Abstract
Micromolar concentrations of porin, purified from the outer membranes of Pseudomonas aeruginosa, induced in vitro the classic morphological and biochemical signs of apoptosis in an epithelial cell line (SVC1) derived from the rat seminal vesicle secretory epithelium. The programmed cell death (PCD) was p53 independent and associated with significant decrease of bcl-2 expression, a marked increase of c-myc transcriptional activity, and an absence of the mRNA coding for tissue transglutaminase. The Ca2+ influx, caused by the porin treatment of SVC1 cells, appears to play an important role in the triggering of apoptosis in our biological model. The possibility that the porin property of inducing PCD plays a role in the infertility of individuals chronically infected by gram-negative bacteria is discussed.
The major toxic components in the outer membranes of gram-negative bacteria are lipopolysaccharides (LPS) (endotoxins) and porins. The latter are hydrophobic proteins (mass, about 35,000 Da) that account for more than 50% (18, 35) of the total membrane proteins and that are named porins for their ability to form transmembrane channels for the passive diffusion of small molecules across the cell membrane (26, 27). Purified porins possess immunomodulatory and procoagulant activities and are considered pathogenicity determinants. Depending on the dose, LPS and porins are either frankly toxic to a number of target cells or significantly alter normal cell functions (10, 45). By acting on human polymorphonuclear leukocytes (PMNs), subtoxic concentrations of porins, for example, inhibit phagocytosis and intracellular killing of Salmonella typhimurium (45), decrease oxidative burst and cell hydrophobicity, and cause significant morphological changes in the target cells (46). Subcutaneous injection of porins in rat hind paws causes local inflammation without complement activation (12, 13), a process that is known to be triggered in vitro by these proteins. Finally, it has recently been demonstrated that nontoxic concentrations of porins stimulate the synthesis and release of platelet-activating factor from different types of human cells (PMNs and mesangial and endothelial cells) (6, 41, 42) and promote proinflammatory and immunomodulatory cytokine release from immunocompetent cells or other cellular sources (11). Porins from different microorganisms have similar effects (8, 23, 43, 44).
The cell damage from severe infections by gram-negative bacteria comes mainly from exotoxins and enterotoxins, even though the occurrence of LPS and porins in the bacterial microenvironment contributes significantly. Exposure of target cells to high concentrations of these substances leads to a rapid lytic death of the cells (1, 5, 10, 34, 45).
In contrast, lower concentrations of LPS produce a less dramatic type of death: programmed cell death (PCD), or apoptosis (49). Classic morphological and biochemical signs of apoptosis in the toxin-injured cells are cell shrinkage, extensive blebbing of the cell surface, disappearance of microvilli, chromatin condensation, internucleosomal DNA fragmentation, presence of large intracytoplasmic vacuoles, increase of intracellular calcium ion and free radical levels, enhancement of endonuclease and protease activities, cell cycle arrest, and modified transcriptional activity of different apoptosis-linked genes (p53, bcl-2, c-myc, etc.) (2, 7). An increase in the expression of type II transglutaminase (tissue transglutaminase [tTGase]) has also been reported to be associated with apoptosis in several cell types, and an important role for tTGase in this process has been proposed (15).
The ability of bacterial endotoxin to kill cells by necrosis and/or apoptosis has been found to play a key role in the pathogenesis of many alterations occurring in subjects with infections by gram-negative bacteria, including disseminated intravascular coagulation, septic shock, degenerative processes in different tissues, and reduced fertility or sterility (4, 10, 24, 48).
The tissue lesions produced by Pseudomonas aeruginosa are of particular interest. P. aeruginosa is an opportunistic pathogen that commonly invades immunocompromised patients. The main targets of the LPS and porin released by this gram-negative rod during its lysis or active growth are the endothelial and epithelial cells present in many anatomical regions, including the skin, eyes, genitourinary tract, and heart valves. In particular, it has been reported that low levels of natural porin or LPS in patients with mild chronic infections severely damage the human spermatozoa in vitro and lead to reduced fertility or sterility (29).
On the basis of these data and considerations, and taking into account the scarce information available on the cytotoxic effects of very low concentrations of LPS or porin on specific target cells, we were prompted to investigate the possible involvement of these substances in the induction of apoptosis in a cell line (SVC1) derived from the rat seminal vesicle secretory epithelium (36, 37, 49).
MATERIALS AND METHODS
Purification of porin.
Porin was purified to homogeneity from P. aeruginosa according to the method of Nurminen (28). The purified porin had a mass of about 37,000 Da (Fig. 1). The purified porin preparations contained only traces (20 pg/ml) of LPS identified by the Limulus test (39) and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (40). Endotoxin-free water and P. aeruginosa LPS (Difco Laboratories) were used as negative and positive controls, respectively.
FIG. 1.

Electrophoretic pattern of the proteins present in a purified sample of P. aeruginosa porin (lane B). The SDS-polyacrylamide gel (12%) was stained with Coomassie blue. The molecular mass markers (daltons) are in lane A.
Cell culture and treatments.
The SVC1 cell line, derived from the rat (Wistar-Fisher) seminal vesicle epithelium (20), has a doubling time of about 20 h. These cells were cultured as monolayers in a standard culture medium (Dulbecco’s modified Eagle’s medium supplemented with 5% fetal calf serum, 2 mM glutamine, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The medium was changed every 2 days. Where required, 106-cell aliquots were plated in petri dishes and treated for different lengths of time (1, 5, or 7 days) with porin (10 μg/ml) or LPS (20 pg/ml), dissolved in standard culture medium or phosphate-buffered saline (PBS), respectively. A dose-response curve was produced to show the effect of different concentrations of porin on SVC1 cells. The cells were treated for 7 days with porin (1, 5, 10, 15, or 30 μg/ml) (Table 1). The purified porin preparations were always preincubated for 1 h at 20°C with polymyxin B (5 μg/ml) to neutralize the very small amount (20 pg/ml) of contaminating LPS. A pool of protein-free gel filtration chromatography fractions was used as a negative control in the experiments designed to demonstrate the apoptotic activity of the porin. Moreover, in the experiments performed to investigate the role played by calcium ions in the molecular mechanism of porin-induced apoptosis, 106 SVC1 cells were grown in standard culture medium for different lengths of time in the presence or absence of 2 mM EGTA (a concentration sufficient to chelate the calcium ions present in the medium without any detectable toxic effect on the proliferating cells) and 10 μg of porin/ml.
TABLE 1.
Apoptosis and necrosis induced by the treatment of SVC1 cells for 7 days with different porin concentrationsa
| Cell treatment with porin (μg/ml) | Apoptotic cellsb (%) | Necrotic cellsb (%) |
|---|---|---|
| None (control) | 2 ± 1 | 0 |
| 1 | 10 ± 3 | 0 |
| 5 | 30 ± 5 | 2 ± 1 |
| 10 | 70 ± 4 | 3 ± 1 |
| 15 | 65 ± 7 | 11 ± 3 |
| 30 | 55 ± 5 | 29 ± 2 |
Experimental details are reported in Materials and Methods.
The percentage of apoptotic and necrotic cells was evaluated by the acridine orange method. The data are reported as means ± standard errors of the mean of determinations performed in triplicate on three different samples.
Morphological analysis.
The morphological features of untreated or porin-treated SVC1 cells were defined by phase-contrast microscopy. The apoptotic cells were identified by the acridine orange assay (33).
DNA fragmentation assay.
To evaluate by agarose gel electrophoresis the possible occurrence of internucleosomal hydrolysis of genomic DNA in the porin-treated cells, 106 cells were incubated in the standard culture medium at 37°C for either 5 or 7 days in the presence or absence of 10 μg of purified porin/ml pretreated with polymyxin B. At the end of incubation, the cells were harvested with a cell scraper, centrifuged at 500 × g, washed in PBS, and finally suspended in 100 μl of TNE buffer (150 mM sodium chloride, 10 mM EDTA, 10 mM Tris-HCl [pH 8]). The cell suspensions were lysed with 3 volumes of lysis buffer (0.2% SDS, 100 μg of RNase/ml in TNE), and the lysate was incubated at 37°C for 1 h. After incubation, 100 μg of proteinase K/ml was added to the lysate, and the mixture was incubated for a further 2.5 h at 56°C. The high-molecular-weight genomic DNA, extracted from the proteinase K-treated lysates according to a published procedure (32), was analyzed by electrophoresis (2 h; 80 V) in 1% agarose gel containing ethidium bromide in TBE (0.045 M Tris-borate, 0.001 M EDTA [pH 8.0]).
DNA fragmentation in individual apoptotic cells was also detected by the technique of terminal deoxytransferase-mediated dUTP nick end labeling (TUNEL) of DNA strand breaks (Apoptosis Detection System, fluorescein; Promega). Treated SVC1 cells adherent to glass slides were rinsed with PBS and fixed in 4% methanol (20 min; 4°C). The slides were washed with PBS and then covered in equilibration buffer. Terminal deoxynucleotidyl transferase in reaction buffer with fluorescein-dUTP was then added to the cells and incubated (37°C; 1 h) in a humidified chamber. The reaction was stopped by immersion in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 15 min, after which the cells were washed and stained with propidium iodide (1 μg/ml; 15 min). The slides were observed under fluorescence microscopy with standard fluorescein excitation. Normal cells were stained red throughout the cytoplasm. Apoptotic cells were identified by yellow-orange and green fluorescence within the nucleus, due to the fluorescein-12-dUTP incorporated at the 3′ OH ends of fragmented DNA. SVC1 cells, partially digested with DNase, were used as a positive control. The negative control consisted of porin-treated SVC1 cells treated by the same protocol but without the addition of terminal deoxynucleotidyl transferase.
tTGase assay.
The cells, washed three times with PBS, were scraped with a rubber policeman from the petri dish in the presence of TE (1 mM EDTA in 10 mM Tris-HCl [pH 7.4]), centrifuged, resuspended at a final concentration of 5 × 106/ml in the same buffer, and then sonicated at 4°C for 20 s. The tTGase activity of the sonicate was assessed by measuring the [14C]spermidine incorporation into N,N′-dimethylcasein (DMC). The incubation mixture contained, in a final volume of 100 μl, 100 mM Tris-HCl (pH 7.5), 10 mM dithiothreitol, 0.25 μCi of [14C]spermidine (Amersham) (101 mCi/mmol), 40 to 80 μg of sonicate protein, and, where indicated, 2.5 mM CaCl2, 20 mg of DMC/ml, and 5 mM EGTA. After 30 min of incubation at 37°C the reaction was terminated by adding 10 μl of 1 M unlabeled spermidine and 1 ml of ice-cold 10% trichloroacetic acid (TCA). The TCA-precipitated material, washed three times with 1 ml of cold 10% TCA, was dissolved in 100 μl of 1 M NaOH, and its radioactivity was measured in a Packard liquid scintillation counter.
Reverse transcription (RT)-PCR analysis.
Total RNA, isolated by RNAZOL B (Biotec Laboratories) from SVC1 cells before and after treatment with porin, was transcribed by reverse transcriptase (Superscript II; 400 U) (GIBCO-BRL) at 37°C for 1.5 h according to the manufacturer’s protocol. Six hundred nanograms of cDNA was amplified in a reaction mixture containing, in a final volume of 50 μl, 10 mM Tris-HCl (pH 8.3); 1.5 mM MgCl2; 50 mM KCl; 100 ng of both sense and antisense primers for bcl-2 (sense, 5′-AACACCAGAATCAAGTGTTC-3′; antisense, 5′-TTCCCTTTGGCAGTAAATAG-3′), p53 (sense, 5′-CCCTTCTCAAAAAACTTACC-3′; antisense, 5′-TCATAACAAGCCCTAAAGTC-3′), human β-actin gene (sense, 5′-ATCCAGGCTGTGTTGTCCCTG-3′; antisense, 5′-AGGAGCCAGGGCAGTAATCTC-3′), tTGase gene (sense, 5′-TCAAGTATGGCCAGTGCTGGGTCTTCGCCG-3′; antisense, 5′-TTAAACTGGCTCCACGAGGA-3′), or c-myc (sense, 5′-AACTTACAATCTGCGAGCCA-3′; antisense, 5′-AGCAGCTCGAATTTCTTCCAGATAT-3′); 200 μM deoxynucleoside triphosphate; and 2.5 U of Taq DNA polymerase (Boehringer Mannheim). The reaction was carried out in a DNA thermal cycler (Perkin-Elmer Cetus Instruments). All PCRs were performed with 35 cycles in the exponential phase of amplification and always started with a 3-min denaturation step at 95°C. The cycle for p53, bcl-2, and the tTGase gene was 95°C, 30 s; 55°C, 1 min; 72°C, 1 min. The cycle for c-myc was 95°C, 45 s; 50°C, 45 s; 72°C, 1 min. A final 7 min at 72°C was used in all cases. The PCR products were analyzed by electrophoresis on a 1.2% agarose gel in TBE. The identities of the amplification products were confirmed by both direct nucleotide DNA sequencing and comparison of their sizes with the sizes expected from the known gene sequences. Coamplification of different cDNA sequences was performed by adding the β-actin gene primers into the amplification reaction mixture 7 cycles later than the other primers, to allow all of the amplifications to occur in the exponential phase of the reaction, well below the plateau conditions (47).
Statistical analysis.
The data for dose-response curves and RT-PCR are reported as means ± standard errors of the mean of determinations performed in triplicate on three different samples. The means were compared by analysis of variance plus Bonferroni’s t test, and a P value of less than 0.05 was considered significant.
RESULTS
Porin treatment leads to morphological changes.
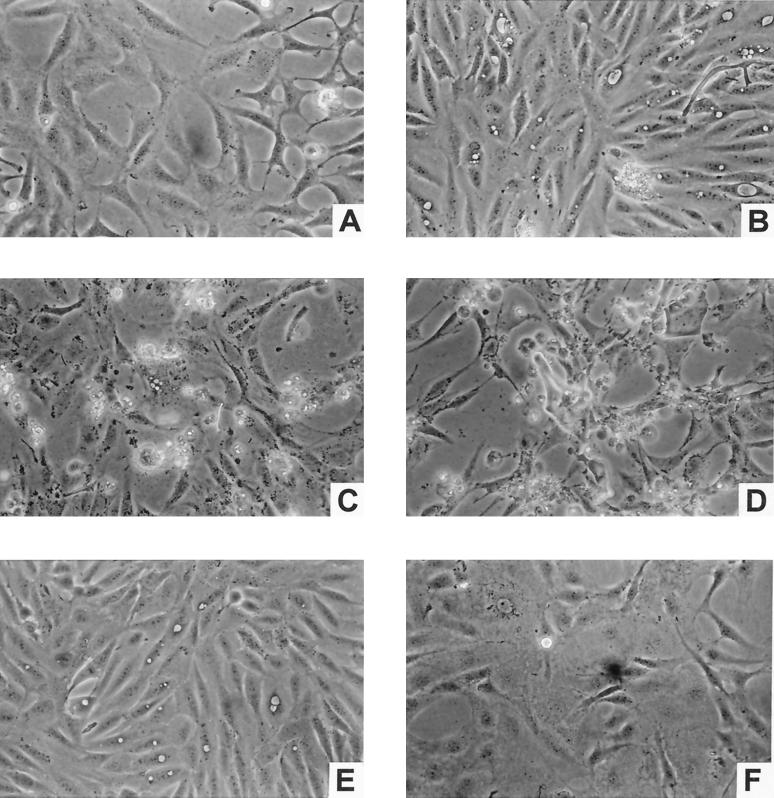
Treatment of SVC1 cells with porin (10 μg/ml for 1, 5, or 7 days) induced a marked change in cell morphology. Intense cytoplasmic vacuolization of the cells was already evident after 1 day of treatment (Fig. 2B). After a 7-day treatment, unequivocal signs of apoptosis (cell shrinkage, nuclear fragmentation, membrane blebbing, and chromatin condensation) could be detected in about 70% of the cells (Fig. 2D). The time required by an apoptogenic stimulus to produce PCD ranges from a few hours to a few days, depending on the type of stimulus and the metabolic and structural characteristics of target cells (22). In our case, the maximum apoptosis was reached only after 7 days of treatment, possibly as a consequence of (i) the type of target cells, which in our case are particularly resistant to apoptotic stimuli (such as hydrogen peroxide and fetal calf serum withdrawal [unpublished data]) or (ii) a possible heterogeneity in the SVC1 membrane proteins more or less capable of interacting with porin in the formation of efficient transmembrane pores, allowing free Ca2+ diffusion.
FIG. 2.
Phase-contrast morphologies of SVC1 cells treated with 10 μg of porin/ml for 1 (B), 5 (C), or 7 (D) days. (A) Untreated cells (control); (E and F) SVC1 cells treated for 7 days with 10 μg of porin/ml under calcium-free conditions and with 20 pg of LPS/ml, respectively. Magnification, ×32.
Porin treatment induces loss of membrane integrity.
The trypan blue exclusion test was negative in the cells treated with porin for 1 day. The same test was positive in 30 and 70% of the cells grown for 5 or 7 days, respectively, in culture medium containing porin. No signs of apoptosis were detected in cells treated with the protein-free chromatographic eluate.
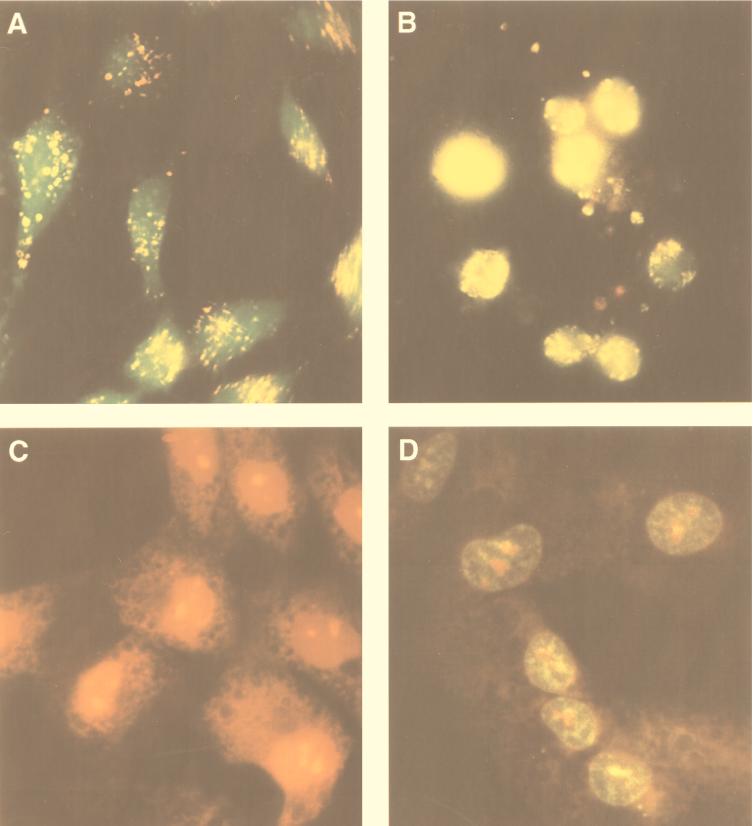
Acridine orange-ethidium bromide has the ability to stain the nuclei of cells in a late phase of apoptosis yellow and to stain the nuclei of necrotic cells orange-red. This is a useful tool to discriminate normal from apoptotic and apoptotic from necrotic cells (33). The data reported in Fig. 3B show that after 7 days of porin treatment about 70% of the treated cells are apoptotic without a significant number of necrotic cells. The treatment of SVC1 cells for 7 days with a higher concentration of porin (30 μg/ml) induced about 30% necrosis and 55% apoptosis. In contrast, the treatment of SVC1 cells for 7 days with 1 μg of porin/ml (Table 1) produced 10% apoptosis without necrosis. The green staining of untreated cells, an expression of membrane integrity, is shown in Fig. 3A.
FIG. 3.
Normal and apoptotic cells stained by acridine orange-ethidium bromide (A and B) or TUNEL assay (C and D). (A) Normal cells are stained green by acridine; the scattered yellow granules visible in the cytoplasm are aggregated RNA granules. Magnification, ×40. (B) Nuclei of cells in late apoptosis are stained bright yellow-orange by ethidium bromide. Magnification, ×50. (C) Normal cells are stained red throughout the cytoplasm. Magnification, ×100. (D) Apoptotic cells; localized green fluorescence within the nuclei is due to the incorporated fluorescein-12-dUTP. Magnification, ×100.
Porin treatment induces DNA fragmentation.
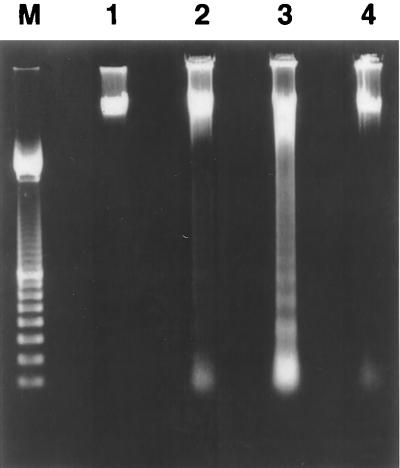
Internucleosomal DNA fragmentation is a classic biochemical event occurring in cells undergoing apoptosis. DNA degradation, characterized by a typical electrophoretic ladder, was found in cells treated for 5 or 7 days with porin, while it did not occur in cells treated with 20 pg of LPS/ml (Fig. 4). The induction of the apoptotic event was time dependent and reached maximum intensity in cells treated with 10 μg of porin/ml for 7 days (Fig. 4). In situ DNA fragmentation was also demonstrated by the TUNEL assay, which showed the occurrence of DNA fragmentation (presence of yellow-green nuclei) in SVC1 cells treated for 7 days with 10 μg of porin/ml (Fig. 3C); the cytoplasm and nuclei of normal cells were stained red (Fig. 3D).
FIG. 4.
Treatment of SVC1 cells with 10 μg of porin/ml induces internucleosomal DNA fragmentation. DNA was extracted after treatment with porins for 5 (lane 2) or 7 (lane 3) days; the treatment of SVC1 with 20 pg of LPS/ml does not induce DNA fragmentation (lane 4). Lane 1, untreated cells; lane M, 100-bp ladder (Boehringer Mannheim) used as a size marker.
Porin-induced apoptosis is p53 independent and is associated with a marked increase of c-myc expression.
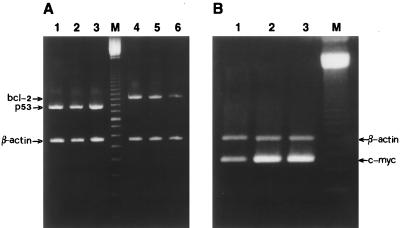
To investigate the roles played by a number of genes involved in PCD, such as p53, bcl-2, and c-myc, we decided to evaluate by semiquantitative RT-PCR the levels of the specific mRNAs produced by these genes in SVC1 cells grown in the presence or absence of micromolar concentrations of porin. In these experiments the cDNA coding for human β-actin was used as an internal control in coamplification reactions. The data obtained demonstrated clearly that the porin-induced apoptosis was p53-independent (Fig. 5A, lanes 1 to 3). In contrast, the c-myc expression markedly increased in porin-treated cells (Fig. 5B), whereas the bcl-2 mRNA levels were found to decrease proportionally with increasing apoptosis (Fig. 5A, lanes 4 to 6). PCD is frequently associated with a marked increase of tTGase expression (15). Therefore, we investigated whether this was also the case in our apoptotic model. Unexpectedly, we found that the amount of tTGase mRNA was unchanged in the porin-treated SVC1 cells. The latter result was confirmed by the lack of changes in the tTGase enzymatic activity measured in the sonicates of porin-treated SVC1 cells in comparison with the activity in those of the untreated ones (data not shown).
FIG. 5.
RT-PCR determination of the relative levels of mRNA coding for p53 (A), bcl-2 (A), and c-myc (B) in the total RNA prepared from SVC1 cells treated or not (control) with 10 μg of porin/ml for 5 or 7 days. (A) β-Actin (the internal control; the 561-bp product in lanes 1 to 6), p53 (lanes 1 to 3), and bcl-2 (lanes 4 to 6) mRNAs. Lanes 1 and 4, untreated-cell mRNA; lanes 2 and 5, mRNA from SVC1 cells treated with 10 μg of porin/ml for 5 days; lanes 3 and 6, mRNA from SVC1 cells treated with 10 μg of porin/ml for 7 days; lane M, 100-bp ladder (Boehringer Mannheim). (B) β-Actin and c-myc mRNAs. Lane 1, untreated-cell mRNA; lanes 2 and 3, mRNA from SVC1 cells treated with 10 μg of porin for 5 and 7 days, respectively. Densitometric analysis of ethidium bromide-stained agarose gel (NIH Image, version 16) was performed to quantitate the amounts of specific mRNAs present in the total RNA prepared from the different cell samples. The p53/β-actin fluorescence intensity (FI) ratios were as follows: lanes 1, 2, and 3, 1.14 ± 0.10, 1.17 ± 0.12, and 1.19 ± 0.16, respectively. The bcl-2/β-actin FI ratios were as follows: lanes 4, 5, and 6, 1.40 ± 0.11, 1.10 ± 0.13, and 0.47 ± 0.15, respectively. The c-myc/β-actin FI ratios were as follows: lanes 1, 2, and 3, 1.80 ± 0.20, 2.80 ± 0.14, and 2.90 ± 0.15, respectively.
Porin-induced apoptosis is a Ca2+-dependent biological event.
To demonstrate that porin-induced apoptosis is a Ca2+-dependent biological event, appropriate experiments in calcium-free conditions were designed. Previous studies have shown that the S. typhimurium porin possesses the ability to induce specific transmembrane channels through which Ca2+ ions enter eukaryotic cells (6, 41, 42). The addition of EGTA to the standard culture medium at a final concentration of 2 mM decreased the Ca2+ ion concentration of the medium from 6.8 mg/ml to 0.4 mg/ml; this was enough to abrogate the apoptotic effects of porin on SVC1 cells (Fig. 2E). In addition, other tests performed under the same conditions on porin-treated SVC1 cells (TUNEL, acridine orange staining, DNA fragmentation, and RT-PCR) were all negative (data not shown).
DISCUSSION
Gram-negative bacteria, during both cell lysis and active growth, release structural components of the outer membrane into the surrounding microenvironment. Such outer membrane fragments are rich in LPS and porins. Our results show that 10 μg of porin/ml can induce PCD in SVC1 cells. The apoptotic event in these cells occurs 7 days after treatment and involves 70% of treated cells. The porin effect is not due to contamination by LPS, because it also occurs with polymyxin B. By binding to the lipid A of LPS, the latter completely inhibits the strong cytopathic effect of this lipid, whereas binding to the porin leaves the biological activity of the protein unmodified.
During gram-negative bacteria-host cell interactions in vivo, the eukaryotic cells come in contact with the LPS-porin complex present on the bacterial surface. We have calculated that the lysis of 108 bacteria, a number of cells easily reached at an infection site, releases porins into the medium in the range of concentrations used to induce apoptosis in our in vitro experiments.
Second messengers, such as intracellular Ca2+, phospholipase A2 (PLA2), and protein kinase activities, can play an important role in the triggering of apoptosis (3, 31). An increment in the intracellular calcium level has been reported as one of the first events to occur in T cells after the ligand-receptor interaction (30). It has also been demonstrated that the formation of small pores induced by the Staphylococcus aureus alpha-toxin in the plasma membrane of its target cells is capable of producing apoptosis in human lymphocytes (19). In addition, data have been reported showing that the neisserial porin PorB causes rapid calcium influx in target epithelial cells and induces apoptosis by the activation of cysteine proteases (25). All these findings are consistent with our results showing that porin-induced apoptosis is a calcium-dependent biological event.
As already mentioned, the porins are released from gram-negative bacteria both during their phase of active growth and during bacteriolysis. These proteins can resist proteolytic enzymes in the host intercellular microenvironment (14, 17) and therefore can persist for a long period of time at the infection site. By interacting with the plasma membranes of the target cells, porins become embedded as hydrophilic pores in the phospholipid bilayer, severely damaging the structure and function of this important part of the cell architecture. With a high porin concentration, a rapid collapse of all membrane functions follows. This is accompanied by a series of biochemical events that leads the cell to a non-genetically controlled necrotic death. In contrast, at lower porin concentrations, the membrane alterations induce a milder cell reaction characterized by a change of cell permeability associated with activation of the apoptotic genetic program that ultimately kills the cell. This hypothesis is supported by a recent finding showing that porins can bind to the plasma membranes of human PMNs (46) with the formation of transmembrane channels. In addition, it has also been demonstrated that porins are able to induce a marked increase in the level of intracellular Ca2+ and in the activity of PLA2 in various human cells (PMNs and endothelial and mesangial cells) (6, 41, 42). In conclusion, by allowing a substantial intracellular influx of Ca2+ associated with an increase of PLA2 activity, pathological embedding of porins in the plasma membranes of eukaryotic target cells may act as an effective mechanism of signal transduction responsible for the activation of the genetic program of apoptotic death. It is well known, in fact, that a sudden intracytoplasmic increase of the Ca2+ level leads to the activation of a number of enzymes (proteases, endonucleases, and tTGase) whose roles have already been demonstrated to be crucial in the molecular mechanism of apoptosis.
It is well known that the appropriate expression of a number of specific genes, such as those encoding p53, bcl-2, c-myc, and tTGase, plays a key role in the molecular mechanism underlying the process of apoptotic cell death. In our experimental model the independence of apoptosis from p53 gene expression is not surprising because this was shown to be the case in a number of other biological systems (7). The gene activity program that most probably controls the apoptotic process in porin-treated SVC1 cells is represented by the down-regulated bcl-2 gene expression associated with the up-regulated c-myc gene transcriptional activity. The absence of tTGase gene expression in the SVC1 cells undergoing apoptosis was unexpected, even though various examples are reported in the literature where PCD is not related to up-regulated tTGase gene expression (2).
Several studies have demonstrated that a modification of the vaginal microbiota involving a prevalence of gram-negative bacteria can induce transitory sterility, probably related to alterations of sperm cell motility (9, 38). LPS, porins, and peptidoglycan fragments have been reported (10) to be toxic for human spermatozoa, and porins have been shown to possess the ability to bind to the sperm cell plasma membrane (16).
In conclusion, we think that the porin-dependent decrease of fertility can probably be explained by a damage of both seminal vesicle epithelial cell secretory activity and sperm cell motility induced by the porin released from the infecting gram-negative bacteria. In particular, the impaired motility of the porin-treated spermatozoa could be related to the inefficiency of the energy-producing biochemical machinery of mitochondria damaged by the binding of porins to the sperm cell plasma membrane. This hypothesis is in good agreement with a number of recent reports showing the importance of mitochondrial damage in PCD (21).
ACKNOWLEDGMENTS
We are grateful to Salvatore Baiano and Francesco Moscatiello for their precious and skillful technical assistance.
This study was supported in part by funds from CNR (9603306) and MURST 1998.
REFERENCES
- 1.Amano F, Karahashi H. A cytotoxic effect of lipopolysaccharide on a macrophage-like cell line, J774.1, in the presence of cycloheximide. J Endotoxin Res. 1996;3:415–423. [Google Scholar]
- 2.Balajthy Z, Kedei N, Nagy L, Davies P J A, Fesus L. Lack of induction of tissue transglutaminase but activation of the preexisting enzyme in c-Myc induced apoptosis of CHO cells. Biochem Biophys Res Commun. 1997;236:280–284. doi: 10.1006/bbrc.1997.6969. [DOI] [PubMed] [Google Scholar]
- 3.Bliska J B, Galan J E, Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993;73:903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- 4.Bone R C. Sepsis and the systemic inflammatory response syndrome (SIRS) J Endotoxin Res. 1995;2:151–155. [Google Scholar]
- 5.Brauner A, Kallenius G, Wrangsell G, Wretlind B, Svenson S B. Antibody responses to Escherichia coli J5 lipopolysaccharide and to Salmonella porin in patients with bacteremia. Microb Pathog. 1986;1:475–481. doi: 10.1016/0882-4010(86)90009-4. [DOI] [PubMed] [Google Scholar]
- 6.Camussi G, Biancone L, Iorio E L, Silvestro L, Da Col R, Capasso C, Rossano F, Servillo L, Balestrieri C, Tufano M A. Porins and lipopolysaccharide stimulate platelet activating factor synthesis by human mesangial cells. Kidney Int. 1992;42:1309–1318. doi: 10.1038/ki.1992.422. [DOI] [PubMed] [Google Scholar]
- 7.Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 8.Cusumano V, Tufano M A, Mancuso G, Carbone M, Rossano F, Mera M T, Ciliberti F A, Ruocco E, Teti G. Porins of Pseudomonas aeruginosa induce release of TNF-α and IL-6 by human leukocytes. Infect Immun. 1997;65:1683–1687. doi: 10.1128/iai.65.5.1683-1687.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friberg J, Fullan N. Attachment of Escherichia coli to human spermatozoa. Am J Obstet Gynecol. 1983;146:465–467. doi: 10.1016/0002-9378(83)90831-1. [DOI] [PubMed] [Google Scholar]
- 10.Galdiero F, Gorga F, Bentivoglio C, Mancuso R, Galdiero E, Tufano M A. The action of LPS, porins and peptidoglycan fragments on human spermatozoa. Infection. 1988;16:349. doi: 10.1007/BF01644545. [DOI] [PubMed] [Google Scholar]
- 11.Galdiero F, Cipollaro de L’Ero G, Benedetto N, Galdiero M, Tufano M A. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993;61:155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galdiero F, Tufano M A, Sommese L, Folgore A, Tedesco F. Activation of complement system by porins extracted from Salmonella typhimurium. Infect Immun. 1984;46:559. doi: 10.1128/iai.46.2.559-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galdiero F, Tufano M A, Galdiero M, Masiello S, Di Rosa M. Inflammatory effect of Salmonella typhimurium porins. Infect Immun. 1990;58:3183. doi: 10.1128/iai.58.10.3183-3186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garten W, Henning U. Cell envelope and shape of Escherichia coli K12. Isolation and preliminary characterization of the major ghost-membrane proteins. Eur J Biochem. 1974;47:343–352. doi: 10.1111/j.1432-1033.1974.tb03699.x. [DOI] [PubMed] [Google Scholar]
- 15.Gentile V, Thomazy V, Piacentini M, Fesus L, Davies P J A. Expression of tissue transglutaminase in Balb-C 3T3 fibroblasts: effects on cellular morphology and adhesion. J Cell Biol. 1992;119:463–474. doi: 10.1083/jcb.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorga F, Ianniello R, Petrillo G, Provenzi E, Tufano M A. The presence in human spermatozoa of sites binding some proteins of the outer membrane of Escherichia coli. Microbiologica. 1992;15:187–190. [PubMed] [Google Scholar]
- 17.Hindennach I, Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975;59:207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- 18.Homma J Y. The protein moiety of the endotoxin of Pseudomonas aeruginosa. Z Allg Mikrobiol. 1968;8:227–230. doi: 10.1002/jobm.3630080310. [DOI] [PubMed] [Google Scholar]
- 19.Jonas D, Walev I, Berger T, Liebertrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kierszenbaum A L, De Philip R M, Spruill W A, Takenaka I. Isolation and culture of rat seminal vesicle epithelial cells. The use of the secretory protein SVS IV as a functional probe. Exp Cell Res. 1983;145:293–304. doi: 10.1016/0014-4827(83)90008-3. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G. The mitochondrion as an integrator/coordinator of cell death pathways. Cell Death Differ. 1998;5:547. doi: 10.1038/sj.cdd.4400387. [DOI] [PubMed] [Google Scholar]
- 22.Lennon S V, Martin S J, Cotter T G. Dose-dependent induction of apoptosis in human tumor cell lines by widely diverging stimuli. Cell Prolif. 1991;24:203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 23.Meghji S, Henderson B, Nair S P, Tufano M A. Bacterial porins stimulate bone resorption. Infect Immun. 1997;65:1313–1316. doi: 10.1128/iai.65.4.1313-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison D C, Bucklin S E, Leeson M C, Norimatsu M. Contribution of soluble endotoxin released from Gram-negative bacteria by antibiotics to the pathogenesis of experimental sepsis in mice. J Endotoxin Res. 1996;3:237–243. [Google Scholar]
- 25.Muller A, Gunther D, Dux F, Naumann M, Meyer T F, Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine protease. EMBO J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976;71:877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- 27.Nakae T. Outer membrane of Salmonella: isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976;251:2176–2178. [PubMed] [Google Scholar]
- 28.Nurminen M. A mild procedure to isolate the 34K, 35K, and 36K porins of the outer membrane of Salmonella typhimurium. FEMS Microbiol Lett. 1978;3:331–334. [Google Scholar]
- 29.Paulson J D, Polakoski K L. Isolation of a spermatozoal immobilization factor from Escherichia coli. Fertil Steril. 1977;28:182–185. doi: 10.1016/s0015-0282(16)42380-0. [DOI] [PubMed] [Google Scholar]
- 30.Penninger J, Mak T W. Signal transduction, mitotic catastrophes, and death in T-cell development. Immunol Rev. 1994;142:231–272. doi: 10.1111/j.1600-065x.1994.tb00892.x. [DOI] [PubMed] [Google Scholar]
- 31.Saito S, Shinomiya H, Nakano M. Protein phosphorylation in murine peritoneal macrophages induced by infection with Salmonella species. Infect Immun. 1994;62:1551–1556. doi: 10.1128/iai.62.5.1551-1556.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Schwartz L M, Osborne B A. Methods in cell biology. 46. Cell death. San Diego, Calif: Academic Press, Inc.; 1995. [Google Scholar]
- 34.Shalygina N B. The role of microbial toxic substances in the pathogenesis of acute intestinal infections. Arkh Patol. 1991;53:3–6. [PubMed] [Google Scholar]
- 35.Smit J, Nikaido H. Outer membrane of gram-negative bacteria. XVIII. Electron microscopic studies on porin insertion sites and growth of cell surface of Salmonella typhimurium. J Bacteriol. 1978;135:687–702. doi: 10.1128/jb.135.2.687-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spina A M, Chiosi E, Naviglio S, Valente F, Marchese M, Furgi A, Metafora S, Illiano G. Ras oncogene-induced transformation of a rat seminal vesicle epithelial cell line produces a marked increase of adenylate cyclase and protein kinase C activities. FEBS Lett. 1993;331:150–154. doi: 10.1016/0014-5793(93)80315-l. [DOI] [PubMed] [Google Scholar]
- 37.Tajana G F, Abrescia P, Locuratolo P, Metafora S, Guardiola J. Synthesis of a testosterone-dependent secretory protein by cultured rat seminal vesicle cells. EMBO J. 1984;3:637–644. doi: 10.1002/j.1460-2075.1984.tb01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teague N S, Boyarsky S, Glenn J F. Interference of human spermatozoa motility by Escherichia coli. Fertil Steril. 1971;22:281–285. doi: 10.1016/s0015-0282(16)38220-6. [DOI] [PubMed] [Google Scholar]
- 39.Thye Yin E, Galanos C, Kinsky S, Bradshaw R A, Wessler S, Luderity O, Sarmiento M F. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphenus blood cell lysate induced by purified lipopolysaccharide and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972;261:284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]
- 40.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;179:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 41.Tufano M A, Biancone L, Rossano F, Capasso C, Baroni A, De Martino A, Iorio E L, Silvestro L, Camussi G. Outer-membrane porins from gram-negative bacteria stimulate platelet-activating-factor biosynthesis by cultured human endothelial cells. Eur J Biochem. 1993;214:685–693. doi: 10.1111/j.1432-1033.1993.tb17969.x. [DOI] [PubMed] [Google Scholar]
- 42.Tufano M A, Tetta C, Biancone L, Iorio E L, Baroni A, Giovane A, Camussi G. Salmonella typhimurium porins stimulate platelet-activating factor synthesis by human polymorphonuclear neutrophils. J Immunol. 1992;149:1023–1030. [PubMed] [Google Scholar]
- 43.Tufano M A, Rossano F, Catalanotti P, Liguori G, Marinelli A, Baroni A, Marinelli P. Properties of Yersinia enterocolitica porins: interference with biological functions of phagocytes, nitric oxide production and selective cytokine release. Res Microbiol. 1994;145:297–307. doi: 10.1016/0923-2508(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 44.Tufano M A, Rossano F, Catalanotti P, Liguori G, Capasso C, Ceccarelli M T, Marinelli P. Immunobiological activities of Helicobacter pylori porins. Infect Immun. 1994;62:1392–1399. doi: 10.1128/iai.62.4.1392-1399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tufano M A, Berlingieri M T, Sommese L, Galdiero F. Immune response in mice and effects on cells by outer membrane porins from Salmonella typhimurium. Microbiologica. 1984;7:353. [PubMed] [Google Scholar]
- 46.Tufano M A, Ianniello R, Galdiero M, De Martino L, Galdiero F. Effect of Salmonella typhimurium porins on biological activities of human polymorphonuclear leukocytes. Microb Pathog. 1989;7:337–346. doi: 10.1016/0882-4010(89)90037-5. [DOI] [PubMed] [Google Scholar]
- 47.Wong H, Anderson W D, Cheng T, Riabowol T. Monitoring mRNA expression by polymerase chain reaction: the “primer-dropping” method. Anal Biochem. 1994;223:251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto Y, He P, Klein T W, Friedman H. Endotoxin induced cytotoxicity of macrophages is due to apoptosis caused by nitric oxide production. J Endotoxin Res. 1994;1:181–187. [Google Scholar]
- 49.Yokochi T, Morikawa A, Kato Y, Sugiyama T, Koide N. Apoptotic cell death in response to LPS. Prog Clin Biol Res. 1998;397:235–242. [PubMed] [Google Scholar]