Abstract
Introduction
Topical ivermectin is an anti-inflammatory and anti-Demodex drug for papulopustular rosacea. Rosacea is a relapsing disease and the time between recurrences should be considered alongside efficacy.
Objectives
The aims of this study were to assess the time of first relapse and relapse rates of Demodex mite infestation and papulopustular rosacea.
Methods
We conducted a prospective study of subjects affected by different degrees of papulopustular rosacea. Patients that achieved a complete response after treatment were monitored every 4 weeks and up to 32 additional weeks. For each patient, we evaluated recording the time to first relapse and relapse rate of Demodex mite infestation and rosacea.
Results
The overall success rate on Demodex infestation was 87.5% only 12.5% relapse. Ivermectin leads to complete response in 70% of patients. Median time to relapse was 140 days, the mean time was 152 days. The global success rate was 54.76%.
Conclusions
Topical ivermectin keeps a remission of Demodex infestation and clinical remission for long time. We proposed a twice weekly ivermectin maintenance therapy to reduce recurrences.
Keywords: ivermectin, rosacea, papulopustular rosacea
Introduction
Rosacea is a chronic, inflammatory skin disease that starts between 30–50 years of age with a large prevalence in the general population [1]. Papulopustular rosacea (PPR) is characterized by multiple small dome-shaped erythematous papules and pustules arising on erythema in a centrofacial distribution [2].
Topical ivermectin cream 1% (IVM) is a drug characterized by both antiparasitic and anti-inflammatory effects [3–7], which gained the Food and Drug Administration (FDA) approval for the treatment of PPR in December 2014, and by European Medicines Agency (EMA) in March 2015 [5].
Demodex mites are standing human ectoparasites that have a higher concentration in PPR (a mean of 12.8 D/cm2) [8] than other people. Demodicosis in humans is associated with the common inflammatory skin condition rosacea but also with pityriasis folliculorum, where it is not accompanied by inflammation, and with other rarer clinical settings, including patients with symptoms of perioral dermatitis [9,10] and granulomatous rosacea [11].
Although is well know the anti-parasitic effect of ivermectin, up to now, there are not studies that consider the time of relapse and relapse rates of Demodex mite infestation in patients affected by rosacea and treated with ivermectin.
Following the onset of the disease, patients with rosacea will experience cycles of relapse and remission of symptoms through their lives [3]. There are only very few studies that have analyzed patterns of relapse in moderate-to-severe and mild-to-moderate PPR treated with ivermectin [3,12].
Objectives
The main aim of this study was to assess the time of first relapse and relapse rates of Demodex mite proliferation after interruption of ivermectin therapy [13]. The secondary goal was to study the time to first clinical relapse and relapse rates in patients affected by almost clear, mild and moderate PPR.
Methods
The prospective study was conducted at the Dermatology Centre of Ospedale Policlinico San Martino IRCCS in Genoa (Italy) from May 1st, 2020 to May 31st, 2021 on subjects suffering from papulopustular rosacea. Diagnosis of rosacea was made by an experienced dermatologist and based on the following National Rosacea Society (NRS) expert panel criteria [1]:
fixed centrofacial erythema in a characteristic pattern that may periodically intensify
papules and pustules (with or without telangiectasias, flushing and ocular manifestations) [14]
Subjects that had been treated with topical and systemic therapies during the previous year were not included in the study. For each patient, disease severity was assessed by Investigator Global Assessment score (IGA score) [11]. IGA score defines disease severity from 0 to 4: IGA 0 = no inflammatory lesions, no erythema; IGA 1 = very few small papules/pustules, very mild erythema; IGA 2 = few small papules/pustules, mild erythema; IGA 3 = several small or large papules/pustules, moderate erythema; IGA 4 = numerous small or large papules/pustules, severe erythema [15].
In order to study Demodex mites count, a standardized skin surface biopsy (SSSB) was performed by a trained dermatologist on the target area [8]. SSSB is a sampling method in which 1 cm2 of the superficial layer of the stratum corneum and its follicular content is recovered for analysis [8]. Samples obtained by SSSB were examined with an optical microscope at × 10 and × 40 magnifications. Samples with ≥5 Demodex/cm2 (D/cm2) was considered positive (D+) [8]. Antiparasitic efficacy assessments were time to first relapse and relapse rate of positive SSSB test.
We defined a successful therapeutic intervention when patients affected by IGA 1, 2 and 3 rosacea at baseline achieved IGA= 0 after 16 weeks of therapy. After this treatment period, patients that achieved IGA=0 were followed every 4 weeks for up to 32 additional weeks (Weeks 16–48) [3].
Efficacy assessments were the time to first relapse and relapse rate [3]. Time to first relapse was defined as the time relapse between Week 16 (end of treatment) and the first relapse (IGA ≥ 1) during follow up. Relapse rate was defined as the percentage of patients who relapsed within the course of the 32 weeks.
Descriptive statistical analyses were performed, and data were shown as median (range), mean (SD), or number (percentage). Relapse rates and time to relapse were evaluated for patients divided for IGA score and analyzed using the Kaplan-Meier method and log-rank test. Patients who relapsed were considered censored in the analysis. For categorical variables, data were analyzed using the χ2 test and Fisher exact test.
The study was approved by the local Ethical Review Board (Comitato Etico Regione Liguria. N. Registro CER Liguria: 467/2021 – DB id 11726)
Results
We recruited 60 Caucasian patients, including 45 women (75%) and 15 men (25%) with a mean age of 57 years (SD 11.2, range 30–81 years). Demographic and clinical data are summarized in Table 1. Thirty-one/60 patients (51.67%) had an IGA score 1, 16/60 patients (26.67%) had an IGA score 2 and 13/60 (21.66%) had an IGA score 3.
Table 1.
Demographic and Clinical Data of Patients Divided by IGA Score.
| IGA 1 (31) | IGA 2 (16) | IGA 3 (13) | Total (60) | |
|---|---|---|---|---|
| Age, years, mean (SD) | 58 (10.1) | 56 (14,0) | 57 (9.5) | 57 (11.2) |
| Gender | ||||
| Female, N (%) | 20 (64.52) | 13 (81.25) | 12 (92.31) | 45 (75) |
| Male, N (%) | 11 (35.48) | 3 (18.75) | 1 (7.69) | 15 (25) |
| Patients reaching IGA= 0 at 16 weeks, N (%) | 24 (77.42) | 11 (68.75) | 7 (53.85) | 42 (70) |
| Patients positive for Demodex at baseline, N (%) | 7 (22.58) | 7 (43.75) | 6 (46.15) | 20 (33.33) |
| Patients negative for Demodex at 16 weeks, N (%) | 5 (16.13) | 6 (37.5) | 5 (38.46) | 16 (26.67) |
IGA= Investigal Global Assessment; SD = standard deviation.
Demodex Mite Density
Using SSSB, Demodex mites were found (D+) in 20/60 (33.33%) patients of whom 7 had IGA 1, 7 had IGA 2 and 6 had IGA3. After 16 weeks of therapy, SSSB resulted negative (D−) in 16/20 (80%) of whom 5/7 (71.43%) had IGA 1, 6/7 (85.71%) had IGA2 and 5/6 (83.33%) had IGA3. There are not statistical differences to become D− (P > 0.999, P > 0.999, P > 0.999) after 16 weeks of therapy.
Relapse Evaluation and Demodex Mite Infestation
Only 2/16 (12.5%) IGA 2 PPR D− relapsed after 224 days. The global success rate of Demodex infestation was 87.5%. Regarding 4 D+ patients positive after 16 weeks of therapy, 2/4 achieved D− at Day 150, ¼ at Day 224 while ¼ remained D+.
IGA Score and Effectiveness
Patients that reached IGA=0 after 16 weeks of treatment are summarized in Table 1. There are not statistical significative differences to achieve IGA = 0 among IGA1-2-3 at baseline (P = 0.725, P = 0.1552, P = 0.47, respectively).
Relapse Evaluation
Kaplan-Meier curves show no different median time of first relapse (IGA ≥ 1) among IGA 1-2-3 (P log rank test = 0.551) (Figure 1). At week 48, the percentages of patients who experienced a relapse (IGA ≥ 1) after discontinuation of ivermectin treatment (relapse rate) was 45.24%, and in particular, they were 45.83% for IGA1, 45.45% for IGA 2 e 28.57% for IGA 3. The global success rate (percentage of patients who reached 0 at the end of treatment and had no relapse in follow up) was 54.76% and in particular, they were 54.17% for IGA1, 54.54% for IGA 2 and 71.43% for IGA3. Median time to relapse was 140 days (84–224 days), in particular, 154 days (range: 84–224) for IGA 1, 196 days (range: 98–224) for IGA 2, 105 days (range: 98–112) for IGA 3. The mean time to relapse was 152.44 ± 51.47 days, in particular, 154 ± 50.47 days for IGA 1, 168 ± 58.57 days for IGA 2 and 105 days ± 9.90 days for IGA 3.
Figure 1.
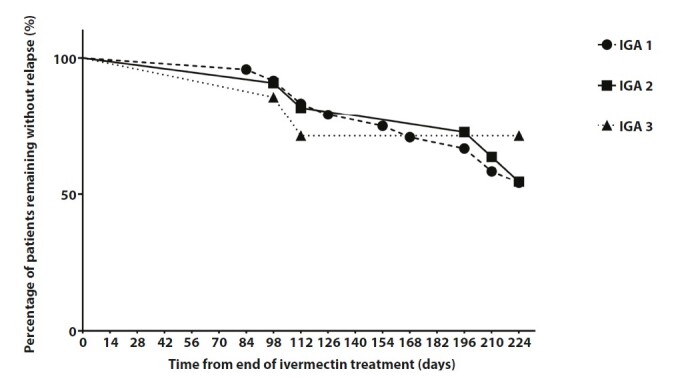
Time to first relapse of patients in ivermectin 1% cream. Kaplan-Meier plot demonstrates that there are not differences among patients treated with ivermectin divided to IGA score (P = 0.551).
Conclusions
Similarly, to previous study, we found 33% of Demodex positive samples at baseline [7]. The great anti-parasitic effect of ivermectin was confirmed by the overall success rate of Demodex infestation (87.5%). In the literature, there are not studies that consider the time of relapse and relapse rates of Demodex mite infestation in patients affected by rosacea and treated with ivermectin. In our study, we observed a low relapse rate of Demodex mite infestation (12.5%) in PPR showing as ivermectin has an excellent antiparasitic effect that last over time. It acts by blocking ligand-gated chloride channels, especially glutamate-gated anion channels in the peripheral nervous system of Demodex mites causing their paralysis and death [16]. The two patients that showed a relapse of Demodex mite infestation presented an erythemato-telangiectatic rosacea (ETR) with few papules (Figure 2). This result is according to the hypothesis that ETR may be associated with non-visible Demodex proliferation, possibly corresponding to a subclinical stage of demodicosis [17–19]. Few papules may be an early sign of relapsed Demodex mite proliferation [20].
Figure 2.
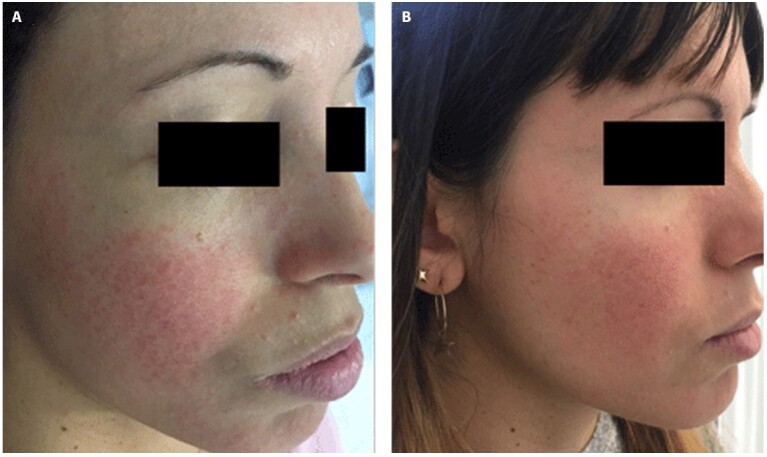
Relapse of erythemato-telangiectatic after 224 days of suspension of therapy.
Our study confirms the efficacy of ivermectin 1% cream leading to complete response in 70% of almost clear, mild and moderate PPR after 16 weeks of therapy. Regarding relapses in patients with rosacea, a study from 2016 showing that patients affected by moderate-severe rosacea and treated with ivermectin had median times of first relapse significantly longer than those treated with metronidazole 0.75% cream (115 days versus 85 days) [3]. In addition, relapse rate was lower for patients previously treated with ivermectin 1% compared with metronidazole 0.75% (62.7% versus 68.4%) [3]. Dall’Oglio found only 2/20 mild and moderate PPR patients that relapsed [12]. In our study, we found a longer median time of first relapse (140 days) with a lower relapse rate (45.24%) than studies in the literature. As we have just shown, relapse rates are difficult to compare between different studies. We suppose that our results are better than those of Shaub et al because we considered mild forms instead of moderate-severe forms of rosacea baseline [3]. In fact, patients with IGA 3–4 rosacea had higher number of inflammatory lesions than patients with IGA 1–2 rosacea that keep clinical remission for longer days. Moreover, differences among studies could be justified by a different definition of relapse; while Shaub et al considered a relapse as IGA ≥ 2 we regarded as IGA ≥ 1 PPR [3]. This is because we take on IGA = 0 as desirable ideal goal of maintenance, crucial for patient’s outcome and for an effective improvement of his quality of life. However, patients that relapsed in our study showed mainly IGA = 1 PPR (22/27) without serious cases after suspension of therapy. Finally, the difficulties may also be due to the different follow-up periods. In fact, while we tracked our patients for 32 weeks, Shaub et al followed them for 36 weeks and Dall’Oglio] for 12-to-24 weeks after treatment: a longer follow-up periods correspond to higher probability of relapse [3,12].
Since our results, ivermectin work with an anti-parasitic effect in Demodex positive PPRs eliminating mites as cause of inflammation and enabling a long remission of PPR with a low risk of relapse. In addition, ivermectin acts as anti-inflammatory drug inducing a downregulation of the pro-inflammatory genes IL-8, human ß-defensin-3 (HBD3), Toll-like receptor-4 (TLR4), and tumor necrotic factor-alpha (TNF-α) [21], and by an inhibition of cathelicidin innate immune mediators, LL-3720 21 and kallikrein-5 (KLK5) in Demodex positive and negative PPRs [21,22]. We propose to keep a twice-weekly ivermectin maintenance therapy to reduce relapses, especially in Demodex negative rosacea where ivermectin only holds an anti-inflammatory effect. However, further studies are needed to evaluate the efficacy of maintenance therapy in patients treated with ivermectin.
Our study has different limitations: we did not have patients with severe rosacea and we did not evaluated patients with object instruments like erythema-directed digital photography.
In conclusion, we confirm the anti-parasitic effect over time of ivermectin in PPR after cessation of the treatment. In addition, we demonstrate an extended clinical remission of PPR and we propose a twice-weekly ivermectin maintenance therapy to reduce clinical recurrences.
Footnotes
Funding: None.
Competing interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.van Zuuren EJ. Rosacea. N Engl J Med. 2017;377(18):1754–1764. doi: 10.1056/NEJMcp1506630. [DOI] [PubMed] [Google Scholar]
- 2.Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46(4):584–587. doi: 10.1067/mjd.2002.120625. [DOI] [PubMed] [Google Scholar]
- 3.Taieb A, Khemis A, Ruzicka T, et al. Ivermectin Phase III Study Group. Maintenance of remission following successful treatment of papulopustular rosacea with ivermectin 1% cream vs. metronidazole 0.75% cream: 36-week extension of the ATTRACT randomized study. J Eur Acad Dermatol Venereol. 2016;30(5):829–836. doi: 10.1111/jdv.13537. [DOI] [PubMed] [Google Scholar]
- 4.Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):465–471. doi: 10.1111/bjd.15173. [DOI] [PubMed] [Google Scholar]
- 5.Steinhoff M, Vocanson M, Voegel JJ, Hacini-Rachinel F, Schäfer G. Topical Ivermectin 10 mg/g and Oral Doxycycline 40 mg Modified-Release: Current Evidence on the Complementary Use of Anti-Inflammatory Rosacea Treatments. Adv Ther. 2016;33(9):1481–501. doi: 10.1007/s12325-016-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendieta Eckert M, Landa Gundin N. Treatment of rosacea with topical ivermectin cream: a series of 34 cases. Dermatol Online J. 2016;22(8) 13030/qt9ks1c48n. [PubMed] [Google Scholar]
- 7.Trave I, Merlo G, Cozzani E, Parodi A. Real-life experience on effectiveness and tolerability of topical ivermectin in papulopustular rosacea and antiparasitic effect on Demodex mites. Dermatol Ther. 2019;32(6):e13093. doi: 10.1111/dth.13093. [DOI] [PubMed] [Google Scholar]
- 8.Forton F, Seys B. Density of Demodex folliculorum in rosacea: a case-control study using standardized skin-surface biopsy. Br J Dermatol. 1993;128(6):650–659. doi: 10.1111/j.1365-2133.1993.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 9.Alniemi DT, Chen DL. Perioral Demodex folliculitis masquerading as perioral dermatitis in the peripartum period. JAAD Case Rep. 2019;5(7):639–641. doi: 10.1016/j.jdcr.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7(1):35–38. doi: 10.5826/dpc.0701a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JY, Hsu CK. Granulomatous rosacea-like demodicidosis. Dermatol Online J. 2007;13(4):9. [PubMed] [Google Scholar]
- 12.Dall’Oglio F, Lacarrubba F, Luca M, Boscaglia S, Micali G. Clinical and erythema-directed imaging evaluation of papulo-pustular rosacea with topical ivermectin: a 32 weeks duration study. J Dermatolog Treat. 2019;30(7):703–707. doi: 10.1080/09546634.2019.1572860. [DOI] [PubMed] [Google Scholar]
- 13.Drago F, Ciccarese G, Herzum A, Rebora A, Parodi A. Rosacea and alcohol intake. J Am Acad Dermatol. 2018;78(1):e25. doi: 10.1016/j.jaad.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 14.Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155. doi: 10.1016/j.jaad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Hopkinson D, Moradi Tuchayi S, Alinia H, Feldman SR. Assessment of rosacea severity: A review of evaluation methods used in clinical trials. J Am Acad Dermatol. 2015;73(1):138–143.e4. doi: 10.1016/j.jaad.2015.02.1121. [DOI] [PubMed] [Google Scholar]
- 16.Laing R, Gillan V, Devaney E. Ivermectin - Old drug, new tricks? Trends Parasitol. 2017;33(6):463–472. doi: 10.1016/j.pt.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52(1):74–87. doi: 10.1016/j.jaad.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Forton F, De Maertelaer V. Erythematotelangiectatic rosacea may be associated with a subclinical stage of demodicosis: a case-control study. Br J Dermatol. 2019;181(4):818–825. doi: 10.1111/bjd.17817. [DOI] [PubMed] [Google Scholar]
- 19.Forton FMN. The Pathogenic Role of Demodex Mites in Rosacea: A Potential Therapeutic Target Already in Erythematotelangiectatic Rosacea? Dermatol Ther (Heidelb) 2020;10(6):1229–1253. doi: 10.1007/s13555-020-00458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trave I, Micalizzi C, Gasparini G, Cozzani E, Parodi A. Dermoscopy of papulopustular rosacea and comparison of dermoscopic features in patients with or without concomitant Demodex folliculorum. Clin Exp Dermatol. 2021;46(8):1434–1440. doi: 10.1111/ced.14731. [DOI] [PubMed] [Google Scholar]
- 21.Schaller M, Gonser L, Belge K, et al. Dual anti-inflammatory and anti-parasitic action of topical ivermectin 1% in papulopustular rosacea. J Eur Acad Dermatol Venereol. 2017;31(11):1907–1911. doi: 10.1111/jdv.14437. [DOI] [PubMed] [Google Scholar]
- 22.Thibaut de Ménonville S, Rosignoli C, Soares E, et al. Topical treatment of rosacea with ivermectin inhibits gene expression of cathelicidin innate immune mediators, LL-37 and KLK5, in reconstructed and ex vivo skin models. Dermatol Ther (Heidelb) 2017;7(8):213–225. doi: 10.1007/s13555-017-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


