Abstract
Non-structural protein 1 (Nsp1) is a main pathogenicity factor of α- and β-coronaviruses. Nsp1 of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) suppresses the host gene expression by sterically blocking 40S host ribosomal subunits and promoting host mRNA degradation. This mechanism leads to the downregulation of the translation-mediated innate immune response in host cells, ultimately mediating the observed immune evasion capabilities of SARS-CoV-2. Here, by combining extensive molecular dynamics simulations, fragment screening and crystallography, we reveal druggable pockets in Nsp1. Structural and computational solvent mapping analyses indicate the partial crypticity of these newly discovered and druggable binding sites. The results of fragment-based screening via X-ray crystallography confirm the druggability of the major pocket of Nsp1. Finally, we show how the targeting of this pocket could disrupt the Nsp1-mRNA complex and open a novel avenue to design new inhibitors for other Nsp1s present in homologous β-coronaviruses.
Research organism: None
Introduction
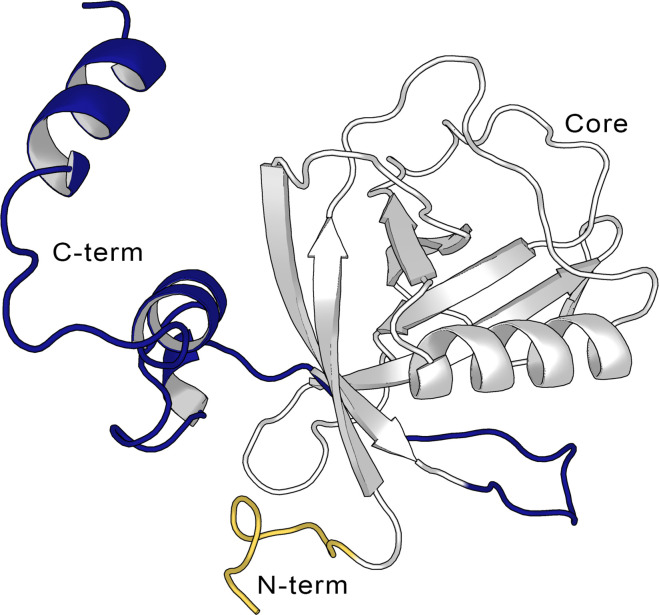
Coronaviruses (CoVs) are the largest family of RNA viruses identified to date. CoVs are members of the subfamily Coronavirinae classified into four genera α-, β-, γ-, and δ-CoV. Common human CoVs belong to the first two genera and include the 229E (α-), NL63 (α-), OC43 (β-), and HKU1 (β-). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which led to the COVID-19 pandemic declared in March 2020 as the severe acute respiratory syndrome-related CoV, SARS-CoV-1, and the Middle East respiratory syndrome-related CoV, MERS-CoV, all belong to the β-CoV genera and are suggested to originate from bats (Andersen et al., 2020; Li et al., 2005; Corman et al., 2014). The genome of different CoVs typically encodes four structural proteins, namely spike, envelope, membrane, and nucleocapsid, and two large polyproteins, pp1a and pp1ab, that are later cleaved into several non-structural proteins (Woo et al., 2010). Due to the SARS-CoV-2 pandemic, significant efforts have been directed to the study and inhibition of these proteins such as the spike protein and the protease Mpro (Owen et al., 2021). Among the different proteins that are involved in the pathogenicity of SARS-CoV-2 is the non-structural protein 1 (Nsp1), a small 180 residue protein Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020 whose function has been studied comparatively less than the rest of the pathogenic proteins. The structure of Nsp1 can be divided into three domains, a structured core, and two disordered domains corresponding to the N- and C-termini of the protein. The structured domain is composed of 117 residues (Glu10-Asn126) and displays an α/β-fold. The disordered C-terminal domain (Gly127-Gly180) was shown to partially fold into a helix-loop-helix pattern when bound to the 40S ribosomal subunit (Figure 1; Schubert et al., 2020).
Figure 1. Structure of the full-length SARS-CoV-2 Nsp1.
Cartoon representation of the full-length non-structural protein 1 (Nsp1) structure from the AlphaFold (Jumper et al., 2021; Varadi et al., 2022) model, showing the N-terminus (in yellow, aa Met1-Asn9), the Nsp1N core (in gray, aa Glu10-Asn126), and the C-terminus (blue, aa Gly127-Gly180).
It is worth noting that Nsp1 is only present in the α- and β-, but not in the γ- and δ-CoVs. The structural analysis of Nsp1N structures from α- (PDB entries 3ZBD and 5XBC) and β- (PDB entry 2HSX) CoVs suggests that, despite the low sequence homology, the Nsp1 of the two genera displays a high structural similarity, with root-mean-square deviation (RMSD) values ranging from 1.8 to 2.4 A (Shen et al., 2018; Min et al., 2020). The structural similarity of the α-CoVs and β-CoVs Nsp1 is reflected by their biological function as both are involved in the regulation of the host and viral gene expression. Multiple studies have shown that the expression of Nsp1 inhibits the translation in host cells by a combination of different mechanisms. For instance, Nsp1 sterically blocks the mRNA tunnel in the 40S ribosomal subunit (Schubert et al., 2020), the 43S preinitiation complex, and the non-translating 80S ribosomes (Schubert et al., 2020; Thoms et al., 2020; Yuan et al., 2020). Moreover, it has been shown that Nsp1 can also trigger the cleavage of the host mRNA and hinder its nuclear export to the cytosol (Zhang et al., 2021). These mechanisms concur in downregulating the translation-mediated innate immune response of the host cell, hence mediating the observed immune evasion capabilities of SARS-CoV-2 (Thoms et al., 2020; Narayanan et al., 2008). Interestingly, only host mRNAs are subjected to Nsp1-mediated endonucleolytic cleavage whereas viral mRNAs escape from this translation suppression mechanism. Experimental results have shown that the interaction of N-terminal Nsp1 with the stem loop 1 (SL1) at the viral mRNA 5’ UTR region is necessary to avoid the Nsp1-mediated translation shutdown and cleavage in infected cells (Tanaka et al., 2012; Vankadari et al., 2020). The proposed mechanism suggests that Nsp1 plays the role of a ribosome gatekeeper by sterically blocking the mRNA tunnel of the ribosome for the host mRNAs and the blockage is lifted upon the interaction of Nsp1 with the 5’-UTR SL1 sequence of the viral mRNA. In this way, the virus can inhibit selectively the translation of the host mRNA and highjack the ribosome to foster the translation of its own mRNA (Tidu et al., 2020; Mendez et al., 2021).
The regulatory role of Nsp1 in viral replication and gene expression has also been demonstrated by mutations in the Nsp1 coding region of the transmissible gastroenteritis virus (TGEV, an α-CoV infecting pigs) and the murine hepatitis virus (MHV, a β-CoV infecting mice) genomes. According to these mutagenesis studies, blocking the function of Nsp1 in different viruses leads to a drastic reduction or elimination of the infectious virus (Shen et al., 2019).
Altogether, the high structural similarity across different α- and β-CoVs from different organisms, the fact that Nsp1 has no homologues outside of the CoVs, as well as its crucial role in mediating immune evasion, make Nsp1 a valuable target for developing antiviral drugs, not only for the ongoing COVID-19 pandemic but also to prevent future pandemic outbreaks caused by new variants. However, the largest folded domain of Nsp1, namely the N-terminal core region (Nsp1N), corresponds to a small compact domain that shows predominantly small superficial cavities, which complicates rational drug design efforts. In spite of Nsp1 being a validated target for therapeutic intervention, very few studies explored Nsp1 for structure-based drug discovery, and only one of them reported the binding of a ligand to the C-terminal domain of SARS-CoV-2 Nsp1 (Afsar et al., 2022). To date, no ligand-bound Nsp1 crystal structures are available, making the ones presented here and in another study from our group the first fragment-bound SARS-CoV-2 Nsp1 crystal structures so far (Ma et al., 2022).
Here, we used a combination of computational and experimental approaches to explore the druggability of Nsp1 in SARS-CoV-2, and the possibility to expand the findings to homologous Nsp1s. In particular, we have used modelling, enhanced sampling simulations, virtual screening, fragment soaking, and X-ray crystallography to identify druggable binding pockets, including hidden (cryptic) ones, and evaluate the potential of ligands to interfere with the formation of Nsp1-RNA complexes. Our enhanced sampling simulations predict four partially cryptic binding pockets of which one is validated by crystallography. Moreover, taking into consideration the 3D similarity between different Nsp1s in α- and β-CoVs, we have extended our analysis to assess the conservation of the pockets across various Nsp1s of α- and β-CoVs infecting humans. The results of this research can be used as a stepping stone for the design of Nsp1 inhibitors for SARS-CoV-2 and potentially for other α- and β-CoVs.
Results and discussion
Structural assessment of Nsp1 pockets
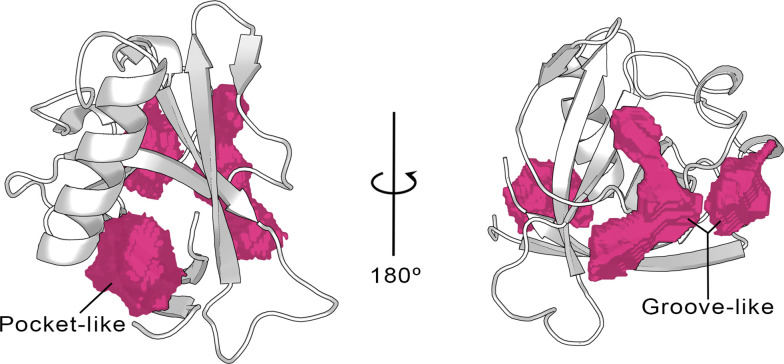
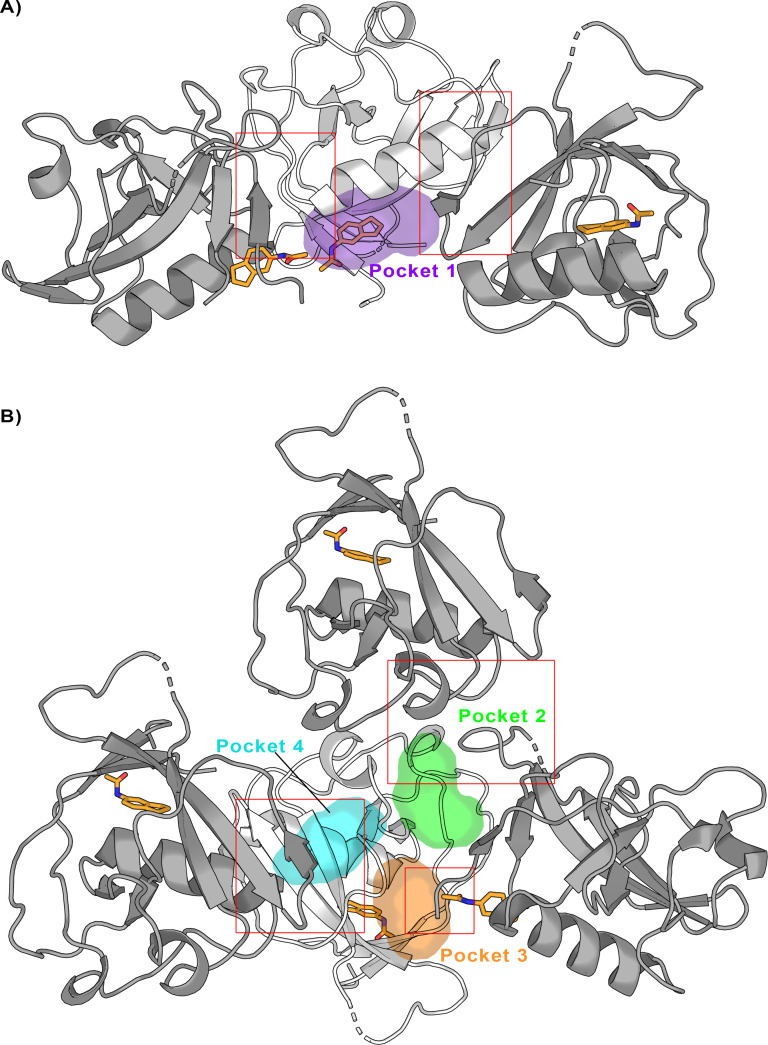
To the date of writing this paper, the only available X-ray structure of apo SARS-CoV-2 Nsp1 contains only the N-terminal region (aa 10–126 PDB entry 7K7P) (Clark et al., 2021), hereafter referred to as Nsp1N system. Starting from this crystal structure, we have characterized the possible binding pockets present in the 3D structure by means of pocket detection algorithms, namely DoGSiteScorer (Volkamer et al., 2012) (available via the ProteinPlus webserver) and Fpocket (Le Guilloux et al., 2009), showing similar results (Figure 2 and Figure 2—figure supplement 1). The analysis indicates the presence of some putative binding sites on the protein surface. Specifically, two different areas were identified to harbor potential binding pockets by both algorithms. The first one is sandwiched between the entrance of the β-barrel and the α-helix displaying a more evident pocket-like structure with a concave topology (Figure 2, left). The second region, located on the opposite side of the β-barrel, is characterized by a groove-like topology and spans a larger area on the protein surface but is very shallow (Figure 2, right).
Figure 2. Cavities identified on the Nsp1N crystal structure (tPDB entry 7K7P) by the ProteinPlus server for the concave pocket-like structure between the β-barrel and the α-helix and the groove-like topology.
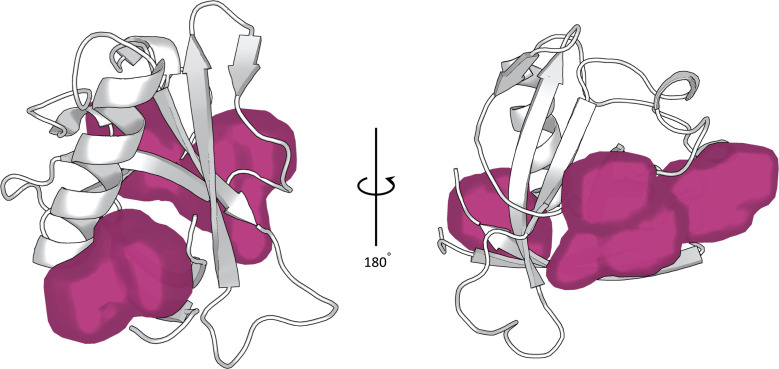
Figure 2—figure supplement 1. Cavities identified by the Fpocket software on the Nsp1N crystal structure (PDB entry 7K7P) (Le Guilloux et al., 2009).
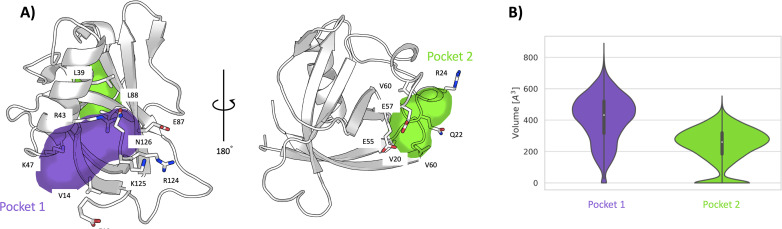
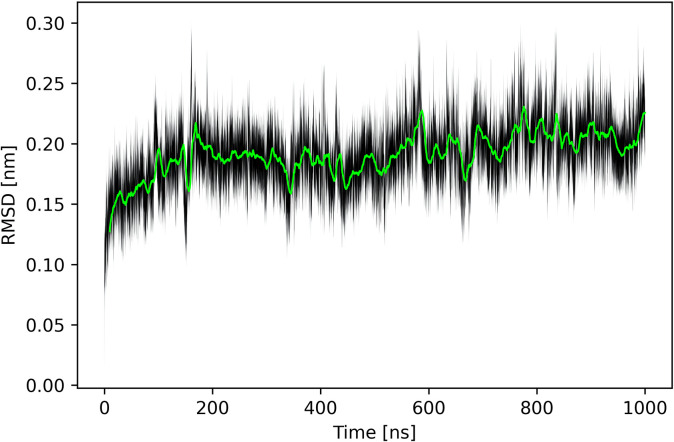
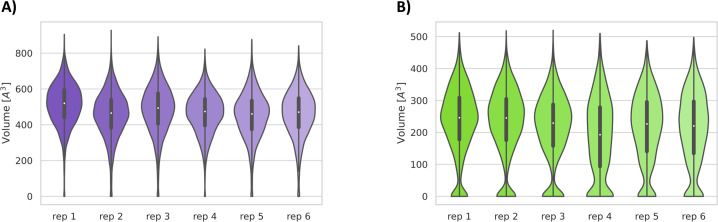
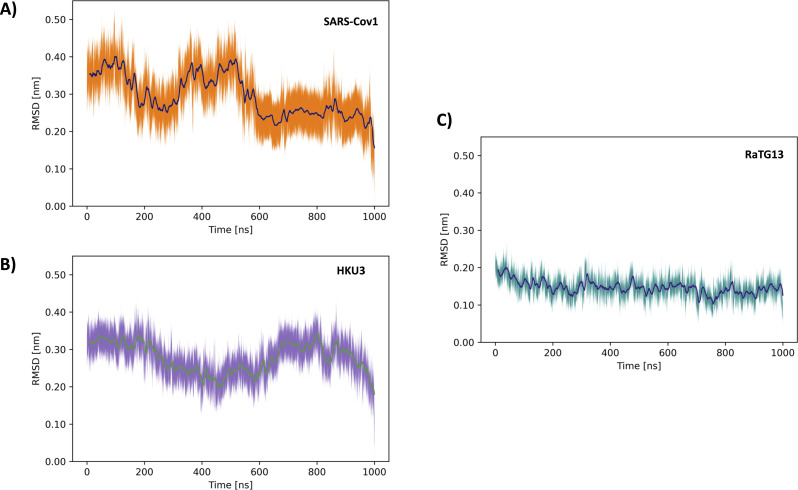
To investigate more in-depth the nature of the pocket-like structure and the possible opening of hidden (cryptic) binding sites in the grooved region, we have carried out a 1-μs-long unbiased molecular dynamics (MD) simulation of Nsp1N in its apo state. The time series of the RMSD shows that the system remains stable throughout the simulation (Figure 3—figure supplement 1). The analysis of the pockets observed along the MD simulation through the MDpocket (Le Guilloux et al., 2009) program confirms the presence of a pocket-like structure, hereafter referred to as pocket 1, at the same place as the one predicted for the X-ray structure by ProteinPlus and Fpocket. A detailed analysis of pocket 1 indicates that the residues forming this pocket are mostly located between the N- and C-termini of the Nsp1N protein, namely between the α-helix and the two β-strands of the β-barrel (Figure 3A, left). The analysis of the pocket’s volume confirms the stability of pocket 1, with an average value of 410±150 Å3 (Figure 3B). Interestingly, the volume obtained during the simulation is significantly larger than the one predicted based on the crystal structure (217 Å3), since during the simulation the structural features of any pocket are subject to fluctuations due to the rearrangements of the amino acid side chains. However, in this case, the difference in volume between the crystal structure and simulations is the result not only of the residue fluctuations, but also of the different residues identified around the pocket. Specifically, the residues close to the β-barrel form a larger cavity in the simulation than the one observed in the Nsp1N crystal structure. This finding suggests that pocket 1 is partially cryptic. It is worth noting that some of these residues are located at the beginning of the C-terminus loop of the protein, suggesting that the binding of a ligand to this region can potentially affect the dynamics of the Nsp1 C-terminus, and possibly release the blockage of host RNA entry into ribosome for translation. Additionally, as pocket 1 is close to the viral RNA binding region (Sakuraba et al., 2021), the binding of a ligand in this pocket could also interfere with the interaction with SL1 of viral RNA, leading to the failure in evasion of translation shutdown and cleavage of viral RNA. The second pocket identified during the MD simulations, namely pocket 2, is located in the grooved region. The structural analysis of pocket 2 over the simulation does not present significant differences with respect to the X-ray structure. In this pocket, the residues are mostly distributed between the loops connecting different β-strands of the β-barrel and the α-helix (Figure 3A, right). Likewise, the volume calculated during the simulation is 240±114 Å3, in agreement with the volume predicted by ProteinPlus for the Nsp1N crystal structure (150 Å3).
Figure 3. Pockets revealed from unbiased simulations.
(A) Cavities identified on Nsp1N along the 1 μs unbiased molecular dynamics (MD) simulation, namely pocket 1 (purple) and pocket 2 (green), with the main residues displayed in sticks. (B) The volume distribution of each pocket along the unbiased MD simulations.
Figure 3—figure supplement 1. Time average of the root-mean-square deviation (RMSD) for Nsp1N.
Crypticity assessment of Nsp1 pockets
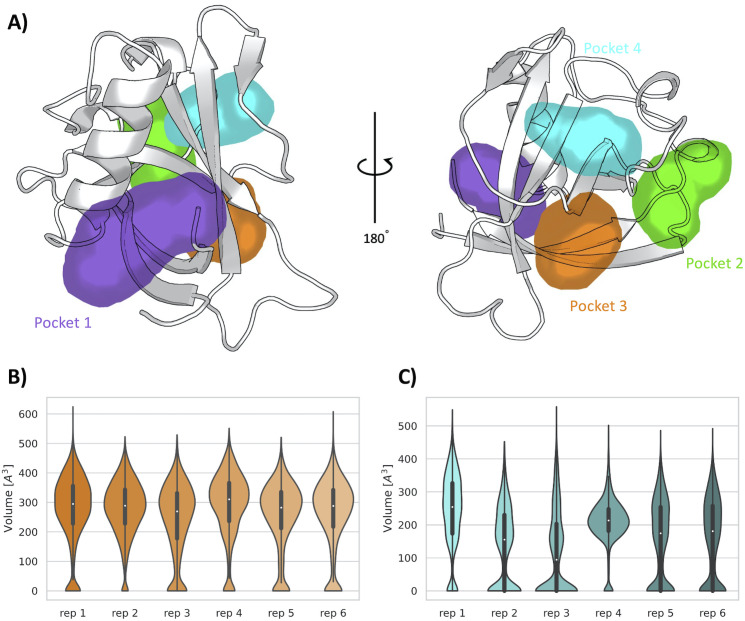
Encouraged by the results obtained from the unbiased MD simulations, we used SWISH (Sampling Water Interfaces through Scaled Hamiltonians) with mixed solvents, an enhanced sampling method developed by our group to explore cryptic binding pockets. SWISH is a Hamiltonian replica-exchange method that improves the sampling of hydrophobic cavities by scaling the interactions between water molecules and protein atoms. It has been shown to be very effective in sampling the opening of hidden (cryptic) cavities in several different targets (Comitani and Gervasio, 2018). We have run six replicas of 500 ns each, considering a concentration of 1 M benzene as co-solvent, the presence of which is expected to stabilize any pocket that will open transiently during the simulations (Figure 4—figure supplement 1). The resulting trajectories have been analyzed with MDpocket (Le Guilloux et al., 2009). It is worth noting that, in addition to confirming the presence of pocket 1 and pocket 2, our analysis shows the presence of two new pockets, namely, pocket 3 and pocket 4. These two pockets are both located on the exterior of the β-barrel, proximal to each other and near pocket 2 (Figure 4A). Most of the residues composing pocket 3 are part of the β-sheets of the β-barrel whereas most of the residues in pocket 4 are part of the loops connecting different β-sheets of the barrel. Interestingly, our previous analysis performed over the X-ray structure identified a shallow groove on the surface of Nsp1 connecting pockets 2, 3, and 4. However, neither of the two pocket detection algorithms used on the X-ray structure detected any evident deep cavity in this region. On the contrary, the analysis of the resulting SWISH trajectories clearly shows the presence of two distinct cavities with deep pocket-like structures. The absence of these sites in the crystal structure of Nsp1N and the fact that these cavities remained closed during the MD simulations indicate the cryptic nature of pocket 3 and pocket 4.
Figure 4. Pockets revealed from SWISH simulations.
(A) Pockets sampled during the 500 ns of replica-exchange SWISH (sampling water interfaces through scaled Hamiltonians) simulations. Volume distributions of the cryptic binding sites pocket 3 (B) and pocket 4 (C) along the six replicas of the SWISH simulations.
Figure 4—figure supplement 1. Time averages of the root-mean-square deviation (RMSD) for Nsp1N across the six SWISH (sampling water interfaces through scaled Hamiltonians) replicas.
Figure 4—figure supplement 2. Violin plots of the pocket volumes.
The average volume of pocket 1 during the SWISH simulations (471±128 Å3) is comparable to the one obtained in the MD simulations (410±150 Å3) for the same pocket (Figure 4—figure supplement 2A). The topology of pocket 1, that is, the residues identified around the pocket, is the same for the MD and SWISH simulations (Figure 3). Likewise, the volume analysis of pocket 2 also indicates that this pocket remains open, and its volume is essentially equal to the one sampled during the unbiased simulation (214±113 Å3, Figure 4—figure supplement 2B). More interestingly, the opening of pocket 3 (268±115 Å3) during the SWISH simulations revealed a pocket of comparable size to pocket 2. The time series of the volume profiles indicates that the pocket starts from a closed-like conformation that opens quickly to a more pocket-like conformation. Once opened, it maintains this open-like conformation in all the replicas (Figure 4B). A different behavior was observed for pocket 4. This pocket, the smallest one sampled during the SWISH simulations (169±120 Å3), displays diverse volume profiles across most SWISH replicas. Nonetheless, we were able to sample an open-like conformation for this pocket in at least two of the six replicas, suggesting that a better combination of molecular probes or higher λ factors could improve the sampling of this cryptic site. Interestingly, as suggested by the preliminary analysis of the X-ray structure, the simulations suggest as well that the shallow groove most likely has a key structural role connecting three cryptic pockets of the β-barrel region of the Nsp1N, spanning this area of the protein. Our findings highlight the potential of this region to be exploited in fragment-based drug design to design larger ligands that could bind to different combinations of these pockets, increasing the specificity of the individual pockets.
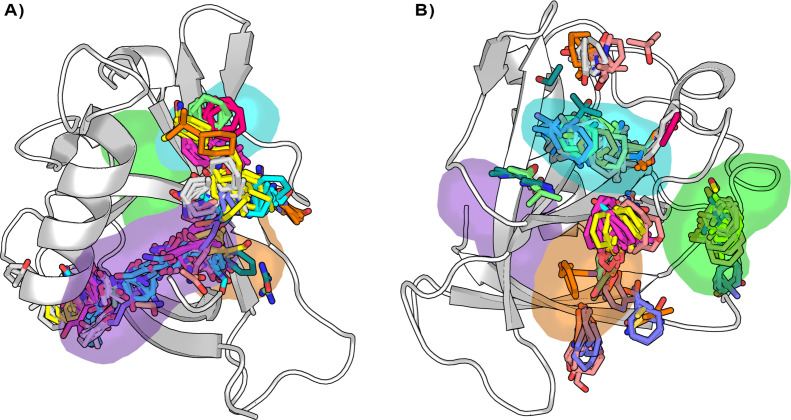
To further investigate the structural features of the predicted pockets, we analyzed the unbiased MD simulations with FTDyn and FTMap programs (Kozakov et al., 2015). These programs have proven to be accurate in locating binding hotspots in proteins. It is based on the fast and accurate distributions of 16 different small organic probes docked and mapped onto the protein surface, retaining the lowest energy probes, which are finally clustered. The clusters obtained for the different probes are clustered together to generate a final consensus cluster, from which the main binding hotspots are then identified. We started by employing the FTDyn webserver, a faster version of the FTMap algorithm without local minimization, to identify the most probable conformation able to bind fragments. We extracted 25 representative conformations from the MD simulation of Nsp1N and determined the median number of interactions between the probes and each protein residue across the conformations selected. This step allows us to identify the most likely binding site residues, that is, the residues with the highest number of probe contacts. From the initially selected conformations, we have retained only 11 structures that present higher-than-average probe-residue contacts. Subsequently, the 11 structures retained were post-processed with FTMap to improve the prediction of binding hotspots on Nsp1N surface (see details in Materials and methods section and Figure 5—source data 1).
Our results are in good agreement with the previous analysis, regarding the location of the binding sites found for 107 consensus clusters obtained for Nsp1N. In pocket 1, 50 out of the 107 consensus clusters are mapped into it, most of them corresponding to protein-fragment complexes with the highest binding energy (Figure 5A and Figure 5—source data 1). Interestingly, the clusters are distributed across the entire MD- and SWISH-sampled volume of pocket 1 and are not only limited to the small region observed in the X-ray structure, suggesting that pocket 1 is partially cryptic. The computational mapping also confirmed the presence of 50 consensus clusters in the vicinity of pockets 2, 3, and 4 (Figure 5B). In all selected bound-like Nsp1N structures, we identified at least two probes consensus clusters located in the region corresponding to the two cryptic sites previously identified by our SWISH simulations. In some cases, the second-best binding hotspot is in the presumably cryptic region, suggesting the ability of this region to accommodate molecular fragments (Figure 5—source data 1; Vajda et al., 2018). Overall, this analysis strongly suggests that the main binding sites on Nsp1N are in the region corresponding to the identified cryptic pockets and highlights their potential use for fragment binding.
Figure 5. Distribution of the binding hotspots on the Nsp1N surface around (A) pocket 1 and (B) pockets 2, 3, and 4.
Multiple consensus clusters are shown in sticks. Each consensus cluster is represented in different colors.
Crystallographic confirmation of Nsp1 cryptic pockets
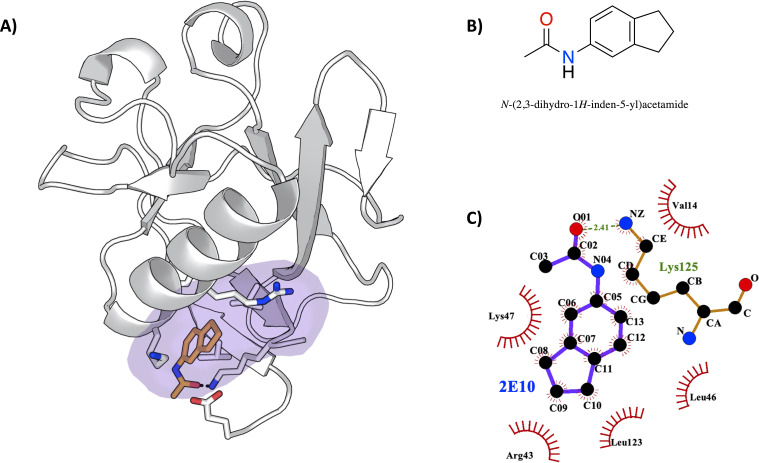
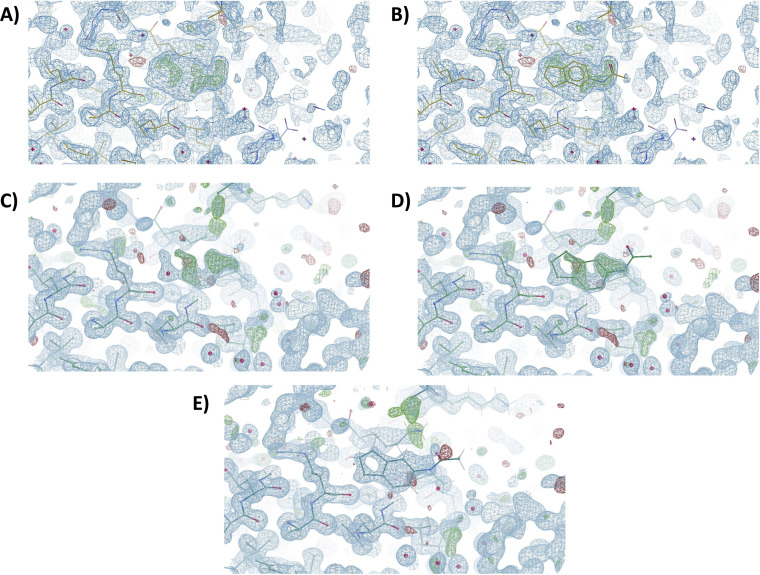
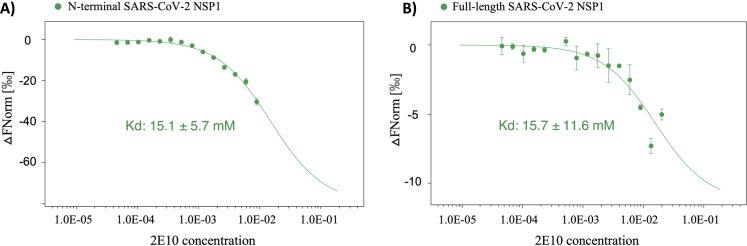
To validate our computational findings, we proceeded with the soaking of Nsp1N crystals with 59 potential fragment hits obtained by computational methods (Table 1). Nsp1N has been crystallized containing only the structured domain, that is, the domain from Glu10 to Asn126, which displays the characteristic α/β-fold (see Materials and methods for details). This structure was used for crystal soaking experiments in which 59 distinct fragment hits, obtained from the Maybridge Ro3 library, were tested and validated through X-ray diffraction experiments using the Pan-Dataset Density Analysis (PanDDA) method, developed to analyze the data obtained from crystallographic fragment screenings (Pearce et al., 2015). Of these 59 fragments, one fragment was found in pocket 1 as previously identified in our simulations. Data collection and refinement statistics are summarized in Figure 6—figure supplement 1 and Figure 6—source data 1. The asymmetric unit contains one molecule of Nsp1. The model covers the sequence from Glu10 to Asn126 (E10 to N126). The chemical structure of the fragment hit is shown in Figure 6B. To study the fragment hit using orthogonal biophysical assays, we employed microscale thermophoresis (MST) and thermal shift (TSA) assays (Figure 6—figure supplement 2 and Table 2). 2E10 binds to Nsp1N pocket 1 with a KD value of 15.1±5.7 mM, indicating a rather good binding for a fragment. In contrast, we did not observe any stabilization of Nsp1N by the fragment. The fragment obeys the rule of 3 (Ro3) with a molecular mass of 172.9 Da, a calculated MolLogP of 2.66 and a total of three hydrogen bond donors and acceptors. Fragment hit 2E10 combines two fused 5- and 6-membered ring systems containing one acetamide substituent at the phenyl group A range of residues located in binding pocket 1, including Glu10, Val14, Arg43, Leu46, Lys47, and Leu123, establish hydrophobic interactions with the ligand (Figure 6C). The acetamide substituent establishes a hydrogen bond interaction with the side chain of Lys125.
Table 1. SMILES and identification number of the 59 tested fragments and corresponding predicted binding.
The predicted pose for each fragment can be found at https://github.com/Gervasiolab/Gervasio-Protein-Dynamics/tree/master/nsp1/virtual_screening (Gervasiolab, 2022;copy archived at swh:1:rev:936e929db11aff39faed53e5fbe6f902f1456a6d). The above reported crystal hit (lig_1427) is highlighted in bold.
| Identification Number | SMILES | Predicted pocket(s) |
|---|---|---|
| lig_30 | C9H7NO2 | 3, 4 |
| lig_83 | C12H13NO2 | 1, 3, 4 |
| lig_113 | C12H13ClF3NO | 1, 3, 4 |
| lig_168 | C13H10O3 | 1, 3, 4 |
| lig_171 | C11H10O3 | 1, 4 |
| lig_194 | C8H6F2O3 | 1, 3, 4 |
| lig_212 | C11H12O2 | 1, 3, 4 |
| lig_243 | C14H17NO2S | 1 |
| lig_262 | C15H16N2O | 1, 3, 4 |
| lig_286 | C13H13NO2S | 1, 3, 4 |
| lig_329 | C9H8F3NOS | 3, 4 |
| lig_335 | C13H11NO2S | 1, 3 |
| lig_349 | C11H9NO2 | 1, 4 |
| lig_355 | C13H13NO2S | 4 |
| lig_369 | C11H12O2 | 1, 3, 4 |
| lig_377 | C12H10O2S | 1, 4 |
| lig_394 | C16H13NO2 | 1, 3, 4 |
| lig_400 | C14H12O2 | 1, 3, 4 |
| lig_422 | C14H9NO2 | 1, 3, 4 |
| lig_490 | C8H5NO2S2 | 1, 4 |
| lig_502 | C12H10O3 | 1, 3, 4 |
| lig_507 | C12H12N2O | 1, 4 |
| lig_552 | C12H15NO2 | 1, 3, 4 |
| lig_570 | C12H9NO2 | 1, 4 |
| lig_575 | C10H8O3 | 1, 4 |
| lig_579 | C12H9NO2 | 1, 3, 4 |
| lig_685 | C14H12O3 | 1, 3, 4 |
| lig_706 | C12H14N2O | 1, 4 |
| lig_752 | C9H7ClFN3S2 | 1, 3, 4 |
| lig_783 | C12H11FO2 | 1, 3, 4 |
| lig_806 | C14H13NO2 | 1, 3 |
| lig_812 | C13H11ClN2O | 1 |
| lig_864 | C15H12O3S | 1, 3, 4 |
| lig_892 | C7H6N2OS | 1, 3 |
| lig_897 | C8H6FNO2S | 1, 3, 4 |
| lig_907 | C10H11NOS2 | 1, 4 |
| lig_910 | C10H6F3NOS | 1, 3, 4 |
| lig_924 | C14H12O3S2 | 1 |
| lig_1009 | C11H10ClNO2 | 1, 4 |
| lig_1037 | C10H7ClO2S | 1, 4 |
| lig_1054 | C12H13NO2 | 1, 3, 4 |
| lig_1057 | C12H16N2O | 1, 3, 4 |
| lig_1064 | C13H12O2 | 1, 3, 4 |
| lig_1149 | C11H10F3NO | 1, 3, 4 |
| lig_1157 | C13H13NO2 | 1, 3, 4 |
| lig_1195 | C11H10N2O | 1, 3, 4 |
| lig_1209 | C9H6F3N3S2 | 1, 3, 4 |
| lig_1216 | C12H11F2NO2 | 1, 3, 4 |
| lig_1220 | C11H12O2S | 3, 4 |
| lig_1223 | C8H9ClN2OS | 1, 3 |
| lig_1228 | C15H13FN2OS | 1, 3, 4 |
| lig_1281 | C9H7F3O3 | 1, 3, 4 |
| lig_1310 | C12H13NO2 | 1, 4 |
| lig_1315 | C13H11F3O2 | 1, 3, 4 |
| lig_1381 | C9H6F3NO | 3, 4 |
| lig_1382 | C12H8F3NO2S | 1, 3, 4 |
| lig_1410 | C9H11NO | 3, 4 |
| lig_1427 | C11H13NO | 1, 4 |
| lig_1428 | C11H11NO2 | 1, 4 |
Figure 6. Characterization of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) non-structural protein 1 (Nsp1)-2E10 complex.
(A) Binding pose of the fragment hit obtained by crystal soaking and structure determination methods. The fragment is located in pocket 1. (B) Chemical structure and name of fragment hit. (C) Magnification of the Nsp1N –2E10 binding pocket showing the interactions the fragment establishes with residues of Nsp1. Hydrophobic interactions are shown by red half-moons and the hydrogen bond interaction is displayed with a dotted green line.
Figure 6—figure supplement 1. Pan-Dataset Density Analysis (PanDDA) event map and standard single dataset map focused on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Nsp1N binding site of fragment hit 2E10.
Figure 6—figure supplement 2. Microscale thermophoresis (MST) measurements of the binding affinity between fragment hit 2E10 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) non-structural protein 1 (Nsp1).
Table 2. Thermal shift assay (TSA) results for 59 potential fragment hits from computational screening using severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Nsp1N.
Fragments showing atypical curves are labelled as atypical curve in the table.
| Identification number | Fragments | Ti ± SD [°C] | ΔTi/ °C |
|---|---|---|---|
| lig_575 | 1B9 | 52.5±0.6 | –2.7 |
| lig_490 | 1C6 | 51.5±0.4 | –3.4 |
| lig_507 | 1H6 | 50.5±0.1 | –4.1 |
| lig_1427 | 2E10 | 53.8±0.3 | –1.3 |
| lig_1428 | 2F10 | 53.5±0.4 | –1.7 |
| lig_1037 | 2F5 | 53.7±0.6 | –1.6 |
| lig_685 | 3E8 | 54.8±0.2 | –0.3 |
| lig_924 | 4B5 | 54.7±0.5 | –0.5 |
| lig_502 | 4C6 | Atypical curve | |
| lig_369 | 4E4 | 53.6±0.4 | –1.4 |
| lig_400 | 5B7 | 53.9±0.1 | –0.9 |
| lig_194 | 5D3 | Atypical curve | |
| lig_335 | 5E8 | 54.8±0.2 | –0.4 |
| lig_30 | 6E3 | Atypical curve | |
| lig_113 | 6G8 | 54.3±0.0 | –0.6 |
| lig_168 | 7A2 | 51.2±0.4 | –3.8 |
| lig_212 | 7A4 | Atypical curve | |
| lig_552 | 7B6 | 55.0±0.2 | 0.1 |
| lig_1220 | 7D10 | 54.7±0.3 | –0.2 |
| lig_171 | 7D2 | 54.6±0.2 | –0.3 |
| lig_570 | 7E6 | 51.5±0.4 | –3.1 |
| lig_579 | 7F11 | 51.8±0.2 | –3 |
| lig_1054 | 7F6 | 54.3±0.3 | –0.6 |
| lig_1310 | 8A4 | Atypical curve | |
| lig_1281 | 8A5 | Atypical curve | |
| lig_422 | 8B4 | 51.2±0.1 | –3.7 |
| lig_349 | 8C4 | Atypical curve | |
| lig_1410 | 8D3 | 53.2±0.3 | –2.4 |
| lig_1009 | 8G7 | 51.5±0.4 | –3.3 |
| lig_355 | 9A11 | 54.9±0.1 | 0 |
| lig_243 | 9C10 | 54.3±0.1 | –0.9 |
| lig_1216 | 9D8 | 54.5±0.4 | –0.7 |
| lig_812 | 9E11 | 53.1±0.1 | –1.9 |
| lig_329 | 9E2 | 54.4±0.3 | –0.5 |
| lig_262 | 9G9 | 53.2±0.3 | –1.7 |
| lig_783 | 10A6 | 54.4±0.2 | –1.2 |
| lig_910 | 10A8 | Atypical curve | |
| lig_806 | 10A9 | 54.6±0.1 | -1 |
| lig_1381 | 10B7 | 50.7±0.3 | –4.9 |
| lig_286 | 10G8 | 54.1±0.2 | –0.7 |
| lig_1209 | 11A5 | 55.8±0.1 | 0.2 |
| lig_1057 | 11B2 | Atypical curve | |
| lig_907 | 11E4 | 55.6±0.0 | 0.7 |
| lig_1064 | 11E8 | 54.6±0.1 | –0.3 |
| lig_377 | 11F10 | 54.8±0.1 | –0.8 |
| lig_1195 | 11F2 | 54.8±0.2 | –0.1 |
| lig_1149 | 11G3 | 55.4±0.5 | 0.5 |
| lig_83 | 12A11 | Atypical curve | |
| lig_1315 | 12A3 | Atypical curve | |
| lig_864 | 12B10 | 55.1±0.2 | –0.1 |
| lig_394 | 12B5 | 55.2±0.2 | 0.1 |
| lig_1382 | 12C4 | 55.5±0.4 | 0.6 |
| lig_752 | 12D5 | 54.9±0.1 | –0.3 |
| lig_1223 | 12F7 | 52.4±0.3 | –2.7 |
| lig_1157 | 12F9 | 55.0±0.2 | –0.2 |
| lig_1228 | 12G7 | 52.6±0.5 | –2.6 |
| lig_892 | 12H2 | 54.8±0.1 | –0.4 |
| lig_706 | 13D6 | 51.8±0.4 | –3.3 |
| lig_897 | 13G5 | 54.3±0.1 | –0.9 |
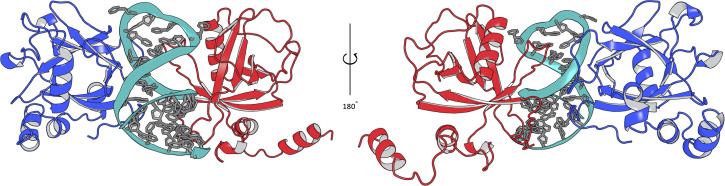
Although, at first, this may seem like a very low number of hits, a detailed analysis of the crystal packing provides an explanation. Figure 7A shows the central Nsp1N structure (white) with the pockets obtained from our SWISH simulations, together with the neighboring Nsp1 structures that interact directly with pocket 1 in the full crystal packing (dark gray). Evidently, the central part of the pocket is completely accessible for fragment binding during the crystal soaking experiments. However, the gray structures are occupying the main hotspots identified by our previous FTMap analysis (Figure 5A) impeding its ability to bind more suitable fragments in pocket 1. Likewise, Figure 7B shows the central Nsp1 structure in cartoon representation (white) with the pockets obtained from our SWISH simulations, along with the neighboring Nsp1 structures directly interacting with pockets 2, 3, and 4 in the full crystal packing (dark gray). The red regions highlighted in Figure 7 involve direct contacts made with the pocket environment. It is worth emphasizing that, in this case, these direct contacts involve regions previously determined by the FTMap analysis as potential and key hotspots for the three cryptic pockets identified by SWISH. Taken altogether, soaking Nsp1N crystals with fragments presents some limitations to implementing an effective computational fragment screening of the Nsp1. Nevertheless, we would like to stress that these results provide a possible explanation for why we did not obtain more fragments crystallized in the positions of the identified cryptic pockets. Moreover, the crystallization of a single fragment in pocket 1 is an encouraging sign of the reliability and efficacy of our computational techniques for cryptic binding pocket detection.
Figure 7. Crystal packing of the Nsp1N-fragment complex (PDB entry 8A4Y) was obtained from X-ray soaking experiments.
The direct crystal contacts around (A) pocket 1 and (B) pockets 2, 3, and 4 are highlighted with squares.
Disrupting Nsp1-RNA complex by means of cryptic pockets
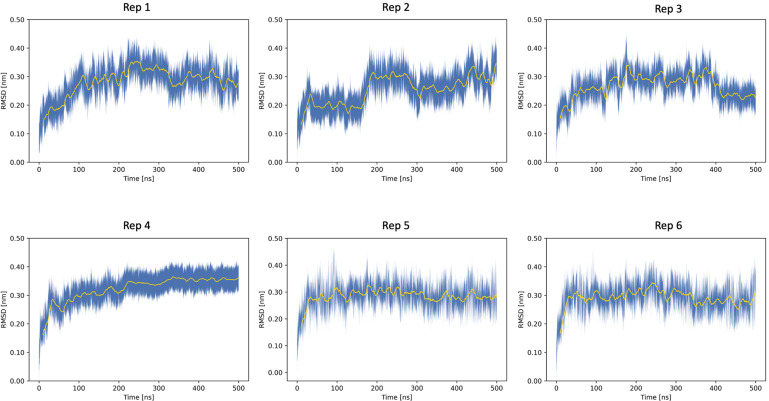
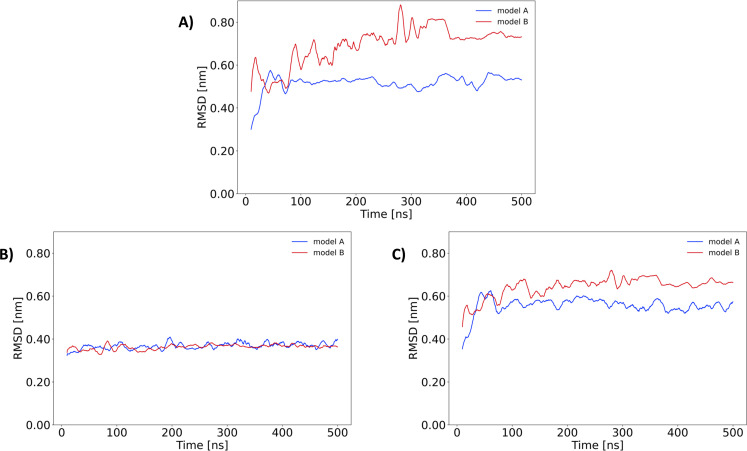
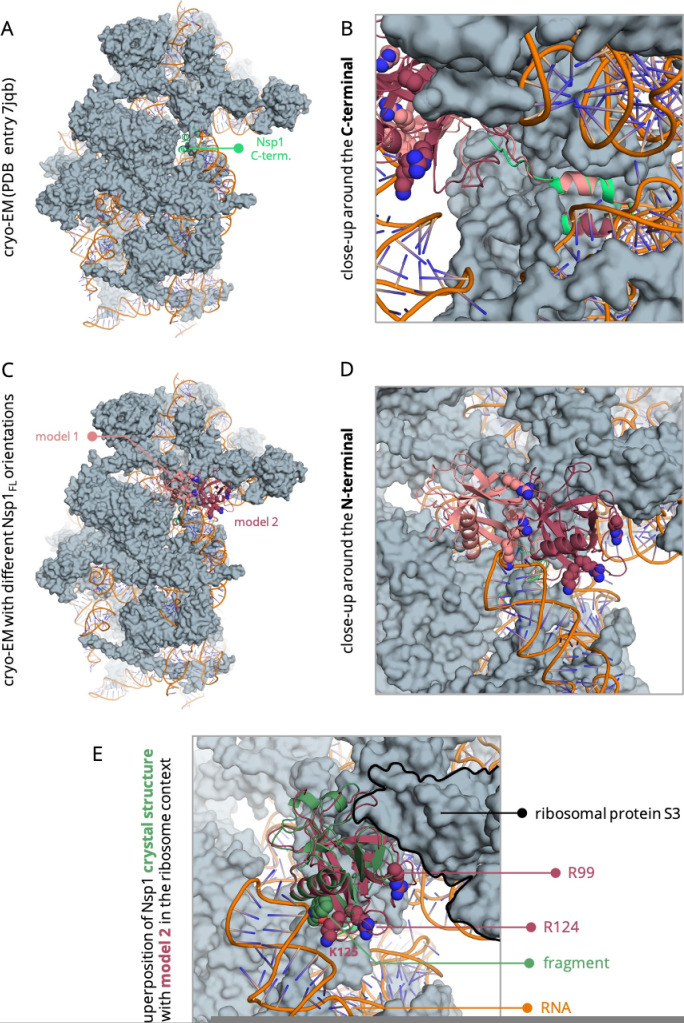
The interaction of Nsp1 with viral RNA can release the inhibition of the viral RNA translation in host cells via the combination of different mechanisms, favoring the survival of the virus. Therefore, the disruption of the Nsp1-RNA complex can affect the life cycle of the virus. With this idea in mind, and taking into consideration the pockets found in Nsp1N, we asked whether the binding of fragments to pocket 1 could hinder the Nsp1-RNA complex formation. To this end, we have modelled two Nsp1-RNA complexes, testing their stability through MD simulations. It has been shown experimentally that the first stem loop (SL1), comprising nucleotides 7–33 of the 5’ UTR region of SARS-CoV-1 and SARS-CoV-2 RNA, is the one that interacts with Nsp1 (Narayanan et al., 2008; Tanaka et al., 2012; Vankadari et al., 2020; Tidu et al., 2020). Therefore, we started by modelling the 3D structure of SL1 RNA. Regarding the Nsp1, we have considered the model of the full-length Nsp1 obtained by AlphaFold (Jumper et al., 2021; Varadi et al., 2022), named Nsp1FL. Using the Nsp1FL and SL1 models, we have run protein-RNA docking using the HADDOCK program (Dominguez et al., 2003; van Zundert et al., 2016). The analysis of the docked Nsp1FL-RNA complexes suggests that two different protein-RNA complexes, named model A and model B, well capture the experimentally validated contacts between the Nsp1FL and SL1. The Nsp1FL in models A and B binds to diametrically opposite positions on the SL1, with Nsp1 in model A interacting predominately with the groove of SL1 (Figure 8A) and in model B with its backbone (Figure 8—figure supplement 1). Since there was no structural or experimental reason to exclude one over the other, we assessed their stability over the course of a 500-ns-long MD simulation. The RMSD of the two Nsp1FL-RNA complexes fluctuates between 5.5 and 6.5 Å for models A and B, respectively (Figure 8—figure supplement 2). However, a more detailed RMSD analysis of each component of the two protein-RNA systems reveals that Nsp1FL is more stable in model A than in model B, with an average RMSD of 5.0 and 7.0 Å, respectively. This result suggests that the Nsp1FL in model A displays a more stable interaction with RNA. However, the observed RMSD difference alone between the two models is not sufficient to let us propose model A over B as the most probable mode of Nsp1-RNA interaction (Figure 8—figure supplement 2).
Figure 8. Nsp1FL-RNA complex obtained from the HADDOCK program.
(A) Residues in the proximity of pocket 1 involved in crucial Nsp1FL-SL1 contacts are displayed in sticks. (B) Crystal structure from soaking experiments (orange transparent cartoon) is superimposed over the Nsp1FL of the Nsp1FL-RNA model. The most important residues for the Nsp1FL-RNA interaction are highlighted in white (Nsp1FL model) and orange (PDB entry 8A4Y from our soaking experiments) sticks. Figures obtained from model A.
Figure 8—figure supplement 1. Different Nsp1FL-RNA models proposed in this work.
Figure 8—figure supplement 2. RMSD for model A and B of the Nsp1-RNA complex.
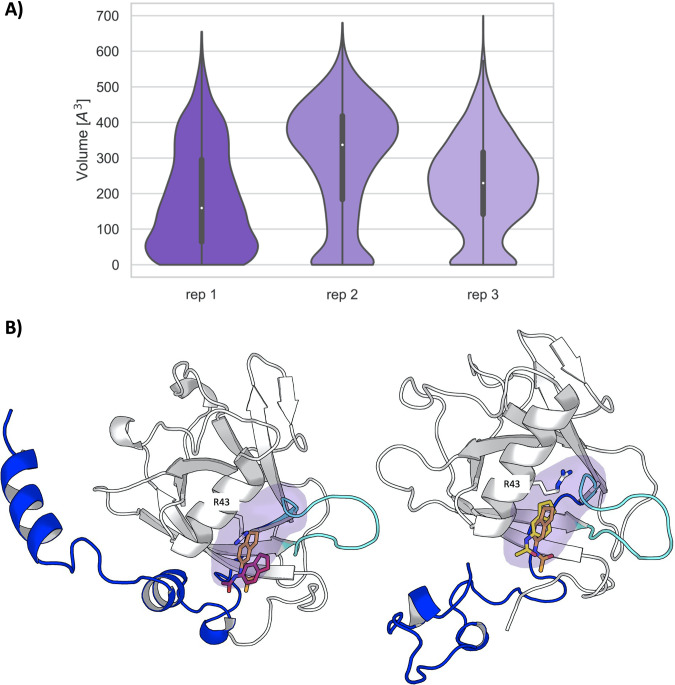
Figure 8—figure supplement 3. Ligand accesibility to pocket 1 in Nsp1FL.
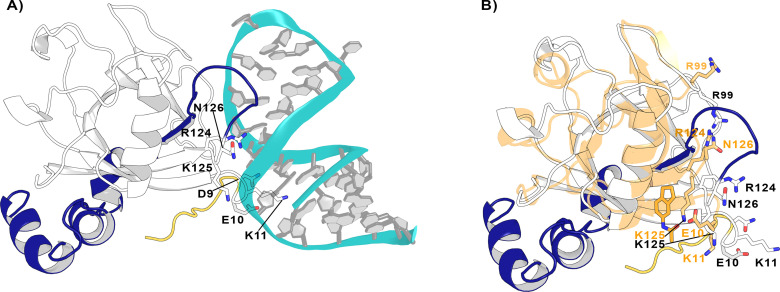
Since both models not only capture the important interactions between Nsp1FL and viral RNA described experimentally but are also stable over the course of the MD simulations, we used them to verify if the previously identified pockets could be exploited to impede the Nsp1FL-RNA complex. Interestingly, out of the four pockets in Nsp1N, pocket 1 is positioned close to the Nsp1FL-RNA interface. More specifically, some residues of the C-terminal loop of Nsp1FL around pocket 1 interact with the viral RNA, namely Arg124, Lys125, and Asn126. Moreover, Glu10 is oriented toward pocket 1 and both Asp9 and Lys11 establish contacts with the SL1 moiety (Figure 8A). Therefore, these results suggest that pocket 1 can be a crucial target for rational drug design since the residues that define this pocket are directly interacting with the viral RNA and the binding of a ligand to this pocket can impair the Nsp1FL-RNA interaction.
To further investigate the effect of ligand binding to pocket 1 and how this would affect the Nsp1-SL1 interface, we superimposed the crystal structure from the soaking experiments on the Nsp1FL-RNA model A (Figure 8B). Interestingly, in the Nsp1FL-RNA complex, some residues of the N-terminus (Asp9, Glu10, and Lys11) and the core (Arg99, Arg124, Lys125, and Asn126) of Nsp1FL are directly interacting with the SL1 of RNA. When compared with the structure obtained from soaking experiments (Glu10 to Asn126), some of these residues have changed orientation. Specifically, Glu10 and Lys11 are pointing toward the fragment, Arg99 is pointing to the surface, and Arg124 and Asn126 are displaced due to the ligand binding. In the orientation that these residues have adopted in the structure obtained from soaking experiments, these residues could not interact with the RNA. As these residues are essential for the Nsp1-SL1 interaction in SARS-CoVs, the binding of a ligand in pocket 1 could diminish the Nsp1-mRNA interaction (Tanaka et al., 2012; Terada et al., 2017). Additionally, a recent study where site-directed mutagenesis was performed on the Nsp1 N-terminal and core region (Nsp1N) has demonstrated the functional role of Arg99, Arg124, and Lys125 residues in host expression shutdown, since the mutation of these residues to Ala compromised the binding of Nsp1 to the host 40S ribosomal subunit and increased the dissociation constants with purified ribosomes (Mendez et al., 2021). Therefore, these findings support the hypothesis that a disruption of the interactions between these residues and SL1 by the presence of a fragment would lead to a decrease in virus-induced host shutoff.
To assess whether the C-terminal end would affect the accessibility of a fragment to pocket 1, we quantified the volume of the pocket throughout three independent replicas. The C-terminal domain indeed affects the accessibility of the identified pocket. The distribution of the pocket volume (Figure 8—figure supplement 3) shows how the volume of pocket 1 reduces to approximately 200 Å3 in the case of Nsp1FL, reaching similar values to the ones found in the crystal structure of the apo Nsp1 (PDB entry 7K7P). The loop that connects the N- to the C-terminal (aa Arg124-Pro153) is flexible and a portion of it (aa Arg124-Gly137) occasionally occludes pocket 1. Nonetheless, we clustered the last 200 ns of the trajectories and extracted representative structures per replica corresponding to the most populated clusters. For the structure representing the second-most populated cluster in replica 2, we were able to dock the fragment within pocket 1, obtaining a similar pose to the one seen in the presented crystal structure (Figure 8—figure supplement 3). This indicates that, even in the setting of full-length Nsp1, pocket 1 is still accessible in the context of the full-length Nsp1. Moreover, we obtained a docked pose also for replica 3, even if the site is partially closed due to the inward orientation of Arg43 (Figure 8—figure supplement 3). A conformation where the pocket was fully closed was instead obtained for replica 1, where no fragment could be docked to the pocket. Taken together, these results indicate that pocket 1 is still accessible for fragment binding in our full-length Nsp1 model in solution. Moreover, the flexible nature of the loop surrounding the pocket supports the hypothesis of the cryptic nature of the site, which is partially detectable in the crystal structures where the C-terminal domain is absent. Ultimately, the accessibility predictions from our simulations are confirmed by the MST measurements conducted on Nsp1FL (Figure 6—figure supplement 2). The measured KD has a larger error bar, but within the error is comparable to the one obtained for Nsp1N, indicating that the C-terminal domain present in Nsp1FL does not prevent the binding of the fragment.
The conservation of Nsp1 in different CoV genera
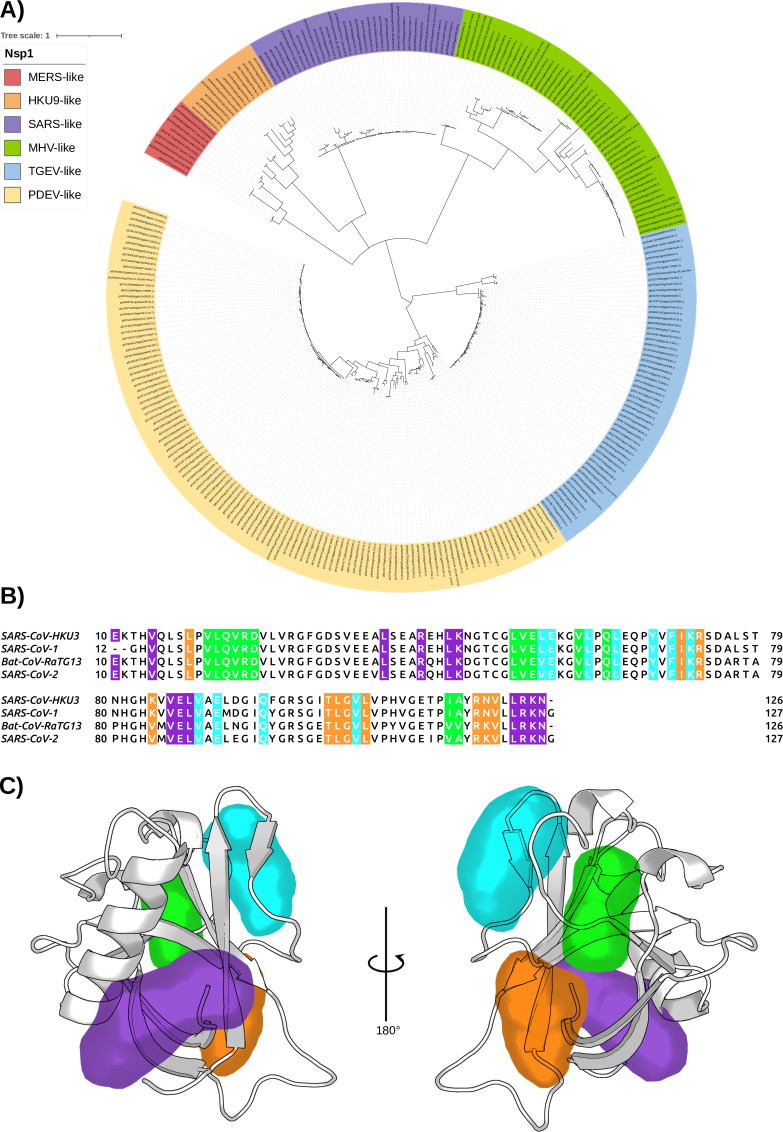
Given the identified cryptic pockets on the Nsp1 of SARS-CoV-2 and their implication in the interaction with the RNA, we sought to analyze thoroughly the structural similarity of the Nsp1 among different CoVs. We asked whether a drug designed for Nsp1 of SARS-CoV-2 could also be useful to inhibit Nsp1 of other CoVs. As mentioned in the Introduction, of the four CoV genera, only α- and β- are common human CoVs, and Nsp1 is expressed only in these two genera. To demonstrate the homology relationship of the Nsp1N domain within different viruses belonging to the α- and β-genera, we have performed a phylogenetic analysis considering this domain, for both α- and β-CoV genera from the Conserved Domain Database (CDD) hosted at the National Center for Biotechnology Information (NCBI) (Lu et al., 2020). The analysis of the 283 Nsp1N sequences available until the end of February 2022 shows the distribution of Nsp1 homologues for different α- (TGEV- and PDEV-like) and β- (MERS-, HKU9-, SARS-, and MHV-like) CoVs (Figure 9A). In particular, we have performed a pair sequence alignment considering one representative Nsp1 protein from each of the α- and β-CoVs subfamilies presented in our phylogenetic tree (Needleman and Wunsch, 1970). The alignment demonstrates that the sequence identity varies widely depending on the homologue proteins under consideration (Table 3). Subsequently, to decrease the high heterogeneity, we have considered the Nsp1N sequence from SARS-CoV-2 and three high identity homologues corresponding to Nsp1 proteins from the human SARS-CoV-1, and two Bat CoVs, namely BatCoV HKU3 and RaTG13 (NCB1 accession numbers: MT782115.1 and MN996532.2, respectively; Min et al., 2020). The selected homologues share a high sequence identity with SARS-CoV-2 Nsp1 ranging from 86% to 93%. Figure 9B shows this high sequence identity between CoVs from different organisms, especially of the residues surrounding the cryptic pockets found in Nsp1 from SARS-CoV-2. Our analysis shows that for the four sequences, all the important residues of the pockets are conserved in the selected β-CoVs.
Figure 9. The conservation of Nsp1 sequence.
(A) Phylogenetic tree based on 283 sequences from distinct α- and β-coronaviruses (CoVs) of different subgenera. The sequences were obtained from the Conserved Domain Database (CDD) with accession number cl41742. The scale bar indicates the number of substitutions per site in the amino acid sequence. Six different Nsp1 domain models can be identified, two for the α-CoVs (transmissible gastroenteritis virus [TGEV]-like and PDEV-like), and four for the β-CoVs genus (MERS-like, HKU9-like, SARS-like, and murine hepatitis virus [MHV]-like). (B) Multi-sequence alignment of the four homologues selected. The alignment was performed with the MUSCLE algorithm (Edgar, 2004). The residues of the different pockets are highlighted in the corresponding color, namely pocket 1 in purple, pocket 2 in green, pocket 3 in orange, and pocket 4 in cyan. (C) Representation of the pockets found in the severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) Nsp1N variant.
Figure 9—figure supplement 1. Time averages of the backbone root-mean-square deviation (RMSD) along the 1 μs unbiased molecular dynamics (MD) simulation for Nsp1N of (A) severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), (B) SARS-HKU3, and (C) Bat-CoV-RatG13.
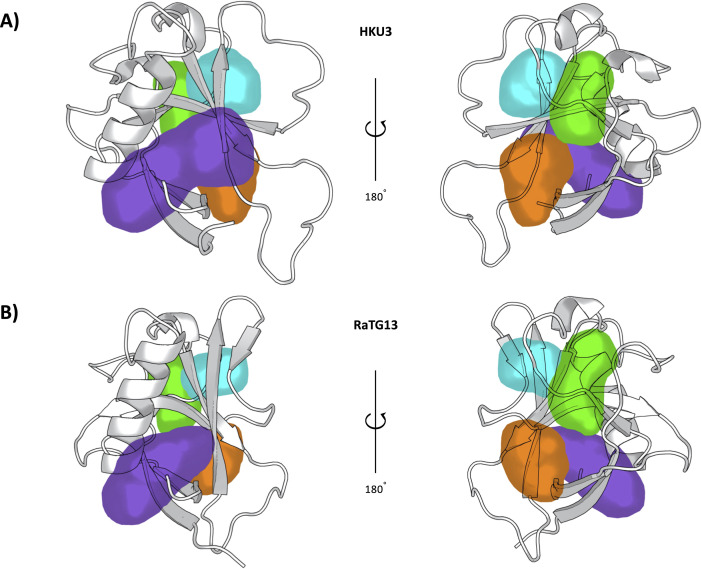
Figure 9—figure supplement 2. Representation of the pockets found in the Bat-CoV-HKU3 (A) and Bat-Cov-RaTG13 (B) Nsp1N variants.
Table 3. Pairwise identity percentages of selected non-structural protein 1 (Nsp1) sequences for representative α- and β-coronaviruses (CoVs) subfamilies.
National Center for Biotechnology Information (NCBI) accession numbers the sequences used for the analysis are as follows: transmissible gastroenteritis virus (TGEV) 6IVC_A, PDEV 5XBC_A, severe acute respiratory syndrome coronavirus 1 (SARS CoV-1) NP_828860.2, severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) YP_009725297.1, HKU9 P0C6T6, MERS YP_009047229, and murine hepatitis virus (MHV) YP_209244.
| CoV-like | SARS-1 | SARS-2 | HKU9 | MERS | MHV | TGEV | PDEV |
|---|---|---|---|---|---|---|---|
| SARS-1 | 100 | ||||||
| SARS-2 | 86.1 | 100 | |||||
| HKU9 | 20.9 | 19.5 | 100 | ||||
| MERS | 20.9 | 17.1 | 24.5 | 100 | |||
| MHV | 16.9 | 16.4 | 4.1 | 18.0 | 100 | ||
| TGEV | 17.4 | 16.9 | 13.3 | 7.9 | 9.0 | 100 | |
| PDEV | 17.9 | 16.1 | 15.2 | 8.7 | 11.5 | 18.2 | 100 |
Finally, taking into account the previous data and the fact that Nsp1 proteins share a high 3D structural core identity across CoV species (Shen et al., 2018; Min et al., 2020), we have run 1 μs of unbiased MD simulations for each of the three Nsp1 homologues considered, which show a stable conformation (Figure 9—figure supplement 1). Additionally, for all of them, we have evaluated the conservation of the cryptic binding pockets via extensive SWISH simulations (six replicas of 500 ns per homologue). The resulting trajectories indicate that the four pockets we reported for Nsp1N of SARS-CoV-2 are conserved in the considered homologues (Figure 9C and Figure 9—figure supplement 2). Taken together, these results suggest that ligands binding to any of the pockets identified in SARS-CoV-2 Nsp1N could also target the corresponding pocket in the evaluated homologues, ultimately paving the way to the development of a drug targeting the Nsp1N of different β-CoVs.
Conclusions
Nsp1 is a promising drug target for CoVs both due to its crucial role in suppressing host immune response and its sequence conservation and structural similarity across the α- and β-CoV families. In this paper, by using a multidisciplinary approach combining modelling, simulations, X-ray crystallography, and fragment screening, we revealed druggable and partially cryptic pockets in the folded main domain of Nsp1. Our enhanced sampling simulations revealed four candidate pockets and predicted that fragments can bind to them. Not all of these are predicted to be accessible in a crystal structure, due to crystallographic contacts. The fragment screening and subsequent crystallographic structures confirm the presence of the deepest and most accessible pocket predicted by the simulations. Interestingly, the pockets are conserved across multiple CoV species. Moreover, we show how the fragment bound to one of these pockets can disrupt the Nsp1-mRNA complex. In particular, the binding of the fragment to the identified pocket interferes with crucial interactions between the 5’-UTR SL1 and Nsp1, namely R124 and K125. This could prevent the viral mRNA to be efficiently translated, ultimately impairing the viral translation strategy. At the same time, the fragment is expected also to lower the affinity of Nsp1 for the ribosome by hindering the interaction mediated by R99, R124, and R125. Altogether, the crucial information arising from our multidisciplinary approach can provide a solid foundation for the rational drug discovery of new inhibitors not only for SARS-CoV-2, but also for other α- and β-CoVs with pandemic potential.
Materials and methods
Setup of the systems
Preparation of Nsp1N
The structure of the N-terminal domain of SARS-CoV-2 Nsp1 (Nsp1N) was obtained from the PDB entry 7K7P (resolution 1.77 Å), comprising residues Glu10-Asn126 (Martínez-Rosell et al., 2017).
Preparation of Nsp1FL
The Nsp1FL structure model was obtained from AlphaFold 2.0 neural network-based structural prediction (Jumper et al., 2021; Varadi et al., 2022), which is based on the NCBI Reference Sequence: YP_009725297.1.
Preparation of Nsp1FL-RNA complex
The SL1 structure corresponding to nucleotides 7–33 at the 5’ UTR of the viral mRNA was modelled with RNAComposer (Popenda et al., 2012; Antczak et al., 2016). The NCBI accession number for the reference viral RNA is NC_045512 (https://www.ncbi.nlm.nih.gov/genome/viruses/). Then, the SL1 structure obtained was docked to the Nsp1FL model from AlphaFold using the HADDOCK software (Dominguez et al., 2003; van Zundert et al., 2016). Two docking poses of Nsp1FL-RNA complexes were selected based on their score and the non-covalent interactions on the interface of Nsp1FL with RNA (Figure 8—figure supplement 1).
Preparation of Nsp1 in different CoV genera
The NMR structure of the SARS-CoV-1 Nsp1N was obtained from PDB entry 2HSX. Since there are no experimentally determined structures for the Nsp1 of Bat CoVs HKU3 nor RatG13, we modelled their structure using Nsp1N of SARS-CoV-2 (PDB entry 7K7P) as a template. The NCBI accession number for HKU3 and RatG13 is QND76032.1 and QHR63299.2, respectively, using the automodel function of MODELLER (Sali and Blundell, 1993).
Each of these systems has been processed in the same way. First, the standard protonation state at physiological pH 7.4 was assigned to ionizable residues with the ProteinPrepare tool of the PlayMolecule server (Martínez-Rosell et al., 2017). Then, the systems were placed in a pre-equilibrated octahedral box using the four-point water model from the a99SB-disp force field (Robustelli et al., 2018), which is a modified version of TIP4P-D (Piana et al., 2015), The final systems considering the full-size enzyme contain the model protein, around 6500 water molecules, and 0.15 M of NaCl, forcing the system to be neutral, leading to simulation systems comprising around 29,000–30,400 atoms for Nsp1N systems, and approximately 96,400 atoms for the Nsp1FL-RNA complex.
All the simulations were performed using the a99SB-disp force field, which is a modified form of the a99SB force field that improves the modelling of intrinsically disordered peptides while retaining the accurate description of folded proteins. To parameterize the SL1 from RNA, the RNA-Shaw force field was used (Tan et al., 2018).
MD simulations
Unbiased MD simulations of Nsp1N
All the atomistic MD simulations were performed using the GROMACS 2021.3 package (Abraham et al., 2015) employing the a99SB-disp force field (Robustelli et al., 2018). Energy minimization was conducted using 50,000 steps of the steepest descent algorithm and setting the tolerance to 100 kJ mol−1 nm−1. The equilibration process was performed in two steps, applying harmonic restraints to all heavy atoms in the system (harmonic constant: 1000 kJ mol−1 nm−1). First, a 5 ns heating in the NVT ensemble was performed, using the V-rescale (τ=0.1 ps) as thermostat (Bussi et al., 2007). Two different groups were used for temperature coupling: one for the protein and one comprising water molecules and ions. The reference temperature was set to 310 K. Second, the system was equilibrated during 15 ns in the NPT ensemble using V-rescale (Bussi et al., 2007) (τ=0.5 ps) and Berendsen (Berendsen et al., 1984) (τ=0.5 ps) as thermostat and barostat, respectively. The same temperature coupling groups were kept during the NPT equilibration step. The final structure from the equilibration process was used as a starting point for the MD simulations. All systems were simulated in the NPT ensemble with periodic boundary conditions using the same parameters as in the equilibration step and removing the harmonic restraints. The particle mesh Ewald method was used for treating long-range electrostatics using a cutoff of 12 A (Darden et al., 1993). A time step of 2 fs was used for all simulations after imposing constraints on the hydrogen stretching modes. We ran one replica of 1 μs of Nsp1N from SARS-CoV-2, and one replica of 1 μs of the three Nsp1N homologues (human SARS-CoV-1 and two Bat CoVs). For the Nsp1FL-RNA complex, we ran 1 replica of 500 ns for each model, A and B. Considering all the systems simulated leads to a total simulation time of 5 ms.
Unbiased MD simulations of Nsp1FL
The AlphaFold 2.0 Nsp1FL structure model that was used to construct the Nsp1FL-RNA complex was isolated from the complex, parameterized with the a99SB-disp force field, and solvated with TIP4P-D water molecules in an octahedral box. We used the same energy minimization and equilibration protocol as the one we applied in the Nsp1N simulations. Post equilibration, we ran three independent replicas of Nsp1FL for 1 μs each starting from the same conformation but with different initial velocities each.
SWISH simulations
SWISH (Woo et al., 2010; Min et al., 2020) is a Hamiltonian replica-exchange enhanced sampling technique that increases the conformational sampling of proteins by scaling the interaction of the apolar atoms of the protein with the water molecules. In this way, water molecules acquire more hydrophobic physicochemical properties that allow them to induce the opening of hydrophobic cavities. Including organic fragments in the solvent during the SWISH simulations has been shown to stabilize the cavities that open up during the simulation (Comitani and Gervasio, 2018; Oleinikovas et al., 2016). All SWISH simulations presented in this work, that is, of the Nsp1N of SARS-CoV-2 and the three CoV homologues, were run employing the same protocol: six different parallel 500 ns replicas, each one at a specific scaling factor (λ, ranging evenly from 1.00 to 1.35) value, in the presence of benzene (1 M concentration) as co-solvent. Since we ran six replicas per SWISH simulation, the total accumulated time is 12 μs. Besides the scaling factors, all other parameters for both equilibration and production are the same as in the ones used for the unbiased MD simulations. Before each production run, six independent equilibration steps were carried out, one for each λ value. A contact-map-based bias was introduced for each replica to prevent the possible unfolding of the protein in high scaling factor replicas. The optimal upper wall value for the contact map was tuned based on the unbiased simulations. The benzene molecules for the mixed-solvent simulations were parametrized using Gaussian 16 (Frisch et al., 2016) with Amber GAFF-2 force field (Mukherjee et al., 2011) and RESP charges.
Pocket detection
Crystal structures
In order to evaluate whether binding pockets exist in the Nsp1 and quantify their physicochemical properties, the crystal structure of Nsp1N (PDB entry 7K7P) was analyzed with Fpocket12 and DoGSiteScorer that is available as part of the modelling server ProteinPlus (Volkamer et al., 2012). Fpocket is a geometry-based cavity detection algorithm that employs Voronoi tessellation and α spheres to identify pockets in the protein structure. In this context, an α sphere is defined as a sphere that contacts four atoms on its boundary and contains no internal atom. Similarly, DoGSiteScorer is an algorithm for pocket and druggability prediction that employs 3D difference of Gaussian filters to detect cavities and a support vector machine to score the identified binding sites.
MD simulations
The trajectories were analyzed with MDpocket (Dominguez et al., 2003), an open source to detect binding pockets along MD simulations. MDpocket was run over down-sampled and reference-aligned trajectories with a time step of 100 ps between each frame. The corresponding minimized Nsp1N structure was chosen as a reference structure for each different system. The outputs let us identify and visualize the pockets observed throughout the whole simulation time with the PyMol software (PyMOL, 2022). This analysis has been performed along all the trajectories resulting from MD and SWISH simulations. Additionally, the software lets us compute the volume of the selected pockets.
Druggability assessment
FTDyn and FTMap algorithms proved to be accurate in locating binding hotspots in proteins, that is, regions of the surface that majorly contribute to the free energy of binding. This approach is based on the fast and accurate distributions of 16 different small organic probes on the protein surface. Each probe is docked billions of times and map onto the target surface and scored according to an energy-based function (Dennis et al., 2002). Hence, the lowest energy probes are retained, locally minimized, and clustered. Ultimately, clusters of different probes are clustered together into a consensus cluster. The main binding hotspot is then identified as the consensus cluster containing the highest number of different probe fragments. We first employed FTDyn, a faster version of the FTMap algorithm without local minimization, to identify the most bound-like conformation in our unbiased MD simulations. We extracted 25 representative conformations from the MD simulation of SARS-CoV-2 Nsp1N, one from each of the first 25 most populated clusters (https://github.com/Gervasiolab/Gervasio-Protein-Dynamics/tree/master/nsp1/ftmap;). The 10,000-frame trajectory was clustered employing the gromos algorithm with a cutoff of 0.1 nm. Hence, we processed these structures with FTDyn server and determined the median number of interactions between probe molecules and each protein residue across the structural ensemble. We then assigned a contact score to each structure in the ensemble. The contact score is calculated as the sum of the number of residues in a given structure with a number of contacts higher than the median. We then calculated an ensemble score averaging over the 25 contact scores of the structures in the ensemble. Finally, we retained the structures in our 25-structure ensemble with a contact score higher than the average, as they were considered to be the best approximations of bound-like conformations. We further processed these 11 structures with FTMap to have a better prediction of binding hotspots on the Nsp1N surface.
Crystallographic data
The N-terminal domain of SARS-CoV-2 Nsp1 was purified and crystallized as previously described (Ma et al., 2022). Fifty-nine potential fragment hits obtained from computational fragment screening of the Maybridge Ro3 library were purchased and fragments soaked into Nsp1N crystals and validated through X-ray diffraction experiments in quasi-automated mode at ESRF beamline MASSIF-1. Data analysis of the 59 datasets was conducted in the multi-crystal system PanDDA (Pearce et al., 2017). One hit was subsequently verified by manual inspection in COOT followed by refinement in Phenix.
Thermal shift assay
The potential 59 fragments hits were tested using TSA at 2 mM containing 1% (v/v) DMSO using SARS-CoV-2 NSP1N at 1.25 mg/mL. The change in inflection temperature (Ti) would indicate a change in protein stability as the result of the protein-fragment interaction. NSP1N in the presence of 1% (v/v) DMSO was used as a control. Each fragment was tested in triplicate. The averaged Ti values for each fragment were calculated from each triplicate group. The change of inflection temperature (∆Ti) for fragments was calculated by subtracting the averaged Ti of the control group from the values of each triplicate group. Some fragments display atypical unfolding curves at 2 mM concentration. The Ti values of these were remeasured at lower concentrations of 1, 0.5, 0.25, or 0.125 mM. Correspondingly, 0.5%, 0.25%, 0.125%, or 0.0625% (v/v) DMSO in 1.25 mg/mL protein were used as control.
Microscale thermophoresis
To label SARS-CoV-2 Nsp1N with a fluorescent dye, 100 nM of RED-TRIS-NTA second-generation dye (MO-L018, NanoTemper, Müchen, Germany) was mixed with 800 nM SARS-CoV-2 Nsp1N and incubated for 30 min on ice. The mixture was centrifuged in a Thermo Scientific Pico 17 MicroCentrifuge, 24-Pl Rotor at 15,000× g for 10 min at 4°C to remove aggregates. The labelled protein was diluted to 100 nM in assay buffer (buffer E supplemented with 0.05% Pluronic(R) F-127) and the fragment hits 2E10 were diluted from 200 to 40 mM with assay buffer. Fifty µL of DMSO was mixed with 200 µL of assay buffer as ligand buffer. The fragment solutions were serially diluted with ligand buffer with a dilution factor of 1.5, obtaining 16 fragment solutions. Then, an equal volume of protein solution was mixed with each diluted fragment solution and incubated for 30 min at 4°C to reach the binding equilibrium. The final fragment concentrations were from 20mM to 45.7 µM, each containing 10% DMSO. The final protein concentration in each sample was 50 nM. The samples were centrifuged at 6000 rpm for 10 min before the supernatant was being loaded into capillaries and detected in the Monolith NT.115 Pico instrument (NanoTemper, Müchen, Germany) under the Pico-RED channel with 20% excitation power and 40% MST power under the Expert mode in the MO.Control software. The temperature was set at 25°C. The fragment hit 2E10 was also tested on SARS-CoV-2 Nsp1FL using the same method. All measurements were performed in triplicate. The data were analyzed, and the figures were generated in the MO.Affinity Analysis software.
Phylogenetic analysis
The 283 sequences of different α- and β-CoVs of different subgenera were obtained from the CDD, family accession number cl41742. The sequences were aligned with MEGA, version 11.0.11 (Tamura et al., 2021). The resulting multi-sequence alignment was used to construct the maximum-likelihood tree with MEGA. The resulting tree was rendered with the iTol webserver (Letunic and Bork, 2021).
Acknowledgements
We acknowledge PRACE and the Swiss National Supercomputing Centre (CSCS) for large supercomputer time allocations on Piz Daint, project IDs: pr126, s1107. FLG acknowledges the Swiss National Science Foundation and Bridge for financial support (projects number: 200021_204795 and 40B2-0_203628). We thank Dr Matthew Bowler at ESRF beamline Massif-1 for his excellent support.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Carolina Estarellas, Email: cestarellas@ub.edu.
Francesco Luigi Gervasio, Email: francesco.gervasio@unige.ch.
Yogesh K Gupta, The University of Texas Health Science Center at San Antonio, United States.
Volker Dötsch, Goethe University, Germany.
Funding Information
This paper was supported by the following grants:
Swiss National Science Foundation 200021_204795 to Francesco Luigi Gervasio.
Swiss National Science Foundation 40B2-0_203628 to Francesco Luigi Gervasio.
Swiss National Supercomputing Centre pr126 to Francesco Luigi Gervasio.
Swiss National Supercomputing Centre s1107 to Francesco Luigi Gervasio.
Additional information
Competing interests
No competing interests declared.
No competing interests declared.
Reviewing editor, eLife.
Author contributions
Data curation, Software, Investigation.
Investigation.
Formal analysis, Writing – original draft.
Formal analysis, Investigation.
Investigation.
Conceptualization, Writing – review and editing.
Supervision, Investigation, Writing – original draft.
Supervision, Investigation, Writing – original draft.
Conceptualization, Resources, Supervision, Funding acquisition, Methodology, Writing – original draft, Project administration, Writing – review and editing.
Additional files
Data availability
The PDB file of the crystallographic structure (8A4Y) is available on the protein data bank: https://www.rcsb.org/structure/8A4Y.
The following dataset was generated:
Gervasio FL. 2022. Revealing druggable cryptic pockets in the Nsp1 of SARS-CoV-2 and other β-coronaviruses by simulations and crystallography. RCSB Protein Data Bank'. 8A4Y
The following previously published dataset was used:
Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz Marc, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer Aron. 2020. Conserved Domain Database (CDD) NCBI. CDD
References
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- Afsar M, Narayan R, Akhtar MN, Das D, Rahil H, Nagaraj SK, Eswarappa SM, Tripathi S, Hussain T. Drug targeting nsp1-ribosomal complex shows antiviral activity against SARS-cov-2. eLife. 2022;11:e74877. doi: 10.7554/eLife.74877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-cov-2. Nature Medicine. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antczak M, Popenda M, Zok T, Sarzynska J, Ratajczak T, Tomczyk K, Adamiak RW, Szachniuk M. New functionality of rnacomposer: an application to shape the axis of mir160 precursor structure. Acta Biochimica Polonica. 2016;63:737–744. doi: 10.18388/abp.2016_1329. [DOI] [PubMed] [Google Scholar]
- Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. doi: 10.1063/1.448118. [DOI] [Google Scholar]
- Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling. J Chem Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- Clark LK, Green TJ, Petit CM. Structure of nonstructural protein 1 from SARS-cov-2. Journal of Virology. 2021;95:e02019-20. doi: 10.1128/JVI.02019-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comitani F, Gervasio FL. Exploring cryptic pockets formation in targets of pharmaceutical interest with SWISH. Journal of Chemical Theory and Computation. 2018;14:3321–3331. doi: 10.1021/acs.jctc.8b00263. [DOI] [PubMed] [Google Scholar]
- Corman VM, Ithete NL, Richards LR, Schoeman MC, Preiser W, Drosten C, Drexler JF. Rooting the phylogenetic tree of middle east respiratory syndrome coronavirus by characterization of a conspecific virus from an african bat. Journal of Virology. 2014;88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-ncov and naming it SARS-cov-2. Nature Microbiology. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden T, York D, Pedersen L. Particle mesh ewald: an N⋅log(N) method for ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. doi: 10.1063/1.464397. [DOI] [Google Scholar]
- Dennis S, Kortvelyesi T, Vajda S. Computational mapping identifies the binding sites of organic solvents on proteins. PNAS. 2002;99:4290–4295. doi: 10.1073/pnas.062398499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C, Boelens R, Bonvin A. Haddock: a protein-protein docking approach based on biochemical or biophysical information. Journal of the American Chemical Society. 2003;125:1731–1737. doi: 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X. Gaussian 16, Revision C.01. Wallingford CT: Gaussian, Inc; 2016. [Google Scholar]
- Gervasiolab Gervasio-protein-dynamics. 4f1bb65GitHub. 2022 https://github.com/Gervasiolab/Gervasio-Protein-Dynamics
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with alphafold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozakov D, Grove LE, Hall DR, Bohnuud T, Mottarella SE, Luo L, Xia B, Beglov D, Vajda S. The ftmap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nature Protocols. 2015;10:733–755. doi: 10.1038/nprot.2015.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guilloux V, Schmidtke P, Tuffery P. Fpocket: an open source platform for ligand pocket detection. BMC Bioinformatics. 2009;10:168. doi: 10.1186/1471-2105-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P. Interactive tree of life (ITOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Research. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Wang H, Crameri G, Hu Z, Zhang H, Zhang J, McEachern J, Field H, Daszak P, Eaton BT, Zhang S, Wang LF. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Research. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Damfo S, Lou J, Pinotsis N, Bowler MW, Haider S, Kozielski F. Two Ligand-Binding Sites on SARS-CoV-2 Non-Structural Protein 1 Revealed by Fragment-Based x-Ray Screening. bioRxiv. 2022 doi: 10.1101/2022.06.12.495816. [DOI] [PMC free article] [PubMed]
- Martínez-Rosell G, Giorgino T, De Fabritiis G. PlayMolecule proteinprepare: a web application for protein preparation for molecular dynamics simulations. Journal of Chemical Information and Modeling. 2017;57:1511–1516. doi: 10.1021/acs.jcim.7b00190. [DOI] [PubMed] [Google Scholar]
- Mendez AS, Ly M, González-Sánchez AM, Hartenian E, Ingolia NT, Cate JH, Glaunsinger BA. The N-terminal domain of SARS-cov-2 nsp1 plays key roles in suppression of cellular gene expression and preservation of viral gene expression. Cell Reports. 2021;37:109841. doi: 10.1016/j.celrep.2021.109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min YQ, Mo Q, Wang J, Deng F, Wang H, Ning YJ. SARS-cov-2 nsp1: bioinformatics, potential structural and functional features, and implications for drug/vaccine designs. Frontiers in Microbiology. 2020;11:587317. doi: 10.3389/fmicb.2020.587317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee G, Patra N, Barua P, Jayaram B. A fast empirical GAFF compatible partial atomic charge assignment scheme for modeling interactions of small molecules with biomolecular targets. Journal of Computational Chemistry. 2011;32:893–907. doi: 10.1002/jcc.21671. [DOI] [PubMed] [Google Scholar]
- Narayanan K, Huang C, Lokugamage K, Kamitani W, Ikegami T, Tseng CTK, Makino S. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. Journal of Virology. 2008;82:4471–4479. doi: 10.1128/JVI.02472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. Journal of Molecular Biology. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Oleinikovas V, Saladino G, Cossins BP, Gervasio FL. Understanding cryptic pocket formation in protein targets by enhanced sampling simulations. Journal of the American Chemical Society. 2016;138:14257–14263. doi: 10.1021/jacs.6b05425. [DOI] [PubMed] [Google Scholar]
- Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ, Dantonio A, Di L, Eng H, Ferre R, Gajiwala KS, Gibson SA, Greasley SE, Hurst BL, Kadar EP, Kalgutkar AS, Lee JC, Lee J, Liu W, Mason SW, Noell S, Novak JJ, Obach RS, Ogilvie K, Patel NC, Pettersson M, Rai DK, Reese MR, Sammons MF, Sathish JG, Singh RSP, Steppan CM, Stewart AE, Tuttle JB, Updyke L, Verhoest PR, Wei L, Yang Q, Zhu Y. An oral SARS-cov-2 mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- Pearce NM, Kelm S, Shi J, Deane CM, von Delft F. PANDDAs: multi-dataset methods for finding hits from fragment screening by X-ray crystallography. Acta Crystallographica Section A Foundations and Advances. 2015;71:s258. doi: 10.1107/S2053273315096072. [DOI] [Google Scholar]
- Pearce NM, Krojer T, Bradley AR, Collins P, Nowak RP, Talon R, Marsden BD, Kelm S, Shi J, Deane CM, von Delft F. A multi-crystal method for extracting obscured crystallographic states from conventionally uninterpretable electron density. Nature Communications. 2017;8:15123. doi: 10.1038/ncomms15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piana S, Donchev AG, Robustelli P, Shaw DE. Water dispersion interactions strongly influence simulated structural properties of disordered protein states. The Journal of Physical Chemistry. B. 2015;119:5113–5123. doi: 10.1021/jp508971m. [DOI] [PubMed] [Google Scholar]
- Popenda M, Szachniuk M, Antczak M, Purzycka KJ, Lukasiak P, Bartol N, Blazewicz J, Adamiak RW. Automated 3D structure composition for large rnas. Nucleic Acids Research. 2012;40:e112. doi: 10.1093/nar/gks339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PyMOL . Schrödinger, LLC; 2022. https://pymol.org/2/ [Google Scholar]
- Robustelli P, Piana S, Shaw DE. Developing a molecular dynamics force field for both folded and disordered protein states. PNAS. 2018;115:E4758–E4766. doi: 10.1073/pnas.1800690115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba S, Qilin X, Kasahara K, Iwakiri J, Kono H. Modeling the SARS-CoV-2 Nsp1–5’-UTR Complex via Extended Ensemble Simulations. bioRxiv. 2021 doi: 10.1101/2021.02.24.432807. [DOI]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. Journal of Molecular Biology. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Schubert K, Karousis ED, Jomaa A, Scaiola A, Echeverria B, Gurzeler LA, Leibundgut M, Thiel V, Mühlemann O, Ban N. SARS-cov-2 nsp1 binds the ribosomal mrna channel to inhibit translation. Nature Structural & Molecular Biology. 2020;27:959–966. doi: 10.1038/s41594-020-0511-8. [DOI] [PubMed] [Google Scholar]
- Shen Z, Ye G, Deng F, Wang G, Cui M, Fang L, Xiao S, Fu ZF, Peng G. Structural basis for the inhibition of host gene expression by porcine epidemic diarrhea virus nsp1. Journal of Virology. 2018;92:e01896-17. doi: 10.1128/JVI.01896-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Wang G, Yang Y, Shi J, Fang L, Li F, Xiao S, Fu ZF, Peng G. A conserved region of nonstructural protein 1 from alphacoronaviruses inhibits host gene expression and is critical for viral virulence. The Journal of Biological Chemistry. 2019;294:13606–13618. doi: 10.1074/jbc.RA119.009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D, Piana S, Dirks RM, Shaw DE. Rna force field with accuracy comparable to state-of-the-art protein force fields. PNAS. 2018;115:E1346–E1355. doi: 10.1073/pnas.1713027115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kamitani W, DeDiego ML, Enjuanes L, Matsuura Y. Severe acute respiratory syndrome coronavirus nsp1 facilitates efficient propagation in cells through a specific translational shutoff of host mrna. Journal of Virology. 2012;86:11128–11137. doi: 10.1128/JVI.01700-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y, Kawachi K, Matsuura Y, Kamitani W. Mers coronavirus nsp1 participates in an efficient propagation through a specific interaction with viral RNA. Virology. 2017;511:95–105. doi: 10.1016/j.virol.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, Hirschenberger M, Kratzat H, Hayn M, Mackens-Kiani T, Cheng J, Straub JH, Stürzel CM, Fröhlich T, Berninghausen O, Becker T, Kirchhoff F, Sparrer KMJ, Beckmann R. Structural basis for translational shutdown and immune evasion by the nsp1 protein of SARS-cov-2. Science. 2020;369:1249–1255. doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidu A, Janvier A, Schaeffer L, Sosnowski P, Kuhn L, Hammann P, Westhof E, Eriani G, Martin F. The viral protein NSP1 acts as a ribosome gatekeeper for shutting down host translation and fostering SARS-cov-2 translation. RNA. 2020;27:253–264. doi: 10.1261/rna.078121.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajda S, Beglov D, Wakefield AE, Egbert M, Whitty A. Cryptic binding sites on proteins: definition, detection, and druggability. Current Opinion in Chemical Biology. 2018;44:1–8. doi: 10.1016/j.cbpa.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert GCP, Rodrigues J, Trellet M, Schmitz C, Kastritis PL, Karaca E, Melquiond ASJ, van Dijk M, de Vries SJ, Bonvin A. The HADDOCK2.2 web server: user-friendly integrative modeling of biomolecular complexes. Journal of Molecular Biology. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Vankadari N, Jeyasankar NN, Lopes WJ. Structure of the SARS-cov-2 nsp1/5’-untranslated region complex and implications for potential therapeutic targets, a vaccine, and virulence. The Journal of Physical Chemistry Letters. 2020;11:9659–9668. doi: 10.1021/acs.jpclett.0c02818. [DOI] [PubMed] [Google Scholar]
- Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, Žídek A, Green T, Tunyasuvunakool K, Petersen S, Jumper J, Clancy E, Green R, Vora A, Lutfi M, Figurnov M, Cowie A, Hobbs N, Kohli P, Kleywegt G, Birney E, Hassabis D, Velankar S. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Research. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkamer A, Kuhn D, Rippmann F, Rarey M. DoGSiteScorer: A web server for automatic binding site prediction, analysis and druggability assessment. Bioinformatics. 2012;28:2074–2075. doi: 10.1093/bioinformatics/bts310. [DOI] [PubMed] [Google Scholar]
- Woo PCY, Huang Y, Lau SKP, Yuen KY. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Peng L, Park JJ, Hu Y, Devarkar SC, Dong MB, Shen Q, Wu S, Chen S, Lomakin IB, Xiong Y. Nonstructural protein 1 of SARS-cov-2 is a potent pathogenicity factor redirecting host protein synthesis machinery toward viral RNA. Molecular Cell. 2020;80:1055–1066. doi: 10.1016/j.molcel.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Miorin L, Makio T, Dehghan I, Gao S, Xie Y, Zhong H, Esparza M, Kehrer T, Kumar A, Hobman TC, Ptak C, Gao B, Minna JD, Chen Z, García-Sastre A, Ren Y, Wozniak RW, Fontoura BMA. Nsp1 protein of SARS-cov-2 disrupts the mrna export machinery to inhibit host gene expression. Science Advances. 2021;7:6. doi: 10.1126/sciadv.abe7386. [DOI] [PMC free article] [PubMed] [Google Scholar]