Abstract
Background
Hand, foot, and mouth disease (HFMD) is a serious threat among children in China. Some studies have found that air pollution is associated with HFMD incidence, but the results showed heterogeneity. In this study, we aimed to explore the heterogeneity of associations between air pollutants and the number of HFMD cases and to identify significant socioeconomic effect modifiers.
Methods
We collected daily surveillance data on HFMD cases in those aged less than 15 years, air pollution variables and meteorological variables from 2015 to 2017 in the basin area of Sichuan Province. We also collected socioeconomic indicator data. We conducted a two-stage multicity time-series analysis. In the first stage, we constructed a distributed lag nonlinear model (DLNM) to obtain cumulative exposure-response curves between each air pollutant and the numbers of HFMD cases for every city. In the second stage, we carried out a multivariable meta-regression to merge the estimations in the first stage and to identify significant socioeconomic effect modifiers.
Results
We found that PM10, NO2 and O3 concentrations were associated with the number of HFMD cases. An inverted V-shaped association between PM10 and the number of HFMD cases was observed. The overall NO2-HFMD association was a hockey-stick shape. For the relationships of PM10, SO2, NO2, O3 and CO with HFMD counts, approximately 58.5%, 48.4%, 51.0%, 55.6% and 52.5% of the heterogeneity could be explained, respectively. The proportion of primary school students, population density, urbanization rate, number of licensed physicians and number of hospital beds explained part of the heterogeneity and modified the relationships.
Conclusion
Our study explored the heterogeneity of associations between air pollutants and HFMD counts. The proportion of primary school students, population density, urbanization rate, number of licensed physicians and number of hospital beds could modify the relationships. The results can serve as a reference for relevant public health decision making.
Author summary
This study constructed a two-stage multi-city time-series analysis to explore the heterogeneity of air pollutants-HFMD associations and identify significant socioeconomic effect modifiers, which may lead to heterogeneity. In the results, we found that PM10, NO2 and O3 concentrations were associated with HFMD. For each air pollutant-HFMD associations between cities, the heterogeneity was found. The proportion of primary school students, population density, urbanization rate, number of licensed physicians and number of hospital beds explained part of the heterogeneity. The results showed that demographic factors, economic factors, and health resource factors may influence the effect of air pollutants on HFMD, which could further the understanding of the associations between air pollutants and HFMD and could be as evidence for relevant public health decision-making.
1. Introduction
Hand, foot, and mouth disease (HFMD), caused by enteroviruses, is a highly prevalent infectious disease mainly occurring in children [1,2]. In recent decades, it has emerged in many Asian countries [1,3–6]. HFMD has a high incidence and imposes a serious disease burden on those in mainland China. From 2008 to 2019, a total of approximately 22.45 million HFMD cases were reported nationwide, ranking first among all notifiable infectious diseases. Although HFMD is usually self-limiting, it may sometimes lead to serious central nervous system and cardiovascular complications and even death [7]. Approximately 75,881 disability-adjusted life years (DALYs) are caused by HFMD annually [8]. Therefore, HFMD is a severe threat among children. However, only inactivated enterovirus A71 vaccines have been developed, and specific therapies are still lacking [9,10]. Thus, nonpharmaceutical interventions are necessary to reduce the risk of HFMD.
Identifying environmental risk factors is important for the implementation of nonpharmaceutical HFMD interventions. Meteorological factors, such as temperature and relative humidity, have been found to be associated with HFMD in many studies [11–14]. In addition, considering persistent air pollution [15], some studies have recently suggested that air pollutants are associated with the HFMD incidence [16–22]. Nonlinear relationships and delayed effects have been shown for most of these associations. However, controversial results have been reported among different studies. For example, a study in Wuhan [16] found that PM10 exposure increased the risk of HFMD on lag10-11 according to a generalized additive model (GAM). Another study in Ningbo [19] demonstrated that there was no significant PM10-HFMD relationship according to a distributed lag nonlinear model (DLNM). A study in Guangxi [23] stated that the PM10-HFMD relationship has a positive linearity according to a principal component regression (PCR). Studies in Chengdu [20] and Shenzhen [21] showed that the cumulative risk curves of PM10 and HFMD were inverted "V"-shaped according to the DLNM. Among these studies, heterogeneity of the correlations and the shapes of the association curves can be observed. This heterogeneity may be attributed to different study methods, the modification effects socioeconomic variables and so on [24]. Exploring heterogeneity can reveal the influence of effect modifiers on air pollutant-HFMD associations, which may guide HFMD prevention strategies. However, all previous studies on air pollutant-HFMD associations were conducted in a single city. Thus, the exploration of heterogeneity is still lacking.
To determine the reasons for the heterogeneity and obtain average air pollutant-HFMD associations, a multiregional study with a common statistical method is needed. Therefore, we conducted a multicity study based on 17 cities in Sichuan Province with a two-stage time-series analysis. We selected this area because HFMD in Sichuan Province has always been ranked among the top three notifiable infectious diseases. In addition, the selected 17 cities, located in the basin area, have serious air pollution because of rapid industrialization [25,26]. Moreover, different socioeconomic development levels can be found among cities. Two-stage time-series analyses, consisting of a DLNM and meta-analysis, have been conducted in many epidemiological studies to explore modification effects [13,14,27,28]. The DLNM can characterize nonlinear associations between two variables in the lag-response and exposure-response dimensions [29,30]. The meta-analysis can merge city-specific associations and identify effect modifiers of heterogeneity among cities [27,31].
In this work, we carried out a two-stage time-series analysis to explore the heterogeneity of air pollutant-HFMD case associations and identify socioeconomic effect modifiers in the basin area of Sichuan Province. In the first stage, we obtained cumulative exposure-response curves between air pollutant concentrations and the numbers of HFMD cases for every city. In the second stage, we merged the estimations in the first stage and included the city-specific socioeconomic indicators to identify significant effect modifiers. Our results can serve as reference data for the prevention and control of HFMD.
2. Methods
2.1 Ethics statement
Our study was approved by the institutional review board of the School of Public Health, Sichuan University. All HFMD surveillance data were collected from Sichuan Center for Disease Control and Prevention. The study methods were carried out in accordance with relevant guidelines and regulations. Our study was constructed at the population level. Therefore, no confidential information was involved in this study and informed consent was not required.
2.2 Study area
The 17 selected cities, with a total area of 185,757 km2, are located in the basin area of Sichuan Province, China. The study area has a subtropical monsoon climate. The western and southern parts of the basin area are heavily polluted, while the northeastern area is less polluted. The western area has a larger population and more health resources than the northeastern area.
2.3 Data collection
Daily HFMD cases in children under the age of 15 in the study areas from January 1, 2015, to December 31, 2017, were collected from Sichuan Center for Disease Control and Prevention. Daily air pollutant data for the same period, including PM10 (μg/m3), PM2.5 (μg/m3), SO2 (μg/m3), NO2 (μg/m3), CO (mg/m3), O3 (μg/m3) and the air quality index (AQI), were obtained from the China National Environmental Monitoring Center by the arithmetic averaging of hourly data. Daily meteorological data for the same period, including mean temperature (tm, °C), mean relative humidity (humid, %), mean wind velocity (win, m/s), precipitation (rain, mm), and sunshine hours (sun, h), were obtained from the China Meteorological Data Sharing Service System. Missing values for PM10, PM2.5, SO2, NO2, O3, CO, AQI, tm, humid and win were imputed for 21 days (0.11%), 11 days (0.06%), 19 days (0.10%), 22 days (0.12%), 33 days (0.18%), 16 days (0.09%), 60 days (0.32%), 33 days (0.23%), 2 days (0.01%) and 34 days (0.24%) by linear interpolation. Missing values for sun and rain were imputed for 31 days (0.22%) and 1141 days (8.00%) by “0".
According to previous studies, 4 types of city-specific indicators were included in our study: (1) demographic variables: the population density (people per km2), birth rate (‰) and proportion of primary school students (‰); (2) economic variables: gross domestic product (GDP) per person (CNY), GDP increase (%) and urbanization rate (%); (3) health resource variables: the number of licensed physicians (per 1000 population) and number of hospital beds (per 1000 population); and (4) a traffic variable: the total number of passengers (trips). City-specific socioeconomic data were collected from the China City Statistical Yearbooks from 2015 to 2017, and each indicator for each city was calculated by arithmetic averaging of the indicators for three years.
2.4 Statistical analysis
A two-stage multicity time-series analysis was carried out to characterize associations between air pollutant concentrations and the number of HFMD cases and identify significant effect modifiers in the study area from 2015 to 2017. First, to obtain the cumulative exposure-response curves of the effects of PM10, PM2.5, SO2, NO2, CO, and O3 concentrations on the number of HFMD cases, a DLNM was constructed for each city. Then, to obtain overall cumulative exposure-response curves, the estimations in the first stage were merged by multivariable meta-analysis. Finally, each city-specific socioeconomic indicator was included to identify whether the indicator could explain the heterogeneity between cities and evaluate its modification effect.
PM10 and PM2.5 concentrations were found to be highly correlated (rs = 0.94) by Spearman rank correlation analysis (S1 Fig), which may lead to multicollinearity. Therefore, considering that both particulate matter and PM10 were analyzed in several studies, PM10, SO2, NO2, O3, and CO were finally included in our study.
2.4.1 First-stage analysis
To measure the cumulative exposure-response curve of the relationship between each air pollutant and the number of HFMD cases for each city, a DLNM was constructed as follows:
| (1) |
| (2) |
where Yt is the number of HFMD cases on day t(t = 1,2,…1096); α is the intercept; cb(·) is the cross-basis function; and Pit is the i(i = 1,2,…5)th pollutant (i = 1,2,…5) on day t. Natural cubic splines with 3 degrees of freedom (df) were applied to characterize the lag-response relationship between each air pollutant concentration and the number of HFMD cases, and natural cubic splines with 2 knots were applied to characterize the exposure-response relationship. The values of the knots were the 33th and 66th percentiles of each air pollutant concentrations for 17 cities (Knots: PM10: 54.3 μg/m3, 86.0 μg/m3; SO2: 11.9 μg/m3, 15.7 μg/m3; NO2: 25.4 μg/m3, 33.5 μg/m3; O3: 45.0 μg/m3, 70.5 μg/m3; CO: 0.7 mg/m3, 0.9 mg/m3). Zero to fourteen days was the lag interval for PM10, SO2, NO2, CO, and O3. To control for confounding factors, the mean temperature was included by calculating the simple moving weighted averages (SMAs) in the same lag interval as the air pollutants and a natural cubic spline with 3 df. Mjt is the j(j = 1,2,…4)th other meteorological confounder, which was incorporated by calculating the SMAs in the same lag interval as the air pollutants. A natural cubic spline with 8 df per calendar year was used to control for seasonal and long-term trends. Holidayt is a binary variable indicating whether day t was a holiday. DOWt is an indicator of the day of a week. At is the autoregressive term to control for autocorrelation. Sensitivity analysis, using the sum of the quasi Akaike Information Criterion (QAIC) of 17 cities as the criterion, was conducted to determine the forms of variables.
2.4.2 Second-stage analysis
The multivariable meta-regression was constructed as follows:
| (3) |
| (4) |
where is a k-dimensional parameter vector representing the estimate of the association between air pollutants and HFMD in the qth city. Sq is a k × k dimensional variance-covariance matrix of . Uq, a k × kp dimensional block-diagonal matrix, is the Kronecker expansion of p area-specific indicators uq = [u1, u2…up]T. When there is no meta-variable, U = I(k) and β denote the overall mean of the parameter vector . Sq and Ψ are variance-covariance matrices representing within-group variation and between-group variation, respectively.
The multivariable meta-regression with only an intercept and restricted maximum likelihood estimation (REML) were adopted to pool the city-specific estimates from the first stage to obtain the overall cumulative exposure-response associations between the number of HFMD cases and air pollutant concentrations. The heterogeneity of these associations was measured by the Cochran Q test and I2 statistic. Then, each city-specific socioeconomic indicator was independently included in the meta-regression to explore whether the indicator could explain the heterogeneity, and the likelihood ratio (LR) test was carried out to identify significant effect modifiers (P≤0.05).
All statistical analyses were conducted in R 4.0.3 using the packages dlnm, splines and mvmeta.
3. Results
3.1. Descriptive analysis
Table 1 shows the summary characteristics of all the variables in our study. A total of 201,035 HFMD cases in individuals aged less than 15 years in 17 cities were reported from 2015 to 2017. The air pollution concentrations were different among cities (S2 Fig). Regarding PM10, PM2.5, SO2, CO and O3, the southern cities had heavier pollution. Regarding NO2, Chengdu was the most polluted city. Variations were also found among city-specific socioeconomic characteristics (S3 Table). The eastern cities had a higher proportion of primary school students, and the northeastern cities had smaller populations and fewer health resources.
Table 1. Descriptions of daily HFMD cases, meteorological factors and air pollutants in the basin area of Sichuan Province from January 1, 2015, to December 31, 2017.
| Variable | Mean± SD | Median (IQR) | Range |
|---|---|---|---|
| HFMD (cases) | 183.4±100.6 | 176.0 (104.0, 244.0) | (3.0, 517.0) |
| Air pollutants | |||
| PM10 (μg/m3) | 79.8±49.1 | 68.0 (45.0, 103.0) | (6.0, 599.0) |
| PM2.5 (μg/m3) | 49.9±35.7 | 40.0 (25.0, 65.0) | (2.0, 370.0) |
| SO2 (μg/m3) | 14.5±8.5 | 13.0 (9.0, 18.0) | (1.0, 112) |
| NO2 (μg/m3) | 30.5±12.7 | 29.0 (21.0, 38.0) | (4.0, 112.0) |
| O3 (μg/m3) | 62.3±35.0 | 56.0 (37.0, 81.0) | (2.0, 242.0) |
| CO (mg/m3) | 0.9±0.3 | 0.8 (0.6, 1.0) | (0.1, 4.0) |
| AQI | 78.1±41.2 | 67.0 (51.0, 94.0) | (14.0, 399) |
| Meteorological factors | |||
| Tm (°C) | 18.1±7.3 | 18.6 (11.3, 24.1) | (-2.2, 36.1) |
| Humid (%) | 77.4±12.1 | 78.0 (69.0, 87.0) | (20.0, 100.0) |
| Win (m/s) | 1.4±0.6 | 1.3 (1.0, 1.7) | (0.0, 6.4) |
| Sun (h) | 3.3±3.9 | 1.2 (0.0, 6.6) | (0.0, 13.3) |
| Rain(mm) | 2.8±9.3 | 0.0 (0.0, 1.3) | (0.0, 266.7) |
| City-specific indicators | |||
| Population density (people per km2) | 449.8±249.1 | 419.1 (269.9, 596.1) | (102.3, 1146.2) |
| Proportion of students (‰) | 62.9±12.9 | 58.1 (54.1, 73.7) | (49.5, 92.9) |
| Birth rate (‰) | 9.9±0.7 | 9.8 (9.5, 10.2) | (8.6, 11.3) |
| GDP per person (CNY) | 37,095.6±13,516.0 | 35676.7(31,106.0, 38,990.3) | (16,543, 79,381.3) |
| GDP increase (%) | 17.0±10.3 | 19.9 (15.9, 21.1) | (-19.5, 28.6) |
| Urbanization rate (%) | 46.6±7.3 | 46.6 (42.4, 48.7) | (38.8, 71.3) |
| Hospital beds(per 1000 population) | 2.6±0.4 | 2.6 (2.3, 2.8) | (1.9, 3.4) |
| Licensed physicians (per 1000 population) | 7.6±1.4 | 7.5 (6.6, 8) | (6.2, 12.1) |
| Passengers (trips) | 3,055,310.9±251,066.8 | 234,231.0(165,834.0, 293,041.0) | (911,55.0, 1,167,754.0) |
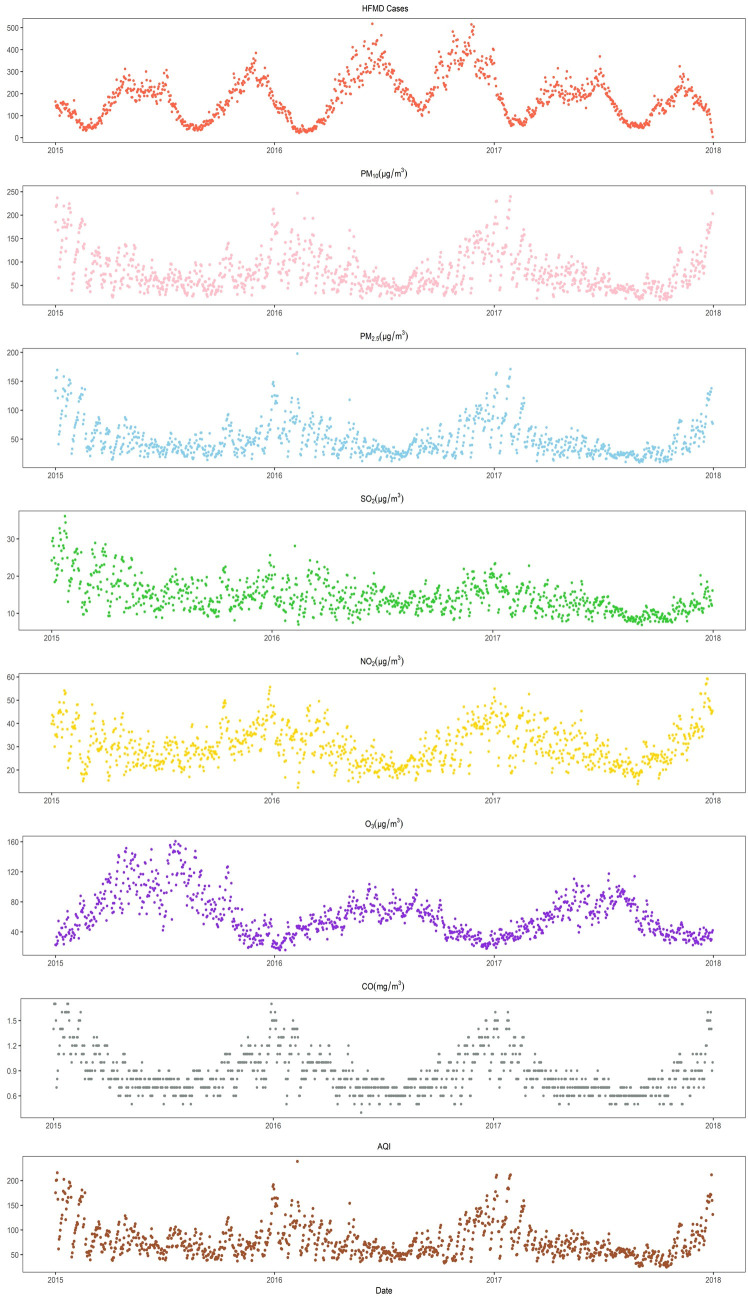
Fig 1 illustrates the seasonal trends of HFMD cases and air pollutants. The number of HFMD cases had two peaks every year, which were concentrated in May-July and November-January. O3 had a peak concentration in summer and a lower concentration in winter. In contrast, the other air pollutant concentrations and the AQI were higher in winter and lower in summer.
Fig 1. Daily distributions of HFMD cases, air pollutants and the AQI in the basin area of Sichuan Province from 2015 to 2017.
3.2 Overall pooled curves of the air pollutant-HFMD relationship
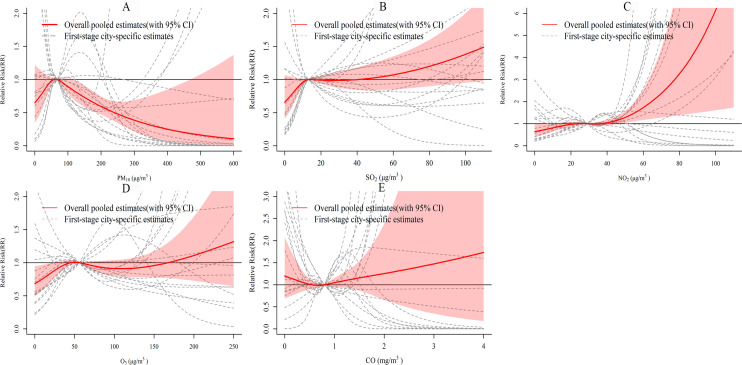
We merged the city-specific estimates for 17 cities to obtain overall pooled cumulative exposure-response curves and 95% confidence intervals (CIs) (Fig 2). Fig 2A indicates that the association between the PM10 concentration and the number of HFMD cases has an approximately inverted V-shape. The relative risk (RR) increased between 0–66 μg/m3 and peaked before decreasing. The curve of NO2 (Fig 2C) was approximately hockey-stick shaped, and the RR reached 4.47 (95% CI: 1.49, 13.40) at an NO2 concentration of 90 μg/m3. For O3 (Fig 2D), the RR increased between 0–50 μg/m3, decreased slightly between 50–108 μg/m3, and then increased with increasing O3 concentrations. For SO2 (Fig 2B) and CO (Fig 2E), the 95% CIs of their curves almost contained 1, meaning that the associations were not statistically significant.
Fig 2. The overall and city-specific cumulative-response curves of the relationships between air pollutant concentrations and the number of HFMD cases.
The reference for all estimates was the median of each pollutant.
3.3 Heterogeneity and effect modifiers of the air pollutant-HFMD relationship
The pooled results showed that the city-specific cumulative exposure-response curves for each pollutant were heterogeneous. Table 2 displays the heterogeneity and significant effect modifiers of the air pollutant-HFMD relationships. The results of the I2 statistic indicated that 58.5%, 48.4%, 51.0%, 55.6% and 52.5% of the variances in the relationships of PM10, SO2, NO2, O3 and CO concentrations with the number of HFMD cases could be explained by differences among cities, respectively.
Table 2. Heterogeneity and effect modifiers of the relationships between pollutant concentrations and the number of HFMD cases.
| Meta-predictor | LR test | Model fit | Cochran Q test | I 2 | ||||
|---|---|---|---|---|---|---|---|---|
| χ2 statistic | df | P | AIC | χ2 statistic | df | P | (%) | |
| PM10 | ||||||||
| Intercept only | - | - | - | 170.1 | 115.5 | 48 | <0.001 | 58.5 |
| Licensed physicians | 8.2 | 3 | 0.042 | 168.0 | 98.4 | 45 | <0.001 | 54.3 |
| SO2 | ||||||||
| Intercept only | - | - | - | 150.0 | 93.0 | 48 | <0.001 | 48.4 |
| Proportion of students | 16.5 | 3 | 0.001 | 139.6 | 72.1 | 45 | 0.006 | 37.5 |
| O3 | ||||||||
| Intercept only | - | - | - | 131.4 | 108.2 | 48 | <0.001 | 55.6 |
| Urbanization rate | 8.7 | 3 | 0.034 | 128.7 | 85.6 | 45 | <0.001 | 47.4 |
| Population density | 7.8 | 3 | 0.050 | 129.6 | 86.9 | 45 | <0.001 | 48.2 |
| Hospital beds | 10.2 | 3 | 0.017 | 127.2 | 98.0 | 45 | <0.001 | 54.1 |
| NO2 | ||||||||
| Intercept only | - | - | - | 154.5 | 98.0 | 48 | <0.001 | 51.0 |
| Proportion of students | 11.6 | 3 | 0.009 | 148.9 | 82.0 | 45 | 0.001 | 45.1 |
| CO | ||||||||
| Intercept only | - | - | - | 151.8 | 101.1 | 48 | <0.001 | 52.5 |
*“-”indicates that the intercept was not tested by the LR test.
For the effect modifiers, some demographic factors, economic factors and health resource factors could partly explain the heterogeneity of the air pollutant-HFMD relationship. The proportion of primary school students explained 10.9% and 5.9% of the heterogeneity of the associations of SO2 and NO2 with the number of HFMD cases, respectively. The number of licensed physicians explained 4.2% of the heterogeneity of the association between PM10 and the number of HFMD cases. The number of hospital beds, urbanization rate and population density explained 1.5%, 8.2% and 7.4% of the heterogeneity of the relationship between O3 and the number of HFMD cases, respectively.
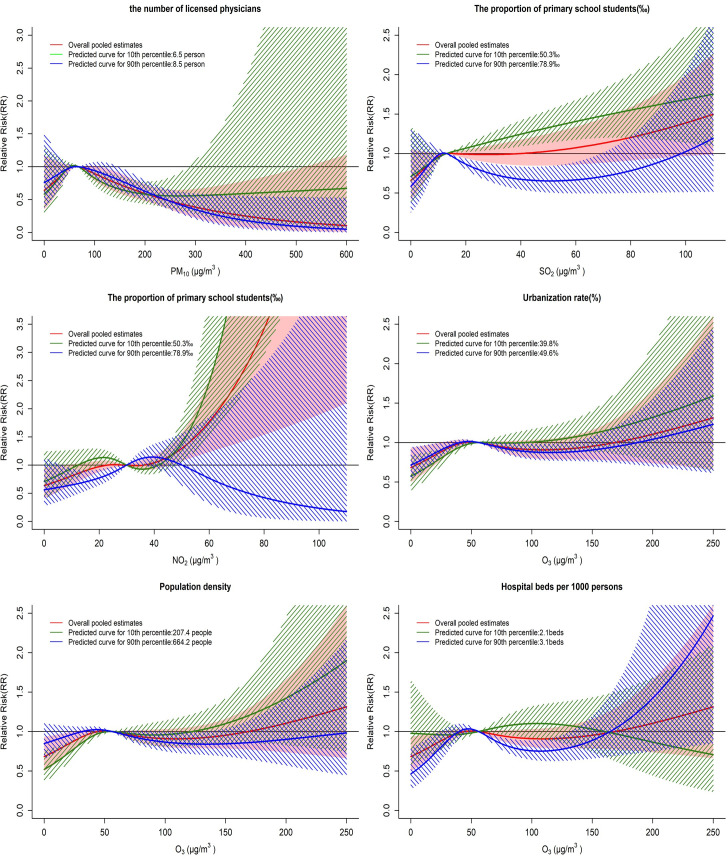
Fig 3 summarizes the estimates of the air pollutant-HFMD associations considering the 10th and 90th percentiles of the effect modifiers. For PM10, the small number of licensed physicians weakened the negative association between the high PM10 concentration and the number of HFMD cases. For SO2, a high proportion of primary school students weakened the positive association with HFMD cases when the concentration exceeded 13 μg/m3. For NO2, a high proportion of primary school students transformed the positive association with the number of HFMD cases into a nonsignificant negative association when the concentrations exceeded 45 μg/m3. For O3, low population density and low urbanization rate strengthened the association between O3 and the number of HFMD cases. A large number of hospital beds per 1000 persons reduced the RR of HFMD at 0–163 μg/m3.
Fig 3. Predicted air pollutant-HFMD associations considering effect modifiers at the 10th and 90th percentiles.
4. Discussion
This study explored the relationships between air pollutant concentrations and the number of HFMD cases and identified significant socioeconomic effect modifiers. Our results revealed that PM10, NO2 and O3 concentrations were associated with the number of HFMD cases in a heavily polluted area with a high HFMD incidence and different socioeconomic levels. Moreover, we found heterogeneity for each pollutant in the cumulative exposure-response associations between cities. Regarding the relationships of PM10, SO2, NO2, O3 and CO with the number of HFMD cases, approximately 58.5%, 48.4%, 51.0%, 55.6% and 52.5% of the variations could be explained by differences among different cities, respectively. The proportion of primary school students, population density, urbanization rate, number of licensed physicians and number of hospital beds could partly explain the heterogeneity and modify the relationships. The exact mechanisms of the effects of air pollutant concentrations on HFMD cases remain unclear and need further exploration. Our results could help to enhance the understanding of the relationships between air pollutant concentrations and the number of HFMD cases and provide a reference for relevant public health decision-making.
For the relationships between air pollutant concentrations and the number of HFMD cases, we found that the overall association between PM10 and the number of HFMD cases had an approximately inverted V-shape. When the PM10 concentration was 0–66 μg/m3, the correlation was positive. One possible explanation is that PM10 may lead to lung function damage and an inflammatory response [32]. Another is that viruses may attach to particles in the air, which may enhance the transmission of the HFMD virus [33–35]. When the PM10 concentration exceeded 66 μg/m3, we found a negative association, which was consistent with results in Chengdu [20], Shenzhen [21], and Guangxi [23]. This phenomenon may be attributed to early warning measures implemented by the government. “The Sichuan Provincial Heavy Pollution Weather Emergency Plan” [36], published in 2014, provides the following warning: when the daily average AQI exceeds 200 for 3 or more days, kindergartens and primary schools are advised to avoid outdoor activities to effectively reduce children’s exposure to air pollution. In addition, people may opt to wear masks, use air purifiers, and close windows on heavy pollution days as a result of increased awareness of the hazards of particulate matter exposure [37,38], which might also reduce the risk of HFMD.
We found that a low O3 concentration had a weakly positive correlation with the number of HFMD cases, which was similar to studies in Guangxi [23] and Ningbo(Gu et al., 2020) and was inconsistent with studies in Guilin(Yu et al., 2019) and Shenzhen(Yan et al., 2019). Some studies have shown that O3 exposure could compromise epithelial defenses, increase transmucosal permeability [39], induce oxidative damage to cells and the lining fluids of the airways [40], and cause an acute effect on child lung function [41], which may increase the susceptibility of children to HFMD. However, some other studies have indicated that an appropriate O3 concentration could reduce virus production and stimulate cytokine production, which may reduce the risk of EV71 HFMD infection [42]. This may lead to a nonsignificant association with HFMD when the O3 concentration exceeds 50 μg/m3. Further research is needed to explore the association between O3 and HFMD cases and the possible mechanism.
The overall relationship between the NO2 concentration and the number of HFMD cases was approximately hockey-stick shaped, and the RR was 4.47 (95% CI: 1.49, 13.40) at 90 μg/m3, suggesting that high NO2 concentrations greatly increase the HFMD risk. A study in Hong Kong found that NO2 may cause peptic ulcer bleeding [43], which might promote the fecal-oral transmission of HFMD. Our results suggest that children should reduce their outdoor exposure on days with high NO2 concentrations. The overall association between the SO2 concentration and HFMD cases was nonsignificant, which was inconsistent with positive associations in studies in Ningbo [44], Shenzhen [21], Hefei [45] and Wuhan [16]. Approximately 95% of the SO2 concentration data in our study were lower than 30 μg/m3, which may lead to a wide confidence interval at high SO2 concentrations. Therefore, our results only suggest that the association between low SO2 concentrations and HFMD cases was not significant. We are unsure whether the nonsignificant result at high SO2 concentrations was due to the wide confidence interval or no actual association between SO2 and HFMD. Further studies are needed in areas with high SO2 concentrations.
Regarding the heterogeneity of the air pollutant-HFMD associations, we found that demographic compositions and health resources could partly explain the heterogeneity. Among these factors, the proportion of primary school students was an important effect modifier that explained 10.9% and 5.9% of the heterogeneity of the associations of SO2 and NO2 concentrations with the number of HFMD cases, respectively. A high proportion of primary school students weakened the positive effect of NO2 on the number of HFMD cases and made the association between SO2 and the number of HFMD cases negative. A possible reason is that the AQI and the concentration of most air pollutants, including SO2 and NO2, are usually higher in winter. During this time, families with children are more likely to reduce the time spent on outdoor activities because of cold weather and early warning measures [37]. Therefore, people in regions with large proportions of primary school students may take more protective measures, which may reduce exposure to HFMD among children. However, the number of licensed physicians, the number of hospital beds, the population density and the urbanization rate were significant effect modifiers. Their effect modifications on the associations between pollutants and the number of HFMD cases were slight, and their CIs on the 10th and 90th percentiles of the modifiers were heavily overlapping. Therefore, it is difficult to say whether these sight modification effects have epidemiological implications. However, these results could still provide clues for future research to explore the modification effects on regions with larger differences in these factors. The results also suggest that children in regions with lower health resources particularly need to reduce their exposure to air pollutants.
Our study explored the heterogeneity of the relationships between air pollutant concentrations and the number of HFMD cases and identified significant effect modifiers. We mainly focused on the modification effects of socioeconomic factors, so we selected cities with different levels of economic development and similar climates to avoid interference from meteorological factors. Therefore, a limitation is that meteorological factors may have modification effects, which were not explored in our study. Additional studies in more regions with diverse climates are needed. For the exposure assignment of each city, we chose the nearest neighbor station to obtain the meteorological exposure data and calculated the arithmetic mean of the monitoring stations to obtain the air pollution exposure data. Therefore, another limitation is that misclassification of exposure may occur in some populations. Further research can consider other better approaches, such as land use regression and random forests, to obtain more accurate exposure data. Finally, when we explored the association between one air pollutant and HFMD cases, other air pollutants were also important confounders. However, some air pollutant data were correlated. Although we controlled for collinearity by not including highly correlated variables in the same model, the moderately correlated variables still influenced the results.
5. Conclusions
Our study found that the air pollutant-HFMD associations between cities showed heterogeneity. Different demographic characteristics and health care resources could partially explain the heterogeneity. Susceptible populations in regions with a smaller proportion of primary school students and fewer medical resources should avoid exposure to SO2 and NO2. Children in regions with lower health resources particularly need to reduce their exposure to air pollutants. The results could be helpful for relevant public health decision-making to prevent HFMD.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Table A. The results of overdispersion test for the HFMD series. Table B. Different confounder model settings. Table C. Different autoregressive term settings. Table D. The values of different knots of splines for the exposure -response structure of air pollutants. Table E. The QAICs of different knots of splines for the exposure -response structure of air pollutants. Fig A. The overall model fit of different values of the degrees of freedom of time splines. Fig B. The overall model fit of different model settings of temperature. Fig C. The overall model fit of different model settings of Humid. Fig D. The overall model fit of different model settings of sunshine hours. Fig E. The overall model fit of different model settings of wind velocity. Fig F. The overall model fit of different model settings of precipitation. Fig G. The autocorrelation analysis on the residuals of the HFMD cases. Fig H. The overall model fit of different autoregressive term settings. Fig I. The ACF and PACF analysis on the residuals of the HFMD cases after controlling autoregressive term. Fig J. The QAICs of different dfs of splines for lag-response structure of air pollutants.
(DOCX)
Data Availability
The HFMD data underlying the results in the study cannot be shared publicly because of the limitation of data availability in the data management rule of Sichuan Center for Disease Control and Prevention. The datasets generated and/or analyzed during the current study are available from Sichuan Center for Disease Control and Prevention (https://www.sccdc.cn/, email: zxbgs@sccdc.cn). The meteorological data can be obtained from the China Meteorological Data Sharing Service System (https://data.cma.cn/data/cdcindex/cid/0b9164954813c573.html). The air pollution data is available from Department of Ecology and Environment of Sichuan Province. (http://www.scaepp.cn/sthjt/c104334/scemc.shtml).
Funding Statement
This work was supported by the National Natural Science Foundation of China received by FY (Grant No. 81872713, Grant No. 81803332), Sichuan Science and Technology Program received by FY (Grant No. 2021YFS0181), Chongqing Science and Technology Program received by FY (grant number: cstc2020jscx-cylhX0003; URL: http://kjj.cq.gov.cn/) and Central government funding items received by FY (grant numbers: 2021zc02; URL: http://www.mca.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Huang J, Liao Q, Ooi MH, Cowling BJ, Chang Z, Wu P, et al. Epidemiology of Recurrent Hand, Foot and Mouth Disease, China, 2008–2015. Emerg Infect Dis. 2018;24(3). 10.3201/eid2403.171303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis. 2018;37(3):391–8. doi: 10.1007/s10096-018-3206-x [DOI] [PubMed] [Google Scholar]
- 3.Koh WM, Bogich T, Siegel K, Jin J, Chong EY, Tan CY, et al. The Epidemiology of Hand, Foot and Mouth Disease in Asia: A Systematic Review and Analysis. Pediatr Infect Dis J. 2016;35(10):e285–300. 10.1097/INF.0000000000001242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14(12):e1076–81. doi: 10.1016/j.ijid.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 5.Tagaya I, Takayama R, Hagiwara A. A large-scale epidemic of hand, foot and mouth disease associated with enterovirus 71 infection in Japan in 1978. Jpn J Med Sci Biol. 1981;34(3):191–6. doi: 10.7883/yoken1952.34.191 [DOI] [PubMed] [Google Scholar]
- 6.Podin Y, Gias EL, Ong F, Leong YW, Yee SF, Yusof MA, et al. Sentinel surveillance for human enterovirus 71 in Sarawak, Malaysia: lessons from the first 7 years. BMC Public Health. 2006;6:180. doi: 10.1186/1471-2458-6-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang LY, Huang LM, Gau SS, Wu YY, Hsia SH, Fan TY, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med. 2007;356(12):1226–34. doi: 10.1056/NEJMoa065954 [DOI] [PubMed] [Google Scholar]
- 8.Koh WM, Badaruddin H, La H, Chen MI, Cook AR. Severity and burden of hand, foot and mouth disease in Asia: a modelling study. BMJ Glob Health. 2018;3(1):e000442. doi: 10.1136/bmjgh-2017-000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao QY, Wang Y, Bian L, Xu M, Liang Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines. 2016;15(5):599–606. doi: 10.1586/14760584.2016.1138862 [DOI] [PubMed] [Google Scholar]
- 10.Aswathyraj S, Arunkumar G, Alidjinou EK, Hober D. Hand, foot and mouth disease (HFMD): emerging epidemiology and the need for a vaccine strategy. Med Microbiol Immunol. 2016;205(5):397–407. doi: 10.1007/s00430-016-0465-y [DOI] [PubMed] [Google Scholar]
- 11.Cheng Q, Bai L, Zhang Y, Zhang H, Wang S, Xie M, et al. Ambient temperature, humidity and hand, foot, and mouth disease: A systematic review and meta-analysis. Sci Total Environ. 2018;625:828–36. doi: 10.1016/j.scitotenv.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Meng Y, Xiang H, Lu Y, Liu S. Association of Short-Term Exposure to Meteorological Factors and Risk of Hand, Foot, and Mouth Disease: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2020;17(21). 10.3390/ijerph17218017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L, Wang X, Guo Y, Xu J, Xue F, Liu Y. Assessment of temperature effect on childhood hand, foot and mouth disease incidence (0-5years) and associated effect modifiers: A 17 cities study in Shandong Province, China, 2007–2012. Sci Total Environ. 2016;551–552:452–9. 10.1016/j.scitotenv.2016.01.173 [DOI] [PubMed] [Google Scholar]
- 14.Yang F, Ma Y, Liu F, Zhao X, Fan C, Hu Y, et al. Short-term effects of rainfall on childhood hand, foot and mouth disease and related spatial heterogeneity: evidence from 143 cities in mainland China. BMC Public Health. 2020;20(1):1528. doi: 10.1186/s12889-020-09633-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and Health Impacts of Air Pollution: A Review. Front Public Health. 2020;8:14. doi: 10.3389/fpubh.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Hao J, Huang S, Yang W, Zhu Z, Tian L, et al. Acute effects of air pollution on the incidence of hand, foot, and mouth disease in Wuhan, China. Atmospheric Environment. 2020;225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiaomin L, Haiyan C, Donglemei W, Zhongyou S. Influence of air pollutants on occurrence risk of hand-foot-mouth disease in Yancheng City Chinese Journal of Woman and Child Health Research. 2018;29(03):276–80. [Google Scholar]
- 18.Huang R, Ning H, He T, Bian G, Hu J, Xu G. Impact of PM10 and meteorological factors on the incidence of hand, foot, and mouth disease in female children in Ningbo, China: a spatiotemporal and time-series study. Environ Sci Pollut Res Int. 2019;26(18):17974–85. doi: 10.1007/s11356-018-2619-5 [DOI] [PubMed] [Google Scholar]
- 19.Huang R, Bian G, He T, Chen L, Xu G. Effects of Meteorological Parameters and PM10 on the Incidence of Hand, Foot, and Mouth Disease in Children in China. Int J Environ Res Public Health. 2016;13(5). 10.3390/ijerph13050481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin F, Ma Y, Zhao X, Lv Q, Liu Y, Li X, et al. Analysis of the effect of PM10 on hand, foot and mouth disease in a basin terrain city. Sci Rep. 2019;9(1):3233. doi: 10.1038/s41598-018-35814-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan S, Wei L, Duan Y, Li H, Liao Y, Lv Q, et al. Short-Term Effects of Meteorological Factors and Air Pollutants on Hand, Foot and Mouth Disease among Children in Shenzhen, China, 2009–2017. Int J Environ Res Public Health. 2019;16(19). 10.3390/ijerph16193639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu G, Li Y, Cai J, Yu D, Tang J, Zhai W, et al. Short-term effects of meteorological factors and air pollution on childhood hand-foot-mouth disease in Guilin, China. Sci Total Environ. 2019;646:460–70. doi: 10.1016/j.scitotenv.2018.07.329 [DOI] [PubMed] [Google Scholar]
- 23.Yanling Z. Analysis of the impact of hand, foot and mouth disease on air quality based on principal component quantile regression. Mathematics in Practice And Theory. 2021;51(02):164–70. [Google Scholar]
- 24.Ji XY, Huang LY, Song J, Fei CN, Liu J, Liu H. Short-term effects of meteorological factors, air pollution, and sunspot on childhood hand, foot, and mouth disease in Tianjin, China: a new time series regression, 2014–2018. Environ Sci Pollut Res Int. 2020;27(29):37022–35. doi: 10.1007/s11356-020-09794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ning G, Wang S, Ma M, Ni C, Shang Z, Wang J, et al. Characteristics of air pollution in different zones of Sichuan Basin, China. Sci Total Environ. 2018;612:975–84. doi: 10.1016/j.scitotenv.2017.08.205 [DOI] [PubMed] [Google Scholar]
- 26.Zhao S, Yu Y, Yin D, Qin D, He J, Dong L. Spatial patterns and temporal variations of six criteria air pollutants during 2015 to 2017 in the city clusters of Sichuan Basin, China. Sci Total Environ. 2018;624:540–57. doi: 10.1016/j.scitotenv.2017.12.172 [DOI] [PubMed] [Google Scholar]
- 27.Gasparrini A, Armstrong B. Reducing and meta-analysing estimates from distributed lag non-linear models. BMC Med Res Methodol. 2013;13:1. doi: 10.1186/1471-2288-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bo Z, Ma Y, Chang Z, Zhang T, Liu F, Zhao X, et al. The spatial heterogeneity of the associations between relative humidity and pediatric hand, foot and mouth disease: Evidence from a nation-wide multicity study from mainland China. Sci Total Environ. 2020;707:136103. doi: 10.1016/j.scitotenv.2019.136103 [DOI] [PubMed] [Google Scholar]
- 29.Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29(21):2224–34. doi: 10.1002/sim.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gasparrini A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J Stat Softw. 2011;43(8):1–20. [PMC free article] [PubMed] [Google Scholar]
- 31.Gasparrini A, Armstrong B, Kenward MG. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat Med. 2012;31(29):3821–39. doi: 10.1002/sim.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013;10(9):3886–907. doi: 10.3390/ijerph10093886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ijaz MK, Zargar B, Wright KE, Rubino JR, Sattar SA. Generic aspects of the airborne spread of human pathogens indoors and emerging air decontamination technologies. Am J Infect Control. 2016;44(9 Suppl):S109–20. doi: 10.1016/j.ajic.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groulx N, Urch B, Duchaine C, Mubareka S, Scott JA. The Pollution Particulate Concentrator (PoPCon): A platform to investigate the effects of particulate air pollutants on viral infectivity. Sci Total Environ. 2018;628–629:1101–7. doi: 10.1016/j.scitotenv.2018.02.118 [DOI] [PubMed] [Google Scholar]
- 35.Cao C, Jiang W, Wang B, Fang J, Lang J, Tian G, et al. Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ Sci Technol. 2014;48(3):1499–507. doi: 10.1021/es4048472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Sichuan Provincial Heavy Pollution Weather Emergency Plan: https://www.sc.gov.cn/10462/10883/11066/2014/1/16/10291214.shtml; 2014 [Available from: https://www.sc.gov.cn/10462/10883/11066/2014/1/16/10291214.shtml.
- 37.Xiong L, Li J, Xia T, Hu X, Wang Y, Sun M, et al. Risk Reduction Behaviors Regarding PM(2.5) Exposure among Outdoor Exercisers in the Nanjing Metropolitan Area, China. Int J Environ Res Public Health. 2018;15(8). 10.3390/ijerph15081728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen RW, Barn P. Individual- and Household-Level Interventions to Reduce Air Pollution Exposures and Health Risks: a Review of the Recent Literature. Curr Environ Health Rep. 2020;7(4):424–40. doi: 10.1007/s40572-020-00296-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2(1):31–86. doi: 10.1080/109374099281232 [DOI] [PubMed] [Google Scholar]
- 40.Zhang JJ, Wei Y, Fang Z. Ozone Pollution: A Major Health Hazard Worldwide. Front Immunol. 2019;10:2518. doi: 10.3389/fimmu.2019.02518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amadeo B, Robert C, Rondeau V, Mounouchy MA, Cordeau L, Birembaux X, et al. Impact of close-proximity air pollution on lung function in schoolchildren in the French West Indies. BMC Public Health. 2015;15:45. doi: 10.1186/s12889-015-1382-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin YC, Juan HC, Cheng YC. Ozone exposure in the culture medium inhibits enterovirus 71 virus replication and modulates cytokine production in rhabdomyosarcoma cells. Antiviral Res. 2007;76(3):241–51. doi: 10.1016/j.antiviral.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 43.Tian L, Qiu H, Sun S, Tsang H, Chan KP, Leung WK. Association between emergency admission for peptic ulcer bleeding and air pollution: a case-crossover analysis in Hong Kong’s elderly population. Lancet Planet Health. 2017;1(2):e74–e81. doi: 10.1016/S2542-5196(17)30021-9 [DOI] [PubMed] [Google Scholar]
- 44.Gu S, Li D, Lu B, Huang R, Xu G. Associations between ambient air pollution and daily incidence of pediatric hand, foot and mouth disease in Ningbo, 2014–2016: a distributed lag nonlinear model. Epidemiol Infect. 2020;148:e46. doi: 10.1017/S0950268820000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Q, Wu J, Zhang Y, Cheng Q, Bai L, Duan J, et al. Short-term exposure to sulfur dioxide and the risk of childhood hand, foot, and mouth disease during different seasons in Hefei, China. Sci Total Environ. 2019;658:116–21. doi: 10.1016/j.scitotenv.2018.11.481 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Table A. The results of overdispersion test for the HFMD series. Table B. Different confounder model settings. Table C. Different autoregressive term settings. Table D. The values of different knots of splines for the exposure -response structure of air pollutants. Table E. The QAICs of different knots of splines for the exposure -response structure of air pollutants. Fig A. The overall model fit of different values of the degrees of freedom of time splines. Fig B. The overall model fit of different model settings of temperature. Fig C. The overall model fit of different model settings of Humid. Fig D. The overall model fit of different model settings of sunshine hours. Fig E. The overall model fit of different model settings of wind velocity. Fig F. The overall model fit of different model settings of precipitation. Fig G. The autocorrelation analysis on the residuals of the HFMD cases. Fig H. The overall model fit of different autoregressive term settings. Fig I. The ACF and PACF analysis on the residuals of the HFMD cases after controlling autoregressive term. Fig J. The QAICs of different dfs of splines for lag-response structure of air pollutants.
(DOCX)
Data Availability Statement
The HFMD data underlying the results in the study cannot be shared publicly because of the limitation of data availability in the data management rule of Sichuan Center for Disease Control and Prevention. The datasets generated and/or analyzed during the current study are available from Sichuan Center for Disease Control and Prevention (https://www.sccdc.cn/, email: zxbgs@sccdc.cn). The meteorological data can be obtained from the China Meteorological Data Sharing Service System (https://data.cma.cn/data/cdcindex/cid/0b9164954813c573.html). The air pollution data is available from Department of Ecology and Environment of Sichuan Province. (http://www.scaepp.cn/sthjt/c104334/scemc.shtml).