Abstract
目的
通过体外生物力学试验,比较经皮骨水泥椎间盘成形术(percutaneous cement discoplasty,PCD)以及经皮骨水泥椎间融合术(percutaneous cement interbody fusion,PCIF)对脊柱稳定性的影响。
方法
生物力学试验分为正常对照(intact,INT)组、经皮腰椎间盘切除(percutaneous lumbar discectomy,PLD)组、PCD组、PCIF组。取6具新鲜成年男性L4、5运动节段标本(包括椎体及椎间盘),分别制作PLD、PCD以及PCIF标本。分别于处理前以及上述处理后,采用MTS多自由度模拟测试系统进行生物力学测试,包括轴向加载300 N压力前后标本高度,并计算差值;加载7.5 Nm扭矩下前屈、后伸、左/右侧弯以及左/右旋转的脊柱活动度(range of motion,ROM)及刚度。
结果
轴向加载压力后,PLD组椎间高度变化大于其他组(P<0.05)。PLD组与INT组比较,各向ROM均增大,刚度均减小(P<0.05)。PCD组与INT组比较,前屈、后伸、左/右旋转ROM均增大,刚度减小(P<0.05);与PLD组比较,前屈、后伸、左/右侧弯ROM均减小,刚度增大(P<0.05)。PCIF组与INT组比较,左/右侧弯ROM均减小,刚度增大(P<0.05);与PLD组比较,各向ROM均减小、刚度增大(P<0.05);与PCD组比较,前屈、左/右侧弯、左/右旋转ROM均减小,刚度增大(P<0.05)。
结论
PCD和PCIF均能减小腰椎运动节段ROM、增加刚度,其中前者主要影响屈伸和侧弯刚度,后者对腰椎各向ROM,尤其是侧弯及旋转方向限制性更强。
Keywords: 腰椎退行性变, 生物力学, 经皮骨水泥椎间盘成形术, 经皮骨水泥椎间融合术, 脊柱稳定性
Abstract
Objective
To investigate the effects of percutaneous cement discoplasty (PCD) and percutaneous cement interbody fusion (PCIF) on spinal stability by in vitro biomechanical tests.
Methods
Biomechanical test was divided into intact (INT) group, percutaneous lumbar discectomy (PLD) group, PCD group, and PCIF group. Six specimens of L4, 5 (including vertebral bodies and intervertebral discs) from fresh male cadavers were taken to prepare PLD, PCD, and PCIF specimens, respectively. Before treatment and after the above treatments, the MTS multi-degree-of-freedom simulation test system was used to conduct the biomechanical test. The intervertebral height of the specimen was measured before and after the axial loading of 300 N, and the difference was calculated. The range of motion (ROM) and stiffness of the spine in flexion, extension, left/right bending, and left/right rotation under a torque of 7.5 Nm were calculated.
Results
After axial loading, the change of intervertebral height in PLD group was more significant than that in other three groups (P<0.05). Compared with INT group, the ROM in all directions significantly increased and the stiffness significantly decreased in PLD group (P<0.05). Compared with INT group, the ROM of flexion, extension, and left/right rotation in PCD group significantly increased and the stiffness significantly decreased (P<0.05); compared with PLD group, the ROM of flexion, extension, and left/right bending in PCD group significantly decreased and the stiffness significantly increased (P<0.05). Compared with INT group, ROM of left/right bending in PCIF group significantly decreased and stiffness significantly increased (P<0.05); compared with PLD group, the ROM in all directions significantly decreased and the stiffness significantly increased (P<0.05); compared with PCD group, the ROM of flexion, left/right bending, and left/right rotation significantly decreased and stiffness significantly increased (P<0.05).
Conclusion
Both PCD and PCIF can provide good biomechanical stability. The former mainly affects the stiffness in flexion, extension, and bending, while the latter is more restrictive on lumbar ROM in all directions, especially in bending and rotation.
Keywords: Lumbar degeneration, biomechanics, percutaneous cement discoplasty, percutaneous cement interbody fusion, spinal stability
腰椎管狭窄症(lumbar spinal stenosis,LSS)常发生于老年人群[1-2],是因椎间盘变性和塌陷、髓核变性消失,导致椎间孔减小以及神经根慢性机械性压迫[3-4],进而引起局部疼痛和放射痛等症状,严重影响患者生活质量。目前LSS主要治疗方法为椎管减压联合椎间融合术。然而,老年患者一般情况大多欠佳,常合并心肺疾病、糖尿病及骨质疏松症等,增加了围术期感染以及术中出血风险[5-8]。
微创手术与开放手术相比,具有手术时间短、术中出血少、感染率低、住院时间短、治疗费用低等优点[9],已是脊柱外科手术的一种趋势。2015年,Varga等[10]率先报道一种微创术式治疗LSS,并将其命名为经皮骨水泥椎间盘成形术(percutaneous cement discoplasty,PCD)。该术式通过向退变椎间盘髓核中注入骨水泥,维持椎间高度,实现椎间孔间接减压,临床应用已获得一定效果[11-15]。然而,由于骨水泥与终板软骨之间无法实现有效融合,且骨水泥材料本身存在高弹性模量的局限性,术后存在终板骨折塌陷、内植物脱落等相关并发症发生风险。本课题组前期研究也显示,尽管PCD能在一定程度上实现脊柱运动节段的生物力学稳定,但手术设计方面仍有改良空间[16]。为此,何成建等[17]提出了经皮骨水泥椎间融合术(percutaneous cement interbody fusion,PCIF),即在PCD基础上增加邻近椎体的经皮椎体成形术(percutaneous vertebroplasty,PVP),实现相邻脊柱运动单元融合[18-19]。田庆华等[20]对比了两种术式,发现与PCD相比,PCIF能明显降低术后邻近椎体骨折发生率,远期疗效更优。本研究拟通过体外生物力学试验比较PCD和PCIF,评价其对腰椎椎体的生物力学影响。报告如下。
1. 材料与方法
1.1. 试验标本
6具新鲜成年男性脊柱标本,由天津市人民医院解剖学实验室以及天津中医药大学解剖实验室提供。年龄58~83岁,平均68.7岁。经X线透视检查,排除骨折、严重骨质疏松(T值≤−4.75SD)、肿瘤等。截取L4、5运动节段,包括椎体及椎间盘,去除标本周围肌肉组织,注意完整保留韧带、关节囊及椎间盘结构,取牙托粉包埋标本上、下椎体后,置于−30℃冰柜保存。实验前将标本取出,置于4℃环境中解冻12 h。
1.2. 生物力学试验方法
1.2.1. 试验分组及模型制备方法
研究分为4组,包括正常对照(intact,INT)组、经皮腰椎间盘切除(percutaneous lumbar discectomy,PLD)组、PCD组、PCIF组。6具标本如图1流程进行处理。① INT组:包埋后正常标本。② PLD组:在INT组基础上,于椎间盘后外侧纤维环作2 mm切口,插入铰刀破坏髓核组织后用髓核钳取出,生理盐水冲洗。经CT检查证实髓核完全去除。③ PCD组:在PLD组基础上,以自制万向磨钻在椎间盘上、下终板处开窗,直径约1 cm。将聚甲基丙烯酸甲酯骨水泥(强生公司,美国)与固化液混合、搅拌至面团期后,通过骨水泥注射器自后外侧纤维环切口处注入,维持体位15 min至骨水泥完全凝固。经CT检查确认终板软骨开窗完全,骨水泥填充均匀,无明显渗漏及充盈缺损。④ PCIF组:在PCD组基础上,于L4及L5左侧关节突处以“人”字嵴方法定位穿刺点,经椎弓根进行穿刺。使用骨水泥填充椎间盘间隙,直至完全凝固。CT检查椎体内骨水泥与椎间盘内骨水泥接触并融合。
图 1.

Schematic diagram of specimens treatment procedure and corresponding sagittal CT images
标本处理流程图及相应矢状位CT图像
1.2.2. 生物力学试验方法
将标本置于MTS多自由度模拟测试系统(MTS公司,美国),设置标本x、y、z轴分别与机器各轴线平行,将标本上、下两端固定于仪器卡具(图2)。以加载速率15 mm/min逐级加载,轴向加载压力设定为300 N,仪器记录标本加载前后高度并计算差值。以7.5 Nm扭矩对标本分别进行前屈、后伸、左/右侧弯以及左/右旋转[21],通过位移传感器自动采集标本各向活动度(range of motion,ROM)。绘制载荷-位移曲线,计算各向刚度(载荷/活动度)。每个加载状态进行3个循环,取第3次作为试验结果,以消除标本松弛、蠕变等时间效应对测量结果的影响。
图 2.
Schematic diagram of biomechanical testing
生物力学测试示意图
箭头示PCD手术椎间盘穿刺骨水泥注射点
Arrow indicated the cement injection point in PCD
1.3. 统计学方法
采用SPSS19.0统计软件进行分析。计量资料经正态性检验,均符合正态分布,数据以均数±标准差表示,组间比较采用方差分析,两两比较采用LSD检验;检验水准α=0.05。
2. 结果
2.1. 轴向载荷下标本高度变化
测试后标本均未出现骨折及植入物脱落或松动迹象。轴向加载300 N压力后,4组标本高度均发生变化,其中PLD组加载前后差值为(0.33±0.08)mm,大于INT组(0.18±0.06)mm、PCD组(0.20±0.02)mm和PCIF组(0.21±0.02)mm,差异均有统计学意义(P<0.05);其余3组间差异均无统计学意义(P>0.05)。
2.2. 各向ROM变化
各组各向ROM见表1。PLD组各向ROM均较INT组增大,差异有统计学意义(P<0.05)。
表 1.
Comparison of ROM in different directions between groups (n=6,  , °)
, °)
各组各向ROM比较(n=6,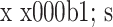 ,°)
,°)
| 组别 Group |
前屈 Flexion |
后伸 Extension |
左侧弯 Left bending |
右侧弯 Right bending |
左旋转 Left rotation |
右旋转 Right rotation |
|
*与INT组比较P<0.05,#与PLD组比较P<0.05,△与PCD组比较P<0.05 *Compared with INT group, P<0.05; #compared with PLD group, P<0.05; △compared with PCD group, P<0.05 | ||||||
| INT | 4.08±0.55#△ | 3.27±0.79#△ | 5.26±0.56# | 5.31±0.59# | 2.99±0.37#△ | 3.00±0.36#△ |
| PLD | 5.49±0.74*△ | 4.21±0.66*△ | 7.16±0.54*△ | 7.14±0.65*△ | 3.62±0.46* | 3.64±0.41* |
| PCD | 4.63±0.76*# | 3.74±0.61*# | 5.43±0.53# | 5.35±0.62# | 3.58±0.46* | 3.62±0.50* |
| PCIF | 3.87±0.79#△ | 3.71±0.70*# | 4.09±0.53*#△ | 4.11±0.37*#△ | 2.78±0.35#△ | 2.82±0.24#△ |
| 统计值 Statistic |
F=252.107 P<0.001 |
F=168.612 P<0.001 |
F=2585.045 P<0.001 |
F=2141.664 P<0.001 |
F=470.095 P<0.001 |
F=532.403 P<0.001 |
PCD组与INT组比较,前屈、后伸、左/右旋转ROM均增大,差异有统计学意义(P<0.05),左/右侧弯ROM差异无统计学意义(P>0.05);与PLD组比较,前屈、后伸、左/右侧弯ROM均减小,差异有统计学意义(P<0.05),而左/右旋转ROM差异无统计学意义(P>0.05)。
PCIF组与INT组比较,除后伸ROM增大外,其余各向ROM均减小,其中前屈、左/右旋转ROM差异无统计学意义(P>0.05),后伸、左/右侧弯ROM差异有统计学意义(P<0.05);与PLD组比较,各向ROM均减小,差异有统计学意义(P<0.05);与PCD组比较,前屈、左/右侧弯、左/右旋转ROM均减小,差异有统计学意义(P<0.05),后伸ROM比较差异无统计学意义(P>0.05)。
2.3. 各向刚度变化
各组各向刚度见表2。PLD组各向刚度均较INT组减小,差异有统计学意义(P<0.05)。
表 2.
Comparison of stiffness in different directions between groups (n=6, 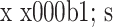 , Nm/°)
, Nm/°)
各组各向刚度比较(n=6, ,Nm/°)
,Nm/°)
| 组别 Group |
前屈 Flexion |
后伸 Extension |
左侧弯 Left bending |
右侧弯 Right bending |
左旋转 Left rotation |
右旋转 Right rotation |
|
*与INT组比较P<0.05,#与PLD组比较P<0.05,△与PCD组比较P<0.05 * Compared with INT group, P<0.05; #compared with PLD group, P<0.05; △compared with PCD group, P<0.05 | ||||||
| INT | 1.87±0.27#△ | 2.42±0.63#△ | 1.44±0.15# | 1.42±0.15# | 2.54±0.27#△ | 2.53±0.26#△ |
| PLD | 1.39±0.21*△ | 1.82±0.30*△ | 1.05±0.08*△ | 1.05±0.10*△ | 2.10±0.26* | 2.08±0.24* |
| PCD | 1.66±0.29*# | 2.07±0.41*# | 1.39±0.14# | 1.42±0.17# | 2.12±0.27* | 2.10±0.27* |
| PCIF | 2.02±0.48#△ | 2.09±0.42*# | 1.86±0.22*#△ | 1.84±0.16*#△ | 2.73±0.36#△ | 2.67±0.23#△ |
| 统计值 Statistic |
F=193.677 P<0.001 |
F=140.622 P<0.001 |
F=1196.934 P<0.001 |
F=1308.112 P<0.001 |
F=554.125 P<0.001 |
F=649.110 P<0.001 |
PCD组与INT组比较,前屈、后伸、左/右旋转刚度均减小,差异有统计学意义(P<0.05),左/右侧弯刚度差异无统计学意义(P>0.05);与PLD组比较,前屈、后伸、左/右侧弯刚度均增大,差异有统计学意义(P<0.05),而左/右旋转刚度差异无统计学意义(P>0.05)。
PCIF组与INT组比较,除后伸刚度减小外,其余各向刚度均增大,其中前屈、左/右旋转刚度差异无统计学意义(P>0.05),后伸、左/右侧弯刚度差异有统计学意义(P<0.05);与PLD组比较,各向刚度均增大,差异有统计学意义(P<0.05);与PCD组比较,各向刚度均增大,其中前屈、左/右旋转、左/右侧弯刚度差异有统计学意义(P<0.05),后伸刚度差异无统计学意义(P>0.05)。
3. 讨论
脊柱运动节段是维持脊柱稳定性的基本功能单位,包括相邻椎体以及其间的椎间盘、关节突关节和韧带结构等。生理条件下,脊柱受到外部以及内部载荷的双重影响,并由此发生形变,包括压缩、扭转(屈伸、侧弯、旋转)等。在椎间融合手术中,经常通过安装大号椎间融合器进行椎间隙撑开,从而扩大椎间孔高度和面积,达到间接减压目的。但椎间融合器安装需要分离软组织,以避开硬膜囊和神经根等重要结构 [22-23]。本研究中的PCD和PCIF是一种间接减压方法,骨水泥可以通过注射方式植入,最大程度地减小了软组织损伤。
Techens等[24]对离体猪脊柱PCD和PLD模型进行生物力学测试,测量在峰值载荷下的椎间高度、椎间活动度和刚度。结果提示PCD可以恢复PLD引起的椎间高度降低,但对脊柱ROM和刚度没有显著影响。由于人体为直立动物,与猪脊柱标本具有较大生物力学特性差异,该研究结果参考价值有限。为此,本研究采用人体标本进行体外生物力学研究。
既往研究表明椎间盘切除后,椎间高度下降60%,椎间孔高度降低24%[25]。本研究结果显示轴向加载300 N压力后,PLD组标本椎间高度变化最大,INT组、PCD组和PCIF组差异无统计学意义,表明完整椎间盘在维持椎间高度中起到重要作用, PCD和PCIF均能维持椎间高度,而这种椎间高度稳定性的实现能缓解神经根受压程度。
在椎体屈伸稳定性方面,本研究中PCD组标本前屈和后伸ROM较PLD组减小、刚度增大,说明植入骨水泥可以有效增加脊柱节段稳定性。PCIF组标本前屈ROM较PCD组进一步减小,刚度增大,说明PCIF能进一步增加脊柱运动节段屈伸稳定性。值得注意的是,PCIF组前屈刚度高于PCD组,而后伸刚度与PCD组相似。结合PCIF组ROM分析,PCIF增加腰椎刚度的作用主要与其限制前屈活动有关。然而,PCIF对前屈活动的限制可能导致更高的局部应力集中,这种效应是否会导致内植物失效需进一步研究。
在椎体侧弯稳定性方面,本研究PCD组和PCIF组侧弯ROM均较PLD组不同程度减小,刚度有所提高。其中,PCD组侧弯刚度低于INT组,而PCIF组上述指标明显增大。说明PCD可以有效改善并缓解髓核切除对脊柱侧弯稳定性的影响,且PCIF作用更强。
在椎体旋转稳定性方面,椎间盘切除术后PLD组脊柱旋转刚度较INT组明显减小。PCD组旋转ROM以及刚度与PLD组相比无明显变化,说明PCD不能提高脊柱旋转稳定性。该现象可能与PCD术中骨水泥与终板之间的滑动摩擦力无法对旋转活动产生影响有关。而PCIF手术后,由于骨水泥与上、下椎体之间融合作用限制了脊柱旋转,脊柱的旋转ROM减小,刚度明显增加。上述结果提示脊柱旋转稳定性差异是PCD和PCIF之间的一个显著区别。
综上述,PCD和PCIF均可以减小腰椎运动节段ROM,增加刚度。其中,PCD主要影响腰椎的屈伸和侧弯刚度,而PCIF对腰椎各向ROM,尤其是侧弯及旋转活动的限制性更强。但本研究存在一些不足:首先,研究采用正常脊柱标本,无法模拟严重腰椎间盘退变患者腰椎失稳情况;其次,本研究结果提示PCD和PCIF可以限制PLD术后腰椎各方向ROM,但腰椎稳定性增加能否改善腰痛症状需要进一步临床研究。
利益冲突 在课题研究和文章撰写过程中不存在利益冲突;经费支持没有影响文章观点和对研究数据客观结果的统计分析及其报道
伦理声明 研究方案经天津市天津医院医学伦理委员会批准(2022医伦审178)
作者贡献声明 李爽:研究设计及实施、文章撰写;邵鹏飞:参与研究及数据分析整理;徐宝山、刘艳成:研究设计、文章审阅及理论指导;刘钢、张洪亮、郭志文、李晓晔:数据收集整理及统计分析;张净宇、胡永成:对文章的知识性内容作批评性审阅及支持性贡献
Funding Statement
国家自然科学基金资助项目(82072491、31900967);天津市自然科学基金项目(20JCYBJC00820);天津市卫生健康委员会科技项目(KJ20211)
National Natural Science Foundation of China (82072491, 31900967); Natural Science Foundation of Tianjin (20JCYBJC00820); Science and Technology Project of Tianjin Health Commission (KJ20211)
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai MKL, Cheung PWH, Cheung JPY A systematic review of developmental lumbar spinal stenosis. Eur Spine J. 2020;29(9):2173–2187. doi: 10.1007/s00586-020-06524-2. [DOI] [PubMed] [Google Scholar]
- 3.Adams MA, Dolan P Intervertebral disc degeneration: evidence for two distinct phenotypes. J Anat. 2012;221(6):497–506. doi: 10.1111/j.1469-7580.2012.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan P, Luo J, Pollintine P, et al Intervertebral disc decompression following endplate damage: implications for disc degeneration depend on spinal level and age. Spine (Phila Pa 1976) 2013;38(17):1473–1481. doi: 10.1097/BRS.0b013e318290f3cc. [DOI] [PubMed] [Google Scholar]
- 5.Puvanesarajah V, Cancienne JM, Werner BC, et al Perioperative complications associated with posterolateral spine fusions: A study of elderly medicare beneficiaries. Spine (Phila Pa 1976) 2018;43(1):16–21. doi: 10.1097/BRS.0000000000001771. [DOI] [PubMed] [Google Scholar]
- 6.Mihailidis HG, Manners S, Churilov L, et al. Is spinal surgery safe in octogenarians? ANZ J Surg, 2017, 87(7-8): 605-609.
- 7.Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ, 2016, 352: h6234. doi: 10.1136/bmj.h6234.
- 8.Deer T, Sayed D, Michels J, et al. A Review of lumbar spinal stenosis with intermittent neurogenic claudication: Disease and diagnosis. Pain Med, 2019, 20(Suppl 2): S32-S44.
- 9.Uddin OM, Haque R, Sugrue PA, et al Cost minimization in treatment of adult degenerative scoliosis. J Neurosurg Spine. 2015;23(6):798–806. doi: 10.3171/2015.3.SPINE14560. [DOI] [PubMed] [Google Scholar]
- 10.Varga PP, Jakab G, Bors IB, et al. Experiences with PMMA cement as a stand-alone intervertebral spacer: Percutaneous cement discoplasty in the case of vacuum phenomenon within lumbar intervertebral discs. Orthopade, 2015, 44 Suppl 1: S1-S7.
- 11.Kiss L, Varga PP, Szoverfi Z, et al Indirect foraminal decompression and improvement in the lumbar alignment after percutaneous cement discoplasty. Eur Spine J. 2019;28(6):1441–1447. doi: 10.1007/s00586-019-05966-7. [DOI] [PubMed] [Google Scholar]
- 12.Tian QH, Lu YY, Sun XQ, et al Feasibility of percutaneous lumbar discectomy combined with percutaneous cementoplasty for symptomatic lumbar disc herniation with Modic type Ⅰ endplate changes. Pain Physician. 2017;20(4):E481–E488. [PubMed] [Google Scholar]
- 13.Sola C, Camino Willhuber G, Kido G, et al Percutaneous cement discoplasty for the treatment of advanced degenerative disk disease in elderly patients. Eur Spine J. 2021;30(8):2200–2208. doi: 10.1007/s00586-018-5547-7. [DOI] [PubMed] [Google Scholar]
- 14.Camino Willhuber G, Kido G, Pereira Duarte M, et al Percutaneous cement discoplasty for the treatment of advanced degenerative disc conditions: A case series analysis. Global Spine J. 2020;10(6):729–734. doi: 10.1177/2192568219873885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camino Willhuber G, Bendersky M, De Cicco FL, et al Development of a new therapy-oriented classification of intervertebral vacuum phenomenon with evaluation of intra- and interobserver reliabilities. Global Spine J. 2021;11(4):480–487. doi: 10.1177/2192568220913006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Xu B, Liu Y, et al Biomechanical evaluation of spinal column after percutaneous cement discoplasty: A finite element analysis. Orthop Surg. 2022;14(8):1853–1863. doi: 10.1111/os.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.何成建, 吴春根, 王涛, 等 经皮椎间融合成形术治疗节段性脊柱恶性溶骨塌陷一例. 介入放射学杂志. 2014;23(2):130–131. doi: 10.3969/j.issn.1008-794X.2014.02.011. [DOI] [Google Scholar]
- 18.田庆华, 卢莹莹, 宋红梅, 等 经皮骨水泥融合术治疗强直性脊柱炎伴假关节形成的邻近椎体应力骨折4例. 介入放射学杂志. 2017;26(6):551–554. doi: 10.3969/j.issn.1008-794X.2017.06.018. [DOI] [Google Scholar]
- 19.Xue YD, Zhang ZC, Dai WX Investigation of preoperative traction followed by percutaneous kyphoplasty combined with percutaneous cement discoplasty for the treatment of severe thoracolumbar osteoporotic vertebral compression fractures. Int J Gen Med. 2021;14:6563–6571. doi: 10.2147/IJGM.S333532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.田庆华, 王涛, 何煜, 等 经皮骨水泥椎间融合术与骨水泥椎间盘成形术治疗老年腰椎间盘突出症的疗效比较. 介入放射学杂志. 2021;30(3):264–269. doi: 10.3969/j.issn.1008-794X.2021.03.010. [DOI] [Google Scholar]
- 21.Wilke HJ, Wenger K, Claes L Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7(2):148–154. doi: 10.1007/s005860050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vadapalli S, Robon M, Biyani A, et al Effect of lumbar interbody cage geometry on construct stability: a cadaveric study. Spine (Phila Pa 1976) 2006;31(19):2189–2194. doi: 10.1097/01.brs.0000232720.23748.ce. [DOI] [PubMed] [Google Scholar]
- 23.Kettler A, Schmoelz W, Kast E, et al In vitro stabilizing effect of a transforaminal compared with two posterior lumbar interbody fusion cages. Spine (Phila Pa 1976) 2005;30(22):E665–E670. doi: 10.1097/01.brs.0000186466.01542.8c. [DOI] [PubMed] [Google Scholar]
- 24.Techens C, Palanca M, Éltes PE, et al Testing the impact of discoplasty on the biomechanics of the intervertebral disc with simulated degeneration: An in vitro study. Med Eng Phys. 2020;84:51–59. doi: 10.1016/j.medengphy.2020.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Grunert P, Moriguchi Y, Grossbard BP, et al. Degenerative changes of the canine cervical spine after discectomy procedures, an in vivo study. BMC Vet Res, 2017, 13(1): 193. doi: 10.1186/s12917-017-1105-5.



