Abstract
Context
DNA demethylation and inhibitory effects of aspirin on pituitary cell proliferation have been demonstrated.
Objective
Our aim was to clarify the molecular mechanisms behind the aspirin-related effects in pituitary cells.
Methods
DNA methylome and whole transcriptome profile were investigated in RC-4B/C and GH3 pituitary cell lines upon aspirin treatment. Effects of aspirin and a demethylation agent, decitabine, were further tested in vitro. PTTG1 expression in 41 human PitNET samples and whole genome gene and protein expression data of 76 PitNET and 34 control samples (available in Gene Expression Omnibus) were evaluated.
Results
Aspirin induced global DNA demethylation and consequential transcriptome changes. Overexpression of Tet enzymes and their cofactor Uhrf2 were identified behind the increase of 5-hydroxymethylcytosine (5hmC). Besides cell cycle, proliferation, and migration effects that were validated by functional experiments, aspirin increased Tp53 activity through p53 acetylation and decreased E2f1 activity. Among the p53 controlled genes, Pttg1 and its interacting partners were downregulated upon aspirin treatment by inhibiting Pttg1 promoter activity. 5hmC positively correlated with Tet1-3 and Tp53 expression, and negatively correlated with Pttg1 expression, which was reinforced by the effect of decitabine. Additionally, high overlap (20.15%) was found between aspirin-regulated genes and dysregulated genes in PitNET tissue samples.
Conclusion
A novel regulatory network has been revealed, in which aspirin regulated global demethylation, Tp53 activity, and Pttg1 expression along with decreased cell proliferation and migration. 5hmC, a novel tissue biomarker in PitNET, indicated aspirin antitumoral effect in vitro as well. Our findings suggest the potential beneficial effect of aspirin in PitNET.
Keywords: pituitary adenoma, pituitary, pitNET, methylation, demethylation, epigenetic, biomarker, pttg1
In previous work we demonstrated the interdependence between DNA demethylation and proliferation behavior and differentiation stage of pituitary neuroendocrine tumors (PitNET) (1). Indeed, the global DNA demethylation level in tumor tissues negatively correlated with Ki-67 proliferation rate and the ratio of 5-hydroxymethylcytosine (5hmC) to 5-methylcytosine (5mC) was higher in less differentiated adenomas (1). Additionally, increased expression of the DNA demethylating enzymes TET2 and TET3 was identified as a potential cause behind increased 5hmC level. TET2-3 exhibited significantly higher expression in adenomas with higher proliferation rate (1). Furthermore, significant positive correlation was detected between TET-cofactor UHRF2 expression and 5hmC level.
Aspirin (acetylsalicylic acid, ASA) is a commonly used nonsteroidal anti-inflammatory drug with antipyretic, analgesic, anti-inflammatory, and antithrombotic effect (2). Its antitumoral effect was demonstrated in several cancer types (3), and epidemiological and clinical studies indicated that aspirin reduced the cancer risk in several tumor types (3–7). While these data are encouraging, the mechanisms behind aspirin antitumoral effect are not entirely clarified. It is partially attributed to the inhibition of cyclooxygenase-2 (COX-2) which is upregulated in various cancer cells (8, 9, 10). Recently, more COX-independent effects of aspirin have been revealed (11, 12). ASA regulates multiple signaling pathways, biological functions, and molecules, including cell cycle, apoptosis, cell differentiation, proteasome and redox-mediated signaling, NF-κΒ, and VEGFs (13–15). Published data indicated that aspirin also had diverse and complex epigenetic influence, which were implicated in its antitumoral effect (9, 13, 16). Indeed, it was suggested that ASA was able to reverse tumor suppressor gene methylation in cancer tissues. In addition, ASA was shown to modulate histone structure through histone acetylase and deacetylase enzymes (13).
Regarding pituitary, it was also shown that ASA exerted significant inhibitory effect on pituitary adenoma cells directly by targeting cell cycle regulator cyclin A, cyclin dependent kinase 2, and indirectly through decreasing expression of survivin on mRNA and protein levels (17).
Based on these previous findings, in this study our aim was to investigate the genomic and epigenomic effects of ASA in pituitary adenomas using high-throughput profiling approaches (global methylation-demethylation analysis by high-performance liquid chromatography–tandem mass spectrometry [HPLC-MS/MS], whole transcriptome sequencing) and validate our findings by targeted methodologies, using in vitro functional assays and by investigating human pituitary tissue samples (mRNA microarray).
Methods
Cell Culture and Treatment
RC-4B/C (CRL-1903) and GH3 (CCL-82.1) pituitary cell lines were obtained from LGC Standards GmbH (Wesel, Germany) in the frame of LGC-ATCC partnership with a corresponding authentication certificate. Cells were cultured as previously reported (1) following ATCC recommendations, and they were used for experiments between 5 and 20 passages.
Aspirin (acetylsalicylic acid, ASA) from Sigma Chemical Co. (#A5376, St. Louis, MO, USA) and decitabine from Adooq (#A10292) were purchased and used similarly to our previous published experiments (ASA in 5 mM, decitabine in 10 μM final concentration, as formerly reported) (1, 17).
Patient Cohorts
As a discovery cohort, we correlated the methylation/demethylation status of 41 human pituitary adenoma samples that we previously reported (1) with currently measured gene expression data on the same samples (Table 1). Tissue samples were collected after surgical removal (National Institute of Clinical Neuroscience, Budapest, Hungary, between 2007 and 2017). Histological evaluation was performed routinely at the First Department of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, Hungary. The histological diagnosis was based on immunohistochemistry for anterior pituitary hormones and transcription factors following the 2017 World Health Organization instructions. Altogether, we analyzed 12 growth hormone (GH)-producing; 25 gonadotropic (18 follicle-stimulating hormone [FSH]/luteinizing hormone [LH]+; 7 hormone-negative [HN] steroidogenic factor-1 [SF-1]+); 3 HN, T-pit+ corticotropic, and one null cell tissues (1). Patients were informed and gave consent in writing; this study was approved by the Scientific and Research Committee of the Medical Research Council of Hungary (0618/15).
Table 1.
Discovery sample cohort
| Sex | Age | Clinical diagnosis | Cell lineage | Immunohistochemistry | Ki 67 (%) | Ellipsoid volume (mm3) | Primary/ recurrent |
|---|---|---|---|---|---|---|---|
| F | 76 | NFPA | gonadotroph | FSH | 2-3% | 1767 | primary |
| M | 44 | NFPA | gonadotroph | FSH | 2-3% | n.a. | primary |
| M | 51 | NFPA | gonadotroph | FSH | 2-3% | 10 | recurrent |
| F | 39 | NFPA | gonadotroph | FSH | 1-2% | n.a. | primary |
| M | 38 | NFPA | gonadotroph | FSH | 3-4% | n.a. | primary |
| F | 49 | NFPA | gonadotroph | FSH, LH | < 1% | 1950 | recurrent |
| F | 74 | NFPA | gonadotroph | FSH, LH | < 3% | n.a. | primary |
| M | 62 | NFPA | gonadotroph | FSH, LH | < 3% | n.a. | primary |
| M | 64 | NFPA | gonadotroph | FSH, LH | < 3% | 87114 | primary |
| M | 63 | NFPA | gonadotroph | FSH, LH | < 1% | 21501 | primary |
| F | 68 | NFPA | gonadotroph | FSH, LH | 3-4% | n.a. | recurrent |
| M | 43 | NFPA | gonadotroph | FSH, LH | 7-10% | n.a. | primary |
| F | 37 | NFPA | gonadotroph | FSH, LH | 7-8% | 1150 | recurrent |
| F | 80 | NFPA | gonadotroph | FSH, LH | 2-3% | 16366 | primary |
| M | 73 | NFPA | gonadotroph | FSH, LH | 2-3% | n.a. | primary |
| M | 38 | NFPA | gonadotroph | FSH, LH | 3-4% | n.a. | primary |
| M | 73 | NFPA | gonadotroph | LH | 2% | 5964 | primary |
| M | 72 | NFPA | gonadotroph | LH | < 2% | 2145 | primary |
| M | 50 | NFPA | gonadotroph | T-Pit-; PIT1-; SF-1+ | < 2% | n.a. | recurrent |
| F | 64 | NFPA | gonadotroph | T-Pit-; PIT1-; SF-1+ | 5% | n.a. | primary |
| F | 60 | NFPA | gonadotroph | T-Pit -; PIT1-; SF-1+ | 5% | n.a. | recurrent |
| M | 58 | NFPA | gonadotroph | T-Pit-; PIT1-; SF-1+ | 5-7% | 14137 | primary |
| F | 64 | NFPA | gonadotroph | T-Pit-; PIT1-; SF-1+ | < 1% | n.a. | primary |
| F | 50 | NFPA | gonadotroph | T-Pit-; PIT1-; SF-1+ | 2% | n.a. | primary |
| F | 43 | NFPA | gonadotroph | T-Pit-; PIT1-; SF-1+ | 1% | n.a. | primary |
| F | 58 | NFPA | corticotroph | T-Pit+; PIT1-; SF-1− | 4% | 1950 | primary |
| F | 65 | NFPA | corticotroph | T-Pit+; PIT1−; SF-1− | 3-4% | 905 | primary |
| F | 49 | NFPA | corticotroph | T-Pit+; PIT1−; SF-1− | 3-4% | n.a. | primary |
| M | 73 | NFPA | null cell | T-Pit−; PIT1−; SF-1− | 3-4% | 6371 | recurrent |
| M | 35 | GH-producing | somatotroph, lactotroph | GH, PRL | 6% | n.a. | primary |
| M | 30 | GH-producing | somatotroph, lactotroph | GH, PRL | 8% | 2185 | primary |
| M | 51 | GH-producing | somatotroph, lactotroph | GH, PRL | < 3% | n.a. | primary |
| F | 22 | GH-producing | somatotroph, lactotroph | GH, PRL | 4-5% | n.a. | recurrent |
| F | 35 | GH-producing | somatotroph, lactotroph | GH, PRL | 5-6% | n.a. | recurrent |
| F | 48 | GH-producing | somatotroph, lactotroph | GH, PRL | 10% | n.a. | primary |
| M | 22 | GH-producing | somatotroph, lactotroph | GH, PRL | 3% | 8181 | primary |
| F | 49 | GH-producing | somatotroph, lactotroph | GH, PRL | 3-4% | 1023 | primary |
| M | 33 | GH-producing | somatotroph, lactotroph | GH, PRL | 3-4% | n.a. | primary |
| F | 60 | GH-producing | somatotroph, lactotroph | GH, PRL | < 3% | n.a. | primary |
| M | 49 | GH-producing | somatotroph | GH | < 1% | n.a. | recurrent |
| F | 43 | GH-producing | somatotroph | GH | 1-3% | n.a. | primary |
Abbreviations: FSH, follicle-stimulating hormone; GH, growth hormone; LH, luteinizing hormone; NFPA, nonfunctioning pituitary adenoma; PRL, prolactin; SF-1, steroidogenic factor-1.
For cross-validation, an independent cohort of human cases was used: altogether pituitary tissue specimens of 76 nonfunctioning adenoma and 34 normal samples (Table 2). Data for gene and protein expression of these samples were obtained from Gene Expression Omnibus and literature mining (Table 2).
Table 2.
Validation sample cohort
| # Pituitary adenoma samples (NFPA) | # Normal control samples (NP) | Platform (used data) | |
|---|---|---|---|
| Gene expression studies | |||
| Morris et al, 2005 (18) | 5 | 5 | Affymetrix HG-U133 Plus 2.0 Array (GEO Acc. Number: GSE2175) |
| Michaelis et al, 2011 (19) | 14 | 9 | Affymetrix HG-U133A Array (GEO Acc. Number: GSE26966) |
| Moreno et al, 2005 (20) | 11 | 3 | Affymetrix HG-U95A V.2 Array (significant gene list published) |
| Elston et al, 2008 (21) | 13 | 3 | Affymetrix HG-U133 Plus 2.0 Array (significant gene list published) |
| Feng et al, 2015 (22) | 7 | 3 | Agilent-014850 Whole Human Genome Microarray (GEO Acc. Number: GSE51618) |
| Protein array studies | |||
| Moreno et al, 2005 (20) | 11 | 3 | 2DGE-MS (significant protein list published) |
| Zhan et al, 2010 (23) | 15 | 8 | 2DGE-MS (significant protein list published) |
Nucleic Acid Isolation
Total RNA was extracted with Qiagen MiRNeasy Mini kit (217004, Qiagen, Hilden, Germany) or Macherey Nagel NucleoSpin miRNA kit (740971.50, Duren, Germany). Genomic DNA was extracted with QIAamp DNA Mini Kit (51104, Qiagen, Hilden, Germany). The isolation protocol was performed strictly following the manufacturer's instructions. Nucleic acid purity and quantity were analyzed with a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
DNA Methylation-Demethylation Analysis by HPLC-MS/MS
Chemicals and reagents
Acetonitrile and formic acid were purchased from VWR International Ltd. (Debrecen, Hungary). Water was prepared by MilliQ Purification System from Millipore (Bedford, MA, USA). The 5-methylcytosine and 5-hydroxymethylcytosine DNA Standard Set was purchased from Zymo Research Corporation (Biocenter Ltd., Szeged, Hungary).
Sample preparation
DNA solution containing 1.0 μg DNA was mixed with 50 µL of 98% formic acid and then hydrolyzed at 140 °C for 90 minutes. The sample was cooled at room temperature and evaporated under nitrogen. The residue was reconstituted in 70 µL acetonitrile-water (50:50, v/v) containing 0.1% formic acid.
The HPLC-MS/MS method was performed as we previously published (18) on an Agilent 1100 Series HPLC coupled with a Sciex 6500 QTRAP tandem mass spectrometer equipped with TurboV ion source instrument.
Transcriptome Sequencing and Data Analysis
PolyA NGS library preparation was performed using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina with Purification Beads (NEB #E7760S/L) strictly following the manufacturer's instructions. Sequencing was run on Illumina NovaSeq platform (NovaSeq 6000 SP 300 cycles [2 × 150 bp]) with data output 100 M PE reads/sample. Fastq file processing was done using R package. For bioinformatic analysis, the Bioconductor package edgeR was applied for investigating differential expression. False discovery rate (FDR) was used for multiple testing correction. Unsupervised cluster analysis was done using Genesis 1.8.1 (https://genome.tugraz.at/genesisclient/genesisclient_news.shtml), t-distributed stochastic neighbor embedding (t-SNE) analysis was performed using iDEP.91 (http://bioinformatics.sdstate.edu/idep/). For pathway analysis, gene set enrichment analysis was performed using Rno Reactome pathway gene sets. Gene ontology analysis was performed using Generic GO Term Finder. For network construction, the String database (https://string-db.org/) was applied. For analyzing transcription factor regulatory relationships, TRRUST was used (https://www.grnpedia.org/trrust/). Raw data was uploaded to NCBI GEO database (GSE202934).
Gene and Protein Array Expression Profile Analysis
Briefly, where raw data were available, we reanalyzed it using Genespring GX 12 Software (Agilent Tech Inc, Santa Clara, CA, USA), avoiding biases originating from different analysis settings and usage of different software packages. Data analysis details were previously published (19). Fold change filter was set to 2-fold, and then unpaired t test was used to identify significant (P < 0.05) gene expression changes with multiple testing correction (Benjamini-Hochberg). When raw data were not available, significant gene and protein lists extracted from manuscripts and supplementary materials were used (Table 2).
In Vitro Functional Assays (Viability, Proliferation, Dead Cell Ratio, Migration, Cell Cycle Analysis, and Ki-67 Staining by Flow Cytometry)
For determining cell viability, Alamar Blue assay (DAL1025, Invitrogen, Thermo Fisher Scientific, Grand Island, NY, USA) was used on 96 well plates, that is a commonly used method for detection of cell viability and in vitro cytotoxicity. Fluorescent signals with excitation at 560 nm and emission at 590 nm were detected using a flash spectral scanning multimode reader (5250040, Varioskan, Thermo Fisher Scientific, Waltham, MA, USA) with SkanIt Software 2.4.5 RE. To investigate cell proliferation and dead cell ratio, cell numbers on 6-well plates were determined using 0.4% Trypan Blue staining (15250061, Gibco, Thermo Fisher Scientific, Waltham, MA, USA).
To assess the effect of ASA on migration, a wound healing assay was performed on 24-well plates. Following ASA treatment, the cell monolayer was wounded using a 200-μL pipette tip and floating cells were washed with phosphate-buffered saline (PBS) (21-040-CV, Corning, Corning, NY, USA). Photos were taken after 0, 24, and 48 hours and analyzed with ImageJ Software (https://imagej.nih.gov/ij/, Bethesda, MD, USA) to calculate cell-free area (CFA %: [(cell-free area 24 or 48 hours/CFA 0 hours) × 100]), and migration rate (cell-covered area: (100-CFA %) (20).
Cell Cycle Analysis by Flow Cytometry
500 000 cells were incubated with 0.5 mL Cycloscope Reagent DNA labeling solution (cat: CYT-CS-R-50; Cytognos, S.L., Salamanca, Spain) for 10 minutes at room temperature in the dark and then measured with a BD FACS Lyric flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). At least 10 000 events were acquired. Flow cytometry data were analyzed using Kaluza 2.1.1 software (Beckman Coulter, Brea, CA, United States).
Ki-67 Staining by Flow Cytometry
500 000 cells were fixed for 15 minutes at RT with 100 µL IntraStain reagent A (Dako-Agilent, CA, USA, cat: K2311). After washing (5 minutes, 400g, room temperature) with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH = 7.4), the pellet was resuspended in 100 µL IntraStain reagent B containing 0.4% Triton X-100 and 5 µL Ki67 antibody (Sony Biotechnology, Weybridge, UK, Cat# 2352515, RRID:AB_2920575). After 15 minutes of incubation at room temperature in the dark, the cells were washed again (5 minutes, 400g, room temperature) and the pellet was resuspended in 0.5 mL PBS containing 0.01 mg/mL 2-(4-amidinophenyl)-6-indolecarbamidine (DAPI) and incubated for 30 minutes at room temperature in the dark. The samples were measured with a BD FACS Lyric flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). At least 50 000 events were acquired. Flow cytometry data were analyzed using Kaluza 2.1.1 software (Beckman Coulter, Brea, CA, United States).
Vector Construction and Luciferase Reporter Gene Assay
The vector construction and luciferase reporter gene assay were performed as we previously reported (21). Briefly, 2090 bp of the Pttg1 5′-UTR 201 ENSRNOT00000005070.5 region was cloned (5′→3′) into pGL3 promoter vector (Promega, Madison, WI) at the 5′ end of the firefly luciferase gene. Activity of Pttg1-luc construction was controlled by direct Sanger sequencing and basic luciferase activity detection. RC-4B/C cells were plated at 104 cells per well in 96-well plates on the day before transfection. Cells were cotransfected with 150 ng Pttg1-luc or control pGL3-promoter plasmid and 150 ng renilla luciferase vector (pRL-TK; Promega, Madison, WI) using Lipofectamine 3000 (Thermo Fisher Scientific, Grand Island, NY, USA) according to the manufacturer's instructions. Luciferase assay was performed 24 hours later using Dual-Glo luciferase assay system (#E2920; Promega, Madison, WI), as previously reported (21).
Targeted Gene Expression Measurements
The measurements were performed as previously described (1) using TaqMan Gene Expression Assays (Thermo Fisher Scientific, Waltham, MA, USA). Dnmt1: Rn007009664_m1; Tet1: Rn01428192_m1; Tet2: Rn01522037_m1; Tet3: Rn01425643_m1; Uhrf1: Rn02346366_m1; Uhrf2: Rn01502134_m1; Pttg1: Rn00574373_m1; Tp53: Rn00755717_m1 and PTTG1: Hs00864094_g1. Glyceraldehyde 3-phosphate dehydrogenase (Rn01775763_g1) or β-actin (Hs01060665_g1) were applied as endogenous control. To calculate the relative gene expression changes we used the fold change formula (fold change [FC] = 2-ΔΔCt).
Protein Extraction and Western Blot
Protein extraction and Western blotting were done as we previously reported (17, 22) using the following antibodies: p53 (1C12) mouse mAb (Cell Signaling Technology Cat# 2524, RRID:AB_331743) (1:1000, Cell Signaling Technology, ZA, Leiden, The Netherlands) and acetyl-p53 (K382) rabbit mAB, (1:500, Bioss Cat# bs-0905R, RRID:AB_10855372) (Bioss Antibodies, Woburn, MA, USA); anti-PTTG antibody (DCS-280) (1:2000, Santa Cruz Biotechnology Cat# sc-56207, RRID:AB_785382) Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-β-actin (1:1000, Cell Signaling Technology Cat# 4967, RRID:AB_330288) (Cell Signaling Technology, ZA, Leiden, The Netherlands) and secondary antibodies as goat anti-mouse HRP-conjugated (1:2000, Agilent Cat# P0447, RRID:AB_2617137) and goat anti-rabbit HRP-conjugated (1:2000, Agilent Cat# P0448, RRID:AB_2617138) (Agilent, Santa Clara, CA, USA).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6.01 software. Results were presented by mean ± SD. For assessing the differences in DNS methylation/demethylation, cell viability, proliferation and dead cell ratio, and mRNA and protein expression, unpaired t test with Welch's correction was used. Investigating correlation between gene expression and methylation/demethylation status Pearson correlation was used. P values < 0.05 were considered significant.
Results
Methylation-Demethylation Alteration Upon Aspirin Treatment
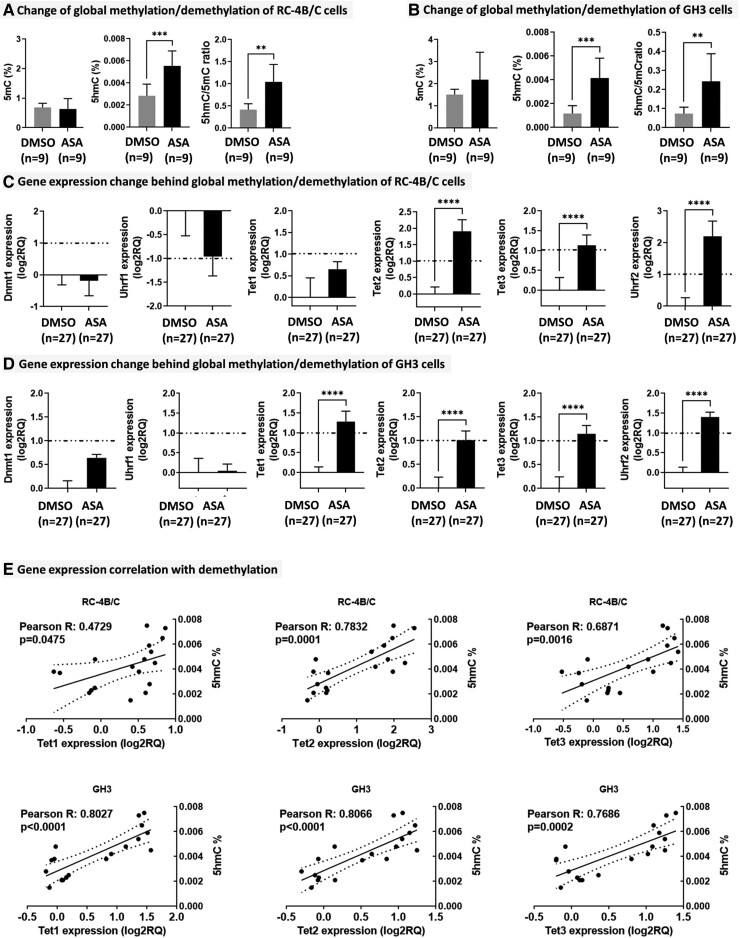
Global methylation-demethylation levels were determined by HPLC-MS/MS following ASA treatment in RC-4B/C and GH3 cells. In RC-4B/C cells, a significant increase of 5hmC (fold change: 1.95; P = 0.0003) and 5hmC/5mC ratio (fold change: 2.49; P = 0.0012) were observed (Fig. 1A). In GH3 cells, similar results were detected: both 5hmC (fold change: 3.5; P = 0.0005) and 5hmC/5mC ratio were elevated (fold change: 3.28; P = 0.0078) (Fig. 1B). Therefore, expression of enzymes and their cofactors regulating the methylation-demethylation cycle was measured by quantitative reverse transcription–polymerase chain reaction (RT-qPCR). In line with the mass spectrometry results, Dnmt1 responsible for cytosine methylation and its cofactor Uhrf1 showed no change in any of the cell lines (Fig. 1C-1D). However, in RC-4B/C cells, among Tet enzymes (converting 5mC to 5hmC) Tet2, Tet3, and their cofactor Uhrf2 were significantly overexpressed in line with increased 5hmC level (Fig. 1C). GH3 cells showed very similar expressional alteration, including increased Tet1 level also (Fig. 1D). Accordingly, Tet1, 2, and 3 showed significant correlation with 5hmC upon ASA treatment in both cell types (Fig. 1E).
Figure 1.
ASA effect on DNA methylation-demethylation. A-B, Change of global methylation-demethylation in RC-4B/C (A) and in GH3 (B) cells. C-D, Gene expression change behind global methylation/demethylation in RC-4B/C (C) and in GH3 (D) cells. E, Correlation between 5hmC and demethylation enzymes.
Global Transcriptome Alteration Upon Aspirin Treatment Indicates Inhibition of Cell Cycle and Proliferation
As global DNA demethylation showed marked alteration after ASA treatment, we investigated its consequence, a change in global transcriptome of pituitary cells following 5 mM ASA treatment.
By next-generation sequencing, 15 655 exons belonging to 3278 genes were identified as significant differentially expressed (< or > fold change 2 and FDR P < 0.05) after 5 mM ASA treatment. Unsupervised hierarchical clustering and t-distributed stochastic neighbor embedding analysis showed that ASA had a significant effect on overall gene expression profile (data not shown).
Pathway and gene ontology analysis indicated that the most affected pathway affected was the cell cycle (Table 3). This was reinforced by network analysis of gene interactions that revealed the top 10 most important genes governing ASA antitumoral effect on transcriptional level (Mad2l1, Pcna, Ube2c, Mcm4, Chek2, Brca1, Smc4, Cdc45, Cdk2, Rbx1), which also influenced proliferation, cell division, DNA damage response, and ubiquitination.
Table 3.
Pathway analysis of transcriptional changes upon ASA treatment (only the first 10 significant pathways are presented)
| Reactome pathway ID | Reactome pathway Name | Observed gene count | Background gene count | False discovery rate |
|---|---|---|---|---|
| RNO-69278 | Cell Cycle, Mitotic | 71 | 387 | 4.82e-06 |
| RNO-1640170 | Cell Cycle | 77 | 449 | 7.19e-06 |
| RNO-1430728 | Metabolism | 162 | 1330 | 0.00035 |
| RNO-194315 | Signaling by Rho GTPases | 56 | 324 | 0.00035 |
| RNO-69306 | DNA Replication | 27 | 102 | 0.00035 |
| RNO-194840 | Rho GTPase cycle | 28 | 117 | 0.00058 |
| RNO-453279 | Mitotic G1-G1/S phases | 26 | 103 | 0.00058 |
| RNO-69002 | DNA Replication Pre-Initiation | 20 | 65 | 0.00058 |
| RNO-69620 | Cell Cycle Checkpoints | 41 | 218 | 0.00058 |
| RNO-68886 | M Phase | 48 | 283 | 0.00097 |
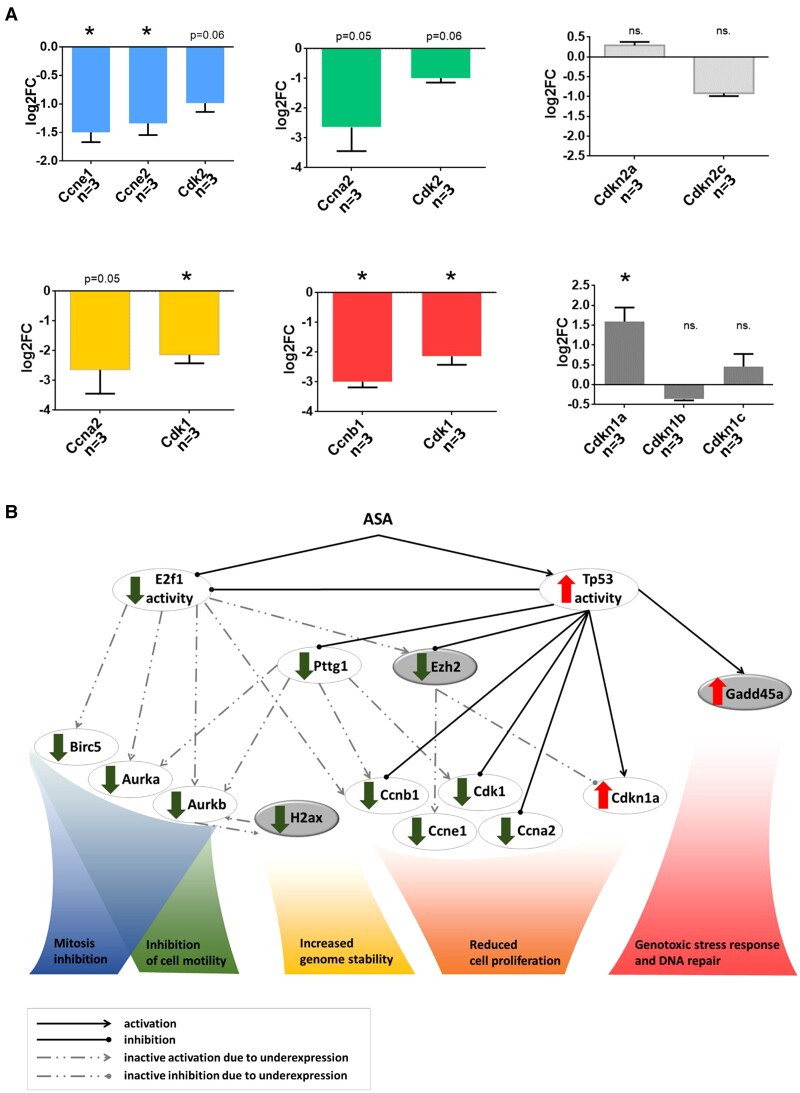
Several cyclins and cyclin dependent kinase were downregulated, while Cdkn1a was upregulated (Fig. 2A). The net outcome of the ASA effect on cell cycle was represented by the marked decrease of Ki-67 (log2FC: −2.66; P = 2.71E-04). In addition, expressional alteration of multiple genes implicated in proliferation, DNA repair, and genome stability were identified (Fig. 2B)
Figure 2.
A, Gene expression change behind cell cycle and proliferation alteration upon aspirin administration. Expression of genes implicated in cell cycle phase G1, S, G2, and M are indicated by blue, green, yellow, and red, respectively. Expressions of genes that inhibit cell cycle are illustrated by gray color. B, ASA genomic effect in pituitary adenoma. Expression changes: Tp53 (log2CPM:-0.403135161, FDR:0.475431307); E2f1 (log2CPM:-1.699322161, FDR:0.017677985); Birc5 (log2CPM:-1.669861341, FDR:0.002634309); Aurka (log2CPM:-2.693827176, FDR:0.000112511); Aurkb (log2CPM:-1.994711397, FDR:0.009565016); Pttg1 (log2CPM:-2.359268022, FDR:3.27562E-05); Ha2 × (log2CPM:-1.59, FDR:5.86E-03); Ezh2 (log2CPM:-1.187080264, FDR:0.067119053); Ccnb1 (log2CPM:-2.995668611, FDR:1.09241E-06); Ccne1 (log2CPM:-1.533242072, FDR:0.015933026); Ccna2 (log2CPM:-2.642928343, FDR:0.050601632); Cdk1 (log2CPM:-2.137090443, FDR:0.000341413); Cdkn1a (log2CPM:1.591983374, FDR:0.003852956); Gadd45a (log2CPM:1.907358455, FDR:0.000777632). Genes indicated by gray represent links between the transcriptional and epigenomic level. See further details in the discussion.
In Vitro Functional Validation of Effects of Aspirin on Viability, Proliferation, and Cell Death
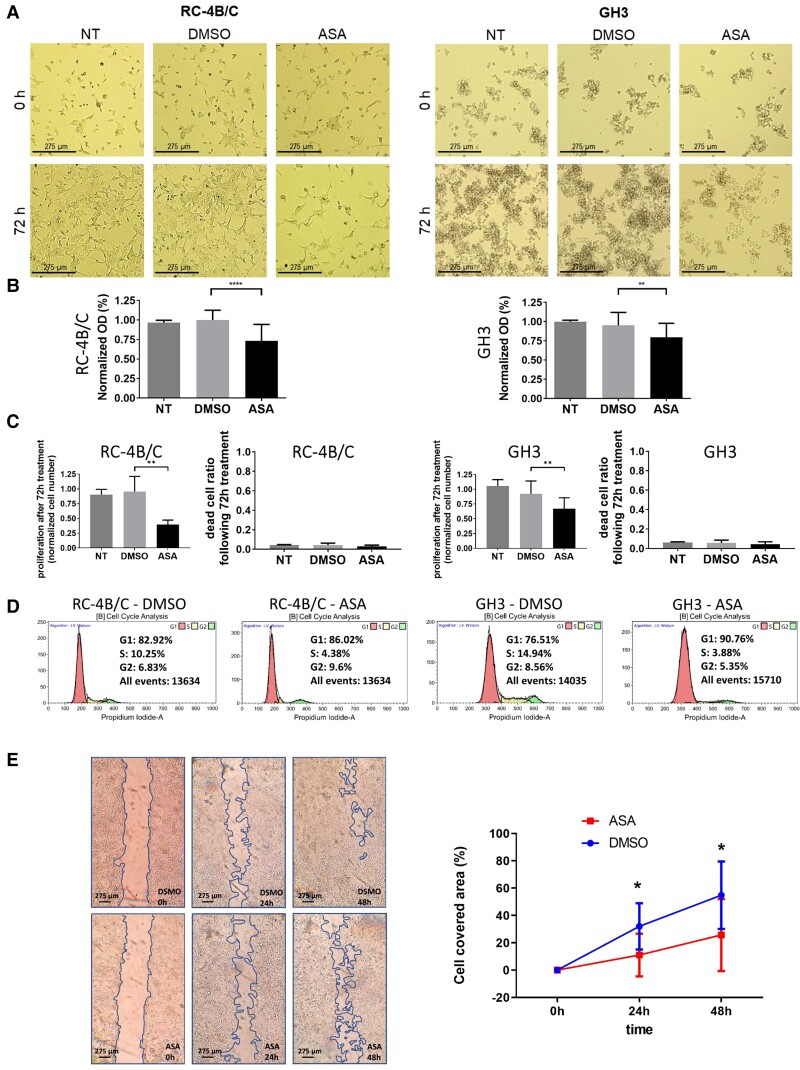
Validation of transcriptional findings using metabolic viability, cell proliferation and cell death of RC-4B/C and GH3 cells by functional in vitro assays were carried out. Treatment with 5 mM ASA led to significant viability decrease of RC-4B/C cells. Inhibition of cell proliferation was also observed, as cell number was decreased by 46.5% (P < 0.0001) (Fig. 3A). Since GH3 cells are characterized by loosely adherent appearance with floating clusters we performed functional assays without media change during ASA treatment. We found slight but significant decrease in viability and cell proliferation by 19.3% (P = 0.0028) upon treatment compared with DMSO control. Upon cell cycle analysis by flow cytometry, reduced number of cells entered to the cell cycle and reduced number of cells in S phase were identified in the ASA-treated group compared with control (4.38% vs 10.25% and 3.88% vs 14.94% in RC-4B/C and GH3 cells, respectively.) This was verified by decreased Ki-67 staining following ASA treatment compared with control: 0.09% vs 0.34% in RC-4B/C cells and 0.34% vs 1.48% in GH3 cells. ASA resulted in only a minor increase (1.66% and 1.11%) of dead cell ratio in RC-4B/C and GH3 cells, respectively (Fig. 3A). Additionally, we observed decreased cellular migration in the scratch assay upon ASA treatment (Fig. 3A).
Figure 3.
In vitro validation. A, microscopic images of RC-4B/C and GH3 cells upon ASA treatment for morphological assessment: significant morphologic changes cannot be observed upon DMSO or ASA treatment, only reduced cell numbers are suggested in ASA group. B, Decreased optical density following ASA treatment was observed compared to controls, due to decreased proliferation. No cytotoxic effect of DMSO was observed vs nontreated (NT) group. C, ASA effect on cell proliferation was further investigated by living cell numbers. Cell proliferation was 46% (P < 0.0001) and 80% (P = 0.028) in ASA group vs DMSO control in RC-4B/C and GH3 cells, respectively. Significant cell death was not observed in either cell line upon treatment. D, Cell cycle analysis by flow cytometry indicated reduced number of cells entering to the cell cycle and reduced number of cells in S phase upon ASA treatment vs control (4.38% vs 10.25% and 3.88% vs 14.94% in RC-4B/C and GH3 cells, respectively). E, ASA decreased RC-4B/C cell migration to 34% (P = 0.01) and 47% (P = 0.02) for 24 and 48 hours, respectively.
Aspirin Effect on Transcription Factor Activity
Transcription factor regulatory relationships were assessed using RNA sequencing data. Following ASA treatment, genomic effect of 12 transcription factors was revealed (Table 4). Among these, Tp53 activity was the most significant. Numerous (25) transcripts regulated by Tp53 showed significant expressional change (Table 5). Among Tp53 controlled genes, Pttg1 was also identified as downregulated (log2FC: −2.36; P = 3.275E-05). The E2F1 transcription factor activity was also modulated by ASA, which was also supported by Birc5 downregulation (log2FC: −1.72; FDR P = 0.052). Furthermore, the expression of Tp53 downstream target Gadd45α was also upregulated (log2FC: 1.90; FDR P = 0.0007) upon ASA treatment (Table 5).
Table 4.
Activity of transcription factors changed following aspirin treatment
| Transcription factor | P value | Adjusted P value | Genes regulated by the transcription factor |
|---|---|---|---|
| TP53 | 9.244E-9 | 5.28E-06 | MCM7; MKI67; FOXM1; AQP3; EGFR; RECQL4; CCNB1; PTTG1; GPNMB; CHEK2; CASP3; E2F7; PDGFRB; PLK3; SMAD3; GADD45A; GDF15; PLK1; CCNA2; PRC1; CDK1; CRYAB; ATF3; MAD1L1; EZH2 |
| E2F1 | 8.646E-8 | 2.47E-05 | TOP2A; RRM1; RRM2; HSPA5; PLK1; FANCA; CDC6; FOXM1; AURKB; AURKA; DHFR; CCNB1; RAD51; CHEK2; DDIT3; CDK1; MYBL2; KIF2C; MCM5; ECT2; ASF1B |
| E2F4 | 1.851E-6 | 3.52E-04 | PLK4; CCNB1; RAD51; PLK1; PCLAF; MCM10; TTK; AURKB |
| E2F3 | 5.498E-6 | 7.85E-04 | CCNA2; CCNB1; PLK1; CDK1; CDC6; AURKA |
| TRP53 | 1.265E-5 | 0.001445 | PDGFRB; DUSP4; PLK3; SMAD3; MCM7; GADD45A; GDF15; INSR; FOXM1; CKS1B; CCNA2; PER2; CCNB1; GAP43; KRT19; COL2A1; CASP3; PCBP2; PTGDS; ATF3 |
| E2F4 | 2.181E-5 | 0.002076 | PLK4; MAP1LC3B; RAD51; E2F2; MYBL2 |
| TFAP2A | 1.144E-4 | 0.009333 | ACHE; CCNB1; CRABP2; GALNT3; TH; CYP11A1; MCAM; CGA; ADRA1A; CRYAB; EGFR |
| ATF1 | 2.953E-4 | 0.021081 | TOP2A; LDHA; MAP1LC3B; TH; SLC20A1; CGA |
| ATF4 | 4.086E-4 | 0.025929 | MAP1LC3B; HSPA5; DDIT3; TRIB3; NDC80; ATF3; DDR2 |
| TFDP1 | 4.440E-4 | 0.025353 | DHFR; RRM1; CDK1; MYBL2 |
| XBP1 | 7.446E-4 | 0.038652 | ERN1; HSPA5; GAD1; DDIT3; ERP29 |
| ARNT | 9.602E-4 | 0.045694 | JUN; GAD1; BHLHE40; AHR; MFSD2A |
Table 5.
Expression changes of genes from Tp53 regulatory network
| GeneName | EnsGeneID | log2FC | P value | FDR |
|---|---|---|---|---|
| Aqp3 | ENSRNOG00000009797 | -2.30422 | 5E-05 | 0.001157 |
| Atf3 | ENSRNOG00000003745 | 3.484486 | 1.43E-08 | 1.8E-06 |
| Casp3 | ENSRNOG00000010475 | -2.04182 | 1.61E-06 | 6.51E-05 |
| Ccna2 | ENSRNOG00000015423 | -2.64293 | 0.02121 | 0.050602 |
| Ccnb1 | ENSRNOG00000058539 | -2.99567 | 9.47E-09 | 1.09E-06 |
| Cdk1 | ENSRNOG00000000632 | -2.13709 | 1.25E-05 | 0.000341 |
| Chek2 | ENSRNOG00000037509 | -1.37363 | 0.0324 | 0.083205 |
| Cryab | ENSRNOG00000010524 | 2.61213 | 0.000107 | 0.002052 |
| E2f7 | ENSRNOG00000026252 | -3.02489 | 0.000156 | 0.002484 |
| Egfr | ENSRNOG00000004332 | 1.951245 | 0.000199 | 0.003023 |
| Ezh2 | ENSRNOG00000006048 | -1.18708 | 0.01809 | 0.067119 |
| Foxm1 | ENSRNOG00000005936 | -2.53223 | 1.17E-06 | 5.55E-05 |
| Gadd45a | ENSRNOG00000005615 | 1.907358 | 3.82E-05 | 0.000778 |
| Gdf15 | ENSRNOG00000019661 | 2.696207 | 2.61E-06 | 0.000124 |
| Gpnmb | ENSRNOG00000008816 | 2.678566 | 3.31E-08 | 2.54E-06 |
| Mad1l1 | ENSRNOG00000001265 | -1.90029 | 0.000841 | 0.009182 |
| Mcm7 | ENSRNOG00000001349 | -2.1472 | 2.97E-05 | 0.000535 |
| Mki67 | ENSRNOG00000028137 | -2.66258 | 1.47E-05 | 0.000271 |
| Pdgfrb | ENSRNOG00000018461 | 2.073018 | 0.002211 | 0.014145 |
| Plk1 | ENSRNOG00000018815 | -2.84073 | 7.04E-09 | 8.9E-07 |
| Plk3 | ENSRNOG00000018484 | 1.98987 | 8.11E-06 | 0.000296 |
| Pprc1 | ENSRNOG00000018561 | -0.15577 | 0.587733 | 0.749968 |
| Pttg1 | ENSRNOG00000003802 | -2.35927 | 5.87E-07 | 3.28E-05 |
| Recql4 | ENSRNOG00000032446 | -1.78421 | 0.000247 | 0.00399 |
| Smad3 | ENSRNOG00000008620 | 1.545729 | 0.001247 | 0.009008 |
| Tp53 | ENSRNOG00000010756 | -0.40314 | 0.300957 | 0.475431 |
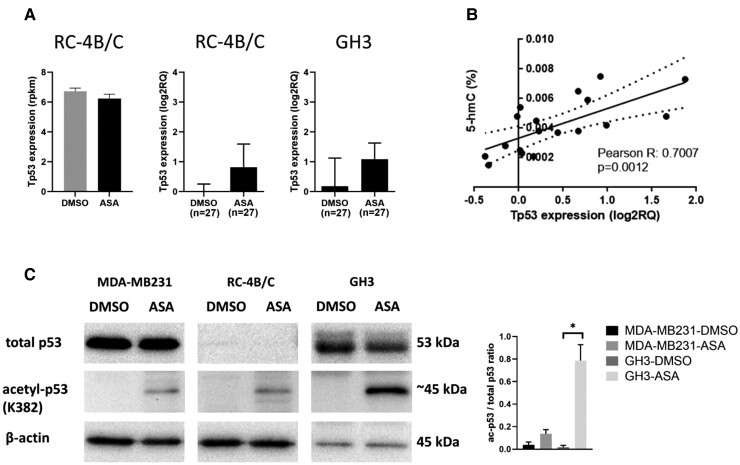
Although Tp53 expression itself did not change (P = 0.475) either in transcriptome data or in validation, ASA led to acetylation at the K382 position and demonstrated significant positive correlation with 5hmC level (Fig 4A-4C).
Figure 4.
A, Tp53 expression in transcriptome and RT-qPCR validation. B, Tp53 significantly correlated with 5hmC upon ASA administration. C, Western blot indicated no total p53 protein change following ASA treatment; however, treatment induced p53 acetylation at K382 residue. Densitometry showing the ratio acetyl-p53/total p53. Due to the lack of total p53 band in RC-4B/C cell, and the lack of acetylated p53 in the untreated cells, the ratio acetyl-p53/total p53 can be given only for MDA-MB231 and GH3 cells and it should be interpreted with caution.
Pttg1 Is Regulated by Aspirin Treatment and Demethylation in Human Pituitary Adenoma
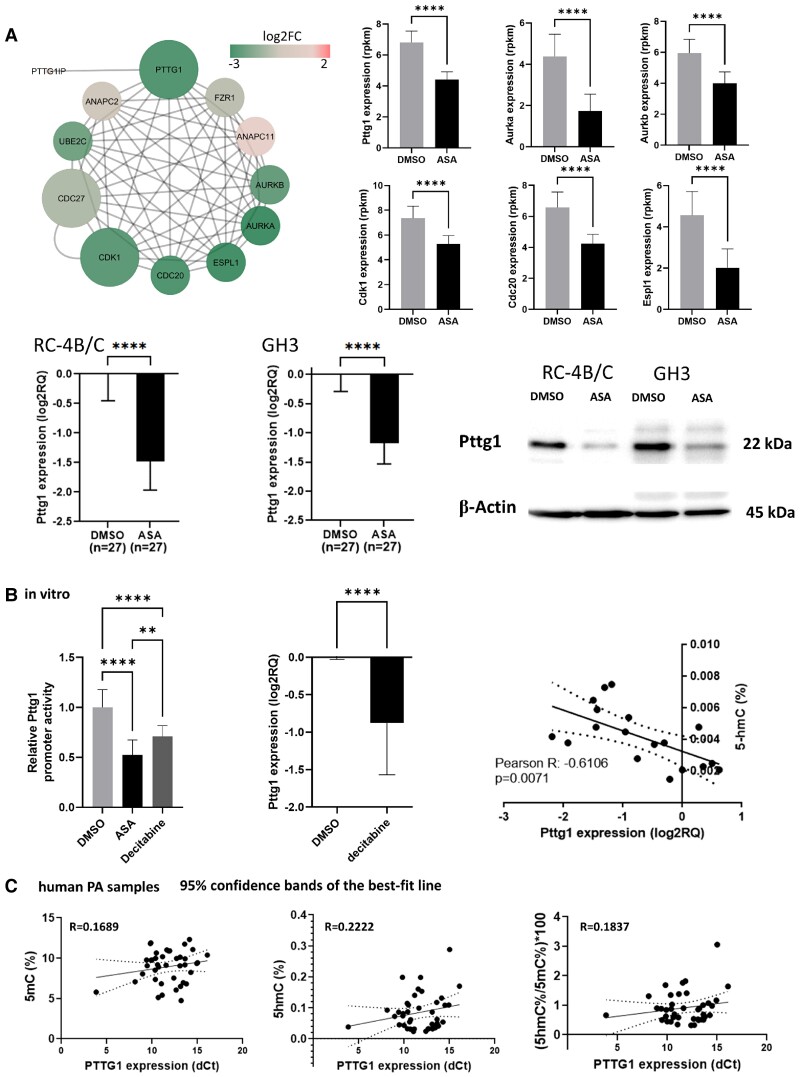
Since Pttg1 is a member in the Tp53 regulatory network and it is a main oncogenic factor in pituitary tumorigenesis, we investigated the expression of Pttg1 further. We found that genes regulated by Pttg1 and Pttg1 interacting partners were downregulated (Fig. 5A) after ASA treatment. Besides Pttg1 interaction partners, Pttg1 expression was significantly decreased on mRNA and protein level as well (Pttg1 protein level decreased to 49% ± 2% in RC-4B/C and to 31% ± 22% in GH3 cells, respectively) (Fig. 5A).
Figure 5.
A, Transcriptional change of Pttg1 and its interaction partners upon ASA effect. Decreased levels of Pttg1 was confirmed during RT-qPCR and Western blot experiments as well. B, In vitro luciferase experiment demonstrated decreased Pttg1 promoter activity after ASA and decitabine treatment. Although in human pituitary adenomas, global demethylation level did not show significant association with PTTG1 expression (C), upon ASA administration significant negative correlation was observed between Pttg1 expression and 5hmC level (B).
By constructing a Pttg1 promoter reporter system, we observed marked decrease in Pttg1 promoter activity upon ASA treatment. Additionally, since upon ASA administration, significant negative correlation was observed between Pttg1 expression and 5hmC level (Fig. 5B), we also investigated the effect of the global demethylation agent decitabine on the Pttg1 promoter. We found that decitabine decreased Pttg1 promoter activity in vitro. However, in human pituitary adenomas global methylation and demethylation level did not show significant association with PTTG1 expression (Fig. 5C)
Aspirin-Regulated Gene Signature Can Be Detected in Human Pituitary Adenoma Samples
To cross-validate the ASA effect, transcriptome and protein expressions of independent human pituitary adenoma samples (76 adenoma and 34 normal control specimen) were used (Table 2). Using the differentially expressed genes between human adenomas and normal specimens (3755 genes), we filtered out those that were also regulated by ASA. A high proportion, 20.15%, of the genes (757/3755) dysregulated in human PitNET samples were common with ASA-regulated genes. The 757 common genes were implicated mostly in cell proliferation and cell cycle, including p53 activity and function, while cellular migration and genome stability were also detected (Table 6).
Table 6.
Pathway analysis of commonly regulated genes between human pituitary adenoma vs normal pituitary and aspirin-regulated genes
| Category | Name | Source* | q-value FDR B&H | # Affected gene nr/# of genes of pathway | Ratio of affected genes in the pathway |
|---|---|---|---|---|---|
| p53-related pathways | p53 Signaling Pathway | 1 | 3.507E-2 | 5/16 | 0.3125 |
| Direct p53 effectors | 2 | 4.360E-2 | 15/132 | 0.113636 | |
| Proliferation and cell division related pathways | Retinoblastoma Gene in Cancer | 1 | 6.484E-6 | 20/88 | 0.227273 |
| DNA strand elongation | 4 | 9.949E-5 | 11/32 | 0.34375 | |
| DNA replication | 3; 1 | 1.188E-3 | 10/36 | 0.277778 | |
| Regulation of nuclear SMAD2/3 signaling | 2; 1 | 2.005E-2 | 12/77 | 0.155844 | |
| G1 to S cell cycle control | 1 | 3.507E-2 | 10/64 | 0.15625 | |
| Genome stability related pathways | Activation of ATR in response to replication stress | 4 | 2.193E-2 | 8/37 | 0.216216 |
Abbreviation: FDR B&H, Benjamini-Hochberg false discovery rate adjustment.
Sources*: 1: MSigDB C2 BIOCARTA (v7.3); 2: BioSystems: Pathway Interaction Database; 3: BioSystems: KEGG; 4: BioSystems: REACTOME.
Discussion
Based on our previous findings that in PitNET DNA demethylation was altered and it showed correlation with proliferative behavior and differentiation (1) and the epigenetic effects of aspirin in other malignancies (13), we investigated the complex genomic and epigenomic consequences of aspirin treatment in PitNET.
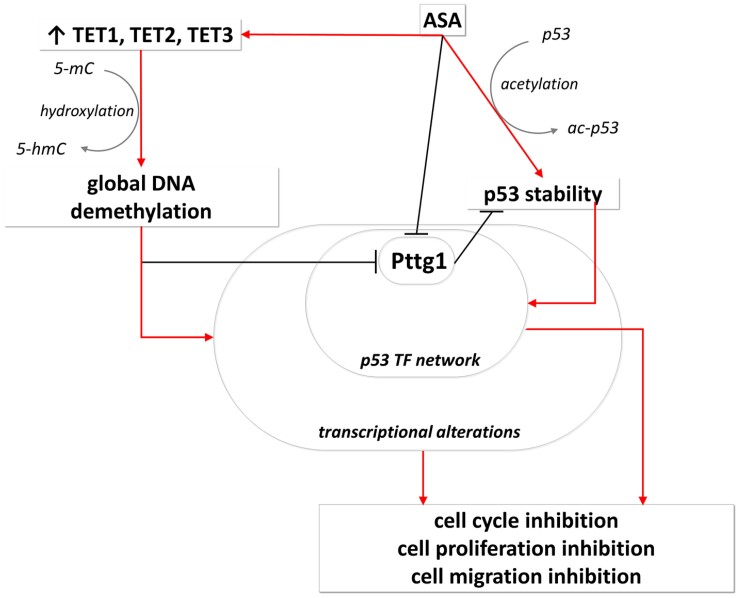
Upon aspirin treatment, increased global DNA demethylation was observed. In line with this, the overexpression of Tet1, 2, and 3 (enzymes responsible for DNA demethylation), along with their cofactor Uhrf2, was confirmed. In addition, DNA methylation machinery did not show significant change (neither the global level of 5mC or Dnmt1 nor the cofactor Uhrf1 exhibited alteration). Since accurate measurement and detection of demethylation (5hmC) is challenging and requires special instrumentation, only scarce information have been available regarding demethylation change upon aspirin treatment. In a study using methylation-specific polymerase chain reaction, Guo et al demonstrated that aspirin induced demethylation of the Forkhead Box D3 (FOXD3) promoter leading to increased FOXD3 expression (23). They also proved that regular use of aspirin dramatically decreased the number of metastatic nodules of cancer cells in immunodeficient mouse lungs through the FOXD3-OLA1P2-STAT3 axis (23). Similarly, we demonstrated that Pttg1 promoter activity and expression were decreased by treatment with aspirin and by the demethylation agent decitabine, respectively (Fig. 6). In addition, a negative correlation was observed between Pttg1 expression and 5hmC level, indicating the link between aspirin, demethylation, and Pttg1 expression change.
Figure 6.
ASA-demethylation-Pttg1-Tp53 regulatory network. In pituitary adenoma ASA increased global demethylation by elevating TET1-3 enzyme level. Global DNA methylation induces global transcriptional alteration including Pttg1 underexpression that was validated by decitabine treatment. Additionally, ASA inhibits Pttg1 promoter activity. Independently, ASA acetylated p53 leading to increased p53 stability and enhanced transcriptional activity (27). Pttg1 inhibits p53 activity as it reduces p53 protein stability and represses its transcriptional activity (30). As a summary, upon ASA treatment global transcription alteration, and enhanced p53 activity results in inhibition of cell cycle, proliferation, viability. See details in the discussion.
As a consequence of global demethylation, significant transcriptome alteration was observed using whole RNA sequencing. Investigating the biological function of the altered transcriptome profile, mainly cell cycle and proliferation related transcriptional changes were observed. Changes in viability, proliferation, and cellular migration of pituitary cells were also validated by multiple in vitro functional assays. All these are in line with our previous finding that the global increase of demethylation correlated with Ki-67 proliferation index measured in human PitNET tissue samples (1). In addition, cell division and pituitary cell migration were also found to be inhibited by aspirin. However, the exact mechanism needs to be further investigated. It could be hypothesized that this occurred through Pttg1 regulated Aurka and Aurkb downregulation (see details below), because Aurka regulates cilia disassembly, neurite extension, and cell motility, while Aurkb has also been described to reduce the cellular migration of tumor cells (24, 25).
When analyzing transcription factor regulatory relationships, Tp53 and E2f1 showed the most significant alteration in activity. Indeed, the transcription factor E2f1, is a key cell cycle regulator and it targets genes encoding proteins that regulate cell cycle progression through the G1/S transition (26). Therefore, its reduced activity upon aspirin treatment was further confirmed by our in vitro functional results. While the expression of Tp53 did not change significantly upon aspirin administration, the change in its activity was experienced by the transcriptional outcome, as genes regulated by Tp53 showed significant enrichment upon aspirin treatment. In the background we detected increased Tp53 acetylation at the lysine residue at 382 position, which was previously shown to stabilize Tp53 protein and increase its transcriptional activity (27) (Fig. 6). Furthermore, Tp53 expression showed positive correlation with the 5hmC level. Since 5hmC is an indicator of decreased proliferation, our findings recapitulated previous data that aspirin acetylated and thereby activated Tp53 and induced Cdkn1a (p21) in colon tumors (28). Additionally, in PitNET cells, ASA also led to elevated Cdkn1a expression, reflecting the increased Tp53 activity.
Since Pttg1 is a well-known oncogene in pituitary tumorigenesis, overexpressed in approximately 90% of pituitary adenomas (29), and it is a member of Tp53 interaction partners, we further investigated its expression and activity following aspirin treatment. Aspirin effectively decreased Pttg1 expression in pituitary adenoma on both the RNA and protein levels, demonstrated by next-generation sequencing, qPCR, and Western blot analysis. Additionally, members of the Pttg1 regulatory network also showed decreased expression indicating decreased Pttg1 activity. Further, upon aspirin treatment of pituitary adenoma cells, a significant negative correlation was observed between Pttg1 and 5hmC, indicating the link between them. Moreover, a high proportion (20.15%) of genes being common between aspirin-regulated genes and dysregulated genes in human pituitary adenomas further indicates that aspirin may have a beneficial effect in this tumor type. These results are in line with previously reported findings, demonstrating that PTTG1 interacted with p53, and in cooperation with PTTG1 binding factor (PBF), it reduced p53 protein stability (30). Altogether, p53 and PTTG1 showed negative correlation, and head and neck squamous cell carcinoma patients with high tumoral PTTG1 had the poorest overall survival, which reflected a marked impairment of p53-dependent signaling (30).
In summary, aspirin has antitumoral effects on multiple levels in pituitary adenoma, summarized in Fig. 6. However, additional links can also be revealed from transcription alterations. For instance, the Tp53 downstream target Gadd45α, which is upregulated upon ASA treatment, not only inhibits proliferation through its interaction with Pcna and regulating DNA repair, but also induces global DNA hypomethylation (31). Aurkb, the mitosis regulator, is induced by Pttg1 and E2f1. Our findings on rat pituitary tumor cells (negative correlation of 5hmC with Ki-67) were in line with previous results described in human PitNET specimens (1); therefore, we can hypothesize that the modulation of 5hmC by ASA or decitabine and associated inhibition of Pttg1 may have antiproliferative effect on human PitNET as well. Nevertheless, our results should be validated in human PitNET samples as well, in order to avoid potential interspecies differences.
Conclusion
Aspirin exerted its antitumoral effects, among others, on genomic and epigenomic levels in pituitary cells. An aspirin-demethylation-Pttg1 regulatory network was described as an antitumoral mechanism in pituitary. The previously identified biomarker 5hmC, correlating with Ki-67 and differentiation of PitNET, also indicated the antitumoral effect of aspirin. In addition, the extensive overlap between the human pituitary transcriptome changes and aspirin-regulated gene signature further suggests the potential beneficial effect of aspirin in PitNET; however, further studies are necessary to strengthen this finding.
Abbreviations
- 5hmC
5-hydroxymethylcytosine
- 5mC
5-methylcytosine
- ASA
aspirin (acetylsalicylic acid)
- COX
cyclooxygenase
- FC
fold change
- FDR
false discovery rate
- FSH
follicle-stimulating hormone
- GH
growth hormone
- HPLC-MS/MS
high-performance liquid chromatography–tandem mass spectrometry
- LH
luteinizing hormone
- PBS
phosphate-buffered saline
- PitNET
pituitary neuroendocrine tumors
- SF-1
steroidogenic factor-1
Contributor Information
Borbála Szabó, Department of Laboratory Medicine, Semmelweis University, H-1089 Budapest, Hungary; Hereditary Tumours Research Group, Hungarian Academy of Sciences—Semmelweis University, H-1089 Budapest, Hungary.
Kinga Németh, Hereditary Tumours Research Group, Hungarian Academy of Sciences—Semmelweis University, H-1089 Budapest, Hungary.
Katalin Mészáros, Hereditary Tumours Research Group, Hungarian Academy of Sciences—Semmelweis University, H-1089 Budapest, Hungary.
Lilla Krokker, Department of Laboratory Medicine, Semmelweis University, H-1089 Budapest, Hungary; Hereditary Tumours Research Group, Hungarian Academy of Sciences—Semmelweis University, H-1089 Budapest, Hungary.
István Likó, Hereditary Tumours Research Group, Hungarian Academy of Sciences—Semmelweis University, H-1089 Budapest, Hungary.
Éva Saskői, Department of Molecular Genetics and the National Tumor Biology Laboratory, National Institute of Oncology, H-1122 Budapest, Hungary.
Krisztina Németh, MS Metabolomics Research Group, Centre for Structural Study, Research Centre for Natural Sciences, Eötvös Loránd Research Network, H-1117 Budapest, Hungary.
Pál Tamás Szabó, MS Metabolomics Research Group, Centre for Structural Study, Research Centre for Natural Sciences, Eötvös Loránd Research Network, H-1117 Budapest, Hungary.
Nikolette Szücs, Department of Endocrinology, Internal Medicine and Oncology, Faculty of Medicine, Semmelweis University, H-1083 Budapest, Hungary.
Sándor Czirják, National Institute of Clinical Neurosciences, H-1145 Budapest, Hungary.
Gábor Szalóki, Department of Pathology and Experimental Cancer Research, Faculty of Medicine, Semmelweis University, H-1085 Budapest, Hungary.
Attila Patócs, Department of Laboratory Medicine, Semmelweis University, H-1089 Budapest, Hungary; Hereditary Tumours Research Group, Hungarian Academy of Sciences—Semmelweis University, H-1089 Budapest, Hungary; Department of Molecular Genetics and the National Tumor Biology Laboratory, National Institute of Oncology, H-1122 Budapest, Hungary.
Henriett Butz, Department of Laboratory Medicine, Semmelweis University, H-1089 Budapest, Hungary; Hereditary Tumours Research Group, Hungarian Academy of Sciences—Semmelweis University, H-1089 Budapest, Hungary; Department of Molecular Genetics and the National Tumor Biology Laboratory, National Institute of Oncology, H-1122 Budapest, Hungary.
Funding
This work was supported by a Hungarian Scientific Research Grant of the National Research, Development and Innovation Office NKFI FK 135065 to H.B. H.B. is a recipient of Bolyai Research Fellowship of the Hungarian Academy of Sciences. H.B. and A.P. acknowledge financial support from the National Laboratories Excellence program (under the National Tumor Biology Laboratory proiect (NLP17)) and the Hungarian Thematic Excellence Programme (TKP2021-EGA-44).
The authors would like to express their acknowledge to the National Program of Bionics (the subtheme medical bioinics led by A.P.). The research was supported by the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the Molecular Biology thematic programme of the Semmelweis University to A.P.
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Szabó B, Németh K, Mészáros K, et al. Demethylation Status of somatic DNA extracted from pituitary neuroendocrine tumors indicates proliferative behavior. J Clin Endocrinol Metab. 2020;105(6):2015–2026. [DOI] [PubMed] [Google Scholar]
- 2. Patrono C, Rocca B. Aspirin and other COX-1 inhibitors. Handb Exp Pharmacol. 2012;210:137–164. [DOI] [PubMed] [Google Scholar]
- 3. Ma J, Cai Z, Wei H, Liu X, Zhao Q, Zhang T. The anti-tumor effect of aspirin: what we know and what we expect. Biomed Pharmacother. 2017;95:656–661. [DOI] [PubMed] [Google Scholar]
- 4. Hua H, Zhang H, Kong Q, Wang J, Jiang Y. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med Res Rev. 2019;39(1):114–145. [DOI] [PubMed] [Google Scholar]
- 5. Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: an updated quantitative review to 2005. Cancer Causes Control. 2006;17(7):871–888. [DOI] [PubMed] [Google Scholar]
- 6. Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–507. [DOI] [PubMed] [Google Scholar]
- 7. Dovizio M, Tacconelli S, Sostres C, Ricciotti E, Patrignani P. Mechanistic and pharmacological issues of aspirin as an anticancer agent. Pharmaceuticals. 2012;5(12):1346–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan MNA, Lee YS. Cyclooxygenase inhibitors: scope of their use and development in cancer chemotherapy. Med Res Rev. 2011;31(2):161–201. [DOI] [PubMed] [Google Scholar]
- 9. Zhang X, Du R, Luo N, Xiang R, Shen W. Aspirin mediates histone methylation that inhibits inflammation-related stemness gene expression to diminish cancer stemness via COX-independent manner. Stem Cell Res Ther. 2020;11(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qadri SSA, Wang JH, Redmond KC, O’Donnell AF, Aherne T, Redmond HP. The role of COX-2 inhibitors in lung cancer. Ann Thorac Surg. 2002;74(5):1648–1652. [DOI] [PubMed] [Google Scholar]
- 11. Alfonso L, Ai G, Spitale RC, Bhat GJ. Molecular targets of aspirin and cancer prevention. Br J Cancer. 2014;111(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Z, Li W, Qiu F, et al. Aspirin cooperates with p300 to activate the acetylation of H3K9 and promote FasL-mediated apoptosis of cancer stem-like cells in colorectal cancer. Theranostics. 2018;8(16):4447–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yiannakopoulou E. Targeting epigenetic mechanisms and microRNAs by aspirin and other non steroidal anti-inflammatory agents—implications for cancer treatment and chemoprevention. Cell Oncol. 2014;37(3):167–178. [DOI] [PubMed] [Google Scholar]
- 14. Yiannakopoulou E. Modulation of lymphangiogenesis: a new target for aspirin and other nonsteroidal anti-inflammatory agents? A systematic review. J Clin Pharmacol. 2012;52(11):1749–1754. [DOI] [PubMed] [Google Scholar]
- 15. Dovizio M, Bruno A, Tacconelli S, Patrignani P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res. 2013;191:39–65. [DOI] [PubMed] [Google Scholar]
- 16. Guo Y, Liu Y, Zhang C, et al. The epigenetic effects of aspirin: the modification of histone H3 lysine 27 acetylation in the prevention of colon carcinogenesis in azoxymethane- and dextran sulfate sodium-treated CF-1 mice. Carcinogenesis. 2016;37(6):616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Németh K, Szücs N, Czirják S, et al. Survivin as a potential therapeutic target of acetylsalicylic acid in pituitary adenomas. Oncotarget. 2018;9(49):29180–29192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris DG, Musat M, Czirják S, Hanzély Z, et al. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur J Endocrinol. 2005;153(1):143–151. doi: 10.1530/eje.1.01937. [DOI] [PubMed] [Google Scholar]
- 19. Michaelis KA, Knox AJ, Xu M, Kiseljak Vassiliades, et al. Identification of growth arrest and DNA-damage-inducible gene beta (GADD45beta) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology. 2011;152(10):3603–3613. doi: 10.1210/en.2011-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moreno CS, Evans CO, Zhan X, Okor M, et al. Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res. 2005;65(22):10214–10222. doi: 10.1158/0008-5472.CAN-05-0884. [DOI] [PubMed] [Google Scholar]
- 21. Elston MS, Gill AJ, Conaglen JV, Clarkson A, et al. Wnt pathway inhibitors are strongly down-regulated in pituitary tumors. Endocrinology. 2008;149(3):1235–1242. doi: 10.1210/en.2007-0542. [DOI] [PubMed] [Google Scholar]
- 22. Feng* et al. 2015: Shi C, Ye Z, Han J, Ye X, et al. BRD4 as a therapeutic target for nonfunctioning and growth hormone pituitary adenoma. Neuro Oncol. 2020;22(8):1114–1125. doi: 10.1093/neuonc/noaa084. (*: Feng J was indicated as contact and contributor: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE51618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhan X, Desiderio DM. Signaling pathway networks mined from human pituitary adenoma proteomics data. BMC Med Genomics. 2010;3:13. doi: 10.1186/1755-8794-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Németh K, Mészáros K, Szabó B, Butz H, Arányi T, Szabó PT. A relative quantitation method for measuring DNA methylation and hydroxymethylation using guanine as an internal standard. Anal Methods. 2021;13(39):4614–4622. [DOI] [PubMed] [Google Scholar]
- 25. Butz H, Szabó PM, Nofech-Mozes R, et al. Integrative bioinformatics analysis reveals new prognostic biomarkers of clear cell renal cell carcinoma. Clin Chem. 2014;60(10):1314–1326. [DOI] [PubMed] [Google Scholar]
- 26. Butz H, Szabó PM, Khella HWZ, Nofech-Mozes R, Patocs A, Yousef GM. miRNA-target network reveals miR-124as a key miRNA contributing to clear cell renal cell carcinoma aggressive behaviour by targeting CAV1 and FLOT1. Oncotarget. 2015;6(14):12543–12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Butz H, Likó I, Czirják S, et al. Down-Regulation of Wee1 kinase by a specific subset of microRNA in human sporadic pituitary adenomas. J Clin Endocrinol Metab. 2010;95(10):E181–E191. [DOI] [PubMed] [Google Scholar]
- 28. Butz H, Németh K, Czenke D, et al. Systematic investigation of expression of G2/M transition genes reveals CDC25 alteration in nonfunctioning pituitary adenomas. Pathol Oncol Res. 2017;23(3):633–641. [DOI] [PubMed] [Google Scholar]
- 29. Guo H, Liu J, Ben Q, et al. The aspirin-induced long non-coding RNA OLA1P2 blocks phosphorylated STAT3 homodimer formation. Genome Biol. 2016;17:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertolin G, Tramier M. Insights into the non-mitotic functions of Aurora kinase A: more than just cell division. Cell Mol Life Sci. 2020;77(6):1031–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Porcelli L, Guida G, Quatrale AE, et al. Aurora kinase B inhibition reduces the proliferation of metastatic melanoma cells and enhances the response to chemotherapy. J Transl Med. 2015;13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sheldon LA. Inhibition of E2F1 activity and cell cycle progression by arsenic via retinoblastoma protein. Cell Cycle (Georgetown, Tex.) 2017;16(21):2058–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reed SM, Quelle DE. P53 acetylation: regulation and consequences. Cancers (Basel). 2014;7(1):30–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ai G, Dachineni R, Kumar DR, Marimuthu S, Alfonso LF, Bhat GJ. Aspirin acetylates wild type and mutant p53 in colon cancer cells: identification of aspirin acetylated sites on recombinant p53. Tumour Biol. 2016;37(5):6007–6016. [DOI] [PubMed] [Google Scholar]
- 35. Tfelt-Hansen J, Kanuparthi D, Chattopadhyay N. The emerging role of pituitary tumor transforming gene in tumorigenesis. Clin Med Res. 2006;4(2):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Read ML, Modasia B, Fletcher A, et al. PTTG And PBF functionally interact with p53 and predict overall survival in head and neck cancer. Cancer Res. 2018;78(20):5863–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamura RE, de Vasconcellos JF, Sarkar D, Libermann TA, Fisher PB, Zerbini LF. GADD45 Proteins: central players in tumorigenesis. Curr Mol Med. 2012;12(5):634–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.