Abstract
Background
Graft macrosteatosis can predispose to a higher risk of graft loss so we sought to redefine acceptable cutoffs for graft steatosis.
Methods
Data of 26,103 donors who underwent liver transplantation (LT) between January 2004 and December 2018 from the UNOS-STAR database were utilized. A high-risk steatotic (HRS) graft and a low-risk steatotic (LRS) graft were defined as ≥20% and <20% macrosteatosis, respectively. High-risk steatotic grafts were further classified as grafts with 20–29% (G1S grafts), 30–39% (G2S grafts), and ≥40% steatosis (G3S grafts). Outcomes between groups were compared.
Results
LRS grafts had excellent graft (93.3 and 87.7%) and overall survival (95.4 and 90.5%) at 90 days and 1 year. Compared to LRS grafts, G1S, G2S, and G3S grafts had worse graft and overall survival at 90 days and 1-year (p <0.001). There was no difference in graft or overall survival of G1S or G3S grafts compared to G2S grafts until after adjustment in which G3S grafts were found to be associated with an increased risk of graft loss—aHR 1.27 (1.03–1.57), p = 0.02.
Discussion
Liver grafts can be categorized into three categories: (1) <20% or “very low risk”, (2) 20–39% or “low-to-moderate risk”, and usually acceptable, and (3) ≥40% steatosis or “moderate-to-high risk”.
How to cite this article
Da BL, Satiya J, Heda RP, et al. Outcomes after Liver Transplantation with Steatotic Grafts: Redefining Acceptable Cutoffs for Steatotic Grafts. Euroasian J Hepato-Gastroenterol 2022;12(Suppl 1):S5–S14.
Keywords: Allografts, Donor selection, Fatty liver, Liver transplantation, Tissue and organ procurement
Introduction
Liver transplantation is currently the only curative option for patients with end-stage liver disease. The performance of LT is limited by the availability of suitable donor grafts and the number of patients on the waiting list for LT continues to outnumber the number of acceptable donors.1 The utilization of steatotic liver allografts is one of the accepted solutions to this organ shortage issue.2 However, the presence of graft macrosteatosis predisposes to a higher risk of ischemic reperfusion injury and has been associated with a wide range of short-term adverse outcomes after LT, including an increased incidence of bile duct injury, higher rates of primary and early graft dysfunction, and worse graft survival.3,4 As such, graft macrosteatosis is also one of the main reasons that potential donor grafts are discarded. In this study, macrosteatosis will be used interchangeably with steatosis and micro-steatosis will not be discussed.
As the prevalence of obesity, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD) is rapidly rising in the United States, the utilization of steatotic liver grafts will continue to be an important topic of debate.5,6 Donors of steatotic grafts are considered extended criteria donors which implies an inherently higher risk of post-LT complications in comparison with a reference donor.7 The degree of acceptable steatosis is controversial and differs greatly between centers.8 Historically, donor hepatic steatosis can be graded into three categories: mild (<30%), moderate (30–60%), and severe (>60%) steatosis.9 However, these cutoffs are largely arbitrary and were formulated decades ago based on small cohorts.10 Nonetheless, most centers are willing to accept grafts up to an upper threshold of approximately 30% macrosteatosis although some centers will be more conservative depending on the presence of other high-risk features such as deceased after cardiac death (DCD) status.
Severely steatotic grafts (>60% steatosis) are rarely used in the present era due to early studies that reported high rates of graft failure.11,12 Meanwhile, grafts with mild steatosis can generally be used safely with graft survival rates similar to grafts without steatosis.13 The tolerability and outcomes of grafts with “moderate” steatosis continue to be heavily debated with some studies reporting decreased graft survival,14–16 overall survival,16 and biliary complications,17 while others have not shown any differences in those outcomes.18,19 It is difficult to make firm conclusions based on existing literature since many of these studies were single center or studies done before direct acting antiviral (DAA) for hepatitis C virus (HCV) infection were available. Moreover, these studies lacked proper graft matching due to a limited understanding of donor/recipient risk factors at the time. In recent years, advances in recipient to donor matching have led to an improvement in outcomes such that the graft survival of moderately steatotic grafts (>30% steatosis) can be similar to that of nonsteatotic donor grafts.3,20,21 Nevertheless, many centers continue to discard a significant portion of their steatotic grafts, especially those with >30% steatosis.
Due to the increasing prevalence of NAFLD, it would also be reasonable to estimate that the degree of steatosis seen in donor liver grafts will also continue to increase, thus posing a threat to organ donation. It is already estimated that the utilization rates of donor grafts will decline from 78 to 44% by 2030 due to declining graft quality, thus representing a serious problem.22 Therefore, every effort should be made to delineate the degree of “tolerable” graft steatosis and create a more precise steatosis categorization system even if it means reclassifying preexisting cutoffs for graft steatosis grading. Thus, in this study, we explored graft and patient survival outcomes with different cutoffs for steatosis using the United Network for Organ Sharing (UNOS) database.
Methods
Data Source and Cohort
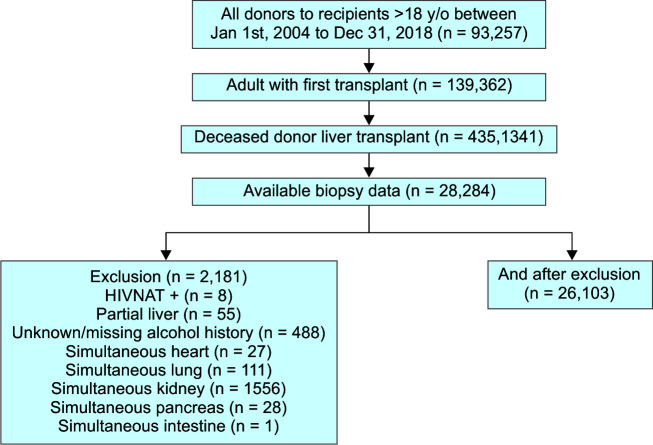
The data used in this study were obtained from the UNOS Standard Transplant Analysis and Research (UNOS-STAR) database. We included all donors for recipients aged 18 and older who underwent LT between January 1, 2004, and December 31, 2018, in the study cohort. As shown in Flowchart 1, we excluded recipients with a history of previous LT (n = 3,895), underwent living donor liver transplant (LDLT) (n = 3,521), LT without available biopsy (n = 57,557), unknown/missing alcohol history (n = 488), underwent partial LT (n = 55), simultaneous multiorgan transplants (n = 1,630), and HIV NAT+ (n = 8). Data from the UNOS-STAR source were used to determine donor and recipient characteristics prior to or at the time of LT. Information on comorbidities, clinical variables, laboratory values, clinical variables, and transplantation-related data of the recipients and donors were also extracted from the UNOS-STAR database.
Flowchart 1.
Study flow diagram
HIV, human immunodeficiency virus; NAT, nucleic acid testing
Definitions
A HRS graft was defined in this study as a donor graft with ≥20% macrosteatosis. High-risk steatotic grafts were further subclassified as grafts with 20–29% (G1S grafts), 30–39% (G2S grafts), and ≥40% macrosteatosis (G3S grafts). Low-risk steatotic grafts, defined as grafts with <20% macrosteatosis, were considered the primary comparison or reference graft group. Body mass was graded at six levels in this study: underweight (BMI <18.5), normal (BMI 18.4–24.9), overweight (BMI 25.0–29.9), class I obesity (BMI 30.0–34.9), class II obesity (BMI 35.0–39.9), and class III obesity (BMI >40.0). Graft loss was defined as patient death or need for re-transplant. Donor risk index (DRI) was calculated for each donor.23 A DRI ≤1 was considered an excellent donor with historical 3-year graft survival of greater than 80%, while a DRI >2 was considered a poor donor with 3-year graft survival of approximately 60%.
Outcomes and Comparisons
The primary outcomes of interest were the 90 days and 1-year graft survival of different steatosis cutoffs of HRS grafts (G1S, G2S, and G3S grafts). Secondary outcomes of interest were the 90 days and 1-year overall patient survival of recipients of different steatosis cutoffs of HRS grafts. The primary comparisons of interest were the risk of graft loss and mortality between different steatosis cutoffs at 1-year.
Statistical Analysis
Baseline characteristics were presented as percentages for categorical variables and mean ± standard deviation (SD) or median (interquartile range [IQR]) for continuous variables. Continuous variables were compared using Student's t test, and categorical variables were compared with the Chi-square test. Cochran–Armitage trend test was used to assess temporal trends in the utilization of the steatotic grafts from 2004 to 2018. To further investigate the overall patient and graft survival within 90 days and 1-year after LT, we censored patients that lived longer than 90 days or 1-year (or have graft survival longer than 90 days or 1-year), respectively. Kaplan–Meier curves were used to assess the overall patient and graft survival within 90 days and 1-year after LT of four separate groups of patients: recipients of (1) LRS grafts, (2) G1S grafts, (3) G2S grafts, and (4) G3S grafts. Log-rank test was used to make comparisons between Kaplan–Meier curves.
The association between increasing grades of HRS grafts and graft loss/mortality after LT was assessed using both univariate and multivariate Cox proportional regression analysis to evaluate the impact of specific graft steatosis group on patients’ survival. For the multivariate Cox proportional hazard model, we adjusted for significant predictors (recipient and donor) of graft loss and mortality (on univariate analysis). Reported p-values were two-sided and reported as significant if <0.05. All analyses were conducted using SAS 9.4 (SAS, Cary, North Carolina). The Institutional Review Boards of the North Shore University Hospital did not require a formal IRB review as the data are publicly available.
Results
Demographic, Clinical, and Biochemical Characteristics of the Entire Cohort Based on Donor Macrosteatosis Subgroup
In this study, 26,103 donors between January 1, 2004, and December 31, 2018, utilized for LT were included. The number of donors in each of the subclassifications of steatotic grafts was as follows: <20% (LRS, n = 21,819), 20–29% (G1S, n = 2,008), 30–39% (G2S, n = 1,298), and ≥40% (G3S, n = 978). Biochemical and donor characteristics for the entire cohort and each subgroup are shown in Table 1. Mean donor age became progressively younger as the steatosis grades increased among the HRS grades: 49.8 ± 13.2 y/o (G1S) versus 48.3 ± 13.1 (G2S) versus 45.5 ± 13.8 y/o (G3S) (p <0.001). Donors of any grade of HRS grafts had a higher BMI than donors of LRS grafts (p <0.0001); however, degree of BMI was not associated with increasing graft steatosis grades.
Table 1.
Comparison of the donor characteristics as grouped by % of macrosteatosis of the donor graft
| Parameters | All donors (n = 26,103) | Groups based on donor graft macrosteatosis % | p-value * | |||
|---|---|---|---|---|---|---|
| <20% (LRS) (n = 21,819) | 20–29% (G1S) (n = 2,008) | 30–39% (G2S) (n = 1,298) | ≥40% (G3S) (n = 978) | |||
| Age, y/o | 49.3 ± 15.0 | 49.5 ± 15.3 | 49.8 ± 13.2 | 48.3 ± 13.1 | 45.5 ± 13.8 | <0.001 |
| Gender, female | 12,086 (46.3) | 10,220 (46.8) | 854 (42.5) | 571 (44.0) | 441 (45.1) | <0.001 |
| Creatinine (mg/dL) | 1.8 ± 1.9 | 1.9 ± 1.9 | 1.8 ± 1.7 | 1.7 ± 1.7 | 1.8 ± 1.6 | 0.71 |
| Total bilirubin (mg/dL) | 0.9 ± 0.9 | 0.9 ± 0.9 | 0.9 ± 0.9 | 0.9 ± 0.8 | 0.9 ± 0.7 | 0.004 |
| ALT (U/L) | 86.5 ± 204.3 | 86.7 ± 208.8 | 85.9 ± 183.9 | 79.41 ± 161.9 | 93.0 ± 191.1 | <0.001 |
| AST (U/L) | 91.7 ± 192.4 | 92.0 ± 196.8 | 89.0 ± 171.0 | 89.0 ± 182.3 | 94.4 ± 140.8 | <0.001 |
| BMI, kg/m2 | 29.7 ± 7.4 | 29.3 ± 7.2 | 32.1 ± 7.3 | 32.4 ± 7.8 | 31.7 ± 7.6 | <0.001 |
| Anti-HCV+, yes | 2,349 (9.0) | 2,129 (9.8) | 120 (6.0) | 50 (3.9) | 50.0 (5.1) | <0.001 |
| HCV NAT+, yes | 745 (2.9) | 679 (3.1) | 32 (1.6) | 16 (1.2) | 18.0 (1.8) | <0.001 |
| HBV NAT+, yes | 37 (0.1) | 35 (0.2) | 1 (0.05) | 1 (0.08) | 0 (0.0) | 0.32 |
| DCD, yes | 940 (3.6) | 851 (3.9) | 40 (1.9) | 30 (2.3) | 19 (1.8) | <0.001 |
| CIT, hours | 6.8 ± 2.5 | 6.8 ± 2.5 | 6.9 ± 2.4 | 6.9 ± 2.5 | 6.9 ± 2.4 | 0.005 |
| Hypertension, yes | 13,312 (51.0) | 11,043 (50.6) | 1,092 (54.4) | 737 (56.8) | 440 (45.0) | <0.001 |
| Diabetes, yes | 5,016 (19.2) | 4,175 (19.1) | 399 (19.9) | 279 (21.5) | 163 (16.7) | 0.03 |
| Heavy alcohol use, yes | 5,372 (20.6) | 4,345 (19.9) | 474 (23.6) | 309 (23.8) | 244 (25.0) | <0.001 |
| Cause of death Anoxia CVA Head trauma CNS tumor Unknown |
8,014 (30.7) 12,012 (46.0) 5,474 (21.0) 102 (0.4) 501 (1.9) |
6,731 (30.9) 10,058 (46.1) 4,525 (20.7) 74 (0.3) 431 (2.0) |
617 (30.7) 923 (46.0) 432 (21.5) 8 (0.4) 28 (1.4) |
353 (27.2) 636 (49.0) 280 (21.6) 10 (0.8) 19 (1.5) |
313 (32.0) 395 (40.4) 237 (24.2) 10 (1.0) 23 (2.4) |
<0.001 |
| DRI coefficient§ | 1.27 (1.11, 1.53) | 1.27 (1.11, 1.54) | 1.27 (1.12, 1.51) | 1.26 (1.12, 1.48) | 1.26 (1.11, 1.51) | 0.59 |
| Race Caucasian Hispanic AA Asian Others |
17,746 (68.0) 2,761 (10.6) 4,515 (17.3) 610 (2.3) 471 (1.8) |
14,751 (67.6) 2,165 (9.9) 4,003 (18.4) 503 (2.3) 397 (1.8) |
1,412 (70.3) 244 (12.2) 244 (12.2) 57 (2.8) 45 (2.2) |
910 (70.1) 167 (12.9) 167 (12.9) 34 (2.6) 20 (1.5) |
673 (68.8) 179 (18.3) 101 (10.3) 16 (1.6) 9 (0.9) |
<0.001 |
Values expressed as mean ± sd or n (%) as appropriate unless otherwise stated.
§Value expressed as medians (IQR).
*Continuous variables across the four groups were compared using Kruskal–Wallis test, while categorical variables were compared using Chi-square test. LRS, low-risk steatosis; G1S, grade I steatosis; G2S, grade II steatosis; G3S, grade III steatosis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HCV, hepatitis C virus; NAT, nucleic acid amplification testing; DCD, donation after cardiac death; CVA, cerebral vascular accident; CNS, central nervous system; DRI, donor risk index; AA, African American
Donors of HRS grafts were less likely to be HCV + (anti-HCV or HCV NAT+) compared to donors of LRS grafts. LRS grafts used in LT were more likely to also be a DCD graft (3.6%) compared to HRS grafts—1.9% (G1S), 2.3% (G2S), and 1.8% (G3S) (p <0.001). However, there was no difference in the liver DRI among grafts from the four steatosis categories (p = 0.59). Recipient characteristics across the four groups (LRS, G1S, G2S, and G3S) are shown in Table 2. Overall, similar recipient characteristics were seen across groups although statistical differences were seen in recipient creatinine, INR, and MELD with increasing graft steatosis grades.
Table 2.
Comparison of recipient characteristics as grouped by % of macrosteatosis of the donor graft
| Parameters | All donors (n = 26,103) | Groups based on donor graft macrosteatosis % | p-value * | |||
|---|---|---|---|---|---|---|
| <20% (LRS) (n = 21,819) | 20–29% (G1S) (n = 2,008) | 30–39% (G2S) (n = 1,298) | ≥40% (G3S) (n = 978) | |||
| Age, years | 55.6 ± 9.7 | 55.6 ± 9.8 | 55.9 ± 9.4 | 55.5 ± 9.9 | 55.9 ± 9.4 | 0.75 |
| Gender, female | 7,815 (29.9) | 6,697 (30.7) | 534 (26.6) | 322 (24.8) | 262 (26.8) | <0.001 |
| BMI, kg/m2 | 28.8 ± 5.7 | 28.8 ± 5.7 | 29.1 ± 5.6 | 28.9 ± 5.7 | 28.9 ± 5.7 | 0.04 |
| BMI group Underweight Normal Overweight Class I obesity Class II obesity Class III obesity |
344 (1.3) 6,668 (25.6) 9,152 (35.1) 6,157 (23.7) 2,752 (10.6) 991 (3.8) |
289 (1.3) 5,628 (25.8) 7,631 (35.0) 5,138 (23.6) 2,288 (10.5) 826 (3.8) |
23 (1.2) 460 (22.9) 710 (35.4) 528 (26.3) 210 (10.5) 76 (3.8) |
15 (1.2) 338 (26.1) 466 (35.9) 286 (22.1) 140 (10.8) 52 (4.0) |
17 (1.7) 242 (24.7) 345 (35.3) 223 (22.8) 114 (11.7) 37 (3.8) |
0.26 |
| Race Caucasian Hispanic AA Asian Other |
19,291 (73.9) 2,262 (8.7) 3,100 (11.9) 1,050 (4.0) 400 (1.5) |
16,049 (73.6) 2,640 (12.1) 1,923 (8.8) 875 (4.0) 332 (1.5) |
1,522 (75.8) 205 (10.2) 157 (7.8) 89 (4.4) 35 (1.7) |
992 (76.4) 137 (10.6) 100 (7.7) 53 (4.1) 16 (1.2) |
728 (74.4) 118 (12.1) 82 (8.4) 33 (3.4) 17 (1.7) |
0.14 |
| Diabetes, yes | 7,152 (27.4) | 5,981 (27.4) | 527 (26.3) | 377 (29.0) | 267 (27.3) | 0.37 |
| Ascites, yes | 19,328 (74.5) | 16,170 (74.6) | 1,483 (74.2) | 951 (73.5) | 724 (74.3) | 0.83 |
| Creatinine, mg/dL | 1.3 ± 1.0 | 1.3 ± 1.0 | 1.3 ± 0.9 | 1.3 ± 0.9 | 1.2 ± 0.8 | <0.001 |
| Total bilirubin, mg/dL | 7.1 ± 9.3 | 7.2 ± 9.4 | 6.9 ± 9.0 | 6.6 ± 8.7 | 6.2 ± 8.3 | 0.008 |
| Albumin, g/dL | 3.1 ± 0.7 | 3.1 ± 0.7 | 3.1 ± 0.7 | 3.1 ± 0.7 | 3.0 ± 0.7 | 0.76 |
| INR | 1.9 ± 1.2 | 1.9 ± 1.2 | 1.9 ± 1.3 | 1.8 ± 1.1 | 1.8 ± 1.1 | 0.007 |
| MELD | 20.7 ± 10.0 | 20.8 ± 10.1 | 20.8 ± 9.9 | 20.2 ± 9.5 | 19.5 ± 10.1 | 0.003 |
| Vent support at LT, yes | 860 (3.3) | 739 (3.4) | 63 (3.1) | 37 (2.9) | 21 (2.2) | 0.13 |
| Etiology of LT Noncholestatic cirrhosis Acute hepatic necrosis Cholestatic liver disease Malignant neoplasms Other |
18,428 (70.6) 775 (3.0) 1,863 (7.1) 3,540 (13.6) 1,496 (5.7) |
15,402 (70.6) 665 (3.1) 1,552 (7.1) 2,933 (13.4) 1,266 (5.8) |
1,421 (70.8) 63 (3.1) 127 (6.3) 280 (13.9) 117 (5.8) |
911 (70.2) 30 (2.3) 103 (7.9) 189 (14.6) 65 (5.0) |
694 (71.0) 17 (1.7) 81 (9.3) 138 (14.1) 48 (4.9) |
0.33 |
Values expressed as mean ± sd or n (%) as appropriate.
*Continuous variables across the four groups were compared using Kruskal–Wallis test, while categorical variables were compared using Chi-square test
Utilization of Steatotic Grafts in the United States
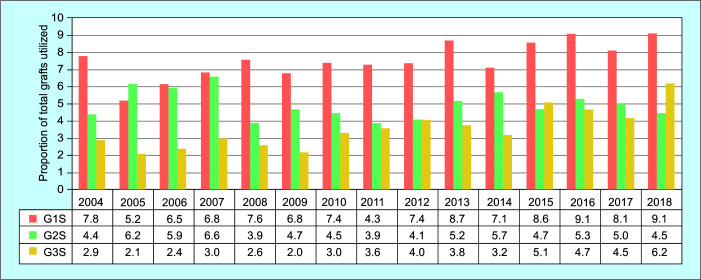
The trend in the proportion of steatotic grafts relative to total liver grafts utilized in the United States from 2004 to 2018 as stratified based on the three HRS groups is depicted in Figure 1. G1S and G3S grafts have been increasingly utilized during the time frame studied (p <0.001), while the utilization of G2S grafts has remained relatively consistent. Meanwhile, the utilization of grafts from obese donors with a BMI >35, >40, >45, and >50 have all increased from 2004 to 2018 as well (p <0.001) (Supplemental Figure 1).
Fig. 1.
Trend for the utilization of HRS liver allografts in the United States. Trends of the proportion of total liver allografts utilized are depicted as three groups. G1S (20–29% macrosteatosis), G2S (30–39% macrosteatosis), and G3S (≥40% macrosteatosis) grafts. Trend in the proportion of G1S and G3S grafts has increased from 2004 to 2018 (p <0.001), while G2S grafts have not (p = 0.23) (Cochran–Armitage trend test). G1S, grade I steatosis, G2S, grade II steatosis, G3S, grade III steatosis
Post-transplant Outcomes for Patients Receiving Steatotic Grafts in the United States
Graft Survival
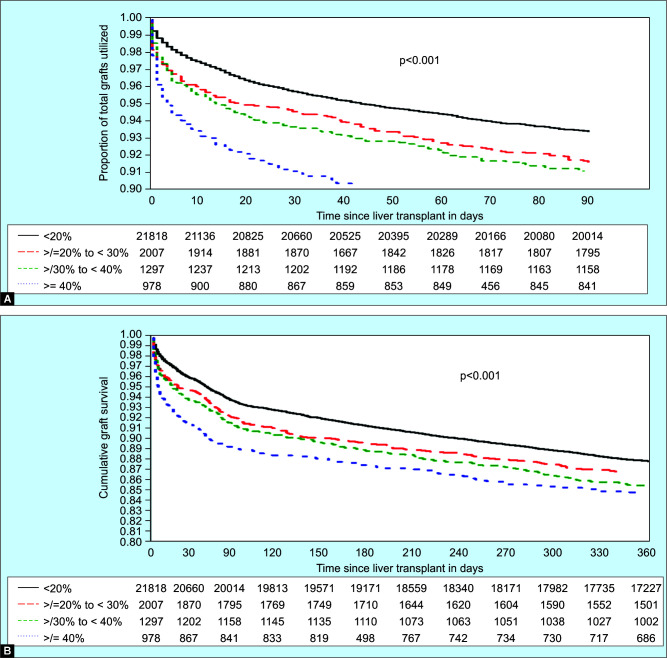
Graft survival post-LT is shown in Figures 2A (90 days) and B (1 year). LRS grafts had a graft survival of 93.3, 91.0, and 87.7% at 90 days, 6 months, and 1 year. G1S grafts had a graft survival of 91.5, 89.4, and 86.6% at 90 days, 6 months, and 1 year. G2S grafts had a graft survival of 90.9, 88.7, and 85.2% at 90 days, 6 months, and 1 year. Finally, G3S grafts had an estimated graft survival of 88.8, 87.0, and 84.5% at 90 days, 6 months, and 1 year. Using LRS grafts as the reference, G1S, G2S, and G3S grafts had worse graft survival at 90 days and 1-year (p <0.001). Using G2S grafts as the reference, there was no difference in 90 days or 1-year graft survival compared to G1S or G3S grafts.
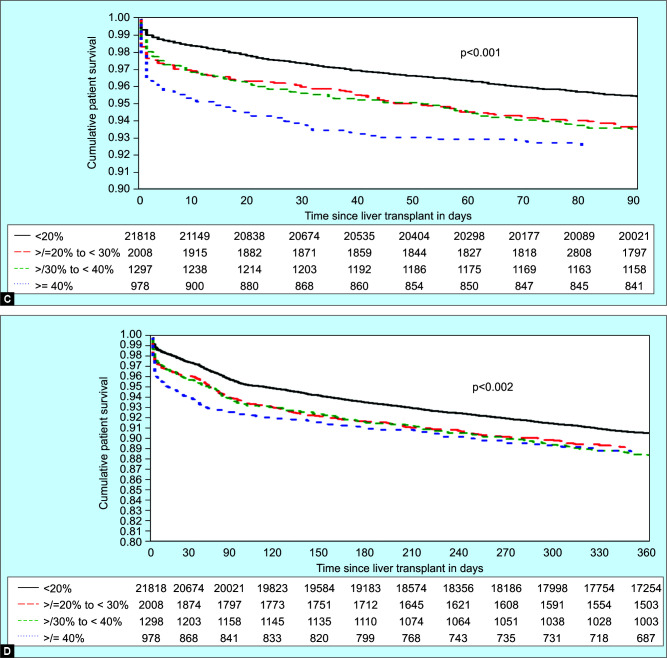
Figs 2A to D.
Overall Graft and patient survival at 90 days and 1 year. (A) Overall graft survival at 90 days, (B) Overall graft survival at 1 year, (C) Overall patient survival at 90 days, (D) Overall patient survival at 1 year. LRS grafts—<20% macrosteatosis; G1S grafts—20–29% macrosteatosis; G2S grafts—30–39% macrosteatosis; and G3S grafts—≥40% macrosteatosis. LRS grafts as the reference: Recipients of G1S, G2S, and G3S grafts had worse graft survival at 90 days and 1 year (p <0.001). Recipients of G2S grafts as the reference: There was no difference in 90 days or 1-year graft survival compared to G1S or G3S grafts
Overall Patient Survival
Patient survival post-LT is shown in Figures 2C (90 days) and D (1-year). Recipients of LRS grafts had an overall survival of 95.4, 93.4, and 90.5% at 90 days, 6 months, and 1 year. Recipients of grafts with G1S had an overall survival of 93.5, 91.6, and 89.1% at 90 days, 6 months, and 1 year. Recipients of grafts with G2S had an overall survival of 93.4, 91.5, and 88.3% at 90 days, 6 months, and 1 year. Recipients of grafts with G3S had an overall survival of 92.4, 90.8, and 88.5% at 90 days, 6 months, and 1 year. Using recipients of LRS grafts as the reference, recipients of G1S, G2S, and G3S grafts had worse overall survival at 90 days and 1-year (p <0.001). Using recipients of G2S grafts as the reference, there was no difference in patient survival between recipients of G1S or G3S grafts compared to recipients of G2S grafts at 90 days or 1 year.
Cause of Death and Reasons for Graft Failure within 1 Year
One-thousand two-hundred and seventy-five and 2,455 patients died within 90 days and 1-year of LT. Cause of death of patients as subdivided based on the degree of steatosis in the donor graft is shown in Supplemental Table 1. Cardiovascular disease was the leading overall cause of death in recipients of LRS grafts at 90 days (27.1%) and the second leading cause of death at 1-year (17.6%) after infection (18.8%). In recipients of G1S, G2S, and G3S grafts, cardiovascular disease was the leading cause of death at 90 days (35.7, 31.0, and 42.5%) and at 1-year (26.7, 19.9, and 30.1%). Graft failure was the cause of death within 90 days and 1-year in 8.9 and 12.0% of recipients of LRS grafts. Meanwhile, graft failure was the cause of death within 1-year in 12.4% of recipients of G1S grafts, 13.7% of recipients of G2S grafts, and 10.4% of recipients of G3S grafts. Across groups, the most common reason for graft failure was primary nonfunction (PNF). Graft failure and PNF usually occurred within 90 days post-LT and occurred more frequently with HRS grafts.
Predictors of Graft Survival and Patient within 1 Year
Using LRS grafts as the reference, all three grades of HRS grafts were associated with a higher likelihood for graft loss and mortality with the exception of G1S grafts relative to LRS grafts for graft loss (p = 0.11) (Supplemental Table 2). Predictors of graft loss and patient mortality at 1-year with HRS grafts (≥20% macrosteatosis) on univariate analysis are shown in Table 3. Significant predictors of graft loss included recipient MELD, recipient need for ventilator support at the time of LT, donor hypertension, donor COD-CVA, donor COD-head trauma, DRI, and CIT. For mortality, significant predictors included recipient age, MELD, and etiology of LT, donor COD-CVA, donor COD-head trauma, and CIT. Table 4 shows the association between steatosis grade and graft loss/mortality at 1-year before and after adjusting for significant predictors on univariate analysis using G1S grafts as the reference. Prior to adjustment, G2S and G3S grafts were not associated with a higher likelihood of graft loss or mortality compared to G1S grafts. After adjusting, G3S grafts demonstrated a higher likelihood of graft loss relative to G1S grafts [aHR 1.27 (1.03–1.57), p = 0.02].
Table 3.
Univariate analysis of variables and effect on outcomes at 1 year in ≥20% steatosis
| Recipient | Graft survival | Mortality | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.00 (0.99–1.01) | 0.69 | 1.01 (1.00–1.02) | 0.05 |
| BMI | 1.01 (0.99–1.02) | 0.27 | 1.01 (0.99–1.03) | 0.77 |
| MELD | 1.02 (1.01–1.03) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Etiology of LT | ||||
| Noncholestatic liver disease | Ref | ref | ||
| Acute liver failure | 1.62 (0.99–2.33) | 0.06 | 1.68 (1.06–2.67) | 0.03 |
| Cholestatic liver disease | 0.87 (0.63–1.22) | 0.42 | 0.87 (0.56–1.21) | 0.32 |
| Malignant neoplasm | 0.86 (0.67–1.10) | 0.24 | 0.85 (0.64–1.13) | 0.26 |
| Other | 1.36 (0.99–1.87) | 0.06 | 1.2 (0.822–1.76) | 0.34 |
| Vent support at LT | 3.01 (2.19–4.13) | <0.001 | 3.04 (2.12–4.36) | <0.001 |
| Donor | ||||
| Age | 1.01 (1.0–1.02) | 0.006 | 1.01 (1.00 –1.01) | 0.1 |
| BMI | 1.0 (0.99–1.01) | 0.85 | 1.00 (0.99–1.01) | 0.95 |
| Gender—female | 0.95 (0.8–1.11) | 0.5 | 0.92 (0.76–1.1) | 0.35 |
| Race | ||||
| Caucasian | Ref | ref | ||
| Hispanic | 1.17 (0.94–1.47) | 0.17 | 1.11 (0.85–1.43) | 0.44 |
| AA | 0.97 (0.75–1.25) | 0.8 | 0.89 (0.66–1.2) | 0.44 |
| Asian | 1.1 (0.67–1.82) | 0.7 | 1.14 (0.66–2.0) | 0.64 |
| Other | 1.31 (0.75–2.27) | 0.34 | 1.27 (0.68–2.39) | 0.45 |
| HCV Ab+ | 0.69 (0.45–1.06) | 0.09 | 0.73 (0.46–1.18) | 0.2 |
| HBV NAT+ | 0.0 (0.0–0.0) | 0.96 | 0.0 (0.0–0.0) | 0.97 |
| Heavy alcohol use | 1.02 (0.85–1.23) | 0.85 | 1.02 (0.83–1.26) | 0.85 |
| Hypertension | 1.24 (1.05–1.46) | 0.001 | 1.09 (0.91–1.31) | 0.35 |
| Diabetes | 1.13 (0.93–1.38) | 0.21 | 1.03 (0.82–1.3) | 0.78 |
| DCD | 1.09 (0.63–1.89) | 0.75 | 0.98 (0.51–1.89) | 0.94 |
| CIT | 1.04 (1.02–1.07) | 0.0003 | 1.03 (1.01–1.06) | 0.02 |
| COD | ||||
| Anoxia | ref | ref | ||
| CVA | 1.83 (1.49–2.25) | <0.001 | 1.88 (1.48–2.39) | <0.001 |
| Head trauma | 1.38 (1.08–1.77) | 0.011 | 1.54 (1.16–2.04) | 0.003 |
| CNS tumor | 1.55 (0.57–4.18) | 0.39 | 1.56 (0.5–4.94) | 0.45 |
| Unknown | 1.36 (0.69–2.67) | 0.38 | 1.83 (0.92–3.63) | 0.084 |
| DRI | 1.18 (1.03–1.35) | 0.018 | 1.16 (0.99–1.35) | 0.07 |
AA, African American; Ab, antibody; BMI, body mass index; CIT, cold ischemic time; CVA, cerebrovascular accident; DCD, deceased after cardiac death; DRI, donor risk index; HBV, hepatitis B virus; HCV, hepatitis C virus; LT, liver transplantation; MELD, model for end-stage liver disease; NAT, nucleic acid amplification
Table 4.
Association between steatosis grade and graft loss/mortality at 1 year
| Graft loss | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted analysis | Adjusted analysis * | Unadjusted analysis | Adjusted analysis ¥ | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| G1S (20–29%) | ref | ref | ref | |||||
| G2S (30–39%) | 1.11 (0.92–1.34) | 0.26 | 1.11 (0.91–1.34) | 0.31 | 1.07 (0.87–1.33) | 0.52 | 1.08 (0.87–1.34) | 0.51 |
| G3S (≥40%) | 1.19 (0.97–1.46) | 0.09 | 1.27 (1.03–1.57) | 0.02 | 1.07 (0.85–1.35) | 0.56 | 1.14 (0.90–1.45) | 0.27 |
*Adjusted for recipient variables: MELD, ventilator at time of LT; donor variables: age, hypertension, CIT, donor cause of death;
¥Adjusted for recipient variables: age, MELD, etiology of LT, ventilator at time of LT; donor variables: CIT, donor cause of death; G1S, grade I steatosis; G2S, grade II steatosis; G3S, grade III steatosis, MELD, model for end-stage liver disease; LT, liver transplantation; CIT, cold ischemic time
Discussion
Our study showed that grafts with <20% macrosteatosis should be classified as “very-low-risk” steatotic grafts since they exhibited excellent graft and patient survival after LT that were better than G1S, G2S, and G3S grafts. Among HRS grafts, G1S and G2S grafts had acceptable and comparable graft and patient survival at 90 days and 1-year. This held true even after multivariate regression modeling adjusting for significant predictors for graft loss and mortality at 1-year. When G1S and G3S grafts were compared, they demonstrated similar patient survival at 1-year but there was a trend for higher graft loss at 1-year in G3S grafts compared to G1S grafts on unadjusted analysis (p = 0.09). After adjustment for significant predictors, we saw a higher rate of graft loss at 1-year, aHR 1.27 (1.03–1.57), p = 0.02, with G3S grafts compared to G1S grafts.
These findings suggest that grafts with 20–39% macrosteatosis (G1S + G2S) should be considered of a similar risk category—termed “low-to-moderate risk”. Meanwhile, grafts with ≥40% macrosteatosis (G3S) grafts should be considered its own risk category—termed “moderate-to-high risk”. This categorization system is different compared to existing categorization systems that subdivides steatotic grafts into either mild (<30%), moderate (30–60%), and severe (>60%),9 or grade 0 (<5%), grade I (5–33%), grade II (>33–66%), and grade III (>66%).24 Ideally, our new categorization system will increase the utilization rate of grafts with 30–39% macrosteatosis as a recent study reported that the discard rate of grafts with >30% macrosteatosis is still approximately 40%.20
Despite our introduction of this new categorization system for steatotic grafts, proper donor-to-recipient matching should still be performed at the physician's discretion as there are likely additional important variates that are difficult to account for or are unaccounted for. For example, although we adjusted for significant predictors on our multivariate regression models, we did not adjust for DRI due to multicollinearity and its nonsignificance as a predictor per our predetermined definition of a two-sided p <0.05.
Interestingly, the rates of graft loss with G3S grafts compared to G1S grafts only became statistically significant on the multivariate regression model. This occurred after adjusting for predictors such as MELD score and ventilator support at the time of transplantation which suggests that these more steatotic grafts are being utilized in healthier recipients at baseline. Of note, hypothermic and normothermic machine perfusion may mitigate some of the risk of graft injury with these higher grade steatotic grafts.25,26 Meanwhile, the higher rates of graft loss but not mortality can likely be attributed to the occurrence of re-transplantation resulting in patient survival.
There are several limitations to our study. First, due to the nature of the UNOS database, characteristics and outcomes are reported by individual centers and may be misreported. Second, we have not reported data regarding the characteristics of the discarded grafts since they are not reported in the UNOS database. Another limitation to our study was that the performance of liver biopsy is not standardized. Instead, liver biopsy was done at the discretion of the transplant center and was not performed in about two-thirds of patients.27 Reasons for performing or not performing a liver biopsy can include physician judgment, which is highly subjective, potential to increase CIT because of need to prepare and interpret the biopsy, and the overall risk tolerance of individual centers to steatotic grafts. All of which could have created a selection bias. Finally, substantial intraobserver and interobserver variation exists among pathologists in the assessment of steatosis severity.28
In summary, we report a new steatosis categorization system in which grafts can be subdivided into three categories in terms of post-LT risk tolerance. The first category includes grafts with <20% macrosteatosis and can be considered “very low risk”. The second category includes grafts with ≥20% but <40% macrosteatosis (G1S + G2S) that should be termed “low-to-moderate risk” and can be usually acceptable for transplantation. The third category includes grafts with ≥40% macrosteatosis (G3S) and should be termed “moderate-to-high risk”. Proper donor-to-recipient matching needs to still be performed taking into account factors such as MELD score, CIT, DRI, DCD, and age. Future studies are needed to evaluate the effectiveness of this practice on existing organ shortage issues.
Author Contributions
SKS is a guarantor of the article; SKS and BLD involved in concept and design; SKS, BLD, JS, and RPH acquired the data; SKS, YJ, and BLD involved in statistical analysis and interpretation of data; all the authors drafted and revised the manuscript; and all the authors approved the final version of the article.
Orcid
Ben L Da https://orcid.org/0000-0002-7750-240X
Jinendra Satiya https://orcid.org/0000-0001-5479-2312
Supplementary Material
All the supplementary materials are available online on the website of www.ejohg.com
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Kwong A, Kim WR, Lake JR, et al. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20(Suppl 1):193–299. doi: 10.1111/ajt.15674. [DOI] [PubMed] [Google Scholar]
- 2.McCormack L, Dutkowski P, El-Badry AM, et al. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54(5):1055–1062. doi: 10.1016/j.jhep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Jackson KR, Motter JD, Haugen CE, et al. Minimizing risks of liver transplantation with steatotic donor livers by preferred recipient matching. Transplantation. 2020;104(8):1604–1611. doi: 10.1097/TP.0000000000003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu MJ, Hickey AJ, Phillips AR, et al. The impact of hepatic steatosis on hepatic ischemia-reperfusion injury in experimental studies: a systematic review. Biomed Res Int. 2013;2013:192029. doi: 10.1155/2013/192029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Bacon BR, Dieterich DT, et al. Disparate access to treatment regimens in chronic hepatitis C patients: data from the TRIO network. J Viral Hepat. 2016;23(6):447–454. doi: 10.1111/jvh.12506. [DOI] [PubMed] [Google Scholar]
- 7.Durand F, Renz JF, Alkofer B, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. 2008;14(12):1694–1707. doi: 10.1002/lt.21668. [DOI] [PubMed] [Google Scholar]
- 8.Hamar M, Selzner M. Steatotic donor livers: where is the risk-benefit maximized? Liver Transpl. 2017;23(Suppl 1):S34–S39. doi: 10.1002/lt.24826. [DOI] [PubMed] [Google Scholar]
- 9.Deroose JP, Kazemier G, Zondervan P, et al. Hepatic steatosis is not always a contraindication for cadaveric liver transplantation. HPB. 2011;13(6):417–425. doi: 10.1111/j.1477-2574.2011.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S, Demetris AJ, Makowka L, et al. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation. 1989;47(5):903–905. doi: 10.1097/00007890-198905000-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nocito A, El-Badry AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol. 2006;45(4):494–499. doi: 10.1016/j.jhep.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 12.D'Alessandro AM, Kalayoglu M, Sollinger HW, et al. The predictive value of donor liver biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation. 1991;51(1):157–163. doi: 10.1097/00007890-199101000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Dutkowski P, Schlegel A, Slankamenac K, et al. The use of fatty liver grafts in modern allocation systems: risk assessment by the balance of risk (BAR) score. Ann Surg. 2012;256(5):861–868. doi: 10.1097/SLA.0b013e318272dea2. discussion 868–869. [DOI] [PubMed] [Google Scholar]
- 14.Chu MJ, Dare AJ, Phillips AR, et al. Donor hepatic steatosis and outcome after liver transplantation: a systematic review. J Gastrointest Surg. 2015;19(9):1713–1724. doi: 10.1007/s11605-015-2832-1. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16(7):874–884. doi: 10.1002/lt.22085. [DOI] [PubMed] [Google Scholar]
- 16.Briceño J, Ciria R, Pleguezuelo M, et al. Impact of donor graft steatosis on overall outcome and viral recurrence after liver transplantation for hepatitis C virus cirrhosis. Liver Transpl. 2009;15(1):37–48. doi: 10.1002/lt.21566. [DOI] [PubMed] [Google Scholar]
- 17.de Graaf EL, Kench J, Dilworth P, et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J Gastroenterol Hepatol. 2012;27(3):540–546. doi: 10.1111/j.1440-1746.2011.06844.x. [DOI] [PubMed] [Google Scholar]
- 18.Angele MK, Rentsch M, Hartl WH, et al. Effect of graft steatosis on liver function and organ survival after liver transplantation. Am J Surg. 2008;195(2):214–220. doi: 10.1016/j.amjsurg.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Fishbein TM, Fiel MI, Emre S, et al. Use of livers with microvesicular fat safely expands the donor pool. Transplantation. 1997;64(2):248–251. doi: 10.1097/00007890-199707270-00012. [DOI] [PubMed] [Google Scholar]
- 20.Jackson KR, Motter JD, Haugen CE, et al. Temporal trends in utilization and outcomes of steatotic donor livers in the United States. Am J Transplant. 2020;20(3):855–863. doi: 10.1111/ajt.15652. [DOI] [PubMed] [Google Scholar]
- 21.Chavin KD, Taber DJ, Norcross M, et al. Safe use of highly steatotic livers by utilizing a donor/recipient clinical algorithm. Clin Transplant. 2013;27(5):732–741. doi: 10.1111/ctr.12211. [DOI] [PubMed] [Google Scholar]
- 22.Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015;21(8):1040–1050. doi: 10.1002/lt.24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.Patrono D, Surra A, Catalano G, et al. Hypothermic oxygenated machine perfusion of liver grafts from brain-dead donors. Sci Rep. 2019;9(1):9337. doi: 10.1038/s41598-019-45843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tchilikidi KY. Liver graft preservation methods during cold ischemia phase and normothermic machine perfusion. World J Gastrointest Surg. 2019;11(3):126–142. doi: 10.4240/wjgs.v11.i3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yersiz H, Lee C, Kaldas FM, et al. Assessment of hepatic steatosis by transplant surgeon and expert pathologist: a prospective, double-blind evaluation of 201 donor livers. Liver Transpl. 2013;19(4):437–449. doi: 10.1002/lt.23615. [DOI] [PubMed] [Google Scholar]
- 28.Cesaretti M, Addeo P, Schiavo L, et al. Assessment of liver graft steatosis: where do we stand? Liver Transpl. 2019;25(3):500–509. doi: 10.1002/lt.25379. [DOI] [PubMed] [Google Scholar]