Abstract
Aim
In this study, we aimed to provide information about transient elastography, a noninvasive method that shows liver steatosis and fibrosis, and to review diagnostic accuracy studies in the literature.
Background
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver diseases. It has a wide clinical spectrum, ranging from asymptomatic steatosis to cirrhosis with complications that can lead to mortality. Although its frequency varies geographically, it is believed that one out of every four people in the world has NAFLD. Recently, the number of studies about the noninvasive diagnosis of NAFLD and liver fibrosis is increasing. Vibration-controlled transient elastography (VCTE) is a method used for about two decades and provides important information in determining steatosis and fibrosis in the liver.
Review results
Area under curve (AUC) levels for ≥S1 are between 0.8 and 0.95 in studies showing the accuracy of the CAP score in detecting steatosis. Sensitivity is between 68 and 87% and specificity is 74 and 91%. AUC levels for steatosis ≥S2 range from 0.73 to 0.88. Sensitivity is between 77 and 85% and specificity is 59 and 81%. For detecting ≥S3, AUC levels were 0.69 to 0.94 and the sensitivity and specificity were 71 to 88%, and 58 to 89%, respectively. In studies, evaluating the effectiveness of elastography in determining the level of fibrosis in patients with NAFLD: AUC was between 0.79 and 0.87, sensitivity was 62 and 94%, and specificity was 61 and 100% for F ≥2. Area under curve was 0.76 to 0.98, sensitivity was 65 to 100% and specificity was 75 to 97% for ≥F3. Area under curve was ranged from 0.91 to 0.99 and sensitivity was 78 to 100% and specificity was 76 to 98% for ≥F4. The studies about the comparison of FibroScan and novel transient elastography device (FibroTouch) reported that results are correlated (r = 0.5–0.6) and the AUC of FibroTouch to detect fibrosis is nearly 0.8.
Conclusion
AUROC in studies are mostly above 0.80 in detecting steatosis and detecting the presence of fibrosis in patients diagnosed with NAFLD indicates the reliability of the data obtained. Transient elastography is suggested by the international guidelines for diagnosing NAFLD, especially the decision of biopsy. FibroTouch was found correlated with FibroScan but further studies are necessary to indicate that FibroTouch can be used instead of FibroScan.
How to cite this article
Ozercan AM, Ozkan H. Vibration-controlled Transient Elastography in NAFLD: Review Study. Euroasian J Hepato-Gastroenterol 2022;12(Suppl 1):S41–S45.
Keywords: Fibrosis, Nonalcoholic fatty liver disease, Steatosis, Transient elastography
Background
Nonalcoholic fatty liver disease refers to a wide spectrum of diseases in the liver, ranging from steatosis to steatohepatitis and advanced fibrosis. Especially after the development of fibrosis, it can also cause serious complications that cause mortality and morbidity, such as hepatic decompensation and hepatocellular carcinoma (HCC).1
Nonalcoholic fatty liver disease is frequently associated with metabolic syndrome components, such as diabetes mellitus, dyslipidemia, and obesity. Recently, it has also been noted that NAFLD is a component of metabolic syndrome.2
Nonalcoholic fatty liver disease is the most common of the chronic liver diseases.3 It is reported that one out of every four people worldwide has NAFLD, and this rate also varies according to geographical regions. The region with the highest prevalence of NAFLD is the Middle East with 31.79%, while the region with the least prevalence of NAFLD is the African continent with 13.48%.3 In a study showing the frequency of NAFLD in Asia, it was found that the prevalence of NAFLD increased from 25.28% between 1999 and 2005 to 33.9% between 2012 and 2017.4
Mortality rates are increasing after the development of fibrosis in NAFLD. It was stated that mortality increased even in the case of stage 1 fibrosis, while in the presence of stage 4 fibrosis, all-cause mortality increased by 6.4 times, liver-related mortality increased by 42.3 times.5
It is important to increase awareness of NAFLD, which is increasing in frequency and can lead to serious morbidity and also mortality. As a result of the screening, only about 20% of the NAFLD detected in 250 patients had a suspicion of NAFLD in their previous medical records, and only 10.4% were referred to a gastroenterology or hepatology specialist due to NAFLD.6
Liver biopsy is an invasive method and is considered the gold standard for the diagnosis of liver steatosis and fibrosis. Due to its invasive nature, life-threatening complications, such as pneumothorax, hemothorax, intestinal perforation, and even death can rarely be seen along with more common complications, such as pain and bleeding.7 Additionally, sampling errors in biopsy and interobserver discordance are also limitations of liver biopsy. In a study evaluating interobserver discordance in liver biopsy examination in patients with fatty liver, it was found that concordance in steatosis stage was 26.7%, 62.7% in inflammation stage, 51.3% in ballooning, 48.7% in fibrosis, and 50.7% in steatohepatitis.8
Because liver biopsy is invasive and has serious complications, it is not appropriate to use it for screening and diagnosing NAFLD, which is thought to occur in approximately one out of every four people. Therefore, especially in recent years, noninvasive methods have been developed to detect NAFLD and, in particular, liver fibrosis. These methods can be grouped into calculations involving blood parameters, imaging methods, and elastography. The noninvasive methods are useful for revealing the presence of advanced fibrosis and cirrhosis, while they are insufficient to distinguish fatty liver from steatohepatitis.9 The first-line imaging method is the abdominal ultrasound (US) in terms of evaluating liver steatosis. In B-mode ultrasonography, the liver parenchyma and the kidney parenchyma are subjectively compared to obtain information about steatosis through the hyperechogenicity of the liver. It may be insufficient to diagnose, especially in mild cases, and it cannot show the presence of any inflammation in the liver.2 The most commonly used and accepted noninvasive methods are transient elastography, fibrosis-4 score (FIB-4), and NAFLD fibrosis score (NFS).10 Methods other than conventional ultrasound are not recommended in guidelines as a noninvasive method for the diagnosis of NAFLD. Methods obtained by serum biomarkers (enhanced liver fibrosis test (ELF), FibroMeter, FibroTest, FIB-4, and NFS) and elastographic measurements (kPa >8) are recommended for evaluating patients followed up for fibrosis due to liver steatosis and guiding them to decide on a biopsy.11 Routine screening is not recommended even in high-risk cases for NAFLD, such as type II diabetes, because the accuracy of diagnostic tests is not considered sufficient and there are no effective treatments that can be used in treatment.2,11
Vibration-controlled Transient Elastography (VCTE)
Vibration controlled transient elastography, ultrasound-based elastography (point shear wave elastography and bidimensional shear wave elastography), and MR elastography are used for measuring liver stiffness. MR elastography and proton density fat fraction (PDFF) is used recently and have the highest accuracy rate among the methods. Despite its high accuracy, high cost, taking a long time, requiring a radiologist for evaluation, and limited availability are the limitations to its use in practice.12
Vibration-controlled transient elastography is the most widely used and valid method among noninvasive liver stiffness measurement methods. The most commonly used, the most studied, and the most validated VCTE device is FibroScan (Echosens, Paris, France).
FibroScan
FibroScan has been used for measuring liver stiffness for about 20 years. Controlled attenuation parameter (CAP) feature was also added to the hand probe, later.13 Europe and Food and Drug Administration (FDA) approved the use of FibroScan in 2003 and 2013, respectively for the evaluation of liver stiffness. It was firstly reported by Yoneda in 2007 that this system can be used to determine the severity of fibrosis in NAFLD.14
In the presence of steatosis of the liver, ultrasound waves quickly attenuate as they pass through the tissue. Controlled attenuation parameter measurement also provides information about the presence of steatosis in the liver by measuring this attenuation.15 The results are expressed in decibels per meter (dB/m), ranging from 100 to 400 dB/m13 (Figs 1A and B). There are three different probe options available and the most commonly used is the standard M probe. XL probe creates a lower frequency (2.5 MHz) ultrasound wave, so it is especially preferred in the presence of obesity, where the skin and subcutaneous tissues are thick. Also, S probe is available, which is usually used in children.16 The method is easy to apply and results in a few minutes. In addition, the person who will perform the procedure does not need to undergo a long training before.
Figs 1A and B.
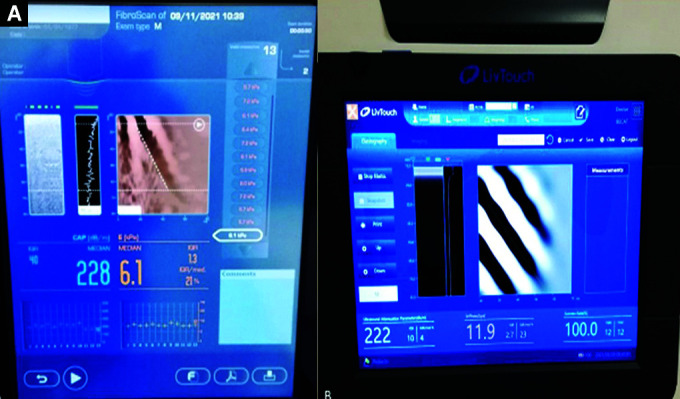
Vibration controlled transient elastography devices. (A) FibroScan 502 touch (Echosens, Paris, France); (B) iLivTouch (Wuxi Hisky Medical Technology, Wuxi, China) (Photos obtained from Hepatology Department of Ankara University)
Vibration controlled transient elastography is highly reproducible (IVV = 0.98) and interobserver variability is low.17 In the presence of inflammation, cholestasis, satiety, venous congestion, liver stiffness is measured at a high level and can be misleading in favor of fibrosis.18
The device spontaneously calculates the interquartile rate (IQR) and IQR/median values of measurements (for CAP and kPa) during measurement, and these values are important in determining the reliability of measurements. Ten valid measurements with IQR/med <30% were considered sufficient as the measurement validity criterion.13 Semmler et al. found that taking CAP IQR <40 dB/m does not make difference in terms of reliability, and it was noted that values such as 60 dB/m, 80 dB/m were also not significant.19 Eddowes et al., shown that having an IQR of <40 dB/m or <30 dB/m does not affect diagnostic performance.20 It was found that FibroScan performance does not increase if Boursier criteria21 (IQR/med <30%, liver stiffness measurement (LSM) ≥7.1 kPa) is used as a reliability criterion for liver stiffness.20 Wong et al. showed that the AUROC value decreased from 0.90 to 0.77 in the case of IQR ≥40, and the difference was found statistically significant in their study, it was also stated that IQR/med value cannot be used for the validity of CAP.22 Unreliable results for liver stiffness were reported nearly 15.8% and associated with operator experience (<500), BMI (>30 kg/m2), age (>52 years), type II diabetes, hypertension, female sex, ALT (>3 × ULN).23 In another study, unreliable results were obtained in 27% of measurements with FibroScan.24
Review Results
Predictive Value of CAP for Steatosis Severity in Meta-analyses (Table 1)
Table 1.
Meta-analyses for accuracy of CAP in assessing liver steatosis
| N | Steatosis grade | AUC | Cut-off (dB/m) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| Petroff (2021) | 1,277a | ≥S1 | 0.80 | 294 | 79 | 74 |
| ≥S2 | 0.73 | 310 | 79 | 59 | ||
| ≥S3 | 0.71 | 331 | 71 | 62 | ||
| Pu (2019) | 1,297b | ≥S1 | 0.95 | – | 87 | 91 |
| ≥S2 | 0.82 | – | 85 | 74 | ||
| ≥S3 | 0.69 | – | 76 | 58 | ||
| Shi (2014) | 1,771c | ≥S1 | 0.85 | 232.5 | 78 | 79 |
| ≥S2 | 0.88 | 255 | 85 | 79 | ||
| ≥S3 | 0.87 | 290 | 83 | 79 | ||
| Karlas (2017) | 2,735c | ≥S1 | 0.82 | 248 | 68 | 82 |
| ≥S2 | 0.86 | 268 | 77 | 81 | ||
| ≥S3 | 0.88 | 280 | 88 | 77 | ||
| Wang (2015) | 2,076c | ≥S1 | 0.86 | 238 | 78 | 79 |
| ≥S2 | 0.88 | 259 | 82 | 79 | ||
| ≥S3 | 0.94 | 290 | 86 | 89 |
aStudy included 2,283 participants with a variety of liver diseases and 1,277 patients with NAFLD analyzed separately;
b1,297patients with biopsy-proven NAFLD were included;
cPatients with variety of liver diseases were included
Evaluation of 1,277 patients with NAFLD, revealed that the AUROC was 0.807 (95% CI, 0.76–0.85) when using an M probe to detect S1 steatosis; the AUROC value was 0.819 (95% CI, 0.77–0.87) when using XL, and no significant difference was found between them. In this study, the cutoff values of S1, S2, and S3 with the M probe were measured as 294, 310, and 331 dB/m, respectively, and the measurements made with the XL probe were similar. It was also shown that the etiology of liver damage, BMI, sex, AST level, and the presence of diabetes can affect the measurement of CAP but the use of an M probe or XL probe does not significantly affect the measurement.25
In a study that included 1,297 patients diagnosed with NAFLD, the AUROC value of the CAP measurement was 0.958 and the sensitivity was 87% and the specificity was 91% in determining the steatosis of S1 and above. In determining steatosis S3 and above, it was shown that the AUROC value decreased by 0.69. Cutoff levels were not specified in this study.26
In another meta-analysis, 1,771 patients who underwent a liver biopsy due to chronic hepatitis were evaluated: The sensitivity of the CAP measurement in the detection of ≥S1 was 78%, the specificity was 79%; the sensitivity in the detection of ≥S2 was 85%, the specificity was 79%; and the sensitivity in the detection of ≥S3 was 83%, the specificity was 79%.27
In a meta-analysis conducted by Karlas et al., optimal cutoff values of 248, 268, and 280 dB/m were found in the detection of steatosis ≥S1, ≥S2, and ≥S3, respectively. With these cutoff values, sensitivity was found to be 68% for ≥S1, 77% for ≥S2, and 88% for ≥S3.28
In Wang et al., the optimal cutoff value for detecting the presence of steatosis (≥S1) was found as 238 dB/m. The level of AUROC was 0.94 in the detection of steatosis ≥S329 (Figs 1A and B).
Diagnostic Accuracy of Elastography on Liver Stiffness in Patients with NAFLD
In studies evaluating the effectiveness of elastography in determining the level of fibrosis in patients with NAFLD, it was noted that if the cutoff value for the diagnosis of advanced fibrosis is 9.9 kPa, the sensitivity is 95%, and the specificity is 77%. AUROC was found to be 0.93 (95% CI, 0.86–0.96) in detecting advanced fibrosis. In this study, no advanced fibrosis was observed in kPa level <7.9. It was also stated that since FibroScan detects advanced fibrosis, it has been predicted that it can eliminate the need for a biopsy in at least 45.1% of patients.24
The analysis of approximately 2,100 NAFLD patients in 11 studies revealed, For F ≥2 fibrosis, AUROC was 0.85 and cutoff values ranged from 6.2 to 11 kPa, with 62 to 90% sensitivity and 74 to 100% specificity. For F ≥3 fibrosis, AUROC was 0.94 and cutoff values ranged from 8 to 12 kPa, with 84 to 100% sensitivity and 83 to 97% specificity. For F4 fibrosis, AUROC was 0.96 and cutoff values ranged from 9.5 to 20 kPa, with 90 to 100% sensitivity and 76 to 98% specificity.18
One thousand and forty-seven patients were evaluated and for F ≥2 fibrosis AUROC values ranged from 0.79 to 0.87, with 67 to 94% sensitivity and 61 to 84% specificity. For F ≥3 fibrosis, AUROC values ranged from 0.76 to 0.98, with 65 to 100% sensitivity and 75 to 97% specificity. For F4 fibrosis, AUROC values range from 0.91 to 0.99, with 78 to 100% sensitivity and 82 to 98% specificity. The overall pooled date showed for F ≥2, sensitivity 79% and specificity 75%; for F ≥3, sensitivity 85% and specificity 85%; for F4, sensitivity 92% and specificity 92%.30
Another study (n = 1,753) reported that overall pooled AUC were 0.85 (95% CI 0.82–0.88), 0.92 (95% CI 0.89–0.94) and 0.94 (95% CI 0.93–0.97) for ≥F2, ≥F3 and F4, respectively.31
Transient elastography measures the steatosis and stiffness of the liver, but can also provide information about the complications of cirrhosis. Especially, the presence of esophageal varices, which is an indicator of decompensation, can be predicted with transient elastography in patients with compensated cirrhosis.18 BAVENO VI consensus stated that LSM <20–25 kPa, platelet count ≥150 × 109/L criteria are associated with a low probability of esophageal varices that requires treatment.32 It is also believed that elastography can also predict the risk of developing HCC in patients with cirrhosis due to the increased risk of developing HCC with the severity of liver fibrosis.18
FibroTouch-iLivTouch
iLivTouch-FibroTouch is a newer device based on similar technical aspects of FibroScan and provides noninvasive liver stiffness and steatosis measurements as kPa and ultrasound attenuation parameter (UAP), respectively. The iLivTouch©—Fibrotouch (Wuxi Hisky Medical Technology Co., Ltd., Wuxi, China) has been used in clinical applications since 2013 and compared with other noninvasive methods for assessment of steatosis and fibrosis in the liver. It has a dynamic hand probe that adjusts positioning and depth of measurement according to skin thickness. The device has a foot button to trigger the vibration impulse. It is necessary to enter the height and weight information before starting the process (Figs 1A and B).
Serra et al. reported good correlation with FibroScan and FibroTouch for stiffness (r = 0.91). Mean overestimation of FibroTouch measurements was found at 3.1 kPa. Ultrasound attenuation parameter was found to strongly depend on BMI and it has been stated that entering the weight and height information before measurement can cause bias.33
In the study Chen et al., a significant positive correlation was found between FibroScan, FibroTouch, and fibrosis scores (r = 0.5 and 0.56). FibroTouch stiffness AUROC was higher than 0.8, especially for mild and severe fibrosis.34
Zeng et al. study enrolled 1,621 patients and correlation between FibroTouch and FibroScan was significant (r = 0.645 for stiffness and r = 0.62 for steatosis).35 Inter and intra-observer reliability of FibroTouch was found higher than FibroScan.36
FibroScan and iLivTouch measurements of 254 consecutive patients with variable liver diseases were compared (unpublished data) in our tertiary hepatology center, Hepatology Department of Ankara University. A positive correlation was found between iLivTouch stiffness measurements and FibroScan, AST to platelet ratio index (APRI), FIB-4, NFS results, and the correlation coefficient between the devices was r = 0.57 (CI 95%, 0.46–0.66). However, Deming regression analysis showed that there may be a proportional bias between the measurements of stiffness. In addition, the mean values of steatosis measurements (UAP and CAP) between the devices were significantly different (247.8 dB/m for iLivTouch and 259 dB/m for FibroScan, p = 0.006).
Discussion and Clinical Significance
Vibration controlled transient elastography is an increasingly used method in the diagnosis and follow-up of NAFLD because of the reliable data and the ease of its applicability. The AUROC in studies are mostly above 0.80 in detecting steatosis and detecting the presence of fibrosis in patients diagnosed with NAFLD indicates the reliability of the data obtained. It has already been emphasized in the international guidelines that it can also be used in the follow-up of patients and especially, in the decision on liver biopsy. Measurement of liver stiffness allows not only to determine the degree of fibrosis but also to predict portal hypertension and varicose veins, which can cause morbidity and even mortality. It is thought that VCTE can be used to monitor the severity of NAFLD and assess the response to treatment, because of obtaining quantitative data on steatosis.
The number of accuracy and comparison studies of the iLivTouch-FibroTouch device are increasing. Although the data obtained in the studies indicate that there is a significant correlation between devices, it is thought that further studies are necessary to state that it can be used instead of FibroScan.
Orcid
Abdullah M Ozercan https://orcid.org/0000-0002-6968-7838
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 5.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blais P, Husain N, Kramer JR, et al. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10–14. doi: 10.1038/ajg.2014.134. [DOI] [PubMed] [Google Scholar]
- 7.Thampanitchawong P, Piratvisuth T. Liver biopsy: complications and risk factors. World J Gastroenterol. 1999;5(4):301–304. doi: 10.3748/wjg.v5.i4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwashiro T, Takahashi H, Hyogo H, et al. Discordant pathological diagnosis of non‐alcoholic fatty liver disease: a prospective multicenter study. JGH Open. 2020;4(3):497–502. doi: 10.1002/jgh3.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–1281. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castera L. Non‐invasive tests for liver fibrosis in NAFLD: creating pathways between primary healthcare and liver clinics. Liver Int. 2020;40:77–81. doi: 10.1111/liv.14347. [DOI] [PubMed] [Google Scholar]
- 11.Berzigotti A, Tsochatzis E, Boursier J, et al. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis–2021 update. J Hepatol. 2021;75(3):659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626–637. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 13.Sirli R, Sporea I. Controlled attenuation parameter for quantification of steatosis: which cut-offs to use? Can J Gastroenterol Hepatol. 2021;2021:6662760, 7. doi: 10.1155/2021/6662760. p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoneda M, Fujita K, Inamori M, et al. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56(9):1330–1331. doi: 10.1136/gut.2007.126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15(5):274–282. doi: 10.1038/nrgastro.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda Y, Yoneda M, Imajo K, et al. Elastography techniques for the assessment of liver fibrosis in non-alcoholic fatty liver disease. Int J Mol Sci. 2020;21(11):4039. doi: 10.3390/ijms21114039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56(7):968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Wong GLH, Wong VWS. Application of transient elastography in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26(2):128–141. doi: 10.3350/cmh.2019.0001n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semmler G, Wöran K, Scheiner B, et al. Novel reliability criteria for controlled attenuation parameter assessments for non-invasive evaluation of hepatic steatosis. United Eur Gastroenterol J. 2020;8(3):321–331. doi: 10.1177/2050640619900820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Boursier J, Zarski JP, De Ledinghen V, et al. Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57(3):1182–1191. doi: 10.1002/hep.25993. [DOI] [PubMed] [Google Scholar]
- 22.Wong VWS, Petta S, Hiriart JB, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017;67(3):577–584. doi: 10.1016/j.jhep.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5‐year prospective study of 13,369 examinations. Hepatology. 2010;51(3):828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 24.Tapper EB, Challies T, Nasser I, et al. The performance of vibration controlled transient elastography in a US cohort of patients with non-alcoholic fatty liver disease. Am J Gastroenterol. 2016;111(5):677–684. doi: 10.1038/ajg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petroff D, Blank V, Newsome PN, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(3):185–198. doi: 10.1016/S2468-1253(20)30357-5. [DOI] [PubMed] [Google Scholar]
- 26.Pu K, Wang Y, Bai S, et al. Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):1–11. doi: 10.1186/s12876-019-0961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi KQ, Tang JZ, Zhu XL, et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta‐analysis of diagnostic accuracy. J Gastroenterol Hepatol. 2014;29(6):1149–1158. doi: 10.1111/jgh.12519. [DOI] [PubMed] [Google Scholar]
- 28.Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Fan Q, Wang T, et al. Controlled attenuation parameter for assessment of hepatic steatosis grades: a diagnostic meta-analysis. Int J Clin Exp Med. 2015;8(10):17654. 26770355 [PMC free article] [PubMed] [Google Scholar]
- 30.Kwok R, Tse YK, Wong GH, et al. Systematic review with meta‐analysis: non‐invasive assessment of non‐alcoholic fatty liver disease–the role of transient elastography and plasma cytokeratin‐18 fragments. Aliment Pharmacol Ther. 2014;39(3):254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Huang S, Teng H, et al. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non-alcoholic fatty liver disease: a meta-analysis. BMJ Open. 2018;8(8):e021787. doi: 10.1136/bmjopen-2018-021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Serra JT, Mueller J, Teng H, et al. Prospective comparison of transient elastography using two different devices: Performance of FibroScan and FibroTouch. Hepat Med. 2020;12:41–48. doi: 10.2147/HMER.S245455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen GF, Ping J, Gu HT, et al. Correlation of liver stiffness measured by FibroTouch and FibroScan with Ishak fibrosis score in patients with chronic hepatitis B. Chin J Hepatol. 2017;25(2):145–150. doi: 10.3760/cma.j.issn.1007-3418.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng J, Sun WL, Chen GY, et al. Efficiency of FibroScan and FibroTouch in liver stiffness measurement and fat quantification: a comparative analysis. Chin J Hepatol. 2016;24(9):652–658. doi: 10.3760/cma.j.issn.1007-3418.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng YZ, Lai LL, Wong SW, et al. Attenuation parameter and liver stiffness measurement using FibroTouch vs Fibroscan in patients with chronic liver disease. PLoS One. 2021;16(5):e0250300. doi: 10.1371/journal.pone.0250300. [DOI] [PMC free article] [PubMed] [Google Scholar]


