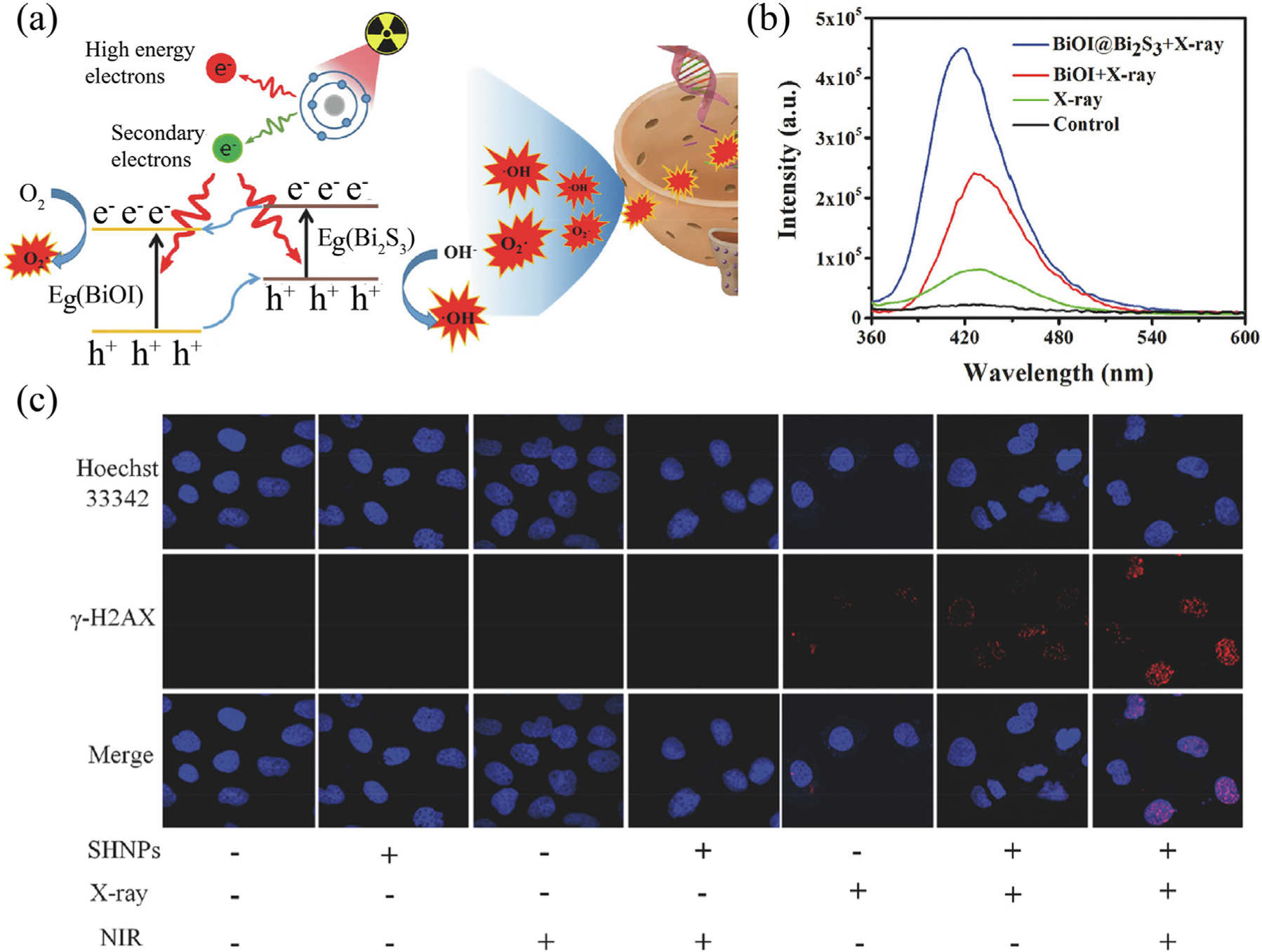
(a) Schematic of BiOI@Bi2S3 heterostructure nanoparticles-induced electron transfer under X-ray irradiation for enhancement of catalytic radiosensitization. (b) Fluorescence spectra of ROS detection by sodium terephthalate. (c) γ-H2AX-stained BEL-7402 cells under different treatments. Reproduced with permission [82]. Copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
In addition, Zhao et al. developed BiP5W30 nanoclusters and reduced graphene oxide (rGO) heterostructures (PVP-PG) [84], which facilitated electron–hole separation to enhance the generation of ROS. Under X-ray irradiation, BiP5W30 absorbed energy to excite electrons from the HOMO to the LUMO, which then moved to rGO, with holes left behind. Through theoretical calculations, the LUMO level of PVP-PG was lower than the potential energy of but higher than that of H2O2/▪OH. Therefore, the electrons generated by X-rays could be selectively captured by H2O2 to generate ▪OH. On the other hand, the HOMO level of PVP-PG was lower than the potential energy of ▪OH/H2O, which also made it easy to catalyze H2O to ▪OH. Furthermore, Tan et al. modified AuPt nanoparticles on the surface of CuS nanosheets (AuPt@CuS NSs) to form plasmonic heterostructure with radiocatalysis and radio-photothermal treatment capabilities [85]. The formation of a heterostructure made electron transfer to CuS convenient, which further catalyzed O2 and H2O2 to produce ROS. At the same time, holes were transferred to the reaction site of AuPt through the interface to eliminate glutathione and ultimately enhance the effect of RT. This design can effectively separate electrons and holes generated by X-ray irradiation, and thus cause tumor cell apoptosis. Undoubtedly, optimizing the efficient separation of electron–hole pairs in heterostructures through interfacial interactions provides a new approach for catalytic radiosensitization.
The close phase interface interaction indeed promotes the separation and transfer of the carriers. However, the migration of carriers is mainly driven by the positions of the valence band and conduction band themselves. Specifically, under the action of an intrinsic electric field formed by the hetero-interface, the separation and transfer efficiency of carriers is greatly improved [86,87]. For this reason, Zhao et al. applied in situ growth method to prepare a BiNPs/graphene oxide (GO) heterostructure functionalized with polyvinylpyrrolidone (PVP) to obtain the resulting nanocomposites [88] (PVP-Bi/GO). Since the work function of graphene oxide (4.46 eV) is lower than that of BiNPs (4.94 eV), electrons flow from GO to BiNPs until their Fermi levels equilibrate under X-ray irradiation. This results in the establishment of an intrinsic electric field, which leads to the bending of the energy band of the graphene/metal interface. According to the energy level, electrons migrated to the surface of BiNPs so that O2 and H2O2 were catalyzed into and ▪OH, respectively. Therefore, this is a new way to control electrons by constructing an intrinsic electric field. Actually, the design of intrinsic electric fields for the improvement of catalytic efficiency is still in its infancy, and a deep understanding of intrinsic electric fields is still lacking [89]. Therefore, an exploration of the mechanisms that involve intrinsic electric fields in nanomaterials for the enhancement in catalytic performance can definitely point out a new direction for enhancing catalytic radiosensitization.
Valence transition
During RT, it is remarkable that X-rays can cause the emission of a large number of secondary electrons from the target material. These electrons possess many important functions, such as directly damaging cellular DNA and activating H2O molecules, to name just two [90]. Another important function is to promote the valence transition of transition metal ions, thereby bringing higher catalytic activity to specific reactions [91]. In addition, changing the valence of metal ions through electron regulation also changes the properties of its compounds from low/nontoxicity to high toxicity, thus damaging tumor cells in a controlled manner. Therefore, an important issue in the field is to maximize the utility of these electrons in RT sensitization.
For example, in a Fenton catalytic reaction, electrons can usually reduce high-valence metal ions to low-valence states, such as reducing Fe3+ to Fe2+, and Cu2+ to Cu+, which greatly improves the catalytic activity of the Fenton reaction. Since Bu et al. first proposed the concept of chemodynamic therapy (CDT) based on a Fe-based Fenton reaction in 2016 [92], CDT has developed rapidly and vigorously [93–97]. However, in a heterogeneous Fenton reaction in tumor, the rapid transformation from Fe2+ to Fe3+ has become a major bottleneck [98]. Therefore, utilizing secondary electrons generated by X-rays to improve the conversion of Fe3+ to Fe2+ not only solves the decisive step of the Fenton reaction but also provides a new extension of X-ray-induced catalytic radiosensitization. Based on the above considerations, Bu et al. constructed Fe3+-coordinated Hf-nMOF (Hf-BPY-Fe) to realize full-process catalytic radiosensitization [20]. The secondary electrons generated by X-ray irradiation relax to a low-energy state in the Hf-nMOF pores to form an electron-rich environment. These accumulated electrons contributed to the reduction of Fe3+ to Fe2+ and further promoted the generation of ▪OH in the process of attacking DNA by the Fenton reaction. In addition, Zhao et al. proposed a nanocomposite (SnS2@Fe3O4 NPs) composed of SnS2 nanoplates and Fe3O4 quantum dots to explore X-ray-facilitated redox cycling for catalytic radiosensitization [99] (Fig. 6a). Due to the matching electronic band structure, SnS2 as an electron donor could trigger electron transfer to Fe3O4 quantum dots under X-ray irradiation, which accelerated the conversion of Fe3+ to Fe2+ on the surface of Fe3O4 quantum dots and promoted the regeneration of Fe2+ active centers (Fig. 6b). Subsequently, it catalyzed the overexpressed H2O2 in the tumor to continuously produce ▪OH and therefore caused serious damage to tumor cells.
FIGURE 6.

(a) The mechanism of X-ray-induced valence transition for enhanced CDT and RT of cancer. (b) X-ray-induced the cycling of Fe2+/Fe3+ testified by selective trapping agent (K3Fe(CN)6) of Fe2+. Reproduced with permission [99]. Copyright 2021, Elsevier Ltd. (c) Schematic of an X-ray-triggered valence transition process of AMQDs and AMNSs. XPS spectra of (d) AMQDs and (e) AMNSs by X-ray irradiation (2 Gy). Reproduced with permission [103]. Copyright 2019, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
In addition to the transformation of Fe3+ to Fe2+, copper ions with Fenton-like reaction activity can also catalyze H2O2 to generate ▪OH. Importantly, Cu+ shows a higher reaction rate than Fe2+ during the catalysis of H2O2 into ▪OH [100]. Therefore, it is essential to accelerate the conversion of Cu2+ to Cu+ to acquire higher catalytic activity. Zhao et al. developed Cu2(OH)PO4 nanocrystals as an intelligent radiation sensitizer [101], which, during X-ray irradiation, triggered a Fenton-like reaction as an approach to improve the efficacy of RT. Under exogenous X-ray irradiation, Cu2+ could be reduced to Cu+ through the process of electron transfer, and then catalyzed H2O2 into highly toxic ▪OH through a Fenton-like reaction, finally inducing apoptosis and necrosis of cancer cells.
The valence transition can also create more active sites, and the active sites will stretch the chemical bonds of the reactant molecules, which in turn deforms the molecules and lowers the energy barrier of chemical reactions. Hence, providing abundant electrons to the surface of radioactive catalysts is important to improve the efficiency of the catalytic reaction. Based on this, Bu et al. proposed a novel strategy named catalytic internal radiotherapy (CIRT) and used an 125I-labelled titanium dioxide nanocatalyst (125I-TiO2), to explore the performance of 125I-TiO2-assisted CIRT [102]. Mechanistically, the low-energy Auger electrons generated by 125I could be captured by Ti4+ on the surface of TiO2 to produce Ti3+ active centers. Then, Ti3+ could further activate the adsorption of H2O by reducing the O–H bond energy of H2O molecules adsorbed on the surface of 125I-TiO2. Finally, with the irradiation of γ-rays produced by the nuclear decay of 125I, the yield of ▪OH catalyzed by activated H2O molecules was significantly improved. By constructing defects on nanomaterials to change the electronic structure of H2O molecules, it is easier to catalyze the conversion of H2O molecules into ▪OH under X-ray irradiation, which is a feasible approach to improve the efficacy of RT.
In addition to using oxidative free radicals, the presence of toxic compounds can also cause irreversible damage to tumor cells. The toxicity of tumor drugs containing transition metals is closely related to their valence. By adjusting the metal valence in the compound, such as adjusting Sb5+ to Sb3+ and Pt4+ to Pt2+, a favorable transition from low/nontoxicity to severe toxicity can be achieved. Zhang et al. demonstrated that ultrathin antimonene nanoparticles (AMNPs) and antimonene quantum dots (AMQDs) were radiosensitizers that could achieve high-efficiency therapeutic effects by inducing strong oxidative stress and radiotoxicity [103] (Fig. 6c). X-ray irradiation could easily oxidize the surface of AMNPs and AMQDs to Sb2O5. With the increasing dose of X-rays, Sb2O5 was further transformed into highly toxic Sb2O3 (Fig. 6d, e). This work significantly expanded the application of antimonene as a next-generation radiosensitizer. Additionally, Bu et al. synthesized a LiLuF4:Ce@SiO2@Ag3-PO4@Pt (IV) (LAPNP) composite, in which the prodrug Pt (IV) could receive photogenerated electrons from the incidence of X-rays on Ag3PO4, which played a vital role in promoting the separation of electrons and holes [104]. At the same time, Pt (IV) was reduced to cisplatin, a clinical chemotherapy drug and a recognized radiosensitizer, by absorbing electrons, and cisplatin acted as a synergistic X-ray sensitizer and further improved the curative effect of ▪OH.
Catalytic radiosensitization based on energy conversion
Clinically, the energy of X-rays is usually in the range of ~keV to ~MeV. However, most conventional photosensitizers (PSs) for cancer treatment can only be activated by accepting energy at the ~eV level [105]. At this point, an energy transducer is required to absorb X-ray radiation energy and transfer it to PSs or a semiconductor catalyst to generate cytotoxic ROS [106]. To overcome this mismatch in energy between X-rays and light absorbed by PSs, scintillators or heavy metals are often used to absorb X-rays and convert them into photons with appropriate wavelengths, exhibiting X-ray-excited optical luminescence (XEOL) [107]. Afterwards, the energy is transferred to the PSs. Through careful design of nanomaterials, energy regulation between X-rays and nanomaterials can be realized, and ROS can be efficiently catalyzed from O2 and H2O [108]. What follows is a detailed discussion of the specific applications and development potential of scintillator-based nanohybrids, persistent luminescence nanoparticles and metal–organic frameworks in energy regulation-based catalytic radiosensitization (Table 2).
TABLE 2.
A summary of nanocatalysts based on energy regulation for catalytic radiosensitization.
| Material types | Nanocatalysts | Mechanism | Products | Ref. |
|---|---|---|---|---|
| Scintillator | Tb3+-doped LaF3 | X-rays convert into UV–Vis to activate PS | 1O2 | [114] |
| Eu2+-doped SrAl2O4 | 1O2 | [124] | ||
| CeF3:Gd3+, Tb3+ | [128] | |||
| Eu3+-doped NaGdF4 | X-rays convert into IR to activate PSs | [125] | ||
| Ce3+:LiYF4@SiO2@ZnO | X-rays convert into UV to activate semiconductor | [129] | ||
| LiLuF4:Ce@SiO2Ag3PO4@Pt (IV) | [104] | |||
| Persistence luminescence materials | Zn3Ga2GeO8:Cr3+, Yb3+, Er3+ | X-rays convert into NIR that glows for about 10 min | 1O2 | [143] |
| W(VI)-doped ZnGa2O4:Cr | X-rays convert into Vis that lasts for more than 6 h | 1O2 | [142] | |
| TiO2-Tf-Tc | PET radionuclides-generated Cerenkov radiation to activate semiconductor | [144] | ||
| 89Zr-MNPs/TCPP | 89Zr-generated Cerenkov radiation to activate PS | 1O2 | [145] | |
| 89Zr-Df-PPN | [146] | |||
| 131I-ZGCs-ZnPcC4 | 131I-generated Cerenkov radiation to activate PS | 1O2 | [148] | |
| Metal-Organic Frameworks | Hf-DBB-Ru | Inelastic scattering induced energy transfer to activate PS | 1O2 | [156] |
| Hf-TCPP NMOFs | [151] | |||
| Hf-BPY-Ir | [113] |
Scintillator-based nanohybrids
When particles or rays with adequately high energy pass through a scintillator, their energy is fully absorbed, and then, the excitation or ionization of the scintillator’s molecules and atoms occurs. The excited molecules and atoms relax to the ground state by releasing energy in the form of one or more photons [109]. Benefiting from the strong penetrating ability of X-rays, the use of X-ray-induced photodynamic therapy (X-PDT) [110–113] to replace traditional photodynamic therapy (PDT) has sprung up in recent years. In principle, X-PDT uses energy transducers, usually nanoscintillators, to convert X-rays into photoluminescence, and then initiates RT and PDT [114]. Importantly, nanoscintillators exhibit XEOL after absorbing X-rays, and the generated XEOL can be further absorbed by the PS, promoting the excitation of PS and then generating 1O2 through energy transfer [115–119]. Because of catalytic radiosensitization ability, X-PDT can significantly reduce the radiation dose compared to clinical RT, consequently decreasing radiation damage in normal tissues and improving the ability to treat deep-seated cancers.
In 2006, Chen et al. reported X-ray activatable nanoparticles for the first time [120]. By coupling nanoscintillators with PSs (e.g., porphyrin and MC540), the nanoparticles could activate PSs after absorbing X-rays and then catalyzed O2 to generate 1O2 to kill cancer cells. Since then, various nanoscintillators have been developed to study radiosensitization based on energy regulation [121–123]. Generally, to realize efficient energy transfer from nanoscintillators to PSs, the absorption spectra of PSs should overlap with the XEOL spectra to the greatest extent. Moreover, nanoscintillators should have high luminous efficiency under X-ray irradiation to ensure the yield of 1O2. Rare-earth element-doped nanomaterials are one of the most studied nanoscintillators at present, as they can absorb incident high-energy radiation and transfer it to the rare-earth luminescent center to realize high-efficiency luminescence in the visible region. Joly et al. reported Tb3+-doped LaF3 nanoscintillators bio-conjugated with meso-tetra (4-carboxyphenyl) porphine (MTCP) and studied its energy transfer process at a relatively low dose of X-ray irradiation [114]. Xie et al. reported that Eu2+-doped SrAl2-O4 nanoscintillators (SAO) could regulate the activation of PSs (MC540) by X-rays [124]. The XEOL of SAO matched well with the excitation wavelength of MC540, which could catalyze O2 to produce cytotoxic 1O2. Importantly, even relatively low doses (0.5 Gy) of X-ray irradiation could cause considerable damage to cancer cells. In addition, Yu et al. designed a Eu3+-doped NaGdF4 nanoscintillator system loaded with s-nitrosothiol group (SNO, NO donor) and indocyanine green (ICG, PS) to achieve radiation-responsive diagnosis and treatment of tumors [125]. Electrons and holes generated by the system after absorbing X-rays could move freely between the lattices, and energy was transferred from electron–hole pairs to luminescent ions or captured in an electron trap. Then, fluorescence emission allowed relaxation from the excited state back to the ground state, mediated by a cascade transition from Gd3+ sensitizers due to neighboring Eu3+ emitters. Finally, the energy transfer of this system efficiently activated ICG to produce 1O2. In vitro and in vivo studies showed that the system could significantly induce cell apoptosis and inhibit tumor growth.
Compared with single rare-earth-element doping, multielement codoping seems to show higher luminous efficiency and stronger scintillation. For example, CeF3 nanoparticles, as traditional nanoscintillators, had a low scintillation yield, which might be because the 4f orbital of Ce was located approximately 3~4 eV above the valence band or because the lack of the Auger cascade led to poor hole-capture probability [126]. In addition, doping Tb3+ in CeF3 NPs also could not improve the energy transfer efficiency from Ce3+ to Tb3+. This may be because 4f transitions are forbidden in most lanthanide ion configurations, which again leads to poor scintillation [127]. In view of this, Li et al. reported that the codoping of Tb3+ and Gd3+ could efficiently enhance the luminescence of CeF3 nanoscintillators (CeF3:Gd3+, Tb3+), which provided a potential complementary method for the clinical treatment of deep tumors [128] (Fig. 7a). As shown in Fig. 7b, the best XEOL could be obtained when the doping amounts of Gd3+ and Tb3+ were 12.3 and 1.24 mol %, respectively, and excellent overlap with the absorption spectrum of rose bengal was realized. Theoretically, the energy level of Gd3+ was between that of Ce3+ and that of Tb3+ in the CeF3:Gd3+, Tb3+ system. Therefore, codoping with Gd3+ could promote the effective migration of trapped energy from the activator (Ce3+) to sensitizer (Tb3+) (Fig. 7c), resulting in efficient energy transfer. Furthermore, X-ray irradiation could successfully generate 1O2 by loading rose bengal (Fig. 7d). In short, this codoping strategy is notable because it allows manipulation of a nanoscintillator to have specific functional characteristics.
FIGURE 7.

(a) Schematic of scintillator-based nanohybrids CGTS-RB for X-ray-induced catalytic radiosensitization based on energy conversion. (b) X-ray-excited optical luminescence of CeF3:Gd3+,Tb3+ and CeF3:Tb3+ (upper); X-ray-excited optical luminescence spectrum of CeF3:Gd3+,Tb3+ and absorbance spectrum of rose bengal (lower). (c) Illustration of the energy levels of Ce3+, Gd3+, and Tb3+ with possible excitation and emission mechanisms. (d) Singlet oxygen in vitro detection by using SOSG under X-ray irradiation. Reproduced with permission [128]. Copyright 2019, American Chemical Society.
The research strategies presented above are all based on energy transfer between nanoscintillators and organic PSs. However, most organic PSs may suffer severe photodestruction during ionizing radiation, leading to loss of their photodynamic efficiency. Therefore, replacing organic PSs with inorganic semiconductors that possess high light stability can prevent light damage to a great extent, which can be regarded as an effective improvement measure. To solve this problem, Bu et al. proposed the strategy of using nanoscintillators as an energy conversion bridge between X-rays and semiconductor PSs. They designed and synthesized Ce3+-doped LiYF4@SiO2@ZnO NPs with a core–shell structure [129] (Fig. 8a). Ce3+-doped LiYF4 nanoscintillators converted X-rays into ultraviolet fluorescence that could excite ZnO to generate electron–hole pairs and then the electron–hole pairs catalyzed O2 and H2O to generate free radicals. Note that ROS produced by this oxygen-independent type-I PDT mechanism could cause severe damage to DNA or proteins, even in a hypoxic environment. In vivo studies showed that tumor growth was significantly inhibited after intratumoral injection and X-ray irradiation (Fig. 8b). However, a problem that semiconductor PSs may not avoid is the recombination and quenching of electron–hole pairs. Following this strategy, Bu et al. further designed a new type of composite nanomaterial whose functional core was a LiLuF4:Ce nanoscintillator [104]. By loading Ag3PO4 semiconductor PSs and the cisplatin prodrug Pt(IV) on the surface of the LiLuF4:Ce nanoscintillator, high-efficiency separation of electron–hole pairs and catalytic radiosensitization could be realized. The specific mechanism was as follows: LiLuF4:Ce converted X-rays into ultraviolet fluorescence that could excite Ag3PO4 to generate electrons and holes, while Pt(IV) loaded on the surface of Ag3PO4 played a role in efficiently separating electron–hole pairs by absorbing photo electrons. In addition, nontoxic Pt (IV) absorbed electrons, which converted it into cisplatin, achieving not only high-efficiency catalytic radiosensitization, but also chemotherapy.
FIGURE 8.

(a) Diagram showing the mechanisms of X-ray-induced energy conversion of SZNPs nanoscintillator. (b) Relative tumor volume changes after exposure of the tissues to different treatments. Reproduced with permission [129]. Copyright 2014, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Notably, reactive nitrogen species (RNS) can also cause oxidative stress similar to ROS or its own unique nitrosative stress to promote tumor cell apoptosis. RNS are formed by the interaction of NO with other compounds including ROS, thus deriving a series of free radicals or nitro compounds (e.g., ONOO− and HOONO) with high oxidation activity [130–132]. In recent years, studies have reported different strategies for achieving controlled release of NO under UV, visible or near-infrared light [133,134]. However, the low tissue penetration degree of UV–Vis and the low efficiency of near-infrared light greatly limit its reasonable application. Based on the above considerations, Bu et al. used X-ray irradiation to realize the directly controlled release of NO [17,32]. In addition, for indirect use of X-rays, Zhao et al. used LiLuF4:Ce3+ nanoscintillators combined with the NO donor Roussin’s black salt (RBS) to form composite materials (RBS-T-SCNPs), which realized the controllable release of ONOO− under X-ray irradiation [135]. LiLuF4:Ce3+ nanoscintillators could convert X-rays into ultraviolet light, which further activated the photoactive RBS to release NO. and NO produced simultaneously at the tumor site could quickly react to generate ONOO−, which further damaged DNA, inhibited the expression of the DNA repair enzyme, and improved the efficacy of RT. Yu et al. used the NaGdF4:Eu3+ nanoscintillator to activate S-nitrosotiol groups under the action of X-rays to produce NO to enhance the tumor-killing effect [125].
In addition, scintillators, as energy sensors, play an important role in drug release. Chen et al. designed biomimetic nanocapsules composed of photoisomerized polyazobenzene (PETAzo) and adenine-modified ZnS (ZnS-A) nanoparticles [136]. ZnS-A NPs convert X-rays into ultraviolet radiation to isomerize the azobenzene groups, thereby achieving controllable diffusion on the bilayer. Additionally, the nucleobase pairing interaction between PETAzo and ZnS-A can prolong circulation in vivo and prevent drug leakage, which not only enhances the accumulation of the drug at the tumor site but also maintains its stability. Such nanocapsules have great potential to become a new anticancer drug delivery system.
Persistent luminescence nanoparticles
Energy transfer based on nanoscintillators is actually a real-time action process meaning that the catalytic reaction no longer occurs after X-rays are removed. Obviously, if the energy donor can still act as the excitation source and continue to excite the energy acceptor after X-ray irradiation is terminated, reactive species can be continuously produced to attack tumor cells [137]. Surprisingly, persistent luminescence nanoparticles can perfectly meet this standard [138,139]. With X-ray excitation, the energy of the afterglow nanoparticles is stored in defects or electron traps, and then the trapped carriers are slowly released [140]. Noncontinuous excitation can emit persistent phosphorescence within minutes, hours or even days. Then, the persistent luminescence can continue to excite the coupled PS. This makes persistent luminescence nanoparticles a promising candidate light source for activating PDT in deep tumors.
Solberg et al. first applied ZnS:Cu,Co afterglow nanoparticles [141] coupled with PS tetrabromorhodamine-123 (TBrRh123) to study the energy transfer process in 2014. After X-ray irradiation was terminated, the ZnS:Cu,Co nanoparticles showed persistent luminescence, which could continue to provide TBrRh123 with long-lasting light energy activation for more than 10 minutes, thereby converting O2 into 1O2. The results proved that the ZnS:Cu,Co nanoparticles had low toxicity to prostate cancer cells in the absence of X-ray irradiation but high toxicity during irradiation. Subsequently, Yang et al. reported low-dose X-ray-activated afterglow nanoparticles for tumor treatment [142] (Fig. 9a). The author synthesized W(VI)-doped ZnGa2O4:Cr persistent luminescence nanoparticles (PLNPs) with stronger sustainable luminescence intensity and longer duration of luminescence (Fig. 9b). By coupling with Zn (II) phthalocyanine tetrasulfonic acid (ZnPcS4), both in vivo and in vitro experiments verified that even a 0.18 Gy dose of X-rays was sufficient to activate ZnPcS4 to produce 1O2 (Fig. 9c), which had a significant inhibitory effect on tumors. Chen et al. employed the meso-porous silica template method to prepare Zn3Ga2GeO8:Cr3+, Yb3+, Er3+ (mZGGOs) afterglow nanoparticles [143] with uniform size and monodisperse spherical morphology. Under low-dose X-ray irradiation, mZGGOs could emit detectable near-infrared persistent afterglow to effectively stimulate PS silicon phthalocyanine (Si-Pc) to produce 1O2 to kill cancer cells. In addition, this afterglow luminescence could penetrate 1 cm of muscle tissue to achieve effective afterglow imaging in mice.
FIGURE 9.

(a) Schematic illustration for the design of X-ray-activated persistent luminescence nanoparticles-mediated PDT nanoplatform. (b) Persistent luminescence spectra of ZGO:Cr/W and ZGO:Cr PLNPs activated by X-rays. (c) DCF fluorescence spectra of ZGO:Cr/W-ZnPcS4 after different X-ray radiation times. Reproduced with permission [142]. Copyright 2018, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (d) Schematic illustration of magnetism-enhanced Cerenkov radiation (CR)-induced PDT. (e) Illustration of tumor-bearing mice with external magnetic field (MF) attaching onto the right tumor side. (f) Quantification uptake of 89Zr-MNP-PEG in the tumor from both sides at various time points. Reproduced with permission [145]. Copyright 2018, American Chemical Society.
Compared with converting external X-rays into ultraviolet light, Cerenkov radiation (CR) can be considered an internal excitation source to deliver light directly to the tumor site. CR radiation induced by radionuclides can activate PS within a certain absorption wavelength range to generate ROS to damage surrounding cells or tissues. As a milestone work, Achilefu et al. envisioned CR as a depth-independent excitation light source to activate the TiO2 semiconductor PS [144], which promoted the separation of electrons and holes of TiO2 under the action of CR and then catalyzed H2O and O2 to produce ROS. Moreover, Cai et al. used the radionuclides 89Zr and meso-tetrakis (4-carboxyphenyl) porphyrin (TCPP) to modify the surface of (Zn0.4Mn0.6) Fe2O4 magnetic nanoparticles (MNPs) to synthesize 89Zr-MNPs/TCPP [145] (Fig. 9d), a therapeutic agent for PDT induced by CR. 89Zr-MNPs/TCPP exhibited high tumor accumulation with the action of an external magnetic field (Fig. 9e, f) and thus exerted an excellent tumor treatment effect under the guidance of multimodal imaging, including fluorescence, CR luminescence (CL) and CR resonance energy transfer (CRET). Cai et al. further reported a “missile detonation” strategy [146]. First, high-dose p-SCN-Bn-deferoxamine-porphyrin-PEG nanocomposite (Df-PPN) was used as a CR energy receiver/missile to passively target tumors, and then low-dose 89Zr-labelled Df-PPN acted as a CR energy donor/detonator. Based on the homologous characteristics, the colocalization of Df-PPN and 89Zr-Df-PPN in tumor sites was maximized, and effective CR energy transfer could be realized. This precise and effective CRIT strategy could lead to significant tumor vascular damage and inhibit tumor growth. Apart from this, Sun et al. previously found that the short half-life radiopharmaceutical 18F-FDG could also be used as a built-in light source to excite ZnGa2O4:Cr3+ (ZGCs) persistent afterglow nanomaterials to produce near-infrared afterglow [147], which achieved high sensitivity, high contrast, and long-term tumor imaging effects. Next, they used the radionuclide 131I to excite ZGCs to produce long-term fluorescence and continuously excite near-infrared PSs (zinc tetra (4-carboxyphenoxy) phthalocyaninate) [148], thus achieving PDT. At the same time, the nanosystem could efficiently deliver the therapeutic nuclide-131I to tumor cells. This combination therapy based on radioactive nanosystems exhibited an outstanding tumor suppression effect while avoiding the use of external excitation light sources.
Metal-organic frameworks
In the past decade, MOFs have developed rapidly as a new type of porous nanomaterials in biomedicine [149,150]. Compared with traditional nanomaterials or porous materials, MOFs enable energy, electrons and reaction intermediates to transfer effectively, resulting in the optimization of the synergistic effect between the host and guest in the catalytic process [151,152]. Therefore, X-ray-induced MOFs-based energy transfer may have unique mechanisms and advantages in catalytic RT. Theoretically, the photosensitive ligands in MOFs are directly excited by photoelectrons generated by X-rays rather than the visible light excited by X-rays incident upon a scintillator, as mentioned above. Therefore, we speculate that the energy transfer of MOFs induced by X-ray irradiation has different damage effects from the energy transfer of nanoscintillator materials previously reported.
It is not difficult to find that nanomaterials based on scintillators are mostly core–shell structures. However, the structural regularity and porosity brought by the unique framework structure of MOFs seem to be more favorable for energy transfer and the occurrence of catalytic reactions [153]. MOFs should be structurally optimal for catalyzing the generation of ROS to sensitize RT. First, Lin et al. used Monte Carlo simulations to demonstrate that nanoscale MOFs (nMOFs) exhibited better radiosensitization than the corresponding nanoparticles under X-rays excitation [154] (Fig. 10a). The three-dimensional array of nMOFs can enhance the scattering of photons and electrons within the lattice, and with more electron collisions in the lattice, energy deposition is significantly improved (Fig. 10b, c). Then, the ordered crystal structure and inherent porosity of nMOFs ensure the construction of heavy metals and PSs so that the PSs maintain the highest density without self-quenching, which is facilitate the diffusion of ROS produced by catalysis. Finally, the pores in nMOFs facilitate easier diffusion of ROS, and the locally abundant O2 concentration in the pore channels allows the occurrence of energy transfer leading to the progression of catalytic reactions, finally achieving effective cytotoxicity [155].
FIGURE 10.
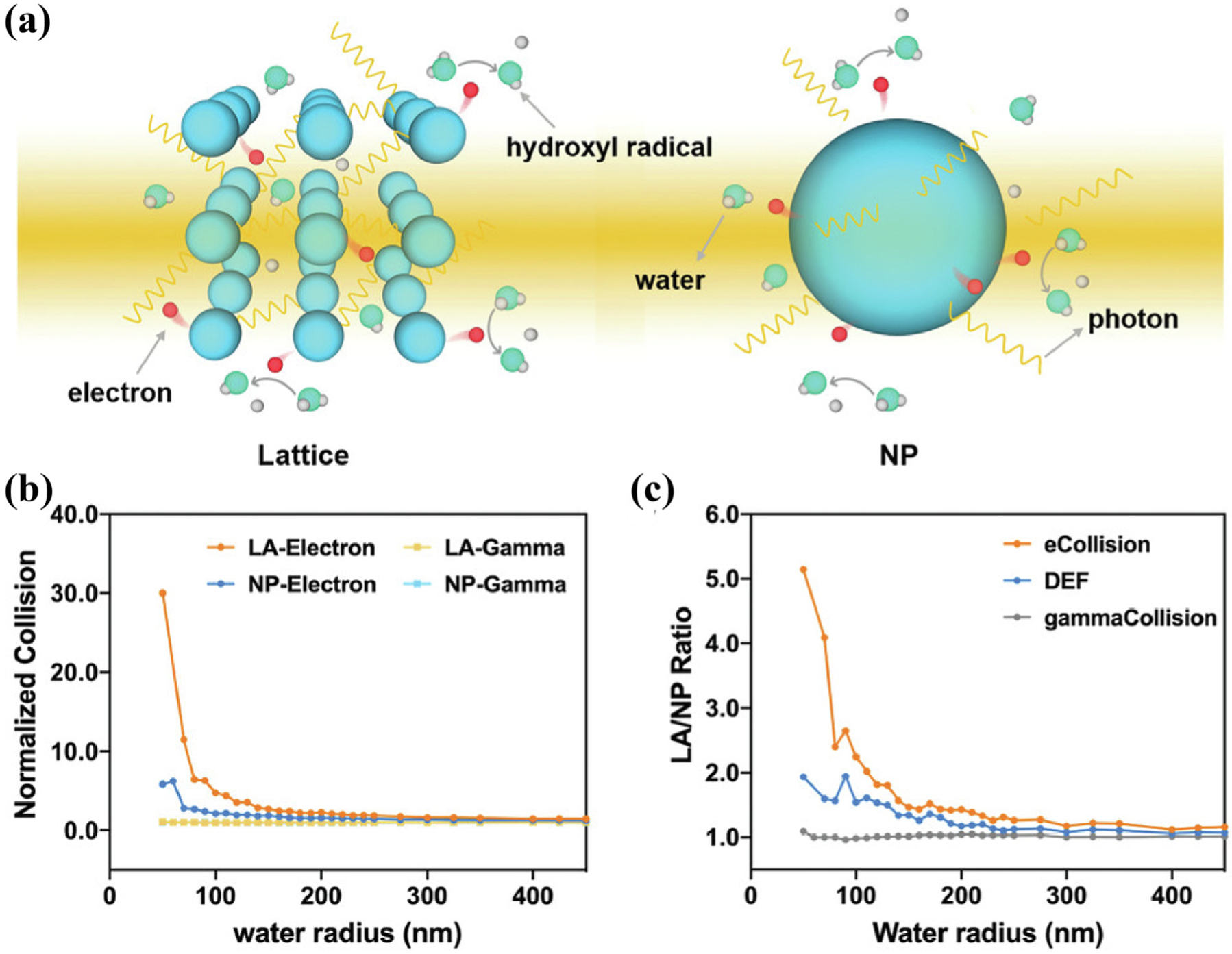
(a) Radiation-induced reactions on a lattice (left) and NP (right). (b) Normalized collision of electrons and photons in a lattice or a NP and (c) their ratios in comparison with dose enhancement factor ratios. Reproduced with permission [154]. Copyright 2021, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Based on the above theoretical conjectures, Lin et al. have made a series of contributions to exploring the application of Hf-based MOFs for catalytic radiosensitization [156,157]. They believed that if the heavy metal clusters in the MOFs structure could act as X-ray absorption antennas, the outer electrons of the heavy metal ions would be ejected in the form of high-energy electrons through the photoelectric effect. Subsequently, the generated photoelectrons could undergo inelastic scattering in MOFs and transfer their energy to the photosensitive ligands, bringing them to an excited state, which in turn catalyzes O2 to 1O2. Following this, Lin et al. designed and synthesized metal organic layers (MOLs) with [Hf6O4(OH)4(HCO2)6] secondary building units (SBUs) as nodes and Ir[bpy(ppy)2]+ or [Ru(bpy)3]2+ as ligands, which were used for X-ray-induced PDT in colon cancer [113]. The synthesized MOLs had an ultrathin two-dimensional structure of approximately 1.2 nm, which allowed for efficient diffusion of ROS and achieved excellent antitumor efficacy. Additionally, Lin et al. designed a new type of electropositive nano-MOFs (Hf-DBB-Ru) [147] with a precisely controllable morphology and size using HfO2 clusters and ruthenium terpyridine derivatives that was capable of targeting the mitochondria of cancer cells (Fig. 11a). Under the excitation of X-rays, Hf-DBB-Ru enriched in mitochondria (Fig. 11d) absorbed the energy of X-rays through the Hf node and excited the terpyridine ruthenium PS in the ligand, thus producing ROS (Fig. 11b, c). The in vivo anticancer effect also showed that Hf-DBB-Ru had an excellent effect of inhibiting tumor growth in mice, and some mice were even completely cured. In addition, Lin et al. fabricated nMOL (Hf-DBP) by using Hf12O8(OH)14 SBUs with high electron density to coordinate with 5,15-di(p-benzoato) porphyrin and achieved a better catalytic RT effect than that reported previously [155]. Importantly, by combining with an anti-PD-L1 antibody, activation of the tumor immune system was achieved, which contributed to the elimination of lung metastasis in the 4T1 in situ model. Overall, the unique advantages of the MOFs structure and its biomedical application potential are definitely advantageous for catalytic radiosensitization, which will help to advance the clinical application of nanomedicines.
FIGURE 11.

(a) Mitochondria-targeted RT-RDT mediated by Hf-DBB-Ru. (b) Schematic showing the energy transfer between Hf6 SBUs and . (c) SOSG fluorescence of Hf-DBB-Ru and Hf-DBA at same concentrations of Hf upon X-ray radiation. (d) Mitochondria co-localization images of Hf-DBB-Ru (upper) and Hf6-DBA (lower). Reproduced with permission [156]. Copyright 2018, Nature Publishing Group.
Conclusion and outlook
In this review, we systematically summarized the concept and progress of the catalytic radiosensitization. Unlike physical enhancement strategy based on high-Z elements, catalytic radiosensitization aims to exploit and utilize the catalytic reaction of nanomaterials under radiotherapy X-rays, thereby achieving more efficient radiotherapy. So far, all strategies for catalytic radiotherapy are based on two basic principles: regulating electron transfer or utilizing energy conversion. Strategies to regulate electron density or interfacial transfer include defect construction, heterostructure design, and valence transition. The highly active catalytic sites generation due to the precise control of the electronic structure of the nanomaterials can realize efficient catalysis involving H2O2, H2O and O2 in tumors. On the other hand, by converting energetic X-rays into low-energy UV–Vis photons or secondary electrons that can be directly utilized by photocatalysts or photosensitizers, the types of catalysts that can be used in catalytic radiosensitization have been greatly expanded. Although nanomaterials have obvious advantages in catalytic radiosensitization, there are still problems that need to be addressed in catalytic radiosensitization based on X-ray-mediated electron and energy regulation. To promote the development of catalytic radiosensitization strategies, follow-up research should consider the following issues:
Although prior researches have greatly improved the efficiency of catalytic radiosensitization, it has not fully met the needs of maximizing the utilization of X-rays in the practical application of nanomaterial-induced catalytic radiosensitization. It is necessary to deeply explore the mechanism at the molecular and atomic levels to provide a solid foundation for the design of new X-ray catalytic materials. In addition, the challenges of X-ray catalytic materials lie in the accurate identification of their active sites and the dynamic reconstruction of the material surface structure under the action of X-rays. Therefore, the use of advanced high-resolution in situ characterization technology may be able to monitor the changes in the material surface state under the action of X-rays, which can provide substantial evidence for understanding the mechanism of catalytic radiosensitization.
Catalytic radiosensitization depends much on ROS to damage tumor biomolecules, especially ▪OH. From the perspective of chemical reactions, the generation of ▪OH mainly comes from the decomposition of H2O and H2O2 in cells. As mentioned above, by lengthening the O–H bond in H2O can reduce the decomposition energy barrier of H2O, thereby increasing the yield of ▪OH under X-rays irradiation. Therefore, designing more effective catalysts with the aid of theoretical calculations to fully activate the O–H bond of H2O can improve the efficiency of H2O decomposition and radical generation under X-rays.
Non-oxygen free radicals, such as alkyl radicals, chlorine radicals, carbonyl radicals and semiquinone radicals, also have great application potential in catalytic radiotherapy, which may give rise to completely different therapeutic mechanisms than ROS. Therefore, it is urgent to design catalytic reactions and catalysts that can generate non-oxygen radicals under X-ray irradiation.
In addition to H2O2, H2O and O2, some metabolic molecules in the tumor microenvironment, such as polyamines, lactic acid and GSH, can also serve as substrates for catalytic radiotherapy. The design and development of related catalytic materials is of great significance, but also challenging.
Converting energetic X-rays into other forms is an ingenious method to trigger catalytic reactions in RT. Other forms of energies other than fluorescence photons converted from X-rays, such as electric energy and thermal energy, may also be used in catalytic radiotherapy. How to improve the energy conversion efficiency is one of the key issues currently.
Moreover, the biological mechanisms and therapeutic pathways involved in catalytic radiosensitization need to be further explored in molecular biology and gene level, which is essential to promote its clinical application.
Acknowledgements
Ya Wang and Huilin Zhang contributed equally to this work. This work was supported in part, by National Funds for Distinguished Young Scientists (51725202), Key Project of Shanghai Science and Technology Commission (19JC1412000), the University of Wisconsin-Madison and the National Institutes of Health (P30CA014520), China National Postdoctoral Program for Innovative Talents (BX2021075). We acknowledge Dr. Kaiyuan Ni (Koch Institute for Integrative Cancer Research at Massachusetts Institute of Technology) for his valuable advice on the application of MOFs in catalytic radiosensitization.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Weibo Cai is a scientific advisor, stockholder, and grantee of Focus-X Therapeutics, Inc. The other authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Miller KD et al. , Ca-a Cancer J. Clin 69 (2019) 363. [Google Scholar]
- [2].Lin BW et al. , Front. Oncol 11 (2021) 644400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Song G et al. , Adv. Mater 29 (2017) 1700996. [Google Scholar]
- [4].Zhang CY et al. , Chem. Sci 10 (2019) 6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen X et al. , Bioact. Mater 7 (2022) 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang C et al. , Adv. Funct. Mater 30 (2020) 2000189. [Google Scholar]
- [7].Son J et al. , Med. Phys 40 (2013). [Google Scholar]
- [8].Wang C et al. , Adv. Mater 34 (2022) e2106520. [DOI] [PubMed] [Google Scholar]
- [9].Guo T, X-ray Nanochemistry, Springer, 2018. [Google Scholar]
- [10].Guo T, Phys. Chem. Chem. Phys 21 (2019) 15917. [DOI] [PubMed] [Google Scholar]
- [11].Zou M et al. , Natl. Sci. Rev 8 (2021) nwaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu W et al. , Pharmaceutics 13 (2021) 1757.34834172 [Google Scholar]
- [13].Lv B et al. , Nano Today 35 (2020) 100988. [Google Scholar]
- [14].Sheng K, Semiconductor Nanomaterials for Radiotherapy. In Cancer Nanotechnology: Principles and Applications in Radiation Oncology, Cho SH, and Krishnan S, (eds.) (2013), pp 137. [Google Scholar]
- [15].Wang H et al. , Nano Today 37 (2021) 101099. [Google Scholar]
- [16].Lu KD et al. , Nat. Biomed. Eng 2 (2018) 600. [DOI] [PubMed] [Google Scholar]
- [17].Li Y et al. , Angew. Chem. Int. Ed 60 (2021) 15472. [DOI] [PubMed] [Google Scholar]
- [18].Davidson RA, Guo T, J. Phys. Chem. Lett 3 (2012) 3271. [Google Scholar]
- [19].Cheng NN et al. , J. Am. Chem. Soc 134 (2012) 1950. [DOI] [PubMed] [Google Scholar]
- [20].Gong T et al. , ACS Nano 14 (2020) 3032. [DOI] [PubMed] [Google Scholar]
- [21].Casta R et al. , J. Nanopart. Res 17 (2015) 3. [Google Scholar]
- [22].Carter JD et al. , J. Phys. Chem. B 111 (2007) 11622. [DOI] [PubMed] [Google Scholar]
- [23].Incerti S et al. , Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 372 (2016) 91. [Google Scholar]
- [24].Ohya K et al. , Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 266 (2008) 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Davidson RA, Guo T, J. Phys. Chem. C 118 (2014) 30221. [Google Scholar]
- [26].Paunesku T et al. , Radiosensitization and nanoparticles, in: Mirkin CA (Ed.), Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer, Springer International Publishing, Cham, 2015, p. 151. [Google Scholar]
- [27].Lee C et al. , J. Phys. Chem. C 116 (2012) 11292. [Google Scholar]
- [28].McMahon SJ et al. , Radiother. Oncol 100 (2011) 412. [DOI] [PubMed] [Google Scholar]
- [29].Yan H et al. , Phys. Med. Biol 61 (2016) 2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fan WP et al. , J. Am. Chem. Soc 135 (2013) 6494. [DOI] [PubMed] [Google Scholar]
- [31].Liu YY et al. , Biomaterials 49 (2015) 1.25725549 [Google Scholar]
- [32].Fan WP et al. , Angew. Chem. Int. Ed 54 (2015) 14026. [DOI] [PubMed] [Google Scholar]
- [33].Xiao QF et al. , J. Am. Chem. Soc 135 (2013) 13041. [DOI] [PubMed] [Google Scholar]
- [34].Yong Y et al. , ACS Nano 9 (2015) 12451. [DOI] [PubMed] [Google Scholar]
- [35].Wang S et al. , Adv. Mater 27 (2015) 2775. [DOI] [PubMed] [Google Scholar]
- [36].Zhang M et al. , Biomaterials 155 (2018) 135. [DOI] [PubMed] [Google Scholar]
- [37].Misawa M, Takahashi J, Nanomed.: Nanotechnol. Bio. Med 7 (2011) 604. [Google Scholar]
- [38].Ye J-H et al. , Acc. Chem. Res 54 (2021) 2518. [DOI] [PubMed] [Google Scholar]
- [39].Witzel S et al. , Chem. Rev 121 (2021) 8868. [DOI] [PubMed] [Google Scholar]
- [40].Fan WP et al. , Biomaterials 35 (2014) 8992. [DOI] [PubMed] [Google Scholar]
- [41].Zhao P et al. , Angew. Chem. Int. Ed 60 (2021) 8905. [DOI] [PubMed] [Google Scholar]
- [42].Liu YS et al. , Sol. Energy Mater. Sol. Cells 208 (2020) 110432. [Google Scholar]
- [43].Isikawa M, Guidelli E, ACS App. Mater. Interfaces 14 (2022) 324. [DOI] [PubMed] [Google Scholar]
- [44].Feng Y et al. , J. Mater. Chem. C 10 (2022) 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wolkenstein T, The Electron Theory of Catalysis on Semiconductors. (1960) pp. 189. [Google Scholar]
- [46].Kibria MG et al. , Nat. Commun 5 (2014) 3825. [DOI] [PubMed] [Google Scholar]
- [47].Yang B et al. , Adv. Mater 31 (2019) 1901778. [Google Scholar]
- [48].Ouyang J et al. , Nano Today 35 (2020) 100949. [Google Scholar]
- [49].Chen W et al. , Angew. Chem. Int. Ed 60 (2021) 7155. [DOI] [PubMed] [Google Scholar]
- [50].Xie J et al. , Adv. Mater 31 (2019) e1802244. [DOI] [PubMed] [Google Scholar]
- [51].Liu T et al. , Pro. Nat. Sci.: Mater. Int 30 (2020) 567. [Google Scholar]
- [52].Chen X et al. , Chem. Soc. Rev 48 (2019) 3073. [DOI] [PubMed] [Google Scholar]
- [53].Dou Y et al. , ACS Nano 10 (2016) 2536. [DOI] [PubMed] [Google Scholar]
- [54].Guo T, Geometry Enhancement of Nanoscale Energy Deposition by X-Rays, The Regents of the University of California, 2017.
- [55].Ran J et al. , Chem. Soc. Rev 43 (2014) 7787. [DOI] [PubMed] [Google Scholar]
- [56].Luo J et al. , ACS Nano 13 (2019) 9811. [DOI] [PubMed] [Google Scholar]
- [57].Nie W et al. , Nano Lett. 21 (2021) 8901. [DOI] [PubMed] [Google Scholar]
- [58].Zeng Z et al. , Nat. Commun 12 (2021) 4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Liu Y et al. , Adv. Energy Mater 6 (2016) 1600436. [Google Scholar]
- [60].Zhang N et al. , J. Am. Chem. Soc 138 (2016) 8928. [DOI] [PubMed] [Google Scholar]
- [61].Xiong J et al. , Adv. Funct. Mater 28 (2018) 1801983. [Google Scholar]
- [62].Zhang M et al. , Mater. Horiz 6 (2019) 1034. [Google Scholar]
- [63].Sugahara A et al. , Nat. Commun 10 (2019) 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Otake KI, et al. , Nat. Commun 11 (2020), 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Selcuk S, Selloni A, Nat. Mater 15 (2016) 1107. [DOI] [PubMed] [Google Scholar]
- [66].Wang H et al. , J. Am. Chem. Soc 140 (2018) 1760. [DOI] [PubMed] [Google Scholar]
- [67].Bentley CL et al. , J. Am. Chem. Soc 141 (2019) 2179. [DOI] [PubMed] [Google Scholar]
- [68].Wang S et al. , Adv. Mater 32 (2020) 2001385. [Google Scholar]
- [69].Liu H et al. , Inorg. Chem 59 (2020) 3482. [DOI] [PubMed] [Google Scholar]
- [70].Ju Y et al. , ACS Nano 11 (2017) 9239. [DOI] [PubMed] [Google Scholar]
- [71].Zhang H et al. , Adv. Sci 5 (2018) 1800271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang C et al. , Nano Lett 9 (2009) 1493. [DOI] [PubMed] [Google Scholar]
- [73].Peng S et al. , Angew. Chem. Int. Ed 50 (2011) 3158. [DOI] [PubMed] [Google Scholar]
- [74].Chan L et al. , ACS Nano 15 (2021) 3047. [DOI] [PubMed] [Google Scholar]
- [75].Zhang Y et al. , Appl. Surf. Sci 521 (2020) 146434. [Google Scholar]
- [76].Tian N et al. , J. Mater. Chem. A 3 (2015) 17120. [Google Scholar]
- [77].Liu H et al. , Biomaterials 226 (2020) 119545. [DOI] [PubMed] [Google Scholar]
- [78].Wang J et al. , Angew. Chem. Int. Ed 57 (2018) 2600. [Google Scholar]
- [79].Zhang Z et al. , ACS App. Mater. Interfaces 4 (2012) 593. [DOI] [PubMed] [Google Scholar]
- [80].Hoertz PG et al. , Radiat. Phys. Chem 84 (2013) 51. [Google Scholar]
- [81].Wang X et al. , ACS Nano 13 (2019) 5947. [DOI] [PubMed] [Google Scholar]
- [82].Guo Z et al. , Adv. Mater 29 (2017) 1704136. [Google Scholar]
- [83].Dong X et al. , ACS Nano. 14 (2020) 5400. [DOI] [PubMed] [Google Scholar]
- [84].Zhou R et al. , Biomaterials 189 (2019) 11. [DOI] [PubMed] [Google Scholar]
- [85].Cai R et al. , J. Am. Chem. Soc 143 (2021) 16113. [DOI] [PubMed] [Google Scholar]
- [86].Xu S et al. , Adv. Funct. Mater 29 (2019) 1808737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tao X et al. , Adv. Energy Mater 9 (2019) 1803951. [Google Scholar]
- [88].Zhou R et al. , ACS Nano 14 (2020) 13016. [DOI] [PubMed] [Google Scholar]
- [89].Guo Y et al. , EcoMat 1 (2019) e12007. [Google Scholar]
- [90].Gao Y et al. , Int. J. Mol. Sci 22 (2021) 7879.34360644 [Google Scholar]
- [91].Zhou R et al. , Nano Today 36 (2021) 101003. [Google Scholar]
- [92].Zhang C et al. , Angew. Chem. Int. Ed 55 (2016) 2101. [DOI] [PubMed] [Google Scholar]
- [93].Tang Z et al. , Adv. Mater 29 (2017) 1701683. [Google Scholar]
- [94].Tang Z et al. , Angew. Chem. Int. Ed 58 (2019) 946. [Google Scholar]
- [95].Ji X et al. , Adv. Sci 6 (2019) 1901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ji X et al. , Nat. Commun 12 (2021) 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yang G et al. , ACS Nano 16 (2022) 2319. [DOI] [PubMed] [Google Scholar]
- [98].Zhao P et al. , Mater. Horiz 6 (2019) 369. [Google Scholar]
- [99].Zhang C et al. , Biomaterials 276 (2021) 121023. [DOI] [PubMed] [Google Scholar]
- [100].Ma B et al. , J. Am. Chem. Soc 141 (2019) 849. [DOI] [PubMed] [Google Scholar]
- [101].Zhang C et al. , Nano Lett. 19 (2019) 1749. [DOI] [PubMed] [Google Scholar]
- [102].Su W et al. , Adv. Sci 7 (2020) 1903585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Duo Y et al. , Adv. Funct. Mater 30 (2019) 1906010. [Google Scholar]
- [104].Wang H et al. , Nano Lett. 18 (2018) 5768. [DOI] [PubMed] [Google Scholar]
- [105].Allison RR et al. , Photodiagn. Photodyn. Ther 1 (2004) 27. [DOI] [PubMed] [Google Scholar]
- [106].Sun W et al. , Theranostics 10 (2020) 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Blasse G, Chem. Mater 6 (1994) 1465. [Google Scholar]
- [108].Gadzhimagomedova Z et al. , Int. J. Mol. Sci 21 (2020) 4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bulin AL et al. , J. Phys. Chem. C 117 (2013) 21583. [Google Scholar]
- [110].Sun W et al. , Adv. Mater 31 (2019) 1808024. [Google Scholar]
- [111].Luo L et al. , ACS App. Mater. Interfaces 12 (2020) 12591. [DOI] [PubMed] [Google Scholar]
- [112].Zhang W et al. , Biomaterials 184 (2018) 31. [DOI] [PubMed] [Google Scholar]
- [113].Lan G et al. , Angew. Chem. In. Ed 56 (2017) 12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu Y et al. , Appl. Phys. Lett 92 (2008) 043901. [Google Scholar]
- [115].Maiti D et al. , Nanoscale Horiz. 5 (2020) 109. [Google Scholar]
- [116].Guo T et al. , Nanoscale 10 (2018) 1607. [DOI] [PubMed] [Google Scholar]
- [117].Zhong X et al. , Nano Lett. 19 (2019) 8234. [DOI] [PubMed] [Google Scholar]
- [118].Kirakci K et al. , Inorg. Chem 55 (2016) 803. [DOI] [PubMed] [Google Scholar]
- [119].Huang H et al. , Biomaterials 171 (2018) 12. [DOI] [PubMed] [Google Scholar]
- [120].Chen W, Zhang J, J Nanosci. Nanotechnol 6 (2006) 1159. [DOI] [PubMed] [Google Scholar]
- [121].Kamkaew A et al. , ACS Nano 10 (2016) 3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Chuang YC et al. , Theranostics 10 (2020) 6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chen H et al. , Mater. Horiz 4 (2017) 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chen H et al. , Nano Lett. 15 (2015) 2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Dou Y et al. , Theranostics 8 (2018) 5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Paul L et al. , Inorganic Scintillators for Detector Systems, Springer, 2016. [Google Scholar]
- [127].Blasse G et al. , Luminescent Materials, Springer, 2016, p. 1994. [Google Scholar]
- [128].Ahmad F et al. , ACS Nano 13 (2019) 10419. [DOI] [PubMed] [Google Scholar]
- [129].Zhang C et al. , Angew. Chem. Int. Ed 54 (2015) 1770. [DOI] [PubMed] [Google Scholar]
- [130].Wang Y et al. , Chem. Sci 9 (2018) 8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Xu Y et al. , Biomaterials 276 (2021) 121017. [DOI] [PubMed] [Google Scholar]
- [132].Fang X et al. , Angew. Chem. In. Ed 60 (2021) 7046. [DOI] [PubMed] [Google Scholar]
- [133].Jiang Y et al. , Sci. Adv 6 (2020) 3513. [Google Scholar]
- [134].Chu X et al. , Adv. Funct. Mater 31 (2021) 2008507. [Google Scholar]
- [135].Du Z et al. , Adv. Mater 30 (2018) e1804046. [DOI] [PubMed] [Google Scholar]
- [136].Deng H et al. , Adv. Mater 31 (2019) e1903443. [DOI] [PubMed] [Google Scholar]
- [137].Liu G et al. , Adv. Funct. Mater 28 (2018) 1804317. [Google Scholar]
- [138].Wu Y et al. , Angew. Chem. Int. Ed 60 (2021) 12868. [DOI] [PubMed] [Google Scholar]
- [139].Liu H et al. , Anal. Chem 91 (2019) 15064. [DOI] [PubMed] [Google Scholar]
- [140].Li Y et al. , Chem. Soc. Rev 45 (2016) 2090. [DOI] [PubMed] [Google Scholar]
- [141].Ma L et al. , Appl. Phys. Lett 105 (2014) 013702. [Google Scholar]
- [142].Song L et al. , Adv. Funct. Mater 28 (2018) 1707496. [Google Scholar]
- [143].Shi T et al. , Adv. Funct. Mater 30 (2020) 2001166. [Google Scholar]
- [144].Kotagiri N et al. , Nat. Nanotechnol 10 (2015) 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ni D et al. , J. Am. Chem. Soc 140 (2018) 14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yu B et al. , Adv. Mater 31 (2019) e1904894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Liu N et al. , Small 16 (2020) e2001494. [DOI] [PubMed] [Google Scholar]
- [148].Wang Q et al. , Adv. Healthc. Mater 10 (2021) e2000802. [DOI] [PubMed] [Google Scholar]
- [149].Zhou Y et al. , Adv. Drug. Deliv. Rev 171 (2021) 199. [DOI] [PubMed] [Google Scholar]
- [150].Lismont M et al. , Adv. Funct. Mater 27 (2017) 1606314. [Google Scholar]
- [151].Liu J et al. , Biomaterials 97 (2016) 1. [DOI] [PubMed] [Google Scholar]
- [152].He Z et al. , Angew. Chem. Int. Ed 58 (2019) 8752. [Google Scholar]
- [153].Lan G et al. , J. Am. Chem. So 141 (2019) 15767. [DOI] [PubMed] [Google Scholar]
- [154].Xu Z et al. , Adv. Mater 33 (2021) e2104249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Ni K et al. , Matter 1 (2019) 1331. [PMC free article] [PubMed] [Google Scholar]
- [156].Ni K et al. , Nat. Commun 9 (2018) 4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ni K et al. , Chem. Sci 11 (2020) 7641. [DOI] [PMC free article] [PubMed] [Google Scholar]


