Abstract
In the present study, we describe the ability of Trypanosoma cruzi trypomastigotes to stimulate the synthesis of β-chemokines by macrophages. In vivo infection with T. cruzi led to MIP-1α, RANTES, and JE/MCP1 mRNA expression by cells from peritoneal inflammatory exudate. In addition, in vitro infection with T. cruzi resulted in expression of β-chemokine MIP-1α, MIP-1β, RANTES, and JE mRNA by macrophages. The expression of the β-chemokine MIP-1α, MIP-1β, RANTES, and JE proteins by murine macrophages cultured with trypomastigote forms of T. cruzi was confirmed by immunocytochemistry. Interestingly, macrophage infection with T. cruzi also resulted in NO production, which we found to be mediated mainly by β-chemokines. Hence, treatment with anti-β-chemokine-specific neutralizing antibodies partially inhibited NO release by macrophages incubated with T. cruzi parasites. Further, the addition of the exogenous β-chemokines MIP-1α, MIP-1β, RANTES, and JE/MCP-1 induced an increased T. cruzi uptake, leading to enhanced NO production and control of parasite replication in a dose-dependent manner. l-NMMA, a specific inhibitor of the l-arginine–NO pathway, caused a decrease in NO production and parasite killing when added to cultures of macrophages stimulated with β-chemokines. Among the β-chemokines tested, JE was more potent in inhibiting parasite growth, although it was much less efficient than gamma interferon (IFN-γ). Nevertheless, JE potentiates parasite killing by macrophages incubated with low doses of IFN-γ. Together, these results suggest that in addition to their chemotactic activity, murine β-chemokines may also contribute to enhancing parasite uptake and promoting control of parasite replication in macrophages and may play a role in resistance to T. cruzi infection.
The infection of inbred mice with Trypanosoma cruzi, the etiological agent of Chagas’ disease, leads to an acute infection characterized by the presence of parasites in the blood, a powerful stimulation of the immune system, and a strong inflammatory reaction, at either the inoculation site or the heart (5). The resistance of mice to infection with T. cruzi has been associated with the production of the proinflammatory cytokine interleukin-12 (IL-12), which triggers the production of gamma interferon (IFN-γ) by NK and T cells (1, 7). The IFN-γ produced in turn activates macrophages to release nitric oxide and kill the obligate intracellular amastigote forms of the parasite (15, 28). Tumor necrosis factor alpha (TNF-α), another cytokine associated with macrophage activation, provides a second signal to induce microbicidal activity in IFN-γ-activated macrophages by stimulating NO production (12). Since T. cruzi-infected macrophages produce TNF-α, this cytokine appears to exert its trypanocidal activity in an autocrine fashion (25).
The inflammatory reaction observed during both the acute and chronic phases of infection appears to play a major role in parasite-elicited pathogenesis. In the affected tissues, there is a local production of inflammatory mediators, which drive an intense migration of leukocytes during the interaction of parasite and host cells (8). Recently, there has been much interest in chemokines, a novel class of inflammatory mediators which appears to play a major role in mediating the extravasation and accumulation of specific leukocyte subsets in acute and chronic inflammatory processes in several diseases. Chemokines can be released by a range of different cell types after activation and have potent chemotactic activity both in vitro and in vivo. Chemokine sequences usually have four conserved cysteine residues, and based on the position of the first two cysteine residues, these proteins can be divided into four subfamilies: the C-X-C (α), C-C (β), C (γ), and C-X3-C (δ) families (4, 9, 16, 19).
In addition to having profound effects on the locomotion of leukocytes, chemokines appear to affect several other biological phenomena, including T-lymphocyte proliferation (27). Th1-Th2 differentiation (14), NK cell migration and activation (22, 23), and macrophage production of IL-1 and IL-6 (18). These effects might be important for the host in mediating resistance to microbial agents, such as virus (10), fungi (11, 14, 17) and helminths (14). Since some of these pathogens are susceptible to killing mediated by NO (2, 20), and a recent report suggests that chemokines activate human macrophages to kill T. cruzi (29), we hypothesized that chemokines may be secreted by infected cells and may mediate resistance to infection. These chemokine-mediated effects may depend not only on the ability of chemokines to induce the attraction of leukocytes but also on their ability to induce NO synthase (NOS) activation.
For this purpose, we examined whether T. cruzi trypomastigotes triggered β-chemokine (MIP-1α, MIP-1β, RANTES, and JE/MCP-1) mRNA expression and protein production and whether these chemokines were involved in the regulation of NO production by infected murine macrophages. The choice of chemokines to be investigated was based on our preliminary studies, which demonstrated the expression of MIP-1α, MIP-1β, RANTES, and JE/MCP-1 mRNAs in the hearts of T. cruzi-infected mice (26a). In the present study, we found mRNA and protein expression of MIP-1α, MIP-1β, RANTES, and JE/MCP-1 in T. cruzi-infected macrophages. Interestingly, macrophage-derived chemokines appeared to drive NO production in infected cells. Moreover, when chemokines were added to the infected cells, they induced NO production and inhibited the intracellular growth of the parasites in an NO-dependent manner. Finally, the β-chemokine JE/MCP-1 synergized with IFN-γ to control parasite replication in vitro.
MATERIALS AND METHODS
Mice.
Female C3H/HeJ or BALB/c mice, 6 to 8 weeks old, were bred and maintained under standard conditions in the animal house of the Department of Immunology, University of São Paulo, Ribeirão Preto, Brazil.
Parasites.
The Y strain of T. cruzi was used in this study. Trypomastigote forms were grown and purified from a monkey fibroblast cell line (LLC-MK2).
Macrophage cultures for RNA extraction.
C3H/HeJ mouse inflammatory macrophages were harvested from peritoneal cavities three days after the injection of 1 ml of 3% sodium thioglycolate (Sigma Chemical Co., St. Louis, Mo.). The cells were washed in Hanks’ medium and resuspended to 106/ml in RPMI-C (RPMI 1640 [Flow Laboratories, Inc., MCLean, Va.] supplemented with 5% fetal bovine serum [HyClone, Logan, Utah], 5 × 10−5 M 2-mercaptoethanol, 2 mM l-glutamine, and antibiotics [all from Sigma Chemical Co.]). The adherent cells were obtained after a 2- to 4-h incubation of single cell suspensions in 24-well tissue culture plates at 37°C. Nonadherent cells were removed by exhaustive washing with Hanks’ medium. Parasites were added in a 1:1 parasite/cell ratio and incubated for 6 h at 37°C in a humidified chamber containing 5% CO2. The cells were then washed three times with Hanks’ medium. One milliliter of Trizol LS reagent (Life Technologies, Grand Island, N.Y.) was added to each 107 cells, incubated at room temperature for 5 min, and stored at −70°C until RNA was extracted. RNA was also purified from peritoneal cavity cells harvested from the mice 6 h after intraperitoneal injection of 5 × 105 trypomastigote forms in 200 μl of phosphate-buffered saline (PBS). As a control, we used cells from mice inoculated with PBS only.
Total RNA extraction and cDNA preparation by reverse transcription (RT).
The extraction of total RNA was performed with the Trizol LS reagent according to the instructions of the manufacturer. Briefly, the samples were homogenized and 0.2 ml of chloroform (Sigma) was added to each 1 ml of Trizol reagent. Samples were then centrifuged at 12,000 × g for 15 min at 4°C, and the aqueous phase was transferred to a clean tube. The same volume of isopropyl alcohol was added, and the samples were mixed in a vortex and incubated for 15 min at −20°C to precipitate the RNA from the aqueous phase. After a further centrifugation, the RNA pellet was washed in 75% ethanol, and samples were then suspended in water at 0.5 μg of RNA/μl. Copy DNA was synthesized with Superscript II reverse transcriptase (Gibco BRL, Gaithersburg, Md.).
Chemokine mRNA detection.
β-Chemokine (MIP-1α, MIP-1β, RANTES, JE/MCP-1, and TCA-3) and β-actin mRNA expression was analyzed by RT-PCR. The primer sequences and PCR product sizes are shown in Table 1. PCRs were performed with Taq polymerase (Gibco) in a PTC-100 thermal cycler (MJ Research, Watertown, Mass.). The reaction conditions were 35 cycles of 1 min at 94°C, 1 min at 54°C, and 2 min at 72°C, with a final extension step of 7 min at 72°C. For each set of primers, a negative sample (water) was run in parallel. The PCR products were separated by acrylamide gel electrophoresis and stained with silver nitrate. The PCR method for the chemokines tested has been validated in the laboratory with plasmids containing the gene for each chemokine (kindly provided by J. Farber, National Institutes of Allergy and Infectious Diseases, National Institutes of Health).
TABLE 1.
Primer sequences and PCR product sizes
| Gene | Primer sequence | PCR product size (bp) |
|---|---|---|
| MIP-1α | CGC GGA TCC CGG AAG ATT CCA CGC CAA TTC | 448 |
| CGC GGA TCC GGT GAG GAA CGT GTC CTG AAG | ||
| MIP-1β | CGC GGA TCC CCC ACT TCC TGC TGT TTC TCT TAC | 444 |
| CGC GGA TCC AGC AGA GAA ACA GCA ATG GTG G | ||
| JE | CCG GAA TTC CAC TCA CCT GCT GCT ACT CAT TCA C | 505 |
| CCG GAA TTC GGA TTC ACA GAG AGG GAA AAA TGG | ||
| RANTES | CGC GGA TCC CCA CGT CAA GGA GTA TTT CTA CAC C | 326 |
| CGC GGA TCC CTG GTT TCT TGG GTT TGC TGT G | ||
| TCA-3 | TGT TAC AGA AAG ATG GGC TCC TCC | 324 |
| TCC AAG AAA CAG AGG CAG CG | ||
| β-Actin | TGG AAT CCT GTG GCA TCC ATG AAA C | 349 |
| TAA AAC GCA GCT CAG TAA CAG TCC G |
Immunohistochemical analysis for β-chemokines.
Thioglycolate-elicited BALB/c or C3H/HeJ macrophages were incubated or not with culture-derived T. cruzi trypomastigotes in a parasite/cell ratio of 5:1 for 2 h. Extracellular parasites were removed, and the cells were incubated at 37°C in 5% CO2 for 18 h, washed with PBS, and fixed with ice-cold acetone for 10 s. The slides were placed in a humidified chamber, and endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 20 min, followed by incubation with a protein-blocking solution. The slides were washed with PBS and incubated overnight with goat anti-mouse MIP-1α, MIP-1β, or RANTES (Santa Cruz Biotechnology, Santa Cruz, Calif.) or rabbit anti-mouse JE/MCP-1 (a kind gift of N. Lukacs, University of Michigan) diluted 100 times in PBS–1% bovine serum albumin. After extensive washes and incubation for 30 min with biotin-labeled rabbit anti-goat or goat anti-rabbit antibody (Vector Laboratories, Burlingame, Calif.), the reaction product was detected with avidin-biotin-peroxidase complex (Vector) and the color was developed with 9-amino-3-ethyl carbazole (Sigma). The slides were counterstained with Mayer hematoxylin. Controls were performed by incubating cells with nonimmune goat or rabbit immunoglobulin G and proceeding as described above.
Macrophage microbiostatic activity.
Peritoneal macrophages were harvested from mice injected 3 days previously with 1 ml of 3% (wt/vol) sodium thioglycolate (Sigma). Chamber slides (Nunc Inc., Naperville, Ill.) were plated with the cells (106/ml) and incubated overnight. Adherent cells were infected at a parasite/cell ratio of 1:1 or 5:1 for 120 min in the presence or absence of different concentrations of chemokines. Extracellular parasites were removed with six washes of RPMI 1640, and the cells were incubated at 37°C in 5% CO2 with or without different concentrations of recombinant murine MIP-1α, MIP-1β, RANTES, JE/MCP-1 (R&D Systems, Minneapolis, Minn.), or IFN-γ (Gibco) or pertussis toxin (PT) (30 ng/ml) (Gibco), l-NMMA (an l-arginine analogue) (Sigma), or goat anti-murine polyclonal antibody to MIP-1α, MIP-1β, RANTES, and JE/MCP-1 (1 μg/ml) (R&D Systems). The supernatants were harvested and assayed for nitrite concentration. The growth of parasites in macrophages was evaluated by counting the trypomastigotes released at various times after infection and by counting the intracellular amastigote forms at 4 and 48 h postinfection, as previously described (25).
Quantification of NO.
The nitrite concentration in the culture supernatants was assayed in a microplate by mixing 0.1 ml of culture supernatant with 0.1 ml of Griess reagent (15). The absorbance at 540 nm was read 10 min later, and the NO2− concentration was determined by reference to a standard curve of 1 to 100 μM NaNO2.
RESULTS
Trypomastigote-induced β-chemokine mRNA expression and protein production in infected macrophages.
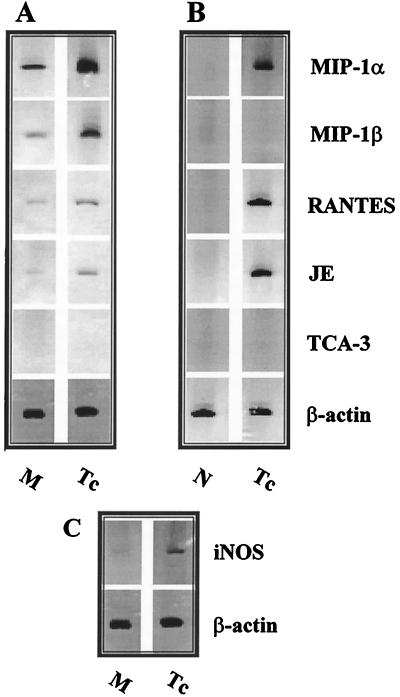
After incubation of inflammatory peritoneal macrophages with T. cruzi trypomastigote forms for 6 h, total RNA was extracted and β-chemokine mRNA expression was assessed by RT-PCR. Message for MIP-1α, MIP-1β, RANTES, and JE, but not for TCA-3, was detected in macrophages incubated with medium only. However, after incubation with parasites, there was a marked increase of message for the β-chemokines MIP-1α, MIP-1β, RANTES, and JE/MCP-1 (Fig. 1A) and for inducible nitric oxide synthase (iNOS) (Fig. 1C). Similar results were obtained when we used an unprimed J774 macrophage cell line (data not shown). Cells harvested from the peritoneal cavities of mice previously injected with T. cruzi trypomastigote forms, but not from control (PBS-treated) animals, also expressed mRNA for MIP-1α, RANTES, and JE/MCP1 (Fig. 1B). In contrast to macrophages infected in vitro, cells from infected animals did not express mRNA message for MIP-1β (Fig. 1). Message for TCA-3 was not detected in either experimental situation (Fig. 1).
FIG. 1.
T. cruzi trypomastigotes induce β-chemokine expression in murine macrophages. Total RNA was extracted from thioglycolate-elicited peritoneal macrophages (A and C) cultured in the presence of medium (M) alone or with T. cruzi trypomastigotes (Tc) in a parasite/cell ratio of 1:1 for 6 h at 37°C in a humidified chamber containing air plus 5% CO2. Total RNA was extracted from C3H/HeJ peritoneal cells (B) harvested from normal (N) mice and from mice injected 6 h before with 105 T. cruzi trypomastigote forms. cDNA was synthesized, and PCR was performed with primers for the β-chemokines and for β-actin. Equal amounts of DNA were loaded into each well. The results shown are representative of five different experiments.
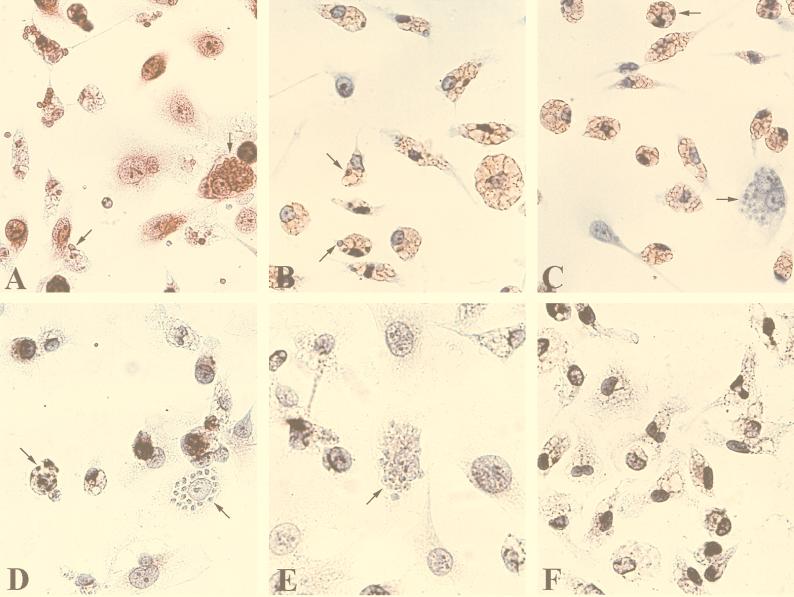
In order to confirm that mRNA message was being translated into protein, the ability of T. cruzi to induce β-chemokine production in mouse macrophages was analyzed by immunoperoxidase staining. JE/MCP-1 (Fig. 2A), MIP-1α (Fig. 2B), MIP-1β (Fig. 2C), and RANTES (Fig. 2D) were detected in macrophage cultures 18 h after infection with T. cruzi. We can see that there are stained cells (indicating the presence of chemokines) and some that are not stained. The majority of infected cells are stained, although some infected cells are not (this represents less than 5% of infected cells and is more commonly observed in heavily infected macrophages). Similarly, most uninfected cells, within the population that was exposed to T. cruzi, are stained. In the absence of the parasite, only a weak staining was detected when the anti-JE/MCP-1 (Fig. 2F), anti-MIP-1α, anti-MIP-1β, and anti-RANTES antibodies were used (data not shown). The addition of normal goat (Fig. 2E) or normal rabbit serum (data not shown) to infected macrophages resulted in the absence of detectable staining. Similar results were obtained when macrophages harvested from BALB/c or C3H/HeJ mice were used.
FIG. 2.
T. cruzi induces β-chemokine production by mouse peritoneal macrophages. Immunoperoxidase staining for β-chemokines of thioglycolate-elicited murine macrophages exposed (A to E) or not (F) to culture-derived T. cruzi trypomastigotes. The cells were incubated with anti-JE/MCP-1 (A and F), anti-MIP-1α (B), anti-MIP-1β (C), and anti-RANTES (D), or normal goat serum (E). 9-Amino-3-ethyl carbazole was used as the peroxidase substrate to generate a brown-staining signal. The arrows indicate intracellular amastigote forms. Magnification, ×364.
β-Chemokines induce NO production by T. cruzi-infected macrophages.
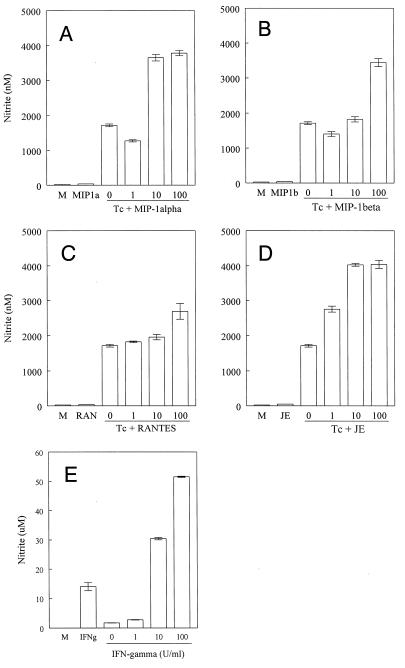
Since T. cruzi induced β-chemokine mRNA expression and protein production, we examined whether β-chemokines could mediate resistance against this infection. Since NO is involved in resistance against the parasite (15, 28), the production of NO by macrophages cultured with recombinant chemokines and trypomastigote forms of T. cruzi was evaluated. The addition of parasites to macrophages induced a small but significant production of NO2− (1.7 μM). Interestingly, the addition of 100 ng of β-chemokines/ml to the macrophage cultures in the presence, but not in the absence, of trypomastigote forms resulted in a significant increase in the release of NO (Fig. 3). JE/MCP-1 was the most potent chemokine tested, as a significant production of NO was detected in concentrations of JE/MCP-1 as low as 1 ng/ml (Fig. 3D). For comparison with the effects of the chemokines and in agreement with our previous observations (15, 28), addition of 10 and 100 U of recombinant murine IFN-γ/ml resulted in high levels of NO production by infected macrophages (Fig. 3).
FIG. 3.
β-Chemokines induce NO production in T. cruzi-infected macrophages. Thioglycolate-elicited murine macrophages were incubated with culture-derived T. cruzi trypomastigotes (Tc) in a parasite/cell ratio of 1:1 for 2 h, and the extracellular parasites were removed. This was followed by 48 h of incubation, with or without the indicated concentrations (in nanograms per milliliter) of recombinant murine MIP-1α (A), MIP-1β (B), RANTES (C), JE/MCP-1 (D), and IFN-γ (E), at 37°C in a humidified chamber containing 5% CO2. The supernatants were harvested, and the nitrite concentration was assayed by the Griess method. The bars represent the means ± standard deviations of triplicate samples. The results shown are representative of three independent experiments.
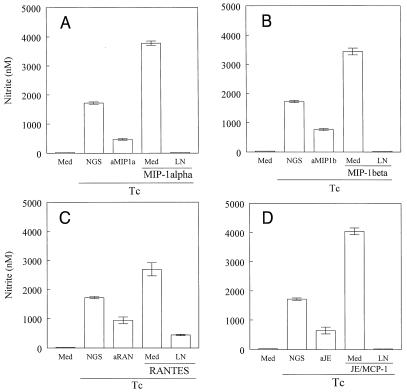
Next, we evaluated the possibility that the small amounts of NO detected in supernatants of T. cruzi-infected macrophages could be a consequence of endogenously produced β-chemokines. To address this possibility, we added neutralizing antibodies against MIP-1α, MIP-1β, RANTES, or JE/MCP-1 to T. cruzi-infected macrophages and assayed the production of NO. Whereas addition of normal goat serum had no effect on macrophage activation, pretreatment with anti-β-chemokine antibodies resulted in a significant inhibition (around 50%) of parasite-induced NO release (Fig. 4). These results suggest that active endogenous chemokines are secreted by T. cruzi-infected cells. To circumvent the possibility that nitrite production could be derived from a nonrelated pathway, we added the iNOS inhibitor (l-NMMA) to infected macrophages cultured with β-chemokines. The results showed that addition of l-NMMA to the culture led to a drastic decrease in the nitrite detected in the supernatants. The addition of a combination of neutralizing antibodies against chemokines did not result in complete inhibition of NO production (data not shown).
FIG. 4.
l-NMMA inhibits β-chemokine-induced NO production by infected macrophages. Thioglycolate-elicited C3H/HeJ macrophages were incubated with culture-derived T. cruzi trypomastigotes (Tc) in a parasite/cell ratio of 1:1 for 2 h, and the extracellular parasites were removed. This was followed by 48 h of incubation with or without MIP-1α (A), MIP-1β (B), RANTES (C), or JE/MCP-1 (D) (all at 100 ng/ml). Specific antibodies against the chemokines (aMIP1a [A], aMIP1b [B], aRAN [C], and aJE [D]) (100 μg/ml) were added. l-NMMA (LN) (200 μM) was added simultaneously with the recombinant chemokines. The bars represent means ± standard deviations of triplicate samples from one of three independent experiments.
β-Chemokine-activated macrophages inhibit parasite growth.
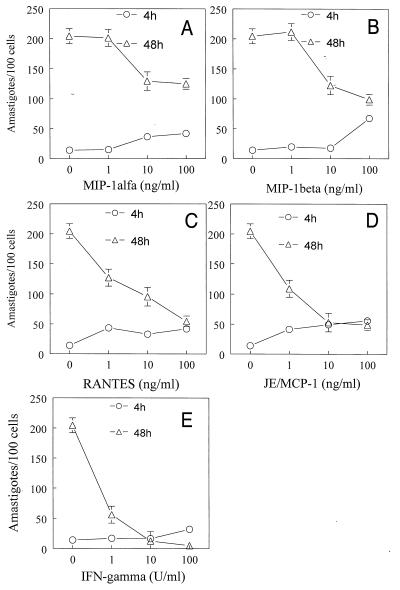
Since T. cruzi trypomastigote forms may induce the synthesis of chemokines which lead to NO production by infected macrophages, the next series of experiments was designed to investigate whether chemokine-derived reactive nitrogen intermediates played a cytotoxic or cytostatic effect on intracellular parasites. T. cruzi-infected macrophages were incubated with chemokines, and parasite uptake and growth were evaluated 4 and 48 h after infection, respectively. The results showed that all of the β-chemokines tested induced a dose-dependent inhibition of parasite growth (Fig. 5). RANTES (Fig. 5C) and JE/MCP-1 (Fig. 5D) were the most potent and effective inhibitors. As a control, the addition of IFN-γ to infected macrophages resulted in a potent microbiostatic activity. In addition to inhibiting parasite growth, pretreatment with MIP-1α (10 and 100 ng/ml), MIP-1β (100 ng/ml), or RANTES or JE/MCP-1 (1 to 100 ng/ml) led to an increased uptake of parasites by macrophages, as assessed by the increased numbers of amastigotes in macrophages at 4 h (Fig. 5).
FIG. 5.
Cytostatic effects of β-chemokines upon intracellular T. cruzi amastigote growth in murine macrophages. C3H/HeJ-derived thioglycolate-elicited peritoneal macrophages were infected with culture-derived T. cruzi trypomastigotes in a parasite/host cell ratio of 1:1, with or without different concentrations of recombinant murine MIP-1α (A), MIP-1β (B), RANTES (C), JE/MCP-1 (D), and IFN-γ (E), at 37°C in a humidified chamber containing 5% CO2. After 2 h, the cells were washed to remove the extracellular parasites, and the chemokines and IFN-γ were again added to the cultures. Two (○) or 48 (▵) h later, the cells were washed, fixed with cold methanol, and stained with Giemsa stain. The intracellular parasites were counted (at ×400 magnification under a light microscope) in 500 cells. Each point represents the mean ± standard deviation of triplicate samples. The results shown are representative of three independent experiments.
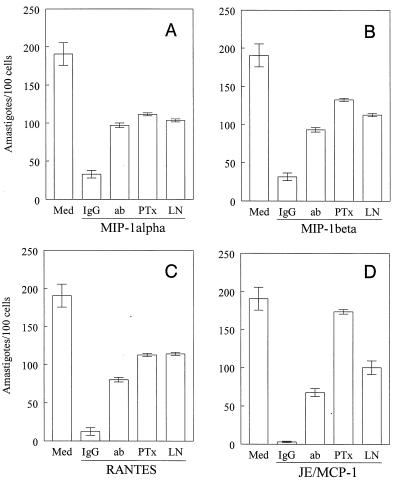
The addition of neutralizing anti-β-chemokine antibodies or l-NMMA caused a significant reversal of the chemokine-induced inhibitory effects on parasite growth (Fig. 6). More importantly, pretreatment of macrophages with PT almost completely inhibited the trypanocidal effect of JE/MCP-1 and significantly reversed the microbiostatic effects of the other chemokines tested (Fig. 6). This is in agreement with the ability of PT to partially reverse the production of NO induced by the same chemokines (data not shown).
FIG. 6.
β-Chemokine-mediated cytostatic effects are reverted with l-NMMA. Thioglycolate-elicited murine macrophages were incubated with culture-derived T. cruzi trypomastigotes in a parasite/cell ratio of 1:1 for 2 h, and the extracellular parasites were removed. This was followed by 48 h of incubation, with or without recombinant murine MIP-1α (A), MIP-1β (B), RANTES (C), or JE/MCP-1 (D) (all at 100 ng/ml) and with or without l-NMMA (LN; 200 μM), PT (30 ng/ml), antibodies (ab) against MIP-1α (A), MIP-1β (B), RANTES (C), JE/MCP-1 (D) (all at 100 μg/ml), or irrelevant antibody (immunoglobulin G). After 48 h, the cultures were washed, fixed, and stained with Giemsa stain. Intracellular amastigotes were counted in 500 cells (at ×400 magnification under a light microscope). The data (means ± standard deviations) are representative of two independent experiments.
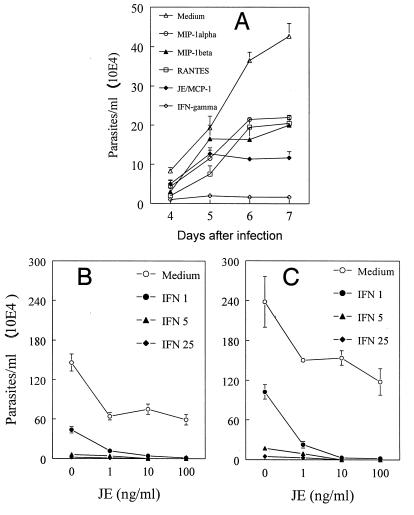
In an attempt to characterize whether addition of β-chemokines results in inhibition of multiplication and the release of viable parasites, infected macrophages were treated with chemokines or IFN-γ and parasite release was counted on days 4 to 7 after infection. As expected, IFN-γ almost completely blocked the release of viable parasites (Fig. 7A). The addition of β-chemokines also led to a decreased number of parasites released in culture supernatants on days 6 and 7 after infection, but they were less effective than IFN-γ. In agreement with its greater effect on NO production, JE/MCP-1 was more effective in controlling parasite growth than the other chemokines tested (Fig. 7A).
FIG. 7.
Effects of β-chemokines on control of T. cruzi replication in macrophages. Thioglycolate-elicited C3H/HeJ macrophages were infected with culture-derived trypomastigotes in a parasite/host cell ratio of 1:1 (A) or 5:1 (B and C) for 2 h, and the extracellular parasites were removed. MIP-1α, MIP-1β, RANTES, JE/MCP-1 (all at 100 ng/ml) or IFN-γ (100 U/ml) (A) or JE/MCP-1 (0, 1, 10, and 100 ng/ml) and/or IFN-γ (0, 1, 5, and 25 U/ml) (B and C) were then added to the cultures. The cells were incubated at 37°C in a humidified chamber containing 5% CO2. The released parasites were counted daily (A) or on days 4 (B) and 6 (C) in a Newbauer chamber. The bars represent means ± standard deviations of triplicate counts. The data are representative of two independent experiments.
IFN-γ is produced soon after parasite injection and plays an important role in resistance to T. cruzi (26). Therefore, it was important to evaluate whether JE/MCP-1 could potentiate the ability of IFN-γ to induce trypanocidal activity. JE/MCP-1 (1 to 100 μg/ml) significantly enhanced the trypanocidal effects of infected macrophages cultured with a low concentration of IFN-γ (1 U/ml), as assessed by the inhibition of parasite release in 4- or 6-day cultures (Fig. 7A and B, respectively).
DISCUSSION
In the acute and chronic phase of T. cruzi infection, there is an intense tissue inflammation in several organs, primarily the heart, leading to one of the most common forms of cardiac disease in Latin America (3). Although it is known that T. cruzi infection induces the production of several proinflammatory and regulatory cytokines (1, 8, 25, 26, 31) that modulate host immunity and pathology, data on early events that take place after the infection are still scarce. The results of this study show that T. cruzi-macrophage interaction leads to mRNA and protein expression of the β-chemokines MIP-1α, MIP-1β, RANTES, and JE/MCP-1.
The importance of the production of these chemotactic cytokines to disease outcome and host immunopathology during infection with T. cruzi is not known, but chemokine expression and release may result in a cascade of inflammatory events leading to leukocyte accumulation in the infected tissue. In addition to being essential in leukocyte recruitment, chemokines also appear to affect several other immunological phenomena, including T-lymphocyte proliferation (27), Th1-Th2 differentiation (14), and NK cell migration and activation (22, 23). In macrophages, β-chemokines, such as MCP-1 (18) or MIP-1α (13), appear to trigger the synthesis of proinflammatory cytokines. Furthermore, a recent study showed the ability of the β-chemokines RANTES, MIP-1α, and MIP-1β to produce NO in human macrophages during in vitro T. cruzi infection (29).
Considering the importance of NO as an effector molecule responsible for the control of T. cruzi replication both in vitro and in vivo (28), it was of interest to examine whether β-chemokines could be involved in the triggering of NO production by infected murine macrophages. Pretreatment of infected macrophages with the β-chemokines RANTES, JE/MCP-1, MIP-1α, and MIP-1β induced a significant amount of NO. Moreover, the inhibitory effects of anti-chemokine antibodies on NO release suggested an autocrine role for chemokines on induction of NO biosynthesis by infected macrophages. Together, these studies demonstrate an important role for chemokines in inducing NO synthesis by murine macrophages exposed to T. cruzi.
A possible explanation for the ability of chemokines to induce NO release is that by triggering intracellular calcium influx, the β-chemokines might augment the expression and function of constitutive NOS, whose activity is calmodulin dependent (21, 24). However, our results suggest that the chemokine-induced NO is mainly due to iNOS activity, since the addition of EGTA to the culture medium did not abolish the production of NO (data not shown). Thus, β-chemokine receptors may be triggering iNOS expression by a yet-undefined signal transduction mechanism. Nevertheless, studies with PT demonstrated that the effects of the chemokines on NO release (and control of parasite replication [see below]) was indeed a Gi protein-mediated and, hence, a receptor-operated event.
It has been shown that NO derived from activated macrophages is cytostatic or cytotoxic for a variety of pathogens, including T. cruzi. We examined whether the NO derived from β-chemokine-activated macrophages was able to control parasite replication. Our results clearly demonstrate that the addition of β-chemokines to infected macrophages significantly inhibited the growth of T. cruzi. In fact, Villalta et al. (29) have recently shown that the same β-chemokines can also trigger NO synthesis and promote T. cruzi killing in human macrophages. Proof that NO was indeed involved in regulation of parasite replication in our system was obtained by experiments demonstrating a reversal of NO release and enhanced parasite replication when cells were pretreated with the iNOS inhibitor, l-NMMA. Although the addition of l-NMMA resulted in a significant inhibition of parasite killing, parasite growth did not reach the levels observed in normal cells. These results indicated that other mechanisms known to control parasite replication (e.g., indoleamine 2,3-dioxygenase pathway or oxidative burst) may also be triggered by the β-chemokines or other molecules released by macrophages exposed to T. cruzi. In addition, the partial inhibitory effects of anti-β-chemokine neutralizing antibodies and PT suggested that chemokines are not the only activators of iNOS during T. cruzi infection. In fact, our previous studies have demonstrated that NO production can also be enhanced by the autocrine production of TNF-α by macrophages exposed to T. cruzi trypomastigotes or their products (6, 25).
As previously described, IFN-γ-activated macrophages develop a remarkable capacity to inhibit the replication of T. cruzi (30). Because treatment of infected macrophages with IFN-γ resulted in a level of NO 10-fold higher than the levels induced by the β-chemokines, the in vivo physiological role of macrophage activation by β-chemokines still has to be established. However, an important finding was the ability of chemokines to enhance the trypanocidal activity of macrophages in the presence of low concentrations of IFN-γ. Thus, it is possible that the early β-chemokine-mediated macrophage activation could play an essential role in the containment of parasite dissemination in the acute phase of infection. On the other hand, the release of parasites from the amastigote nests within the site of infection and/or cardiac tissue may induce chemokine production. The released chemokines may in turn induce the recruitment of further leukocytes to the tissue that may act locally to enhance the control of parasite replication and spread in the host tissues. A side effect of such β-chemokine-induced inflammatory infiltrate would be myocarditis, often found in acute and chronic Chagas’ disease. However, these points are still obscure and require further experiments, which are being performed in our laboratory.
ACKNOWLEDGMENTS
This study was supported by grants from FAPESP (96/4118-9 and 97/11640-6) and FAPEMIG (CBS-1208/95) and by fellowships from CAPES (F.S.M. and J.T.S.) and CNPq (R.T.G., M.M.T., and J.S.S.).
REFERENCES
- 1.Aliberti J C S, Cardoso M A, Martins G A, Gazzinelli R T, Vieira L Q, Silva J S. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–1967. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alspaugh J A, Granger D L. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991;59:2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade Z A. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found Symp. 1983;99:214–233. doi: 10.1002/9780470720806.ch12. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 5.Brener Z, Gazzinelli R T. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas’ disease. Int Arch Allergy Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 6.Camargo M M, Andrade A C, Almeida I C, Travassos L R, Gazzinelli R T. Glycoconjugates isolated from Trypanosoma cruzi but not from Leishmania species membranes trigger nitric oxide synthesis as well as microbicidal activity in IFN-γ-primed macrophages. J Immunol. 1997;159:6131–6139. [PubMed] [Google Scholar]
- 7.Cardillo F, Voltarelli J C, Reed S G, Silva J S. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrasekar B, Melby P C, Troyer D A, Colston J T, Freeman G L. Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute Chagasic cardiomyopathy. Am J Pathol. 1998;152:925–934. [PMC free article] [PubMed] [Google Scholar]
- 9.Clark-Lewis I, Kim K-S, Rajarathnam K, Gong J-H, Dewald B, Moser B, Baggiolini M, Sykes B D. Structure-activity relationships of chemokines. J Leukoc Biol. 1995;57:703–711. doi: 10.1002/jlb.57.5.703. [DOI] [PubMed] [Google Scholar]
- 10.Cook D N, Beck M A, Coffmann T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 11.Doyle H A, Murphy J W. MIP-1 alpha contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans. J Leukoc Biol. 1997;61:147–155. doi: 10.1002/jlb.61.2.147. [DOI] [PubMed] [Google Scholar]
- 12.Drapier J C, Wietzerbin J, Hibbs J B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988;18:1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- 13.Fahey T J, III, Tracey K J, Tekamp-Olson P, Cousens L S, Jones W G, Shires G T, Cerami A, Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- 14.Gao J-L, Wynn T A, Chang Y, Lee E J, Broxmeyer H E, Cooper S, Tiffany H L, Westphal H, Kwon-Chung J, Murphy P M. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 16.Howard O M Z, Ben-Baruch A, Oppenheim J J. Chemokines: progress toward identifying molecular targets for therapeutic agents. Trends Biotechnol. 1996;14:46–51. doi: 10.1016/0167-7799(96)80920-6. [DOI] [PubMed] [Google Scholar]
- 17.Huffnagle G B, Strieter R M, McNeil L K, McDonald R A, Burdick M D, Kunkel S L, Toews G B. Macrophage inflammatory protein-1alpha (MIP-1alpha) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J Immunol. 1997;159:318–327. [PubMed] [Google Scholar]
- 18.Jiang Y, Beller D I, Frendl G, Graves D. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–2428. [PubMed] [Google Scholar]
- 19.Kelner G S, Kennedy J, Bacon K B, Kleyensteuber S, Largaespada D A, Jenkins N A, Copeland N G, Bazan J F, Moore K W, Schall T J, Zlotnik A. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 20.Lovchik J A, Lyons C R, Lipscomb M F. A role for gamma interferon-induced nitric oxide in pulmonary clearance of Cryptococcus neoformans. Am J Respir Cell Mol Biol. 1995;13:116–124. doi: 10.1165/ajrcmb.13.1.7598935. [DOI] [PubMed] [Google Scholar]
- 21.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 22.Maghazachi A A, Al-Aoukaty A, Schall T J. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J Immunol. 1994;153:4969–4977. [PubMed] [Google Scholar]
- 23.Maghazachi A A, Al-Aoukaty A, Schall T J. CC chemokines induce the generation of killer cells from CD56+ cells. Eur J Immunol. 1996;26:315–319. doi: 10.1002/eji.1830260207. [DOI] [PubMed] [Google Scholar]
- 24.Nathan C, Xie Q-W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 25.Silva J S, Vespa G N R, Cardoso M A G, Aliberti J C S, Cunha F Q. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Talvani, A., et al. Unpublished data.
- 27.Taub D D, Turcovski-Corrales M, Key M L, Longo D L, Murphy W J. Chemokines and T lymphocyte activation. I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 28.Vespa G N R, Cunha F Q, Silva J S. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villalta F, Zhang Y, Bibb K E, Kappes J C, Lima M F. The cysteine-cysteine family of chemokines RANTES, MIP-1α, and MIP-1β induce trypanocidal activity in human macrophages via nitric oxide. Infect Immun. 1998;66:4690–4695. doi: 10.1128/iai.66.10.4690-4695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirth J J, Kierszenbaum F, Sonnenfeld G, Zlotnik A. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect Immun. 1998;49:61–66. doi: 10.1128/iai.49.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Tarleton R L. Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infections. Exp Parasitol. 1996;84:203–213. doi: 10.1006/expr.1996.0106. [DOI] [PubMed] [Google Scholar]