Abstract
The 2022 edition of the IAS-USA drug resistance mutations list updates the Figure last published in September 2019. The mutations listed are those that have been identified by specific criteria for evidence and drugs described. The Figure is designed to assist practitioners to identify key mutations associated with resistance to antiretroviral drugs, and therefore, in making clinical decisions regarding antiretroviral therapy.
Keywords: HIV, antiretroviral, drug resistance, TAM, therapy, mutations
The 2022 edition of the International Antiviral Society-USA (IAS-USA) drug resistance mutations list updates the Figure last published in September 2019.1 In this update:
Cabotegravir, fostemsavir, and ibalizumab have now been approved by regulatory agencies in many countries are all now included. The capsid inhibitor lenacapavir (GS 6207) has been added to the Figure.2
A new section on specific drugs and details has been added to this update for information on recently approved drugs, that may not been added to the Figure.
Several changes were made to drugs already on the Figure. Several changes were made to the Figure Bars of the integrase strand transfer inhibitors (InSTIs) cabotegravir and dolutegravir, the protease inhibitors atazanavir and lopinavir, and the nonnucleoside analogue reverse transcriptase (NNRTI) inhibitor doravirine.
The user notes for tenofovir have been modified as recent clinical data suggest that the K65R plus M184V mutational profile is of less clinical relevance if tenofovir with either lamivudine or emtricitabine is prescribed in combination with a boosted protease inhibitor or one of the second generation InSTIs bictegravir or dolutegravir.
For antiretroviral drugs that are no longer recommended, the associated Figure Bars are listed at the bottom of the drug class and are shaded in gray. Their user notes are retained for historical significance.
Specific Drugs and Details
Cabotegravir (formerly GSK-1265744) was approved by the US Food and Drug Administration (FDA) in December 2021 in combination with rilpivirine for the treatment of HIV-1 infection in adults who are virologically suppressed on a stable antiretroviral regimen with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine. Cabotegravir is available for the treatment of HIV-1 infection as oral formulation or as an extended-release injectable suspension copackaged with rilpivirine.3,4 Cabotegravir suspension was also approved as a long-acting injectable for the use of preexposure prophylaxis (PrEP).5
Fostemsavir (formerly GSK-3684934) was approved by the FDA in February 2020 as a first-in-class oral attachment inhibitor binding to gp120.6 It is licensed for the treatment of HIV-1 infection in combination with other antiretro-viral drugs in heavily treatment-experienced adults with multidrug-resistant HIV-1 infection in whom their current regimen has failed due to resistance, intolerance, or safety considerations.2,7 Fostemsavir shows high variation of in vitro susceptibility, but susceptibility is not dependent on tropism or on subtype with the exception of CRF01_AE, which shows intrinsic resistance.8,9 In areas where CRF01_AE is prevalent, subtyping is recommended. No correlation between baseline resistance and treatment success has yet been established. For this reason, resistance testing for gp120 is not currently recommended. Fostemsavir-associated resistance does not cause cross-resistance to other entry or attachment inhibitors such as ibalizumab and maraviroc.10
Ibalizumab, a humanized monoclonal antibody and noncompetitive CD4 post-attachment inhibitor, is approved for treatment in patients with multiclass drug-resistant virus.11 Since the mechanism of action of ibalizumab requires a previous attachment of HIV-gp120 to the CD4 receptor, ibalizumab does not interfere with the functional capacity of CD4 receptors unbound to HIV-1. Loss of N-linked glycosylation sites in the V5 loop reduce the activity of this compound by preventing HIV-1 gp120 conformational changes and gp41 rearrangements required for the virus to enter target cells.12-14 There are no mutations depicted on the Figure Bars for fostemsavir, ibalizumab, or maraviroc. As such, genotypic testing to predict resistance to these drugs is not recommended in clinical practice. In rare occasions phenotypic testing may be performed, if available.
Methods
The IAS-USA Drug Resistance Mutations Group is an independent, volunteer panel of experts charged with delivering accurate, unbiased, and evidence-based information on drug resistance-associated mutations for HIV clinical practitioners. The group reviews new data on HIV drug resistance to maintain a current list of mutations associated with clinical resistance to HIV-1. This list includes mutations that may contribute to a reduced virologic response to a drug.
The group considers only data that have been published or have been presented at a scientific conference. Table 1 provides the list of amino acids and the abbreviations used. Drugs that have been approved by the US FDA and are generally recommended, as well as any drugs available in development with expectation of approval in the next few years are included (listed in alphabetic order by drug class). Drugs that are no longer recommended are listed at the bottom of the class and are shaded in gray. User notes provide additional information. Although the Drug Resistance Mutations Group works to maintain a complete and current list of these mutations, it cannot be assumed that the list presented here is definitive.
Table 1.
Amino acids and their abbreviations.
| Alanine | A | Metdionine | M |
| Cysteine | C | Asparagine | N |
| Aspartate | D | Proline | P |
| Glutamate | E | Glutamine | Q |
| Phenylalanine | F | Arginine | R |
| Glycine | G | Serine | S |
| Histidine | H | Threonine | T |
| Isoleucine | I | Valine | V |
| Lysine | K | Tryptophan | W |
| Leucine | L | Tryosine | Y |
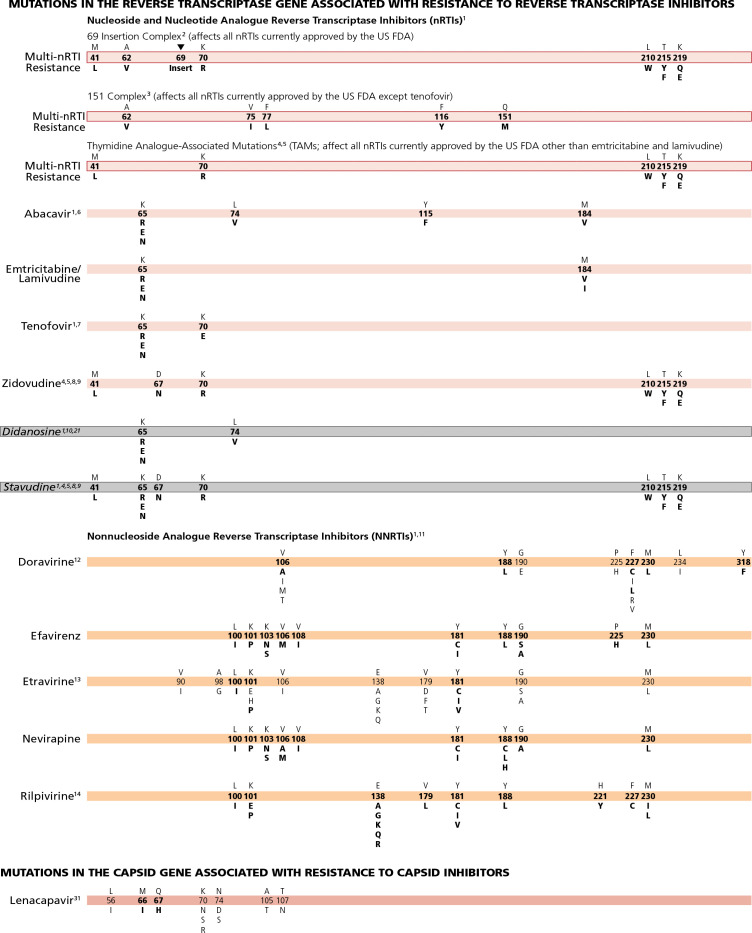
The magnitude of the reduction in susceptibility conferred by drug resistance mutations varies widely, and is modulated by the genetic context of the HIV sequence in which the mutation occurs. Despite the fact that mutations result in a spectrum of degrees of resistance, mutations have been arbitrarily designated as major (bolded) or minor (not bolded) (see Figure 1). Those defined as major tend to occur earlier during treatment failure and generally confer larger reductions in susceptibility. Those defined as minor tend to accrue after the emergence of a major mutation, confer some incremental resistance, may occur as well as polymorphisms in wild-type virus, and in some cases do not reduce susceptibility but restore replication fitness to viruses with resistance mutations that impair fitness. In general, a major mutation should raise concern that a drug is at least partially compromised; a minor mutation on its own may not raise such a concern, but it should add concern in the presence of other mutations. The delineation between major and minor is often not clear-cut.
Figure 1.
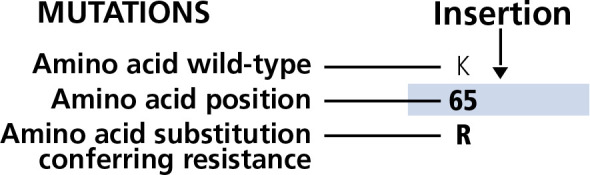
Display of the Figure Bar: Amino acid position, wild type, mutation conferring resistance, and indication of insertion mutation.
Identification of Mutations
The mutations listed are those that have been identified by 1 or more of the following criteria: (1) in vitro passage experiments with validation of contribution to resistance by using site-directed mutagenesis; (2) susceptibility testing of laboratory or clinical isolates; (3) nucleotide sequencing of viruses from patients in whom the drug is failing; (4) association studies between genotype at baseline and virologic response in patients exposed to the drug.
The development of more recently approved drugs that cannot always be tested as monotherapy precludes assessment of the impact of resistance on antiretroviral activity that is not seriously confounded by the activity of other drug components in the background regimen. Readers are encouraged to consult the literature and experts in the field for clarification or more information about specific mutations and their clinical impact. Polymorphisms associated with impaired treatment responses that occur in otherwise wildtype viruses should not be used in epidemiologic analyses to identify transmitted HIV-1 drug resistance. Consequently, only some of the resistance mutations depicted on the Figure can be used to identify transmitted drug resistance.15
Clinical Context
The Figure is designed for practitioners to use in identifying key mutations associated with antiretroviral drug resistance and in selecting therapeutic regimens. In the context of making clinical decisions regarding antiretroviral therapy, evaluating the results of HIV-1 genotypic testing includes: (1) assessing whether the pattern or absence of a pattern in the mutations is consistent with the patient’s history of antiretroviral therapy; and (2) recognizing that resistant strains may be present at levels below the limit of detection of the test after discontinuation or during poor adherence of the regimen that conferred the selection pressure. Analyzing stored samples, collected under selection pressure, could be useful in this setting; and (3) recognizing that virologic failure of a first-line regimen typically involves HIV-1 isolates with resistance to only 1 or 2 of the drugs in the regimen. In this setting, resistance emerges most commonly to lamivudine or emtricitabine, NNRTIs, or first-generation InSTIs (elvitegravir, raltegravir).
The absence of detectable viral resistance after treatment failure may result from any combination of the following factors: the presence of drug-resistant minority viral populations, a prolonged interval between the time of antiretroviral drug discontinuation and genotypic testing, nonadherence to medications, laboratory error, lack of current knowledge of the association of certain mutations with drug resistance, the occurrence of relevant mutations outside the regions targeted by routine resistance assays, drug-drug interactions leading to subtherapeutic drug levels, and possibly the consequence of drugs not reaching optimal levels in specific anatomic compartments.
For more in-depth reading and an extensive reference list, see the 2018 IAS-USA panel recommendations for resistance testing16 and 2020 IAS-USA panel recommendations for antiretroviral therapy.17 Updates to the Figure are posted periodically at www.iasusa.org.
Comments
Please send your evidence-based comments, including relevant reference citations, to journal@iasusa.org.
Reprint Requests
The Drug Resistance Mutations Group welcomes interest in the Figure as an educational resource for practitioners and encourages dissemination of the material to as broad an audience as possible. However, permission is required to reprint the Figure and no alterations in format or content may be made.
Requests to reprint the material should include the name of the publisher or sponsor, the name or a description of the publication in which the material will be reprinted, the funding organization(s), if applicable, and the intended audience. Requests to make any minimal adaptations of the material should include the former, plus a detailed explanation of the adaptation(s) and, if possible, a copy of the proposed adaptation. To ensure the integrity of the Figure, IAS-USA policy is to grant permission for only minor, preapproved adaptations of the Figure (eg, an adjustment in size). Minimal adaptations only will be considered; no alterations of the content of the Figure or user notes will be permitted.
Permission will be granted only for requests to reprint or adapt the most current version of the Figure as they are posted at www.iasusa.org. Because scientific understanding of HIV drug resistance evolves and the goal of the Drug Resistance Mutations Group is to maintain the most up-to-date compilation of mutations for HIV clinicians and researchers, publication of out-of-date figures is counterproductive. If you have any questions about reprints or adaptations, please contact IAS-USA.
The IAS-USA has identified and resolved ahead of time any possible conflicts of interest that may influence CME activities with regard to exposition or conclusion. All financial relationships with ineligible companies for the authors and planners/reviewers are below.
Footnotes
Financial relationships with ineligible companies within the past 24 months: Dr Calvez has served as an advisor or consultant to and has received research grants from Bristol-Myers Squibb, Johnson & Johnson, Merck Sharp & Dohme, Inc, ViiV Healthcare, and Gilead Sciences, Inc. Dr Ceccherini-Silberstein has been a consultant to ViiV Healthcare, Gilead Sciences, Inc, and Merck Sharp & Dohme, Inc, and has received research grants from ViiV Healthcare, Gilead Sciences, Inc, and Merck Sharp & Dohme, Inc. Dr Charpentier serves as an advisor for ViiV Healthcare, Gilead Sciences, Inc, Janssen Therapeutics, Theratechnologies, and Merck Sharp & Dohme, Inc, and has received research grants from ViiV Healthcare. Dr Günthard has served as a consultant to Merck & Co, Inc, ViiV Healthcare, GlaxoSmithKline, Novartis, Johnson and Johnson Inc, and Gilead Sciences, Inc, and has received research grants from Gilead Sciences, Inc. Dr Paredes has received research grants from ViiV Healthcare and Merck Sharp & Dohme, Inc and has been a consultant for Gilead Sciences, Inc, ViiV Healthcare, Pfizer, Inc, Theratechnologies, Inc, and Eli Lilly and Company. Dr Richman has been a consultant to Antiva Biosciences, Assembly Biosciences, Generate Biomedicines, and IGM Biosciences, and serves as Chair of the Data Management Committee of Gilead Sciences, Inc. Dr Shafer has received research grants from Janssen Therapeutics, Vela Diagnostics, and InSilixa, Inc, and personal consulting fees from Abbott Diagnostics. Dr Wensing has served on advisory boards for ViiV Healthcare, GlaxoSmithKline, Janssen Therapeutics, and Gilead Sciences, Inc, and has received investigator-initiated research grants from Gilead Sciences, Inc. Ms Jacobsen has no relevant financial relationships with ineligible companies to disclose. All relevant financial relationships with ineligible companies have been mitigated.
Funding/Support: This work w as funded by IAS-USA. No commercial company or government funding was used to support the effort. Panel members are not compensated.
References
- 1. Wensing AM, Calvez V, Ceccherini-Silberstein F, et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2019;27(3):111–121. [PMC free article] [PubMed] [Google Scholar]
- 2. Dvory-Sobol H, Shaik N, Callebaut C, Rhee MS. Lenacapavir: a first-in-class HIV-1 capsid inhibitor. Curr Opin HIV AIDS. 2022;17(1):15–21. [DOI] [PubMed] [Google Scholar]
- 3. ViiV Healthcare. Vocabria [prescribing information]. 2021. Research Triangle Park, NC, Viiv Healthcare. [Google Scholar]
- 4. ViiV Healthcare. Cabenuva [prescribing information]. 2021. Research Triangle Park, Viiv Healthcare. [Google Scholar]
- 5. ViiV Healthcare. Apretude [prescribing information]. 2021. Research Triangle Park, NC, ViiV Healthcare. [Google Scholar]
- 6. Lataillade M, Lalezari JP, Kozal M, et al. Safety and efficacy of the HlV-1 attachment inhibitor prodrug fostemsavir in heavily treatment-experienced individuals: week 96 results of the phase 3 BRIGHTE study. Lancet HIV. 2020;7(11):e740–e751. [DOI] [PubMed] [Google Scholar]
- 7. ViiV Healthcare. Rukobia [prescribing information]. 2020. Research Triangle Park, NC, ViiV Healthcare. [Google Scholar]
- 8. Nowicka-Sans B, Gong YF, McAuliffe B, et al. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012; 56(7):3498–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montaner LJ, Lynn K, Azzoni L, et al. Susceptibility to 3BNC117 and 10-1074 in ART-suppressed chronically infected persons [CROI Abstract 503]. In Special Issue: Abstracts From the 2022 Conference on Retro-viruses and Opportunistic Infections. Top Antivir Med. 2022;30(1s):192–193. [Google Scholar]
- 10. Rose R, Gartland M, Li Z, et al. Clinical evidence for a lack of cross-resistance between temsavir and ibalizumab or maraviroc. AIDS. 2022;36(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canada Theratechnologies Inc. Trogarzo [prescribing information]. 2018. Montréal, Québec Canada, Canada Theratechnologies Inc. [Google Scholar]
- 12. Emu B, Fessel J, Schrader S, et al. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med. 2018;379(7):645–654. [DOI] [PubMed] [Google Scholar]
- 13. Pace CS, Fordyce MW, Franco D, Kao CY, Seaman MS, Ho DD. Anti-CD4 monoclonal antibody ibalizumab exhibits breadth and potency against HlV-1, with natural resistance mediated by the loss of a V5 glycan in envelope. JAIDS. 2013;62(1):1–9. [DOI] [PubMed] [Google Scholar]
- 14. Toma J, Weinheimer SP, Stawiski E, et al. Loss of asparagine-linked glycosylation sites in variable region 5 of human immunodeficiency virus type 1 envelope is associated with resistance to CD4 antibody ibalizumab. J Virol. 2011;85(8):3872–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pingen M, Nijhuis M, de Bruijn JA, Boucher CA, Wensing AM. Evolutionary pathways of transmitted drug-resistant HIV-1. J Antimicrob Chemother. 2011;66(7): 1467–1480. [DOI] [PubMed] [Google Scholar]
- 16. Gunthard HF, Calvez V, Paredes R, et al. Human immunodeficiency virus drug resistance: 2018 recommendations of the International Antiviral Society-USA panel. Clin Infect Dis. 2018;67:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324(16):1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
User Notes
1. Mutations at the C-terminal reverse transcriptase domains (amino acids 293-560) outside of regions depicted on the Figure Bar may contribute to nucleoside (or nucleotide) analogue reverse transcriptase inhibitor (nRTI) and nonnucleoside analogue reverse transcriptase inhibitors (NNRTI) HIV-1 drug resistance. The clinical relevance of these connection domain mutations arises mostly in conjunction with thymidine analogue-associated mutations (TAMs) and M184V and they have not been associated with increased rates of virologic failure of etravirine or rilpivirine in clinical trials.1-3 K65E/N/R variants are reported in patients experiencing treatment failure of tenofovir (ie, tenofovir disoproxil fumarate [TDF] or tenofovir alafenamide [TAF]), stavudine, or didanosine. The K65R/N variants may be selected by tenofovir, didanosine, abacavir, or stavudine and are associated with decreased viral susceptibility to these drugs.4-8 The K65R may be more easily selected in subtype C clades.9 K65E usually occurs in mixtures with wild-type virus. Patient-derived viruses with K65E and site-directed mutations replicate very poorly in vitro; as such, no susceptibility testing can be performed.10,11 Some nRTI mutations, like T215Y and H208Y,12 may lead to viral hypersusceptibility to NNRTIs, including etravirine.13 The presence of these mutations may improve subsequent virologic response to NNRTI-containing regimens (nevirapine or efavirenz) in NNRTI-naive individuals;14-18 no clinical data exist for improved response to etravirine in NNRTI-experienced individuals.
2. The 69 insertion complex consists of a substitution at codon 69 (typically T69S) and an insertion of 2 or more amino acids (S-S, S-A, S-G, or others). The 69 insertion complex is associated with resistance to all nRTIs currently approved by the US Food and Drug Administration (FDA) when present with 1 or more TAMs at codons 41, 210, or 215.4 Some other amino acid changes from the wild-type T at codon 69 without the insertion may be associated with broad nRTI resistance.
3. Since no differences in resistance patterns have been observed between TDF and TAF, both drugs are referred to as “tenofovir” on the Figure Bar.19 Tenofovir retains activity against the Q151M complex of mutations.4 Q151M is the most important mutation in the complex (ie, the other mutations in the complex [A62V, V75I, F77L, and F116Y] in isolation may not reflect multi-nucleoside resistance).
4. Mutations known to be selected by TAMs (ie, M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E) also confer reduced susceptibility to all currently approved nRTIs20 except emtricitabine and lamivudine, which in fact reverse the magnitude of resistance and are recommended with tenofovir or zidovudine in the presence of TAMs. The degree to which cross-resistance is observed depends on the specific mutations and number of mutations involved.21-24
5. Although reverse transcriptase changes associated with the E44D and V118I mutations may have an accessory role in increased resistance to nRTIs in the presence of TAMs, their clinical relevance is very limited.25-27
6. The M184V mutation alone does not appear to be associated with a reduced virologic response to abacavir in vivo. When associated with TAMs, M184V increases abacavir resistance.5,28
7. K65R is the most common drug resistance mutation to emerge in patients with virologic failure on a tenofovircontaining regimen. It is associated with about 2-fold reduced tenofovir susceptibility, which is clinically significant. However, when K65R occurs in combination with the lamivudine/emtricitabine resistance mutation M184V/I, the reduction in tenofovir susceptibility is less than 1.5 fold, a reduction in susceptibility that is less clinically significant. This is particularly the case in patients who are treated with the combination of tenofovir, a cytosine analogue, and a highly potent third drug such as the integrase strand transfer inhibitors (InSTIs) bictegravir and dolutegravir or a boosted protease inhibitor (PI).29,30
A reduced response also occurs in the presence of 3 or more TAMs inclusive of either M41L or L210W.4 The presence of TAMs or combined treatment with zidovudine prevents the emergence of K65R in the presence of tenofovir.31-33
8. The presence of M184V appears to delay or prevent emergence of TAMs.34 This effect may be overcome by an accumulation of TAMs.
9. The T215A/C/D/E/G/H/I/L/N/S/V substitutions are revertant mutations at codon 215 that confer increased risk of virologic failure of zidovudine or stavudine in antiretroviral-naive patients.35,36 The T215Y mutant may emerge quickly from one of these mutations in the presence of zidovudine or stavudine.37
10. The presence of 3 of the following mutations—M41L, D67N, L210W, T215Y/F, K219Q/E—is associated with resistance to didanosine.38 The presence of K70R or M184V alone does not decrease virologic response to didanosine.39 However, the mutations depicted on the Figure Bar cannot be considered comprehensive because little relevant research has been reported in recent years to update the resistance and cross-resistance patterns for this drug.
11. There is no evidence for the utility of efavirenz, nevirapine, or rilpivirine in patients with NNRTI resistant virus.40
12. Doravirine is active in vitro against variants containing the common NNRTI mutations K103N, E138K, Y181C, and G190A.41,42 Doravirine selects for mutations at positions 106, 108, 227, and 234, with more than 1 mutation usually required for substantial levels of resistance.43 Mutations V106A, Y188L, and M230L are associated with a 10- or greater fold reduced susceptibility to doravirine. V106A and Y188L have also been selected in vivo.44,45 In 1 clinical isolate, G190E was associated with about 20-fold reduced susceptibility to doravirine.42 Furthermore, the double and triple mutants V106A and F227L; V106A and L234I; V106A and F227L and L234I; and V106A and 190A and F227L, are all associated with substantial resistance to doravirine.41,43,46
13. Resistance to etravirine has been extensively studied only in the context of coadministration with ritonavir-boosted darunavir. Mutations associated with virologic outcome were assessed and their relative weights (or magnitudes of impact) assigned. Phenotypic cutoff values were calculated, and assessments of genotype-phenotype correlations from a large clinical database have determined the relative importance of the various mutations. These 2 approaches are in agreement for many, but not all, mutations and weights.47-49 The single mutations L100I, K101P, and Y181C/I/V have high relative weights with regard to reduced susceptibility and reduced clinical response compared with other mutations.50,51 The presence of K103N alone does not affect etravirine response.51 Accumulation of several mutations results in greater reductions in susceptibility and virologic response than do single mutations.52-54
14. Sixteen mutations have been associated with decreased rilpivirine susceptibility (K101E/P, E138A/G/K/Q/R, V179L, Y181C/I/V, Y188L, H221Y, F227C, and M230I/L).55-57 The K101P and Y181I/V mutations reduce rilpivirine susceptibility approximately 50 fold and 15 fold, respectively, but are not commonly observed in patients receiving rilpivirine.58-60 Mutations at position 138 (most notably E138A) may occur as natural polymorphisms, especially in non-B subtype virus.61 The K101E, E138K, and Y181C mutations, each of which reduces rilpivirine susceptibility 2.5 fold to 3 fold, occur commonly in patients receiving rilpivirine. E138K and to a lesser extent K101E usually occur in combination with the nRTI resistance-associated mutation M184I, which alone does not reduce rilpivirine susceptibility. When M184I is combined with E138K or K101E, rilpivirine susceptibility is reduced about 7 fold and 4.5 fold, respectively.60,62-64 The combinations of reverse transcriptase-associated mutations L100I plus K103N/S and L100I plus K103R plus V179D were strongly associated with reduced susceptibility to rilpivirine; however, for isolates harboring the K103N/R/S or V179D as single mutations, no reduction in susceptibility was detected.57,65
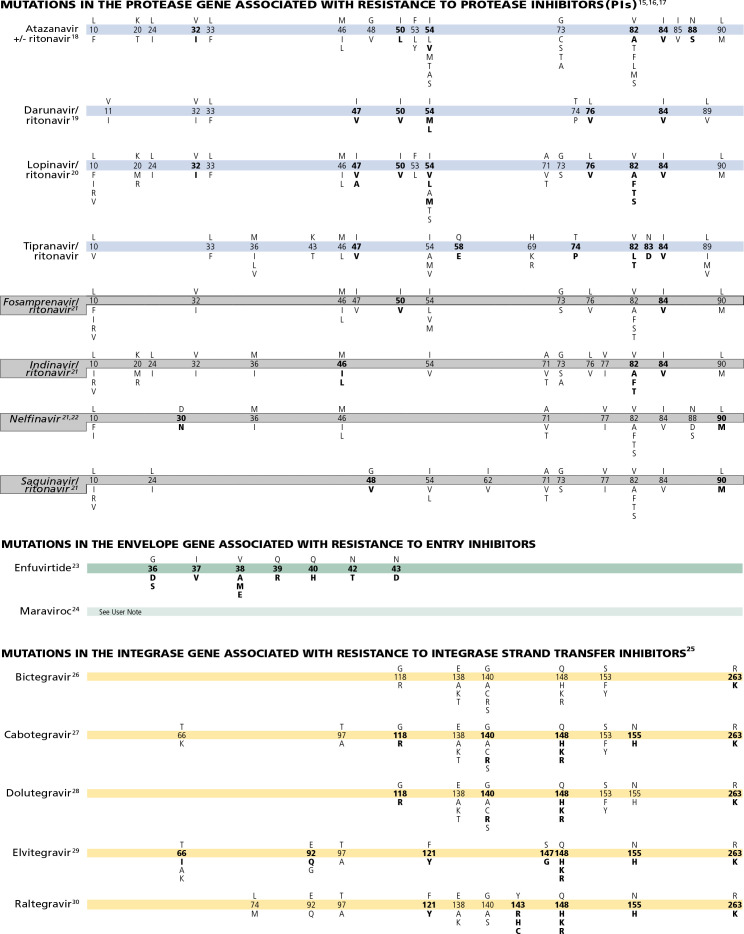
15. Often, several mutations are necessary to substantially impact virologic response to a ritonavir-boosted PI.66
16. Mutations in Gag cleavage sites may confer or contribute to resistance to PIs and may even emerge before mutations in protease.67 A large proportion of virus samples from patients with confirmed virologic failure on a PI-containing regimen is not found to have PI resistance-associated mutations, attributable to poor adherence.
17. Ritonavir is not listed separately, as it is currently used only at low doses as a pharmacologic booster of other PIs.
18. Several mutations are associated with atazanavir resistance. Their impacts differ, with I50L, I84V, and N88S having the greatest effect. Mutations that are selected during unboosted atazanavir are not different from those selected during boosted atazanavir, but the relative frequency of mutations may differ. Higher atazanavir levels obtained with ritonavir boosting increase the number of mutations required for loss of activity. The presence of M46I plus L76V might increase susceptibility to atazanavir when no other related mutations are present.68
19. Virologic response to ritonavir-boosted darunavir correlates with baseline susceptibility and the presence of several specific PI resistance-associated mutations. Reductions in response are associated with increasing numbers of the mutations indicated on the Figure Bar. The negative impact of the protease mutations I47V, I54M, T74P, and I84V and the positive impact of the protease mutation V82A on virologic response to ritonavir-boosted darunavir were shown independently in 2 data sets.69,70 Some of these mutations appear to have a greater effect on susceptibility than others (eg, I50V vs V11I). The presence at baseline of 2 or more of the substitutions V11l, V32I, L33F, I47V, I50V, I54L/M, T74P, L76V, I84V, or L89V was associated with a decreased virologic response to ritonavir-boosted darunavir.71
20. Virologic response to ritonavir-boosted lopinavir is affected by the presence of 3 or more of the following amino acid substitutions in protease at baseline: L10F/I/R/V, K20M/N/R, L24I, L33F, M36I, I47V, G48V, I54L/T/V, V82A/C/F/S/T, and I84V. In addition, the combination of 47A/V with V32I is associated with high-level resistance.68,72-78 I50V is only occasionally selected in vivo but has a clear impact on susceptibility.12,79-81 Subtype C patterns with M46L, I54V, L76V, and V82A are frequently observed in patients receiving ritonavir-boosted lopinavir.
21. The mutations depicted on the Figure Bar cannot be considered comprehensive because little relevant research has been reported in recent years to update the resistance and cross-resistance patterns for this drug.
22. In some nonsubtype-B HIV-1, D30N is selected less frequently than are other PI resistance-associated mutations.82
23. Resistance to enfuvirtide is associated primarily with mutations in the first heptad repeat (HR1) region of the gp41 envelope gene. However, mutations or polymorphisms in other regions of the env (eg, the HR2 region or those yet to be identified), as well as coreceptor usage and density, may affect susceptibility to enfuvirtide.83-85
24. The activity of CC chemokine receptor 5 (CCR5) antagonists is limited to patients with virus that use only CCR5 for td (R5 virus). Viruses that use both CCR5 and CXC chemokine receptor 4 (CXCR4; termed dual/mixed [D/M] virus) or only CXCR4 (X4 virus) do not respond to treatment with CCR5 antagonists. Virologic failure of these drugs is frequently associated with outgrowth of D/M or X4 virus from a preexisting minority population present at levels below the limit of assay detection. Mutations in HIV-1 gp120 that allow the virus to bind to the drug-bound form of CCR5 have been described in viruses from some patients whose virus remained RUN5 after virologic failure of a CCR5 antagonist. Most of these mutations are found in the V3 loop, the major determinant of viral tropism.86 There is as yet no consensus on specific signature mutations for CCR5 antagonist resistance, so they are not depicted on the Figure Bar. Some CCR5 antagonist-resistant viruses selected in vitro have shown mutations in gp41 without mutations in V3;87 the clinical significance of such mutations is not yet known.
25. With their low genetic barrier to resistance and the high level of cross-resistance, the InSTIs elvitegravir and raltegravir are no longer generally recommended in an initial therapy for most people with HIV.88 A second-generation InSTIs (dolutegravir, bictegravir, and cabotegravir) is recommended for most treatment situations.
26. In vitro susceptibility data indicate relatively small quantitative reductions in most cases for dolutegravir and bictegravir for single mutations in integrase.89-91 Consequently, the Figure Bar listing the mutations or indicating them as bold is somewhat arbitrary in the absence of clinical data. The listing of mutations is based in most cases on in vitro selection data and testing single mutations seen mostly with first-generation InSTI failure in vitro. Several mutations were selected by dolutegravir, primarily during monotherapy trials or as add-on therapy to failing regimens.92 Failure with the emergence of resistance to bictegravir, which is only available as a fixed-dose formulation with TAF and emtricitabine for individuals with no known InSTI resistance, has not been well documented. The only clinical data for treatment of individuals with InSTI resistance comes from the VIKING Study, in which even double doses of dolutegravir combined with the best available background regimen had higher failure rates against Q148K with 2 or more additional mutations in integrase.93 Failure with emergence of resistance to bictegravir in a first-line regimen has been very rarely observed.94 In vitro data suggest that these double mutants might have compromised the efficacy of bictegravir in one study but not another.90,91 Multiple mutations are not displayed in the Figure Bar.
27. Cabotegravir is a long-acting InSTI. In clinical trials in individuals receiving HIV treatement or PrEP, several resistance mutations were observed in integrase associated with in vitro cabotegravir resistance.95-97 A multivariate analysis showed that the presence of at least 2 factors among archived rilpivirine resistance-associated mutations at baseline, HIV-1 subtype A6/A1, or body mass index of at least 30 kg/m2, was associated with increased risk of confirmed virologic failure.98 The A6/A1 subtype frequently harbors the L74I polymorphism. A recent study showed that L74I conferred greater replication capacity to recombinant viruses expressing HIV-1 A6 integrase when present together with InSTI resistance mutations at positions 118, 140, 148, and 263. This finding may explain in part the association of this subtype to virologic failures of long-acting cabotegravir/rilpivirine.99
Although knowledge from clinical studies thus far is limited, in vitro studies indicate that multiple integrase substitutions including compensatory mutations enhance resistance to cabotegravir.100
28. Several mutations are required in HIV integrase to confer high-level resistance to dolutegravir.100,101 Cross-resistance studies with raltegravir- and elvitegravir-resistant viruses indicate that Q148H/R and G140S in combination with mutations L74I/M, E92Q, T97A, E138A/K, G140A, or N155H are associated with 5-fold to 20-fold reduced dolutegravir susceptibility102 and reduced virologic suppression in patients.103-106
29. Seven elvitegravir codon mutations have been observed in InSTI treatment-naive and -experienced patients in whom therapy is failing.107-113 T97A, which may occur as a polymorphism,114 results in only a 2-fold change in elvitegravir susceptibility and may require additional mutations for resistance.112,113 The sequential use of elvitegravir and raltegravir (in either order) is not recommended because of cross-resistance between these drugs.112
30. Raltegravir failure is associated with integrase mutations in at least 3 distinct, but not exclusive, genetic pathways defined by 2 or more mutations including (1) a mutation at Q148H/K/R, N155H, or Y143R/H/C; and (2) 1 or more additional minor mutations. Minor mutations described in the Q148H/K/R pathway include L74M plus E138A, E138K, or G140S. The most common mutational pattern in this pathway is Q148H plus G140S, which also confers the greatest loss of drug susceptibility. Mutations described in the N155H pathway include this major mutation plus either L74M, E92Q, T97A, E92Q plus T97A, Y143H, G163K/R, V151I, or D232N.115 The Y143R/H/C mutation is uncommon.116-120 E92Q alone reduces susceptibility to elvitegravir more than 20 fold and causes limited (<5 fold) cross-resistance to raltegravir.121-123 N155H mutants tend to predominate early in the course of raltegravir failure, but are gradually replaced with continuing raltegravir treatment by viruses with higher resistance, often bearing mutations G140S plus Q148H/R/K.
31. The emergence of resistance with lenacapavir was characterized with in vitro selection, which identified several variants in the capsid (CA) portion of Gag (L56I, M66I, Q67H, K70N, N74D/S, and T107N), with 20-fold to 1000-fold reduced susceptibility in vitro with Q67H+N74S, Q67H+T107N, L56I (204), Q67H+M66I, Q67H+N74D, M66I (>2,700), and reduced replication capacity for most mutant viruses.124-126
None of these mutations were found to be polymorphic suggesting there is no need for resistance testing before treatment with lenacapavir.127 In a phase Ib study, post-monotherapy analyses revealed the emergence of mutation Q67H at the lowest lenacapavir doses.125,126 In highly treatment-experienced patients with lenacapavir failure, M66I was observed alone or in combination with other mutations. In all cases, the failures were initially associated with the selection of M66I.30,128
In highly treatment-experienced patients experiencing treatment failure in the CAPELLA study, the M66I mutation was most frequently observed.129 In treatment-naive individuals in the CALIBRATE trial mutations 67H (fold change 7) and 70R were selected.130,131
References to the User Notes
- 1. von Wyl V, Ehteshami M, Demeter LM, et al. HIV-1 reverse transcriptase connection domain mutations: dynamics of emergence and implications for success of combination antiretroviral therapy. Clin Infect Dis. 2010;51(5):620–628. [DOI] [PubMed] [Google Scholar]
- 2. Gupta S, Vingerhoets J, Fransen S, et al. Connection domain mutations in HIV-1 reverse transcriptase do not impact etravirine susceptibility and virologic responses to etravirine-containing regimens. Antimicrob Agents Chemother. 2011;55(6):2872–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rimsky L, Van Eygen V, Vingerhoets J, Leijskens E, Picchio G. Reverse transcriptase connection domain mutations were not associated with virological failure or phenotypic resistance in rilpivirine-treated patients from the ECHO and THRIVE Phase III trials (week 96 analysis). Antivir Ther. 2012;17(Suppl 1):A36. [Google Scholar]
- 4. Miller MD, Margot N, Lu B, et al. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis. 2004; 189(5):837–846. [DOI] [PubMed] [Google Scholar]
- 5. Harrigan PR, Stone C, Griffin P, et al. Resistance profile of the human immunodeficiency virus type 1 reverse transcriptase inhibitor abacavir (1592U89) after monotherapy and combination therapy. CNA2001 Investigative Group. J Infect Dis. 2000;181(3):912–920. [DOI] [PubMed] [Google Scholar]
- 6. Winters MA, Shafer RW, Jellinger RA, Mamtora G, Gingeras T, Merigan TC. Human immunodeficiency virus type 1 reverse transcriptase genotype and drug susceptibility changes in infected individuals receiving dideoxyinosine monotherapy for 1 to 2 years. Antimicrob Agents Chemother. 1997;41(4):757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Svarovskaia ES, Margot NA, Bae AS, et al. Low-level K65R mutation in HIV-1 reverse transcriptase of treatment-experienced patients exposed to abacavir or didanosine. JAIDS. 2007;46(2):174–180. [DOI] [PubMed] [Google Scholar]
- 8. Hawkins CA, Chaplin B, Idoko J, et al. Clinical and genotypic findings in HIV-infected patients with the K65R mutation failing first-line antiretroviral therapy in Nigeria. JAIDS. 2009;52(2):228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brenner BG, Oliveira M, Doualla-Bell F, et al. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS. 2006; 20:F9–F13. [DOI] [PubMed] [Google Scholar]
- 10. Fourati S, Visseaux B, Armenia D, et al. Identification of a rare mutation at reverse transcriptase Lys65 (K65E) in HIV-1-infected patients failing on nucleos(t)ide reverse transcriptase inhibitors. J Antimicrob Chemother. 2013;68(10): 2199–2204. [DOI] [PubMed] [Google Scholar]
- 11. Chunduri H, Crumpacker C, Sharma PL. Reverse transcriptase mutation K65N confers a decreased replication capacity to HIV-1 in comparison to K65R due to a decreased RT processivity. Virology. 2011;414(1):34–41. [DOI] [PubMed] [Google Scholar]
- 12. Clark SA, Shulman NS, Bosch RJ, Mellors JW. Reverse transcriptase mutations 118I, 208Y, and 215Y cause HIV-1 hypersusceptibility to non-nucleoside reverse transcriptase inhibitors. AIDS. 2006; 20(7):981–984. [DOI] [PubMed] [Google Scholar]
- 13. Picchio G, Vingerhoets J, Parkin N, Azijn H, de Bethune MP. Nucleoside-associated mutations cause hypersusceptibility to etravirine. Antivir Ther. 2008;13(Suppl 3):A25. [Google Scholar]
- 14. Shulman NS, Bosch RJ, Mellors JW, Albrecht MA, Katzenstein DA. Genetic correlates of efavirenz hypersusceptibility. AIDS. 2004;18(13):1781–1785. [DOI] [PubMed] [Google Scholar]
- 15. Demeter LM, DeGruttola V, Lustgarten S, et al. Association of efavirenz hypersusceptibility with virologic response in ACtG 368, a randomized trial of abacavir (ABC) in combination with efavirenz (EFV) and indinavir (IDV) in HIV-infected subjects with prior nucleoside analog experience. HIV Clin Trials. 2008;9(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haubrich RH, Kemper CA, Hellmann NS, et al. The clinical relevance of non-nucleoside reverse transcriptase inhibitor hypersusceptibility: a prospective co hort analysis. AIDS. 2002;16(15): F33–F40. [DOI] [PubMed] [Google Scholar]
- 17. Tozzi V, Zaccarelli M, Narciso P, et al. Mutations in HIV-1 reverse transcriptase potentially associated with hypersusceptibility to nonnucleoside reverse-transcriptase inhibitors: effect on response to efavirenz-based therapy in an urban observational cohort. J Infect Dis. 2004;189(9):1688–1695. [DOI] [PubMed] [Google Scholar]
- 18. Katzenstein DA, Bosch RJ, Hellmann N, et al. Phenotypic susceptibility and virological outcome in nucleoside-experienced patients receiving three or four antiretroviral drugs. AIDS. 2003;17(6): 1821–830. [DOI] [PubMed] [Google Scholar]
- 19. Margot N, Cox S, Das M, Mc-Callister S, Miller MD, Callebaut C. Infrequent development of drug resistance in HIV-1-infected treatment-naive subjects after 96 weeks of treatment with elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide or elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate. Antivir Ther. 2017;22(5):443–446. [DOI] [PubMed] [Google Scholar]
- 20. Whitcomb JM, Parkin NT, Chappey C, Hellman NS, Petropoulos CJ. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J Infect Dis. 2003;188(7):992–1000. [DOI] [PubMed] [Google Scholar]
- 21. Larder BA, Kemp SD. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989;246(4934):1155–1158. [DOI] [PubMed] [Google Scholar]
- 22. Kellam P, Boucher CA, Larder BA. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high-level resistance to zidovudine. Proc Natl Acad Sci USA. 1992;89(5): 1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Calvez V, Costagliola D, Descamps D, et al. Impact of stavudine phenotype and thymidine analogues mutations on viral response to stavudine plus lamivudine in ALTIS 2 ANRS trial. Antivir Ther. 2002;7(3):211–218. [PubMed] [Google Scholar]
- 24. Kuritzkes DR, Bassett RL, Hazelwood JD, et al. Rate of thymidine analogue resistance mutation accumulation with zidovudine- or stavudine-based regimens. JAIDS. 2004;36(1):600–603. [DOI] [PubMed] [Google Scholar]
- 25. Romano L, Venturi G, Bloor S, et al. Broad nucleoside-analogue resistance implications for human immunodeficiency virus type 1 reverse-transcriptase mutations at codons 44 and 118. J Infect Dis. 2002;185(7):898–904. [DOI] [PubMed] [Google Scholar]
- 26. Walter H, Schmidt B, Werwein M, Schwingel E, Korn K. Prediction of abacavir resistance from genotypic data: impact of zidovudine and lamivudine resistance in vitro and in vivo. Antimicrob Agents Chemother. 2002;46(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mihailidis C, Dunn D, Pillay D, Pozniak A. Effect of isolated V118I mutation in reverse transcriptase on response to first-line antiretroviral therapy. AIDS. 2008;22(3): 427–430. [DOI] [PubMed] [Google Scholar]
- 28. Lanier ER, Ait-Khaled M, Scott J, et al. Antiviral efficacy of abacavir in antiretroviral therapy-experienced adults harbouring HIV-1 with specific patterns of resistance to nucleoside reverse transcriptase inhibitors. Antivir Ther. 2004; 9(1):37–45. [DOI] [PubMed] [Google Scholar]
- 29. Paton NI, Musaazi J, Kityo C, et al. Dolutegravir or darunavir in combination with zidovudine or tenofovir to treat HIV. N Engl J Med. 2021;385(4):330–341. [DOI] [PubMed] [Google Scholar]
- 30. Segal-Maurer S, DeJesus E, Stellbrink HJ, et al. Capsid inhibition with lenacapavir in multidrug-resistant HIV-1 infection. N Engl J Med. 2022;386(19):1793–1803. [DOI] [PubMed] [Google Scholar]
- 31. Parikh UM, Zelina S, Sluis-Cremer N, Mellors JW. Molecular mechanisms of bidirectional antagonism between K65R and thymidine analog mutations in HIV-1 reverse transcriptase. AIDS. 2007;21(11):1405–1414. [DOI] [PubMed] [Google Scholar]
- 32. Parikh UM, Barnas DC, Faruki H, Mellors JW. Antagonism between the HIV-1 reverse-transcriptase mutation K65R and thymidine-analogue mutations at the genomic level. J Infect Dis. 2006;194(5):651–660. [DOI] [PubMed] [Google Scholar]
- 33. von Wyl V, Yerly S, Böni J, et al. Factors associated with the emergence of K65R in patients with HIV-1 infection treated with combination antiretroviral therapy containing tenofovir. Clin Infect Dis. 2008;46(8):1299–1309. [DOI] [PubMed] [Google Scholar]
- 34. Kuritzkes DR, Quinn JB, Benoit SL, et al. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. AIDS. 1996;10(9):975–981. [DOI] [PubMed] [Google Scholar]
- 35. Violin M, Cozzi-Lepri A, Velleca R, et al. Risk of failure in patients with 215 HIV-1 revertants starting their first thymidine analog-containing highly active antiretroviral therapy. AIDS/ 2004;18(2): 227–235. [DOI] [PubMed] [Google Scholar]
- 36. Chappey C, Wrin T, Deeks S, Petropoulos CJ. Evolution of amino acid 215 in HIV-1 reverse transcriptase in response to intermittent drug selection. Antivir Ther. 2003;8):S37. [Google Scholar]
- 37. Garcia-Lerma JG, MacInnes H, Bennett D, Weinstock H, Heneine W. Transmitted human immunodeficiency virus type 1 carrying the D67N or K219Q/E mutation evolves rapidly to zidovudine resistance in vitro and shows a high replicative fitness in the presence of zidovudine. J Virol. 2004; 78(14):7545–7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marcelin AG, Flandre P, Pavie J, et al. Clinically relevant genotype interpretation of resistance to didanosine. Antimicrob Agents Chemother. 2005;49(5):1739–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Molina JM, Marcelin AG, Pavie J, et al. Didanosine in HIV-1-infected patients experiencing failure of antiretroviral therapy: a randomized placebo-controlled trial. J Infect Dis. 2005;191(6):840–847. [DOI] [PubMed] [Google Scholar]
- 40. Antinori A, Zaccarelli M, Cingolani A, et al. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res Hum Retroviruses. 2002;18(12):835–838. [DOI] [PubMed] [Google Scholar]
- 41. Feng M, Wang D, Grobler JA, Hazuda DJ, Miller MD, Lai MT. In vitro resistance selection with doravirine (MK-1439), a novel nonnucleoside reverse transcriptase inhibitor with distinct mutation development pathways. Antimicrob Agents Chemother. 2015; 59(1):590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin EA, Lai MT, Ngo W, et al. Review of doravirine resistance patterns identified in participants during clinical development. J Acquir Immune Defic Syndr. 2020; 85(5):635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lai MT, Feng M, Falgueyret JP, et al. In vitro characterization of MK-1439, a novel HIV-1 non-nucleoside reverse transcriptase inhibitor. Antimicrob Agents Chemother. 2014;58(3): 1652–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Orkin C, Squires KE, Molina JM, et al. Doravirine/lamivudine/tenofovir disoproxil fumarate is noninferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis. 2019;68(4):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Molina JM, Squires K, Sax PE, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVEFORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV. 2018;5(5):e211–e220. [DOI] [PubMed] [Google Scholar]
- 46. Smith SJ, Pauly GT, Akram A, et al. Rilpivirine and doravirine have complementary efficacies against NNRTI-resistant HIV-1 mutants. JAIDS. 2016;72(5):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benhamida J, Chappey C, Coakley E, Parkin NT. HIV-1 genotype algorithms for prediction of etravirine susceptibility: novel mutations and weighting factors identified through correlations to phenotype. Antivir Ther. 2008;13(Suppl 3):A142. [Google Scholar]
- 48. Coakley E, Chappey C, Benhamida J, et al. Biological and clinical cut-off analyses for etravirine in the PhenoSense HIV assay. Antivir Ther. 2008;13(Suppl 3):A134. [Google Scholar]
- 49. Vingerhoets J, Tambuyzer L, Azijn H, et al. Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled Phase Ill clinical studies. AIDS. 2010;24(4):503–514. [DOI] [PubMed] [Google Scholar]
- 50. Haddad M, Stawiski E, Benhamida J, Coakley E. Improved genotypic algorithm for predicting etravirine susceptibility: comprehensive list of mutations identified through correlation with matched phenotype. Poster presented at: 17th Conference on Retroviruses and Opportunistic Infections (CROI); February 16-19, 2010; San Francisco, CA.
- 51. Janssen Therapeutics. Etravirine [prescribing information]. 2013. Titusville, NJ, Janssen Therapeutics. [Google Scholar]
- 52. Scherrer AU, Hasse B, Von Wyl V, et al. Prevalence of etravirine mutations and impact on response to treatment in routine clinical care: the Swiss HIV Cohort Study (SHCS). HIV Med. 2009;10(10):647–656. [DOI] [PubMed] [Google Scholar]
- 53. Tambuyzer L, Nijs S, Daems B, Picchio G, Vingerhoets J. Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. JAIDS. 2011; 58(1):18–22. [DOI] [PubMed] [Google Scholar]
- 54. Tudor-Williams G, Cahn P, Chokephaibulkit K, et al. Etravirine in treatment-experienced, HIV-1-infected children and adolescents: 48-week safety, efficacy and resistance analysis of the phase II PIANO study. HIV Med. 2014;15(9): 513–524. [DOI] [PubMed] [Google Scholar]
- 55. Janseen Therapeutics. Rilpivirine [prescribing information]. 2015. Titusville, NJ, Janseen Therapeutics. [Google Scholar]
- 56. Azijn H, Tirry I, Vingerhoets J, et al. TMC278, a next-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010;54(2):718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Picchio GR, Rimsky LT, Van Eygen V, et al. Prevalence in the USA of rilpivirine resistance-associated mutations in clinical samples and effects on phenotypic susceptibility to rilpivirine and etravirine. Antivir Ther. 2014;19(8):819–823. [DOI] [PubMed] [Google Scholar]
- 58. Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011;378(9787):229–237. [DOI] [PubMed] [Google Scholar]
- 59. Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378(9787):238–246. [DOI] [PubMed] [Google Scholar]
- 60. Rimsky L, Vingerhoets J, Van Eygen V, et al. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. JAIDS. 2012;59(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 61. Hofstra LM, Sauvageot N, Albert J, et al. Transmission of HIV drug resistance and the predicted effect on current first-line regimens in Europe. Clin Infect Dis. 2016; 62(5):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kulkarni R, Babaoglu K, Lansdon EB, et al. The HIV-1 reverse transcriptase M184I mutation enhances the E138K-associated resistance to rilpivirine and decreases viral fitness. JAIDS. 2012;59(1): 47–54. [DOI] [PubMed] [Google Scholar]
- 63. Hu Z, Kuritzkes DR. Interaction of reverse transcriptase (RT) mutations conferring resistance to lamivudine and etravirine: effects on fitness and RT activity of human immunodeficiency virus type 1. J Virol. 2011;85(21):11309–11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu HT, Asahchop EL, Oliveira M, et al. Compensation by the E138K mutation in HIV-1 reverse transcriptase for deficits in viral replication capacity and enzyme processivity associated with the M184I/V mutations. J Virol. 2011; 85(21):11300–11308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haddad M, Napolitano LA, Frantzell A, et al. Combinations of HIV-1 reverse transcriptase mutations L100I + K103N/S and L100I + K103R + V179D reduce susceptibility to rilpivirine. Poster presented at: 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); September 10-13, 2013; Denver, CO.
- 66. Hirsch MS, Günthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008; 47(2):266–285. [DOI] [PubMed] [Google Scholar]
- 67. Fun A, Wensing AM, Verheyen J, Nijhuis M. Human immunodeficiency virus gag and protease: partners in resistance. Retrovirology. 2012;9):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Young TP, Parkin NT, Stawiski E, et al. Prevalence, mutation patterns, and effects on protease inhibitor susceptibility of the L76V mutation in HlV-1 protease. Antimicrob Agents Chemother. 2010; 54(11):4903–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. De Meyer S, Descamps D, Van Baelen B, et al. Confirmation of the negative impact of protease mutations I47V, I54M, T74P and I84V and the positive impact of protease mutation V82A on virological response to darunavir/ritonavir. Antivir Ther. 2009;14(Suppl 1):A147. [Google Scholar]
- 70. Descamps D, Lambert-Niclot S, Marcelin AG, et al. Mutations associated with virological response to darunavir/ritonavir in HlV-1-infected protease inhibitor-experienced patients. J Antimicrob Chemother. 2009;63(3):585–592. [DOI] [PubMed] [Google Scholar]
- 71. Janssen Therapeutics. Darunavir [prescribing information]. 2015. Titusville, NJ, Janssen Therapeutics. [Google Scholar]
- 72. Masquelier B, Breilh D, Neau D, et al. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavirritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2002;46(9):2926–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kempf DJ, Isaacson JD, King MS, et al. Identification of genotypic changes in human immunodeficiency virus protease that correlate with reduced susceptibility to the protease inhibitor lopinavir among viral isolates from protease inhibitor-experienced patients. J Virol. 2001;75(16):7462–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. AbbVie Inc. Lopinavir/ritonavir [prescribing information]. 2015. Abbott Park, IL, AbbVie Inc. [Google Scholar]
- 75. Mo H, King MS, King K, Molla A, Brun S, Kempf DJ. Selection of resistance in protease inhibitor-experienced, human immunodeficiency virus type 1-infected subjects failing lopinavir- and ritonavir-based therapy: mutation patterns and baseline correlates. J Virol. 2005;79(6):3329–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kagan RM, Shenderovich M, Heseltine PN, Ramnarayan K. Structural analysis of an HIV-1 protease I47A mutant resistant to the protease inhibitor lopinavir. Protein Sci. 2005;14(7):1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. AbbVie Inc. KALETRA (lopinavir and ritonavir) [prescribing information]. 2018. Abbott Park, IL, AbbVie Inc. [Google Scholar]
- 78. Friend J, Parkin N, Liegler T, Martin JN, Deeks SG. Isolated lopinavir resistance after virological rebound of a ritonavir/lopinavir-based regimen. AIDS. 2004;18(14):1965–1966. [DOI] [PubMed] [Google Scholar]
- 79. Lam E, Parkin NT. Amprenavir resistance imparted by the I50V mutation in HIV-1 protease can be suppressed by the N88S mutation. Clin Infect Dis. 2003;37(9):1273–1274. [DOI] [PubMed] [Google Scholar]
- 80. Rhee SY, Taylor J, Fessel WJ, et al. HIV-1 protease mutations and protease inhibitor cross-resistance. Antimicrob Agents Chemother. 2010;54(10):4253–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hermans LE, Steegen K, ter Heine R, et al. PI drug-level testing as screening tool for drug resistance in 2nd-line ART failure. Top Antivir Med. 2019;27(1s):169s. [Google Scholar]
- 82. Gonzalez LM, Brindeiro RM, Aguiar RS, et al. Impact of nelfinavir resistance mutations on in vitro phenotype, fitness, and replication capacity of human immunodeficiency virus type 1 with subtype B and C proteases. Antimicrob Agents Chemother. 2004;48(9):3552–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reeves JD, Gallo SA, Ahmad N, et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci USA. 2002; 99(25):16249–16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Reeves JD, Miamidian JL, Biscone MJ, et al. Impact of mutations in the coreceptor binding site on human immunodeficiency virus type 1 fusion, infection, and entry inhibitor sensitivity. J Virol. 2004;78(10):5476–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xu L, Pozniak A, Wildfire A, et al. Emergence and evolution of enfuvirtide resistance following long-term therapy involves heptad repeat 2 mutations within gp41. Antimicrob Agents Chemother. 2005;49(3):1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. ViiV Healthcare. Maraviroc [prescribing information]. 2015. Research Triangle Park, NC, ViiV Healthcare. [Google Scholar]
- 87. Anastassopoulou CG, Ketas TJ, Sanders RW, Klasse PJ, Moore JP. Effects of sequence changes in the HIV-1 gp41 fusion peptide on CCR5 inhibitor resistance. Virology. 2012;428(2):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324(16): 1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Oliveira M, Ibanescu RI, Anstett K, et al. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology. 2018; 15(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tsiang M, Jones GS, Goldsmith J, et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother. 2016;60(12): 7086–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smith SJ, Zhao XZ, Burke TR, Jr., Hughes SH. Efficacies of cabotegravir and bictegravir against drug-resistant HIV-1 integrase mutants. Retrovirology. 2018;15(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rhee SY, Grant PM, Tzou PL, et al. A systematic review of the genetic mechanisms of dolutegravir resistance. J Antimicrob Chemother. 2019;74(11):3135–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir-and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 Study. J Infect Dis. 2014;210(3):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chamberlain N, Mena L, Brock JB. Case report: emergent resistance in a treatment-naive person with human immunodeficiency virus under bictegravir-based therapy. Open Forum Infect Dis. 2021;8(6): ofab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet. 2021; 396(10267):1994–2005. [DOI] [PubMed] [Google Scholar]
- 96. Marzinke MA, Grinsztejn B, Fogel JM, et al. Characterization of Human Immunodeficiency Virus (HIV) Infection in Cisgender Men and Transgender Women Who Have Sex With Men Receiving Injectable Cabotegravir for HIV Prevention: HPTN 083. J Infect Dis. 2021;224(9):1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir + rilpivirine every 2 months: ATLAS-2M week 152 results [CROI Abstract 479]. In Special Issue: Abstracts From the 2022 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2022;30(1s):183. [Google Scholar]
- 98. Cutrell AG, Schapiro JM, Perno CF, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS. 2021;35(9): 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hu Z, Cordwell T, Jeffrey J, Kuritzkes D. Effect of L74I polymorphism on fitness of IHV-1 subtype A6 resistant to cabotegravir [CROI Abstract 506]. In Special Issue: Abstracts From the 2022 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2022;30(1s):193–194. [Google Scholar]
- 100. Cheung PK, Shahid A, Dong W, of clinically observed HIV integrase mutations on phenotypic resistance to integrase strand transfer inhibitors (INSTIs): a molecular study. J Antimicrob Chemother. 2022;77(4):979–988. [DOI] [PubMed] [Google Scholar]
- 101. Frantzell A, Petropoulos C, Huang W. Dolutegravir resistance requires multiple primary mutations in HIV-1 integrase. Top Antivir Med. 2015; 23(e-1):51. [Google Scholar]
- 102. Kobayashi M, Yoshinaga T, Seki T, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55(2):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, doubleblind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868): 735–743. [DOI] [PubMed] [Google Scholar]
- 104. Eron JJ, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Seki T, Suyama-Kagitani A, Kawauchi-Miki S, et al. Effects of raltegravir or elvitegravir resistance signature mutations on the barrier to dolutegravir resistance in vitro. Antimicrob Agents Chemother. 2015;59(5): 2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. DeAnda F, Hightower KE, Nolte RT, et al. Dolutegravir interactions with HIV-1 integrase-DNA: structural rationale for drug resistance and dissociation kinetics. PLoS One. 2013;8(10):e77448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Goodman D, Hluhanich R, Waters J, et al. Integrase inhibitor resistance involves complex interactions among primary and second resistance mutations: a novel mutation L68V/I associates with E92Q and increases resistance. Antivir Ther. 2008; 13(Suppl 3):A15. [Google Scholar]
- 108. Waters J, Margot N, Hluhanich R, et al. Evolution of resistance to the HIV integrase inhibitor (INI) elvitegravir can involve genotypic switching among primary INI resistance patterns. Fort Myers, FL. Antivir Ther. 2009; 14(Supp 1):A137. [Google Scholar]
- 109. Doyle T, Dunn DT, Ceccherini-Silberstein F, et al. Integrase inhibitor (INI) genotypic resistance in treatment-naive and raltegravir-experienced patients infected with diverse HIV-1 clades. J Antimicrob Chemother. 2015; 70(11):3080–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835): 2439–2448. [DOI] [PubMed] [Google Scholar]
- 111. DeJesus E, Rockstroh J, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–2438. [DOI] [PubMed] [Google Scholar]
- 112. Abram ME, Hluhanich RM, Goodman DD, et al. Impact of primary elvitegravir resistance-associated mutations in HIV-1 integrase on drug susceptibility and viral replication fitness. Antimicrob Agents Chemother. 2013;57(6):2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. White K, Kulkarni R, Miller MD. Analysis of early resistance development at the first failure timepoint in elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate-treated patients. J Antimicrob Chemother. 2015;70(9):2632–2638. [DOI] [PubMed] [Google Scholar]
- 114. Scherrer AU, Yang WL, Kouyos RD, et al. Successful prevention of transmission of integrase resistance in the Swiss HIV Cohort Study. J Infect Dis. 2016;214(3):399–402. [DOI] [PubMed] [Google Scholar]
- 115. Hazuda DF, Miller MD, Nguyen BY, Zhao J, for the P005 Study Team. Resistance to the HIV-integrase inhibitor raltegravir: analysis of protocol 005, a phase II study in patients with triple-class resistant HIV-1 infection. Antivir Ther. 2007;12):S10. [Google Scholar]
- 116. Gatell JM, Katlama C, Grinsztejn B, et al. Long-term efficacy and safety of the HIV integrase inhibitor raltegravir in patients with limited treatment options in a Phase II study. JAIDS. 2010; 53(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fransen S, Gupta S, Danovich R, et al. Loss of raltegravir susceptibility by human immunodeficiency virus type 1 is conferred via multiple nonoverlapping genetic pathways. J Virol. 2009;83(22):11440–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hatano H, Lampiris H, Fransen S, et al. Evolution of integrase resistance during failure of integrase inhibitor-based antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;54(4): 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wittkop L, Breilh D, Da Silva D, et al. Virological and immunological response in HIV-1-infected patients with multiple treatment failures receiving raltegravir and optimized background therapy, ANRS CO3 Aquitaine Cohort. J Antimicrob Chemother. 2009; 63(6):1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Armenia D, Vandenbroucke I, Fabeni L, et al. Study of genotypic and phenotypic HIV-1 dynamics of integrase mutations during raltegravir treatment: a refined analysis by ultra-deep 454 pyrosequencing. J Infect Dis. 2012;205(4):557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359(4):355–365. [DOI] [PubMed] [Google Scholar]
- 122. Malet I, Delelis O, Valantin MA, et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob Agents Chemother. 2008;52(4):1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Blanco JL, Varghese V, Rhee SY, Gatell JM, Shafer RW. HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis. 2011;203(9):1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Link JO, Rhee MS, Tse WC, et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature. 2020;584(7822):614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Margot N, VanderVeen L, Naik V, et al. Phenotypic resistance to lenacapavir and monotherapy efficacy in a proof-of-concept clinical study. J Antimicrob Chemother. 2022;77(4):989–995. [DOI] [PubMed] [Google Scholar]
- 126. Callebaut CVL, Margot N, Naik V, Rhee M. Activity and resistance characterization of the HIV CAPSID inhibitor lenacapavir [CROI Abstract 128]. In Special Issue: Abstracts From the 2021 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2021;29(1):35. [Google Scholar]
- 127. Marcelin AG, Charpentier C, Jary A, et al. Frequency of capsid substitutions associated with GS-6207 in vitro resistance in HIV-1 from antiretroviral-naive and -experienced patients. J Antimicrob Chemother. 2020; 75(6):1588–1590. [DOI] [PubMed] [Google Scholar]
- 128. Margot N, VaderVeen L, Naik V, et al. Resistance analysis of long-acting lenacapavir in highly treatment-experienced people with HIV after 26 weeks of treatment. Poster presented at: 18th European AIDS Conference (EACS); October 27-30, 2021; London, UK.
- 129. Ogbuagu O, Segal-Maurer S, Brinson C, et al. Long-acting lenacapavir in people with multidrug resistant HIV-1: week 52 results [CROI Abstract 491]. In Special Issue: Abstracts From the 2022 Conference on Retro-viruses and Opportunistic Infections. Top Antivir Med. 2022; 30(1s):188. [Google Scholar]
- 130. VanderVeen L, Margot N, Naik V, et al. Interim-resistance analysis of long-acting lenacapavir in treatment-naive people with HIV at 28 weeks (CALIBRATE). Poster presented at: IDWeek 2021 Virtual Conference; September 29-October 3, 2021.
- 131. Gupta S, Sims J, Brinson C, et al. Lenacapavir as part of a combination regimen in treatment naive PWH: week 54 results [CROI Abstract 138]. In Special Issue: Abstracts From the 2021 Conference on Retroviruses and Opportunistic Infections. Top HIV Med. 2022;30(1s):53. [Google Scholar]