Abstract
Introduction
Hidradenitis suppurativa (HS) is a severe chronic skin disease. Although the pathogenesis remains unclear, at the basis of HS there is an enhancement of the immune and inflammatory response together with a susceptibility to environmental factors. Cytokine dysregulation is crucial in HS severity and progression.
Objectives
The aim of this study was to analyze serum levels of different cytokines focusing on adiponectin concentration and its oligomers in HS patients compared to both obese and healthy subjects.
Methods
The concentrations of adiponectin and cytokines were measured using enzyme-linked immunosorbent assay (ELISA); the oligomeric distribution of adiponectin (low molecular weight (LMW), medium molecular weight (MMW) and high molecular weight (HMW) oligomers)was evaluated through Western Blotting analysis.
Results
Total adiponectin is statistically higher in HS patients compared to matched controls and obese subjects. Interestingly, Adiponectin oligomerization state is altered in HS, with an increase of HMW oligomers. Serum levels of PDGF-BB, IL-1β, IL-5, Il-6, IL12, IL13, IL15, IL-17, GMCSF, INFγ, VEGF and MCP-1 are statistically higher while IL-1ra and RANTES levels are statistically lower in HS patients compared to healthy controls. Interestingly, adiponectin positively correlates with PDGF-BB, and IL-13.
Conclusions
Our data confirmed that the complex network that links metabolism to immune homeostasis is dysregulated in HS and that adiponectin and its HMW oligomers are actively involved in this disease. In addition, the correlation between adiponectin and PDGF-BB, and IL-13 extends the role of this adipokine in modulation of the immune response, in particular regulating the innate immune system rather that the adaptive one. Further researches are needed to clarify the complex inflammatory milieu that characterizes HS syndrome.
Keywords: adiponectin, hidradenitis suppurativa, cytokines, inflammation, immune system
Introduction
Hidradenitis suppurativa (HS) is a complex, chronic inflammatory skin disease characterized primarily by a dysregulation of the innate immune system and by a chronic inflammation that is not only restricted to skin but, especially in severe cases, affects different tissues and organs [2,3]. In particular, the over-activated immune cell infiltration, that is at the basis of HS disease, enhances the inflammatory response through the secretion of a considerable quantity of pro-inflammatory (IL-1β, TNFα, IL-17, INF γ) as well as anti-inflammatory cytokines (IL-10) and chemokines [4–6]. On the other hand, an immunological “priming” in HS comes also from environmental factors such as, smoking-related inflammatory mediators and obesity related pro-inflammatory signals [7]. In particular, in obesity, the increased adipose tissue determines a pro-inflammatory environment due to the imbalance in production of adipokines that contributes to severity and progression of HS disease [8,9]. Recently, it was described a functional interplay among adipose tissue and other organs and tissues whom dysregulation has a key role in inflammation. Indeed, adipose tissue is an endocrine organ that produces several adipocytokines among which adiponectin exerts multivalent beneficial functions; it is abundantly secreted in serum where it circulates as oligomers of different molecular weight: low molecular weight (LMW), medium molecular weight (MMW) and high molecular weight (HMW) [10]. The HMW are the most biologically active oligomers [11]. Adiponectin is involved in the regulation of energy homeostasis, insulin sensitivity and inflammation [12]; interestingly, adiponectin expression is up-regulated in different inflammatory diseases and in some auto-immune diseases, while is down-regulated in metabolic diseases [13]. Regarding hidradenitis, serum adiponectin concentrations were found to be significantly lower, while the levels of the other adipocytokines have been found significantly higher than in controls [14,23].
The aim of this study was to analyze the serum concentrations of 27 cytokines and the most abundant adipocytokine, the adiponectin, in patients affected by HS to investigate the potential relationships with metabolic parameters, disease severity and the risk of HS. We examined cytokines, adiponectin as potential biomarkers of inflammation in HS.
Objectives
To better understand the nature of inflammation in HS and the potential cross link with adipose tissue (AT), the aim of our study was to analyze the expression of different cytokines focusing on adiponectin concentrations and its oligomeric distribution in serum of HS patients respect to both obese and healthy subjects.
Methods
Participants
Fifty-three patients (31 females, 22 males), aged 30.0 ± 13.0 years, were recruited from the Dermatology Unit of the Università degli Studi della Campania “Luigi Vanvitelli”. Subjects were excluded from our study if they met any of the following criteria: age < 18 years, body mass index (BMI) < 17 or > 35, major metabolic disorders (type 2 diabetes, cardiovascular disorders, metabolic syndrome), the presence of concomitant inflammatory cutaneous or systemic disorders and the presence of cancer; were excluded also the patients receiving any systemic treatment which could interfere with the studied parameters. Disease staging was based on the three-degree scale proposed by Hurley. The mean BMI of 29.67 ± 6.1 kg/m2 qualified our patients as overweight. The smoker rate amounted to 62.3%. Forty-two healthy volunteers were recruited from the CEINGE staff, they aged 33 ± 12.0 years old and constituted the control group (BMI = 23.3 ± 3.0); 53 obese subjects, aged 33±12 years old (BMI = 48.4 ± 9.4), were recruited from the Foundation “Salvatore Maugeri” Telese, Italy [15]. All HS patients fulfilled the established HS diagnostic criteria. All subjects signed an informed consent form. The study was approved by the Ethic Committee of the Università degli Studi della Campania “Luigi Vanvitelli” (Prot. 12478/20).
Anthropometric and Biochemical Measurements
Blood samples from 53 HS patients, 42 healthy subjects and 53 obese subjects were collected after a 12-hours overnight fasting period and centrifuged to collect serum. Serum aliquots were immediately frozen in liquid nitrogen and stored at −80°C. For all participants total cholesterol, triglycerides, glycemia, C-Reactive Protein were measured (Table 1). The concentration of total adiponectin was measured in triplicate by an enzyme-linked immunosorbent assay (ELISA) as previously described [16].
Table 1.
Clinical, biochemical and anthropometrical characteristics of HS, obese patients and healthy subjects.
| HS patients (N 53) | Obese subjects (N. 53) | Controls (N 42) | P | |
|---|---|---|---|---|
| Sex (F), N (%) | 31 (53.8) | 33 (62.3) | 20 (47.6) | 0.38 |
| Age mean (±SD), years | 30±13 | 33±12 | 33±12 | 0.37 |
| BMI mean (±SD) | 29.67 ± 6.12 | 48.4 ± 9.4 | 23.3 ± 3.04 | 0.00 |
| Cholesterol mean (±SD) (mg/dL) | 203.54 ± 44.16 | 169.94 ± 37.21 | 191.48 ± 35.22 | 0.00 |
| Triglycerides mean (±SD) (mg/dL) | 103.62 ± 36.97 | 146.81 ± 102.54 | 82.86 ± 50.31 | 0.00 |
| Glycemia mean (±SD) (mg/dL) | 94 ± 19 | 87 ± 28 | 86 ± 17 | 0.17 |
| C-Reactive Protein mean (±SD) (mg/L) | 6.87 ± 8.36 | 8.23 ± 8.48 | - | 0.41 |
| Hurley IN (%) | 16 (30.2) | - | - | - |
| Hurley II N (%) | 28 (52.8) | - | - | - |
| Hurley III N (%) | 9 (17.0) | - | - | - |
| Adiponectin mean (±SD) (γg/mL) | 28.54 ± 4.49 | 20.06 ± 4.71 | 24.67 ± 3.35 | 0.00 |
BMI = body mass index; HS = hidradenitis suppurativa; SD = standard deviation.
The levels of 27 cytokine species (PDGF-BB, IL1β, IL1ra, IL2, IL4, IL5, IL6, IL7, IL8, IL9, IL10, IL12, IL13, IL15, IL17, Eotaxin, FGF, GCSF, GMCSF, INFγ, IP10, MCP-1, IP1α IP1β, RANTES, TNFa, VEGF) were measured in 30 HS patients and in 39 healthy controls using a commercially available kit (Bio-Plex Pro™ Human Cytokine 8-plex Assay). The assay was performed according to the manufacturer’s instructions and the concentrations of cytokines were calculated by comparing reads with a 5-parameter logistic standard curve using a Bioplex-200 instrument (Bio-Rad).
Western Blotting Analysis of Serum Adiponectin
Five micrograms of total serum proteins were treated and subjects to electrophoresis as previously described [17]. The blots were developed by ECL (Amersham Biosciences) with the use of Kodak BioMax Light film and digitalized with a scanner (1.200 dpi) and analyzed by densitometry with the ImageJ software. Each serum sample was tested 2 times in duplicate.
Statistical Analysis
Data is expressed as average ± standard deviation (SD) and median. The meanings of the differences in biochemical parameters between the groups were determined using the Mann-Whitney test and the Chi-square test. To evaluate the relationship with median adiponectin levels, multiple logistic regression was performed. A P value <0.05 was considered to indicate statistically significant results.
Results
Baseline Features and Serum Levels of Adiponectin in HS Patients
The anthropometric and biochemical characteristics of HS patients, sex and age-matched obese and healthy subjects are shown in Table 1. We found statistically significant difference in BMI between HS patients and controls (29.67 ± 6.12 versus 23.3 ± 3.04, P < 0.00) as well as for total adiponectin serum levels (28.25 μg/ml ± 4.49 versus 24.67 μg/ml ± 3.35, P < 0.01); both parameters result significantly higher in HS patients compared to controls. The statistical analysis indicated that the increase of adiponectin levels in HS is independent from BMI and sex, 2 potential confounding factors. Statistical analysis did not reveal significant difference in adiponectin concentrations among the HS groups based on the three Hurley degrees of disease severity. HS Hurley degree of HS patients are reported in Table 1: 30.2% of the patients have a Hurley I, 52.8% Hurley II and 17.0% Hurley III degree of disease severity.
As in literature was reported an opposite trend and to further validate the findings on concentration of adiponectin, we measured the levels of this adipokine in a cohort of 53 obese patients; as shown in Table 1, the comparison of biochemical parameters between HS patients and obese subjects showed a statistically differences in BMI (29.67 ± 6.12 versus 48.4 ± 9.4, P < 0.00) as well as in adiponectin levels; these latter are higher in HS than in obese patients (28.25 μg/ml ± 4.49 versus 20.06 μg/ml ± 4.71, P < 0.00).
Oligomeric Distribution of Adiponectin in HS Patients
To better investigate the involvement of adiponectin in HS patients, we examined the oligomeric profile of this adipokine through the visualization of HMW; MMW and LMW oligomers. Western blot evidenced that the HMW and MMW adiponectin oligomers are increased in serum of HS patients if compared to obese and healthy subjects (Figure 1, P < 0.05).
Figure 1.
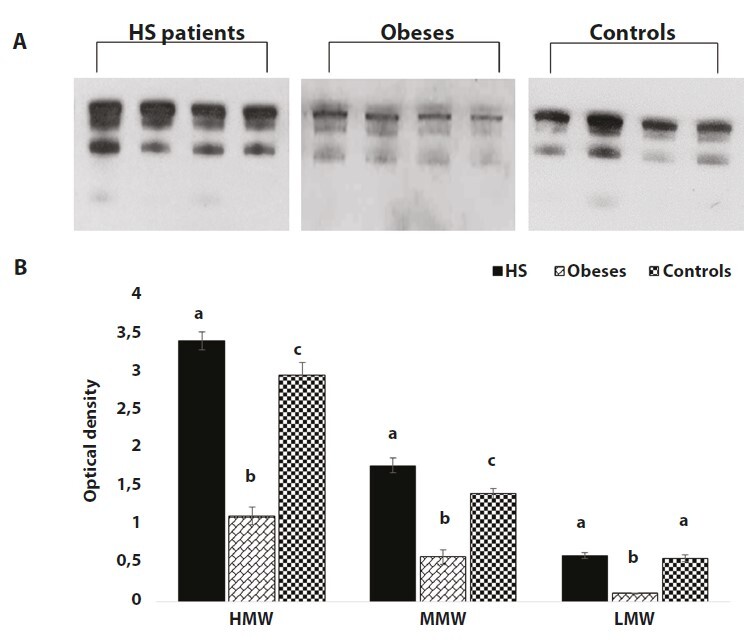
Western blotting analysis shows that adiponectin HMW and MMW oligomers are statistically higher in serum from HS patients compared to obese and healthy subjects. (A) Representative WB image of adiponectin different oligomers (HMW, MMW, LMW) from four HS patients, four obese subjects and four controls. (B) Graphical representation of pixel quantization of adiponectin oligomers analysed in 53 HS patients, 53 obese subjects and 42 controls. For other details see materials and methods. P < 0.05.
Cytokine Concentration in HS Patients and Healthy Controls
To better explore the inflammatory milieu in serum from HS patients, we analyzed a panel of 27 different cytokines (PDGF-BB, IL1β, IL1ra, IL2, IL4, IL5, IL6, IL7, IL8, IL9, IL10, IL12, IL13, IL15, IL17, Eotaxin, FGF, GCSF, GMCSF, INFγ, IP10, MCP-1, IP1α, IP1β, RANTES, TNFα, VEGF). We tested 30 HS patients and 39 controls. The results demonstrated that, among the others, PDGF-BB, IL-1β, IL-5, Il-6, IL12, IL13, IL15, IL-17, GMCSF, INFγ, VEGF and MCP-1 levels were statistically higher while IL-1ra and RANTES levels were statistically lower in the serum of HS patients compared to healthy controls (Table 2).
Table 2.
Cytokine levels (pg/ml) in HS patients and healthy subjects.
| Parameters | Controls (N 39) | HS patients (N 30) | P | |
|---|---|---|---|---|
| Sex N (%) | F | 17 (44%) | 19 (63%) | 0.10 |
| M | 22 (56%) | 11 (37%) | ||
| Age, mean (±SD) | 33.8 (6.8) | 29.6 (13.5) | 0.096 | |
| BMI, mean (±SD) | 25.5 (3.7) | 26.6 (3.8) | 0.23 | |
| IL-1β, mean (±SD) | 2.0 (0.5) | 2.3 (0.5) | 0.014 | |
| IL-12, mean (±SD) | 11.3 (1.3) | 13.5 (3.9) | 0.002 | |
| IL-13, mean (±SD) | 5.5 (1.1) | 8.4 (4.1) | <0.001 | |
| IL-15, mean (±SD) | 347.2 (25.0) | 390.3 (38.4) | <0.001 | |
| IL-17, mean (±SD) | 25.0 (3.9) | 27.2 (5.2) | 0.048 | |
| GM-CSF, mean (±SD)) | 12.8 (0.7) | 14.5 (1.5) | <0.001 | |
| IFNγ, mean (±SD) | 13.3 (3.1) | 18.6 (4.7) | <0.001 | |
| MCP-1 (MCAF), mean (±SD) | 36.9 (15.0) | 56.1 (31.7) | 0.001 | |
| RANTES, median (IQR) | 6833.4 (5469.2, 8214.1) | 45145.1 (20829.6, 93670.0) | <0.001 | |
| PDGF-BB, median (IQR) | 1638.5 (1180.5, 1991.2) | 2306.6 (1701.6, 3208.0) | <0.001 | |
| IL-1ra, median (IQR) | 371.5 (333.0, 446.4) | 333.0 (257.0, 400.0) | 0.036 | |
| IL-5, median (IQR) | 52.3 (49.0, 55.5) | 58.5 (52.3, 67.1) | 0.002 | |
| IL-6, median (IQR) | 7.6 (7.0, 8.1) | 9.0 (7.8, 10.2) | <0.001 | |
| VEGF, mean (SD) | 417.6 (40.2) | 452.3 (52.7) | 0.003 | |
BMI = body mass index; HS = hidradenitis suppurativa; SD = standard deviation. Acronym list: IL (interleukin); Granulocyte-Macrophage Colony-Stimulating (GM-CSF); Interferon (INF); Monocytes Chemoattractant Protein(MCP); Platelet-Derived Growth Factor-BB (PDGF-BB); Vascular Endothelial Growth Factor (VEGF).
Next, to investigate whether adiponectin is functionally related with any tested cytokines, we divided the HS patients in two subgroups using the median value of adiponectin concentration (27.8 μg/mL) as an arbitrary cut-off. According to adiponectin concentrations, patients with higher adiponectin concentrations (ie with values above the median) represented subgroup 1 and patients with lower adiponectin concentrations (ie with values under the median) represented subgroup 2. Statistical analysis performed using the univariate model showed that the patients with higher levels of adiponectin (subgroup 1) have also significantly higher PDGF-BB and a similar trend versus IL-13 (Table 3).
Table 3.
Univariate analysis of anthropometric, clinical parameters and cytokines levels (pg/ml) on the basis of adiponectin levels: median value of adiponectin (27.8 μg/ml) was used as an arbitrary cut-off.
| Parameters | Adiponectin ≤ 27.8 | Adiponectin > 27.8 | P | |
|---|---|---|---|---|
| N = 14 | N = 16 | |||
| Sex | M, N (%) | 7 (50%) | 4 (25%) | 0.16 |
| F, N (%) | 7 (50%) | 12 (75%) | ||
| Age, mean (±SD) | 30.1 (12.1) | 29.3 (15.1) | 0.87 | |
| BMI, mean (±SD) | 27.3 (3.7) | 26.0 (4.0) | 0.36 | |
| PDGF-BB, (median) (IQR) | 2984.9 (2226.8, 3463.2) | 1823.9 (1306.3, 2507.5) | 0.020 | |
| IL-5, median (IQR) | 61.5 (55.5, 65.7) | 55.5 (48.1, 68.1) | 0.18 | |
| IL-13, median (IQR) | 8.8 (7.3, 9.7) | 5.8 (4.9, 9.6) | 0.05 | |
| GM-CSF, median (IQR) | 14.7 (13.9, 15.5) | 13.5 (12.7, 15.8) | 0.15 | |
| Hurley | I | 5 (36%) | 6 (38%) | 0.98 |
| II | 5 (36%) | 6 (38%) | ||
| III | 4 (29%) | 4 (25%) |
Conclusions
Hidradenitis (HS) is a severe chronic inflammatory skin disease primarily due to the alteration of immunity and to chronic inflammation [2,18]. A functional interconnection between immune system and adipose tissue, link observed in patients affected by metabolic disorders in which the dysregulation of energy metabolism negatively affects the immune system and viceversa. Indeed, in obese or overweight people there is a greater frequency of autoimmune diseases such as rheumatoid arthritis, type I diabetes and HS [24]. This functional interconnection is guaranteed by the hormonal activity of the adipose tissue through the secretion of adipokines such as adiponectin. In literature, numerous studies support the hypothesis that high levels of adiponectin represent a key component of the body protective systemic response to the chronic inflammatory processes and immune system alterations [17,18,21].
In this study, to investigate the nature of the inflammatory milieu in HS and the potential contribute of adipose tissue, we analyzed several cytokines focusing on the most abundant adipokine, ie adiponectin and its oligomeric profile. Interestingly, we found increased levels of adiponectin and HMW oligomers in HS patients compared to both healthy and obese subjects independently from BMI. To our knowledge, there are two studies describing adiponectin concentration in HS that found decreased serum level of adiponectin in the patients [22,23]. The discrepancy with our results may be traced back to clinical and biochemical differences of the considered patients: the study by Malara et al. analyzed patients with a very high BMI (33 versus 29.6 of our cohort), while Gonzalez-Lopez considered a cohort of patients with a more severe clinical phenotype of HS [22,23]. It is to notice, however, that we excluded that BMI might represent a confounding factor for adiponectin expression in HS patients; indeed, HS patients are more likely to have obesity and metabolic syndrome and overweight people have a greater incidence of HS [24].
In addition, although the significance of the molecular distribution of adiponectin is still largely unknown, it has been shown that the HMW oligomers have a stronger biological meaning and is the most important contributor to adiponectin functions [10]. Our findings that HS is associated with high circulating adiponectin levels, combined with a shift towards the HMW forms reinforce the hypothesis that adiponectin has a strong functional role in regulating inflammation in HS. The specificity of adiponectin role in HS is confirmed also by the significant difference of its concentrations that we found in the two populations, HS and obese subjects.
Next, in this study, we analyzed different cytokine expression previously reported to spill-over from the skin lesions into the systemic circulation resulting in heightening risk for systemic inflammation in HS patients [1]. Among the others, we found that PDGF-BB, IL-1β, IL-5, Il-6, IL12, IL13, IL15, IL-17, GMCSF, INFγ, VEGF and MCP-1 levels are statistically higher in HS patients while IL-1ra and RANTES levels are statistically lower in the serum of HS patients compared to healthy controls. Serum cytokine levels are very often altered in HS patients [25,23]. In accordance with our data, the levels of the pro-inflammatory IL-17 cytokine, whose production is made by neutrophils and Th17 cells, is increased in HS patients [26,27]. IL-17 is crucial in determining the inflammatory process of HS, inducing the expression of other pro-inflammatory cytokines, such as IL1β and TNFα and stimulating the activation of adaptive immune cells [1,28]. On the other hand, one study found no differences in IL-17 levels between patients and controls [29]. Regarding IFN-γ, no statistically decrease was found in the serum of HS patients while a significant difference was found in another study [7].
Although not significant, our data also evidenced that IL-10 is higher in HS patients than in controls suggesting that the immune system is compensating the dysregulation in Th/Treg ratio typical of HS compatible with the mild phenotype of most of our patients. In accordance with our results, increased serum IL-10 levels compared to control was reported in one study [30]; in two other studies no statistical difference was found [31,32].
Finally, for the first time, we correlated cytokines expression level to adiponectin concentration. We found that adiponectin correlates with PDGF-BB, and IL-13 but not with IL-17, IL-1β, and INFγ suggesting that adiponectin function might be related to the innate immune system activation rather that the adaptive one, exerting anti-inflammatory actions. Previously, adiponectin has been demonstrated to directly and specifically bind PDGF-BB in smooth muscle cells suppressing their proliferation and migration [33] suppressing the development of atherosclerosis, promoting inflammation. Arita et al. demonstrated that the inhibitory effects of adiponectin towards PDGF-BB result in suppression of vasculogenesis and inflammation [33]. The association between adiponectin and PDGF-BB in HS patients suggests that the adipokine probably counteracts the inflammatory process triggered by the disease.
As IL-13 has been described as anti-inflammatory factor in the adipose tissue, the direct correlation with adiponectin further confirms that adiponectin is acting as anti-inflammatory molecule [34]. In addition, IL-13 has been involved in the maintenance of macrophages in an anti-inflammatory state as M2 phenotype supporting the hypothesis that adiponectin might participate in the regulation of the innate immune response [35].
There are two main limitations in the present study, one is the relatively small number of patients and the other is the absence of a large cohort of severe patients. In addition, the great heterogeneity in age, gender, environmental factors and potential comorbidities among the HS analyzed patients in different studies may be at the basis of the variability found in the expression of cytokines.
In conclusion, our data confirmed that the complex network that links together metabolism to immune homeostasis is dysregulated in HS and that adiponectin and its HMW oligomers are not only actively involved in the HS but that interacts with the complex inflammatory systemic milieu made by pro-inflammatory cytokines. In addition, the correlation between adiponectin and PDGF-BB, and IL-13 extends the role of this adipokine in modulation of the immune response suggesting that adiponectin might act regulating the innate immune system rather that the adaptive one. Further researches are needed to clarify the complex inflammatory milieu that characterizes HS syndrome.
Footnotes
Funding: POR CAMPANIA FESR 2014/2020. project-CUP: B21C17000030007
Competing interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways Into a Cohesive Pathogenic Model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabat R, Jemec GBE, Matusiak Ł, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. doi: 10.1038/s41572-020-0149-1. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TV, Damiani G, Orenstein LAV, Hamzavi I, Jemec GB. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35(1):50–61. doi: 10.1111/jdv.16677. [DOI] [PubMed] [Google Scholar]
- 4.Constantinou CA, Fragoulis GE, Nikiphorou E. Hidradenitis suppurativa: infection, autoimmunity, or both? Ther Adv Musculoskelet Dis. 2019;11 doi: 10.1177/1759720X19895488. 1759720X19895488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher JM, Moran B, Petrasca A, Smith CM. IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa. Clin Exp Immunol. 2020;201(2):121–134. doi: 10.1111/cei.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay C, Kuraitis D, Murina A. Serum cytokine levels in patients with hidradenitis suppurativa vary with race. J Am Acad Dermatol. 2021;84(5):1405–1406. doi: 10.1016/j.jaad.2020.08.084. [DOI] [PubMed] [Google Scholar]
- 7.Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Res. 2018;7:1923. doi: 10.12688/f1000research.17268.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiricozzi A, Raimondo A, Lembo S, et al. Crosstalk between skin inflammation and adipose tissue-derived products: pathogenic evidence linking psoriasis to increased adiposity. Expert Rev Clin Immunol. 2016;12(12):1299–1308. doi: 10.1080/1744666X.2016.1201423. [DOI] [PubMed] [Google Scholar]
- 9.Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47–56. doi: 10.2147/ITT.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuerq C, Morineau G, Dufour-Rainfray D, et al. Rôles biologiques à multiples facettes de l’adiponectine [Mutltifaceted biological roles of adiponectin] Ann Biol Clin (Paris) 2020;78(3):243–252. doi: 10.1684/abc.2020.1562. [DOI] [PubMed] [Google Scholar]
- 11.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nigro E, Scudiero O, Monaco ML, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi HM, Doss HM, Kim KS. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int J Mol Sci. 2020;21(4):1219. doi: 10.3390/ijms21041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang SW, Whitley MJ, Mariottoni P, Jaleel T, MacLeod AS. Hidradenitis Suppurativa: Host-Microbe and Immune Pathogenesis Underlie Important Future Directions. JID Innovations. 2021;1(1):100001. doi: 10.1016/j.xjidi.2021.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbi G, Polito R, Monaco ML, et al. Adiponectin Expression and Genotypes in Italian People with Severe Obesity Undergone a Hypocaloric Diet and Physical Exercise Program. Nutrients. 2019;11(9):2195. doi: 10.3390/nu11092195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigro E, Scudiero O, Ludovica Monaco M, Polito R, Schettino P, Grandone A, Perrone L, Miraglia Del Giudice E, Daniele A. Adiponectin profile and Irisin expression in Italian obese children: Association with insulin-resistance. Cytokine. 2017;94:8–13. doi: 10.1016/j.cyto.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Polito R, Nigro E, Fei L, et al. Adiponectin Is Inversely Associated With Tumour Grade in Colorectal Cancer Patients. Anticancer Res. 2020;40(7):3751–3757. doi: 10.21873/anticanres.14364. [DOI] [PubMed] [Google Scholar]
- 18.Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9 doi: 10.12688/f1000research.26083.1. F1000 Faculty Rev-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signoriello E, Lus G, Polito R, et al. Adiponectin profile at baseline is correlated to progression and severity of multiple sclerosis. Eur J Neurol. 2019;26(2):348–355. doi: 10.1111/ene.13822. [DOI] [PubMed] [Google Scholar]
- 20.Pecoraro A, Nigro E, Polito R, et al. Total and High Molecular Weight Adiponectin Expression Is Decreased in Patients with Common Variable Immunodeficiency: Correlation with Ig Replacement Therapy. Front Immunol. 2017;8:895. doi: 10.3389/fimmu.2017.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380(1–2):24–30. doi: 10.1016/j.cca.2007.01.026. Epub 2007 Feb 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-López MA, et al. Circulating levels of adiponectin, leptin, resistin and visfatin in non-diabetic patients with hidradenitis suppurativa (2020) Arch Dermatol Res. 312:595–600. doi: 10.1007/s00403-019-02018-4. [DOI] [PubMed] [Google Scholar]
- 23.Malara A, Hughes R, Jennings L, et al. Adipokines are dysregulated in patients with hidradenitis suppurativa. Br J Dermatol. 2018;178(3):792–793. doi: 10.1111/bjd.15904. [DOI] [PubMed] [Google Scholar]
- 24.Kraszula L, Jasińska A, Eusebio M, Kuna P, Głąbiński A, Pietruczuk M. Evaluation of the relationship between leptin, resistin, adiponectin and natural regulatory T cells in relapsing-remitting multiple sclerosis. Neurol Neurochir Pol. 2012;46(1):22–28. doi: 10.5114/ninp.2012.27211. [DOI] [PubMed] [Google Scholar]
- 25.Wolk K, Sabat R. Adipokines in psoriasis: An important link between skin inflammation and metabolic alterations. Rev Endocr Metab Disord. 2016;17(3):305–317. doi: 10.1007/s11154-016-9381-0. [DOI] [PubMed] [Google Scholar]
- 26.Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174(3):514–521. doi: 10.1111/bjd.14214. [DOI] [PubMed] [Google Scholar]
- 27.Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Bernardini N, Skroza N, Tolino E, et al. IL-17 and its role in inflammatory, autoimmune, and oncological skin diseases: state of art. Int J Dermatol. 2020;59(4):406–411. doi: 10.1111/ijd.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomi R, Schlapbach C, Yawalkar N, Simon D, Yerly D, Hunger RE. Elevated levels of the antimicrobial peptide LL-37 in hidradenitis suppurativa are associated with a Th1/Th17 immune response. Exp Dermatol. 2018;27(2):172–177. doi: 10.1111/exd.13482. [DOI] [PubMed] [Google Scholar]
- 30.Kanni T, Tzanetakou V, Savva A, et al. Compartmentalized Cytokine Responses in Hidradenitis Suppurativa. PLoS One. 2015;10(6):e0130522. doi: 10.1371/journal.pone.0130522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiménez-Gallo D, de la Varga-Martínez R, Ossorio-García L, Albarrán-Planelles C, Rodríguez C, Linares-Barrios M. The Clinical Significance of Increased Serum Proinflammatory Cytokines, C-Reactive Protein, and Erythrocyte Sedimentation Rate in Patients with Hidradenitis Suppurativa. Mediators Inflamm. 2017;2017:2450401. doi: 10.1155/2017/2450401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolk K, Wenzel J, Tsaousi A, et al. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br J Dermatol. 2017;177(5):1385–1393. doi: 10.1111/bjd.15424. [DOI] [PubMed] [Google Scholar]
- 33.Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105(24):2893–8. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 34.Kwon H, Laurent S, Tang Y, Zong H, Vemulapalli P, Pessin JE. Adipocyte-specific IKKβ signaling suppresses adipose tissue inflammation through an IL-13-dependent paracrine feedback pathway. Cell Rep. 2014;9(5):1574–1583. doi: 10.1016/j.celrep.2014.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirola L, Ferraz JC. Role of pro- and anti-inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World J Biol Chem. 2017;8(2):120–128. doi: 10.4331/wjbc.v8.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett. 2008;582(12):1719–1724. doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]


