Abstract
Despite substantial advances in the field, liver disease morbidity and mortality remain serious issues among people with HIV. The causes of liver disease are often multifactorial and include hepatitis viruses, hepatic steatosis and oxidative stress, bacterial translocation with activation of hepatic macrophages and stellate cells, and direct toxicities from alcohol and drugs of abuse. Biopsychosocial factors including a high prevalence of psychiatric disorders, food insecurity, insufficient access to care and medications, and social stigma all play roles in the persistence of liver injury and hepatic fibrosis development among people with HIV. Rising rates of hepatocellular carcinoma have been observed, suggesting that the epidemiology of liver disease is evolving.
Keywords: HIV, HCV, HBV, hepatitis, NASH, NAFLD, fatty liver, pathogenesis, opioid
Introduction
Liver disease was initially recognized as a major contributor to morbidity and mortality among people with HIV in the early 1990s and became fully manifest as a major health issue in the mid-1990s following the introduction of effective combination antiretroviral therapy (ART). The subsequent 3 decades witnessed tremendous progress in HIV care, as well as the stubborn persistence of liver disease threatening survival and quality of life. Microelimination of the hepatitis C virus (HCV) infection in people with HIV seems achievable, yet new infections and reinfections threaten progress. Hepatitis B virus (HBV) infection can be suppressed by nucleotide and nucleoside therapy, but a cure remains elusive. Limited HBV vaccine responses in people with HIV who use older vaccine products remain problematic. Newly developed HBV vaccines may help people with HIV but have not been widely studied yet in the populations that would most benefit. Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) have increased in prominence as HCV treatment reduces the threat of HCV-related liver disease. ART drugs have improved in their hepatic safety, but some trends like the use of 2-drug regimens with limited HBV activity may represent a step backward for people coinfected with HBV, and weight gain associated with many integrase strand transfer inhibitors (InSTIs) is problematic as well. Barriers to treatment and prevention remain, in part because of the presence of major psychiatric comorbidities that are more prevalent in people with HIV. Emergence of the COVID-19 pandemic added complexity to the prevention and management of liver disease among people with HIV and continues to affect care and disease outcomes.
To address these issues and encourage collaboration among researchers to investigate emerging issues, the National Institute of Allergy and Infectious Diseases, in association with industry partners, has provided support for a biennial meeting to discuss the research agenda. Held in September 2021, the 8th Biennial HIV and Liver Disease Conference included key representatives from infectious diseases, hepatology, psychiatry, nutrition, and pharmacology, as well as policymakers, regulators, and basic and translational scientists focused on liver-related issues in this unique, at-risk population.
Epidemiologic Considerations
Liver Disease and Hepatocellular Carcinoma
Few studies have addressed the epidemiology of and trends in liver disease over time in large cohorts that are diverse and have sufficient geographic representation to fully represent the at-risk populations. The NA-ACCORD (North American AIDS Cohort Collaboration on Research and Design), a US and Canadian cohort study, examined morbidity and mortality of liver disease across 3 distinct time periods that correspond broadly to eras in available choices of ART. Its results clearly documented the lack of longitudinal changes in disease mortality and progression to end-stage liver disease (ESLD) associated with coinfection with HCV, HBV, or both in people with HIV.1 Despite this finding, more recent analyses in the NA-ACCORD identified a clear increase in rates of hepatocellular carcinoma (HCC), culminating in an incidence of 0.75 cases per 1000 person-years compared with an HCC rate in the general US population of 0.23 per 1000 person-years.2 Interestingly, people affected by triple infection with HBV/HCV/HIV are at highest risk.
High HCC rates were also observed in the VACS (Veterans Aging Cohort Study) among nearly 35,000 veterans. Data adjusted for age, sex, race, body mass index, alcohol use, diabetes, and HBV and HCV serostatus revealed HIV virus detection and viral load as key factors associated with this outcome.3 Presence of fatty liver disease was also identified as an important factor. In a combined analysis of 4 European cohorts of individuals coinfected with HBV/HIV on tenofovir disoproxil fumarate (TDF), cumulative time on TDF treatment was associated with a stable-to-decreased risk of HCC development, but the time off TDF therapy was highly associated with an increase in the incidence rate ratio of HCC.4 In the same study, HCC surveillance strategies were evaluated to determine the optimal screening paradigm. In people with cirrhosis, as in HBV monoinfection, age was not found to be a factor, meaning all persons with HBV/HIV coinfection should be screened. In people without cirrhosis, an age threshold of 45 years was associated with a predetermined screening threshold of 2 events/1000 person-years.
The association of HCC development with HBV viral titer was previously well established in the Taiwanese REVEAL (Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus) study.5 More recently, this relationship was also noted in people with HBV/HIV coinfection; higher HCC risk was noted for persons with HBV DNA levels greater than 200 IU/mL than those with lower quantities. Longer duration of complete HBV suppression was likewise associated with decreased HCC risk.6 Additional risk for HCC attributable to hepatitis D virus (HDV) infection was described in results from the Swiss HIV Cohort Study. The hazard ratio for HCC-free survival was 9.3 (95% CI, 3-28.6), representing a significant decrease in cancer-free survival for persons with HDV/HBV/HIV infection.7 Recently, bulevirtide was approved in the European Union for treatment of hepatitis D virus (HDV) infection and may soon be approved in the USA, providing one tool to combat this serious challenge.
Not all news related to liver disease trends in people with HIV is negative, however. Among the first groups to document the salutary effects of effective HCV treatment in this population, Mocroft and colleagues reported significant reductions in the incidence of ESLD in people with HIV in the EuroSIDA cohort whose HCV was cleared spontaneously, versus those whose HCV was not. The lowest rates of ESLD were observed in those with spontaneous clearance, and those with treatment failure had the highest observed rates of ESLD. Successfully treated persons were normalized at an incidence rate of 1.8 Similarly, the national US database known as the Nationwide Inpatient Sample was used to examine trends in mortality over a 15-year period within a subset of people with liver disease, namely, those with cirrhosis, either with or without HIV. Higher inpatient mortality (10.9%) was observed among people with cirrhosis and HIV than among those without HIV (9.2%; P <.001). However, the rate declined in both groups over the 15-year period. Liver-decompensating events decreased but infections increased in people with HIV. Thus, HIV remains an independent predictor of mortality in people with cirrhosis.9
Trends in people with NAFLD and NASH remain unclear and are difficult to discern because of substantial differences in reporting methods used to define the presence or absence of disease. Varying definitions within and between diagnostic categories sharply limit the ability to draw conclusions about disease progression and make comparisons between people with HIV and cohorts without HIV. For example, the histologic definition of steatosis is the presence of 5% or more hepatocytes containing fat droplets, and the biomarker MRI-derived proton density fat fraction appears to have excellent reliability and reproducibility for defining steatosis at this level.10 However, most studies utilize other, substantially less sensitive methodologies including controlled attenuation parameter or even echogenicity on ultrasound. The definition used for NAFLD is also variable, requiring abnormal levels of alanine aminotransferase (and with studies using varying cutoff levels) or the presence of fibrosis as determined by liver-stiffness measurement, which is itself a surrogate for fibrosis. NASH is a purely histologic diagnosis, but few studies perform enough liver biopsies to allow meaningful cross-sectional comparisons between people with HIV and control participants. This area remains key for future investigation and is especially important because of the putative relationship between some ART drugs and fat accumulation in the liver and elsewhere. This point is discussed later in more detail.
HIV Epidemiology
In 2018, several key issues in HIV epidemiology were identified. At that time, not only had progress toward ending the epidemic of new HIV infections in the United States stalled, but the opioid epidemic was driving a resurgence of new infections. Indeed, the US Centers for Disease Control and Prevention (CDC) estimated that the next decade would yield a net gain of nearly 400,000 HIV infections, based upon an incidence of greater than 38,000 new cases/year.11 This modeling led the CDC to begin a new initiative targeting a 75% reduction in new HIV infections within 5 years and a 90% reduction in 10 years. The cornerstones of this effort included increased use of HIV preexposure prophylaxis (PrEP) and syringe exchange service programs (SSPs). Furthermore, early diagnosis and treatment with the goal of sustained viral suppression would reduce the pool of HIV index cases. Targeting was achieved by identifying 57 jurisdictions with the highest rates of HIV transmission, many in 7 rural states.
Unfortunately, data available in 2021 show little progress made to date. There were 34,800 new HIV infections in 2019, the majority (82%) among males. Black/African American and Hispanic/Latino persons account for the majority (69%) of new infections. Male-to-male sexual contact represents the highest proportion of transmissions (66%). Because 80% of infections are transmitted by people unaware they have HIV, this group remains a key priority for intervention.12 Self-testing strategies may have an important role in increasing early diagnosis.13 Large gaps in PrEP use between whites and other racial or ethnic groups remain, representing an area of opportunity. However, SSPs have lost ground in areas with high opioid-use risk, which poses a threat to achievement of lower HIV incidence targets. Results of several economic modeling studies suggest that SSPs are cost-effective, but political considerations may continue to limit implementation.14
Immunopathogenesis
The higher risk of cirrhosis in people living with HIV than in those without HIV underscores the importance of understanding the mechanisms of liver fibrosis. Most of the focus and disease burden has been on individuals with underlying HCV or HBV infection. However, some data suggest that even without a primary liver disease, HIV itself may cause liver steatosis and fibrosis. For example, results of a study of 432 people with HIV revealed that 10% of those without HBV infection, HCV infection, or self-reported excessive alcohol use had elevated values of liver stiffness (>7.1 kPa), a finding associated with HIV viral load and metabolic dysfunctions like diabetes.15 Thus, even though the apparent net effect of HIV on liver fibrosis is most evident in the presence of a second contributor such as HCV infection, HBV infection, excessive alcohol use, or metabolic liver disease, HIV infection itself biases the liver toward fibrosis and synergistically promotes these other processes. Notably, some mechanisms are reversed by ART, and others continue to contribute (or are even caused by) the ART medications (discussed later).
Various mechanisms have been proposed through which HIV may potentiate or cause liver fibrosis; many overlap, and all converge on the central role of stellate cells (Table). Results of pivotal in vitro studies have shown that HIV cooperatively enhances the fibrogenesis caused by HCV accentuating reactive oxygen species and transforming growth factor (TGF)-beta induction, thereby inducing the secretion of type 1 collagen and tissue inhibitor of metalloprotease (TIMP)-1 in hepatic stellate cells as well as hepatocyte apoptosis.16,17 Likewise, with HBV coinfection, recent evidence suggests that HIV and HBV cooperatively promote fibrosis by upregulation of hypoxia-inducible factor 1-alpha, which in turn might increase TGF-beta production.18 Emerging evidence also suggests a role for the Hippo-yes-associated protein (YAP) pathway in hepatic fibrogenesis. When Hippo is turned off (directly or indirectly by infection), YAP is unphosphorylated and free to translocate to the stellate cell nuclei to induce genes promoting fibrosis in a manner attenuated by recognized inhibitors of fibrogenesis such as lysophosphatidic acid (LPA) and epidermal growth factor receptor (EGFR) inhibitors. Interestingly, in a murine model the YAP-mediated fibrogenesis is also attenuated by ART.
Table.
Proposed Overlapping Mechanisms for HIV Potentiation of Liver Disease
| Enhanced oxidative stress and TGF-beta induction |
| HIF-1, Hippo, YAP, LPA signaling effects |
| Adaptive immune dysfunction (eg, CD4+ T-cell depletion) |
| Alterations in Kupffer cell physiology |
| Lipodystrophy and adipocyte effects |
| Enhanced microbial translocation |
Abbreviations: HIF-1, hypoxia-inducible factor-1; LPA, lysophosphatidic acid; TGF-beta, transforming growth factor-beta; YAP, yes-associated protein.
Other data point to a role for liver macrophages (Kupffer cells) in the pathogenesis of HIV-related liver disease. HIV can infect Kupffer cells and alter their cellular physiology, even though the macrophage is not thought to contribute to the latent reservoir.19 The M2 Kupffer cell phenotype is especially relevant, in that it can activate stellate cells, a process that is correlated with the net production of a soluble protein, CD163.20,21 Soluble CD163 was highly associated with evidence of liver injury in people with HIV and with development of hepatic fibrosis in a human cohort study.22
HIV infection also has myriad effects on adipocyte biology that might coordinately impact liver disease. The most obvious connection is with the accumulation of additional liver fat (steatosis), which in some instances also is associated with disease (inflammation or fibrosis). One example is the HIV accessory protein Vpr, which can inhibit peroxisome proliferator-activated receptor (PPAR)-gamma, increasing lipolysis and fat accumulation in liver.23 Although inhibition of HIV replication would be expected to reduce that mechanism, some ART medications themselves are associated with fatty liver. Older ART medications such as stavudine (d4T) were directly toxic to cells, but even newer drugs like the InSTIs cause weight gain and possibly increased hepatic steatosis.
Chemokines and Their Receptors
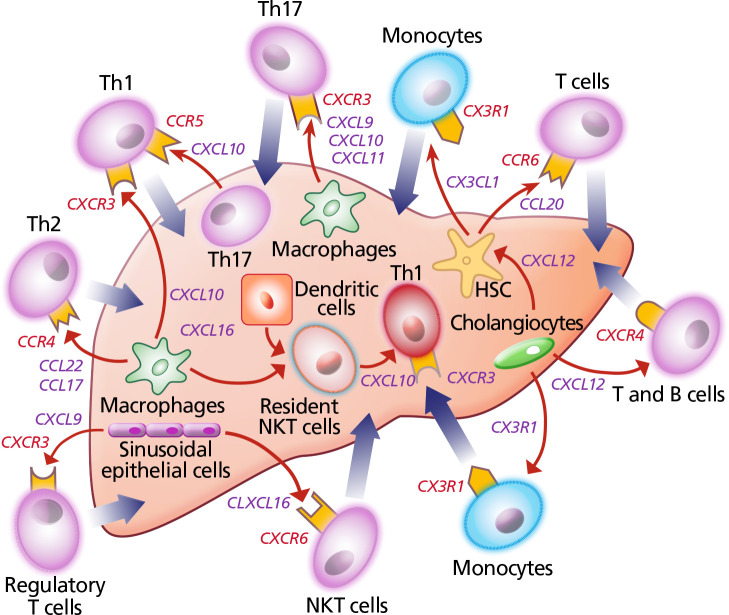
Chemokines are molecules that regulate inflammation and, not surprisingly, are dysregulated in people with HIV. For example, the peripheral circulation of people with HIV/HCV coinfection contains fewer CD4+ T cells than that of individuals monoinfected with HCV, and these cells are disproportionately in an activated and “exhausted” state (PD1+, CD38+, HLADR+). Compared with T cells from people with HCV monoinfection, cells isolated from individuals with HIV/HCV coinfection are also more likely to express CXCR3, a liver-homing molecule, and to secrete chemokines and cytokines that stimulate stellate cells to produce extracellular matrix proteins and accelerate liver fibrosis (Figure 1).24 Interestingly, in contrast to the findings for circulating blood, opposite trends occur in liver tissue (ie, more CD4+ T cells from individuals with monoinfection express CXCR3). This paradigm suggests that blocking chemokine receptor 2 (CCR2) or chemokine receptor 5 (CCR5) might attenuate the increase in liver fibrosis associated with HIV.
Figure 1.
Schematic diagram depicting cytokines and chemokines that modulate hepatic injury and fibrosis.53 Red arrows indicate chemokine secretion; thick arrows denote pro-inflammatory infiltration.
Abbreviations: CCL, chemokine ligand; CCR, chemokine receptor; CXCL, CXC chemokine ligand; CXCR, CXC chemokine receptor; CX3CL, CX3C chemokine ligand; CX3R, CX3 chemokine receptor; HSC, hepatic stellate cell; NKT, natural killer T cell; Th, helper T cell.
Evidence supporting the pharmacologic blockade of these chemokine pathways comes from murine models of liver inflammation and NASH.25,26 Some, but not all, human epidemiologic data also support the paradigm. For example, people with hemophilia and HIV/HCV coinfection who have 1 copy of the CCR5 allele known as the delta 32 mutation have lower serum liver fibrosis scores than those who are homozygous for the wild type.27 Likewise, in a study in which people with HIV but not HCV were randomly assigned 2:1 to receive either cenicriviroc or efavirenz (plus TDF and emtricitabine), the cenicriviroc group had lower serum fibrosis scores than the efavirenz group.27 This observational research set the stage for clinical trials to test the hypothesis. Thus far, a phase 2b study was reported in which people with NASH were randomly assigned to receive cenicriviroc either immediately or after a delay, and results of each group were compared with those of a placebo group.28 In this relatively small study, no difference was detected in the number of participants who had both an improvement of at least 1 stage of fibrosis and no worsening of NASH.
Microbiome
The microbiome refers to all the microorganisms (bacteria, viruses, fungi, and archaea) that live within an ecosystem. In humans, the gut is the most studied ecosystem, and in health, homeostasis exists between the microbiome and intestinal mucosa. HIV disrupts this homeostasis, and evidence indicates that HIV affects the mucosal interface. Specifically, during acute HIV infection, there is marked depletion of the CD4+ T cells that line the intestinal lumen.29 More pronounced effects are the depletion of a subset of Th17+ T cells and disruption of mucosal barriers. This breach in the gut mucosa is thought to promote translocation of bacteria from the gut microbiome, and correlates of that process (and macrophage activation) such as soluble CD14 are associated with HIV-related immune activation and even mortality.30
There is also evidence that the components of the microbiome are altered in people with HIV. Results of some studies adjusting for HIV risk exposure and other confounders show differences linked to HIV infection itself. Specifically enriched in people with HIV are some Proteobacteria and Enterobacteria species, with a corresponding reduction in Bacteroides species.31 A dynamic metabolic mechanism has been proposed, with increased production of kynurenine from tryptophan associated with disease in a manner that contributes to depletion of Th17+ cells and disruptions in mucosal protection. Results of a recent study provided evidence for this paradigm in peripheral blood. In people with HIV receiving ART, fragments of enteric microbes and specifically Serratia species were correlated with proinflammatory cytokines and CD4+ T-cell increases in the first treatment year. Subsequently, lower Serratia species DNA abundance was associated with more favorable outcomes. DNA fragments traced to Pseudomonas species had the opposite associations.32
The link with liver pathogenesis is assumed to be related to the drainage of organisms that are translocated from the gut to the liver via the portal blood.33,34 Collectively, this work raises the question of whether the microbiome could be manipulated therapeutically to improve HIV and liver outcomes. There is precedence in the use of rifaximin and lactulose to reduce encephalopathy in people with cirrhosis. Although probiotics and synbiotics have been studied in NAFLD, none has yet been convincingly translated into improving patient care.35
Prevention and Treatment of Liver Disease in People With HIV
Prevention and treatment of HIV-related liver disease logically targets the underlying causes, beginning with prevention and treatment of HIV itself. The clear medical benefits of PrEP shift the focus to implementation, overcoming barriers, and reaching those at risk. Notably, prevention of disease need not be siloed. Harm-reduction strategies to reduce the risks of illicit drug use also prevent HCV and HIV infections. These benefits were observed in Scott County, Indiana, where changes in behavior were reported after introduction of SSPs as part of the public health response to an HIV outbreak associated with shared injection paraphernalia among opioid users.36 Unfortunately, county officials ended the program in mid-2021, following ongoing local and national debate over providing such services.37
As mentioned, ART improves some drivers of liver disease and is indicated for all people with HIV. Individuals for whom specific components of ART clearly need to differ are those with HBV/HIV coinfection, for whom compounds that are also active against HBV, such as tenofovir analogues, are recommended. This principle became especially relevant with the 2021 US Food and Drug Administration (FDA) approval of a long-acting HIV regimen composed of cabotegravir and rilpivirine, which lacks activity against HBV. People with HIV should not be switched from a TDF-based regimen without knowledge of each individual’s HBV serostatus.38 Similarly, a dolutegravir/lamivudine combination is not effective for long-term suppression of HBV, as resistance emerges quickly when HBV is present. An additional recent consideration concerns the association of marked steatosis with the weight gain that may accompany InSTI treatment. If confirmed, this association might justify switching people with NAFLD from an InSTI-based treatment to alternative regimens (eg, ritonavir boosted or darunavir based).39
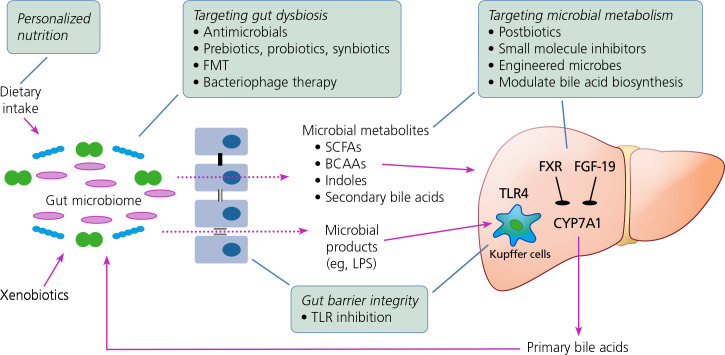
Improved understanding of the immunopathogenesis of liver disease in people with HIV has yet to translate into HIV-specific treatments other than ART. Medical manipulation and restoration of the specific molecular pathways described earlier are theoretically possible; various potential approaches are shown in Figure 2. However, large, randomized clinical trials are still needed to establish their efficacy.
Figure 2.
Schematic showing potential targets for decreasing liver injury (boxed text) that are not disease specific and may find application in people with HIV.54 Solid arrows indicate impact on disease pathogenesis; dotted arrows denote gut microbiome metabolite leakage into portal circulation. BCAA, branched-chain amino acid; CYP, cytochrome P450; FGF, fibroblast growth factor; FMT, fecal microbiota transplantation; FXR, farnesoid X receptor; LPS, lipopolysaccharide; SCFA, short-chain fatty acid; TLR, toll-like receptor.
Beyond treatment of HIV, the current approach for preventing and treating liver disease in people with HIV is essentially the same as in those without HIV: reducing or eliminating alcohol ingestion, reducing weight in people with high body mass index values, and updating vaccinations as needed to protect against other forms of liver disease like that related to HBV or hepatitis A virus.
Since 2017, 2 new HBV vaccines have been approved by the FDA. The first was a 2-dose recombinant, adjuvanted HBV vaccine that is more immunogenetic than historic recombinant vaccines and is undergoing testing in people with HIV in a large multicenter, multinational clinical trial. Single-center retrospective results have been reported that suggest improved efficacy compared with older vaccine products.40,41 The second vaccine, approved in November 2021, is a recombinant hepatitis B vaccine that is composed of recombinant forms of surface and 2 larger presurface envelope proteins and is more immunogenetic than historic recombinant vaccines when given in 3 doses.42 Studies in people with HIV are needed to determine whether these new products (1) overcome the lower responses to historic recombinant vaccines in people with HIV, and (2) permit revaccination with desirable responses among people who did not respond to older vaccine preparations.
Although interferon alfa-based treatments for HCV originally differed for people coinfected with HIV, the current era of direct-acting antiviral (DAA) HCV therapies now provides similarly high efficacy and effectiveness for sustained virologic response in both groups.43 The same treatments are recommended for HCV without regard for an individual’s HIV serostatus. With available DAA treatments, even liver transplantation outcomes are similar between people coinfected with HIV and those with HCV monoinfection.44
Biopsychosocial Barriers and Responses
The prevention and treatment of liver disease in people with HIV are inhibited by the presence of numerous barriers to care, some of which are intrinsic to this population and others that are more broadly distributed in the general population and thus impact those with HIV as well. These barriers have their basis in socioeconomic and political processes that affect equal application of current scientific principles of prevention and care.45 Cultural and religious differences among people at risk also lead to imbalances in the application of interventions. For example, hepatitis and HIV harm-reduction programs, including SSPs and health-education and screening programs, are limited or not available in many jurisdictions in the United States, despite evidence from Australia and Europe that they reduce spread of chronic viral infections.46,47 Funding opportunities vary between state and local levels. Even use of Ryan White HIV/AIDS Program funds for HCV treatment is allowed only in some states for people coinfected with HIV. Rules for the use of DAAs vary by state as well, with some requiring evaluation by a subspecialist before prescriptions for treatment can be filled. Although liver transplantation has been shown with few caveats to be safe and efficacious for people with HIV, only a subset of transplant centers will consider this population for transplant.47,48
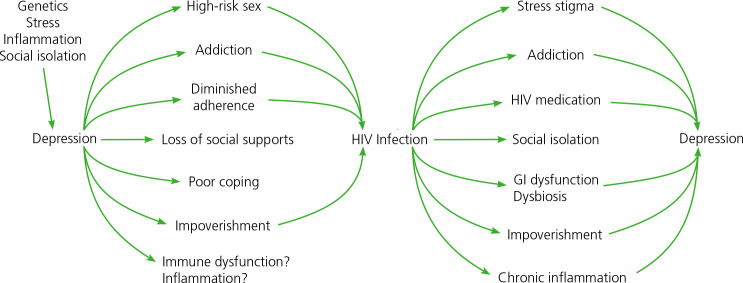
Comorbid psychiatric and behavioral disorders are common among people with HIV. Axis 1 disorders may be present in nearly 50% of individuals with HIV.49 These disorders include psychiatric diseases such as depression, bipolar disorder, and schizophrenia; behavior disorders like addictions; problems of endowment including personality and cognitive disorders; and problems of lived experiences including poor coping, limited choices, and poverty. A complex interaction exists among factors that lead to HIV, systemic immune activation, and depression and other psychiatric manifestations of disease (Figure 3).
Figure 3.
Diagram summarizing the complex interplay between HIV infection and depression and other manifestations of psychiatric and behavior disease processes. (Diagram courtesy of Glenn Treisman, MD, PhD.)
Behavioral interventions appear to represent a key step in limiting the epidemic and managing individual patients. Implementing this step requires the development of integrated care systems that include medical, psychiatric, and substance-use expertise.50,51 Economic models suggest that this approach is cost-effective, but it requires investment and infrastructure, and sadly, implementation of such systems has been absent from most care venues. Randomized trials are needed to confirm the efficacy of these approaches.52
Research Agenda: 2022 and Beyond
A key deliverable from the 8th Biennial HIV and Liver Disease Conference was the identification of a research agenda encompassing near- and longterm priorities for HIV and liver disease; a synthesis is presented herein. For HIV infection itself, targeted programs have been put in place as part of disease-elimination plans in the United States, but ongoing assessments of their efficacy and adjustments of implementation methods are still needed. HIV and viral hepatitis continue to be spread via parenteral exposure. Syringe exchange service programs work as an element of risk reduction, but backsliding is observed in many parts of the country. Economic modeling and education are needed to provide information on the cost-effectiveness of SSPs and other early-intervention programs that limit disease transmission.
Highly effective treatment regimens for HIV are readily available but are not curative and may engender adverse effects with long-term implications for liver disease. Additional research is clearly needed to clarify the role of InSTIs in weight gain and the linkage to NAFLD, NASH, and other complications of metabolic syndrome. Up to 35% of people with HIV may have NAFLD, including NASH, yet data are limited regarding new treatment modalities for NAFLD and NASH in this group, and many researchers are still using diagnostic modalities like ultrasound that lack both sensitivity and specificity for disease identification. Lean disease appears to be more prevalent than NAFLD and NASH in people with HIV and may have a different mechanism that requires different treatment. Despite many efforts to utilize large electronic medical record databases to study these issues, more refined and integrated tools to do so are needed. The use of International Classification of Diseases (ICD)-10 codes is at best a blunt instrument when trying to determine disease incidence and prevalence.
Basic and translational science research continues to identify new pathways for liver injury and fibrosis (eg, hypoxia-inducible factor-1, yes-associated protein-1). These pathways represent potential new targets for therapeutic intervention. To this end, results of several clinical studies suggest a central role for chemokines as mediators of liver injury, stellatecell activation, and remodeling. Thus far, however, the results are mixed for blockade of receptors like CCR5 and CCR2, and whether this lack of clarity stems from efficacy or study design is unclear.
Results of other studies point toward manipulation of the microbiome as a promising intervention strategy, but progress is hindered by the lack of agreement on the best methods for identifying microbial populations as well as clearly defined risks of such manipulation. Data suggest that a conceptually simple idea like addressing food insecurity can impact the microbiome and reduce downstream hepatic injury by affecting translocation and macrophage activation. However, any beneficial strategies for changing diet would require an understanding of the economic and social drivers of dietary choice and lack of choice.
Hepatic viral infections continue as ongoing sources of injury and progressive liver disease, even in 2022. HBV cure still seems remote despite the availability of valid targets and medications. Longstanding definitions of treatment response need to be updated, and current measures used to define response require revalidation with newer endpoints or replacement with newer biomarkers. The ability to separate host-integrated HBV DNA from covalently closed circular DNA (cccDNA) remains a challenge and may be key to definitions of functional cure. HBV vaccination outcomes remain suboptimal. Studies are needed to identify optimal vaccine strategies, which may rely on newer vaccine products and better population-based adherence to useful preventive vaccine regimens. Infection with HCV is now easily curable with DAAs, but new infections continue to occur, and men who have sex with men, with or without HIV infection, remain at high risk. An effective HCV vaccine is still elusive; development of new RNA vaccine technologies such as those used for SARS-CoV-2 may lead to development of new HCV vaccines. In addition, human challenge studies such as used for SARS-CoV-2, dengue, malaria, and other vaccines may promote HCV vaccine testing. Infection with HDV currently receives little attention but may emerge as a key issue with new treatment interventions approaching approval. However, availability of testing for HDV RNA is limited, and studies are needed to confirm the value of reflex-testing strategies following HBV detection.
Long-acting treatments for HCV and HBV infections may well transform their treatment and prevention, as they have for HIV. For HCV in particular, long-acting treatments might provide the ability to cure infection in a single encounter, opening the paradigm to test-and-cure public health approaches to elimination. In contrast, long-acting treatments for HBV infection might provide another “pill-free” option for maintaining treatment, especially useful when adherence is challenging. The dual activity of tenofovir against HIV and HBV makes development of those long-acting approaches of interest.
Although use of illicit drugs and high alcohol intake are known as key factors in the promotion and maintenance of HIV disease and viral hepatitis, recent data suggest they have substantial direct effects on liver injury and fibrosis progression. Cocaine may promote fibrosis independently of its effects on HIV. Fentanyl and other opioids may increase viral loads of HCV and HIV and thus alter both disease epidemiology and clinical presentation in individuals with either or both infections. Studies of these cofactors are needed to further elucidate their roles in individuals who use drugs.
The linkage between HIV infection and liver disease is multifactorial and remains a key driver of morbidity and mortality. Ongoing research in a variety of areas provides the opportunity to change the current landscape and improve the health of people with HIV.
Footnotes
This article was prepared by Dr Sherman and Dr Thomas in March 2022 and accepted for publication in Topics in Antiviral Medicine, Volume 30, Issue 4.
Financial relationships with ineligible companies within the past 24 months: Dr Sherman has received grant support or contracts awarded to his institution from AbbVie, Gilead Sciences, Inc, Intercept Pharmaceuticals, Inc, Zydus, and Merck & Co, Inc; served as an advisor or consultant to Inovio Pharmaceuticals, Inc, and Gilead; and served on data and safety monitoring boards for MedPace, Inc, and Horizon. Dr Thomas has served as an advisor to Merck & Co, Inc, and Excision BioTherapeutics, Inc (updated July 22, 2022).
Funding for the Eighth Biennial HIV and Liver Disease Conference was provided [in part] by the National Institutes of Health under Award Number R13AI071925 from the National Institute of Allergy and Infectious Diseases (NIAID). The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the US Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the US Government. The Conference was also supported by educational grants from Abbott Laboratories, Gilead Sciences, Inc, Theratechnologies Inc, and ViiV Healthcare.
References
- 1. Klein MB, Althoff KN, Jing Y, et al. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis. 2016;63(9):1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun J, Althoff KN, Jing Y, et al. Trends in hepatocellular carcinoma incidence and risk among persons with HIV in the US and Canada, 1996-2015. JAMA Netw Open. 2021;4(2):e2037512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torgersen J, Kallan MJ, Carbonari DM, et al. HIV RNA, CD4+ percentage, and risk of hepatocellular carcinoma by cirrhosis status. J Natl Cancer Inst. 2020;112(7): 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wandeler G, Mauron E, Atkinson A, et al. Incidence of hepatocellular carcinoma in HIV/HBV-coinfected patients on tenofovir therapy: relevance for screening strategies. J Hepatol. 2019;71(2):274–280. [DOI] [PubMed] [Google Scholar]
- 5. Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11(4):797–816, viii. [DOI] [PubMed] [Google Scholar]
- 6. Kim HN, Newcomb CW, Carbonari DM, et al. Risk of HCC with hepatitis B viremia among HIV/HBV-coinfected persons in North America. Hepatology. 2021;74(3):1190–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Béguelin C, Moradpour D, Sahli R, et al. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol. 2017;66(2):297–303. [DOI] [PubMed] [Google Scholar]
- 8. Mocroft A, Lundgren J, Gerstoft J, et al. Clinical outcomes in persons coinfected with human immunodeficiency virus and hepatitis C virus: impact of hepatitis C virus treatment. Clin Infect Dis. 2020;70(10): 2131–2140. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan A. Declining mortality for patients with human immunodeficiency virus (HIV) and cirrhosis: an analysis of national trends. Presented at the Eighth Biennial HIV and Liver Disease Conference. September 2021; Teton Village, WY.
- 10. Qu Y, Li M, Hamilton G, Zhang YN, Song B. Diagnostic accuracy of hepatic proton density fat fraction measured by magnetic resonance imaging for the evaluation of liver steatosis with histology as reference standard: a meta-analysis. Eur Radiol. 2019;29(10):5180–5189. [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention (CDC). Estimated HIV incidence and prevalence in the United States, 2014-2018. https://stacks.cdc.gov/view/cdc/87841. Accessed August 12, 2022.
- 12. Dailey AF, Hoots BE, Hall HI, et al. Vital signs: human immunodeficiency virus testing and diagnosis delays - United States. MMWR Morb Mortal Wkly Rep. 2017; 66(47):1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacGowan RJ, Chavez PR, Mermin JH. Implementation of HIV self-testing program in New York City-reply. JAMA Intern Med. 2020;180(4):616–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruiz MS, O’Rourke A, Allen ST, et al. Using interrupted time series analysis to measure the impact of legalized syringe exchange on HIV diagnoses in Baltimore and Philadelphia. J Acquir Immune Defic Syndr. 2019;82 Suppl 2:S148–S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohr R, Schierwagen R, Schwarze-Zander C, et al. Liver fibrosis in HIV patients receiving a modern cART: which factors play a role? Medicine (Baltimore). 2015; 94(50):e2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin W, Tsai WL, Shao RX, et al. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology. 2010;138(7):2509–2518, 2518.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salloum S, Holmes JA, Jindal R, et al. Exposure to human immunodeficiency virus/hepatitis C virus in hepatic and stellate cell lines reveals cooperative profibrotic transcriptional activation between viruses and cell types. Hepatology. 2016;64(6):1951–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu PJ, Harris JM, Marchi E, et al. Author Correction: Hypoxic gene expression in chronic hepatitis B virus infected patients is not observed in state-of-the-art in vitro and mouse infection models. Sci Rep. 2020; 10(1):19332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kandathil AJ, Sugawara S, Goyal A, et al. No recovery of replication-competent HIV-1 from human liver macrophages. J Clin Invest. 2018;128(10):4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kazankov K, Barrera F, Møller HJ, et al. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology. 2014;60(2):521–530. [DOI] [PubMed] [Google Scholar]
- 21. Lidofsky A, Holmes JA, Feeney ER, et al. Macrophage activation marker soluble CD163 is a dynamic marker of liver fibrogenesis in human immunodeficiency virus/hepatitis C virus coinfection. J Infect Dis. 2018;218(9):1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sherman KE, Meeds HL, Rouster SD, et al. Soluble CD163 identifies those at risk for increased hepatic inflammation & fibrosis. Open Forum Infect Dis. 2021; 8(6):ofab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agarwal N, Iyer D, Gabbi C, et al. HIV-1 viral protein R (Vpr) induces fatty liver in mice via LXRalpha and PPAR-alpha dysregulation: implications for HIV-specific pathogenesis of NAFLD. Sci Rep. 2017;7(1):13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shrivastava S, Kottilil S, Sherman KE, Masur H, Tang L. CCR5+ T-cells homed to the liver exhibit inflammatory and profibrogenic signatures in chronic HIV/HCV-coinfected patients. Viruses. 2021;13(10):2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bartneck M, Koppe C, Fech V, et al. Roles of CCR2 and CCR5 for hepatic macrophage polarization in mice with liver parenchymal cell-specific NEMO deletion. Cell Mol Gastroenterol Hepatol. 2021;11(2):327–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016;11(6):e0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sherman KE, Abdel-Hameed E, Rouster SD, et al. Improvement in hepatic fibrosis biomarkers associated with chemokine receptor inactivation through mutation or therapeutic blockade. Clin Infect Dis. 2019 May. 17;68(11):1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ratziu V, Sanyal A, Harrison SA, et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology. 2020;72(3):892–905. [DOI] [PubMed] [Google Scholar]
- 29. Schuetz A, Deleage C, Sereti I, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10(12):e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12): 1365–1371. [DOI] [PubMed] [Google Scholar]
- 31. Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5(193):193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nganou-Makamdop K, Talla A, Sharma AA, et al. Translocated microbiome composition determines immunological outcome in treated HIV infection. Cell. 2021;184(15):3899–3914.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135(1):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kardashian A, Ma Y, Yin MT, et al. High kynurenine:tryptophan ratio is associated with liver fibrosis in HIV-monoinfected and HIV/hepatitis C virus-coinfected women. Open Forum Infect Dis. 2019; 6(7):ofz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. 2019;110(1):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dasgupta S, Broz D, Tanner M, et al. Changes in reported injection behaviors following the public health response to an HIV outbreak among people who inject drugs: Indiana, 2016. AIDS Behav. 2019;23(12): 3257–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knowles H. Rural Indiana county ends needle swap that helped fight HIV — sparking fears of another outbreak. The Washington Post. June 5, 2021.
- 38. Pintado C, Delaugerre C, Molina JM. Acute hepatitis B infection after a switch to long-acting cabotegravir and rilpivirine. Open Forum Infect Dis. 2020;7(9):ofaa367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bischoff J, Gu W, Schwarze-Zander C, et al. Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART). EClinicalMedicine. 2021;40):101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khaimova R, Fischetti B, Cope R, Berkowitz L, Bakshi A. Serological response with Heplisav-B® in prior Hepatitis B vaccine non-responders living with HIV. Vaccine. 2021;39(44):6529–6534. [DOI] [PubMed] [Google Scholar]
- 41. Schnittman SR, Zepf R, Cocohoba J, Sears D. Brief report: heplisav-B seroprotection in people with HIV: a single-center experience. J Acquir Immune Defic Syndr. 2021;86(4):445–449. [DOI] [PubMed] [Google Scholar]
- 42. Vesikari T, Langley JM, Segall N, et al. Immunogenicity and safety of a tri-antigenic versus a mono-antigenic hepatitis B vaccine in adults (PROTECT): a randomised, double-blind, phase 3 trial. Lancet Infect Dis. 2021;21(9):1271–1281. [DOI] [PubMed] [Google Scholar]
- 43. Naggie S, Cooper C, Saag M, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8):705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shimada S, Ivanics T, Kitajima T, et al. Improvements in liver transplant outcomes in patients with HCV/HIV coinfection after the introduction of direct-acting antiviral therapies. Transpl Infect Dis. 2022;24(2)e13808. [DOI] [PubMed] [Google Scholar]
- 45. Saab S, Le L, Saggi S, Sundaram V, Tong MJ. Toward the elimination of hepatitis C in the United States. Hepatology. 2018;67(6):2449–2459. [DOI] [PubMed] [Google Scholar]
- 46. Bretaña NA, Gray RR, Cunningham EB, et al. Combined treatment and prevention strategies for hepatitis C virus elimination in the prisons in New South Wales: a modelling study. Addiction. 2020;115(5):901–913. [DOI] [PubMed] [Google Scholar]
- 47. van Santen DK, Boyd A, Matser A, et al. The effect of needle and syringe program and opioid agonist therapy on the risk of HIV, hepatitis B and C virus infection for people who inject drugs in Amsterdam, the Netherlands: findings from an emulated target trial. Addiction. 2021; 116(11):3115–3126. [DOI] [PubMed] [Google Scholar]
- 48. Wall A, Lee GH, Maldonado J, Magnus D. Medical contraindications to transplant listing in the USA: a survey of adult and pediatric heart, kidney, liver, and lung programs. World J Surg. 2019;43(9):2300–2308. [DOI] [PubMed] [Google Scholar]
- 49. Ahmed S, Algarin AB, Thadar H, et al. Comorbidities among persons living with HIV (PLWH) in Florida: a network analysis. AIDS Care. doi: 10.1080/09540121.2022.2038363. [DOI] [PMC free article] [PubMed]
- 50. Seval N, Frank CA, Litwin AH, et al. Design and methods of a multi-site randomized controlled trial of an integrated care model of long-acting injectable buprenorphine with infectious disease treatment among persons hospitalized with infections and opioid use disorder. Contemp Clin Trials. 2021;105):106394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sudjaritruk T, Aurpibul L, Songtaweesin WN, et al. Integration of mental health services into HIV healthcare facilities among Thai adolescents and young adults living with HIV. J Int AIDS Soc. 2021;24(2):e25668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weaver MR, Conover CJ, Proescholdbell RJ, et al. Cost-effectiveness analysis of integrated care for people with HIV, chronic mental illness and substance abuse disorders. J Ment Health Policy Econ. 2009;12(1):33–46. [PubMed] [Google Scholar]
- 53. Czaja AJ. Review article: chemokines as orchestrators of autoimmune hepatitis and potential therapeutic targets. Aliment Pharmacol Ther. 2014;40(3):261–279. [DOI] [PubMed] [Google Scholar]
- 54. Sharpton SR, Schnabl B, Knight R, Loomba R. Current concepts, opportunities, and challenges of gut micro-biome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metab. 2021;33(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]