Abstract
Background:
Malawi’s PMTCT Option B+ program has expanded the reach of ART services amongst pregnant and breastfeeding women, but retention in lifelong HIV care remains challenging. Given that depression can undermine retention, it is important to understand how depression changes over the perinatal period, varies across treatment and retention groups, and could be buffered by social support.
Methods:
Data are from an observational study conducted among women enrolled in Malawi’s PMTCT Option B+ program. We used multilevel generalized linear models to estimate the odds of probable depression by time, treatment and retention group, and social support. Probable depression was assessed with the Edinburgh Postnatal Depression Scale and Patient Health Questionnaire-9.
Results:
Of 468 women, 15% reported probable depression at antenatal enrollment and prevalence differed across newly diagnosed individuals, second line therapy users, and previous defaulters (18%, 21 %, 5%, p = 0.001). Odds of probable perinatal depression decreased over time (OR per month: 0.87, 95% CI: 0.82–0.92) but were higher among those newly diagnosed (OR: 3.25, 95% CI: 1.59–6.65) and on second line therapy (OR: 3.39, 95% CI: 1.44–7.99) as compared to previous defaulters. Odds of probable postpartum depression were lower for participants with high social support (OR: 0.19, 95% CI: 0.09–0.39).
Limitations:
Lack of diagnostic psychiatric evaluation precludes actual diagnosis of depression.
Conclusions:
Probable depression varied across the perinatal period and across treatment and retention groups. Social support was protective for postpartum depression among all participants. Depression screening and provision of social support should be considered in PMTCT programs.
Keywords: perinatal depression, HIV, social support
INTRODUCTION
In sub-Saharan Africa (SSA), women living with HIV (WLWH) experience a high burden of depression during the perinatal period, which includes both pregnancy and the year following childbirth(Dadi et al., 2020; Gavin et al., 2005; James et al., 2018; Sawyer et al., 2010; Sowa et al., 2015; Stringer et al., 2014). The prevalence of antenatal and postnatal depression in the region may be as high as 49% and 36%, respectively (Mokhele et al., 2019; Peltzer et al., 2018; Sowa et al., 2015). Depressive symptoms among WLWH are associated with HIV disease progression and are strong predictors of non-adherence to antiretroviral therapy (ART) (Evans et al., 2002; Ickovics et al., 2001; Nakimuli-Mpungu et al., 2012; Stringer et al., 2014). Depressive symptoms during the perinatal period can additionally undermine engagement in antenatal care (ANC), prevention-of-mother-to-child-transmission (PMTCT), and attainment of optimal child outcomes (Grede et al., 2014; Psaros et al., 2014; Stein et al., 2014; Stringer et al., 2014). Taken together, these findings suggest a crucial need to address perinatal depression amongst this population and minimize downstream effects on HIV transmission, maternal health, and child development.
Existing literature suggests heterogeneity in how women experience depression during pregnancy and postnatal months, particularly in the onset and progression of symptoms. A recent systematic review of perinatal depression in high-income countries identified three common trajectories across studies: 1) a low risk trajectory, characterized by stable low depressive symptoms throughout the perinatal period; 2) a moderate-high trajectory, with persistently elevated depressive symptoms throughout the perinatal period; and 3) transient trajectories with either increasing, decreasing, or episodic depressive symptoms (Baron et al., 2017). The few studies conducted in SSA have documented similar trajectories, but have rarely investigated patterns of perinatal depression among WLWH (Barthel et al., 2016; Familiar et al., 2019; Fellmeth et al., 2017; Garman et al., 2019; Gelaye et al., 2016; Pellowski et al., 2019; Sawyer et al., 2010; Sowa et al., 2015).
In 2001, Malawi launched “Option B+”, a prevention-of-mother-to-child-transmission (PMTCT) program which offers pregnant and breastfeeding women free lifelong ART at diagnosis, regardless of CD4 count or clinical stage (Rosenberg and Pettifor, 2018; Schouten et al., 2011). The program has been largely considered a success, having achieved high rates of ART initiation and a reduction in mother-to-child transmission of HIV (Haas et al., 2017; Hoffman et al., 2017; Kim et al., 2015). Despite this progress, retention in HIV care has been suboptimal: nearly 25% of women are lost to follow-up within one year of initiating ART (Haas et al., 2016, 2016). In addition, Option B+ has resulted in a range of HIV treatment groups as pregnant women presenting for ANC are at varied stages of their HIV treatment course, from newly diagnosed to second and advanced lines of therapy. These stages have serious implications for depression as women are navigating the perinatal period as well as the potential distress of receiving an HIV diagnosis, managing a chronic illness, disease exacerbation, or treatment failure. Understanding patterns of perinatal depression across treatment groups can further inform how and when to identify subgroups of WLHW who are at high risk as well as opportunities to increase ART adherence amongst this population.
Social support is a well-established protective factor for both perinatal depression and ART adherence (Biaggi et al., 2016; Bisetegn et al., 2016; Elwell, 2016; Garman et al., 2019; Gelaye et al., 2016; Grede et al., 2014; Kelly et al., 2014; Ncama et al., 2008a; Pellowski et al., 2019; Peltzer et al., 2018; Psaros et al., 2014; Sawyer et al., 2010; Stewart et al., 2015; Umuziga et al., 2020). Social support is the provision of tangible, emotional, instrumental, or informational assistance leading one to perceive that they are cared for and an accepted member of a network of mutual obligations (Cobb, 1976). Social support may directly protect against perinatal depression or function by buffering the impact of stressful life circumstances that lead to perinatal depression (Cobb, 1976; Cohen and Wills, 1985; Ozbay et al., 2007). Taken together, effectively addressing the burden of perinatal depression among WLWH in Malawi requires an improved understanding of the progression of perinatal depression, identifying the women at heightened risk for depression, and an investigation into social factors – such as social support – that may buffer depression.
In this secondary analysis, we utilize longitudinal data from pregnant women enrolled in Malawi’s Option B+ program to investigate three research aims. First, we examine perinatal depression trajectories among three HIV treatment and retention groups (newly diagnosed, those on second line therapy, and previous defaulters). Second, we describe levels of social support among the HIV treatment and retention groups. Third, we investigate the association between social support and perinatal depression and how the association varies across the HIV treatment and retention groups.
METHODS
Study Overview
Data are from the Safety, Suppression, Second-Line, Survival (S4) study, an observational cohort study conducted among pregnant women living with HIV enrolled in Malawi’s Option B+ prevention of maternal to child transmission of HIV program. Participants were recruited from a government antenatal clinic in Lilongwe, Malawi from 2015 to 2018 (ClinicalTrials.gov identifier: NCT02249962). Per Malawi standard of care for opt-out HIV testing, all women who seek antenatal care are offered HIV testing with two rapid tests. A convenience sample of women who tested positive for HIV at their first antenatal visit, in any trimester of pregnancy, were invited to enroll in the S4 study. Eligibility criteria included being pregnant, 18 years (or 16–17 and married), and planning to give birth in Lilongwe. Women who chose not to participate or were ineligible received routine antenatal and HIV care through the government clinic. Additional details on recruitment and enrollment have been previously published (Harrington et al., 2020, 2019b, 2019a, 2018).
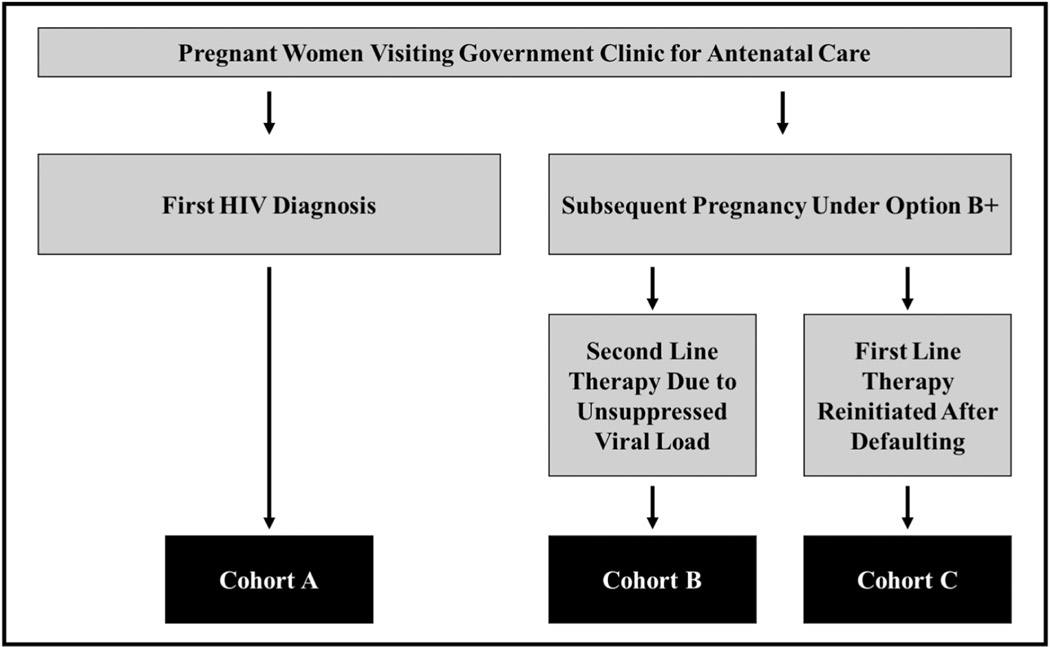
Mirroring HIV standard of care in Malawi, S4 study visits after enrollment occurred monthly for 6 months and then quarterly thereafter. At each S4 study visit, clinical data were collected and S4 nurses conducted one-on-one interviews with participants in Chichewa, the local language. Interview questions examined demographics, HIV care, infant health, and mental health. Study nurses received training on how to administer all study questionnaires, including the mental health assessments, but none of the nurses had mental health backgrounds. All women received ART through the study, as well as primary care for themselves and their infants. S4 participants were divided into three cohorts: Cohort A) women newly diagnosed with HIV and initiating standard first line ART with tenofovir/lamivudine/efavirenz (TDF/3TC/EFV); Cohort B) women on ART during a subsequent pregnancy requiring second line therapy due to unsuppressed viral load; and Cohort C) women who experienced a gap in ART care but returned for subsequent pregnancy management (Figure 1). The same demographic characteristics and mental health outcomes were collected for each cohort.
Figure 1.
Study Cohort Recruitment
The S4 study enrolled 545 pregnant women with HIV across three cohorts (Cohort A: N = 300, Cohort B: N = 95, Cohort C: N = 150). Given our interest in examining both antenatal and postpartum depression, we excluded participants who did not have an antenatal depression measurement (N = 23) or a postpartum depression measurement (N = 54). Thus, our analytical sample included 468 women with HIV (Cohort A: N = 268, Cohort B: N = 77, Cohort C: N = 123).
The S4 study received approval from both the National Health Science Research Committee in Malawi and the University of North Carolina at Chapel Hill Institutional Review Board. All participants provided informed consent.
Measures
Probable depression was defined as screening positive on the Edinburgh Postnatal Depression Scale (EPDS-10) or the Patient Health Questionnaire-9 (PHQ-9) at a study visit. The EPDS-10 is a 10-item instrument designed to identify probable depression among women who are pregnant or postpartum (Cox et al., 1987; Gibson et al., 2009; Kozinszky and Dudas, 2015; Stewart et al., 2013). Each item in the EPDS-10 asks about depressive symptoms in the past 7 days, is scored from 0 to 3, for an overall score range of 0 to 30. The EPDS-10 has been previously validated in Chichewa among pregnant women in Malawi. A score threshold of 6, rather than the typical 13, was recommended as a dichotomous cut-point to indicate probable depression.(Stewart et al., 2013) Accordingly, participants who scored ≥6 on the EPDS-10 screened positive for probable depression in our analyses. The PHQ-9 is a 9-question instrument designed to assesses probable depression in adults. Responses to each item range from 0 (symptom occurred zero days in the past two weeks) to 3 (nearly every day), for an overall score range of 0 to 27. The PHQ-9 has been validated in Malawi among persons with diabetes and has been extensively utilized (including translation and back-back translation in Chichewa) in published studies among antenatal populations in Malawi and neighboring countries (Cholera et al., 2014; Hanlon et al., 2015; Pence et al., 2012; Udedi et al., 2019). Traditionally, a PHQ-9 score of 10 or more is indicative of major depression that requires treatment and a score of 5–9 is suggestive of mild depression (Yawn et al., 2009; Zhong et al., 2014). In this analysis we utilized a PHQ-9 score of 5 or greater to be consistent with the lower threshold used for the EPDS-10 and previous analyses among S4 study participants (Harrington et al., 2018). Participants who scored above the threshold on one instrument but not the other were still included in our analysis as having probable depression.
Social support was measured using the positive social interaction subscale of the Medical Outcomes Study Social Support Survey instrument, which has been previously utilized in studies among people living with HIV in Sub-Saharan Africa (Bajunirwe et al., 2009; Casale et al., 2014; Epino et al., 2012; Gaede et al., 2006; Ncama et al., 2008b; Sherbourne and Stewart, 1991). At each postpartum visit, the three subscale items asked participants how often they had someone to “have a good time with”, “get together with for relaxation”, and “do something enjoyable with”. Responses to each item ranged from 0 (none of the time) to 2 (all of the time), for an overall score range of 6. Based on preliminary descriptive analyses, a binary variable was created to indicate high social support (score of 6) versus low social support (lower than 6) for each study visit.
Our covariates included age (in continuous single years), primary school completion (standard 8), ever being married, sufficient monthly income to support one’s family, and self-reported history of depression. These variables were measured at baseline and have been shown to be associated with perinatal mental health in Malawi (Harrington et al., 2019b; Stewart et al., 2014).
Data Analysis
We first examined demographic characteristics and probable depression at baseline and compared across cohorts using chi-square tests. We then used multilevel generalized linear models (MLM) with a binomial distribution and logit link function to examine the odds of probable depression by time in months, cohort, and levels of social support. MLM was chosen for two main reasons (Singer et al., 2003). First, our hierarchical data structure included multiple assessments nested within participants and MLM accounts for interdependencies in repeated measures data by specifying a random intercept for each person in the sample. Second, MLM can simultaneously model between-person differences and within-person differences across assessments. Between-person differences are estimated using variables that do not change across assessments (level one, fixed effects, baseline covariates) and within-person differences are estimated using variables that can change across assessments (level two, random effects, time (months) and social support) (Kwok et al., 2008).
We fit two models. In our first model, we examined the odds of probable depression across the entire perinatal period with regard to time and cohort. The model was fit to time since enrollment (in months, mean centered at child’s birth month) at Level 2 and cohort and covariates at Level 1. The model included the main effects of each independent variable as well as a cross-level interaction (cohort*time) to examine whether cohort modified the relationship between time and probable depression. In the second model, we examined the odds of probable postpartum depression with regard to time, social support, and cohort. The model was fit to time (in months, mean centered at birth month) and high social support (vs. low) at Level 2 and cohort and covariates at Level 1. The model included main effects of each independent variable as well as cross-level interactions (cohort*time and cohort*social support) to examine whether cohort modified the relationship between time and probable postpartum depression and the relationship between social support and probable postpartum depression. All models were fit using a maximum likelihood approach in XTMELOGIT in StataSE version 14.2 (College Station, TX).
RESULTS
Demographics at Baseline
The sample include 468 women living with HIV in three cohorts: newly diagnosed participants (Cohort A: N = 268, 57%), second line therapy participants (Cohort B: N = 77, 16%), and previously defaulting participants (Cohort C: N = 123, 26%). Overall, participants had a median age of 27 years (IQR 23–32 years) and primarily presented to antenatal care during their third trimester (57%, N = 269). Most women were ever married (90%, N = 421) and completed primary school (51%, N = 240) while less than a quarter reported having enough monthly income to support their families (21%, N = 97). Many women had a self-reported history of depression or anxiety (40%, N = 185) (Table 1).
Table 1.
Demographics by Cohort at Enrollment
| Total (N = 468) | Cohort A: Newly Diagnosed (N = 268) | Cohort B: Second Line (N = 77) | Cohort C: Defaulters (N = 123) | Chi-Square | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | % | N | % | N | % | N | % | p-value | |
| Median Age (IQR) | 27.0 | (23–32) | 26.6 | (23–30) | 29.8 | (26–33) | 26.1 | (23–32) | 0.00 |
| Ever Married | 421 | 90% | 242 | 90% | 68 | 88% | 111 | 90% | 0.87 |
| Completed Primary School | 240 | 51% | 150 | 56% | 39 | 51% | 51 | 41% | 0.03 |
| Sufficient Monthly Income to Support Family | 97 | 21% | 66 | 25% | 14 | 18% | 17 | 14% | 0.04 |
| Current Trimester | |||||||||
| First | 6 | 1% | 4 | 1% | 1 | 1% | 1 | 1% | |
| Second | 193 | 41% | 118 | 44% | 30 | 39% | 45 | 37% | |
| Third | 269 | 57% | 146 | 54% | 46 | 60% | 77 | 63% | 0.63 |
| Previously Depressed | 185 | 40% | 104 | 39% | 31 | 40% | 50 | 41% | 0.63 |
Perinatal Depression
The prevalence of probable perinatal depression decreased from the antenatal period to the postnatal period. At antenatal enrollment, 15% of women (N=69) reported current probable depression and prevalence differed across Cohorts A, B, and C (18%, 21 %, 5%, p = 0.001). Postpartum, the prevalence of probable postpartum depression was 4% (N=21) at the first postnatal visit and 3% (N=13) at the second postnatal visit. Reported probable depression was lower at all postpartum time points than at antenatal enrollment (Table 2).
Table 2.
Probable Depression and Social Support by Cohort Across Perinatal Period
| Antenatal Care Visit 1 | Postnatal Care Visit 1 | Postnatal Care Visit 2 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | N | % | |
| Probable Depression | ||||||
| Total (N = 468) | 69 | 15 % | 21 | 4% | 13 | 3% |
| Cohort A (N = 268) | 47 | 18 % | 12 | 4% | 8 | 3% |
| Cohort B (N = 77) | 16 | 21% | 7 | 9% | 3 | 4% |
| Cohort C (n = 123) | 6 | 5% | 2 | 2% | 2 | 2% |
| Social Support Items | ||||||
| Have Someone to Have a Good Time With | ||||||
| Never | 28 | 6% | 33 | 7% | ||
| Some Times | 133 | 28% | 115 | 25% | ||
| Most Times | 307 | 66% | 300 | 64% | ||
| Have Someone to Do Something Enjoyable With | ||||||
| Never | 63 | 13 % | 57 | 12 % | ||
| Some Times | 184 | 39% | 172 | 37% | ||
| Most Times | 221 | 47% | 219 | 47% | ||
| Have Someone to Get Together With | ||||||
| Never | 75 | 16 % | 84 | 18 % | ||
| Some Times | 154 | 33% | 141 | 30% | ||
| Most Times | 239 | 51% | 223 | 48% | ||
| High Social Support | ||||||
| Total (N = 468) | 307 | 66 % | 300 | 64 % | ||
| Cohort A (N = 268) | 176 | 66 % | 168 | 63 % | ||
| Cohort B (N = 77) | 48 | 62% | 52 | 68% | ||
| Cohort C (N = 123) | 83 | 67% | 80 | 65% | ||
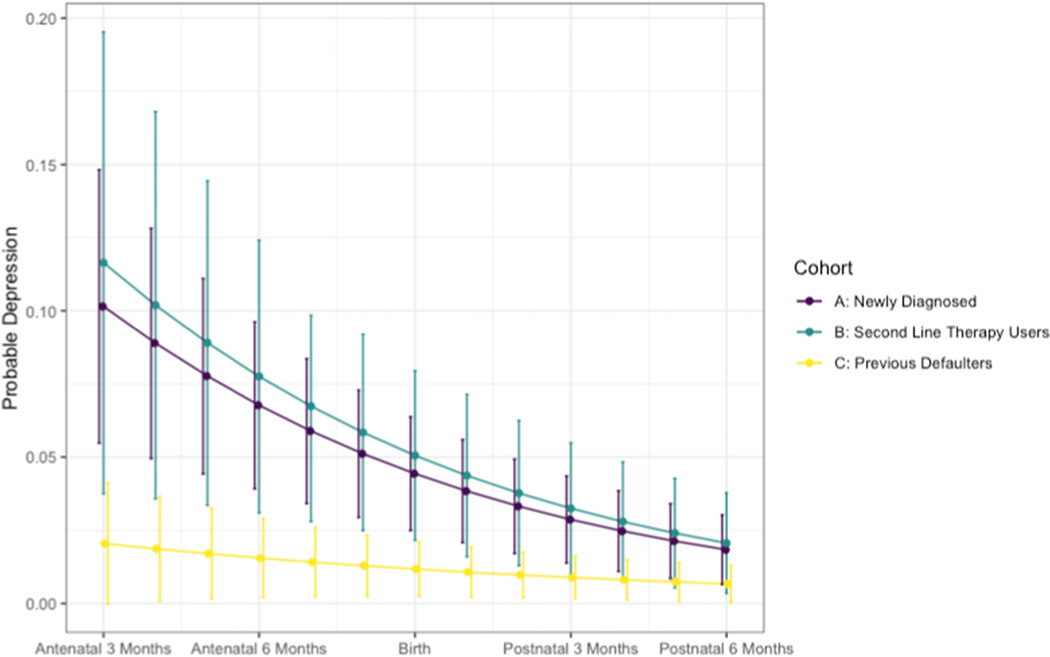
Table 3 contains the results of the model examining the odds of probable depression across the entire perinatal period in regard to time and cohort. We found a decreasing time trend for probable depression across our sample. The odds ratio 0.87 (95% CI: 0.82, 0.92) represents the effect of time on the likelihood of probable depression and there was a decrease of 13% in the odds of probable depression for each additional month since study enrollment. Probable depression was higher in Cohort A (OR: 3.25, 95% CI: 1.59, 6.65) and Cohort B (OR: 3.39, 95% CI: 1.44, 7.99) as compared to Cohort C. Study cohort significantly modified the relationship between time and probable depression (β: −0.09, 95% CI: −0.15, −0.02) and is illustrated in Figure 2. The three lines differ in both intercept and slope, as is indicated by the parameter estimates in Table 3. The difference in intercepts suggests that participants in Cohort A and B were more likely to have probable depression during the antenatal period as compared to Cohort C. The difference in slopes indicates that depression declined more rapidly in Cohort A and B as compared to Cohort C.
Table 3.
Probable Perinatal Depression*
| β | SE | OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Level 2 Effects | |||||
| Time (Months) | −0.14 | 0.03 | 0.87 | (0.82, 0.92) | < 0.001 |
| Level 1 Effects | |||||
| Cohort | |||||
| Cohort A (Initiating ART) | 1.18 | 0.36 | 3.25 | (1.59, 6.65) | 0.001 |
| Cohort B (Second Line Therapy) | 1.22 | 0.44 | 3.39 | (1.44, 7.99) | 0.002 |
| Cohort C (Defaulters) - | |||||
| Reference | |||||
| Cross Level Interaction | |||||
| Cohort*Time | −0.09 | 0.02 | 0.91 | (0.86, 0.98) | 0.048 |
Adjusted for age, marital status, primary school completion, sufficient monthly income, and depression history
Figure 2.
Probable Perinatal Depression by Cohort
Postpartum Depression and Social Support
At the first postnatal visit, 4% of women (N=21) reported current probable depression and prevalence differed across Cohorts A, B, and C (4%, 9 %, 2%, p = 0.046) (Table 2). Table 5 contains the results of the model examining the odds of probable postpartum depression in regard to time, social support, and cohort. There was a very small decreasing time trend for probable postpartum depression, the odds of probable postpartum depression decreased by 3% for each additional month since giving birth (OR: 0.97, 95% CI: 0.94, 0.99). However, there were no differences in probable postpartum depression between Cohort A (OR: 1.87, 95% CI: 0.69, 5.06) and Cohort B (OR: 2.72, 95% CI: 0.82, 9.05), as compared to Cohort C. As such, study cohort did not moderate the relationship between time and probable postpartum depression (β: 0.01, 95% CI: −0.03, 0.05) (Table 4)
Table 4.
Probable Postpartum Depression*
| β | SE | OR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Level 2 Effects | |||||
| Time (Months) | −0.03 | 0.02 | 0.97 | (0.94 ,0.99) | 0.038 |
| High Social Support | −1.65 | 0.61 | 0.19 | (0.06 ,0.64) | < 0.001 |
| Level 1 Effects | |||||
| Cohort | |||||
| Cohort A (Initiating ART) | 0.63 | 0.51 | 1.87 | (0.69 ,5.06) | 0.237 |
| Cohort B (Second Line Therapy) | 1.00 | 0.61 | 2.72 | (0.82 ,9.05) | 0.096 |
| Cohort C (Defaulters) - Reference | |||||
| Cross Level Interaction | |||||
| Cohort*Time | 0.01 | 0.02 | 1.01 | (0.97 ,1.05) | 0.691 |
| Cohort*High Social Support | 0.06 | 0.40 | 1.06 | (0.49 ,2.33) | 0.876 |
Adjusted for age, marital status, primary school completion, sufficient monthly income, and depression history
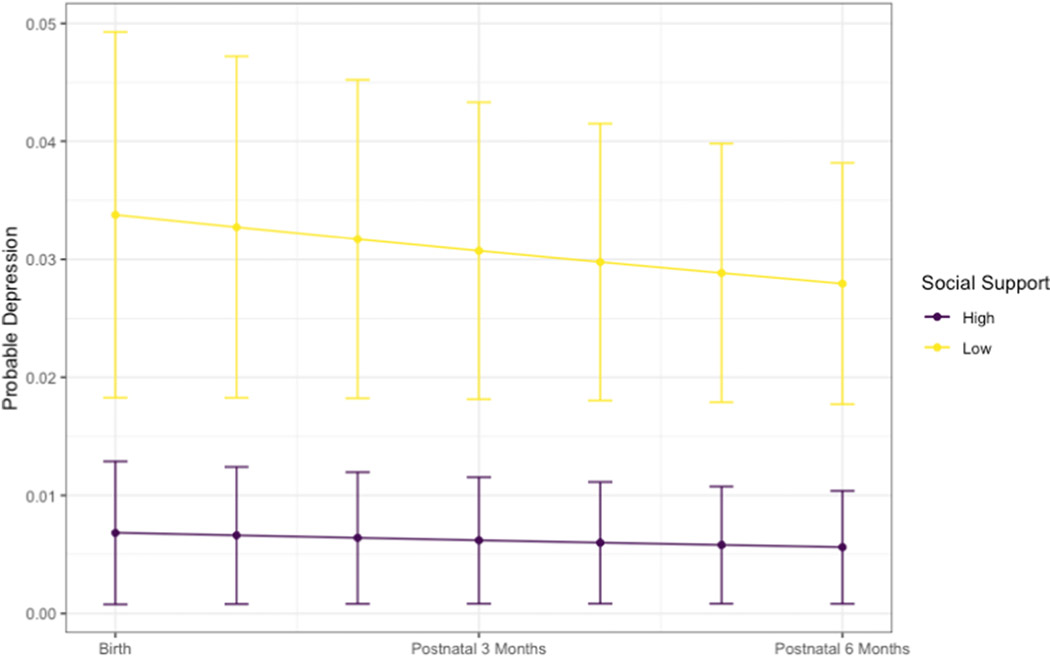
In terms of social support, half of all participants reported having someone to have a good time with (N= 307, 66%), do something enjoyable with (N = 221, 47%), and get together with (N = 239, 51%) at most times. When aggregated, 66% of participants reported high levels of social support at the first postnatal visit but high social support did not differ across Cohort A, B, and C (66%, 62%, 67%, p = 0.757) (Table 2). Figure 3 illustrates the change in probable depression during the postpartum period by level of social support. The odds of probable postpartum depression were 81% lower for participants who had high social support as compared to those who had low social support (OR: 0.19, 95% CI: 0.06, 0.64). Study cohort did not moderate the relationship between social support and probable postpartum depression (β: 0.06, 95% CI: −0.10, 0.85) (Table 4).
Figure 3.
Probable Postpartum Depression by Social Support
DISCUSSION
Among our observational cohort, we found probable perinatal depression to significantly decrease from the antenatal period to the postpartum period. Probable depression differed across cohorts during the antenatal period with those newly diagnosed and those on second line therapy more likely to have probable depression when compared to previous defaulters. During the postpartum period, there were no significant differences between cohorts. At the first postnatal visit, most women reported high levels of social support. Yet, those with high social support were less likely to have probable postpartum depression than those with low social support.
The prevalence of antenatal depression (15%) and postpartum depression (3–4%) differed from other published estimated in sub-Saharan Africa. A literature review of studies in the region produced higher weighted mean prevalence estimates of antenatal depression (23.4%), suspected antenatal depression (43.5%), postnatal depression (22.5%), and suspected postnatal depression (31.1%) among women living with HIV (Sowa et al., 2015). A meta-analysis including studies from the United States and sub-Saharan Africa estimated a mean 36% (95% CI: 27, 45%) prevalence of antenatal depression and a mean 21% (95% CI: 14, 27%) prevalence of postpartum depression among women living with HIV (Zhu et al., 2019). The primary analysis of this observational cohort data posited several hypotheses to explain the low prevalence in our data including: loss-to-follow-up of more depressed women, remarkable resilience of women who chose to participate and stay engaged in the study, social desirability bias, and possible poor performance and understanding of the EPDS and PHQ-9 (Harrington et al., 2019b). A qualitative investigation of these phenomena found that women from the study reported some confusion around the wording of the screening questions and concern that the tools failed to capture culturally relevant symptoms, such as ‘thinking too much (Harrington et al., 2020). There is a need to ensure that appropriate and valid measures are used to continue build understanding around the progression of perinatal depression in sub-Saharan Africa (Tsai et al., 2013).
Our study found a higher likelihood of probable depression during the antenatal period among those newly diagnosed and on second line therapy than those who had previously defaulted, though these differences were attenuated over time. Few studies have compared perinatal depression between these groups, though one study in South Africa found a similar prevalence of antenatal depression between groups diagnosed during the current pregnancy and those who already knew their status (Peltzer et al., 2018). It is possible that the cohorts in our study experienced different psychological stressors. For example, those newly diagnosed must manage an HIV diagnosis during pregnancy and those on second line therapy might be experiencing concerns related to modifying their HIV medication during pregnancy (LeMasters et al., 2020). The low prevalence of probable depression among out previous defaulter cohort was unexpected given that depression has been associated with low engagement in care and adherence to ART among WLWH in SSA. It might be possible that previous defaulters in our study potentially had time to accept their status and may be driven to reengage in care to protect themselves and their future child. Fortunately, early mental health intervention has been shown to improve maternal mental and social wellbeing and positively impact birth outcomes and child development (Austin, 2004; Cena et al., 2020; Rahman et al., 2013). In settings such as Malawi where mental health resources are limited, it will continue to be essential to identify women at highest risk of perinatal depression.
During the postpartum period, the majority of participants reported high levels of social support and those with high levels of social support had lower odds of probable depression. Our results align with existing literature documenting the buffering effects of social support on perinatal depression among women living with HIV in Malawi and across sub-Saharan Africa (Harrington et al., 2019b; Kapetanovic et al., 2014; LeMasters et al., 2020; Stewart et al., 2013). However, we did not observe significant differences in social support among our three cohorts (newly diagnosed, second line therapy users, and previous defaulters) or significant changes in social support across the postpartum period. These findings might be due to our measure of social support and timing of when it was measured. The positive social interaction items of the Medical Outcomes Study Social Support Survey instrument assess the frequency of supportive interactions, but not the type or source of support provided. It is possible that cohorts varied in terms of their access to emotional, instrumental, informational, and appraisal support or support from social network members such as partners, family members, and peers. In addition, we did not measure social support during the antenatal period, a vulnerable time where there might be variation in perceptions and needs of support. Future work to understand social support amongst this population is important as social support, similar to perinatal depression, is a well-established correlate of adherence to PMTCT programs (Ambia and Mandala, 2016; Biaggi et al., 2016; Elwell, 2016; Grede et al., 2014). Taken together, interventions that improve depression and provide social support have the potential to help women living with HIV cope with the stress of chronic illness as they navigate pregnancy, childbirth, and engagement in lifelong treatment.
Limitations
Despite the strengths of using longitudinal data there are several limitations that warrant discussion. First, depression assessments were intended to occur at enrollment (antenatal), week 6, and at months 3, 6, and 12 postpartum, but many participants’ study visits did not align with this schedule. In addition, most women enrolled in the study towards the end of their second trimester or early in their third trimester. This limited our ability to measure depression in early pregnancy and it is possible that this variability in assessment impacted our results related to changes in probable depression over time. Second, we only included participants with antenatal and postnatal depression measurements in our sample. Participants who were missing these measurements or WLWH who chose not to enroll in our study, and thus excluded from our analysis, may be different across mental health, social support, and HIV treatment and retention outcomes. Third, the PHQ-9 and EPDS-10 thresholds used in our study were much lower than thresholds utilized in studies conducted in neighboring countries in SSA and limits comparability to other study populations. We used lower thresholds to be consistent with other papers published amongst our study population in Malawi (Harrington et al., 2020, 2019b, 2018; Udedi et al., 2019).
CONCLUSION
Our results suggest that amongst our sample of WLWH enrolled in Malawi’s Option B+ program, the prevalence of probable depression was higher during the antenatal period than the postpartum period and decreased over time. In addition, probable depression significantly varied among women newly diagnosed with HIV, second line therapy users, and previous defaulters. Social support was protective for postpartum depression across all treatment and retention groups. This evidence serves as a starting point for developing and tailoring interventions that improve mental health outcomes and their HIV-related clinical correlates in WLWH during the perinatal period.
HIGHLIGHTS.
Probable depression was higher in the antenatal period than the postpartum period
Probable depression varied across treatment and retention groups
Social support was protective for postpartum depression among all participants
Lack of diagnostic psychiatric evaluation precluded actual diagnosis of depression
Depression screening and social support should be considered in PMTCT programs
ACKNOWLEDGEMENTS:
We are appreciative of our S4 study collaborators (the Malawi Ministry of Health HIV/AIDS Unit, Baobab Health, Lighthouse Trust, Baylor College of Medicine), Bwaila Hospital Family Health Unit, and UNC Project-Malawi. Special thanks to the S4 study participants and the S4 study team.
SOURCES OF FUNDING:
NLB and BLD were supported by the National Institute of Allergy and Infectious Diseases (T32 AI00700140). MAS was supported by National Institute of Mental Health (T32MH096724). BJH was supported by National Institutes of General Medical Sciences (T32GM008719) and Mental Health (F30MH111370). NLB, BJH, and MAS were supported by the National Institutes of Health Fogarty International Center (R25TW009340). MBC was supported by the Malawi HIV Implementation Research Scientist Training Program (D43TW01006). Regulatory support was provided through the UNC Center for AIDS Research (P30AI50410). The parent study was funded by the National Institute for Child Health and Human Development (R01HD080485). The funding sources had no role in the study design, data collection and analysis, interpretation of results, or preparation of the manuscript for publication.
Footnotes
CONFLICT OF INTEREST:
All authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ambia J, Mandala J, 2016. A systematic review of interventions to improve prevention of mother-to-child HIV transmission service delivery and promote retention. J. Int. AIDS Soc. 19, 20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin M-P, 2004. Antenatal screening and early intervention for “perinatal” distress, depression and anxiety: where to from here? Arch. Womens Ment. Health 7, 1–6. 10.1007/s00737-003-0034-4 [DOI] [PubMed] [Google Scholar]
- Bajunirwe F, Tisch DJ, King CH, Arts EJ, Debanne SM, Sethi AK, 2009. Quality of life and social support among patients receiving antiretroviral therapy in Western Uganda. AIDS Care 21, 271–279. 10.1080/09540120802241863 [DOI] [PubMed] [Google Scholar]
- Baron E, Bass J, Murray SM, Schneider M, Lund C, 2017. A systematic review of growth curve mixture modelling literature investigating trajectories of perinatal depressive symptoms and associated risk factors. J. Affect. Disord. 223, 194–208. 10.1016/j.jad.2017.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel D, Kriston L, Barkmann C, Appiah-Poku J, Te Bonle M, Esther Doris KY, Carine Esther BK, Jean Armel KE, Mohammed Y, Osei Y, Fordjour D, Owusu D, Eberhardt KA, Hinz R, Koffi M, N’Goran E, Nguah SB, Tagbor H, Schoppen S, Ehrhardt S, Bindt C, 2016. Longitudinal course of ante- and postpartum generalized anxiety symptoms and associated factors in West-African women from Ghana and Côte d’Ivoire. J. Affect. Disord. 197, 125–133. 10.1016/j.jad.2016.03.014 [DOI] [PubMed] [Google Scholar]
- Biaggi A, Conroy S, Pawlby S, Pariante CM, 2016. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J. Affect. Disord. 191, 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisetegn TA, Mihretie G, Muche T, 2016. Prevalence and Predictors of Depression among Pregnant Women in Debretabor Town, Northwest Ethiopia. PloS One 11, e0161108. 10.1371/journal.pone.0161108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale M, Wild L, Cluver L, Kuo C, 2014. The relationship between social support and anxiety among caregivers of children in HIV-endemic South Africa. Psychol. Health Med. 19, 490–503. 10.1080/13548506.2013.832780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cena L, Palumbo G, Mirabella F, Gigantesco A, Stefana A, Trainini A, Tralli N, Imbasciati A, 2020. Perspectives on Early Screening and Prompt Intervention to Identify and Treat Maternal Perinatal Mental Health. Protocol for a Prospective Multicenter Study in Italy. Front. Psychol. 11, 365. 10.3389/fpsyg.2020.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholera R, Gaynes BN, Pence BW, Bassett J, Qangule N, Macphail C, Bernhardt S, Pettifor A, Miller WC, 2014. Validity of the Patient Health Questionnaire-9 to screen for depression in a high-HIV burden primary healthcare clinic in Johannesburg, South Africa. J. Affect. Disord. 167, 160–166. 10.1016/j.jad.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb S, 1976. Social support as a moderator of life stress. Psychosom. Med. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA, 1985. Stress, social support, and the buffering hypothesis. Psychol. Bull. 98, 310–357. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R, 1987. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Dadi AF, Miller ER, Bisetegn TA, Mwanri L, 2020. Global burden of antenatal depression and its association with adverse birth outcomes: an umbrella review. BMC Public Health 20, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell K, 2016. Facilitators and barriers to treatment adherence within PMTCT programs in Malawi. AIDS Care 28, 971–975. [DOI] [PubMed] [Google Scholar]
- Epino HM, Rich ML, Kaigamba F, Hakizamungu M, Socci AR, Bagiruwigize E, Franke MF, 2012. Reliability and construct validity of three health-related self-report scales in HIV-positive adults in rural Rwanda. AIDS Care 24, 1576–1583. [DOI] [PubMed] [Google Scholar]
- Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, Brinker-Spence P, Job C, Mercer DE, Wang YL, 2002. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am. J. Psychiatry 159, 1752–1759. [DOI] [PubMed] [Google Scholar]
- Familiar I, Sikorskii A, Murray S, Ruisenor-Escudero H, Nakasujja N, Korneffel C, Boivin M, Bass J, 2019. Depression Symptom Trajectories Among Mothers Living with HIV in Rural Uganda. AIDS Behav. 23, 3411–3418. 10.1007/s10461-019-02465-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellmeth G, Fazel M, Plugge E, 2017. Migration and perinatal mental health in women from low-and middle-income countries: a systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 124, 742–752. [DOI] [PubMed] [Google Scholar]
- Gaede BM, Majeke SJ, Modeste RRM, Naidoo JR, Titus MJ, Uys LR, 2006. Social support and health behaviour in women living with HIV in KwaZulu-Natal. SAHARA J J. Soc. Asp. HIVAIDS Res. Alliance 3, 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman EC, Schneider M, Lund C, 2019. Perinatal depressive symptoms among low-income South African women at risk of depression: trajectories and predictors. BMC Pregnancy Childbirth 19, 202. 10.1186/s12884-019-2355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T, 2005. Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol. 106, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Gelaye B, Rondon MB, Araya R, Williams MA, 2016. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry 3, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R, 2009. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr. Scand. 119, 350–364. [DOI] [PubMed] [Google Scholar]
- Grede N, de Pee S, Bloem M, 2014. Economic and social factors are some of the most common barriers preventing women from accessing maternal and newborn child health (MNCH) and prevention of mother-to-child transmission (PMTCT) services: a literature review. AIDS Behav. 18, 516–530. [DOI] [PubMed] [Google Scholar]
- Haas AD, Tenthani L, Msukwa MT, Tal K, Jahn A, Gadabu OJ, Spoerri A, Chimbwandira F, van Oosterhout JJ, Keiser O, 2016. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV 3, e175–182. 10.1016/S2352-3018(16)00008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AD, van Oosterhout JJ, Tenthani L, Jahn A, Zwahlen M, Msukwa MT, Davies M-A, Tal K, Phiri N, Spoerri A, Chimbwandira F, Egger M, Keiser O, 2017. HIV transmission and retention in care among HIV-exposed children enrolled in Malawi’s prevention of mother-to-child transmission programme. J. Int. AIDS Soc. 20, 21947. 10.7448/IAS.20.1.21947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C, Medhin G, Selamu M, Breuer E, Worku B, Hailemariam M, Lund C, Prince M, Fekadu A, 2015. Validity of brief screening questionnaires to detect depression in primary care in Ethiopia. J. Affect. Disord. 186, 32–39. 10.1016/j.jad.2015.07.015 [DOI] [PubMed] [Google Scholar]
- Harrington BJ, DiPrete BL, Jumbe AN, Ngongondo M, Limarzi L, Wallie SD, Chagomerana MB, Hosseinipour MC, Team SS, 2019a. Safety and efficacy of Option B+ ART in Malawi: few severe maternal toxicity events or infant HIV infections among pregnant women initiating tenofovir/lamivudine/efavirenz. Trop. Med. Int. Health 24, 1221–1228. [DOI] [PubMed] [Google Scholar]
- Harrington BJ, Hosseinipour MC, Maliwichi M, Phulusa J, Jumbe A, Wallie S, Gaynes BN, Maselko J, Miller WC, Pence BW, 2018. Prevalence and incidence of probable perinatal depression among women enrolled in Option B+ antenatal HIV care in Malawi. J. Affect. Disord. 239, 115–122. 10.1016/j.jad.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington BJ, Klyn LL, Ruegsegger LM, Thom A, Jumbe AN, Maliwichi M, Stockton MA, Akiba CF, Go V, Pence BW, 2020. Locally contextualizing understandings of depression, the EPDS, and PHQ-9 among a sample of postpartum women living with HIV in Malawi. J. Affect. Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington BJ, Pence BW, John M, Melhado CG, Phulusa J, Mthiko B, Gaynes BN, Maselko J, Miller WC, Hosseinipour MC, 2019b. Prevalence and factors associated with antenatal depressive symptoms among women enrolled in Option B+ antenatal HIV care in Malawi: a cross-sectional analysis. J Ment Health 28, 198–205. 10.1080/09638237.2018.1487542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RM, Phiri K, Parent J, Grotts J, Elashoff D, Kawale P, Yeatman S, Currier JS, Schooley A, 2017. Factors associated with retention in Option B+ in Malawi: a case control study. J. Int. AIDS Soc. 20, 21464. 10.7448/IAS.20.01.21464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J, Group HERS, 2001. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. Jama 285, 1466–1474. [DOI] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 392, 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic S, Dass-Brailsford P, Nora D, Talisman N, 2014. Mental health of HIV-seropositive women during pregnancy and postpartum period: a comprehensive literature review. AIDS Behav. 18, 1152–1173. 10.1007/s10461-014-0728-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JD, Hartman C, Graham J, Kallen MA, Giordano TP, 2014. Social support as a predictor of early diagnosis, linkage, retention, and adherence to HIV care: Results from the Steps Study. J. Assoc. Nurses AIDS Care JANAC 25, 405–413. 10.1016/j.jana.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Ahmed S, Hosseinipour MC, Yu X, Nguyen C, Chimbwandira F, Paul ME, Kazembe PN, Abrams EJ, 2015. Brief Report: Impact of Option B+ on the Infant PMTCT Cascade in Lilongwe, Malawi. J. Acquir. Immune Defic. Syndr. 1999 70, 99–103. 10.1097/QAI.0000000000000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinszky Z, Dudas RB, 2015. Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. J. Affect. Disord. 176, 95–105. [DOI] [PubMed] [Google Scholar]
- Kwok O-M, Underhill AT, Berry JW, Luo W, Elliott TR, Yoon M, 2008. Analyzing Longitudinal Data with Multilevel Models: An Example with Individuals Living with Lower Extremity Intra-articular Fractures. Rehabil. Psychol. 53, 370–386. 10.1037/a0012765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasters K, Dussault J, Barrington C, Bengtson A, Gaynes B, Go V, Hosseinipour MC, Kulisewa K, Kutengule A, Meltzer-Brody S, Midiani D, Mphonda S, Udedi M, Pence B, 2020. “Pain in my heart”: Understanding perinatal depression among women living with HIV in Malawi. PLOS ONE 15, e0227935. 10.1371/journal.pone.0227935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhele I, Nattey C, Jinga N, Mongwenyana C, Fox MP, Onoya D, 2019. Prevalence and predictors of postpartum depression by HIV status and timing of HIV diagnosis in Gauteng, South Africa. PLOS ONE 14, e0214849. 10.1371/journal.pone.0214849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakimuli-Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, Katabira E, Nachega JB, 2012. Depression, alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 16, 2101–2118. [DOI] [PubMed] [Google Scholar]
- Ncama BP, McInerney PA, Bhengu BR, Corless IB, Wantland DJ, Nicholas PK, McGibbon CA, Davis SM, 2008a. Social support and medication adherence in HIV disease in KwaZulu-Natal, South Africa. Int. J. Nurs. Stud. 45, 1757–1763. 10.1016/j.ijnurstu.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Ncama BP, McInerney PA, Bhengu BR, Corless IB, Wantland DJ, Nicholas PK, McGibbon CA, Davis SM, 2008b. Social support and medication adherence in HIV disease in KwaZulu-Natal, South Africa. Int. J. Nurs. Stud. 45, 1757–1763. 10.1016/j.ijnurstu.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Ozbay F, Johnson DC, Dimoulas E, Morgan CA III, Charney D, Southwick S, 2007. Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry Edgmont 4, 35. [PMC free article] [PubMed] [Google Scholar]
- Pellowski JA, Bengtson AM, Barnett W, DiClemente K, Koen N, Zar HJ, Stein DJ, 2019. Perinatal depression among mothers in a South African birth cohort study: Trajectories from pregnancy to 18 months postpartum. J. Affect. Disord. 259, 279–287. 10.1016/j.jad.2019.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, Rodriguez VJ, Lee TK, Jones D, 2018. Prevalence of prenatal and postpartum depression and associated factors among HIV-infected women in public primary care in rural South Africa: a longitudinal study. AIDS Care 30, 1372–1379. 10.1080/09540121.2018.1455960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Gaynes BN, Atashili J, O’Donnell JK, Tayong G, Kats D, Whetten R, Whetten K, Njamnshi AK, Ndumbe PM, 2012. Validity of an interviewer-administered patient health questionnaire-9 to screen for depression in HIV-infected patients in Cameroon. J. Affect. Disord. 143, 208–213. 10.1016/j.jad.2012.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaros C, Mosery N, Smit JA, Luthuli F, Gordon JR, Greener R, Bennett K, Bangsberg DR, Safren SA, 2014. PMTCT adherence in pregnant South African women: the role of depression, social support, stigma and structural barriers to care. AIDS Res. Hum. Retroviruses 30, A61–A61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Fisher J, Bower P, Luchters S, Tran T, Yasamy MT, Saxena S, Waheed W, 2013. Interventions for common perinatal mental disorders in women in low- and middle-income countries: a systematic review and meta-analysis. Bull. World Health Organ. 91, 593–601I. 10.2471/BLT.12.109819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg NE, Pettifor AE, 2018. Taking Malawi’s option B+ programme from a B+ to an A. Lancet HIV 5, e672–e673. 10.1016/S2352-3018(18)30320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer A, Ayers S, Smith H, 2010. Pre- and postnatal psychological wellbeing in Africa: a systematic review. J. Affect. Disord. 123, 17–29. 10.1016/j.jad.2009.06.027 [DOI] [PubMed] [Google Scholar]
- Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, Chirwa Z, Harries AD, van Oosterhout JJ, Meguid T, Ben-Smith A, Zachariah R, Lynen L, Zolfo M, Van Damme W, Gilks CF, Atun R, Shawa M, Chimbwandira F, 2011. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet Lond. Engl. 378, 282–284. 10.1016/S0140-6736(10)62303-3 [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL, 1991. The MOS social support survey. Soc. Sci. Med. 1982 32, 705–714. 10.1016/0277-9536(91)90150-b [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB, Willett JB, 2003. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford university press. [Google Scholar]
- Sowa NA, Cholera R, Pence BW, Gaynes BN, 2015. Perinatal depression in HIV-infected African women: a systematic review. J. Clin. Psychiatry 76, 1385–1396. 10.4088/JCP.14r09186 [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM, 2014. Effects of perinatal mental disorders on the fetus and child. The Lancet 384, 1800–1819. [DOI] [PubMed] [Google Scholar]
- Stewart RC, Umar E, Gleadow-Ware S, Creed F, Bristow K, 2015. Perinatal distress and depression in Malawi: an exploratory qualitative study of stressors, supports and symptoms. Arch. Womens Ment. Health 18, 177–185. 10.1007/s00737-014-0431-x [DOI] [PubMed] [Google Scholar]
- Stewart RC, Umar E, Tomenson B, Creed F, 2014. A cross-sectional study of antenatal depression and associated factors in Malawi. Arch. Womens Ment. Health 17, 145–154. 10.1007/s00737-013-0387-2 [DOI] [PubMed] [Google Scholar]
- Stewart RC, Umar E, Tomenson B, Creed F, 2013. Validation of screening tools for antenatal depression in Malawi—A comparison of the Edinburgh Postnatal Depression Scale and Self Reporting Questionnaire. J. Affect. Disord. 150, 1041–1047. [DOI] [PubMed] [Google Scholar]
- Stringer EM, Meltzer-Brody S, Kasaro M, Stuebe AM, Wiegand S, Paul R, Stringer JS, 2014. Depression, pregnancy, and HIV: the case to strengthen mental health services for pregnant and post-partum women in sub-Saharan Africa. Lancet Psychiatry 1, 159–162. [DOI] [PubMed] [Google Scholar]
- Tsai AC, Scott JA, Hung KJ, Zhu JQ, Matthews LT, Psaros C, Tomlinson M, 2013. Reliability and validity of instruments for assessing perinatal depression in African settings: systematic review and meta-analysis. PloS One 8, e82521. 10.1371/journal.pone.0082521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udedi M, Muula AS, Stewart RC, Pence BW, 2019. The validity of the patient health Questionnaire-9 to screen for depression in patients with type-2 diabetes mellitus in non-communicable diseases clinics in Malawi. BMC Psychiatry 19. 10.1186/s12888-019-2062-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umuziga MP, Adejumo O, Hynie M, 2020. A cross-sectional study of the prevalence and factors associated with symptoms of perinatal depression and anxiety in Rwanda. BMC Pregnancy Childbirth 20, 68. 10.1186/s12884-020-2747-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn BP, Pace W, Wollan PC, Bertram S, Kurland M, Graham D, Dietrich A, 2009. Concordance of Edinburgh Postnatal Depression Scale (EPDS) and Patient Health Questionnaire (PHQ-9) to assess increased risk of depression among postpartum women. J. Am. Board Fam. Med. JABFM 22, 483–491. 10.3122/jabfm.2009.05.080155 [DOI] [PubMed] [Google Scholar]
- Zhong Q, Gelaye B, Rondon M, Sánchez SE, García PJ, Sánchez E, Barrios YV, Simon GE, Henderson DC, Cripe SM, Williams MA, 2014. Comparative Performance of Patient Health Questionnaire-9 and Edinburgh Postnatal Depression Scale for Screening Antepartum Depression. J. Affect. Disord. 162, 1–7. 10.1016/j.jad.2014.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q-Y, Huang D-S, Lv J-D, Guan P, Bai X-H, 2019. Prevalence of perinatal depression among HIV-positive women: a systematic review and meta-analysis. BMC Psychiatry 19, 330. 10.1186/s12888-019-2321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]