Abstract
The formation of EspA-containing surface appendages in pathogenic Escherichia coli strains, both enteropathogenic E. coli (EPEC) and Shiga toxin-producing E. coli strains, is essential for critical events in the infective process, e.g., localized bacterial adherence to host cells with formation of microcolonies and induction of attaching and effacing lesions. It has been reported that EPEC mutants deficient in the production of EspD, which is encoded by the esp operon, are unable to accumulate actin underneath adherent bacteria but exhibit an attachment similar to that of the wild type. Here, we report the construction and characterization of an in-frame espD deletion mutant of the enterohemorrhagic E. coli (EHEC) strain EDL933. In contrast to what was observed in EPEC mutants, the EDL933 espD mutant not only lacked the capacity to accumulate actin but also exhibited an impaired attachment to HeLa cells. The synthesis of the EspD protein was also essential for the formation of EspA-containing filaments. Finally, localization studies demonstrated that the EspD protein is transferred to the cytoplasm and integrated into the cytoplasmic membranes of infected cells. These results help to elucidate the underlying molecular events in infections caused by EHEC.
Many pathogenic Escherichia coli share a conserved region inserted into the chromosome known as the locus of enterocyte effacement (LEE). This pathogenicity island encodes bacterial products which are required for the production of the attaching and effacing (A/E) lesions (24). Recently, Perna et al. published the DNA sequence of the LEE of enterohemorrhagic E. coli (EHEC) EDL933, which comprises 41 non-prophage 933L open reading frames (ORFs) (30). The comparison between the LEE of the enteropathogenic E. coli (EPEC) strain E2348/69 (10) and that of the EHEC strain EDL933 shows that they are highly conserved, containing ORFs with identities ranging from 100% (escS and escF) to 66.48% (tir and espE). While all the identified components of the type III secretion apparatus encoded within the LEE, except the sepZ gene, reveal fairly high homologies (98 to 100%), the secreted proteins EspA, EspB, EspD, and EspE are more diverse (84.63, 74.01, 80.36, and 66.48% homology, respectively).
Type III secretion systems are widespread in a variety of pathogenic bacteria and encoded by at least 20 genes (for reviews, see references 4 and 12). The disruption of any of these genes results in the abolishment of signal transduction events that are essential for bacterial interactions with eukaryotic cells during the infection process (11, 22, 32). However, differences have been observed between EPEC and Shiga toxin-producing E. coli (STEC) when the involvement of secreted proteins in the infection of eukaryotic cells was assessed. While the attachment of strains of EPEC and rabbit EPEC (REPEC) with mutations in espA to the host cells was only slightly affected, if at all (1, 19), the adhesion of strains of STEC with mutations in espA was strongly reduced (3, 9), suggesting that EspA plays a key role in the pathogenicity of STEC. The secreted protein EspA is a major component of the recently described surface appendages which are required for localized bacterial adherence, the formation of microcolonies, and the induction of A/E lesions (9, 21). In addition, EspA is necessary for the translocation of the potential effector protein EspB, which in turn is found in the cytosol and the cytoplasmic membranes of infected cells (38).
The esp operon encodes EspA, EspB, and a third protein, EspD (3). Although the ability to accumulate actin underneath bacteria of an EPEC espD mutant was abolished, the ability to adhere to eukaryotic cells was maintained (23). In order to assess the role played by EspD in the pathogenesis of EHEC, a nonpolar espD deletion derivative of the prototype EHEC strain EDL933 was generated. The characterization of this clone demonstrated that, as has been demonstrated for EspA (3, 9), EspD plays a more significant role in the pathogenic process of EHEC than in that of EPEC. The obtained results suggest that EspD is essential for the formation of surface appendages, is integrated in the cytoplasmic membranes of target cells, and might participate in the translocation of effector molecules.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The strains and plasmids used in this study are described in Table 1. Bacteria were grown in Luria-Bertani (LB) broth (33) or LB agar and in serum-free Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Karlruhe, Germany) supplemented with 100 mM HEPES (pH 7.4). Plasmids were maintained in E. coli DH5α, and the INVαF′ strain was used as a recipient for cloning fragments amplified by PCR into the pCR2.1 vector. Media were supplemented with chloramphenicol (50 μg ml−1), ampicillin (200 μg ml−1), or nalidixic acid (50 μg ml−1) when required.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant genotype or serotype | Reference or source |
|---|---|---|
| Strains | ||
| EDL933 | Prototypic O157:H7 EHEC strain | 27 |
| E32511/0 | O157 EHEC strain | 37 |
| E32511/0 Nalr | Nalr, spontaneous derivative of E32511/0 | This study |
| DH5α | endA1 recA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA96 φ80d lacZΔM15 Δ(lacZYA-argF) U169 d− | 33 |
| INVαF′ | F′ endA1 recA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA96 relA1 φ80 lacZΔM15 Δ(lacZYA-argF) U169 deoR λ− | Invitrogen |
| S17-1 (λpir) | Tpr SmrrecA thi pro, hsdR mutant, M+ RP4:2-Tc:Mu:Km Tn7 λpir | 7 |
| Plasmids | ||
| pCR 2.1 | Apr Kmr, high-copy-number vector for cloning PCR products | Invitrogen |
| pMAK700oriT | Cmr, thermosensitive positive-selection suicide vector | 35 |
| pANK1 | Cmr Kmr, pMAK700oriT derivative with a multiple cloning site and an aph-A3 cassette in the HindIII site | This study |
| pANK84 | Apr Kmr, pCR 2.1 derivative containing the PCR fragment generated with primers ANK7191 and AE19, which encompass the region between eaeA (position 1891) and espB (position 7544) | This study |
| pAKSK78 | Derivative of pANK84 encompassing the region between eaeA (position 1891) and the start codon of espD (position 3334) | This study |
| pANK155 | Cmr Kmr, pANK1 derivative with a ΔespDEDL933 fragment | This study |
DNA manipulations.
Plasmid DNA isolation, restriction endonuclease digestion, ligation, transformation, agarose gel electrophoresis, and other standard DNA techniques were carried out as described by Sambrook et al. (33). Oligonucleotides (Table 2) were synthesized by GIBCO. Colony PCR, extraction of PCR products, and cloning experiments were performed according to standard protocols (33). DNA sequencing was performed with a Taq dyedeoxy terminator cycle sequencing kit and an automatic DNA sequencer (model 373A; Applied Biosystems) according to the manufacturer’s instructions. Restriction and modification enzymes were purchased from New England Biolabs (Schwalbach, Germany). Electroporation was carried out with a gene pulser (Bio-Rad Laboratories) as described by O’Callaghan and Charbit (28). Searches in databases for nucleotide and amino acid sequence homologies were performed with the BLASTP (2), BLASTP plus BEAUTY (2, 39), NNPP (31), and PSORT (26) algorithms.
TABLE 2.
Oligonucleotides used for PCR and sequencing
| Oligonucleotide | Sequence (5′→3′) | Positionsa |
|---|---|---|
| ANKA288 | CACGTTCTGATGTGCAATCTC | 3258–3278 |
| ANKA289 | CAACCCGGGCTAAGGACATCCTCAGCAGC | 3549–3530 |
| ANKA290 | GTCCTTAGCCCGGGTTGGATGTCCGATTCAGCACGGG | 4405–4424 |
| ANK7191 | GCTTTATTCTGGCTCTCAAAAACG | 4739–4716 |
| ANK14 | CCAGGATCCATGGATACATCAAATGCAAC | 2741–2760 |
| ANK16 | CTTTATTTGCAACCTCAGAAGCC | 4924–4902 |
| AE19b | CAGGTCGTCGTGTCTGCTAAA |
Construction of a nonpolar mutation.
By overlap extension PCR (16) an in-frame deletion in the espD gene from the EHEC strain EDL933 was generated (Fig. 1). Two PCR fragments were obtained by colony PCR with an Expand High Fidelity kit (Boehringer, Mannheim, Germany) with the primer pairs ANKA288-ANKA289 and ANKA290-ANK7191, followed by mixing of the PCR products and a second PCR with the primer pair ANKA288-ANK7191. The resulting 875-bp fragment contained the first 218 bp and the last 52 bp of the espD ORF and coded for a polypeptide (99 amino acids [aa]) in which 275 aa of the wild-type EspD protein (374 aa) are deleted, herein called the ΔespD gene. After being cloned into the vector pCR2.1 and after the DNA sequence was checked, the ΔespD fragment was subcloned into KpnI- and XbaI-digested pANK1 (a pMAK700oriT derivative [35]), thereby generating pANK155. This plasmid was transformed into the S17-1 (λpir) strain and then transferred by conjugation (15) into the recipient EHEC strain E32511/0 Nalr. Plasmid pANK155 was recovered from E32511/0 and subsequently electroporated into the EHEC strain EDL933. The cointegration and excision of the suicide vector were performed as previously described (22). The in-frame deletion contained in the EDL933ΔespD mutant resulting from the allelic exchange was confirmed by PCR analysis with the primer pair ANKA288-ANK7191 or ANK14-ANK16, which are homologous to adjacent external sequences (data not shown).
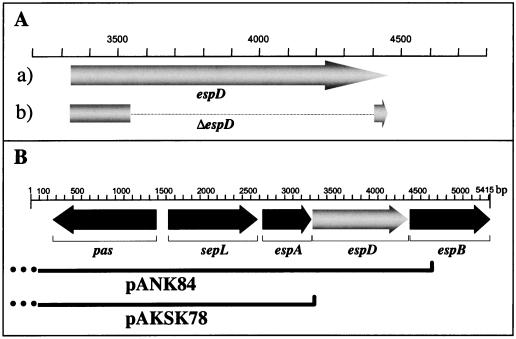
FIG. 1.
Construction of an in-frame deletion mutant of the espD gene in EHEC. (A) The ORF of espD in the wild-type EHEC strain EDL933 (arrow a) and the recombinant ORF of the espD mutant EDL933ΔespD (broken arrow b) are schematically shown. The corresponding positions (according to the published sequence [EMBL accession no. Y13068]) are also shown. (B) Plasmids used for the complementation studies performed with the EHEC strain EDL933ΔespD. Plasmid pANK84 contains an insert from the 3′ end of eaeA (dotted line) to the 5′ end of espB, and plasmid pAKSK78 contains a sequence from the 3′ end of eaeA (dotted line) to the 5′ end of espD.
For complementation studies of the EDL933ΔespD mutant, plasmid pANK84 (22), which harbors a region from the 3′ end of the eaeA gene to the 5′ end of the espB gene, was electroporated into EDL933ΔespD, thereby generating EDL933ΔespD[pANK84]. As a control to exclude spurious effects of plasmid pANK84 resulting from the nucleotide sequence upstream of espD, a derivative of pANK84 (pAKSK78), which had been generated by exonuclease III digestion (22) and contained the region from the 3′ end of the eaeA gene to the start codon of the espD gene, was transferred into EDL933ΔespD, thereby generating EDL933ΔespD[pAKSK78].
Tissue culture methods and analysis by immunofluorescence microscopy.
HeLa cells (ATCC CCL2) were maintained in DMEM supplemented with 25 mM HEPES, 10% (vol/vol) fetal calf serum (FCS), and glutamine (GIBCO) in an atmosphere containing 5% CO2 at 37°C. To study the reorganization of cellular actin underneath bacteria upon EHEC infection, cells were seeded at a concentration of approximately 5 × 104 per well onto 12-mm-diameter glass coverslips (InterMed Nunc, Roskilde, Denmark) in 24-well Nunclon Delta tissue culture plates (InterMed Nunc). Cell monolayers were infected with bacteria grown overnight and resuspended in DMEM-HEPES at a cell/bacterium ratio of 1:100. After 4 h of incubation, monolayers were washed to remove unattached bacteria, fixed with 3.7% (vol/vol) p-formaldehyde in phosphate-buffered saline (PBS), and permeabilized with 0.2% Triton X-100 in PBS and bacteria were stained with a rabbit polyclonal antiserum against O157 K− (Behring, Marburg, Germany). After being washed, the primary antibody was labeled with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibodies (Dianova, Hamburg, Germany), and F-actin was stained (20) with tetramethyl rhodamine isothiocyanate (TRITC)-labeled phalloidin (Sigma, Deisenhofen, Germany). Then coverslips were washed and mounted and cells were examined by epifluorescence with a Zeiss axiophot microscope (Carl Zeiss, Jena, Germany).
Detection of secreted and cellular proteins in and cellular fractioning of infected HeLa cells.
To enhance expression and secretion of Esp proteins, bacteria were grown in DMEM-HEPES until they reached an absorbance at 600 nm of 0.6 (18). Then the proteins present in the supernatant fluids were precipitated by the addition of 10% (vol/vol) trichloroacetic acid, overnight incubation at 4°C, and subsequent centrifugation at 4,000 × g for 30 min. The dry pellet was resuspended in 1.5 M Tris (pH 8.8). To obtain whole-cell extracts, bacteria were pelleted, and after resuspension in electrophoresis sample buffer (33), they were boiled at 100°C for 10 min. Bacteria were fractionated to obtain cytoplasmic and periplasmic and outer- and inner-membrane extracts according to standard protocols (34). Proteins (30 μg/lane) were fractionated by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 12.5% separating gel (33). Then they were transferred onto a positively charged Biodyne B nylon membrane (Pall, Dreieich, Germany) with a semidry device (Bio-Rad Laboratories). Nonspecific binding sites were saturated with 5% (vol/vol) low-fat milk (1.5%) in PBS-Tween 20 (0.1%, vol/vol). The EspA, EspB, EspD, and EspE proteins were detected with mouse monoclonal antibodies (MAbs) specific for EspA (MAb B71), EspB (MAb A289), EspD (MAb anti-EspD), and EspE (MAb B51) (6, 8, 9) as first antibodies and with horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G and immunoglobulin M as second antibodies (Bio-Rad Laboratories). Antigen-antibody complexes were visualized by chemiluminescence with the ECL system (Amersham Life Science, Braunschweig, Germany). Cellular fractioning of infected HeLa cells was carried out according to the methods described by Wolff et al. (38).
SEM.
For scanning electron microscopy (SEM), infected cells grown on round 12-mm-diameter Thermanox glass coverslips were fixed in cacodylate buffer (0.1 M cacodylate, 0.01 M MgCl2, 0.01 M CaCl2 [pH 6.9]) containing 3% (vol/vol) glutaraldehyde and 5% (vol/vol) p-formaldehyde for 45 min on ice, washed with PBS, dehydrated in a graded series of acetone, and subjected to critical-point drying with CO2. Samples were then sputtered with a 10-nm-thick gold film and examined with a Zeiss DSM 982 Gemini field emission SEM.
Quantitative determination of bacterial attachment and invasion.
HeLa cells were seeded into 24-well plates (5 × 104 cells/well) and grown overnight in DMEM-HEPES with 10% FCS. Prior to infection, each well was washed and the medium was replaced by DMEM-HEPES supplemented or not supplemented with FCS. Monolayers were infected at a bacterium/cell ratio of 100:1 for 3.5 h. Supernatant fluids were subsequently discarded, the wells were washed with PBS to remove nonadherent bacteria, DMEM supplemented with gentamicin (100 μg ml−1) was added, and cells were further incubated for 2.5 h. Then the wells were washed with PBS, HeLa cells were lysed by adding 500 μl of 0.25% (vol/vol) Triton X-100, and the number of CFU recovered from each well was determined by plating appropriate dilutions on LB agar plates with a spiral plater (Autoplate model 3000; Spiral Biotech, Inc., Bethesda, Md.). For the quantification of attached bacteria, cells were infected for 6 h with antibiotic-free DMEM-HEPES (during this period monolayers were washed several times to remove nonadherent bacteria). Then cells were washed and lysed and the total number of bacteria recovered per well was determined. Values were corrected by subtracting the number of viable intracellular bacteria, as determined from matching controls pretreated with gentamicin. Reported results are mean values from three independent experiments. The statistical significance of the obtained results has been evaluated by the Student t test, and differences were considered significant at a P of ≤0.05.
Nucleotide sequence accession number.
The nucleotide sequence of espD (22) is available in the EMBL database under the accession no. Y13068. This sequence is identical to the one deposited and published by Perna et al. (30) (EMBL accession no. AF071034), with the exception of cytosin residues at positions 751 and 967 of the espD gene.
RESULTS AND DISCUSSION
Sequence analysis of espD from EHEC EDL933.
The gene encoding the secreted protein EspD from the EHEC strain EDL933 is localized 13 bp downstream of the stop codon of espA and 21 bp upstream of espB, exposing a potential Shine-Dalgarno sequence 5 bp upstream of espD (GGAGA). The 1,124 bp of the espD gene encodes a protein product of 374 aa with an isoelectric point of 5.23, and no signal peptide cleavage site was detected. The analysis using the algorithm of the topology prediction program TMPred (17) resulted in the identification of three certain transmembrane (TM) stretches (I171 to N191, L194 to M214, and V228 to S248) and three putative TM stretches (D7 to S27, T48 to A68, and P108 to S128), whereas the PSORT algorithm (26) detected two certain TM regions (V181 to M205 and V228 to G244). Based on the predicted topology, the EspD protein is localized to either the bacterial inner membrane (P = 0.215) or the eukaryotic plasma membrane (P = 0.440). Using the BLASTP plus BEAUTY algorithm (2, 39) we found homologies to the EspD protein in EPEC and diffusely adhering E. coli strains (between 73 and 85% identity and 82 and 90% similarity; EMBL accession no. Y09228, Y13859, Y17875, and Y17874), the translocator protein YopB from Yersinia pestis and Yersinia enterolitica (25 and 24% identity and 42 and 40% similarity, respectively; EMBL accession no. Q06114 and P37131), and flagellin from Pseudomonas putida (EMBL accession no. L15366). With the FASTA algorithm (29), homologies were also detected for IpaB from Shigella flexneri and Shigella dysenteriae (22% identity and 39% similarity, respectively; EMBL accession no. P18011 and Q03945). The degree of homology between EspD and the YopB protein, as well as their conserved structural features (36), suggests that EspD might be involved in the formation of a translocation apparatus.
Generation and characterization of an espD deletion mutant.
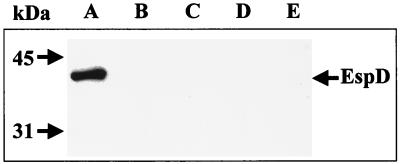
In former studies, we have given proof that EHEC EDL933 secretes EspD via the type III secretion system, because a mutant which was defective in an essential component of the type III secretion apparatus (pas) no longer secreted EspD (22). To characterize the role played by EspD in the pathogenicity of EHEC, a mutant with an in-frame deletion in espD (EDL933ΔespD) was generated as described in Materials and Methods (Fig. 1). The deletion of the espD gene in EDL933ΔespD was expected to result in the loss of production and secretion of EspD but not that of the proteins EspA and EspB, which are encoded by ORFs located upstream and downstream of espD, respectively. Therefore, EDL933 and its ΔespD derivative were grown in DMEM-HEPES; bacterial cultures were fractionated into supernatant, cytoplasmic and periplasmic, and outer- and inner-membrane fractions; and the resulting extracts were separated by SDS-PAGE and analyzed by immunoblotting with MAb anti-EspD to determine the expression and secretion of EspD. EDL933 produced and secreted EspD as expected (only secretion shown in Fig. 2A), whereas no signals were obtained in any of the fractions for EDL933ΔespD, showing that the mutant was deficient in the production of EspD (Fig. 2B to E).
FIG. 2.
Expression and secretion of EspD. The supernatant fluids of the EHEC strain EDL933 (lane A) and its espD derivative EDL933ΔespD (lane B) were concentrated with trichloroacetic acid, and cultures from EDL933ΔespD were fractionated into the cytoplasmic and periplasmic (lane C), inner-membrane (lane D), and outer-membrane (lane E) fractions. Samples (30 μg) of proteins were separated by SDS-PAGE and analyzed by immunoblotting with MAb anti-EspD. The expected size of the EspD protein (40 kDa) is indicated.
Although quantitative studies were not carried out, the generation of an espD deletion mutant in EPEC led to a reduced expression of EspA and EspB, which was not restored by complementation (23). Since espA, espD, and espB constitute a single operon (3), we were concerned about the potential effects resulting from the deletion of espD in the transcriptional unit. Therefore, the expression and secretion of EspA and EspB in supernatants and whole-cell extracts were assessed by Western blot analysis. Both proteins were expressed and secreted, and no significant differences could be observed between the wild-type strain and the EDL933ΔespD mutant (not shown).
The EDL933ΔespD mutant exhibits an impaired attachment to HeLa cells.
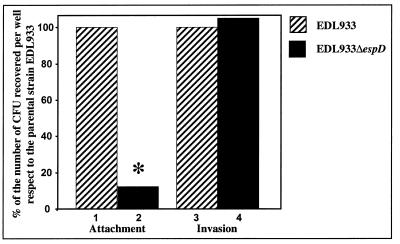
Knutton et al. found that an espA mutant of the EPEC strain E2348/69 exhibited a significant reduction in its adherence to epithelial cells (21). However, the deletion of espA had a less dramatic effect on the attachment of EPEC or REPEC (1, 19) than on that of STEC (3, 9). It seems that in EPEC and REPEC the presence of another adhesin(s) compensates for the lack of EspA-containing filaments, resulting in conserved attachment. To assess the role played by EspD in the adherence of STEC strains to epithelial cells, the attachment of the EDL933ΔespD mutant to HeLa cells was analyzed. The adhesion of EDL933ΔespD was reduced by 88% when compared to that of the parental strain (Fig. 3). Interestingly, the invasiveness of the ΔespD derivative was not affected (Fig. 3). This suggests that bacterial invasion is EspD independent.
FIG. 3.
Adherence and invasiveness of the EHEC strain EDL933ΔespD. The ability of EHEC strains EDL933 and EDL933ΔespD to attach to and invade HeLa cells was determined as described in Materials and Methods. As a control, the attachment and invasion of E. coli K-12 were also analyzed (0.1 and 0%, respectively, of the values for EDL933 [not shown]). Differences between EDL933 and EDL933ΔespD were statistically significant at a P of ≤0.05 (∗).
EspD is required for actin accumulation underneath adherent bacteria.
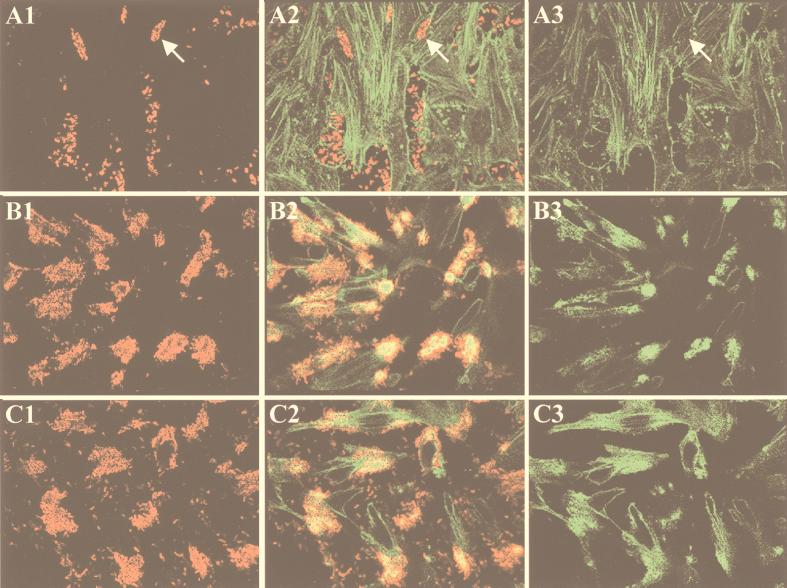
The infection of eukaryotic cells with the EHEC strain EDL933 does not result in the tyrosine phosphorylation of the translocated receptor for intimin EspE (6). Therefore, to investigate whether the signal transduction pathway was impaired in the mutant strain, actin accumulation underneath adherent bacteria was studied by immunofluorescence microscopy. Staining of F-actin with TRITC-labeled phalloidin revealed that in contrast to the parental strain EDL933, the mutant strain EDL933ΔespD was unable to trigger actin accumulation (Fig. 4). Therefore, the involvement of the EspD protein from EHEC in the generation of A/E lesions appears to be similar to that of the EspD protein from EPEC (23). The capacity to accumulate the actin of the EDL933ΔespD mutant was restored when this strain was transformed with a plasmid containing the espD gene under the control of its natural promoter (pANK84) (Fig. 4C1 to C3). The fact that the wild-type phenotype was not restored by providing in trans a pANK84 derivative (pAKSK78) in which the espD gene was deleted (not shown) ruled out any contribution of the upstream sequences in the observed complementation.
FIG. 4.
Immunofluorescence microscopy of HeLa cells infected with EHEC. HeLa cells were infected for 4 h with EDL933ΔespD (A1 to A3), EDL933 (B1 to B3), or EDL933ΔespD[pANK84] (C1 to C3). Bacteria were labeled with O157-specific antiserum and TRITC-conjugated secondary antibodies (column 1), and actin was labeled with FITC-conjugated phalloidin (column 3). An overlay of series 1 and 3 is shown (column 2). Arrows indicate adherent EDL933ΔespD bacteria, which do not accumulate actin.
Influence of EspD in the production of EspA filaments.
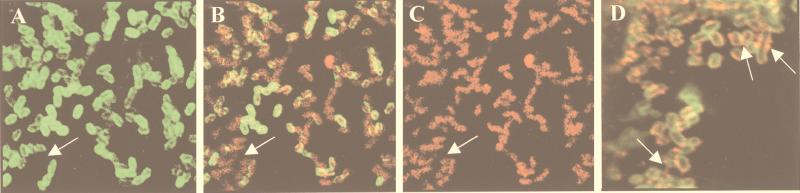
Recently, our group and others (9, 21) have described the production of surface appendages by STEC and EPEC. These filaments contain EspA as a major component and seem to be translocation channels to deliver effector molecules into the cytosol of host cells. Knutton et al. reported that an espD mutant of EPEC produces shorter variants of these filaments and hypothesized that EspD might be a constituent of EspA surface appendages (21). However, experimental data supporting this speculation were not provided and whether the reported effect was due to the direct involvement of EspD in the formation of the filaments or to its indirect involvement in the synthesis, export, or assemblage of structural components of the appendages was not analyzed. Thus, we carried out initial immunofluorescence studies with MAb B71 to detect EspA filaments during EHEC infection of HeLa cells. The wild-type strain EDL933 produced EspA-containing surface appendages which established a link between bacteria and host cells, whereas the ΔespD derivative lacked the filamentous structures and exhibited poor staining with anti-EspA antibodies (Fig. 5). However, the appendages produced by EHEC were less numerous and thinner than those produced by EPEC (30 versus 50 nm in thickness [not shown]). Interestingly, some members of the EDL933ΔespD population showed focal accumulation of EspA on the bacterial surface, suggesting the presence of aggregates of structural proteins following an aborted formation of surface appendages (Fig. 5A to C).
FIG. 5.
Immunofluorescence microscopy of EspA filaments from EDL933. After 4 h of infection of HeLa cells with EDL933ΔespD (A to C) or EDL933 (D), bacteria were labeled with O157-specific antiserum and FITC-conjugated secondary antibodies (A and D) and EspA was labeled with MAb B71 and TRITC-conjugated secondary antibodies (C and D). An overlay of panels A and C (B) is shown. The arrows indicate the secreted EspA protein aggregated and localized between EDL933ΔespD bacteria (A to C) or EspA filaments of EDL933 (D).
The bacterial cultures used in the infection studies were grown overnight in LB medium at 37°C (5% CO2) in 15-ml Falcon tubes without shaking to preserve the filaments. Under these conditions, the wild-type strain and its Δpas (22) and ΔespA derivatives (3) remained in suspension whereas EDL933ΔespD sedimented (not shown). The growth rates in LB medium were similar for both EDL933 and EDL933ΔespD, and the motility of the ΔespD mutant was only slightly reduced (not shown). Interestingly, when the ΔespD derivative was grown at 37°C in DMEM-HEPES with shaking (200 rpm), round bacterial aggregates and precipitated proteins, as confirmed by SDS-PAGE, were detected (not shown). This result recalls the inducible precipitation of Yop proteins previously observed in Yersinia spp. (25).
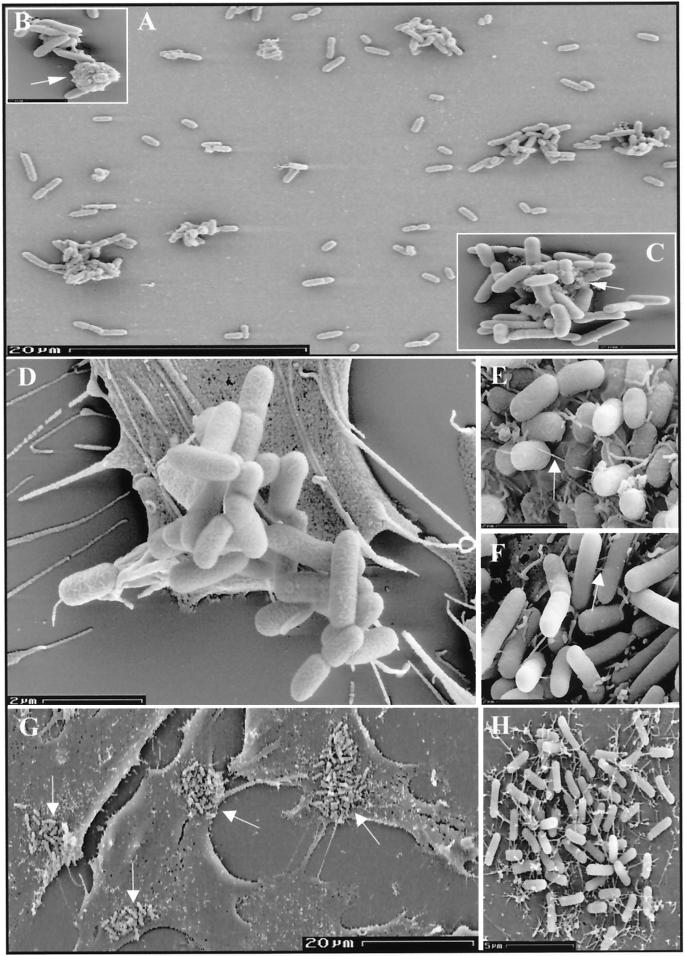
SEM studies were then performed to analyze the aggregates. The EDL933ΔespD mutant formed clumps often containing more than 30 bacteria, which seemed to be held together by the presence of irregular aggregates of a solid material between individual cells (Fig. 6A to C). Similar bacterial aggregates were visualized on the surfaces of infected cells (Fig. 6D). Considering the nonstructured accumulation of EspA on the surface of EDL933ΔespD (Fig. 5), we can speculate that in the ΔespD mutant, although the EspA protein is still secreted, the lack of an EspD or EspD-dependent product(s) results in an abortive synthesis of the surface appendages. The resulting protein accumulation on the bacterial surface leads to the aggregation of bacteria, since promotion of adherence is one of the properties of the EspA-containing filaments. The SEM studies also revealed that in contrast to what occurs after infection with the parental strain, attached bacteria did not synthesize surface appendages and were not enveloped by microvilli when HeLa cells were infected with the ΔespD mutant (Fig. 6D). When the EDL933ΔespD strain was complemented in trans with the espD gene, the resulting clone, EDL933ΔespD[pANK84], produced EspA filaments similar to those synthesized by the wild-type strain (Fig. 6E and F), formed microcolonies on the surfaces of infected cells (Fig. 6G), and was also enveloped by microvilli (Fig. 6H).
FIG. 6.
SEM analysis of EDL933ΔespD-infected HeLa cells. Aggregates of bacteria (A to C), which contained amorphous material (indicated by arrows) between individual bacterial cells (B and C), could be visualized. The surface appendages observed in EDL933-infected cells after 4 h (E) were not produced by EDL933ΔespD (D). The wild-type phenotype was restored in the complemented strain EDL933ΔespD[pANK84] (F), which also formed microcolonies (G; arrows) covered by microvilli (H) on the surfaces of HeLa cells. Arrows in panels E and F indicate EspA filaments.
Localization of the product encoded by the espD gene during EHEC infection of HeLa cells.
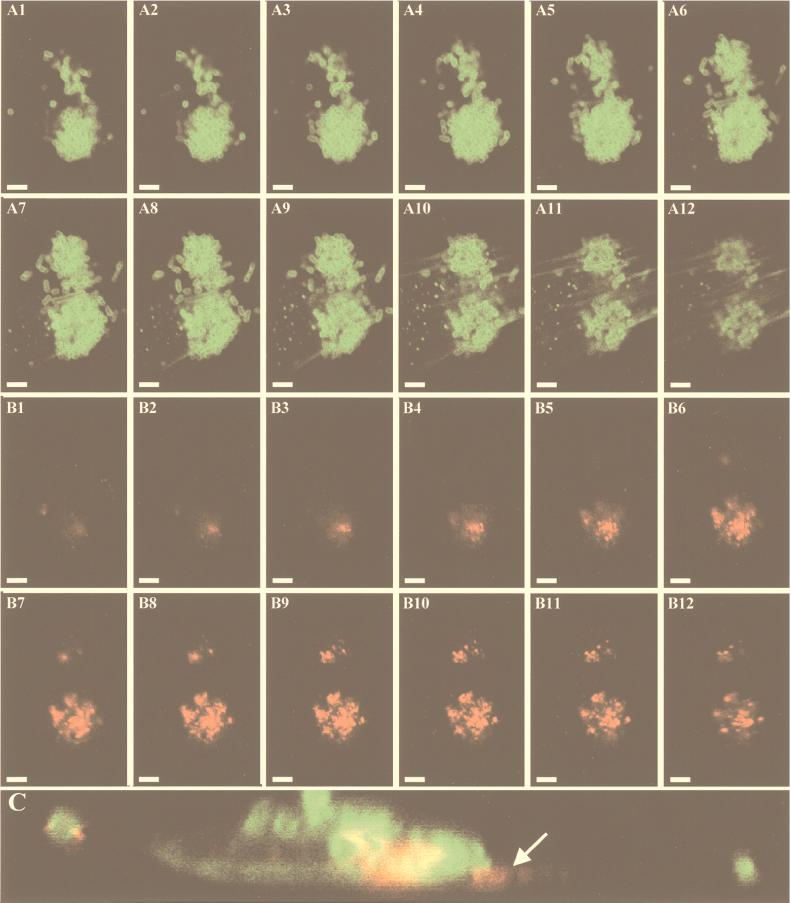
To investigate the potential involvement of EspD in the synthesis of the surface appendages, immunofluorescence studies were performed. In contrast to what was expected, EspD was not found as a second component of the EspA filaments but rather was found to form patches underneath attached bacteria (Fig. 7A and B). However, this finding seems to be in agreement with the fact that EspD exhibits homology with the secreted protein YopB of Yersinia spp., which is delivered into the membranes of target cells to function as a translocator for effector proteins (5), and with the predicted topology of EspD in the cytoplasmic membranes of eukaryotic cells (see above).
FIG. 7.
Detection of EspD by immunofluorescence microscopy. After 4 h of infection of HeLa cells, bacteria were labeled with O157-specific antiserum and FITC-conjugated secondary antibodies (A1 to A12), actin was labeled with FITC-conjugated phalloidin (A1 to A12), and EspD was labeled with MAb anti-EspD and TRITC-conjugated secondary antibodies (B1 to B12). The analytical sectioning by confocal laser microscopy was performed from the top to the bottom of the microcolony (panels 1 to 12) in 0.02-μm steps. (C) The x,z sectioning with the same labeling shows EspD underneath attached bacteria. The bar in panel A1 indicates 5 μm, and the arrow in panel C indicates EspD within the eukaryotic cells.
Wolff et al. have shown that the EspB protein of EPEC is translocated into the cytosol as well as inserted into the cytoplasmic membranes of infected cells (38). It was suggested that both EspD and EspB can be homologues of YopB (14, 38). This possibility might indicate similar modes of action for both proteins. In Yersinia spp., YopB interacts with YopD to form a pore in the membranes of target cells (5). Therefore, EspB and EspD may perform an analogous function in EHEC. To confirm the localization of EspD, HeLa cells were fractionated 6 h after infection with EDL933 into supernatant fluids, cytosol, cytoplasmic membrane, and a fraction containing nuclei, cytoskeletal proteins, and proteins of adherent bacteria. Because of the small amount of EspD, proteins were concentrated by methanol precipitation and fractionated by SDS-PAGE and EspD was detected by Western blotting.
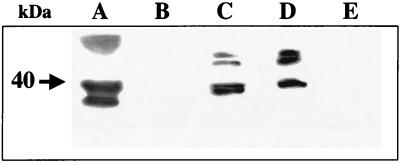
The obtained results confirmed that most of the EspD protein was localized within the cytoplasmic membrane fractions of infected cells but that smaller amounts of EspD were found in the cytosol and in supernatant fluids (Fig. 8). These results are in agreement with observations published by Wachter et al. during the revision of the manuscript of this article; they reported that the EspD protein from diffusely adhering E. coli is inserted into the host cell membrane (36). However, they did not detect the EspD protein in the cytoplasmic fraction. The lack of EspD protein associated with the fraction containing cytoskeletal proteins suggests that EspD is efficiently secreted by bacteria and does not interact with cytoskeletal proteins. On the other hand, the localization of EspD in association with the membranes and the cytosol of infected cells corresponds to the topology of EspB during infection with EPEC (38). The presence of reacting bands with higher electrophoretic mobility in both the cytosolic and cytoplasmic membrane fractions of infected cells than in the supernatant fluids suggests that EspD is posttranslationally modified following transfer to the eukaryotic cells.
FIG. 8.
Localization of the EspD protein within HeLa cells after infection with EDL933. Cells were infected with the EHEC strain EDL933 for 6 h. Eukaryotic cells were fractionated as described in Materials and Methods, and EspD was detected by Western blotting. (A) Secreted proteins of bacteria grown in DMEM-HEPES; (B) secreted protein of bacteria during infection of HeLa cells; (C) cytosolic fraction; (D) cytoplasmic membrane fraction; (E) fraction containing nuclei, cytoskeletal proteins, and proteins of adherent bacteria. The molecular mass of the main EspD protein is indicated by an arrow.
These results demonstrate that EspD also plays an essential role in the pathogenicity of EHEC. In fact, the EspD protein is required to obtain efficient bacterial attachment to target cells. Its main role in the infection process appears to be the establishment of a direct link between bacteria and eukaryotic cells via EspA-containing surface appendages and perhaps to facilitate the translocation of the effector proteins required for A/E lesions and intimate attachment. By analogy to the proposed model for YopB and YopD proteins from Yersinia spp., EspB and EspD might be inserted into the cytoplasmic membranes of the target cells, interacting as pore-forming components of the translocation apparatus.
ACKNOWLEDGMENTS
We gratefully acknowledge F. Ebel for the provision of monoclonal antibodies and helpful discussions and K. N. Timmis for his generous support and encouragement during this study. A.U.K. appreciates the excellent technical assistance of Ellruth Mueller in electron microscopy.
This work was, in part, supported by a Lower Saxony-Israel Cooperation Grant funded by the Volkswagen Foundation (21.45-75/2).
REFERENCES
- 1.Abe A, Kenny B, Stein M, Finlay B B. Characterization of two virulence proteins secreted by rabbit enteropathogenic Escherichia coli, EspA and EspB, whose maximal expression is sensitive to host body temperature. Infect Immun. 1997;65:3547–3555. doi: 10.1128/iai.65.9.3547-3555.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basis local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Beltrametti F, Kresse A U, Guzmán C A. Transcriptional regulation of the esp genes of enterohemorrhagic Escherichia coli. J Bacteriol. 1999;181:3409–3418. doi: 10.1128/jb.181.11.3409-3418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdanove A J, Wei Z M, Zhao L, Beer S V. Erwinia amylovora secretes hairpin via a type III pathway and contains a homolog of yopN of Yersinia spp. J Bacteriol. 1996;178:1720–1730. doi: 10.1128/jb.178.6.1720-1730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 6.Deibel C, Krämer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 7.De Lorenzo V, Eltis L, Kessler B, Timmis K N. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 8.Ebel F, Deibel C, Kresse A U, Guzmán C A, Chakraborty T. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect Immun. 1996;64:4472–4479. doi: 10.1128/iai.64.11.4472-4479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebel F, Podzadel T, Rohde M, Kresse A U, Kramer S, Deibel C, Guzmán C A, Chakraborty T. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol Microbiol. 1998;30:147–161. doi: 10.1046/j.1365-2958.1998.01046.x. [DOI] [PubMed] [Google Scholar]
- 10.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic E. coli. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 11.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galán J E. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Dev Biol. 1997;12:221–255. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 13.Gannon V P, Rashed M, King R K, Thomas E J. Detection and characterization of the eae gene of Shiga-like toxin-producing Escherichia coli using polymerase chain reaction. J Clin Investig. 1993;31:1268–1274. doi: 10.1128/jcm.31.5.1268-1274.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensel M, Shea J E, Waterman S R, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang F C, Holden D W. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann K, Stoffel W. TMbase—a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;374:166. [Google Scholar]
- 18.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 20.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kresse A U, Schulze K, Deibel C, Ebel F, Rohde M, Chakraborty T, Guzmán C A. Pas, a novel protein required for protein secretion and attaching and effacing activities of enterohemorrhagic Escherichia coli. J Bacteriol. 1998;180:4370–4379. doi: 10.1128/jb.180.17.4370-4379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai L C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michiels T, Wattiau P, Brasseur R, Ruysschaert J M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in Gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien A D, Lively T A, Chen M E, Rothman S W, Formal S B. Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (Shiga) like cytotoxin. Lancet. 1983;i:702. doi: 10.1016/s0140-6736(83)91987-6. [DOI] [PubMed] [Google Scholar]
- 28.O’Callaghan D, Charbit A. High efficiency transformation of Salmonella typhimurium and Salmonella typhi by electroporation. Mol Gen Genet. 1990;223:156–158. doi: 10.1007/BF00315809. [DOI] [PubMed] [Google Scholar]
- 29.Pearson W R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- 30.Perna N T, Mayhew G F, Posfai G, Elliott S, Donnenberg M S, Kaper J B, Blattner F R. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1998;66:3810–3817. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reese M G, Harris N L, Eeckman F H. Large scale sequencing specific neural networks for promoter and splice recognition. In: Hunter L, Klein T, editors. Biocomputing: Proceedings of the 1996 Pacific Symposium. Singapore: World Scientific Publishing Co.; 1996. [Google Scholar]
- 32.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Schnaitman C A. Cell fractionation. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. p. 60. [Google Scholar]
- 35.Viret J F, Cryz S J, Jr, Favre D. Expression of Shigella sonnei lipopolysaccharide in Vibrio cholerae. Mol Microbiol. 1996;19:949–963. doi: 10.1046/j.1365-2958.1996.435967.x. [DOI] [PubMed] [Google Scholar]
- 36.Wachter C, Beinke C, Mattes M, Schmidt M A. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1999;31:1695–1707. doi: 10.1046/j.1365-2958.1999.01303.x. [DOI] [PubMed] [Google Scholar]
- 37.Willshaw G A, Smith H R, Scotland S M, Rowe B. Cloning of genes determining the production of Vero cytotoxin by Escherichia coli. J Gen Microbiol. 1985;131:3047–3053. doi: 10.1099/00221287-131-11-3047. [DOI] [PubMed] [Google Scholar]
- 38.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–156. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]
- 39.Worley K C, Wiese B A, Smith R F. BEAUTY: an enhanced BLAST-based search tool that integrates multiple biological information resources into sequence similarity search results. Genet Res. 1995;5:173–184. doi: 10.1101/gr.5.2.173. [DOI] [PubMed] [Google Scholar]