Abstract
Introduction
Patients with serious comorbidities are traditionally excluded from clinical trials. Apremilast is not contraindicated in active infections, malignancy and serious hepatic or renal impairment, but real-life data is needed to support this recommendation.
Objectives
The aim of this paper is to present our personal as well as literature-sourced real-world evidenced on apremilast use in psoriasis patients with serious baseline comorbidities.
Methods
A case-series and systematic literature review were performed. The psoriasis archives of a tertiary-care hospital, four electronic databases (MEDLINE, ScienceDirect, Cochrane Library, Google scholar) and other sources were searched (January 2014 – July 2021). Identified records were considered eligible, if they reported on the use of apremilast monotherapy in psoriasis patients with chronic infections, history of malignancy, serious liver, renal, psychiatric, or other disease(s).
Results
At least 841 psoriasis patients with serious baseline diseases received apremilast. Only 3 cases of cancer progression and no infection reactivations or worsening of other diseases were documented. No increased frequency/severity of adverse events or reduced drug efficacy were noted. Main limitations of this study are the exclusion of a few reports due to inappropriately documented data and the fact that at least some patients might have been counted more than once.
Conclusions
Apremilast is a safe and adequately efficacious option for psoriasis that cannot be treated/is challenging to treat with classic systemic agents and/or biologics.
Keywords: apremilast, comorbidities, medical dermatology, psoriasis, biologics
Introduction
Psoriasis is a chronic cutaneous disease of inflammatory nature, with a worldwide prevalence ranging between 1–3%; it has a substantial negative effect on patient physical well-being and quality-of-life, as well as on national health expenditure [1–3]. Apremilast (Otezla®, Amgen), a small molecular inhibitor of phosphodiesterase 4 (PDE4), has been used for the treatment of psoriatic arthritis and psoriasis since 2014 (first US Food and Drug Administration [FDA] approval). Contrary to biologics, it does not target any one specific component of the inflammatory process involved in the pathophysiology of psoriasis, but rather achieves some sort of equilibrium of pro-inflammatory and anti-inflammatory agents [4,5].
Apremilast comes with a set of favorable attributes, among which are the oral distribution, lower cost comparing to biologics and good safety profile [3,6]. It is not contraindicated in patients with active infections or serious liver impairment, nor is routine lab monitoring necessary. Similarly, it can be administered to patients with past or current malignancy [8]. Patients with severe renal impairment can still receive a reduced dose of apremilast [9]. A pooled analysis (≥ 156 weeks) from the 2 apremilast-approving (ESTEEM) trials showed that serious infection rate was low among patients receiving apremilast, while no serious opportunistic infections or cases of tuberculosis reactivation were noted [10]. However, as patients with chronic infections, cancers and serious co-existing diseases are traditionally excluded from clinical trials testing new drugs, conclusions regarding the use of said drugs in these occasions cannot always be safely drawn [7,11].
Objectives
The purpose of this study is to present our five-year experience in administering apremilast to psoriasis patients with serious comorbidities in the setting of a tertiary-care center in terms of drug safety and efficacy, as well as to concisely portray relevant real-life evidence sourced through a systematic literature search.
Methods
Case Series
The March 2016 (apremilast approval in Greece) to June 30th, 2021 archives of both the Psoriasis Outpatient Clinic and Afternoon Private Clinics of the First Dermatology Department, Aristotle University, Thessaloniki, Greece were consecutively searched for all psoriasis patients having received at least one dose of apremilast. Patients with an appropriately documented chronic/latent infection, recent (past 10 years) malignancy excluding basal cell carcinoma, serious kidney (stage IV and V) or liver (Child-Pugh C) disease, severe psychiatric disorder or other serious illness as was defined by Kelley [12] were included in the study. The following data were retrieved by two collaborating authors (AT and NS): age, gender, comorbidity(-ies), year of comorbidity diagnosis, apremilast dose, baseline Psoriasis Area and Severity Index (PASI), treatment outcome in terms of efficacy, duration of apremilast treatment (weeks) and adverse events (AEs) including adverse outcomes related to comorbidity(-ies) in question. Written informed consent was obtained by all participants. This project was designed and conducted based on the declaration of Helsinki and was approved by the Ethics Committee of the Hospital of Venereal and Cutaneous Diseases, Thessaloniki, Greece.
Systematic Review
Eligibility Criteria
We conducted this systematic review as stated by Meta-analyses Of Observational Studies in Epidemiology (MOOSE) statement. Published and unpublished prospective or retrospective observational studies reporting on the use of apremilast for the treatment of psoriasis patients with serious baseline comorbidities (chronic/latent infections such as tuberculosis, hepatitis and HIV, cancer diagnosis within past ten years excluding basal cell carcinoma, stage IV and V chronic kidney disease hemodialysis, Child-Pugh class C liver disease, serious psychiatric disorders or other serious illness as was defined by Kelley [12]) were considered eligible for inclusion in our study. Clinical trials or studies not presenting real-life data, as well as studies reporting on combination therapy of apremilast and other systemic agents aside from phototherapy, systemic corticosteroids or other medication administered systemically for existing comorbidities were excluded from our review. Only studies performed after 2014 (apremilast first FDA approval) were considered. No language restrictions were placed.
Information Sources
Three electronic databases were searched (MEDLINE, ScienceDirect, and the Cochrane Library electronic databases). Google scholar (https://scholar.google.com/) was also browsed. Abstract compendia of the World Congresses of Dermatology, World Psoriasis and Psoriatic Arthritis Congresses, American Academy of Dermatology Annual Meetings and European Academy of Dermatology and Venereology Annual Congresses of the last five years were examined (online browsing). Last search date for all above mentioned platforms was July 4th, 2021. Amgen® was contacted and kindly asked to supply our team with any published or unpublished data abiding by our search criteria. The “Reference” section of studies included in our review was hand searched for additional eligible work.
Search Strategy
The following search strategy was used for MEDLINE database and modified accordingly for the rest of searched platforms: (apremilast[Title]) AND (psoriasis[Title/Abstract]) filtered by year of publication (2014 to 2021). The search was performed independently by two authors (AT and NS).
Study Endpoints
The primary endpoint of this study was the documentation of any safety-related outcomes in patients with serious comorbidities having received apremilast (for example comorbidity progression, AEs commonly related to apremilast use or other events). Secondary endpoint was treatment efficacy, without any limitations imposed on efficacy measures used.
Selection Process and Data Collection
Duplicate records were independently manually removed by two reviewers (NS and AT). Subsequently, two reviewers (AT and NS) independently screened titles and abstracts for relevance to the study objective. The full text of remaining records was read, and eligible studies were included in the review. AT and NS separately extracted the following data from included studies according to a pre-formulated sheet: first author, year of publication / research completion, comorbidity(-ies), age and gender of patient(-s), apremilast dose, baseline PASI, AEs including comorbidity-related events. Any disagreements were resolved in consultation with a third author (ES).
Quality Assessment
Two authors (AT and ES) independently assessed included reports based on two different tools, namely the Joanna Briggs Institute (JBI) critical appraisal tool for case reports/case series and the JBI critical appraisal tool for analytical cross-sectional studies, each comprising eight questions (Supplement). Each study was assigned an overall rating of poor, good or fair, if 0–5, 6–7 or 8 criteria were met respectively.
Results
The psoriasis-archives search returned 16 eligible cases, one of which was not included in the analysis due to incompletely documented patient data (Table 1). No progression of malignancy, reactivation of chronic / latent infection or deterioration of already deficient renal or hepatic function were noted. Apremilast was generally well-tolerated and only mild transient AEs were reported in 6 patients. Patient compliance to treatment was high (three cases of temporary drug discontinuation, < 14 days, due to Covid-19-related restrictions and consequent difficulties in drug prescription). Desired response (PASI50) was not achieved in 1 case and apremilast was therefore discontinued (primary drug failure).
Table 1.
Cases of psoriasis patients with serious baseline comorbidities treated with apremilast.
| Case | Sex | Age | Comorbidity (diagnosis) | APR Dose | Bas PASI | Tx outcome | APR duration | AEs |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 69 | Prostate cancer (2013) | 30 mg bid | 10.3 | PASI50 week 16 | 53 (LOoE) | None |
| 2 | M | 76 | Lung tuberculosis (1968) | 30 mg bid | 9 | PASI50 week 16 | 52 (Lost to f/u) | None |
| 3 | M | 73 | Latent tuberculosis | 30 mg bid | 13.4 | PASI75 week 16 | 72 (LOoE) | None |
| 4 | M | 79a | Lung cancer (2015) | 30 mg bid | 15.9 | PASI50 not achieved | 21 (LAoE) | Abdominal pain |
| 5 | F | 65 | Breast cancer (2016), hepatitis B | 30 mg bid | 5.3 | PASI100 24 weeks | 35 (clear skin) | Dyspepsia |
| 6 | M | 63 | Hepatitis C and liver fibrosis | 30 mg bid | 6.7 | PASI50 week 16 | 37 (LOoE) | None |
| 7 | M | 44 | Hemodialysis (Stage 5 Chronic kidney disease – IgA nephropathy) | 30 mg od | 13.2 | PASI50 week 24 | 40 (LOoE) | None |
| 8 | F | 66 | Lung tuberculosis (1980) | 30 mg bid | 11.1 | PASI50 week 16 | 4 (AEs) | Headache |
| 9 | M | 33 | HIV positive (2011) (CD4+ 602 cells/mm3) | 30 mg bid | 15.8 | PASI75 week 16 | 149 (SoD) | None |
| 10 | M | 65 | Urinary bladder cancer (2015), Stage 2 chronic kidney disease | 30 mg bid | 9.1 | PASI50 week 16 | 31 (LOoE) | None |
| 11 | F | 56 | Cervical cancer (2013) | 30 mg bid | 10.8 | PASI75 week 24 | 154 (SoD) | None |
| 12 | M | 37 | HIV positive (2009) (CD4+ 590 cells/mm3) | 30 mg bid | 24.5 | PASI50 week 24 | 56 (SoD) | Transient nausea |
| 13 | M | 44 | HIV positive (2002) (CD4+ 550 cells/mm3) | 30 mg bid | 33.6 | PASI90q week 52 | 104 (SoD) | Transient diarrhea |
| 14 | M | 32 | HIV positive (2009) (CD4+ 702 cells/mm3) | 30 mg bid | 19.3 | PASI90 week 24 | 34 (SoD) | Transient diarrhea |
| 15 | M | 88 | Hepatitis B | 30 mg bid | 28.6 | PASI90 week 8 | 16 (SoD) | None |
AEs = adverse events; APR = apremilast; Bas = baseline; F = female; f/u = follow-up; HIV = human immunodeficiency; LAoE = lack of efficacy; LOoE = loss of efficacy; M = male; o.d. = once daily; PASI = Psoriasis Area and Severity Index;; bid = twice daily (bis in die); PASI50 = 50% reduction of baseline PASI; PASI75 = 75% reduction of baseline PASI; virus; PASI90 = 90% reduction of baseline PASI; SoD = still on drug; Tx = treatment.
Deceased.
The systematic literature search yielded 52 studies eligible for inclusion (Figure 1). One additional study was identified after the last search date (published July 8th 2021) and does not appear in the flow diagram (Figure 1) [13]. A total of at least 826 psoriasis patients with serious comorbidities (various malignancies – at least 456 patients –, latent / past tuberculosis – 49 patients –, hepatitis B, C and HIV infections – at least 83 patients –, serious renal – 7 patients – or liver impairment – at least 110 patients –, serious psychiatric disorders – 49 patients – or other serious illness as was defined by Kelley [12]) were prescribed apremilast twice or once daily (Table 2). The exact number of patients with serious comorbidities prescribed apremilast was not reported in a few studies, therefore, the numbers mentioned above are a conservative underestimation of the studied population. Included patients manifested all types of psoriasis and/or nail psoriasis. Overall, apremilast was hardly ever associated with negative comorbidity-related outcomes and reported AEs were apparently not more severe or frequent than those experienced by the average psoriasis patient. Sufficient response of psoriasis to apremilast was recorded in most cases. Overall, the quality of included studies was good (Table 3).
Figure 1.
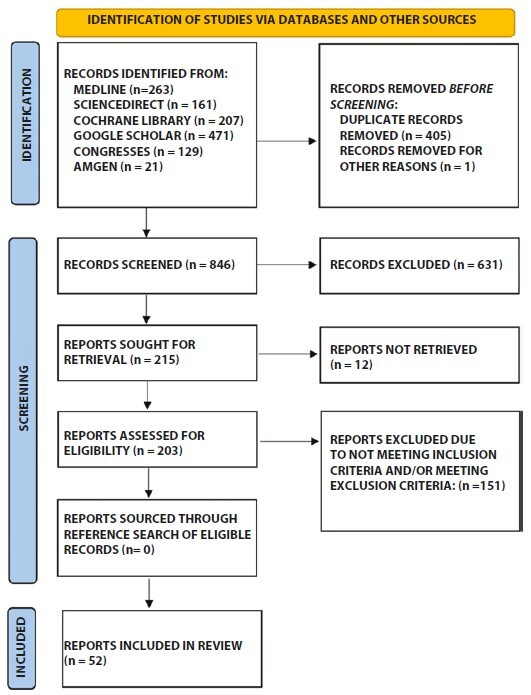
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram detailing the number and origin of records identified, included in and excluded from this systematic review, as well as the reasons for exclusion.
Table 2.
Literature cases of apremilast administered to psoriasis patients with serious baseline comorbidities.
| Report | Comorbidity | Age/Sex | APR dose | Bas PASI | Tx outcome | AEs |
|---|---|---|---|---|---|---|
| Mugheddu 2020 [22] | Recurrent brain oligodendroglioma under temozolomide and prednisone | 45/M | 30 mg bid | 45 | Improvement | N/R COVID-19 pneumonia, full recovery |
| Manfreda 2019 [15] | HIV positive (CD4+ 628 cells/mm3), Hepatitis B, drug addiction history | 41/M | 30 mg bid | 12 | PASI90W24 | None, stable CD4+ count |
| Zarbafian 2019 [5] | HIV positive (CD4+ >1000 cells/mm3) | 54/M | 30 mg bid | 10.2 | 73% PASI reduction M7 | Transient diarrhea, frequent URTIs, stable CD4+ count |
| Reddy 2017 [4] | HIV positive, Hepatitis C | 46/M | 30 mg bid | BSA 8% | BSA 1.5% M5 | None |
| Shah 2019 [18] | HIV positive (CD4+ 742/μl) | 35/M | 30 mg bid, then od | 14.7 | PASI100 W6 | None, stable CD4+ count |
| Sacchelli 2018 [32] | HIV positive (2002) (CD4+ 566/mc), past Hepatitis B and C | 55/M | 30 mg bid | 8 NAPSIq 21 |
PASI75M6 NAPSI 7 M6 |
None |
| Apalla 2019 [11] | Metastatic lung adenocarcinoma, stage IIIB (2013) | 67/F | 30 mg bid | 18 | PASI75 W16 | None, cancer course not affected by APR |
| Reddy 2019 [17] | HIV positive (CD4+ 460 cells/mm3) | 50/M | 30 mg bid and then od | BSA 10% | BSA 0% M3 | N/R |
| Fotiadou 2018 [33] | Chronic hepatitis B, undetectable viral load | 52/F | 30 mg b.i.d. | 13.2 | PASI90 W24 | None, normal HBload and LFTs |
| Jeon 2017 [34] | Active hepatitis C, decompensated cirrhosis, metastatic HCC | 55/M | 30 mg bid | N/R | PASI100 W6 | Initial nausea |
| González-Cantero 2018 [35] | Malignancy: 3, liver disease: 1, alcoholism: 1, infectious disease: 1, renal insufficiency: 1, demyelinating disease: 1 | N/R | N/R | N/R | N/R | No changes in the course of comorbidities |
| Gottlieb 2021 [36] | Malignancy: 92, serious infection: 53 | N/R | N/R | N/R | N/R | N/R |
| Kahn 2019 [37] | 1. renal cell carcinoma 2. melanoma |
63, N/R 64, N/R |
N/R | BSA 10% BSA 5% |
N/R | No clinical/radiographical signs of cancer recurrence |
| Nagata 2019 [38] | Hemodialysis | 60, M | 30 mg od | PSSI 36 | PSSI 10 W12 | Nausea (transient) |
| Uvais 2020 [39] | Bipolar affective disorder | 30, F | 30 mg/day | N/R | N/R | N/R |
| Vico-Alonso 2020 [40] | Chronic myeloid leukemia under imatinib, latent tuberculosis, alcoholism, steatohepatitis | 58, M | 30 mg bid | 22.4 | PASI90 W24 | None, stable tumoral state, normal lab tests |
| Melis 2020 [41] | Malignancy (excl. NMSC): 13 (breast, bladder, colorectal), severe infection: 7 (HIV, HBV, HCV), psychiatric disorder: 6, liver disease: 3, latent tuberculosis: 2 | N/R | 30 mg bid | N/R | N/R | N/R |
| Perrone 2017 [42] | Chronic anemia and thrombocytopenia | 71, M | N/R | N/R | N/R | Fanconi Syndrome |
| Carpentieri 2020 [43] | Malignancy (excl. NMSC): 10 | N/R | N/R | N/R | Most patients showed improvement | no clinical or radiographic recurrence or progression of their cancer |
| Peitsch 2019 [44] | Mantel cell lymphoma under rituximab, hepatic and pulmonary aspergillosis | 60, M | 30 mg bid | 17.2 | PASI90 M5 | None, lymphoma in remission |
| Takama 2020 [45] | Urinary bladder cancer under pembrolizumab | 74, M | 30 mg bid, then od | 2.9 | PASI50 W2, PASI90 M2 | Nausea |
| Foti 2021 [46] | Melanoma with lymph node metastasis under nivolumab | 62, M | 30 mg bid | 44 | PASI50 M6, PASI90 M12 | None, no worsening of melanoma |
| Di Lernia 2021 [47] | Malignancy: 3 colorectal, 2 GI stromal, 1 leukemia, 1 lymphoma,1 kidney, 1 uterus and thyroid, 2 melanoma,1 urinary bladder,1 metastatic SCC, 1 prostate | 5 F, 9 M (age N/R) | 30 mg bid | N/R | N/R | Diarrhea, headache (3 patients, disc. APR), urinary bladder cancer and metastatic SCC recurrence after APR disc. |
| Aragon-Miguel 2019 [48] | Malignancy hx: 6, latent tuberculosis: 16, hepatitis C or B (chronic or past): 7 | N/R | N/R | N/R | N/R | N/R No infection reactivations |
| Tampouratzi 2019 [49] | Chronic hepatitis B, under entecavir | 64, M | N/R | N/R | Excellent response | None |
| Papadavid 2018 [50] | Malignancy hx: 1, latent tuberculosis: 1, chronic latent hepatitis B: 1 | N/R | N/R | N/R | N/R | N/R |
| Sahuquillo-Torralba 2020 [51] | Latent tuberculosis: 1, active hepatitis B: 1, spontaneous bacterial peritonitis: 1, immune hepatitis: 1 | N/R | N/R | N/R | N/R | N/R |
| Siciliano 2020 [52] | Metastatic melanoma (2018) | 75, M | 30 mg bid | N/R | Improvement | None, melanoma remission |
| Lanna 2020 [53] | Tumors: 5 | N/R | N/R | N/R | N/R | N/R |
| Lanna 2019 [54] | Hepatitis E | 55, M | 30 mg bid | 18 | PASI90 M6 | None |
| Balato 2020 [55] | Malignancy: 40 | N/R | N/R | N/R | N/R | Lower PASI50 and PASI75 response rates |
| Queiro Silva 2020 [21] | Hairy cell leukemia | 55, M | N/R | N/R | N/R | Severe COVID-19 infection |
| Ighani 2018 (1) [56] | Malignancy hx: 15, liver disease: 8, psychiatric disorder: 9 | N/R | N/R | N/R | N/R | N/R |
| Ighani 2018 (2) [57] | Malignancy hx: 31, liver disease: 27, psychiatric disorder: 29 | N/R | N/R | N/R | N/R | N/R |
| Ighani 2018 (3) [58] | Malignancy hx: 5, liver disease: 4, psychiatric disorder: 4 | N/R | N/R | N/R | N/R | N/R |
| Phan 2020 [59] | Malignancy: 40 | >65 y | N/R | N/R | N/R | N/R |
| Ighani 2018 (4) [60] | hepatitis C: 2, breast cancer: 2, renal disease (unspecified): 2 | N/R | N/R | N/R | N/R | N/R |
| Megna 2020 [61] | Malignancy: 9, hepatitis C: 4, latent tuberculosis: 3 | N/R | N/R | N/R | N/R | N/R |
| Del Alcázar 2020 [62] | Lung cancer: 20, latent tuberculosis: 20, hepatitis C: 17, hepatitis B: 13, hepatitis B and C: 2, malignancy hx: 92, liver disease: 33 | N/R | N/R | N/R | N/R | No cases of infection reactivation or cancer recurrence |
| Fremlin 2017 [63] | Cases of alcohol excess, alcoholic liver disease, previous malignancy (unspecified number) | N/R | N/R | N/R | N/R | N/R |
| Foulkes 2017 [64] | Cases of malignant melanoma and HIV (unspecified number) | N/R | N/R | N/R | N/R | N/R |
| Malara 2018 [65] | Latent tubercular skin infection: 2, previous hepatitis: 1, endocarditis-related cardiac valve failure: 1, malignancy: 4 | N/R | N/R | N/R | N/R | None |
| Daudén 2020 [14] | Malignancy hx: 14 (6 in last 5 years), hepatitis B:12, hepatitis C: 5, chronic liver disease: 20, renal insufficiency: 3 | N/R | N/R | N/R | N/R | N/R |
| Kungurov 2019 [66] | Hepatitis B: 1, chronic pancreatitis, cholecystitis and colitis: 1, hepatitis C: 1 | 47, F 38, F 31, M |
N/R | 27.3 29.9 33 |
PASI75 W24 PASI75 W6 PASI75 W28 |
None |
| Aragon-Miguel 2019 [67] | breast cancer: 1, gallbladder cancer: 1, treated latent tuberculosis: 3 | N/R | N/R | N/R | N/R | N/R |
| Bulic 2019 [68] | 1. laryngeal SCC (2013) 2. papillary thyroid cancer (2014) 3. liver cirrhosis (Child-Pugh B)/portal hypertension/ hepatocellular cancer (pT2pNxpMx)/ liver transplant (2017) 4. melanoma (pT1a, Breslow 0.81mm) (2013) |
51, M 50, M 51, M 58, F |
N/R | N/R | N/R | No recurrency of malignancy over a two-year follow-up |
| Fattore 2019 [69] | Non-metastatic non-small-cell lung cancer under nivolumab | 74, F | 30 mg bid | N/R | PASI100 W6 | Transient nausea |
| Magdaleno 2019 [70] | Chronic liver disease: 13, severe infection: 11, previous malignancy: 8 | N/R | N/R | N/R | N/R | N/R |
| Magdaleno-Tapial 2019 [71] | Chronic active alcoholism and hyper-transaminasemia | 61, M | N/R | N/R NAPSI 32 |
PASI<5 M6 | No serious AEs |
| Gioe 2021 [72] | Chronic hepatitis B, bipolar disorder, acute MRSAee bacteremia, pulmonary embolus | 34, F | N/R | N/R (>95% BSA) | BSA< 15% M1 | N/R |
| Ibarguren 2021 [73] | 1. Stage IV uveal melanoma 2. Stage IV laryngeal carcinoma 3. Stage IV squamous cell lung carcinoma all 3 under nivolumab |
50, M 70, M 60, M |
30 mg bid | 12 13.8 6.4 |
PASI50 M12 PASI75 (time N/R) PASI50 not achieved |
1. diarrhea 2. headache, cancer progression after 10 months 3. cancer progression after 10 months |
| Kurata 2021 [74] | Colorectal cancer within past year | 82, F | N/R | 5.5 NAPSI 77 |
PASI90 and NAPSI50 W8 | γ-GT increase after 8 weeks & drug disc. |
| Cohen-Sors 2021 [13] | Hematologic malignancy: 10 | N/R | N/R | N/R | N/R | Evolution of previously stable CLL (1 case) |
AEs = adverse events; APR = apremilast; Bas = baseline; bid = twice daily; BSA = body surface area; F = female; CLL = chronic lymphocytic leukemia; disc. = discontinuation; HBV = hepatitis B virus; HCC = hepatocellular carcinoma; HCV = hepatitis C virus; HIV = human immunodeficiency virus; hx = history; LFTs = liver function tests; M = male; M = month; MRSA = methicillin resistant staphylococcus aureus; NAPSI = nail psoriasis severity index; NMSC = non-melanoma skin cancer; N/R = not reported; od = once daily; PASI = Psoriasis Area and Severity Index; PASI50 = 50% reduction of baseline PASI; PASI75 = 75% reduction of baseline PASI; PASI90 = 90% reduction of baseline PASI; PASI100 = 100% reduction of baseline PASI; PSSI = psoriasis scalp severity index; SCC = squamous cell carcinoma; Tx = treatment;y = years old; URTIs = upper respiratory tract infections; W = week.
Table 3.
Methodological quality assessment of included reports.
| Report | Mugheddu 2020 [22] | Manfreda 2019 [15] | Zarbafian 2019 [5] | Reddy 2017 [4] | Shah 2019 [18] | Sacchelli 2018 [32] | Apalla 2019 [11] | Reddy 2019 [17] | Fotiadou 2018 [33] | Jeon 2017 [34] |
|---|---|---|---|---|---|---|---|---|---|---|
| Quality | Fair | Fair | Fair | Fair | Fair | Fair | Fair | Fair | Fair | Fair |
| Report | Gonzalez-Cantero 2018 [35] | Gottlieb 2021 [36] | Kahn 2019 [37] | Nagata 2019 [38] | Uvais 2020 [39] | Vico-Alonso 2020 [40] | Melis 2020 [41] | Perrone 2017 [42] | Carpentieri 2020 [43] | Peitsch 2019 [44] |
| Quality | Good | Good | Good | Good | Good | Fair | Good | Fair | Good | Fair |
| Report | Takama 2020 [45] | Foti 2021 [46] | Di Lernia 2021 [47] | Aragon-Miguel 2019 [48] | Tampouratzi 2019 [49] | Papadavid 2018 [50] | Sahuquillo-Torralba 2020 [51] | Siciliano 2020 [52] | Lanna 2020 [53] | Lanna 2019 [54] |
| Quality | Fair | Fair | Good | Good | Good | Good | Good | Good | Good | Good |
| Report | Balato 2020 [55] | Queiro Silva 2020 [21] | Ighani 2018 (1) [56] | Ighani 2018 (2) [57] | Ighani 2018 (3) [58] | Phan 2020 [59] | Ighani 2018 (4) [60] | Megna 2020 [61] | Del Alcazar 2020 [62] | Fremlin 2017 [63] |
| Quality | Good | Good | Good | Good | Good | Good | Good | Good | Good | Good |
| Report | Foulkes 2017 [64] | Malara 2018 [65] | Daudén 2020 [14] | Kungurov 2019 [66] | Aragon-Miguel 2019 [67] | Bulic 2019 [68] | Fattore 2019 [69] | Magdaleno 2019 [70] | Magdaleno-Tapial 2019 [71] | Gioe 2021 [72] |
| Quality | Good | Good | Good | Good | Good | Good | Good | Good | Good | Good |
| Report | Ibarguren 2021 [73] | Kurata 2021 [74] | Cohen-Sors 2021 [13] | |||||||
| Quality | Good | Good | Good |
Case reports have been assessed through the JBI critical appraisal checklist for case reports. Cross-sectional studies have been assessed through the NIH quality assessment tool for observational cohort and cross-sectional studies. Each report has been assigned an overall quality marking of poor, good or fair.
Conclusions
This case series and systematic review reports on the use of apremilast in 841 psoriasis patients with serious baseline comorbidities, which would have made the use of classic systemic agents and/or biologics inappropriate or challenging. According to our study, the use of apremilast in this group of patients apparently neither leads to deterioration / exacerbation of severe pre-existing comorbidity(-ies) nor is it associated with an increased risk of adverse events. What is more, drug efficacy does not seem to be affected by the underlying comorbidity, with cases of remarkable response even in erythrodermic patients with very serious underlying diseases.
A drug safety profile, especially in the context of pre-existing comorbidities, is one of its major attributes to be taken into consideration, when deciding on a treatment regimen [14]. Psoriasis patients receiving apremilast, as opposed to other systemic agents, seem to have a lower infection risk [15]. Rates of herpes zoster infection were lowest for users of apremilast among a cohort of psoriasis patients treated with biologics and/or small molecules (5.4, 95% CI 1.7–12.6) [16]. Comparing to methotrexate (MTX), as investigated in a cohort of 2845 psoriasis patients treated with nine systemic agents in Spain, apremilast had a lower infection (incidence rate 0.3 [95% CI 0.1–0.9]) and malignant neoplasm risk (incidence rate 0.1 [95% CI 0–0.7]) [14].
Psoriasis may be more severe or difficult to treat in patients with HIV infection [17,18]. What is more, HIV-positive patients are immuno-compromised and prone to reactivation of latent infections [17]. According to the 2020 Belgian practical recommendations for the treatment of psoriasis in HIV-positive patients, apremilast and acitretin are considered first-line choices ]. Furthermore, many biologics (adalimumab, certolizumab pegol, etanercept, infliximab, ustekinumab, guselkumab, risankizumab, brodalumab, secukinumab, ixekizumab) can be used in patients receiving highly active antiretroviral therapy (HAART) and having undetectable viral load [19]. In case of detectable viral load, discussion with an infectious-disease specialist is warranted, with apremilast and acitretin being the preferred options [19]. Based on the same recommendations, the preferred options for short-term systemic treatment of psoriasis patients with chronic hepatitis C infection are adalimumab and etanercept, as there is not enough evidence to support the use of other biologics and apremilast [19]. As far as chronic hepatitis B infection is concerned, ustekinumab, apremilast, cyclosporine and acitretin are preferred [19]. Apremilast has shown potential in the treatment of psoriasis patients with a history of malignancy. It may even help treat lung cancer, as PDE4 is expressed in lung cancer cells [20].
In the current pandemic era, it is important to examine a drug safety with regards to the COVID-19 infection. According to the World Health Organization, psoriasis patients on small molecules like apremilast are thought to be immunosuppressed [6]. There have been reports of asymptomatic, as well as of fast and uneventful resolution of COVID-19 infections in psoriasis patients under apremilast, even in the case of serious comorbidities [21–23 ]. What is more, it seems that apremilast use does not hinder the formation of antibodies against SARS-CoV-2 [6]. It is important to remember that common apremilast AEs like taste alteration and gastrointestinal symptoms can mimic COVID-19 manifestations [6].
A relatively new, special population of psoriatic patients are those receiving therapy with immune checkpoint inhibitors (ICPIs) for various types of cancer. ICPIs have revolutionized cancer treatment and their use expands constantly. They include monoclonal antibodies that target cytotoxic T lymphocyte–associated antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed death ligand 1 (PD-L1) [24]. Due to the unique nature of ICPIs, a new category of AEs emerged concurrently with their clinical use [24]. They are known as “immune-related adverse events” (irAEs) and although they can affect any organ, skin is the one most frequently involved [24]. Morbilliform exanthems, pruritus, vitiligo and lichenoid eruptions are by far the most common cutaneous irAEs [25,26]. Numerous others have been reported, among which newly occurring or exacerbating previous psoriasis.
In the majority of cases, cutaneous irAEs are mild-to-moderate (grade 1–2) and anti-cancer treatment is not interrupted, although severity may vary, up to life-threatening Stevens-Johnson syndrome/toxic epidermal necrolysis [27,28]. Similarly, psoriasis is usually managed with topical treatment [24,29]. In moderate/severe cases, treatment is more complicated since immunosuppression by anti-psoriatic drugs can theoretically lead to tumor escape. In those patients, apremilast seems to be a relatively safe and effective choice, however its use is supported only by case reports/ small case series. Finally, an algorithm published recently by the ENCADO (European Network for Cutaneous Adverse Event to Oncologic Drugs) also suggests apremilast if the patient does not respond to phototherapy and/or acitretin [30].
Our study is not without its limitations. A few large studies like Armstrong and Levi were excluded from this review and potentially significant data was missed, because results were not reported separately for patients receiving apremilast monotherapy and those receiving combination treatment or other systemic agents [31]. On the other hand, it is fairly possible that some patients included in this review have been counted more than once, as they might have been sourced from the same databases or research centers (eg multiple publications by the same authors, Table 2). What is more, in studies reporting on multiple patients, efficacy and safety outcome measures were usually presented indistinguishably for all included patients and not individually, based on the comorbidity status, therefore the relevant fields of Table 2 could not be filled in. Last but not least, baseline comorbidity cases such as chronic infections were sometimes presented as a total number, without distinguishing among different types of eg infections.
All in all, according to this case series and systematic review, real-life use of apremilast so far suggests that the latter is indeed a safe and adequately efficacious option for moderate-to-severe psoriasis that cannot be treated/ is challenging to treat with classic systemic agents and/or biologics. What is more, there seems to be no increased risk of COVID-19 infection in patients receiving apremilast, with evidence suggesting a smoother course of the disease.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Gottlieb AB, Strober B, Krueger JG, et al. An open-label, single-arm pilot study in patients with severe plaque-type psoriasis treated with an oral anti-inflammatory agent, apremilast. Curr Med Res Opin. 2008;24(5):1529–1538. doi: 10.1185/030079908X301866. [DOI] [PubMed] [Google Scholar]
- 2.Shutty B, West C, Pellerin M, Feldman S. Apremilast as a treatment for psoriasis. Expert Opin Pharmacother. 2012;3(12):1761–1770. doi: 10.1517/14656566.2012.699959. [DOI] [PubMed] [Google Scholar]
- 3.Palfreeman AC, McNamee KE, McCann FE. New developments in the management of psoriasis and psoriatic arthritis: a focus on apremilast. Drug Des Devel Ther. 2013;7:201–210. doi: 10.2147/DDDT.S32713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31(11):e481–e482. doi: 10.1111/jdv.14301. [DOI] [PubMed] [Google Scholar]
- 5.Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: A case report. S. AGE Open Med Case Rep. 2019;7 doi: 10.1177/2050313x19845193. 2050313X1984519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacifico A, D’Arino A, Pigatto PDM, Malagoli P, Young D, Damiani G. COVID-19 vaccines do not trigger psoriasis flares in patients with psoriasis treated with apremilast. Clin Exp Dermatol. 2021;46(7):1344–1346. doi: 10.1111/ced.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piaserico S, Messina F, Russo FP. Managing Psoriasis in Patients with HBV or HCV Infection: Practical Considerations. Am J Clin Dermatol. 2019;20(6):829–845. doi: 10.1007/s40257-019-00457-3. [DOI] [PubMed] [Google Scholar]
- 8.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Zhou S, Assaf M, Nissel J, Palmisano M. Impact of Renal Impairment on the Pharmacokinetics of Apremilast and Metabolite M12. Clin Pharmacol Drug Dev. 2016;5(6):469–479. doi: 10.1002/cpdd.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grekin SK, Robinson DM, Berk DR, Plc A, Ahluwalia G. Low serious infection rates in patients with psoriasis and psoriatic arthritis treated with apremilast for 156 weeks and beyond: Pooled analysis of the phase 3 ESTEEM 1 and 2 and PALACE 1–3 trials. J Am Acad Dermatol. 2017;76(2):AB162. doi: 10.1016/j.jaad.2017.04.630. [DOI] [Google Scholar]
- 11.Apalla Z, Psarakis E, Lallas A, Koukouthaki A, Fassas A, Smaragdi M. Psoriasis In Patients With Active Lung Cancer: Is Apremilast a Safe Option? Dermatol Pract Concept. 2019;9(4):300–301. doi: 10.5826/dpc.0904a11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley AS. Defining “serious illness.”. J Palliat Med. 2014;17(9):985. doi: 10.1089/jpm.2014.0164. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Sors R, Fougerousse A-C, Reguiai Z, et al. Biological Therapies or Apremilast in the Treatment of Psoriasis in Patients with a History of Hematologic Malignancy: Results from a Retrospective Study in 21 Patients. Clin Cosmet Investig Dermatol. 2021;14:845–854. doi: 10.2147/CCID.S320098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daudén E, Carretero G, Rivera R, et al. Long-term safety of nine systemic medications for psoriasis: A cohort study using the Spanish Registry of Adverse Events for Biological Therapy in Dermatological Diseases (BIOBADADERM) Registry. J Am Acad Dermatol. 2020;83(1):139–150. doi: 10.1016/j.jaad.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Manfreda V, Esposito M, Campione E, Bianchi L, Giunta A. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131(3):239–240. doi: 10.1080/00325481.2019.1575613. [DOI] [PubMed] [Google Scholar]
- 16.Jick S, Wilcox K, Persson R, et al. The rates of herpes zoster, hepatitis c, and tuberculosis among patients with psoriasis treated with apremilast, biologics, conventional systemics, and corticosteroids in the U.S. MarketScan database. J Am Acad Dermatol. 2018;79:AB292. doi: 10.1016/j.jaad.2018.05.1152. [DOI] [Google Scholar]
- 17.Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103(5):E6–E7. [PubMed] [Google Scholar]
- 18.Shah B, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: A case report. Indian J Dermatol. 2019;64(3):242–244. doi: 10.4103/ijd.IJD_633_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert JLW, Segaert S, Ghislain PD, et al. Practical recommendations for systemic treatment in psoriasis in case of coexisting inflammatory, neurologic, infectious or malignant disorders (BETA-PSO: Belgian Evidence-based Treatment Advice in Psoriasis; part 2) J Eur Acad Dermatol Venereol. 2020;34(9):1914–1923. doi: 10.1111/JDV.16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pullamsetti SS, Banat GA, Schmall A, et al. Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by cross-talk with HIF. Oncogene. 2013;32(9):1121–1134. doi: 10.1038/onc.2012.136. [DOI] [PubMed] [Google Scholar]
- 21.Queiro Silva R, Armesto S, González Vela C, Naharro Fernández C, González-Gay MA. COVID-19 patients with psoriasis and psoriatic arthritis on biologic immunosuppressant therapy vs apremilast in North Spain. Dermatol Ther. 2020;33(6) doi: 10.1111/dth.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugheddu C, Pizzatti L, Sanna S, Atzori L, Rongioletti F. COVID-19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: safety and compatibility of apremilast with critical intensive care management. J Eur Acad Dermatol Venereol. 2020;34(8):e376–e378. doi: 10.1111/jdv.16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olisova OY, Anpilogova EM, Svistunova DA. Apremilast as a potential treatment option for COVID-19: No symptoms of infection in a psoriatic patient. Dermatol Ther. 2020;33(4):e13668. doi: 10.1111/dth.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors : Skin Toxicities and Immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 25.Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: The role of the dermatologist. Yale J Biol Med. 2020;93(1):123–132. [PMC free article] [PubMed] [Google Scholar]
- 26.Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor– related dermatologic adverse events. J Am Acad Dermatol. 2020;83(5):1255–1268. doi: 10.1016/j.jaad.2020.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. Dermatologic toxicities to immune checkpoint inhibitor therapy: A review of histopathologic features. J Am Acad Dermatol. 2020;83(4):1130–1143. doi: 10.1016/j.jaad.2020.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rovers J, Bovenschen H. Dermatological side effects rarely interfere with the continuation of checkpoint inhibitor immunotherapy for cancer. Int J Dermatol. 2020;59(12):1485–1490. doi: 10.1111/IJD.15163. [DOI] [PubMed] [Google Scholar]
- 29.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Current Opinion in Oncology. 2016;28(4):254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 30.Nikolaou V, Sibaud V, Fattore D, et al. Immune checkpoint-mediated psoriasis: A multicenter European study of 115 patients from the European Network for Cutaneous Adverse Event to Oncologic Drugs (ENCADO) group. J Am Acad Dermatol. 2021;84(5):1310–1320. doi: 10.1016/j.jaad.2020.08.137. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong A, Levi E. Real-World Clinical Experience With Apremilast in a Large US Retrospective Cohort Study of Patients With Moderate to Severe Plaque Psoriasis. J Drugs Dermatol. 2017;16(12):1240–1245. [PubMed] [Google Scholar]
- 32.Sacchelli L, Patrizi A, Ferrara F, Bardazzi F. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31(6):e12719. doi: 10.1111/dth.12719. [DOI] [PubMed] [Google Scholar]
- 33.Fotiadou C, Trakatelli M, Papathemeli D, Lazaridou E. Safety of Apremilast in the treatment of psoriasis patient with chronic hepatitis B. Acta Derm Venereol. 2018;98(Suppl 219):40–41. [Google Scholar]
- 34.Jeon C, Nakamura M, Sekhon S, et al. Generalized pustular psoriasis treated with apremilast in a patient with multiple medical comorbidities. JAAD Case Rep. 2017;3(6):495–497. doi: 10.1016/j.jdcr.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González-Cantero Á, Sánchez-Moya AI, Arias-Santiago S, Schoendorff-Ortega C. Apremilast en el tratamiento de la psoriasis, experiencia clínica real. Piel Form Contin en dermatología. 2018;33:144–145. doi: 10.1016/J.PIEL.2017.05.009. [DOI] [Google Scholar]
- 36.Gottlieb AB, Merola JF, Cirulli J, et al. Characteristics of Patients with Psoriasis Treated with Apremilast in the Corrona Psoriasis Registry. Dermatol Ther (Heidelb) 2021;11(1):253–263. doi: 10.1007/s13555-020-00479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn JS, Casseres RG, Her MJ, Dumont N, Gottlieb AB, Rosmarin D. Treatment of Psoriasis With Biologics and Apremilast in Patients With a History of Malignancy: A Retrospective Chart Review. J Drugs Dermatol. 2019;18(4) S1545961619P0387X. [PubMed] [Google Scholar]
- 38.Nagata M, Kamata M, Ohtsuki M, Sato S, Tada Y. Scalp psoriasis in a haemodialysis patient successfully treated with a half-dose of apremilast. Eur J Dermatol. 2019;29(3):341–342. doi: 10.1684/ejd.2019.3588. [DOI] [PubMed] [Google Scholar]
- 39.Uvais NA, Rakhesh SV, Afra TP, Hafi NAB, Razmi TM. Comorbid psoriasis-bipolar disorder successfully treated with apremilast: Much more than a mere coincidence? Gen Psychiatr. 2020;33(3):e100181. doi: 10.1136/gpsych-2019-100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vico-Alonso C, Sánchez-Velázquez A, Pinilla-Martin B, et al. Psoriasis and chronic myeloid leukemia: treatment with Apremilast. Int J Dermatol. 2020;59(4):e102–e103. doi: 10.1111/ijd.14598. [DOI] [PubMed] [Google Scholar]
- 41.Melis D, Mugheddu C, Sanna S, Atzori L, Rongioletti F. Clinical efficacy, speed of improvement and safety of apremilast for the treatment of adult Psoriasis during COVID-19 pandemic. Dermatol Ther. 2020;33(4):e13722. doi: 10.1111/dth.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrone D, Afridi F, King-Morris K, Komarla A, Kar P. Proximal Renal Tubular Acidosis (Fanconi Syndrome) Induced by Apremilast: A Case Report. Am J Kidney Dis. 2017;70(5):729–731. doi: 10.1053/j.ajkd.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Carpentieri A, Frisario R, Loconsole F. 18264 Malignancy and psoriasis treatment with apremilast: Retrospective chart review. J Am Acad Dermatol. 2020;83:AB96. [Google Scholar]
- 44.Peitsch WK. Apremilast in a patient with psoriasis and mantle cell lymphoma under maintenance treatment with rituximab. J Dtsch Dermatol Ges. 2019;17(3):330–332. doi: 10.1111/ddg.13771. [DOI] [PubMed] [Google Scholar]
- 45.Takama H, Shibata T, Ando Y, et al. Pembrolizumab-induced psoriasis vulgaris successfully treated with apremilast. Eur J Dermatol. 2020;30(2):188–190. doi: 10.1684/ejd.2020.3723. [DOI] [PubMed] [Google Scholar]
- 46.Foti C, Tucci M, Stingeni L, et al. Successful treatment with apremilast of severe psoriasis exacerbation during nivolumab therapy for metastatic melanoma. Dermatol Ther. 2021;34(1):e14653. doi: 10.1111/dth.14653. [DOI] [PubMed] [Google Scholar]
- 47.Di Lernia V, Casanova DM, Ricci C. Apremilast in patients with history of malignancy: a real-life, single-center experience. Int J Dermatol. 2021;60(1):e22–e24. doi: 10.1111/ijd.15093. [DOI] [PubMed] [Google Scholar]
- 48.Aragon-Miguel R, Calleja-Algarra A, Calleja-Algarra B, et al. Apremilast in plaque psoriasis: A retrospective, real-life study up to 12-month observation. J Am Acad Dermatol. 2019;81(4):AB21. doi: 10.1016/j.jaad.2019.06.115. [DOI] [Google Scholar]
- 49.Tampouratzi E, Vrakas S, Koutoufaris G, Xourgias V, Katsantonis J. Successful treatment with apremilast psoriatic patient with underlying chronic hepatitis B. 24th World Congress of Dermatology Milan, Abstracts Book; 2019. [Google Scholar]
- 50.Papadavid E, Rompoti N, Theodoropoulos K, Kokkalis G, Rigopoulos D. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32(7):1173–1179. doi: 10.1111/jdv.14832. [DOI] [PubMed] [Google Scholar]
- 51.Sahuquillo-Torralba A, de Unamuno Bustos B, Rodríguez Serna M, Monte Boquet E, Botella Estrada R. Treatment Persistence and Safety of Apremilast in Psoriasis: Experience With 30 Patients in Routine Clinical Practice. Actas Dermosifiliogr (Engl Ed) 2020;111(5):415–418. doi: 10.1016/j.ad.2018.10.031. English, Spanish. [DOI] [PubMed] [Google Scholar]
- 52.Siciliano MA, Dastoli S, d’Apolito M, et al. Pembrolizumab-Induced Psoriasis in Metastatic Melanoma: Activity and Safety of Apremilast, a Case Report. Front Oncol. 2020;10:579445. doi: 10.3389/fonc.2020.579445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanna C, Cesaroni GM, Mazzilli S, et al. Apremilast as a target therapy for nail psoriasis: a real-life observational study proving its efficacy in restoring the nail unit. J Dermatolog Treat. 2022;33(2):1097–1101. doi: 10.1080/09546634.2020.1801976. [DOI] [PubMed] [Google Scholar]
- 54.Lanna C, Cesaroni GM, Mazzilli S, Bianchi L, Campione E. Small Molecules, Big Promises: Improvement of Psoriasis Severity and Glucidic Markers with Apremilast: A Case Report. Diabetes Metab Syndr Obes. 2019;12:2685–2688. doi: 10.2147/DMSO.S229549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balato A, Campione E, Cirillo T, et al. Long-term efficacy and safety of apremilast in psoriatic arthritis: Focus on skin manifestations and special populations. Dermatol Ther. 2020;33(3):e13440. doi: 10.1111/dth.13440. [DOI] [PubMed] [Google Scholar]
- 56.Ighani A, Georgakopoulos JR, Walsh S, Shear NH, Yeung J. A comparison of apremilast monotherapy and combination therapy for plaque psoriasis in clinical practice: A Canadian multicenter retrospective study. J Am Acad Dermatol. 2018;78(3):623–626. doi: 10.1016/j.jaad.2017.09.060. [DOI] [PubMed] [Google Scholar]
- 57.Ighani A, Georgakopoulos JR, Shear NH, Walsh S, Yeung J. Short-term reasons for withdrawal and adverse events associated with apremilast therapy for psoriasis in real-world practice compared with in clinical trials: A multicenter retrospective study. J Am Acad Dermatol. 2018;78(4):801–803. doi: 10.1016/j.jaad.2017.09.067. [DOI] [PubMed] [Google Scholar]
- 58.Ighani A, Georgakopoulos JR, Shear NH, Walsh S, Yeung J. Maintenance of therapeutic response after 1 year of apremilast combination therapy compared with monotherapy for the treatment of plaque psoriasis: A multicenter, retrospective study. J Am Acad Dermatol. 2018;79(5):953–956. doi: 10.1016/j.jaad.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 59.Phan C, Beneton N, Delaunay J, et al. Real-World Effectiveness and Safety of Apremilast in Older Patients with Psoriasis. Drugs Aging. 2020;37(9):657–663. doi: 10.1007/s40266-020-00781-y. [DOI] [PubMed] [Google Scholar]
- 60.Ighani A, Georgakopoulos JR, Zhou LL, Walsh S, Shear N, Yeung J. Efficacy and Safety of Apremilast Monotherapy for Moderate to Severe Psoriasis: Retrospective Study. J Cutan Med Surg. 2018;22(3):290–296. doi: 10.1177/1203475418755982. [DOI] [PubMed] [Google Scholar]
- 61.Megna M, Fabbrocini G, Camela E, Cinelli E. Apremilast efficacy and safety in elderly psoriasis patients over a 48-week period. J Eur Acad Dermatol Venereol. 2020;34(11):e705–e707. doi: 10.1111/jdv.16443. [DOI] [PubMed] [Google Scholar]
- 62.del Alcázar E, Suárez-Pérez JA, Armesto S, et al. Real-world effectiveness and safety of apremilast in psoriasis at 52 weeks: a retrospective, observational, multicentre study by the Spanish Psoriasis Group. J Eur Acad Dermatol Venereol. 2020;34(12):2821–2829. doi: 10.1111/jdv.16439. [DOI] [PubMed] [Google Scholar]
- 63.Fremlin G, Bedlow A. Real-world experience of apremilast. (2017), Bristol Cup Posters. Br J Dermatol. 177:25–77. doi: 10.1111/bjd.15426. [DOI] [Google Scholar]
- 64.Foulkes A, Nemazee L, Griffiths C, Warren R. Apremilast, an oral phosphodiesterase 4 inhibitor, in a tertiary-referral psoriasis service. (2017), Bristol Cup Posters. Br J Dermatol. 177:25–77. doi: 10.1111/bjd.15426. [DOI] [Google Scholar]
- 65.Malara G, Micelli GF, Arceri F. Is apremilast a promising treatment for psoriasis and psoriatic arthritis? J Am Acad Dermatol. 2018;79:AB171. doi: 10.1016/J.JAAD.2018.05.699. [DOI] [Google Scholar]
- 66.Kungurov NV, Zilberberg NV, Kokhan MM, Keniksfest JV, Grishaeva EV. Experience in the treatment of psoriasis patients using Apremilast, a selective signalling pathway inhibitor. Vestn Dermatol Venerol. 2019;94:67–76. [Google Scholar]
- 67.Aragon-Miguel R, Calleja-Algarra A, Andres-Lencina J, et al. Generalized and palmoplantar pustular psoriasis and acrodermatitis continua of Hallopeau: a case series of 8 patients treated with apremilast. 28th EADV Congress; 2019; p. P1758. [Google Scholar]
- 68.Bulic S, Dediol I. Apremilast in psoriasis patients with a history of malignancy - case series. 28th EADV Congress; 2019; p. P1682. [Google Scholar]
- 69.Fattore D, Annunziata MC, Panariello L, Marasca C, Fabbrocini G. Successful treatment of psoriasis induced by immune checkpoint inhibitors with apremilast. Eur J Cancer. 2019;110:107–109. doi: 10.1016/j.ejca.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Magdaleno J, Valenzuela C, Ortiz-Salvado J, et al. Experience with Apremilast for the treatment of psoriasis in real-world clinical practice. 28th EADV Congress; 2019; p. P1814. [Google Scholar]
- 71.Magdaleno-Tapial J, Valenzuela-Oñate C, Hernández-Bel P. Tratamiento efectivo de la psoriasis ungueal con apremilast. Piel Form Contin en dermatología. 2019;34:74–76. doi: 10.1016/J.PIEL.2018.05.006. [DOI] [Google Scholar]
- 72.Gioe OA, Savoie C, Grieshaber EB, Hilton DC. Treatment of erythrodermic psoriasis with apremilast. JAAD Case Rep. 2021;11:36–37. doi: 10.1016/j.jdcr.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mayor Ibarguren A, Enrique EA, Diana PL, Ana C, Pedro HP. Apremilast for immune checkpoint inhibitor-induced psoriasis: A case series. JAAD Case Rep. 2021;11:84–89. doi: 10.1016/j.jdcr.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurata M, Ohyama M. Long-term remission of severe nail psoriasis after discontinuation of apremilast in a colorectal cancer survivor. J Dermatol. 2021;48(6):e248–e249. doi: 10.1111/1346-8138.15831. [DOI] [PubMed] [Google Scholar]


