Abstract
Yersinia pestis is the causative agent of bubonic plague and possesses a set of plasmid-encoded, secretable virulence proteins termed LcrV and Yops which are essential for survival in mammalian hosts. Yops and LcrV are secreted by a type III mechanism (Ysc), and Yops are unidirectionally targeted into the cytosol of associated eukaryotic cells in a tissue culture infection model. LcrV is required for Yops targeting, and recent findings have revealed that it can localize to the bacterial surface; however, its fate in this infection model has not been investigated in detail. In this study, we compared the localization of LcrV to that of the targeted proteins YopE and YopM by immunoblot analysis of fractions of Yersinia-infected HeLa cultures or by laser-scanning confocal microscopy of infected monolayers. Both LcrV and YopE were secreted by contact-activated, extracellularly localized yersiniae and were targeted to the HeLa cell cytosol. Although a significant amount of LcrV partitioned to the culture medium (unlike YopE), this extracellular pool of LcrV was not the source of the LcrV that entered HeLa cells. Unlike targeting of YopE and YopM, targeting of LcrV occurred in the absence of a functional Ysc apparatus and other virulence plasmid (pCD1)-expressed proteins. However, the Ysc is necessary for LcrV to be released into the medium, and our recent work has shown that localization of LcrV on the bacterial surface requires the Ysc. These results indicate that two mechanisms exist for the secretion of LcrV by Y. pestis, both of which are activated by contact with eukaryotic cells. LcrV secreted by the Ysc reaches the bacterial surface and the surrounding medium, whereas the second is a novel, Ysc-independent pathway which results in localization of LcrV in the cytosol of infected cells but not the surrounding medium.
The genus Yersinia contains three medically significant species: Y. pestis, the etiologic agent of bubonic plague; and the enteropathogenic species Y. pseudotuberculosis and Y. enterocolitica. The ability of these facultative intracellular, gram-negative pathogens to establish an infection in a mammalian host requires a set of antihost proteins termed LcrV and Yops (6, 40) which are encoded on a common ca. 70-kb virulence plasmid termed pCD1 in Y. pestis (18, 42). LcrV and Yops are secreted by a Yop secretion (Ysc) type III mechanism and function during an infection to prevent or evade a host innate immune response (7). Delivery of these proteins to host targets in vivo is most likely tightly regulated, since strains that deploy excess Yops (17, 26) or LcrV (60) are attenuated for virulence in a mouse model. Therefore, Yersinia have evolved an elaborate mechanism to couple secretion to regulation of expression of LcrV and Yops.
Expression of LcrV and Yops is thermally induced by the transcriptional activator LcrF (24). In vitro, in media containing sodium and glutamate, maximal thermal induction is prevented by cultivation of Yersinia in the presence of millimolar concentrations of Ca2+ (12, 65). Under these conditions, LcrV and Yops are minimally expressed and their secretion is blocked. Growth at 37°C in a Ca2+-deficient medium results in secretion of maximally induced LcrV and Yops (29, 67, 73). This in vitro, Ca2+-absence signal has been proposed to mimic an in vivo signal manifested by intimate association with eukaryotic cells (45, 53, 66). Y. pestis lacks several adhesin proteins present in the enteropathogenic Yersinia, and it is not known what promotes binding of Y. pestis to the surface of infected cells (63). Contact induction results in expression and secretion of LcrV and Yops, even when Ca2+ is present in the growth medium. According to a currently accepted model, Ca2+ in the absence of contact prevents thermal induction by stabilizing a closed conformation of Ysc channels. Blockage of channels mediated by outer-surface-bound LcrE (also called YopN [11]) and TyeA (20) and cytoplasmic LcrG (38) causes accumulation of LcrQ and YopD, two proteins involved in negative feedback control of LcrF-mediated induction (45, 72). The LcrE-, TyeA-, and LcrG-mediated block is displaced during cultivation in the absence of Ca2+ or upon contact with eukaryotic cells, resulting in secretion of LcrQ and YopD and dissipation of negative feedback control.
At least six effector Yops (YopE, YopH, YopJ, YopM, YpkA, and YopT) are delivered to the cytoplasm of infected eukaryotic cells, where they function to disrupt cellular processes (reviewed in reference 6). Delivery entails secretion of Yops across both membranes of extracellularly localized (51, 62), cell-associated (44) Yersinia and subsequent translocation or targeting across the eukaryotic plasma membrane. Secretion from Yersinia is mediated by a Ysc secretion system composed of at least 22 gene products (reviewed in references 6 and 40), including the inner membrane protein YscV (formerly termed LcrD [46, 47]) and the outer membrane secretin YscC (21).
The translocator Yops (YopB and YopD) and LcrV, all of which are encoded within the polycistronic lcrGVH-yopBD operon (2, 33, 50), are indispensable for the targeting of Yops (16, 37, 44, 53, 57) and may form a structure which mediates transfer of Yops from bacteria to an associated cell (5, 9). Although a Yersinia Yops targeting apparatus has not yet been characterized, Cornelis (5) speculated that it may resemble the needle-like structure discovered in Salmonella typhimurium (22). Purified preparations of YopB have membrane-disruptive activity, and YopB expressed by Yersinia is hemolytic for sheep erythrocytes (16), indicating that YopB probably forms part of a translocation pore in the eukaryotic cell plasma membrane. The size of this pore is modulated by the accessory protein YopK (17). YopD has also been suggested to be a pore component, based on the presence of hydrophobic domains in the peptide sequence (36), but is not essential for the hemolytic activity of YopB (16). Although LcrV can interact with YopB and YopD (57), there is no direct evidence that LcrV is a pore component. Instead, findings described by Nilles et al. (37) and Sarker et al. (57) suggest that LcrV is required for assembly of the YopB-containing pore.
Yop secretion into eukaryotic cells is thought to occur only through Ysc channels that make direct contact with the plasma membrane. Hence, in tissue culture infection assays, secretion is unidirectional or polarized, and effector Yops are targeted directly to eukaryotic cells and not released into the medium in significant amounts. Targeting of Yops has been demonstrated indirectly by assaying enzyme activity of Yop-Cya reporter fusions (62) or by observing cytotoxicity manifested as rounding up of the eukaryotic cells (7). Direct assays for targeting have used confocal microscopy (3, 53, 58) and immunoblot analysis of fractionated cultures (25, 37).
One primary function of intracellular Yops is to impair the phagocytic capabilities of host cells, including professional phagocytes such as macrophages and neutrophils (56, 70). YopE (51, 52) and YopT (19) function independently to disrupt actin microfilaments, whereas YopH dephosphorylates structural components of focal adhesions (1, 43). YopJ interferes with host cell signal cascades, with resulting inhibition of tumor necrosis factor alpha expression (39, 54, 55), and induces apoptosis in infected macrophages (31, 32). Specific intracellular functions of YpkA and YopM have not yet been determined, but they localize to the inner face of the eukaryotic plasma membrane (15) and nucleus (58), respectively.
LcrV is essential to virulence of Yersinia (49) and, unlike the effector YopS, functions in multiple ways during infection. Within yersiniae, LcrV indirectly induces Yops expression and secretion by inactivating the inner-membrane, LcrG-mediated Ysc block (37, 38). LcrV is also essential for targeting of secreted Yops (37, 57), and LcrV located on the extracellular surface of Yersinia may act as a pilot for assembly of a targeting apparatus or may function as part of a Yop targeting complex (9). Once released from Yersinia, LcrV may function as a diffusible modulator of host immune responses. An LcrV-containing fusion protein was able to prevent production of the proinflammatory cytokines tumor necrosis factor alpha and gamma interferon (34). Preparations containing LcrV have also been shown to enhance production of the T helper type 2-inducing cytokine interleukin-10 (35) and inhibit chemotaxis of neutrophils (70). In previous reports, we had noted that LcrV, like Yops, becomes associated with eukaryotic cells (37). Although a tissue culture infection model has been used to characterize localization and function of Yops, partitioning of LcrV among compartments in that model has not previously been studied. In this study we investigated LcrV localization and found that LcrV partitioned differently from the vectorially targeted protein YopE. Unlike Yops, significant levels of LcrV were detected in the medium fraction. We also demonstrate that the HeLa cell-associated LcrV first reported by Nilles et al. (37) corresponded to LcrV localized to the HeLa cell cytoplasm, and LcrV did not require YopB or YopD to attain this intracellular localization. Instead, LcrV enters eukaryotic cells by a novel, virulence plasmid-independent pathway.
MATERIALS AND METHODS
Growth conditions, bacterial strains, and eukaryotic cell lines.
Strains and plasmids used in this study are listed in Table 1. Unless otherwise noted, Escherichia coli DH5α was grown in Luria-Bertani (LB) (30) medium or on LB agar at 37°C. Y. pestis strains were cultivated in heart infusion broth (Difco Laboratories, Detroit, Mich.) at 26°C prior to infection of eukaryotic cells. Y. pestis was also cultivated at 26°C on tryptose blood agar (Difco) during construction of the Y. pestis lcrE strain KIM8-3233.1 and on tryptose blood agar supplemented as described previously (28), with modifications by Nilles et al. (37), during construction of the yopB strain KIM8-3002.1. In an experiment not shown, LcrV, HT-VN68, and YopM were expressed from plasmids and tested for their secretion from pCD1− Y. pestis KIM8 growing in the defined medium TMH (64) at 37°C without added Ca2+. When appropriate, bacteria were grown in the presence of antibiotics used at 15 μg/ml for tetracycline, 50 μg/ml for kanamycin, or 100 μg/ml for ampicillin and streptomycin. The HeLa human epithelial cell line was used in this study and was maintained at 37°C with 5% CO2 in RPMI 1640 medium (RPMI; GIBCO-BRL, Grand Island, N.Y.) supplemented with 10% (vol/vol) fetal bovine serum (FBS; GIBCO-BRL). For experiments examining partitioning of LcrV and Yops within infected eukaryotic cells, RPMI supplemented with FBS was replaced with RPMI lacking FBS.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Properties | Source or reference |
|---|---|---|
| E. coli K-12 | ||
| DH5α | F− f80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− rM+) phoA supE44 λ− thi-1 gyrA96 relA1 | GIBCO-BRL |
| DH5α λpir | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− rM+) phoA supE44 thi-1 gyrA96 relA1 λpir | Laboratory stock |
| Y. pestisa | ||
| KIM6 | pPCP1 (40), pMT1 (40) | R. R. Brubaker |
| KIM10 | pMT1 | Laboratory stock |
| KIM8-3241 | pCD1 (lcrV [LcrVΔ18–215]byopJ::MudI1734 [Kmr Lac+]),c pMT1, pOS50 | 9 |
| KIM8-3002 | Smr; pCD1 (Lcr+), pMT1 | 37 |
| KIM8-3002.1 | Smr; pCD1 (yopB [YopBΔ8-390), pMT1 | This study |
| KIM8-3002.2 | Smr; pCD1 (yopD [YopDΔ1–305]), pMT1 | 72 |
| KIM8-3233 | Smr; pCD1 (yopM::lacZYA), pMT1 | 58 |
| KIM8-3233.1 | Smr; pCD1 (yopM::lacZYA lcrE [LcrEΔ48–197]), pMT1 | This study |
| KIM5-3001.2.1 | Smr; pCD1 (yscV [YscVΔ192–343], yopD [YopDΔ1–305]), pPCP1, pMT1 | 72 |
| Plasmids and constructs | ||
| pLD55 | Apr Tcr; suicide vector for allelic exchange | 28 |
| pΔyopB | PCR-amplified DNA from pAW161 containing ΔyopB (deleting nucleotides 23–1169) in pLD55 | This study |
| pUK4134.6 | PstI deletion of lcrE cloned into pUK4134 | 48 |
| pHT-V | PCR-amplified lcrV cloned in pPRoEX-1 in-frame with a 23-residue leader sequence encoding His6 (lcrV) | 9 |
| pHT-VN68 | Subcloned lcrV in pPRoEX-1 with a 29-residue, His6-containing leader sequence fused at the EcoRV site in lcrV (′lcrV [LcrVΔ1–67]) | 9 |
| pTrcV | BbvI/NcoI fragment of pES6–1 cloned into SmaI-cut pTrc99A (lcrV) | 9 |
| TrcM.2 | Subcloned yopM in pTrc99A (yopM) | 48 |
| pAW161 | PCR-amplified lcrH and yopD cloned into pBAD33 (14) expression vector (lcrH, ′yopB [YopBΔ8–390], yopD) | 72 |
| pOS50 | Tcr; competitive plasmid for pPCP1 | J. Goguen |
All strains are Pgm− (69).
Numbers in brackets list residues deleted from gene product.
Descriptions in parentheses give relevant genes present on construct.
DNA methods and strain construction.
Plasmid DNA was purified by midi or spin-prep columns (Qiagen, Inc., Studio City, Calif.), and cloning was performed essentially as described elsewhere (27). Selected DNA was amplified by PCR in a Perkin-Elmer Cetus (Foster City, Calif.) GeneAmp model 2400 thermocycler using Pfu DNA polymerase (Stratagene, La Jolla, Calif.) and oligonucleotide primers synthesized by Genosys Biotechnologies (The Woodlands, Tex.). Typical PCR conditions included a 10-min preincubation at 94°C followed by 30 amplification cycles. Denaturation, annealing, and extension reactions were carried out for 30 s each at 94, 55, and 72°C, respectively. PCR fragments were resolved on agarose gels and extracted by using a Qiaquick gel extraction kit (Qiagen). E. coli and Y. pestis were transformed by the CaCl2 method (27) and by electroporation (41), respectively. pΔyopB was generated by PCR amplification of Y. pestis DNA from pAW161, using synthetic oligonucleotides lcrH1SDA (5′ GCTAGAGCTCAGGAGGAACATATGCAACAAGAGACGACAGAC 3′) and yopD921B (5′ GCTCTAGATCAGACAACACCAAAAGCGGC 3′) constructed by Williams and Straley (72). The PCR product contained the coding sequence for LcrH (also called SycD), the first 7 and last 11 residues of YopB, and YopD. The purified ca. 1.6-kb PCR fragment and SmaI-digested pLD55 were ligated by using T4 DNA ligase (Promega, Madison, Wis.) and used to transform E. coli DH5αλpir. pΔyopB was used to create Y. pestis KIM8-3002.1 by allelic exchange of pCD1-encoded yopB as described previously (37), and gene replacement was confirmed by PCR. The resulting strain was confirmed to have normal expression and secretion of YopD, which is encoded by the gene immediately downstream of yopB in the lcrGVH-yopBD operon (data not shown). pUK4134.6, carrying an in-frame deletion within lcrE (codons 48 to 197), was used to replace the pCD1-encoded lcrE by allelic exchange in the yopM insertion mutant KIM8-3233. Selection for homologous recombination and subsequent gene replacement were done as described elsewhere (59). Digestion of pCD1 with BamHI was used to verify the correct deletion.
Infection assays.
Protein localization during infection of HeLa cell monolayers was assayed essentially as described previously by immunoblotting (9, 37) or laser-scanning confocal microscopy (58). Briefly, HeLa cells were subcultured either into six-well 35-mm-diameter tissue culture plates or on 12-mm-diameter glass coverslips in 24-well cluster dishes (Costar, Cambridge, Mass.) in RPMI with 10% (vol/vol) FBS. Monolayers were incubated at 37°C in 5% CO2 for roughly 72 h in 6-well dishes to a density of 5 × 105 to 8 × 105 per well for fractionation or 48 h in 24-well dishes to a density of ca. 105 for confocal analysis. Y. pestis strains were cultivated at 26°C in heart infusion broth, harvested at an optical density at 620 nm of ca. 1.0, and diluted directly into 37°C RPMI lacking FBS. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to 0.1 mM for treatments with Y. pestis strains harboring constructs with inducible promoters. Some wells were treated with 5 μg of cytochalasin D (Sigma) per ml 30 min before infection and then throughout the infection period (58). This treatment has been shown to have no effect on targeting of YopM or YopE by Y. pestis (58) or on viability of the bacteria for at least 1 h (8). It has been confirmed to abolish invasion (to below detection) of HeLa cells (8). Immediately before infection, HeLa cells were washed twice with RPMI lacking FBS, and bacteria were added to wells at a multiplicity of infection (MOI) of 10. Plates were then centrifuged at 200 × g for 5 min to achieve contact between bacteria and target cells and incubated at 37°C in 5% CO2 for 4 h. Gentamicin (GIBCO-BRL) was added to wells to 7.5 μg/ml 30 min postinfection for experiments in which extracellular bacteria were killed. After infection, cultures were either fractionated for immunoblot analysis or fixed and stained for microscopic analysis. For culture fractionation, one replicate per infecting strain was treated for 5 min at 37°C in 5% CO2 with 100 μg of trypsin (Sigma) per ml. The trypsin treatment was terminated by addition of a protease inhibitor cocktail (Pefabloc, leupeptin, and aprotinin; all from Boehringer Mannheim Corp., Indianapolis, Ind.) added to each well such that each inhibitor was present at 20 μg/ml. Media were then removed from wells and passed through 0.2-μm-pore-size filters into tubes on ice, and proteins were precipitated by treatment with 10% (vol/vol) (final concentration) trichloroacetic acid (TCA) on ice for 2 h to overnight. Infected monolayers were washed twice with phosphate-buffered saline (PBS; 135 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4 [pH 7.4]) and lysed by treatment as previously described with ice-cold H2O containing protease inhibitors at 2 μg/ml each (58) or by disruption in RPMI with a Kontes homogenizer (B pestle, 100 strokes). Lysed cell fractions were then centrifuged at 4°C at 20,800 × g for 15 min. The liquid supernatant, corresponding to the eukaryotic cell cytoplasmic and nuclear soluble fraction, was removed, and proteins were precipitated by the addition of TCA to 10% (vol/vol). The 20,800 × g debris and precipitated proteins from media and cellular soluble fractions were dissolved in polyacrylamide gel electrophoresis (PAGE) sample buffer containing 2.3% (wt/vol) sodium dodecyl sulfate (SDS), 5% (vol/vol) β-mercaptoethanol, 60 mM Tris (pH 6.8), and 25% (vol/vol) glycerol.
To test for the extent of induction of Yops expression when the parent Pla− Y. pestis KIM8-3002 infects HeLa cells (contact induction), HeLa cells were infected as described above except that the MOI was 5. Additional wells containing RPMI but no HeLa cells received the same number of bacteria. After 2 h incubation at 37°C in 5% CO2, the entire contents of each well was harvested and the proteins were precipitated with TCA. Twofold serial dilutions of samples representing equal fractions of the original well contents were subjected to SDS-PAGE and analyzed by immunoblotting. LcrV and YopE were assayed by specific antibodies, and the loading of wells with Yersinia was compared by probing lanes with an anti-Yersinia antibody.
For confocal analysis, media were removed, and infected monolayers were washed with 1.0-ml volumes of Hanks’ balanced salt solution (GIBCO-BRL). Samples were then treated sequentially at room temperature with 2% (wt/vol) paraformaldehyde pH 7.4 in PBS for 30 min to fix proteins, with buffer containing 0.5% Triton X-100 for 20 min (52) to permeabilize membranes, and with PBS supplemented with 10% (vol/vol) FBS and 1% (vol/vol) mouse serum (Sigma) for 60 min to block nonspecific antigenic sites. Where appropriate, LcrV and YopM were detected by probing with LcrV- or YopM-specific polyclonal rabbit antibodies followed by incubation with Oregon green-coupled anti-rabbit immunoglobulin G (IgG; Molecular Probes, Eugene, Oreg.) as described elsewhere (58). Coverslips were mounted using SlowFade mounting medium (Molecular Probes) and analyzed by laser-scanning confocal microscopy using a Leica TSC NT confocal system (Ar-Kr laser) and a 63× objective. Differential interference contrast (DIC) images were generated by a Leica DM IRB/E inverted microscope with Nomarski optics.
Protein purification.
A purified preparation of a His6-tagged LcrV (HT-V) was used to test the ability of exogenous LcrV to enter HeLa cells and the specificity of LcrV-specific antibodies. Purification was performed essentially as described elsewhere (10). Briefly, E. coli DH5α expressing pHT-V was cultivated at 37°C in LB to an optical density at 620 nm of ca. 1.0. IPTG was added to 0.1 mM, and the culture was incubated for an additional 3 h. Bacterial lysates were generated by French press as described previously (10), and HT-V was purified by passage of clarified lysates over a Talon (Clontech Laboratories, Inc., Palo Alto, Calif.) metal affinity resin according to the manufacturer’s instructions. Pooled samples containing pure HT-V were dialyzed into PBS, and protein concentration was quantitated by the bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.).
Protein electrophoresis and immunoblot analysis.
Proteins from fractionated, infected HeLa cultures were resolved in 12% (wt/vol) polyacrylamide gels by SDS-PAGE (23). Samples were loaded so that lanes represented equivalent volume fractions of the original cultures. Once resolved, proteins were transferred to Immobilon-P (Millipore, Corp., Bedford, Mass.) in Tris-glycine buffer (25 mM Tris, 192 mM glycine [pH 8.3] containing 10% [vol/vol] methanol) as described previously (67). Specific proteins were detected by using polyclonal rabbit antibodies specific for His-tagged LcrV (α-HTV) or His-tagged YopE (α-YopE; gift of G. Plano, University of Miami). In one experiment, the loading of wells with Yersinia was compared by using a polyclonal rabbit antibody raised against Y. pestis KIM6 whole cells grown at 26°C (anti-Yersinia). Proteins were visualized by treatment of immunoblots with alkaline phosphatase- or horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma), followed by development with nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP; GIBCO-BRL) or enhanced chemiluminescence (ECL) substrate (Pierce), respectively.
RESULTS
Contact-activated entry of LcrV into infected HeLa cells.
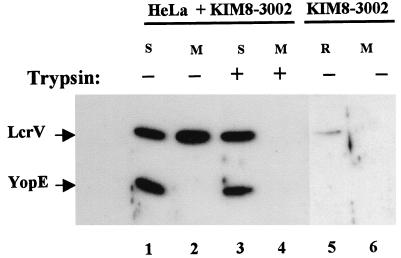
A tissue culture infection model has been used to characterize the localization and function of certain Yop proteins once they are secreted by yersiniae. We have hypothesized that LcrV can function in intimate association with infected eukaryotic cells to help mediate transfer of Yops into the cell cytoplasm. The goal of this study was to investigate the localization of LcrV in this model system. We had previously found that unlike the vectorially targeted Yops, LcrV is secreted into the medium and also becomes cell-associated in a tissue culture infection model (37). The cell-associated LcrV was intriguing, since LcrV is required for the targeting of Yops and may function at the interface between a bacterium and associated eukaryotic cell. Furthermore, YopD, an additional protein involved in Yops targeting, may itself be targeted (13), raising the possibility that LcrV localized in a translocation apparatus can also gain access to the eukaryotic cell cytoplasm. We therefore tested whether LcrV could enter infected cells. HeLa cell monolayers were infected with our Pla− parent Y. pestis KIM8-3002 for 4 h, and secreted proteins in one replicate culture were tested for accessibility to proteolysis by exogenously added trypsin as an indicator of extracellular localization. Cultures were then fractionated into cell-free medium (containing proteins secreted into the culture medium), cellular soluble (representing HeLa cell cytoplasm and released nuclear proteins), and debris (containing yersinia bound to cell membrane) fractions. We then compared the localization of LcrV to that of the vectorially targeted YopE by immunoblot analysis (Fig. 1). As previously noted (37), LcrV, but not YopE, was released into the culture medium during infection (lane 2) and was completely susceptible to proteolysis by trypsin (lane 4). LcrV, like YopE, was present in HeLa soluble fractions (lanes 1 and 3) and was protected from digestion by trypsin (lane 3), suggesting an intracellular localization for LcrV. Interestingly, quantities of cell-associated LcrV were significant when compared to levels secreted into the medium (lane 2). A similar distribution of LcrV was detected after infection of the macrophage-like cell line J774 (data not shown). The cell-associated LcrV did not arise from yersiniae internalized by the HeLa cells, because separate gentamicin protection studies indicate that less than 1% of Y. pestis KIM8-3002 enter HeLa cells in a 4-h infection (8). An alternative possibility for cell-associated LcrV was that intracellular LcrV was an artifact of the fractionation protocol. We have shown that LcrV can localize in deposits on the extracellular surface of Y. pestis (9), raising the possibility that the cold water lysis treatment had dissociated surface LcrV, releasing it into the HeLa cytoplasmic fraction. To test this possibility, we incubated equivalent numbers of Y. pestis KIM8-3002 in the absence of HeLa cells in a parallel assay. The bacteria were then subjected to the cold water treatment used to lyse eukaryotic cells, and the cell-free medium (lane 6) and water-released fraction (lane 5) were analyzed by immunoblotting with LcrV- and YopE-specific antibodies. As with YopE, LcrV was not secreted by yersiniae in the absence of HeLa cell contact (lane 6). No YopE and only a small amount of LcrV were released by the water treatment (lane 5), indicating that the strong LcrV-specific signal detected in lanes 1 and 3 was not derived from release of surface LcrV into the cytoplasmic fraction.
FIG. 1.
LcrV partitions to the cell-free medium and HeLa soluble fractions. Duplicate wells containing HeLa monolayers were infected with Y. pestis KIM8-3002 at an MOI of 10 (lanes 1 to 4). As a control, an equal dose of Y. pestis KIM8-3002 was also added to a well lacking HeLa cells (lanes 5 and 6). After incubation at 37°C-5% CO2 for 4 h, trypsin was added to 100 μg/ml to one replicate of infected HeLa cells. Yersinia-infected HeLa cultures were fractionated, and the cell-free medium (M) and water-lysate soluble (S) fractions were further analyzed. Cell-free medium (M; lane 6) from the Yersinia-only well corresponded to the culture supernatant after removal of bacteria by centrifugation. The bacteria were then treated with cold water and pelleted, and the resulting supernatant corresponding to the water-released (R; lane 5) fraction was removed. Samples from each fraction representing 0.3% of the original culture were resolved in a 12% polyacrylamide gel. LcrV and YopE were detected by probing immunoblots with α-HTV and α-YopE followed by secondary antibodies conjugated to horseradish peroxidase and development with ECL reagent.
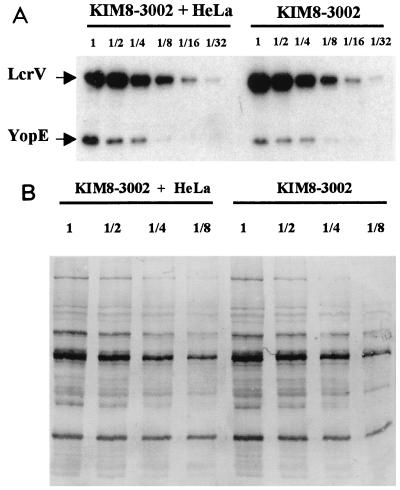
A concern was whether this control test of yersiniae in the absence of HeLa cells might have been misleading if yersiniae not in the presence of HeLa cells contained only basal amounts of LcrV and YopE. To address this question, we infected HeLa cells or allowed an equal number of Y. pestis KIM8-3002 bacteria to incubate in RPMI in a well lacking HeLa cells. After 2 h, the entire contents of each well was recovered, and the TCA-precipitated proteins were compared for their relative contents of LcrV and YopE by analyzing serial dilutions in an immunoblot (Fig. 2). Figure 2A shows that whether HeLa cells were present or not, LcrV was present in comparable amounts, and the same was true for YopE. A similar blot probed with a polyclonal anti-Yersinia antibody (Fig. 2B) showed that the samples from wells with and without HeLa cells had contained equal amounts of bacteria (this had also been verified for replicate wells by CFU determinations). These data show that incubation of Y. pestis in a cluster dish at 37°C-5% CO2 in RPMI is sufficient to induce LcrV and YopE expression and that contact with HeLa cells does not cause further induction, unlike the situation for Y. pseudotuberculosis (45). However, Y. pestis clearly is contact activated for Yops and LcrV secretion and targeting, as these proteins are not secreted without HeLa cells (Fig. 1).
FIG. 2.
Y. pestis is contact activated for secretion and targeting but not contact induced for expression of LcrV and YopE. HeLa cells were infected with Y. pestis KIM8-3002 for 2 h at an MOI of 5, or an equal number of yersiniae were incubated in RPMI without HeLa cells. The total proteins harvested from each well were recovered by TCA precipitation and analyzed in serial twofold dilutions by immunoblotting. In panel A, the blot was probed with a mixture of antibodies to LcrV and YopE; in panel B, a polyclonal anti-Yersinia antibody was used. Proteins were visualized by probing immunoblots with IgG coupled to horseradish peroxidase followed by ECL detection (A) or with alkaline phosphatase-coupled IgG followed by development with NBT-BCIP (B). Fold dilutions of the samples are given above the lanes.
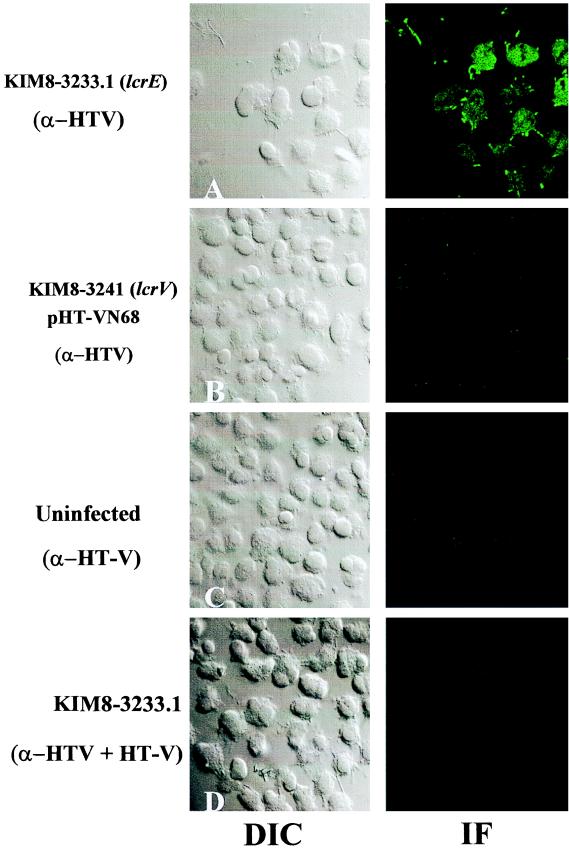
YopE has been demonstrated by multiple experimental techniques to localize to the cytoplasm of infected cells (62). The observation that LcrV, like YopE, was trypsin resistant in our assay suggested that LcrV also was cytoplasmic. We directly analyzed LcrV localization in infected HeLa cells by laser-scanning confocal microscopy to formally test this prediction (Fig. 3). Infection with our parent strain Y. pestis KIM8-3002 did not yield a signal detectable by this assay (data not shown). We therefore chose to test localization of LcrV expressed by an lcrE mutant of Y. pestis, a strain that has been shown to hypersecrete LcrV (60). We replaced wild-type lcrE in yopM Y. pestis KIM8-3233 by allelic exchange with a subcloned lcrE lacking codons 48 to 197 to create Y. pestis KIM8-3233.1. The yopM mutation was present for a control test needed later and was not expected to influence the targeting of LcrV, as there is considerable precedent for use of strains lacking even multiple Yops in targeting studies (e.g., references 15 and 19). HeLa cells were infected in the presence of cytochalasin D with the lcrE yopM mutant or the lcrV yopJ mutant KIM8-3241 expressing HT-VN68 (as a negative control). HT-VN68 is an N-terminally truncated, His-tagged version of LcrV which fails to be secreted by Y. pestis (9). LcrV-specific staining of uninfected HeLa cells or HeLa cells infected with Y. pestis expressing HT-VN68 did not yield a significant immunofluorescence signal (Fig. 3B and C). Significant staining, however, was detected in the cytoplasm of lcrE yopM Y. pestis-infected HeLa cells (Fig. 3A; compare immunofluorescence and DIC images). LcrV appeared to be localized throughout the cell cytoplasm, and examination of multiple optical planes (data not shown) revealed that intracellular LcrV may also be present in the cell nucleus. The detected signal was LcrV specific, since it could be competed by addition of excess, pure HT-V during staining of monolayers with α-HTV (Fig. 3D). These results confirm that LcrV does gain access to the cytoplasm of infected HeLa cells.
FIG. 3.
LcrV can localize to the cytoplasm of infected HeLa cells. HeLa cell monolayers cultivated on glass coverslips in the presence of cytochalasin D were uninfected (C), infected with Y. pestis KIM8-3233.1 (lcrE) (A), or infected with Y. pestis KIM8-3241 expressing HT-VN68 as a negative control (B) for 4 h at 37°C-5% CO2. Monolayers were then fixed, permeabilized, and stained with α-HTV. During staining of one replicate (D), 10 μg of pure HT-V (representing a fivefold molar excess over antigen-binding sites of α-HTV) was added during incubation with α-HTV as an antibody specificity control. LcrV was detected by secondary staining with Oregon green-conjugated anti-rabbit IgG followed by visualization by confocal laser-scanning microscopy. A 63× objective was used; immunofluorescence (IF) and DIC images are shown.
LcrV does not require components of the Yops targeting apparatus to enter HeLa cells.
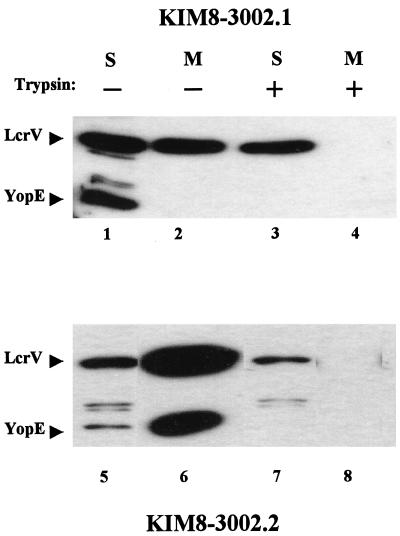
Although our results show that LcrV can enter HeLa cells, it was unclear whether LcrV entry occurred by the Yops targeting mechanism. Targeting of YopE and other Yops requires secretion through a Ysc channel followed by YopB- and YopD-dependent translocation across the eukaryotic plasma membrane (53, 54). Secretion of both LcrV and Yops is Ysc dependent (6, 40), and we predicted that LcrV may also require YopB and YopD to gain access to the HeLa cell cytoplasm. To test this hypothesis, we created Y. pestis KIM8-3002.1 (yopB) by allelic exchange of parent yopB with a subcloned in-frame yopB deletion carried on pΔyopB, which removed essentially all of the 401-residue YopB coding sequence (codons 8 to 390). Consistent with the properties of a nonpolar yopB deletion constructed by Håkansson et al. (16), our yopB mutation did not affect regulation of Yop expression or secretion and did not have a polar effect on the downstream gene for YopD (data not shown). We infected HeLa monolayers with yopB Y. pestis (lanes 1 to 4) or a previously constructed yopD (lanes 5 to 8) mutant KIM8-3002.2 (72) and assayed LcrV and YopE localization in fractionated cultures by immunoblot analysis (Fig. 4). In these experiments, infection with the yopD strain was carried out in the presence of cytochalasin D to prevent any internalization of the yersiniae which would be unable to target antiphagocytic Yops. This was important here, because yopD strains are constitutively strongly induced for Yops and LcrV expression (72), and a few intracellular yersiniae might give an artifactual signal for YopE in the HeLa soluble fraction. As expected, trypsin-inaccessible YopE was not detected in the soluble fractions of HeLa cells infected by yopB (lane 3) or yopD (lane 7) Y. pestis, consistent with the inability of these mutants to target Yops. Instead, YopE was detected in the culture medium (lane 6) or in soluble fractions (lanes 1 and 5) of nontrypsinized cultures. Interestingly, partitioning of LcrV in cultures was unchanged by either the yopB or yopD mutation in Y. pestis: LcrV was detected in the cell-free medium (lanes 2 and 6) as well as soluble fractions from both trypsinized (lanes 3 and 7) and nontrypsinized cultures (lanes 1 and 5). These results indicate that unlike YopE, LcrV does not require YopB or YopD to traverse the plasma membrane of infected HeLa cells.
FIG. 4.
Entry of LcrV into HeLa cells does not require the translocator Yops, YopB and YopD. HeLa cell monolayers were infected, in duplicate, for 4 h at 37°C-5% CO2 with Y. pestis KIM8-3002.1 (yopB) (lanes 1 to 4) or KIM8-3002.2 (yopD) (lanes 5 to 8) at an MOI of 10. Infection with yopD Y. pestis was done in the presence of cytochalasin D at 5 μg/ml. One replicate of each treatment was treated with trypsin at 100 μg/ml prior to harvest. Fractionation of cultures was done as previously described, and proteins in samples corresponding to 0.3% of original culture volume were resolved in 12% polyacrylamide gels. LcrV and YopE in cell-free medium (M) and soluble (S) HeLa cell water-lysate fractions were detected by immunoblotting with α-HTV and α-YopE. Proteins were visualized by probing with horseradish peroxidase-conjugated anti-rabbit IgG followed by development with ECL reagent.
Extracellular yersiniae directly target LcrV to the HeLa cytoplasm.
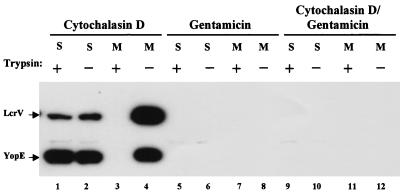
Yersinia can invade eukaryotic cells and survive even within phagolysosomes of professional phagocytes (65). YopE and other Yops, however, are targeted across the eukaryotic cell plasma membrane by extracellular, peripherally associated yersiniae and not by intracellular bacteria (44, 51, 62). Since LcrV did not seem to enter HeLa cells by the Yops targeting pathway, we began to investigate the route of LcrV entry by testing the localization of bacteria from which the intracellular pool of LcrV was derived. We infected HeLa cells with Y. pestis KIM8-3002 and either prevented bacterial internalization with cytochalasin D or killed extracellular bacteria with gentamicin to test the source of LcrV detected in these assays (Fig. 5). As expected, YopE was trypsin nonsusceptible (lane 1) and, therefore, targeted when extracellular localization of yersiniae was ensured by the addition of cytochalasin D. In the presence of cytochalasin D (lanes 1 to 4), LcrV was detected in the medium (lane 4), and trypsin-resistant LcrV was detected in the soluble (lane 1) fraction. Neither YopE nor LcrV was detected in samples from gentamicin-treated infections (lanes 5 to 8). As a control, extracellular bacteria were also killed with gentamicin after infection of cytochalasin-treated HeLa cells (lanes 9 to 12) to verify that the amount of cytochalasin D used was sufficient to prevent internalization of the bacteria. Under these conditions, no YopE or LcrV was detected, indicating that sufficient levels of cytochalasin D were used in this assay. Although these results do not rule out low-level secretion of LcrV by intracellular bacteria, these observations do indicate that the LcrV detected in these assays originates from extracellular yersiniae.
FIG. 5.
Extracellular Y. pestis targets LcrV. Prior to infection, HeLa monolayers were treated with 5 μg of cytochalasin D per ml for 30 min (lanes 1 to 4 and 9 to 12). These monolayers were then infected with Y. pestis KIM8-3002 in the presence of cytochalasin D. Some wells (lanes 5 to 12) received 7.5 μg of gentamicin per ml 30 min after infection to kill extracellular bacteria. After treatment of replicate wells with trypsin, cultures were harvested and fractionated, and samples of cell-free medium (M) and HeLa soluble (S) fractions corresponding to 0.3% of the original culture volume were resolved in 12% polyacrylamide gels. Immunoblots were probed with α-HTV and α-YopE, and LcrV and YopE were detected by incubating immunoblots with horseradish peroxidase-conjugated anti-rabbit IgG followed by development with ECL reagent.
Yersiniae are required to mediate entry of LcrV into HeLa cells.
The significant amount of YopE detected in the culture medium in Fig. 5 (lane 4) was not seen in repetitions of that experiment. Secretion of LcrV in this tissue culture infection model, however, consistently occurred; more than half of LcrV was released into the culture medium during infection. Given that LcrV-containing fusion proteins have an immunomodulatory effect in the absence of yersiniae, and since entry of LcrV into HeLa cells was YopB and YopD independent, we wondered whether this pool of LcrV in the medium was the source of intracellular LcrV. We have previously demonstrated that HT-V can enter HeLa cells when expressed in Y. pestis (9). We therefore tested whether HT-V added exogenously to subconfluent monolayers could enter HeLa cells (Fig. 6). Uninfected and Y. pestis KIM8-3002-infected HeLa monolayers were incubated for 4 h in the presence of 1 μg of HT-V (500 ng/ml) purified from E. coli. In the test with infected cultures, we wanted to determine whether the interaction between Yersinia and HeLa cells in some way caused or stimulated the internalization of exogenous LcrV. The HT-V migrated more slowly in SDS-PAGE than native, Yersinia-derived LcrV (because of the extra mass in the 23-amino-acid His6-containing leader) and could be distinguished from native LcrV in immunoblots. After incubation, cultures were fractionated and analyzed as before. We did not detect significant amounts of HT-V in HeLa soluble fractions when HT-V was added to uninfected monolayers. Instead, essentially all of the fusion protein was detected in the culture medium. Presence of HT-V during infection of monolayers did not appreciably alter this distribution. Although some HT-V was detected in the soluble fraction, it was susceptible to digestion by trypsin prior to cell lysis, indicating an extracellular localization. In contrast, native, Yersinia-derived LcrV in the soluble fraction was not susceptible to trypsin. According to these results, intracellular LcrV must be delivered directly by Yersinia and does not result from entry of LcrV that is free in the medium.
FIG. 6.
Delivery by Y. pestis is required for LcrV to gain access to the HeLa cell cytoplasm. Purified HT-V was added at 500 ng/ml to HeLa monolayers alone (left) or with Y. pestis KIM8-3002 (right), and the cultures were incubated for 4 h at 37°C-5% CO2. After trypsin treatment and fractionation of cultures, native LcrV and HT-V were detected in cell-free medium (M) and HeLa soluble (S) fractions by immunoblot analysis using α-HTV. Proteins were visualized using horseradish peroxidase-conjugated anti-rabbit IgG and development with ECL reagent.
A functional Ysc secretion apparatus is not required to mediate entry of LcrV into HeLa cells.
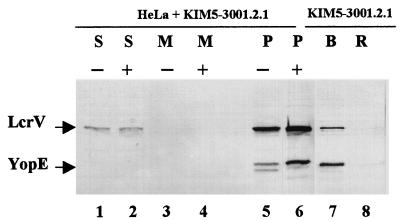
Our data suggested that LcrV may enter eukaryotic cells by an entirely novel pathway. It is well established that in vitro secretion of LcrV and Yops requires the Ysc apparatus, and we have recently shown that YscC is required for LcrV to become deposited on the surface of Y. pestis (9). We extended our analysis of the LcrV entry pathway by testing whether Y. pestis lacking an essential component of the Ysc apparatus could deliver LcrV to the cytoplasm of infected HeLa cells. We used Y. pestis KIM5-3001.2.1, which lacks both yscV and yopD. The absence of yopD served to prevent any downregulation of expression of LcrV and Yops that otherwise could result from the inactivation of the Ysc by the yscV mutation. (Recall that YopD is required to prevent maximal thermal induction of the LCR, and yopD strains express fully induced levels of both LcrV and Yops [72].) HeLa monolayers were infected with Y. pestis KIM5-3001.2.1 as done previously (Fig. 7); as expected, infected cells were not cytotoxic, because Yops could not be secreted and targeted (data not shown). For this experiment, we chose to lyse the infected monolayers by mechanical disruption in a Kontes homogenizer, because as shown in Fig. 1 (lane 5), treatment of yersiniae with cold water released small quantities of LcrV which could contaminate the HeLa soluble fractions. Treatment with a Kontes homogenizer, however, seemed to limit this effect (61). Indeed, immunoblot analysis of proteins released from a Yersinia-only control revealed that negligible amounts of LcrV and YopE were released from the bacteria by this method (lane 8), even though easily detectable levels of LcrV and YopE were found in the pelleted bacteria (lane 7). The Yersinia-containing low-speed pellets from lysed, infected HeLa cells were analyzed and found to contain readily detectable levels of LcrV and YopE, showing that strong expression of LcrV and Yops had occurred in the yscV yopD Y. pestis (lanes 5 and 6). As expected, neither LcrV nor YopE was detected in the cell-free medium fraction (lane 3). Surprisingly, LcrV was detected in the HeLa soluble fraction (lanes 1 and 2) and was not susceptible to proteolysis by trypsin (lane 2). This localization was specific for LcrV, since YopE was not detected in either of these fractions. Although these results do not rule out the possibility that other Ysc components are required for LcrV to gain access to the HeLa cell cytoplasm, they do suggest that different pathways mediate release of LcrV into the medium and entry of LcrV into eukaryotic cells.
FIG. 7.
Entry of LcrV into the HeLa cell cytoplasm does not require a functional Ysc secretion apparatus. Y. pestis KIM5-3001.2.1 (yopD yscV) was incubated in RPMI for 4 h in the presence (lanes 1 to 6) or absence (lanes 7 and 8) of HeLa cell monolayers. Infected monolayers were treated with trypsin where indicated, and the cell-free medium (M) fraction was harvested as before. Infected HeLa monolayers were scraped, suspended in ice-cold RPMI, and disrupted in a Kontes homogenizer (at least 100 strokes). Disruption was confirmed by analysis of homogenates by phase-contrast microscopy. Subsequent centrifugation resulted in the HeLa soluble (S) fraction and a low-speed pellet (P) containing yersiniae and large HeLa cell debris. Bacteria from the Yersinia-only culture were pelleted by centrifugation, suspended in ice-cold RPMI, and subjected to the Kontes homogenizer. Samples were centrifuged to yield the bacterial pellet (B) and supernatant corresponding to Kontes-released (R) proteins. Samples representing 0.5% of original culture volume were resolved in 12% polyacrylamide gels, and immunoblots were incubated with α-HTV and α-YopE. Proteins were visualized by probing immunoblots with alkaline phosphatase-conjugated anti-rabbit IgG and development with NBT-BCIP.
LcrV enters HeLa cells by a novel, pCD1-independent pathway.
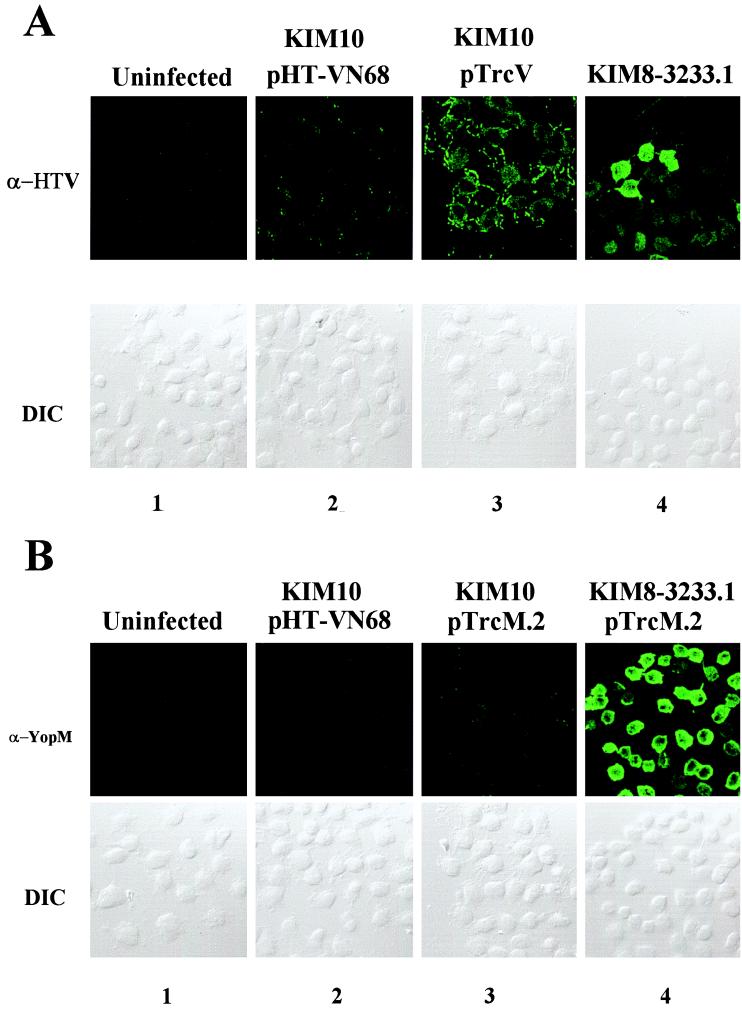
All components of the Y. pestis Ysc secretion apparatus are encoded, along with LcrV and Yops, on the virulence plasmid pCD1. We tested for an alternative LcrV-specific export pathway by infecting HeLa cells with pCD1− Y. pestis KIM10 expressing LcrV under the control of an inducible promoter (Fig. 8A). In preliminary test, Y. pestis KIM10 expressing LcrV or YopM did not secrete either protein into culture supernatant when cultivated in defined medium under inductive conditions, even though both proteins were strongly expressed within the bacteria (data not shown). For the experiments with infected HeLa cells, we used YopM, expressed in Y. pestis KIM10 under control of the same promoter used for lcrV, as a negative control Yop that is dependent on YopB and YopD to be targeted (Fig. 8B). HeLa monolayers were also infected with Y. pestis KIM8-3233.1 (lcrE yopM) or Y. pestis KIM8-3233.1 expressing YopM, as positive controls that cause cellular internalization of LcrV and targeting of YopM, respectively. Uninfected monolayers were used as a specificity control, and HeLa cells infected with Y. pestis KIM10 expressing the nonsecretable HT-VN68 served as an additional negative control. All infections were done in the presence of cytochalasin D, since Y. pestis KIM10 lacks all Yop-mediated antiphagocytic properties; IPTG was used to induce expression of trans copies of lcrV and yopM. To avoid any possible fractionation artifacts, we directly assayed for cytoplasmic LcrV and YopM by staining fixed, permeabilized monolayers with LcrV-specific (Fig. 8A) or YopM-specific (Fig. 8B) antibodies, followed by visualization using laser-scanning confocal microscopy. DIC images showed the position of HeLa cells and associated yersiniae. As expected, YopM was detected within HeLa cells infected with Y. pestis KIM8-3233.1 expressing YopM (Fig. 8B, column 4) after staining with α-YopM. Conversely, no significant signal was detected in HeLa cells infected with pCD1− Y. pestis KIM10 expressing YopM (Fig. 8B, column 3) or the nonsecretable HT-VN68 (Fig. 8B, column 2). Some YopM-specific staining of permeabilized bacteria was detected (Fig. 8B, column 3). When LcrV was expressed in Y. pestis KIM10, LcrV-specific staining was detected in the cytoplasm of infected HeLa cells (Fig. 8A, column 3), although the staining was weaker than that detected in some cells of the positive control culture infected with lcrE yopM Y. pestis. Although permeabilized bacteria were stained, the signal detected in HeLa cells infected with Y. pestis KIM10/pHT-VN68 (Fig. 8A, column 2) was comparable to that in an uninfected negative control (Fig. 8, column 1). Taken together, these results indicate that Y. pestis lacking the virulence plasmid pCD1 can target LcrV but not YopM. LcrV, therefore, enters eukaryotic cells by a novel, virulence plasmid-independent mechanism.
FIG. 8.
Entry of LcrV into the HeLa cell cytoplasm does not require pCD1-encoded proteins. Cytochalasin D-treated (5 μg/ml) HeLa cell monolayers were uninfected (A and B, column 1), infected with Y. pestis KIM8-3233.1 (lcrE yopM) (A, column 4) or KIM8-3233.1 expressing YopM from pTrcM.2 (B, column 4), and infected with pCD1− Y. pestis KIM10 expressing HT-VN68 (A and B, column 2), LcrV (A, column 3), or YopM (B, column 3). IPTG was added to 0.1 mM to cultures for activation of inducible promoters. After 4 h, monolayers were fixed, permeabilized, and stained with α-HTV (A) or α-YopM (B). Proteins were detected by secondary staining with Oregon green-conjugated anti-rabbit IgG followed by visualization by confocal laser-scanning microscopy. A 63× objective was used; immunofluorescence (IF) and DIC images are shown.
DISCUSSION
In this study, we characterized the partitioning of LcrV in a tissue culture infection model. We chose infection of HeLa cells as a model system, since the deployment of Yops by Yersinia has been investigated using this model. Previous reports by this lab showed that LcrV, like the Yops, was detected in culture fractions representing the cytoplasm of infected HeLa cells (37), suggesting that LcrV may be targeted into infected eukaryotic cells by the Yops targeting mechanism. In this report, we showed that LcrV does enter infected HeLa cells, but by a mechanism distinct from that which mediates Yops targeting. Our evidence also indicates that at least two pathways exist for the export of LcrV from Y. pestis. The first is manifested as Ysc-dependent secretion of LcrV into the tissue culture medium and localization at the bacterial surface (9), while the second, pCD1-independent pathway results in delivery of LcrV directly into the cytoplasm of infected eukaryotic cells.
In vitro in defined medium, both LcrV and YopE are secreted by the Ysc type III mechanism (40). In the tissue culture infection model, Ysc-dependent secretion of YopE and subsequent unidirectional targeting is induced by contact with a target eukaryotic cell (6, 45). In the case of Y. pseudotuberculosis, contact also concomitantly induces increased Yops expression (45). This appears not to be the case for Y. pestis at 37°C-5% CO2 in RPMI: thermal induction in the absence of cell contact in this culture condition is sufficient to induce Yops and LcrV expression, and cell contact provides no further induction. However, cell contact clearly causes activation of the Ysc for secretion and targeting. Consistent with this observation, we failed to detect YopE release into tissue culture medium when Y. pestis was incubated in tissue culture medium in the absence of HeLa cells. LcrV was also not detected, indicating that its release is probably triggered by the same mechanism that initiates delivery of Yop effectors. Conversely, LcrV was released when Y. pestis was incubated in the presence of HeLa cells. Consistent with previous observations (37), significant amounts of LcrV were detected in the tissue culture medium and HeLa soluble fractions, in contrast to YopE, which was unidirectionally targeted into the HeLa cells and not released into the medium in significant amounts. Secretion of LcrV into the medium and targeting of YopE both require a functional Ysc apparatus, and LcrV, but not YopE, can localize to the extracellular surface of Y. pestis (9). This is true also for the Ysc outer-gate protein LcrE (11), so there already was precedent prior to this study for the surface localization of Ysc accessory proteins that themselves are secreted by the Ysc. Moreover, these proteins are surface localized under conditions that are not permissive for strong Yop expression or secretion (i.e., absence of cell contact). These data suggest that Yersinia has the ability to differentially sort proteins secreted by the same pathway. Further investigation is needed to determine how and where this differential sorting occurs.
Although we detected trypsin-resistant LcrV in the HeLa soluble fraction, it was important to rule out several possible sources of artifactual introduction of LcrV into this fraction. For instance, we have shown that LcrV can exist on the extracellular surface of Y. pestis and have proposed that LcrV may be a component of a Yops delivery apparatus (9). Accordingly, it is possible that trypsin could not gain access to LcrV incorporated into a structure, and as a result, LcrV would enter the HeLa soluble fraction and appear to be trypsin resistant if some of the structures were released from the bacteria and into the HeLa soluble fraction during the fractionation procedure. Skzrypek et al. (58) have also documented that the treatment with 0.1% Triton X-100 that often is used to lyse eukaryotic cells results in release of Yops from Y. pestis. In our hands, even water lysis caused small amounts of LcrV to be released from Y. pestis (Fig. 1, lane 5). Similarly, our lab has found that treatment with 10 μg of digitonin per ml results in nonspecific release of Yops from Y. pestis (61). We therefore turned to confocal microscopy to directly demonstrate intracellular LcrV, and we easily detected it when HeLa cells were infected with Y. pestis lacking the Ysc outer-gate protein LcrE (Fig. 3 and 8). However, we were unable to detect LcrV targeted from parent Y. pestis by this method (data not shown), even though we believe that it was present (Fig. 1 and 5). This difference between parent and lcrE yersiniae may arise from a difference in the way LcrV is partitioned within the bacteria for the two strains. Previous studies with Y. pestis grown in defined medium lacking Ca2+ have shown that essentially all of the LcrV made by lcrE yersiniae is released into the medium, whereas in parent Y. pestis about half of LcrV stays in the bacterial soluble fraction (60). It may be that LcrE normally exerts a retentive effect on LcrV, which also has a cytoplasmic Ysc-related secretion-regulatory function (38, 60). In the absence of LcrE, this internal pool of LcrV may be freed from its association with the Ysc and be available for targeting by the putative, novel Ysc-independent pathway. In the parent Y. pestis, a much smaller amount of LcrV may be available to that pathway, and this might be below the sensitivity of detection by immunofluorescence. We found that the intracellular LcrV has a diffuse distribution. Interestingly, LcrV appeared to be present throughout the cell, including the interior of the nucleus, when Yops also were being targeted (Fig. 3 and 8, KIM8-3233.1), whereas it appeared to be mainly cytoplasmically located when it alone was being targeted (Fig. 8A, KIM10 pTrcV). This could reflect a cellular response to a targeted Yop, that allows LcrV to enter the nucleus, an idea that will be important to test in the future.
We initially thought that LcrV gained access to the HeLa cytoplasm by a mechanism similar to that used by Yops. YopE, for example, lacks the intrinsic ability to enter eukaryotic cells and therefore must be directly delivered by extracellularly associated yersiniae (52). Y. pestis used in our experiments was pregrown at 26°C and as a result lacked the thermally inducible, antiphagocytic capsule (4). Such bacteria are readily phagocytosed by macrophages (65), and in preliminary experiments, there was significantly less LcrV within infected J774 macrophage-like cells unless cytochalasin D was present to prevent phagocytosis (data not shown). A similar observation has previously been reported for YopM targeting by Y. pestis (58) as well as for Y. pseudotuberculosis internalized into HeLa cells (44, 51). We therefore predicted that the LcrV detected in our experiments was derived from extracellular yersiniae. Indeed, we found that extracellular yersiniae were the source of both cytoplasmic and medium-localized LcrV in infected HeLa cells (Fig. 5). In addition, exogenously added pure LcrV (HT-V) could not enter HeLa cells (Fig. 6). One interpretation of these results is that LcrV must be delivered directly to HeLa cells by associated yersiniae and does not gain access to the cytoplasm by diffusing to HeLa cells in a paracrine fashion from the medium. However, we then found that unlike entry of YopE, entry of LcrV does not require either YopB or YopD. These results indicate that a separate mechanism must exist for the transfer of LcrV from Yersinia to infected cells.
Our next test showed that this mechanism does not require a functional Ysc apparatus, because LcrV still entered HeLa cells and became trypsin resistant when the infecting strain was a yscV yopD double mutant (Fig. 7). This Y. pestis lacked functional YscV, an inner membrane Ysc component that is essential for secretion of LcrV and Yops under inductive conditions in a defined medium (47). The yopD mutation counteracted any downregulation that otherwise might have resulted from the inability to secrete the negative regulator LcrQ (45), because YopD is required for LcrQ to have its negative regulatory effect (72). Accordingly, the double mutant expressed LcrV and Yops strongly but failed to secrete either protein into the tissue culture medium and failed to target YopE into the HeLa cells, whereas LcrV was targeted by this strain. We believe that this targeting was real and that it was not complicated by contamination of the HeLa soluble fraction during the fractionation process. Surface localization of LcrV, one potential source of contamination, would not have occurred, because it requires a functional Ysc; second, treatment of bacteria in a Kontes homogenizer did not induce release of LcrV or YopE from the bacteria. Since all Ysc components are encoded on the virulence plasmid pCD1 (18, 42), we tested whether strains lacking pCD1 could mediate targeting of LcrV. LcrV, but not YopM, gained access to the HeLa cytoplasm, indicating that a separate targeting mechanism may be encoded elsewhere in the Y. pestis genome. One concern was that because the virulence plasmid pCD1 possesses several insertion sequence elements (42) and has been observed to integrate into the Yersinia chromosome (40), it was possible that Y. pestis KIM10 was not truly pCD1−. We believe that this was not the case, because we were unable to amplify any ysc-specific DNA by probing for multiple pCD1-encoded genes by PCR of Y. pestis KIM10 DNA (data not shown). Our findings have not proven that no LcrV enters HeLa cells through the Ysc-associated targeting mechanism; in fact, there appeared to be more LcrV targeted by the pCD1+ Y. pestis KIM8-3233.1 (for some cells in the population) than by the pCD1− Y. pestis KIM10 pTrcV (Fig. 8A, columns 4 versus 3). However, our data do show that LcrV is targeted by a Ysc-dependent mechanism that is contact activated (Fig. 4, 7, and 9).
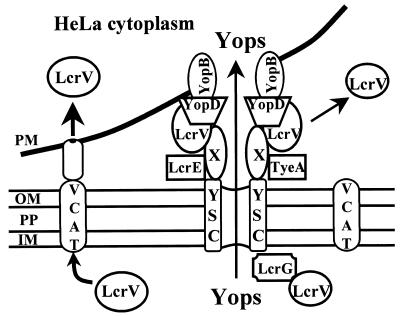
FIG. 9.
Model for Ysc-dependent and independent secretion of LcrV. LcrV, YopB, YopD, and possibly other proteins (X) are secreted by contact-activated Ysc secretion channels and assemble into an apparatus that mediates targeting of secreted Yops across the plasma membrane and into the cytoplasm of eukaryotic cells. LcrV shed from these structures diffuses into the surrounding medium. The LcrV-transporting contact-activated translocator (VCAT) can also secrete LcrV from Yersinia. Upon contact with the plasma membrane, LcrV not involved in secretion or targeting of Yops is secreted across the inner membrane (IM), periplasm (PP), and outer membrane (OM) of Y. pestis through the VCAT mechanism composed of non-pCD1-encoded proteins. LcrV secreted in this manner is delivered directly to associated eukaryotic cells.
This study raises many fascinating, unanswered questions. However, there is some information available to refine our thinking about two of these: how does this secondary mechanism mediate transfer of LcrV to infected cells, and what is the function of intracellular LcrV. The secondary pathway for LcrV targeting requires cell contact but not secretion by the Ysc type III mechanism. We have recently shown that LcrV truncated at the C terminus gains access to the HeLa cytosol but is not secreted by the Ysc, as it is not detected in the tissue culture medium and does not localize to the surface of Y. pestis (9). LcrV’s N-terminal 67 residues can be replaced by the first 15 amino acids of YopE, and this protein enters HeLa cells even though it also is not secreted by the Ysc (9). Perhaps the N and C termini are required for Ysc-dependent secretion, while an internal domain is required for the secondary pathway of LcrV targeting. Sequence analysis predicted a Sec signal-like sequence 100 residues from the N terminus (50). However, this sequence is not required for LcrV targeting, since an LcrV lacking this region (LcrV Δ108–125 [60]) can gain access to the cytoplasm of infected HeLa cells (data not shown). Further investigation is needed to elucidate a novel export and delivery mechanism that recognizes an internal domain in LcrV.
Although we have not identified a specific function for intracellular LcrV, we propose that transfer of LcrV to eukaryotic cells is not a spurious process. In a separate report, we suggest that LcrV may be involved in Yops targeting by acting as an essential component of a Yops targeting apparatus that is assembled at the interface between yersiniae and associated eukaryotic cells (9). We propose that intracellular LcrV is not related to this Yops targeting role, because LcrV entry is independent of the Ysc, whereas Yops targeting, including surface localization of LcrV, requires the Ysc. An immunomodulatory role for free LcrV has also been proposed, based on studies using pure LcrV-containing fusion proteins (34, 35, 71). The target and mechanism by which this occurs remain to be determined. Our purified preparation of LcrV was unable to enter HeLa cells, and although these data do not rule out a role in cytokine regulation, they suggest that intracellular LcrV may not function in the activities defined using LcrV-containing proteins. Finally, we did not notice a cytotoxic effect of LcrV during infections with yopB or yopD Y. pestis; HeLa cells remained flat and adherent to plastic culture dishes. Future work, therefore, will examine whether intracellular LcrV has a specific function in interrupting cellular processes required to eliminate Yersinia during an infection.
In this report, we have demonstrated that LcrV partitions differently than the vectorially targeted Yops in a tissue culture infection assay and is able to enter eukaryotic cells by a novel pathway. We propose a model (Fig. 9) in which contact of Y. pestis with eukaryotic cells can simultaneously activate two separate pathways in Y. pestis for secretion of LcrV. Secretion by the previously characterized Ysc mechanism results in deposition of LcrV on the surface of yersiniae in structures that mediate Yops targeting. These structures may be somewhat dynamic, promoting the release of LcrV into the surrounding medium. We suggest that it is this released pool of LcrV that is free to diffuse away and function as an immunomodulator. The second pathway is a novel, Ysc-independent mechanism that mediates direct targeting of LcrV to the cytosol of associated eukaryotic cells without release into the surrounding medium. Future work will focus on determining the composition of this novel LcrV-transporting contact-activated translocator, assessing whether additional substrates exist, and elucidating a function for intracellular LcrV.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant AI21017.
We gratefully thank Greta Fowler for providing the HT-V used in the experiment of Fig. 3D and Gregory Plano (University of Miami) for the kind gift of α-YopE.
REFERENCES
- 1.Black D S, Bliska J B. Identification of p130cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman T, Håkansson S, Forsberg Å, Norlander L, Macellaro A, Backman A, Bölin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland A, Sory M P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanaugh D C, Randall R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959;83:348–363. [PubMed] [Google Scholar]
- 5.Cornelis G R. The Yersinia deadly kiss. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 8.Cowan, C., Y. Kaya, H. Jones, R. D. Perry, and S. C. Straley. Unpublished data.
- 9.Fields, K. A., and S. C. Straley. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 10.Fields K A, Williams A W, Straley S C. Failure to detect binding of LcrH to the V antigen of Yersinia pestis. Infect Immun. 1997;65:3954–3957. doi: 10.1128/iai.65.9.3954-3957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsberg Å, Viitanen A M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 12.Fowler J M, Brubaker R R. Physiological basis of the low calcium response in Yersinia pestis. Infect Immun. 1994;62:5234–5241. doi: 10.1128/iai.62.12.5234-5241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis M S, Wolf-Watz H. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol Microbiol. 1998;29:799–813. doi: 10.1046/j.1365-2958.1998.00973.x. [DOI] [PubMed] [Google Scholar]
- 14.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Håkansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 16.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 17.Holmström A, Pettersson J, Rosqvist R, Håkansson S, Tafazoli F, Fallman M, Magnusson K E, Wolf-Watz H, Forsberg Å. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 18.Hu P, Elliott J, McCready P, Skowronski E, Garnes J, Kobayashi A, Brubaker R R, Garcia E. Structural organization of the virulence-associated plasmids of Yersinia pestis. J Bacteriol. 1998;180:5192–5202. doi: 10.1128/jb.180.19.5192-5202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iriarte M, Cornelis G R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:915–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 20.Iriarte M, Sory M P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koster M, Bitter W, Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 22.Kubori T, Matsushima Y, Uralil J, Lara-Tejero M, Sukhan A, Galán J E, Aizawa S-I. Supramolecular structure of the Salmonella typhimurium type III secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lambert de Rouvroit C L, Sluiters C, Cornelis G R. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol Microbiol. 1992;6:395–409. [PubMed] [Google Scholar]
- 25.Lee V T, Anderson M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 26.Leung K Y, Reisner B S, Straley S C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect Immun. 1990;58:3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 28.Metcalf W W, Jiang W, Daniels L L, Kim S-K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Mol Microbiol. 1996;24:73–91. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 29.Michiels T, Wattiau P, Brasseur R, Ruysschaert J M, Cornelis G R. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 31.Mills S D, Boland A, Sory M P, Van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulder B, Michiels T, Simonet M, Sory M P, Cornelis G. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect Immun. 1989;57:2534–2541. doi: 10.1128/iai.57.8.2534-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nedialkov Y A, Motin V L, Brubaker R R. Resistance to lipopolysaccharide mediated by the Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect Immun. 1997;65:1196–1203. doi: 10.1128/iai.65.4.1196-1203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neyt C, Cornelis G R. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopB. Mol Microbiol. 1999;31:143–156. doi: 10.1046/j.1365-2958.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 37.Nilles M L, Fields K A, Straley S C. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J Bacteriol. 1998;180:3410–3420. doi: 10.1128/jb.180.13.3410-3420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer L E, Hobbie S, Galán J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNFα production and downstream regulation of the MAP kinase p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 40.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry R D, Pendrak M, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry R D, Straley S C, Fetherston J D, Rose D J, Gregor J, Blattner F. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect Immun. 1998;66:4611–4623. doi: 10.1128/iai.66.10.4611-4623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persson C, Carballeira N, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130cas and FAK, and the associated accumulation of these proteins in focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Persson C, Nordfelth R, Holmström A, Håkansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 45.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 46.Plano G V, Barve S S, Straley S C. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J Bacteriol. 1991;173:7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plano G V, Straley S C. Multiple effects of lcrD mutations in Yersinia pestis. J Bacteriol. 1993;175:3536–3545. doi: 10.1128/jb.175.11.3536-3545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price S B, Cowan C, Perry R D, Straley S C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991;173:2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Price S B, Leung K Y, Barve S S, Straley S C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989;171:5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosqvist R, Forsberg Å, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 52.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruckdeschel K, Harb S, Roggenkamp A, Hornef M, Zumbihl R, Kohler S, Heesemann J, Rouot B. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of macrophage TNF-α production. J Exp Med. 1998;187:1069–1079. doi: 10.1084/jem.187.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruckdeschel K, Machold J, Roggenkamp A, Schubert S, Pierre J, Zumbihl R, Liautard J P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. J Biol Chem. 1998;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 56.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neurophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarker M, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skrzypek E, Cowan C, Straley S C. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol Microbiol. 1998;30:1051–1065. doi: 10.1046/j.1365-2958.1998.01135.x. [DOI] [PubMed] [Google Scholar]
- 59.Skrzypek E, Haddix P L, Plano G V, Straley S C. New suicide vector for gene replacement in yersiniae and other gram-negative bacteria. Plasmid. 1993;29:160–163. doi: 10.1006/plas.1993.1019. [DOI] [PubMed] [Google Scholar]
- 60.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on the secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skrzypek, E., and S. C. Straley. Unpublished data.
- 62.Sory M P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 63.Straley S C. Adhesins in Yersinia pestis. Trends Microbiol. 1993;1:285–286. doi: 10.1016/0966-842x(93)90001-8. [DOI] [PubMed] [Google Scholar]
- 64.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Straley S C, Harmon P A. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect Immun. 1984;45:655–659. doi: 10.1128/iai.45.3.655-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Straley S C, Perry R D. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 1995;3:310–317. doi: 10.1016/s0966-842x(00)88960-x. [DOI] [PubMed] [Google Scholar]
- 67.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ of the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 68.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Une T, Brubaker R R. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Visser L G, Annema A, van Furth R. Role of Yops in inhibition of phagocytosis of opsonized Yersinia enterocolitica by human granulocytes. Infect Immun. 1995;63:2570–2575. doi: 10.1128/iai.63.7.2570-2575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welkos S, Friedlander A, McDowell D, Weeks J, Tobery S. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb Pathog. 1998;24:185–196. doi: 10.1006/mpat.1997.0188. [DOI] [PubMed] [Google Scholar]
- 72.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zahorchak R J, Charnetsky W T, Little R V, Brubaker R R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979;139:792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]