Abstract
The remarkable ability of Mycobacterium tuberculosis to survive attacks from the host immune response and drug treatment is due to the resilience of a few bacilli rather than a result of survival of the entire population. Maintenance of mycobacterial subpopulations with distinct phenotypic characteristics is key for survival in the face of dynamic and variable stressors encountered during infection. Mycobacterial populations develop a wide range of phenotypes through an innate asymmetric growth pattern and adaptation to fluctuating microenvironments during infection that point to heterogeneity being a vital survival strategy. In this Review, we describe different types of mycobacterial heterogeneity and discuss how heterogeneity is generated and regulated in response to environmental cues. We discuss how this heterogeneity may have a key role in recording memory of their environment at both the single-cell and population level to give mycobacterial populations plasticity to withstand complex stressors.
Graphical Abstract
In this Review, Aldridge and colleagues describe different types of mycobacterial heterogeneity and discuss how cell-to-cell and environmental heterogeneity is generated and regulated in response to environmental cues.
Introduction
Tuberculosis (TB) is caused by infection with Mycobacterium tuberculosis and remains a major cause of death. Every year, there are 10 million new cases of TB and 1.5 million deaths globally 1. TB requires a lengthy antibiotic treatment of a minimum of 6 months1. The primary reason for this long treatment duration is that M. tuberculosis generates phenotypically diverse subpopulations during pathogenesis, and some of these subpopulations are drug-tolerant. This variation in drug response is due to two predominant sources of heterogeneity that develop during the disease. One is the ability of the pathogen to adapt to different microenvironments within granulomas [G]. M. tuberculosis bacteria adapt their metabolic and physiological states to these different lesions, resulting in altered growth properties and antimicrobial susceptibilities2. Therefore, heterogeneity among lesions is a major cause of variation wherein distinct M. tuberculosis subpopulations are compartmentalized into different microenvironments. The other primary source of this variation in drug susceptibility is inherent bacterial heterogeneity that arises among closely related bacteria in the same environment, in part, through an asymmetric growth and division pattern3-9. By overlaying the sources of phenotypic variation, mycobacteria amplify bacterial heterogeneity within just a few generations. Recently, a strong link between this bacterial heterogeneity and drug tolerance in stressful host microenvironments has been demonstrated by several studies on clinical strains10-14.
Technological and analytical advancements have provided researchers with the tools to uncover the numerous facets of heterogeneity in TB in the past few decades. Fluorescence-activated cell sorting coupled with innovative use of live and dead bacterial reporters or a replication reporter and RNA-sequencing have revealed the immense variation of macrophage activation [G] and its effect on M. tuberculosis growth15-17. Use of microfluidics and the development of staining and rapid imaging technologies have enabled high-definition time-lapse studies on mycobacteria at the single-cell level under precisely controlled environments18-20. Recent technological improvements have also expanded our understanding of the host response to M. tuberculosis infection. Heterogeneity in TB lesion structure, metabolic activity and drug penetrance is being interrogated through laser-capture microdissection, immunofluorescence and mass spectrometry21-23. In the clinic, positron emission tomography and computed tomography are now often used to evaluate patients’ disease progression and response to anti-TB treatment24. Images of mycobacteria, especially M. tuberculosis, are more challenging to automatically segment and track compared to other model organisms due to their irregular shape and tendency to form aggregates25,26. The application of machine learning has led to substantial improvements in image analysis software for bacteria in recent years; these advances may enable high-throughput analysis of microscopy experiments for mycobacteria23,25-27. These technologies have the potential to reveal the immense diversity in M. tuberculosis phenotypes and how this variation affects treatment and disease outcomes.
In this Review, we describe how heterogeneous M. tuberculosis subpopulations form from cell-to-cell and environmental variation (that is; the microenvironments that M. tuberculosis faces during infection, such as acidic phagosomes or hypoxic necrotic caseum [G]). Although heterogeneity refers to the variation between single cells, each microenvironment affects heterogeneity differently by causing M. tuberculosis populations to adapt within a compartment in the host tissue. Therefore, in response to changes in the environment, both the average (central part of the population distribution) and distribution (variation and density) shift. Despite the crucial role of the microenvironment on M. tuberculosis heterogeneity in virulence and treatment outcome, studies on mycobacterial phenotypes have concentrated on either population-level changes in different growth environments28-36 or single-cell analyses in nutrient-rich conditions3,4,42,5,7,8,37-41. Understanding how variation is controlled at both the population and single-cell levels in the host niche environments is critical to rationally designing improved TB interventions that mitigate relapse. This Review frames the most recent research around the idea that these layers of heterogeneity function as a survival mechanism against the host immune response and drug treatment.
Bacterial heterogeneity
Cell-to-cell variation in a clonal population, which occurs even within the same microenvironment, has been observed in various cell types, including mammalian and bacterial cells. The etiology of this heterogeneity has been studied extensively in model organisms and attributed to stochastic gene expression, mistranslations and random partitioning of cell components43-46. As a bet-hedging strategy, model bacterial organisms, such as Escherichia coli and Bacillus subtilis, rapidly expand the cellular population to survive. By contrast, M. tuberculosis is a slow-growing organism so that a population-level strategy for survival may be to rapidly expand variation in cellular state rather than to achieve a large population size. Below, we describe mechanisms whereby M. tuberculosis controls multiple aspects of cell physiology to generate and compound bacterial heterogeneity (Fig. 1).
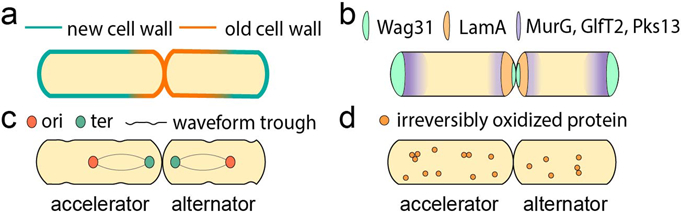
Figure 1. Mycobacterial asymmetry.
(a) In mycobacteria, growth is asymmetric: the old pole elongates more than the new pole. The sister that inherits the old pole from the mother called the accelerator cell) is born longer and elongates more than the sister that inherits the new pole (the alternator cell). (b) The cell elongation and division protein Wag31 and growth inhibitor LamA are regulators of asymmetric growth in mycobacteria. Inhibition of growth at the new pole by LamA is relieved after a time lag. MurG, GlfT2 and Pks13 are cell wall synthesis complexes in Mycobacterium smegmatis that are localized in the subpolar regions with their highest local concentration at the old pole. (c) In M. smegmatis, chromosome positioning is asymmetric with the terminus (ter) closer to the new pole than the origin of replication (ori) is to the old pole. The asymmetric positioning of the chromosome is proportional to cell size. The future sites of asymmetric division are determined in mother and grandmother cells and can be observed by surface wave troughs that are inherited from previous generations. (d) Asymmetric distribution of irreversibly oxidized proteins (IOPs) in Mycobacterium tuberculosis creates sister cells with different growth properties and sensitivities against antibiotic stresses depending on their IOP-burdens. Specifically, sister cells with a high amount of IOPs are more sensitive to drugs.
Asymmetric growth and division.
Mycobacterial growth and division patterns are unlike the model species E. coli and B. subtilis, and mycobacteria elongate from the cell poles, which provides a mechanism to grow and divide asymmetrically. Asymmetric growth and division generate uneven partitions of cell components, and different growth behaviors and drug susceptibilities between sister cells3-5.
Through cell-wall labeling experiments with time-lapse and fixed-cell imaging, the old pole was observed to grow more than the new pole in Mycobacterium smegmatis3,7,8,38,42 (FIG. 1a). Additionally, the abundance of cell wall elongation machinery at the old pole relative to the new pole suggests that regulation of protein localization may have an essential role in growth asymmetry37-40. For example, key proteins in cell wall synthesis, such as MurG, GlfT2 and Pks13, are at a higher concentration at the old pole relative to the new pole38. This may confer asymmetry of the mycobacterial cell wall under nutrient-rich conditions38. However, more studies are needed to understand the relationship between the concentrations of cell wall elongation machinery at each pole and polar growth asymmetry. Recent studies suggest that the new pole of mycobacteria may grow less over the course of one cell cycle period because there is a lag before the new pole is licensed to elongate8,20. Using a creative genetic screen, a group identified a key mediator of asymmetric growth, LamA, which is a member of the mycobacterial divisome [G] complex that actively inhibits growth at the new pole8 (FIG. 1b). Interestingly, lamA-deleted M. tuberculosis mutants were killed more rapidly compared to the wild type by rifampicin and vancomycin, which suggests that asymmetric growth may contribute to mycobacterial survival against antibiotic treatment8. Additionally, it was demonstrated that in nutrient-rich growth conditions, the new pole (but not the old pole) has a lag phase before growth, which accounts for an apparent slower elongation rate throughout the division cycle20. The authors suggested that the ratio between the lag time and inter-division time may affect the heterogeneity in cell wall age distribution20. However, some studies performed in nutrient-rich conditions suggest that M. tuberculosis growth is less asymmetric compared to that of M. smegmatis41. By using fluorescent D-alanine analog (FDAA) to visualize the elongation of the new cell wall, the study found that the FDAA is incorporated at both poles in larger cells41. Further studies are necessary to determine the asymmetry of cell growth in M. tuberculosis by time-lapse imaging and in the context of host-relevant growth environments.
Mycobacteria do not divide perfectly mid-cell. This asymmetric division creates sister cells that are born at varying sizes and with different growth properties3-5,7,8,42. In M. smegmatis, the septal site is localized at a 45:55 position from the new:old pole such that the larger daughter inherits the old growth pole from the mother cell (accelerator cell [G]) and the smaller sister (alternator cell) inherits the slower growing new pole from the mother cell. Cell size is correlated with linear growth rate, so cells born larger (such as the accelerator cells [G]) elongate more per hour and before division than cells born small5,7,37,42,47,48. Asymmetry of the septal site is mirrored by an asymmetry in sub-cellular organization, including the origin of replication (ori) and the chromosomal terminus (ter). The chromosome is in a mid-cell position closer to the new pole and organized with the ori near the old pole in young cells and an ori-ter-ter-ori orientation in cells at later cell cycle stages (FIG. 1c). The asymmetric positioning of the chromosome is proportional to cell size, which is consistent regardless of different cell age or growth pole7. This proportional ‘ruler’ of cellular organization may be established for a daughter cell by the grandmother cell. For example, it was demonstrated with time-lapse atomic-force microscopy that waveform troughs on the surface of M. smegmatis cells are at the future cell division sites6 (FIG. 1c). Additionally, the results suggest that the coordination of wave troughs and chromosome partitioning restricts cell division at the mid-cell position, causing asymmetric cell division6.
Genetic and epigenetic bacterial heterogeneity.
Whereas asymmetric growth and division generate heterogeneity on a rapid time scale (approximately the duration of one cell cycle), phenotypic heterogeneity also involves heritable (inheritable genetic mutations) and non-heritable (epigenetic changes which occur temporally within a generation) mechanisms (Table 1).
Table 1.
Genetic, transcriptional, translational and post-translational modifications involved in bacterial heterogeneity
| Type of variation | Cause | Outcome | Reference |
|---|---|---|---|
| Genetic variation | INDEL and SNPs in mps1-mps2-gap, mmpl4b | Switch of colony morphology from smooth to rough form, leading to more severe, persistent infections | 52 |
| SNPs in prpR | Multidrug tolerance to INH, RIF, OFX under propionate-rich condition | 12 | |
| Gene mutations in Rv0565c | Tolerance to ETH and PTH | 13 | |
| Frameshift mutations in glpk | Slower growth, reduced sensitivity to drug regimens containing PZA | 53,54 | |
| Transcriptional level | Induced expression of rpoB promoter II in response to RIF | Tolerance to RIF | 57 |
| Various expression levels of KatG | Better survival following treatment with INH | 56 | |
| Translational level | Substitution of amino acids in RNA polymerase | Phenotypic resistance to RIF | 58 |
| Substitution of amino acid (S315T) in KatG | Resistance to INH | 59-62 | |
| Post-translational level | Lysine acetylation and methylation of HupB | Phenotypic resistance to INH | 63-65 |
| Phosphorylation at Y102 in MtrA | Tolerance to INH and VAN | 66 | |
| Phosphorylation at T34 in PonA1 | Increased MIC of RIF | 39 |
ETH, ethambutol; INDEL, insertion or deletion;INH, isoniazid;MIC, minimum inhibitory concentration; OFX, ofloxacin; PTH, prothionamide; PZA, pyrazinamide; RIF, rifampicin; SNP, single nucleotide polymorphism; VAN, vancomycin.
One of the heritable phenotypes in mycobacteria is the switch of colony morphology between smooth (S) and rough (R) forms. Colony morphology is highly correlated with disease severity in some mycobacterial species including M. tuberculosis, Mycobacterium avium and Mycobacterium abscessus, with R morphotypes being associated with more severe and persistent infections relative to the S morphotypes49-52. Colony morphological changes can be mediated by genetic variations. For example, small insertions and deletions and single nucleotide polymorphisms (SNPs) within the gene cluster mps1-mps2-gap or mmpl4b, which are involved in the synthesis and transport of glycopeptidolipids [G] (GPLs), converts the S form into the R forms in M. abscessus52. Clinically relevant SNPs in mycobacteria are not only related to changes in morphology and virulence; several mutations in clinical isolates have an impact on drug tolerance and thus treatment outcomes12,13,53,54. Through whole-genome sequencing, SNPs in prpR, which encodes a transcriptional regulator involved in propionate metabolism, were detected among clinical strains12. These genetic differences conferred multi-drug tolerance to isoniazid, rifampicin and ofloxacin under propionate-rich conditions within the macrophage12. This drug tolerance could not be detected in standard in vitro assays12, highlighting the importance of mimicking host microenvironment conditions to unveil drug-tolerant mutations. It was also found that clinical strains with genetic mutations in Rv0565c, which functions as an activator of ethionamide and prothionamide, were substantially more tolerant to ethionamide and prothionamide treatment13. High-frequency mutations in carbon metabolic pathways, such as frameshift mutations in glpK, which encodes glycerol kinase, led to slower growth and reduced sensitivity to drug regimens containing pyrazinamide in clinical strains53,54.
Single amino-acid variants, which can be caused by nonsynonymous single nucleotide variants and insertions and deletions, may also contribute to genetic heterogeneity among strains55. For example, an analysis of clinical isolates with integrated whole-genome sequencing and mass spectrometry-based proteomics identified that proteome characteristics vary from strain-to-strain55. It was shown that two clinical Latin American-Mediterranean isolates contained 59 peptides with single amino-acid variants, along with 29 distinct peptides that induce phenotypic variation in clinical M. tuberculosis strains55.
Mycobacterial variation, and the resulting drug tolerance, are also generated at the transcriptional, translational and post-translational levels. Differences in gene expression within a population can result in semi-heritable drug tolerance in mycobacteria56,57. For example, rifampicin treatment inhibited rpoB promoter I expression but induced expression from rpoB promoter II, leading to a maximal production of RpoB in a subpopulation of M. smegmatis57. This differential response within the population to RNA polymerase-targeting drugs, even among isogenic sister cells [G], can permit tolerance to first-line antimicrobials. Using time-lapse imaging, it was found that individual cells expressed different levels of catalase-peroxidase (KatG), which activates isoniazid. The expression level of KatG was negatively correlated with cell survival, which suggests that drug tolerance is influenced by epigenetic heterogeneity at the single-cell level56.
Reversible phenotypic errors can generate drug tolerance in subpopulations during translation, such as the substitution of amino acids58. Substitution of glutamate for glutamine and aspartate for asparagine of the β-subunit of RNA polymerase occurs in mycobacteria under nutrient-rich conditions, which induces phenotypic resistance to rifampicin58. In addition, the rate of these errors increases under host-relevant stress conditions such as in stationary-phase, which suggests that this is an intentional strategy for adaptation to stressors58. KatG can be modified by the substitution of serine to threonine at codon 315 (S315T), which is most frequently found in isoniazid-resistant mutants59. This alteration enables the mutant to retain KatG activity but decreased capacity to activate isoniazid, possibly due to subtle alterations in the binding of isoniazid to KatG 59-62.
Post-translational modifications involved in cell growth, drug activation and signal transduction systems can also regulate phenotypic resistance to drugs in mycobacteria. The mycobacterial histone-like protein, HupB, is post-translationally modified by lysine acetylation and methylation, altering mycobacterial chromatin structure63,64. This change in chromatin structure can cause phenotypic resistance to isoniazid due to modified genome organization 64,65. It was also suggested that chemical modifications after translation may induce reversible phenotypic resistance to antibiotics65. For example, two-component regulatory signal transduction system MtrAB, which enables mycobacteria to respond to stressors caused by toxic radicals through chemical modifications, can control tolerance to antimicrobials in mycobacteria66. Phosphotransfer to the MtrA response regulator at Y102 causes changes in cell division and cell wall synthesis, and increases tolerance to isoniazid and vancomycin66. Phosphorylation of another enzyme involved in M. tuberculosis growth, peptidoglycan synthase (PonA1) increases the cell length as well as the minimum inhibitory concentration [G] (MIC) of rifampicin39.
Environmental heterogeneity
M. tuberculosis readily adapt to their local environment through metabolic flexibility, entering growth arrest, or changing physical attributes including the thickness of the cell wall67. During pulmonary infection, M. tuberculosis resides within granulomas, which consist primarily of various immune cells and caseum. The composition of this granulomatous tissue can vary substantially among patients, within a single patient, and within a granuloma68-70 (FIG. 2). Moreover, these compartments change over time so that the composition and abundance of nutrients and stressors are dynamic. Because M. tuberculosis adapts into distinct states (and population structures) in different granulomas, compartmentalization of the M. tuberculosis population into physically separated granulomas imposes an additional layer of variation21,28,71-78.
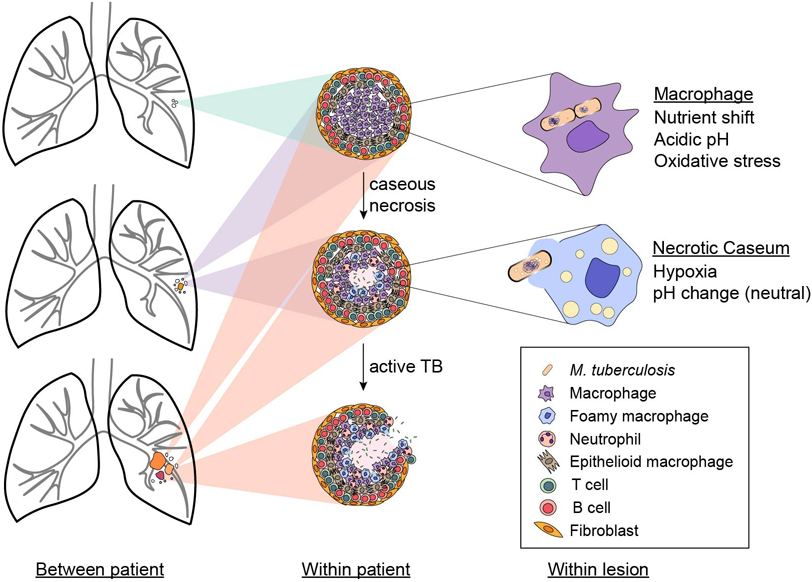
Figure 2. Environmental heterogeneity.
Microenvironments in tuberculosis lesions (granulomas) vary among patients, within one patient, and even within a lesion as shown with examples in the first, second, and third columns, respectively. Lesion composition is dependent on the stage of disease progression and the network of immune cells present. Many types of cells respond to infection with Mycobacterium tuberculosis, including macrophages, neutrophils, T and B cells, epithelioid cells, and fibroblasts, and populate cellular granulomas. M. tuberculosis in infected macrophages encounter an environment characterized by low pH, oxidative stress and abundant lipids. As M. tuberculosis utilizes these host lipids, it secretes lipid vesicles into the macrophage cytoplasm, inducing some of the infected macrophages to become foamy with lipid droplets. Cytoplasmic debris from necrotic macrophages form the granuloma's caseous center, which may be acidic or neutral and vary in oxygen content.
Because adaptation to different types of lesions induces major phenotypic changes in M. tuberculosis, studies of M. tuberculosis pathogenesis and drug response that include many in vitro and animal models can better capture the range of key states79-83. For example, integrating studies of two mouse models (for example, C3HeB/FeJ mice may have caseating lesions and BALB/c mouse lesions are primarily cellular) broadens the types of M. tuberculosis populations that are being evaluated68,81. Using panels of diverse mice for TB studies (through collaborative crosses [G] ) expands our ability to interrogate a range of lesion types84. In this Review, we do not thoroughly discuss lesion types in different animal models, as this is well described in other reviews68,85. Instead, we focus on aspects of the microenvironment in TB lesions, such as nutrient shifts, acidic pH, hypoxia and oxidative stress, and describe how these conditions alter M. tuberculosis subpopulations and their behaviors21,68,76,86. We highlight these well-characterized host environments and their contributions in establishing and maintaining mycobacterial heterogeneity.
Nutrient shift.
M. tuberculosis can derive energy from many different carbon sources but, during the course of infection, the bacteria mainly encounter lipids. The type of lipid environment M. tuberculosis experiences can vary substantially, ranging from cholesterol, cholesterol esters, triacylglycerols (TAGs) and lactosylceramide87. Remarkably, the pathogen can readily use or co-metabolize these various lipids as carbon sources which differentially affect growth state, morphologies and drug susceptibility patterns28,29,88-90. When M. tuberculosis encounters a fatty acid-rich environment, for example, in a propionate-rich medium, it induces enzymes such as isocitrate lyase to sustain citric acid cycle activity30,91,92. Infected macrophages accumulate lipid droplets induced by M. tuberculosis, gradually converting into foamy macrophages [G] 31,32,88,93 (FIG. 2). As M. tuberculosis adapts to utilize these lipids they develop a remarkable nongrowing dormant state, a phenotype consistently observed in hypoxic, lipid-loaded macrophages31,88. Nearly half of all M. tuberculosis inside lipid-rich macrophages developed phenotypic tolerance to isoniazid and rifampicin by accumulating lipid droplets containing host TAG31,32. A group sought to target this subpopulation by treating infected macrophages with vitamin D, which reduces the accumulation of lipid droplets94. Bacterial burden was reduced, which suggests that obstruction of lipid metabolism in the macrophage may be an essential determinant of M. tuberculosis killing94. Nutrient shifts that result in altered bacterial metabolism can enable M. tuberculosis to endure immune-mediated killing as well88. Activation of interferon-γ in the macrophages induces a cholesterol-rich intracellular environment which in turn enables mycobacteria to utilize this lipid as a carbon source and persist88.
Acidic pH.
M. tuberculosis encounters a range of pH levels during infection. Caseum pH ranges from pH ~6-8, whereas macrophages impose acidic conditions in phagolysosomal compartments77,95-98 (FIG. 2). Differences in pH not only affect the metabolic state of M. tuberculosis, but also determine drug treatment responses, as has been well described for pyrazinamide, ethambutol, isoniazid and linezolid99-101.
The acidic microenvironment within the macrophage influences growth rate, metabolism, and induction of virulence factors [G] 33,102,103. M. tuberculosis slows its growth at a pH below 6.4 and arrests growth at pH 5.0 in a nutrient-rich medium, but this trend can change depending on the carbon source that M. tuberculosis Mtb utilizes inside macrophages33. Recently, it was found that M. tuberculosis can grow in an acidic environment, as low as pH 4.5, if it alters its carbon metabolism to utilize lipids, such as oleic acid, instead of carbohydrates104. In addition, the M. tuberculosis PhoPR two-component system is induced as a response to low pH, reducing growth33,34,105 and modulating virulence factor and lipid anabolism106,107.
Production of virulence factors, such as cell surface lipids and secretion systems [G] , protects M. tuberculosis from acidic stress in macrophages by inhibiting the formation of phagosomes and preventing the acidification of phagolysosomes108-111. Trehalose-6,6’-dimycolate, also known as cord factor, is a toxic glycolipid that delays maturation of phagosomes by preventing acidification and reducing hydrolytic activity109,110. ESX-1 and ESX-3, the type VII secretion systems, and lipoarabinomannan also inhibit phagosomal maturation by phagosomal rupture and/or blocking the delivery of lysosomal constituents to the phagosome and interfering with endosomal sorting, respectively108,111-114.
Adaptation to the acidic environment and expression of virulence factors can have a profound impact on the fitness of M. tuberculosis in the host and affect drug sensitivity107,115. For example, ethoxzolamide, a carbonic anhydrase inhibitor, downregulates the PhoPR regulon, which impairs the ability of M. tuberculosis to induce the accumulation of lipids in the macrophage107. M. tuberculosis growth was notably diminished in mouse lungs as a result of PhoPR downregulation. Treatment with the compound AC2P36 induced thiol stress at acidic pH, which enhanced M. tuberculosis killing and potentiated the activity of isoniazid, clofazimine and diamide115.
Oxidative stress.
As a part of the innate immune response, macrophages generate reactive oxygen species (ROS) to slow M. tuberculosis growth or kill it. Macrophages produce ROS through the action of Nox enzymes, the mitochondrial matrix and acidification of the phagosome116-118. Oxidative stress is highly toxic to M. tuberculosis as it damages DNA, protein and lipids119, and impedes thiol redox homeostasis120,121. ROS-mediated damage can cause cell death by the oxidation of the guanine nucleotide pool and its incorporation into DNA and RNA122. Also, thiol-specific oxidizing stress can be caused by the impaired thiol redox homeostasis, which results in growth disruption and cell death120,121. To combat oxidative stress, M. tuberculosis has developed a distinctly thick cell wall consisting primarily of mycolic acid, which acts as a robust physical barrier to protect from ROS35,123. Additionally, some mycobacterial genes have dedicated roles to counteract DNA damage, particularly those involved in Fe–S cluster biogenesis36,124. Many genes related to the oxidative stress response can protect against the harmful effects of ROS upon detection, but others are responsible for repair after damage125-129. In this Review, we do not discuss the genetic mechanisms involved in the responses, as a recent review thoroughly explains how M. tuberculosis responds to oxidative stress130. Some of the virulence factors secreted by M. tuberculosis play a part in protecting M. tuberculosis from ROS. A phosphate transporter, PstS, is one of the glycolipoproteins that induces phagocytosis and reduces ROS production by binding to the macrophage mannose receptor131,132. M. tuberculosis nucleoside diphosphate kinase (Ndk) also contributes to decreased ROS production in the host macrophage by attenuating NADPH oxidase-mediated host innate immunity133. Thus, M. tuberculosis responds to host stressors by both adapting their own state and manipulating the host response.
The importance of the oxidative stress response for M. tuberculosis pathogenesis is well studied, and thus, drug treatments that curtail these protective mechanisms have been designed. For example, a compound associated with thiol stress, AC2P36 (see above), enhances the accumulation of ROS, selectively killing M. tuberculosis at low pH115. As AC2P36 depletes free thiol pools of M. tuberculosis, it synergizes with isoniazid, clofazimine and diamide by causing a ROS burst and thwarts defense of M. tuberculosis against oxidative damage115. Along with new drug development, the use of nutritional supplements for the host, such as vitamin C, in combination with antibiotics is also being investigated as a way to potentiate the bactericidal ability of treatments134,135. It was found that M. tuberculosis is more sensitive to vitamin C than other bacterial pathogens because it promotes high ferrous ion levels and ROS production through the Fenton reaction134. Moreover, when vitamin C is used in combination with pyrazinamide, bacterial heterogeneity is reduced by the killing of subpopulations that are tolerant to other drugs such as isoniazid and rifampicin135.
Hypoxia.
Drug tolerance is enhanced in M. tuberculosis in hypoxic granulomas68. Hypoxic conditions have been detected in lesions where cellular necrosis occurred, notably within the necrotic caseum136,137 (FIG. 2). Even though M. tuberculosis is an aerobe, it can survive hypoxia by transcriptionally transitioning to an anaerobic dormant state138-140. Because M. tuberculosis requires oxygen for its growth, hypoxia rapidly establishes a non-replicating, drug-tolerant state12,138,141,142 (Table 2). Hypoxia induces the deacetylation of DosR, a dormancy survival regulator, promoting anaerobic dormancy by inhibiting aerobic respiration and preventing replication138,140. During this anaerobic dormancy, M. tuberculosis alters its morphology and metabolism, which results in changes in cell wall structure, lipid metabolism and gene expression, and increasing tolerance to rifampicin and clofazimine31,67,142,143. For example, the outer layer of the cell wall of M. tuberculosis is uneven and thicker in low-oxygen growth conditions compared to normoxia67. This morphological change restricts the entry of rifampicin, conferring tolerance. Moreover, this population of M. tuberculosis had increased survival rates against clofazimine treatment in the lungs of C3HeB/FeJ mice which form hypoxic, necrotic granulomas compared to the lungs of BALB/c mice, which only present cellular granulomas143. These data suggest that hypoxic environments promote the accumulation of drug-tolerant M. tuberculosis subpopulations as a consequence of their survival mechanisms to this host-mediated stress. The susceptibility of M. tuberculosis in hypoxia to treatment is dependent on the drug mechanism of action, as hypoxia induces susceptibility of non-replicating M. tuberculosis to metronidazole in both in vitro and non-human primate models136,144-146. Also, it was found that the combination of rifampicin and nitazoxanide showed enhanced M. tuberculosis killing in hypoxia compared to the first-line treatment regimen of rifampicin, isoniazid, pyrazinamide and ethambutol146.
Table 2.
Mycobacterium tuberculosis in different host microenvironments and effective drugs
| Host environment |
Specific lesion | Impact on M. tuberculosis and adaptation of M. tuberculosis |
Effective drugs and supplements |
Refs |
|---|---|---|---|---|
| Nutrient shift | Macrophages and caseum (lipid-rich environment) | Altered metabolism and growth rate | Vitamin D | 28,29,88,32 |
| Changed growth control and morphology | NA | 7,89,164 | ||
| Induces enzymes to utilize lipids | 30,91,92 | |||
| Accumulation of lipid droplets | 31,32,88 | |||
| Acidic pH | Macrophage | Growth arrest | Ethoxzolamide | 33,107 |
| Altered physiology by regulating phoPR two component system | AC2P36 | 33,34,105,115 | ||
| Modulation of virulence factors and lipid anabolism | NA | 106-111,114 | ||
| Oxidative stress | Macrophage | Thickening of the cell wall as a physical barrier | AC2P36 | 35, 115,123 |
| Altered gene regulation to protect after ROS recognition | Vitamin C | 36,124,134,135 | ||
| Damage repair | NA | 125-129 | ||
| Regulation of virulence factors | NA | 131-133 | ||
| Hypoxia | Caseous center of necrotic lesions and cavities | Growth arrest and dormancy | Metronidazole | 136, 139, 144,145, 165 |
| Phenotypic drug tolerance | Rifampicin-nitazoxanide | 140-142,146 | ||
| Altered morphology and metabolism; changes in cell wall structure, lipid metabolism and gene expression | NA | 31,67,142,143 |
NA, not applicable; ROS, reactive oxygen species.
Consequences of heterogeneity.
Population structure.
The different sources and types of variation arising from innate bacterial heterogeneity and adaptations to the local environment generate a range of physiological states in mycobacteria. Subpopulations of M. tuberculosis differ from each other in many measurable factors (such as growth, metabolism, damage and gene expression) so that they exhibit different phenotypes. One example is persisters; that is, members of a drug-tolerant subpopulation survive in the presence of an antibiotic without genetic resistance and can regrow after removing antibiotics147,148. As understanding of the mechanisms underlying heterogeneity improves, mycobacteriologists are beginning to transition away from investigating gross changes in population response towards interrogating subpopulation and single-cell behaviors. This approach is well established in other fields including immunology, for example, in their use of flow cytometry to measure a population of cells as the aggregate of many multivariate single-cell measures. Flow cytometry plots, which plot individual cells over physiological measurements, can then be used to determine the distributions of subpopulations. In this section, we propose interrogating physiological states of M. tuberculosis through a conceptual framework similar to that of a flow cytometry plot. We refer to this as a ‘population structure’, which is a map of individual cells across multiple measurable states in a phase diagram format (FIG. 3). We illustrate how population structure can be visualized to enable parsing of subpopulations of M. tuberculosis with similar physiological states and phenotypes as they vary with host microenvironments. For example, one well-characterized subpopulation of replicating M. tuberculosis is formed in response to the oxidative stress of the phagosome117. This group of cells can be mapped onto a diagram of population structure to help understand the parameters of drug tolerance in the phagosome (FIG. 3). In this example, the growth state (property A) is high because the M. tuberculosis are replicating (x-axis), their metabolic state (property B) is chemically reduced (y-axis), and these bacilli are tolerant to isoniazid and rifampicin treatment117.
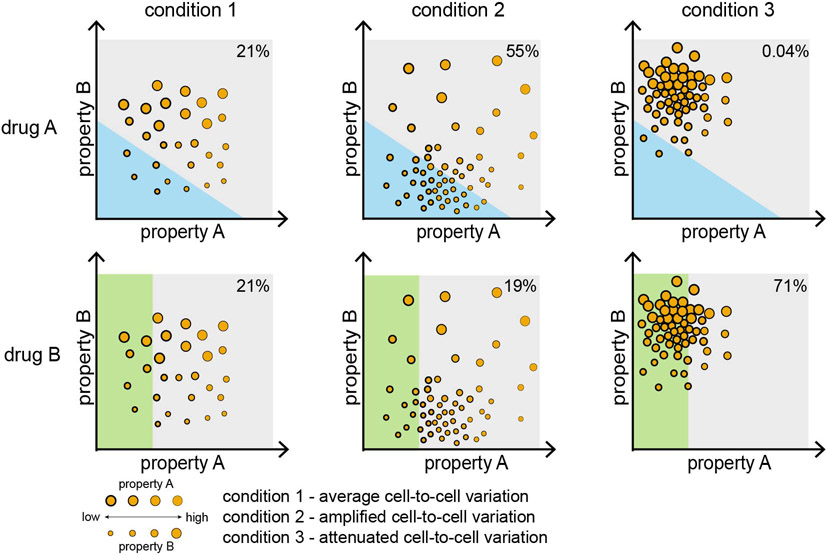
Figure 3. Population structure and complex Mycobacterium tuberculosis phenotypes.
A hypothetical population structure framework maps single-cell and population-level cellular states and phenotypes across multiple processes. The locations, dense regions and spread in this phase diagram change due to adaptation to different environmental conditions, including drug treatment. Each axis (property A and property B) can represent cellular states, such as cell size, gene expression or virulence. Abstracted axes, as being used for illustrative examples here, may be made quantitative using weighted sums of specific cellular features through dimensionality reduction techniques such as principal component analysis. Condition 1 is an example wherein cells are evenly distributed throughout the range of properties A and B. During adaptation to new environments and stressors, the population structure can be shifted and/or concentrated to other regions of the phase diagram. The degree of bacterial heterogeneity can become amplified (condition 2) or attenuated (condition 3). Following antibiotic treatment, the size of the tolerant subpopulation may vary depending on which condition Mycobacterium tuberculosis has adapted to (for example, 21% in condition 1, 55% in condition 2, 0.04% in condition 3 following treatment with drug A). The region that encompasses drug-tolerant cell states will be dependent on the activity and mechanism of action of the drug (compare drug A versus drug B), highlighting that there are different kinds of persister cells.
In principle, exhaustive measurement of every feature of a population of M. tuberculosis is the only way to comprehensively describe the subpopulations that form in these environments; this is clearly not feasible, even with different -omic profiling techniques. Thus, we can only probe subsets of properties, and we must identify those most pertinent for reporting on the physiology relevant to disease and treatment response. Composites of measurements, derived from dimensionality reduction algorithms such as uniform-manifold approximation (UMAP) or principal component analysis (PCA) may be used to probe cellular properties that distinguish subpopulations from each other. As an abstract example, we map population structure using two measurements: property A and property B which can be replaced with any cellular states such as cell size, gene expression or virulence (FIG. 3). Some environments may shift the average cellular states (condition 1) and may either amplify (condition 2) or attenuate (condition 3) cell-to-cell variation. Through population structures, we aim to understand how bacterial and environmental heterogeneity intersects to generate distinct M. tuberculosis subpopulations and single-cell behaviors.
Consequences of bacterial heterogeneity.
Mycobacteria produce bacterial heterogeneity by asymmetric cell growth and division, genetic mutations, and through variation at the transcriptional and post-translational levels. To date, studies of cell-to-cell heterogeneity in M. tuberculosis and mycobacteria indicate that there are distinct subpopulations of M. tuberculosis that can be characterized by different cellular states, such as growth rate, asymmetry in growth and division and metabolism. Although these features correlate with drug susceptibility among subpopulations, we have yet to comprehensively describe the specific states and growth properties that propagate this complex landscape of responses.
Asymmetric growth, division and cellular organization give rise to sister cells that inherit different cell wall ages, lipid bodies, growth properties and protein aggregates from their mother. Together, these differences stratify cellular states within a single population, which we attempt to correlate with phenotypes such as drug tolerance so that we can understand how M. tuberculosis adapt at population- and single-cell levels to tolerate stressors. For example, asymmetric distribution of cellular components such as irreversibly oxidized proteins (IOPs) causes different drug susceptibility owing to different levels of oxidative stress between sister cells149 (FIG. 1d). IOPs are extensively oxidized proteins that irreversibly accumulate carbonyls on amino acid side chains and gain toxicity through illicit binding150,151. Researchers have discovered that the sister cells with a high amount of IOPs are more sensitive to drugs such as kanamycin, streptomycin and isoniazid, which promote oxidative damage149,155,156. Thus, presenting the population structure of M. tuberculosis and the measurement of cellular IOP concentration in a diagram would reveal a subset of drug-susceptible bacteria characterized by high amounts of IOPs (FIG. 3).
Though the mechanisms are not understood, cell size correlates with drug susceptibility at a single-cell level3,8,11,42. It may be that cell size is an informative and measurable factor of individual cells that integrates several elements of growth behavior and is variable from cell-to-cell. Long cells (such as accelerator cells) are more sensitive to cell wall-acting drugs, such as cycloserine, meropenem and isoniazid, relative to smaller cells (alternator cells)3. However, cells born small are more susceptible to rifampicin, which targets transcription8,42. Using the framework of a population structure, if we plot cell size, we would expect the low end of the distribution to be susceptible to transcriptional inhibitors and the high end to be susceptible to cell wall-acting agents (FIG. 3). The breadth of this cell size distribution is also a determinant of drug tolerance as knockdown of lamA, which reduces cell size variation, increases rifampicin and vancomycin susceptibility in M. smegmatis and M. tuberculosis8. Depending on the degree of such asymmetry, the distribution of heterogeneity may also vary from population-to-population. In a population structure framework, decreasing asymmetry (and heterogeneity) may reduce the proportion of the population in the ‘survivor’ region (for example, shifting from the structure in condition 2 to 3 with drug A) (FIG. 3). However, we have yet to understand how drug-tolerant subpopulations may be characterized using patterns of growth state in different host-relevant growth conditions. Rational approaches to designing shortened treatment therapies for TB must address the origins and vulnerabilities of persisters, and how growth and division asymmetry in these conditions promote tolerance.
Consequences of environmental heterogeneity.
M. tuberculosis are versatile, with the flexibility to readily adapt to different host environments. We illustrate examples of how adaptation to different host environments changes the population structure. One example is a simple shift to cholesterol as a carbon source. In this example, one axis (property A) in FIG. 3 can be used to represent the expression level of mce4, which encodes the cholesterol import system88. We presume that the population would shift to the right on a cholesterol diet as mce4 expression increases. Growth state can be used as property B in which population structure movement relative to the y-axis will depend on the cholesterol levels, as M. tuberculosis doubles faster in higher concentrations of cholesterol28. Changes to specific carbon sources may increase density in the population structure plot (FIG. 3), whereas others may cause scattering.
The parameters of the population structure of M. tuberculosis in the acidified phagosome have yet to be thoroughly defined. However, data suggests that we can map drug and stress tolerance to metabolism and growth state. For example, a strong link between non-growing cells (for example, a growth state as property A) and increased use of the glyoxylate shunt (metabolism as property B) at low pH was identified, which confers rifampicin and isoniazid tolerance157 (FIG. 3). The subpopulations formed in this environment are gradually being characterized and ongoing work focuses on understanding the changes in metabolism caused by M. tuberculosis altering its carbon sources104.
The framework of a population structure may also explain the physiological state of M. tuberculosis in the hypoxic conditions of the granuloma. As one example, expression of genes related to lipid catabolism is upregulated in hypoxia, increasing energy stores in a non-replicating state. Together, these changes create phenotypic drug tolerance142. Applying this example to the framework, if one axis presents lipid catabolism-related gene expression, the population will move to a greater value on this axis in hypoxia. On another axis, which represents growth state, the population would move to a smaller value as they are non-replicating (Fig. 3). Further studies are necessary to define these dynamic population structures in complex lesion conditions and their interplay with drug tolerance.
Consequence of heterogeneity in the clinic.
In patients with TB, subpopulations resulting from both bacterial and environmental heterogeneity are observed. One example is differentially detectable (DD) Mtb/differentially culturable tubercule bacteria (DCTB) found in the patient’s sputum. DD Mtb/DCTB are subpopulations of M. tuberculosis bacilli that are undetectable in standard in vitro culture medium but can grow and therefore be detected in medium supplemented with culture filtrate10,158. DD Mtb/DCTB has been identified in fresh clinical isolates and culture filtrate-added media conditions in vitro10,159. Because standard medical laboratory assays do not detect DD Mtb/DCTB, this hidden subpopulation may be responsible for disease relapse in patients after treatment10,158,160. We have yet to understand how DD Mtb/DCTB cells are generated and the relative proportions of DD Mtb/DCTB in M. tuberculosis populations from different types of lesions. It may also be that other clinically important subpopulations that have yet-to-be defined are undetectable in either medium. Once we understand the factors that foster growth of these subpopulations we can begin to characterize their growth and metabolic properties in the format of a population structure (FIG. 3).
Different microenvironments not only affect the formation of heterogeneous M. tuberculosis subpopulations, including DD Mtb/DCTB, but may also affect the pharmacokinetics–pharmacodynamics [G] (PK–PD). For example, complex granuloma structure and poor vascularization obstruct drug distribution in specific sites78. As we learn more about drug penetrance and the microenvironment of different lesion compartments, we may improve multi-drug regimen designs to contain effective antibiotics and access all M. tuberculosis niches23,28,161-163.
The range of environmental conditions and stressors imposed in different granulomas generates a heterogeneous M. tuberculosis population with remarkable differences in growth and metabolic states and drug susceptibility patterns (FIG. 2). However, population adaptation to different microenvironments does not necessarily make one M. tuberculosis subpopulation tolerant to all types of antibiotics. Persistence is condition- and drug-dependent. We should take advantage of our current knowledge on the range of persisters to rationally design multi-drug therapies that comprehensively target this ensemble. To do so, we must map M. tuberculosis state to patterns of drug efficacy and understand what subpopulations are formed in each lesion type at the single-cell level.
Conclusions and outlook:
Variation in mycobacterial virulence and drug response is achieved by subpopulations that can survive and thrive in the presence of host immune and drug stressors. The characteristics of the stress-tolerant bacilli are dependent on the stressor. Therefore, to have a flexible response to a range of stressors, mycobacteria use variation as a survival strategy. Mycobacterial heterogeneity is generated, in part, through stochastic processes as in other species, but it is amplified in mycobacteria through asymmetric growth and division patterns, and genetic and epigenetic modifications. The pathology of TB also organizes M. tuberculosis into physically separate subpopulations via granulomas, causing resident M. tuberculosis to encounter different and dynamic microenvironments. The adaptation of M. tuberculosis in these physically separate and compositionally distinct lesions forces heterogeneity in the population. In addition, this heterogeneity can be further compounded by innate bacterial variation.
The lengthy, multi-drug treatment regimen for TB is necessary to sterilize every niche of the lung, underscoring how important it is to focus on developing new therapies against the vulnerabilities of the resident drug-tolerant subpopulations. However, we still do not understand how this variation is developed and maintained from both bacterial and lesional sources. This task is difficult not only due to technical challenges in working with a slow-growing pathogen that clumps readily in culture, but also because of the complexity of M. tuberculosis heterogeneity. Cell state and variation are multivariate, dynamic, and condition-specific. For this reason, we propose that mycobacteriologists utilize an integrated approach to aggregate and interpret these data in future studies. These population structures (for example, phase diagrams) will enable us to define different features (axes) of importance and track how single-cell and population-level behaviors can be reconciled (FIG. 3). In the physical sciences (and increasingly in biology), phase diagrams are used to navigate multivariate spaces to understand critical points where qualitative behaviors change. We anticipate that M. tuberculosis will not only shift between different regions on the phase diagram as they adapt to changing host environments but that the degree of heterogeneity (that is; scatter) will also be regulated. In particular, the phase diagram may help specify the changes and effects of these factors that make TB treatment difficult. As TB scientists make more discoveries, visualizing the population structure in this way will help us characterize the relevant features of the M. tuberculosis subpopulations that lead to treatment failure and subsequently inform new treatment strategies to target them.
Technological improvements are poised to accelerate our understanding of heterogeneity in TB and how variation affects disease and treatment outcomes. For example, we expect more widespread use of new imaging methods, including deep immunophenotyping and higher throughput time-lapse imaging accompanied by deep-learning-assisted image segmentation and analysis approaches. Genetic tools such as CRISPR interference, collaborative cross mice, and sets of clinical M. tuberculosis isolates will enable us to understand variation in both the host and pathogen. Together, the increase in depth and breadth in TB studies will help us design improved TB interventions by understanding how M. tuberculosis creates and exploits heterogeneity.
Text Box 1: Mycobacterium tuberculosis heterogeneity and drug susceptibility testing.
Antimicrobial susceptibility testing (AST) is widely implemented in the treatment and management of tuberculosis (TB) cases that fail the frontline treatment of rifampicin and isoniazid166,167. Such testing is required for surveillance and designing an effective regimen of second-line drugs for individual patients to prevent poor treatment outcomes168,169. There are two central methods for diagnosing drug-resistant TB infection: genotypic testing and phenotypic culture-based AST. Though it may take more than 3 weeks before final interpretation, phenotypic testing is often the preferred method for determining drug susceptibility as it is quantitative and overcomes the failure of molecular assays to detect under-appreciated resistance mutations170,171.
Current phenotypic testing methods are nevertheless affected by the extensive heterogeneity of Mycobacterium tuberculosis. Phenotypic AST relies on assessing the growth of a clinical isolate in traditional solid media in the presence of a critical concentration of a drug170. To determine critical concentration and set antimicrobial breakpoints [G] for the classification of isolates as resistant, international committees use data from minimum inhibitory concentration (MIC) distributions of wild-type M. tuberculosis in nutrient-rich growth medium172-174. However, growth in standard conditions does not accurately represent the drug response of M. tuberculosis in vivo21,28,139,160,71-78.
Using results from current AST methods as guidance for clinical regimens has caveats because standard in vitro conditions do not foster the growth of the complete array of M. tuberculosis subpopulations present in the lung. One such example is differentially detectable (DD) Mtb/ differentially culturable tubercle bacilli (DCTB)175-179. Such bacilli require additional, unknown resuscitation-promoting factors not present in rich-growth medium and, thus, these bacilli are not tested for susceptibility using current methods159.
Other subpopulations such as persister cells, or phenotypically drug-tolerant cells, may also confound efforts to utilize AST results to guide clinical regimens. Bulk population growth inhibition metrics like MIC used in AST fail to evaluate the killing of these persisters, the cells that eventually lead to regrowth of M. tuberculosis148. Thus, two M. tuberculosis isolates might have similar MICs but disparate proportions of persisters, which may lead to different treatment outcomes in patients147. A growing appreciation of all of these factors may prompt a re-evaluation of the methods used to determine antimicrobial breakpoints, critical concentrations, and susceptibility of clinical isolates.
Acknowledgements
Work in the author’s laboratory was funded, in part, by the Bill and Melinda Gates Foundation (OPP1204444) and NIH (R01 AI143611-01).
Glossary:
- Granulomas
Complex lesion structures that form during TB pathogenesis and consist of immune cells, epithelioid cells, and necrotic tissue
- Macrophage activation
A change in physiology in response to signals from adaptive or innate immune mechanisms that enable macrophages to perform specialized effector functions
- Necrotic caseum
Lipid-rich foci within granulomas primarily derived from necrotic cellular debris
- Divisome
Protein complex responsible for performing membrane- and cell wall-division functions
- Accelerator cell
Cells that inherit the pole from which its mother cell grew and usually elongate at a higher rate than alternator cells
- Alternator cells
Cells that generate a new growth pole upon birth and elongate at a lower rate than accelerator cells
- Glycopeptidolipid (GPL)
A type of lipid present on the outer leaflet mycobacterial envelopes that influences biofilm formation, growth, and pathogenicity
- Isogenic sister cells
Cells born from the same mother and share identical genotypes
- Minimum inhibitory concentration (MIC)
The lowest concentration of a drug that will inhibit growth
- Collaborative crosses
Panels of mouse strains with significant genomic variation designed to mimic the heterogeneity of the human population and its spectrum of disease phenotypes
- Foamy macrophages
Macrophages that are abundant in lipid droplets formed from pathogen-induced perturbation of lipid biosynthesis
- Virulence factors
Components of a pathogen that enable it to invade and colonize a host
- Secretion systems
Molecular nanomachines present on the outer surface of a pathogen that secrete substrates to promote pathogenicity
- Pharmacokinetics–pharmacodynamics (PK–PD)
The relationship between drug distribution, absorption, metabolism, and concentration in the human body over time
- Antimicrobial breakpoints
The lowest concentration of a drug that will inhibit the growth of almost all (95%) wild type strains of the organism but does not inhibit clinical strains that are resistant
Footnotes
Competing interests
The authors declare no competing interests.
REFERENCES
- 1.WHO. Global Tuberculosis Report. vol. 148 (2020). [Google Scholar]
- 2.Xie YL et al. Fourteen-day PET/CT imaging to monitor drug combination activity in treated individuals with tuberculosis. Sci. Transl. Med 13, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aldridge BB et al. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science 529, 100–104 (2012). This study shows a asymmetric mycobacterial growth and division and finds that sister cells show differential drug susceptibility.
- 4.Joyce G et al. Cell division site placement and asymmetric growth in Mycobacteria. PLoS One 7, e44582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh B et al. Asymmetric growth and division in Mycobacterium spp.: Compensatory mechanisms for non-medial septa. Mol. Microbiol (2013) doi: 10.1111/mmi.12169. This study presents that Mycobacterium marinum and Mycobacteirum smegmatis cells often form division sites at off-centre, which may be compensated by post-septal DNA transport and unequal polar growth.
- 6. Eskandarian HA et al. Division site selection linked to inherited cell surface wave troughs in mycobacteria. Nat. Microbiol 2, 1–6 (2017). This study shows that mycobacteria present morphological landmark on the surface of the cells which corresponds to future sites of division.
- 7.Logsdon MM et al. A Parallel Adder Coordinates Mycobacterial Cell-Cycle Progression and Cell-Size Homeostasis in the Context of Asymmetric Growth and Organization. Curr. Biol 27, 3367–3374.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hesper Rego E, Audette RE & Rubin EJ Deletion of a mycobacterial divisome factor collapses single-cell phenotypic heterogeneity. Nature 546, 153–157 (2017). This study presents that LamA is a member of the mycobacterial division complex by showing deletion of lamA decreases asymmetric polar growth in mycobacteria.
- 9.Hannebelle MTM et al. A biphasic growth model for cell pole elongation in mycobacteria. Nat. Commun 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito K et al. Rifamycin action on RNA polymerase in antibiotic tolerant Mycobacterium tuberculosis results in differentially detectable populations. Proc. Natl. Acad. Sci. U. S. A 114, E4832–E4840 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vijay S et al. Influence of stress and antibiotic resistance on cell-length distribution in Mycobacterium tuberculosis clinical isolates. Front. Microbiol 21, 2296 (2017) doi: 10.3389/fmicb.2017.02296. This study shows that increased cell size and variation in cell length were found in sputum and infected macrophages compared to liquid culture. It also shows that increased cell length was associated with pulmonary TB disease severity.
- 12.Hicks ND et al. Clinically prevalent mutations in Mycobacterium tuberculosis alter propionate metabolism and mediate multidrug tolerance. Nat. Microbiol 3, 1032–1042 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks ND, Carey AF, Yang J, Zhao Y & Fortunea SM Bacterial genome-wide association identifies novel factors that contribute to ethionamide and prothionamide susceptibility in Mycobacterium tuberculosis. MBio 10, e00616–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Advani J et al. Whole genome sequencing of Mycobacterium tuberculosis clinical isolates from India reveals genetic heterogeneity and region-specific variations that might affect drug susceptibility. Front. Microbiol 10, 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginhoux F, Schultze JL, Murray PJ, Ochando J & Biswas SK New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol 17, 34–40 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Mouton JM, Helaine S, Holden DW & Sampson SL Elucidating population-wide mycobacterial replication dynamics at the single-cell level. Microbiol. (United Kingdom) 162, 966–978 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryson BD et al. Heterogeneous GM-CSF signaling in macrophages is associated with control of Mycobacterium tuberculosis. Nat. Commun 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y et al. Rapid and specific labeling of single live Mycobacterium tuberculosis with a dual-targeting fluorogenic probe. Sci. Transl. Med 10, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potvin-Trottier L, Luro S & Paulsson J Microfluidics and single-cell microscopy to study stochastic processes in bacteria. Curr. Opin. Microbiol 43, 186–192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannebelle MTM et al. A biphasic growth model for cell pole elongation in mycobacteria. Nat. Commun 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prideaux B et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat. Med (2015) doi: 10.1038/nm.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerman M et al. Ethambutol partitioning in tuberculous pulmonary lesions explains its clinical efficacy. Antimicrob. Agents Chemother 61, e00924–17 (2017) doi: 10.1128/AAC.00924-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCaffrey EF et al. The immunoregulatory landscape of human tuberculosis granulomas. Nat. Immunol (2020) doi: 10.1038/s41590-021-01121-x. (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu WY et al. Updates on 18F-FDG-PET/CT as a clinical tool for tuberculosis evaluation and therapeutic monitoring. Quant. Imaging Med. Surg 9, 1132–1146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stylianidou S, Brennan C, Nissen SB, Kuwada NJ & Wiggins PA SuperSegger: robust image segmentation, analysis and lineage tracking of bacterial cells. Mol. Microbiol 102, 690–700 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Vicar T et al. Cell segmentation methods for label-free contrast microscopy: Review and comprehensive comparison. BMC Bioinformatics 20, 1–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J et al. Spatiotemporal localization of proteins in mycobacteria. Cell Rep. 37, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkins-Ford J et al. Systematic measurement of combination-drug landscapes to predict in vivo treatment outcomes for tuberculosis. Cell Syst. 1–18 (2021) doi: 10.1016/j.cels.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JJ et al. Transient drug-tolerance and permanent drug-resistance rely on the trehalose-catalytic shift in Mycobacterium tuberculosis. Nat. Commun 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinney JD et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735 (2000). [DOI] [PubMed] [Google Scholar]
- 31. Daniel J, Maamar H, Deb C, Sirakova TD & Kolattukudy PE Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 7, (2011). This study shows that Mtb develops phenotypic tolerance to isoniazid under hypoxic and lipid-loaded macrophages. They also suggest that Mtb utilize host TAG for their lipid metabolism.
- 32.Santucci P et al. Nitrogen deprivation induces triacylglycerol accumulation, drug tolerance and hypervirulence in mycobacteria. Sci. Rep 9, 8667 (2019) doi: 10.1038/s41598-019-45164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker JJ, Johnson BK & Abramovitch RB Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Mol. Microbiol 94, 56–69 (2014) doi: 10.1111/mmi.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng L, Chen S & Hu Y PhoPR positively regulates whiB3 expression in response to low pH in pathogenic mycobacteria. J. Bacteriol 200, e00766–17 (2018) doi: 10.1128/JB.00766-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shastri MD et al. Role of oxidative stress in the pathology and management of human tuberculosis. Oxidative Medicine and Cellular Longevity 2018, 7695364 (2018) doi: 10.1155/2018/7695364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voskuil MI, Bartek IL, Visconti K & Schoolnik GK The response of Mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front. Microbiol 2, 105 (2011) doi: 10.3389/fmicb.2011.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kang CM, Nyayapathy S, Lee JY, Suh JW & Husson RN Wag31, a homologue of the cell division protein DivIVA, regulates growth, morphology and polar cell wall synthesis in mycobacteria. Microbiology 154, 725–735 (2008) doi: 10.1099/mic.0.2007/014076-0. This study shows Wag31, a homologue of the cell division protein DivIVA, is localized to the cell pole, regulating cell wall synthesis and cell shape.
- 38. Meniche X et al. Subpolar addition of new cell wall is directed by DivIVA in mycobacteria. Proc. Natl. Acad. Sci 111, E3243–E3251 (2014). This study shows that the tropomyosin-like protein, DivIVA, is loaced at the tip of the growing cell pole, interacting with enzymes that are involved in the cell wall precursor synthesis.
- 39.Kieser KJ et al. Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria. PLoS. Pathog 11, e1005010 (2015) doi: 10.1371/journal.ppat.1005010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jani C et al. Regulation of polar peptidoglycan biosynthesis by Wag31 phosphorylation in mycobacteria. BMC Microbiol. 10, 327 (2010) doi: 10.1186/1471-2180-10-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Botella H et al. Distinct Spatiotemporal Dynamics of Peptidoglycan Synthesis between Mycobacterium smegmatis and Mycobacterium tuberculosis. MBio 8, e01183–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson K et al. Temporal and intrinsic factors of rifampicin tolerance in mycobacteria. Proc. Natl. Acad. Sci. U. S. A 113, 8302–8307 (2016) doi: 10.1073/pnas.1600372113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elowitz MB, Levine AJ, Siggia ED & Swain PS Stochastic gene expression in a single cell. Science (80-. ) 297, 1183–1186 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Huh D & Paulsson J Random partitioning of molecules at cell division. Proc. Natl. Acad. Sci. U. S. A 108, 15004–15009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohler K & Ibba M Translational fidelity and mistranslation in the cellular response to stress. Nature Microbiology (2017) doi: 10.1038/nmicrobiol.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen MMK, Desai RV, Simpson ML & Weinberger LS Cytoplasmic Amplification of Transcriptional Noise Generates Substantial Cell-to-Cell Variability. Cell Syst. 7, 384–397.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kieser KJ & Rubin EJ How sisters grow apart: mycobacterial growth and division. Nat. Rev. Microbiol 12, 550–562 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Logsdon MM & Aldridge BB Stable regulation of cell cycle events in mycobacteria: Insights from inherently heterogeneous bacterial populations. Front. Microbiol 9, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torrelles JB et al. Characterization of virulence, colony morphotype and the glycopeptidolipid of Mycobacterium avium strain 104. Tuberculosis 82, 293–300 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Giovannini D et al. A new Mycobacterium tuberculosis smooth colony reduces growth inside human macrophages and represses PDIM Operon gene expression. Does an heterogeneous population exist in intracellular mycobacteria? Microb. Pathog 53, 135–146 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Supply P, Marceau M, Mangenot S & Roche D Genome analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of the etiologic agent of tuberculosis. Nat. Genet 45, 172–179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pawlik A et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. (2013) doi: 10.1111/mmi.12387. [DOI] [PubMed] [Google Scholar]
- 53.Bellerose MM et al. Common Variants in the Glycerol Kinase Gene Reduce Tuberculosis Drug Efficacy. mBio 10, e00663–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Safi H et al. Phase variation in Mycobacterium tuberculosis glpK produces transiently heritable drug tolerance. Proc. Natl. Acad. Sci. U. S. A 116, 19665–19674 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heunis T et al. Proteogenomic investigation of strain variation in clinical Mycobacterium tuberculosis isolates. J. Proteome Res 16, 3841–3851 (2017) doi: 10.1021/acs.jproteome.7b00483. [DOI] [PubMed] [Google Scholar]
- 56. Wakamoto Y et al. Dynamic persistence of antibiotic-stressed mycoacteria. Science 339, 91–95 (2013). This study shows that stochastic processes contribute to population heterogeneity in mycobacteria and cause drug persistence.
- 57.Zhu JH et al. Rifampicin can induce antibiotic tolerance in mycobacteria via paradoxical changes in rpoB transcription. Nat. Commun 9, 4218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Javid B et al. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc. Natl. Acad. Sci. U. S. A 111, 1132–1137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pym AS, Saint-Joanis B & Cole ST Effect of katG mutations on the virulence of Mycobacterium tuberculosis and the implication for transmission in humans. Infect. Immun 70, 4955–4960 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wengenack NL, Todorovic S, Yu L & Rusnak F Evidence for differential binding of isoniazid by Mycobacterium tuberculosis KatG and the isoniazid-resistant mutant KatG(S315T). Biochemistry 37, 15825–15834 (1998). [DOI] [PubMed] [Google Scholar]
- 61.Gagneux S et al. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog. 2, 0603–0610 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie L et al. First succinyl-proteome profiling of extensively drug-resistant Mycobacterium tuberculosis revealed involvement of succinylation in cellular physiology. J. Proteome Res 14, 107–119 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Gupta M et al. HupB, a nucleoid-associated protein of Mycobacterium tuberculosis, is modified by serine/threonine protein kinases in vivo. J. Bacteriol 196, 2646–2657 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh S, Padmanabhan B, Anand C & Nagaraja V Lysine acetylation of the Mycobacterium tuberculosis HU protein modulates its DNA binding and genome organization. Mol. Microbiol 100, 577–588 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Sakatos A et al. Posttranslational modification of a histone-like protein regulates phenotypic resistance to isoniazid in mycobacteria. Sci. Adv 4, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorla P et al. MtrA response regulator controls cell division and cell wall metabolism and affects susceptibility of mycobacteria to the first line antituberculosis drugs. Front. Microbiol 9, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jakkala K & Ajitkumar P Hypoxic Non-replicating Persistent Mycobacterium tuberculosis Develops Thickened Outer Layer That Helps in Restricting Rifampicin Entry. Front. Microbiol 10, 2339 (2019) doi: 10.3389/fmicb.2019.02339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lenaerts A, Barry CE & Dartois V Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol. Rev 264, 288–307 (2015) doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qualls JE & Murray PJ Immunometabolism within the tuberculosis granuloma: amino acids, hypoxia, and cellular respiration. Semin. Immunopathol 38, 139–152 (2016) doi: 10.1007/s00281-015-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cadena AM, Fortune SM & Flynn JL Heterogeneity in tuberculosis. Nat. Rev. Immunol 17, 691–702 (2017) doi: 10.1038/nri.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barry CE et al. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol 7, 845–855 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santucci P et al. Intracellular localisation of Mycobacterium tuberculosis affects efficacy of the antibiotic pyrazinamide. Nat. Commun 12, 1–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin PL et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat. Med 20, 75–79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manina G, Dhar N & McKinney JD Stress and host immunity amplify Mycobacterium tuberculosis phenotypic heterogeneity and induce nongrowing metabolically active forms. Cell Host Microbe 17, 32–46 (2015) doi: 10.1016/j.chom.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Subbian S et al. Lesion-specific immune response in granulomas of patients with pulmonary tuberculosis: A pilot study. PLoS One 10, e0132249 (2015) doi: 10.1371/journal.pone.0132249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marakalala MJ et al. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat. Med 22, 531–538 (2016) doi: 10.1038/nm.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarathy JP et al. Extreme drug tolerance of Mycobacterium tuberculosis in Caseum. Antimicrob. Agents Chemother 62, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strydom N et al. Tuberculosis drugs’ distribution and emergence of resistance in patient’s lung lesions: A mechanistic model and tool for regimen and dose optimization. PLoS Med. 16, 1–26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsai MC et al. Characterization of the tuberculous granuloma in murine and human lungs: Cellular composition and relative tissue oxygen tension. Cell. Microbiol 8, 218–232 (2006) doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 80.Ryan GJ et al. Multiple M. tuberculosis phenotypes in mouse and guinea pig lung tissue revealed by a dual-staining approach. PLoS One 5, e11108 (2010) doi: 10.1371/journal.pone.0011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Driver ER et al. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother 56, 3181–3195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blanc L et al. High-resolution mapping of fluoroquinolones in TB rabbit lesions reveals specific distribution in immune cell types. Elife 7, 1–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rifat D et al. Pharmacokinetics of rifapentine and rifampin in a rabbit model of tuberculosis and correlation with clinical trial data. Sci. Transl. Med 10, eaai7786 (2018) doi: 10.1126/scitranslmed.aai7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith CM et al. Functionally overlapping variants control TB susceptibility in Collaborative Cross mice. mBio 10, 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gold B & Nathan C Targeting Phenotypically Tolerant Mycobacterium tuberculosis. Microbiol. Spectr (2017) doi: 10.1128/microbiolspec.tbtb2-0031-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guerrini V et al. Storage lipid studies in tuberculosis reveal that foam cell biogenesis is disease-specific. PLoS Pathog. 14, e1007223 (2018) doi: 10.1371/journal.ppat.1007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim MJ et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol. Med 2, 258–274 (2010) doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandey AK & Sassetti CM Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci 105, 4376–4380 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith TC II. et al. Morphological profiling of tubercle bacilli identifies drug pathways of action. Proc. Natl. Acad. Sci. U. S. A 117, 18744–18753 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borah K et al. Metabolic fluxes for nutritional flexibility of Mycobacterium tuberculosis. Mol. Syst. Biol 17, 1–16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gould TA, Van De Langemheen H, Muñoz-Elías EJ, McKinney JD & Sacchettini JC Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Mol. Microbiol 61, 940–947 (2006) doi: 10.1111/j.1365-2958.2006.05297.x. [DOI] [PubMed] [Google Scholar]
- 92.Eoh H & Rhee KY Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids. Proc. Natl. Acad. Sci. U. S. A 111, 4976–4981 (2014) doi: 10.1073/pnas.1400390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mali PC & Meena LS Triacylglycerol: nourishing molecule in endurance of Mycobacterium tuberculosis. J. Biosci 43, 149–154 (2018) doi: 10.1007/s12038-018-9729-6. [DOI] [PubMed] [Google Scholar]
- 94.Salamon H et al. Cutting Edge: Vitamin D Regulates Lipid Metabolism in Mycobacterium tuberculosis Infection. J. Immunol 193, 30–34 (2014) doi: 10.4049/jimmunol.1400736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schaible UE, Sturgill-Koszycki S, Schlesinger PH & Russell DG Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol 160, 1290–1296 (1998). [PubMed] [Google Scholar]
- 96.Pethe K et al. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. U. S. A 101, 13642–13647 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tan S, Sukumar N, Abramovitch RB, Parish T & Russell DG Mycobacterium tuberculosis Responds to Chloride and pH as Synergistic Cues to the Immune Status of its Host Cell. PLoS Pathog. 9, e1003282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarathy JP & Dartois V Caseum: A niche for Mycobacterium tuberculosis drug-tolerant persisters. Clin. Microbiol. Rev 33, 1–19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Irwin SM et al. Bedaquiline and Pyrazinamide Treatment Responses Are Affected by Pulmonary Lesion Heterogeneity in Mycobacterium tuberculosis Infected C3HeB/FeJ Mice. ACS Infect. Dis 2, 251–267 (2016) doi: 10.1021/acsinfecdis.5b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanoix JP et al. Selective inactivity of pyrazinamide against tuberculosis in C3HeB/FeJ mice is best explained by neutral pH of caseum. Antimicrob. Agents Chemother 60, 735–743 (2016) doi: 10.1128/AAC.01370-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Early JV, Mullen S & Parish T A rapid, low pH, nutrient stress, assay to determine the bactericidal activity of compounds against non-replicating Mycobacterium tuberculosis. PLoS One 14, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raynaud C et al. Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol. Microbiol 45, 203–217 (2002). [DOI] [PubMed] [Google Scholar]
- 103.Vandal OH, Nathan CF & Ehrt S Acid resistance in Mycobacterium tuberculosis. J. Bacteriol 191, 4714–4721 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gouzy A, Healy C, Black KA, Rhee KY & Ehrt S Growth of Mycobacterium tuberculosis at acidic pH depends on lipid assimilation and is accompanied by reduced GAPDH activity. Proc. Natl. Acad. Sci. U. S. A 118, 1–9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parish T Two-Component Regulatory Systems of Mycobacteria. Microbiol. Spectr 2, (2014) doi: 10.1128/microbiolspec.mgm2-0010-2013. [DOI] [PubMed] [Google Scholar]
- 106.Walters SB et al. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol 60, 312–330 (2006) doi: 10.1111/j.1365-2958.2006.05102.x. [DOI] [PubMed] [Google Scholar]
- 107.Johnson BK et al. The carbonic anhydrase inhibitor ethoxzolamide inhibits the Mycobacterium tuberculosis PhoPR regulon and Esx-1 secretion and attenuates virulence. Antimicrob. Agents Chemother 59, 4436–4445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fratti RA, Chua J, Vergne I & Deretic V Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. U. S. A 100, 5437–5442 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Indrigo J, Hunter RL & Actor JK Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology 149, 2049–2059 (2003). [DOI] [PubMed] [Google Scholar]
- 110.Axelrod S et al. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell. Microbiol 10, 1530–1545 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Tinaztepe E et al. Role of metal-dependent regulation of ESX-3 secretion in intracellular survival of Mycobacterium tuberculosis. Infect. Immun 84, 2255–2263 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.MacGurn JA & Cox JS A genetic screen for Mycobacterium tuberculosis mutants defective for phagosome maturation arrest identifies components of the ESX-1 secretion system. Infect. Immun 75, 2668–2678 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Augenstreich J et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell. Microbiol 19, 1–19 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Tufariello JAM et al. Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. Proc. Natl. Acad. Sci. U. S. A 113, E348–E357 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coulson GB et al. Targeting Mycobacterium tuberculosis Sensitivity to Thiol Stress at Acidic pH Kills the Bacterium and Potentiates Antibiotics. Cell Chem. Biol 24, 993–1004 (2017) doi: 10.1016/j.chembiol.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goyal N, Kashyap B, Singh NP & Kaur IR Neopterin and oxidative stress markers in the diagnosis of extrapulmonary tuberculosis. Biomarkers 22, 648–653 (2017) doi: 10.1080/1354750X.2016.1265005. [DOI] [PubMed] [Google Scholar]
- 117. Mishra R et al. Targeting redox heterogeneity to counteract drug tolerance in replicating Mycobacterium tuberculosis. Sci. Transl. Med (2019) doi: 10.1126/scitranslmed.aaw6635. This study presents that drug tolerant Mtb population increases during phagosomal acidification by altering the redox physiology.
- 118.Herb M & Schramm M Functions of ros in macrophages and antimicrobial immunity. Antioxidants 10, 1–39 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Acker H & Coenye T The Role of Reactive Oxygen Species in Antibiotic-Mediated Killing of Bacteria. Trends Microbiol. 25, 456–466 (2017) doi: 10.1016/j.tim.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 120.Lin K et al. Mycobacterium tuberculosis Thioredoxin Reductase Is Essential for Thiol Redox Homeostasis but Plays a Minor Role in Antioxidant Defense. PLoS Pathog. 12, 1–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ezraty B, Gennaris A, Barras F & Collet JF Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol 15, 385–396 (2017) doi: 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- 122.Foti JJ, Devadoss B, Winkler J, Collins J & Walker G Oxidation of the guanine nucleotide poll underlies cell death by bactericidal antibiotics. Science (80-. ) 336, 315–319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Portevin D et al. Lipidomics and genomics of Mycobacterium tuberculosis reveal lineage-specific trends in mycolic acid biosynthesis. Microbiologyopen 3, 823–835 (2014) doi: 10.1002/mbo3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu Y, Gulbins E & Grassmé H Crosstalk between sphingomyelinases and reactive oxygen species in mycobacterial infection. Antioxid. Redox Signal 28, 935–948 (2018) doi: 10.1089/ars.2017.7050. [DOI] [PubMed] [Google Scholar]
- 125.Bryk R, Griffin P & Nathan C Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215 (2000) doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 126.Springer B et al. Silencing of oxidative stress response in Mycobacterium tuberculosis: Expression patterns of ahpC in virulent and avirulent strains and effect of ahpC inactivation. Infect. Immun 69, 5967–5973 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin DM et al. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 6, e1001230 (2010) doi: 10.1371/journal.ppat.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu J & Holmgren A The thioredoxin antioxidant system. Free Radic. Biol. Med 66, 75–87 (2014) doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 129.Nambi S et al. The Oxidative Stress Network of Mycobacterium tuberculosis Reveals Coordination between Radical Detoxification Systems. Cell Host Microbe 17, 829–837 (2015) doi: 10.1016/j.chom.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mishra R, Yadav V, Guha M & Singh A Heterogeneous Host–Pathogen Encounters Coordinate Antibiotic Resilience in Mycobacterium tuberculosis. Trends Microbiol. 29, 606–620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Astarie-Dequeker C et al. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect. Immun 67, 469–477 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Esparza M et al. PstS-1, the 38-kDa Mycobacterium tuberculosis glycoprotein, is an adhesin, which binds the macrophage mannose receptor and promotes phagocytosis. Scand. J. Immunol 81, 46–55 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Sun J et al. Mycobacterium tuberculosis Nucleoside Diphosphate Kinase Inactivates Small GTPases Leading to Evasion of Innate Immunity. PLoS Pathog. 9, 1–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vilchèze C, Hartman T, Weinrick B & Jacobs WR Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun 4, 1881 (2013) doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sikri K et al. Multifaceted remodeling by vitamin C boosts sensitivity of Mycobacterium tuberculosis subpopulations to combination treatment by anti-tubercular drugs. Redox Biol. 15, 452–466 (2018) doi: 10.1016/j.redox.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Via LE et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun 76, 2333–2340 (2008) doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harper J et al. Mouse Model of Necrotic Tuberculosis Granulomas Develops Hypoxic Lesions. J. Infect. Dis 205, 595–602 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leistikow RL et al. The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J. Bacteriol 192, 1662–1670 (2010) doi: 10.1128/JB.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oehlers SH et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature 517, 612–615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang H et al. Lysine acetylation of DosR regulates the hypoxia response of Mycobacterium tuberculosis. Emerg. Microbes Infect 7, 34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schnappinger D et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: Insights into the phagosomal environment. J. Exp. Med 198, 693–704 (2003) doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Galagan JE et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499, 178–183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Irwin SM et al. Limited activity of clofazimine as a single drug in a mouse model of tuberculosis exhibiting caseous necrotic granulomas. Antimicrob. Agents Chemother 58, 4026–4034 (2014) doi: 10.1128/AAC.02565-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin PL et al. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc. Natl. Acad. Sci. U. S. A 109, 14188–14193 (2012) doi: 10.1073/pnas.1121497109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Y Metronidazole validates drugs targeting hypoxic bacteria for improved treatment of tuberculosis. Proc. Natl. Acad. Sci. U. S. A 109, 13890–13891(2012) doi: 10.1073/pnas.1211081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Iacobino A, Giannoni F, Pardini M, Piccaro G & Fattorini L The combination rifampin-nitazoxanide, but not rifampin-isoniazid-pyrazinamide-ethambutol, kills dormant Mycobacterium tuberculosis in hypoxia at neutral pH. Antimicrob. Agents Chemother 63, e00273–19 (2019) doi: 10.1128/AAC.00273-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Torrey HL, Keren I, Via LE, Lee JS & Lewis K High persister mutants in Mycobacterium tuberculosis. PLoS One 11, 1–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Balaban NQ et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol 17, 441–448 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vaubourgeix J et al. Stressed mycobacteria use the chaperone ClpB to sequester irreversibly oxidized proteins asymmetrically within and between cells. Cell Host Microbe 17, 178–190 (2015). This study shows that irreversibly oxidized protein (IOP) aggregates are distributed asymmetrically within bacteria. It shows that progeny with various IOP burdens show different growth rate and survival against antibiotic stress.
- 150.Dukan S & Nyström T Bacterial senescence: Stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev. 12, 3431–3441 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hartl FU, Bracher A & Hayer-Hartl M Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 (2011). [DOI] [PubMed] [Google Scholar]
- 152.Barak Z, Gallant J, Lindsley D, Kwieciszewki B & Heidel D Enhanced ribosome frameshifting in stationary phase cells. J. Mol. Biol 263, 140–148 (1996). [DOI] [PubMed] [Google Scholar]
- 153.Dukan S et al. Protein oxidation in response to increased transcriptional or translational errors. Proc. Natl. Acad. Sci. U. S. A 97, 5746–5749 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ballesteros M, Fredriksson Å, Henriksson J & Nyström T Bacterial senescence: Protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO J. 20, 5280–5289 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grant SS, Kaufmann BB, Chand NS, Haseley N & Hung DT Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc. Natl. Acad. Sci. U. S. A 109, 12147–12152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nandakumar M, Nathan C & Rhee KY Isocitrate lyase mediates broad antibiotic tolerance in Mycobacterium tuberculosis. Nat. Commun 5, 4306 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Baker JJ & Abramovitch RB Genetic and metabolic regulation of Mycobacterium tuberculosis acid growth arrest. Sci. Rep 8, 4168 (2018) doi: 10.1038/s41598-018-22343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Mukamolova GV, Turapov O, Malkin J, Woltmann G & Barer MR Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am. J. Respir. Crit. Care Med 181, 174–180 (2010) doi: 10.1164/rccm.200905-0661OC. This study found a Mtb population from human sputum samples that only grows in the presence of resuscitation-promotic factors.
- 159.Gordhan BG et al. Detection of differentially culturable tubercle bacteria in sputum using mycobacterial culture filtrates. Sci. Rep 11, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Turapov O et al. Phenotypically adapted Mycobacterium tuberculosis populations from sputum are tolerant to first-line drugs. Antimicrob. Agents Chemother 60, 2476–2483 (2016) doi: 10.1128/AAC.01380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Singh V & Mizrahi V Identification and validation of novel drug targets in Mycobacterium tuberculosis. Drug Discov. Today 22, 503–509 (2017). [DOI] [PubMed] [Google Scholar]
- 162.Aldridge BB et al. The Tuberculosis Drug Accelerator at year 10: what have we learned? Nat. Med 27, 1329–1333 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ernest JP et al. Development of New Tuberculosis Drugs: Translation to Regimen Composition for Drug-Sensitive and Multidrug-Resistant Tuberculosis. Annu. Rev. Pharmacol. Toxicol 61, 495–516 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Priestman M, Thomas P, Robertson BD & Shahrezaei V Mycobacteria modify their cell size control under sub-optimal carbon sources. Front. Cell Dev. Biol 5, 64 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jennewein J et al. Low-oxygen tensions found in Salmonella-infected gut tissue boost Salmonella replication in macrophages by impairing antimicrobial activity and augmenting Salmonella virulence. Cell. Microbiol 17, 1833–1847 (2015) doi: 10.1111/cmi.12476. [DOI] [PubMed] [Google Scholar]
- 166.Bloemberg GV et al. Acquired Resistance to Bedaquiline and Delamanid in Therapy for Tuberculosis. N. Engl. J. Med 373, 1986–1988 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schön T et al. Mycobacterium tuberculosis drug-resistance testing: challenges, recent developments and perspectives. Clin. Microbiol. Infect 23, 154–160 (2017). [DOI] [PubMed] [Google Scholar]
- 168.Georghiou SB et al. Guidance for Studies Evaluating the Accuracy of Rapid Tuberculosis Drug-Susceptibility Tests. J. Infect. Dis 220, S126–S135 (2019). [DOI] [PubMed] [Google Scholar]
- 169.World Health Organization. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. 1–96 (2014). [Google Scholar]
- 170.Gilpin C, Korobitsyn A & Weyer K Current tools available for the diagnosis of drug-resistant tuberculosis. Ther. Adv. Infect. Dis 3, 145–151 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dookie N, Rambaran S, Padayatchi N, Mahomed S & Naidoo K Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother 73, 1138–1151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Juréen P et al. Wild-type MIC distributions for aminoglycoside and cyclic polypeptide antibiotics used for treatment of Mycobacterium tuberculosis infections. J. Clin. Microbiol 48, 1853–1858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ängeby K, Juréen P, Kahlmeter G, Hoffner SE & Schönd T Challenging a dogma: antimicrobial susceptibility testing breakpoints for Mycobacterium tuberculosis. Bull. World Health Organ 90, 693–698 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.EUCAST. SOP for calibrating surrogate MIC methods for M. tuberculosis against the EUCAST reference MIC method. 2–4 (2019). [Google Scholar]
- 175.Hett EC et al. A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis. Mol. Microbiol 66, 658–668 (2007) doi: 10.1111/j.1365-2958.2007.05945.x. [DOI] [PubMed] [Google Scholar]
- 176.Hett EC, Chao MC, Deng LL & Rubin EJ A mycobacterial enzyme essential for cell division synergizes with resuscitation-promoting factor. PLoS Pathog. 4, e1000001 (2008) doi: 10.1371/journal.ppat.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nikitushkin VD et al. A product of RpfB and RipA joint enzymatic action promotes the resuscitation of dormant mycobacteria. FEBS J. 282, 2500–2511 (2015) doi: 10.1111/febs.13292. [DOI] [PubMed] [Google Scholar]
- 178.Chengalroyen MD et al. Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am. J. Respir. Crit. Care Med 194, 1532–1540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dusthackeer A et al. Differential culturability of Mycobacterium tuberculosis in culture-negative sputum of patients with pulmonary tuberculosis and in a simulated model of dormancy. Front. Microbiol 10, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]