Objectives:
Timing of tracheostomy in patients with COVID-19 has attracted substantial attention. Initial guidelines recommended delaying or avoiding tracheostomy due to the potential for particle aerosolization and theoretical risk to providers. However, early tracheostomy could improve patient outcomes and alleviate resource shortages. This study compares outcomes in a diverse population of hospitalized COVID-19 patients who underwent tracheostomy either “early” (within 14 d of intubation) or “late” (more than 14 d after intubation).
Design:
International multi-institute retrospective cohort study.
Setting:
Thirteen hospitals in Bolivia, Brazil, Spain, and the United States.
Patients:
Hospitalized patients with COVID-19 undergoing early or late tracheostomy between March 1, 2020, and March 31, 2021.
Interventions:
Not applicable.
Measurements and Main Results:
A total of 549 patients from 13 hospitals in four countries were included in the final analysis. Multivariable regression analysis showed that early tracheostomy was associated with a 12-day decrease in time on mechanical ventilation (95% CI, −16 to −8; p < 0.001). Further, ICU and hospital lengths of stay in patients undergoing early tracheostomy were 15 days (95% CI, −23 to −9 d; p < 0.001) and 22 days (95% CI, −31 to −12 d) shorter, respectively. In contrast, early tracheostomy patients experienced lower risk-adjusted survival at 30-day post-admission (hazard ratio, 3.0; 95% CI, 1.8−5.2). Differences in 90-day post-admission survival were not identified.
Conclusions:
COVID-19 patients undergoing tracheostomy within 14 days of intubation have reduced ventilator dependence as well as reduced lengths of stay. However, early tracheostomy patients experienced lower 30-day survival. Future efforts should identify patients most likely to benefit from early tracheostomy while accounting for location-specific capacity.
Keywords: airway management, length of stay, mechanical ventilators, pandemics, survival
KEY POINTS.
Question: Do outcomes differ between COVID-19 patients undergoing tracheostomy within 14 days of intubation and those undergoing later tracheostomy?
Findings: In this retrospective cohort study of 549 patients from 13 hospitals in four countries, patients undergoing early tracheostomy spent 12 fewer days on mechanical ventilation and 15 fewer days in the ICU. However, early tracheostomy patients experienced worse 30-day survival in multivariable analysis.
Meanings: Early tracheostomy in COVID-19 patients reduces ventilator dependence and length of stay without improving short-term survival; intensivists should identify patients most likely to benefit from early tracheostomy while accounting for location-specific capacity.
Tracheostomy timing attracted substantial attention throughout the COVID-19 pandemic. Between 8% and 20% of patients admitted to the hospital for symptomatic COVID-19 required intubation, with many requiring prolonged mechanical ventilation (1–5). Data from before the pandemic indicates that early tracheostomy may reduce ventilator dependence (6) and sedation use (7), decrease ICU length of stay (LOS) (8), improve survival (9), and be cost-effective (10, 11).
In the context of the COVID-19 pandemic, however, potential benefits of early tracheostomy must be weighed against theoretical risk to providers. Severe acute respiratory syndrome coronavirus-2, responsible for COVID-19, is transmitted through aerosol inhalation or mucous membrane contact (12). Since invasive airway procedures could increase exposure risk (12, 13), initial COVID-19 guidelines recommended delaying or avoiding tracheostomy to reduce risk to healthcare providers (14–17).
Nonetheless, many providers safely performed tracheostomies in COVID-19 patients (18–20). Studies in COVID-19 patients suggested that early tracheostomy reduces ventilator dependence (21–25) and ICU LOS (20, 24, 26); however, they also demonstrated inferior (27), superior (28), and equivalent survival (21, 23, 29). Additionally, this research is largely based in the United States and Europe, though South America had the third greatest number of COVID-19 cases relative to population (30).
We therefore evaluated outcomes after tracheostomy in a diverse patient population from Europe, South America, and North America. We examined ventilator dependence, LOS, and survival in hospitalized COVID-19 patients undergoing tracheostomy within 14 days of intubation (“early”) compared with those who underwent “late” tracheostomy.
MATERIALS AND METHODS
Patient data were collected from 10 hospitals in the United States and three centers in Bolivia, Brazil, and Spain. Data were gathered via chart review and recorded in Research Electronic Data Capture (31). The Emory University institutional review board (IRB) approved the study entitled “Outcomes after tracheostomy in COVID-19 patients” (Study Number 00001633) on November 10, 2020. Due to the retrospective nature of the study, informed consent was waived. Study protocol was provided to collaborators, and individual IRBs approved the study internally. Procedures were followed in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Helsinki Declaration of 1975.
Hospitalized adult patients with COVID-19 who underwent tracheostomy between March 1, 2020, and March 31, 2021, were eligible for inclusion. Data were collected up to 90 days posttracheostomy. Inclusion criteria were age greater than or equal to 18 years, diagnosis of active COVID-19 infection, admission for inpatient services, need for invasive mechanical ventilation, and receipt of tracheostomy during the index admission. Patients who underwent tracheostomy prior to COVID-19 diagnosis and those diagnosed during a previous admission were excluded.
Early tracheostomy was defined as tracheostomy within 14 days of intubation, inclusive of the 14th day. Late tracheostomy was defined as tracheostomy performed more than 14 days after intubation. Primary outcomes assessed were number of ventilator-dependent days (VDDs), hospital LOS, and ICU LOS. Secondary outcomes assessed were 30- and 90-day survivals, with day 1 designated as the first day of admission to the hospital. VDDs were determined as the number of days between intubation and the first complete 24-hour period without mechanical ventilation. Reventilation of patients after the first 24-hour ventilation-free period was not captured. Patients who died while still on mechanical ventilation were excluded from VDD analysis. Those who died while still in the hospital or ICU were excluded from the respective LOS analyses. Additional details of data collection and statistical analyses performed are presented in the Supplemental Methods (http://links.lww.com/CCX/B88).
RESULTS
Patient Population
We included 549 hospitalized COVID-19 patients who underwent tracheostomy (Table 1). Most patients were male (63%) and younger than 65 years (59%). Tracheostomy timing differed by country (p < 0.001), with early tracheostomies accounting for 18% (n = 38/206) of tracheostomies in Brazil, 24% (n = 72/302) in the United States, 25% (n = 2/8) in Spain, and 61% (n = 20/33) in Bolivia.
Table 1.
Demographic, Anthropometric, and Clinical Characteristics of COVID-19 Patients Undergoing Early or Late Tracheostomy
| Variable | Overalla (n = 549) | Earlya (n = 132) | Latea (n = 417) | p b | |
|---|---|---|---|---|---|
| Country | United States | 302 (55%) | 72 (55%) | 230 (55%) | < 0.001 |
| Spain | 8 (2%) | 2 (2%) | 6 (1%) | ||
| Bolivia | 33 (6%) | 20 (15%) | 13 (3%) | ||
| Brazil | 206 (38%) | 38 (29%) | 168 (40%) | ||
| Sex | Female | 204 (37%) | 51 (39%) | 153 (37%) | 0.687 |
| Male | 345 (63%) | 81 (61%) | 264 (63%) | ||
| Age | <65 yr | 326 (59%) | 87 (66%) | 239 (57%) | 0.080 |
| 65+ Years | 223 (41%) | 45 (34%) | 178 (43%) | ||
| Ethnicity or race | Hispanic or Latino | 61(19%) | 18 (22%) | 43 (18%) | 0.339 |
| Black or African American | 134 (42%) | 35 (43%) | 99 (42%) | ||
| White | 101 (32%) | 26 (32%) | 75 (32%) | ||
| Other | 21 (6.6%) | 2 (2.5%) | 19(8.1%) | ||
| Missing | 232 | 51 | 181 | ||
| Chronic respiratory disease | 140 (26%) | 29 (22%) | 111 (27%) | 0.286 | |
| Heart disease | 106 (19%) | 18 (14%) | 88 (21%) | 0.058 | |
| Chronic kidney disease | 75 (14%) | 18 (14%) | 57 (14%) | 0.992 | |
| Cancer | 61 (11%) | 17 (13%) | 44 (11%) | 0.458 | |
| Obesity | 159 (50%) | 34 (39%) | 125 (54%) | 0.015 | |
| Missing | 229 | 44 | 185 | ||
| Number of Comorbidities | 1–2 | 336 (61%) | 86 (65%) | 250 (60%) | 0.285 |
| 3+ | 213 (39%) | 46 (35%) | 167 (40%) | ||
| Sequential Organ Failure Assessment Scorec | Median (IQR) | 9 (7, 10) | 9 (7, 11) | 9 (7, 10) | 0.471 |
| Missing | 339 | 74 | 265 | ||
| Simplified Acute Physiology Score 3c | Median (IQR) | 69 (57, 78) | 70 (60, 79) | 69 (57, 78) | 0.806 |
| Missing | 343 | 94 | 249 | ||
| Pao2:Fio2 ratioc | < 100 | 37 (8%) | 15 (14%) | 22 (6%) | 0.057 |
| 100–199 | 205 (45%) | 44 (41%) | 161 (46%) | ||
| 200–299 | 125 (27%) | 31 (29%) | 94 (27%) | ||
| 300+ | 89 (20%) | 17 (16%) | 72 (21%) | ||
| Missing | 93 | 25 | 68 | ||
| Vasopressorsd | None | 181 (53%) | 47 (51%) | 134 (54%) | 0.541 |
| Any | 159 (47%) | 46 (49%) | 113 (46%) | ||
| Missing | 209 | 39 | 170 | ||
IQR = interquartile range.
aData are shown as n (%) or median (IQR).
bPearson χ2 or Fisher exact test, or Wilcoxon rank-sum test.
cClosest available calculation within 24 hr prior to tracheostomy.
dUse or not of any vasopressor within 24 hr prior to tracheostomy.
Bivariate analysis of demographic, anthropometric, and clinical characteristics of COVID-19 patients undergoing tracheostomy within 14 d of intubation (“early”) vs “late” tracheostomy. Country of treatment and obesity frequency differ by tracheostomy timing (p < 0.050). Percents may not sum to 100 due to rounding.
Critical illness severity within 1 day pretracheostomy was defined by the Sequential Organ Failure Assessment (SOFA) score (32) or Simplified Acute Physiology Score III (SAPS3) (33). No statistically significant difference in SAPS3, SOFA scores, or vasopressor use was observed by timing (Table 1).
Procedural Factors
Most (73%) tracheostomies were performed earlier in the pandemic (prior to October 2020; Table 2). Early tracheostomies accounted for 20% (n = 80/397) of those performed prior to October 2020, increasing to 34% (n = 52/152) after October 2020 (p = 0.001). Use of percutaneous versus open technique did not differ between cohorts (p = 0.289; Table 2). Early tracheostomies were more frequently performed outside the ICU (early: 16% vs late: 25%; p = 0.005).
Table 2.
Tracheostomy Procedural Details for COVID-19 Patients by Tracheostomy Timing
| Variable | Overall (n = 549)a | Early (n = 132)a | Late (n = 417)a | p b | |
|---|---|---|---|---|---|
| Pandemic timeline | March–June 2020 | 234 (43%) | 39 (30%) | 195 (47%) | < 0.001 |
| July–September 2020 | 163 (30%) | 41 (31%) | 122 (29%) | ||
| October–December 2020 | 48 (9%) | 12 (9%) | 36 (9%) | ||
| January–March 2021 | 104 (19%) | 40 (30%) | 64 (15%) | ||
| Location | ICU | 449 (82%) | 98 (75%) | 351 (84%) | 0.005 |
| Operating room | 78 (14%) | 23 (17%) | 55 (13%) | ||
| Otherc | 22 (4%) | 11 (8%) | 11 (3%) | ||
| Technique | Percutaneous | 332 (61%) | 84 (65%) | 248 (60%) | 0.289 |
| Open | 211 (39%) | 45 (35%) | 166 (40%) | ||
| Missing | 6 | 3 | 3 | ||
| Bronchoscopy use | Bronchoscopy minimized | 222 (45%) | 43 (37%) | 179 (47%) | 0.052 |
| Standard bronchoscopy | 276 (55%) | 74 (63%) | 202 (53%) | ||
| Missing | 51 | 15 | 36 | ||
| Mechanical ventilation management | Ventilator pause | 405 (81%) | 85 (73%) | 320 (84%) | 0.006 |
| Standard ventilation | 93 (19%) | 32 (27%) | 61 (16%) | ||
| Missing | 51 | 15 | 36 | ||
| Electrocautery use | Electrocautery minimized | 328 (66%) | 66 (56%) | 262 (69%) | 0.014 |
| Standard electrocautery | 170 (34%) | 51 (44%) | 119 (31%) | ||
| Missing | 51 | 15 | 36 | ||
| Patient-provider barriers | Additional barriers used | 81 (16%) | 22 (19%) | 59 (15%) | 0.395 |
| Standard barriers | 417 (84%) | 95 (81%) | 322 (85%) | ||
| Missing | 51 | 15 | 36 | ||
| Aerosol-reduction techniques | Aerosol reduction used | 439 (88%) | 97 (83%) | 342 (90%) | 0.045 |
| Standard methods used | 59 (12%) | 20 (17%) | 39 (10%) | ||
| Missing | 51 | 15 | 36 | ||
| Periprocedural complications | None | 481 (92%) | 116 (93%) | 365 (91%) | 0.639 |
| ≥ 1 | 43 (8.2%) | 9 (7.2%) | 34 (8.5%) | ||
| Missing | 25 | 7 | 18 | ||
| 30-D airway-related complications | ≥ 1 | 21 (8%) | 9 (11%) | 12 (6%) | 0.130 |
| Missing | 272 | 53 | 219 | ||
| 30-d non-airway complications | ≥ 1 | 179 (65%) | 45 (57%) | 134 (68%) | 0.092 |
| Missing | 272 | 53 | 219 | ||
aData are shown as n (%) or median (range).
bPearson χ2 or Fisher exact test.
cTracheostomy performed in a location other than the ICU or operating room.
Bivariate analysis of tracheostomy procedural details for COVID-19 patients undergoing tracheostomy within 14 d of intubation (“early”) vs “late” tracheostomy. Date of procedure, procedure location, and use of aerosol reduction methods, including pausing mechanical ventilation and minimizing electrocautery, differed according to tracheostomy timing (p < 0.050).
Special methods to reduce aerosolization included temporarily discontinuing mechanical ventilation (81%, n = 405/499), use of additional barriers (16%, n = 81/499), minimizing electrocautery (66%, n = 328/499), and minimizing bronchoscopy (45%, n = 222/499). Use of aerosol-minimizing methods was more common in the late tracheostomy cohort (early: 83% vs late: 90%; p = 0.045; Table 2).
Ventilator-Dependent Days
Fifty-five percent of patients (n = 300/549) were liberated from mechanical ventilation. In bivariate analysis, early tracheostomy patients spent approximately 14 fewer days on mechanical ventilation (median of 18 d [interquartile range {IQR}, 14–31] vs 32 d [IQR, 26–40]; p < 0.001). In multivariable analysis, early tracheostomy remained significantly associated with fewer VDD (–11.8 d; 95% CI, –15.6 to –8.0; p < 0.001; Table 3). Additional factors associated with reduced VDD included tracheostomy performed outside the United States (Spain: –28.8 d; 95% CI, –53.4 to –4.1; p = 0.020; Bolivia: –11.6 d; 95% CI, –18.3 to –5.0; p < 0.001; and Brazil: –17.5 d; 95% CI, –22.6 to –12.4; p < 0.001) and use of aerosol-reducing methods (–6.4 d; 95% CI, –12.4 to –0.5; p = 0.035; Table 3).
Table 3.
Analyses of Ventilator Dependence, ICU Length of Stay, and Survival in COVID-19-Positive Patients Undergoing Tracheostomy
| Variable | Level | Ventilator-Dependent Daysa | ICU Length of Staya | 30-d Survivalb | |||
|---|---|---|---|---|---|---|---|
| p | p | p | |||||
| Timing (unadjusted)c | Early | 18 (14–31) | < 0.001 | 25 (19–41) | < 0.001 | 2.1 (1.3–3.3) | 0.002 |
| Late | 32 (26–40) | 38 (30–48) | Ref | ||||
| Timing (adjusted)d; Ref: late | Early | –11.8 (–15.6 to –8.0) | < 0.001 | –15.7 (–22.5 to –8.9) | < 0.001 | 3.0 (1.8–5.2) | < 0.001 |
| Aged; Ref: <65 | 65+ yr | 1.5 (–1.8 to 4.7) | 0.377 | –3.1 (–8.4 to 5.0) | 0.625 | 1.9 (1.1–3.0) | 0.014 |
| Countryd; Ref: United States | Spain | –28.8 (–53.4 to –4.1) | 0.020 | –9.7 (–43.8 to 24.4) | 0.577 | 2.7 (0.3–20.7) | 0.348 |
| Bolivia | –11.6 (–18.3 to –5.0) | < 0.001 | –3.9 (–14.5 to 7.6) | 0.508 | 2.7 (0.4–21.0) | 0.339 | |
| Brazil | –17.5 (–22.6 to –12.4) | < 0.001 | –4.8 (–11.5 to 4.2) | 0.297 | 12.6 (2.9–54.6) | 0.001 | |
| Comorbiditiesd; Ref: <3 | 3+ | –3.2 (–6.7 to 0.3) | 0.073 | –4.6 (–10.5 to 1.4) | 0.132 | 1.6 (1.0–2.6) | 0.065 |
| Pretracheostomy Pao2:Fio2 ratiod; Ref: 300+ | < 100 | 12.0 (3.1–20.9) | 0.008 | 1.1 (–14.5 to 16.7) | 0.894 | 4.6 (1.7–12.5) | 0.003 |
| 100–199 | 4.3 (0.1–8.4) | 0.043 | 0.4 (–6.9 to 7.7) | 0.919 | 2.2 (0.9–5.2) | 0.088 | |
| 200–299 | –0.1 (–4.4 to 4.2) | 0.962 | –1.3 (–9.1 to 6.5) | 0.173 | 2.6 (1.0–6.7) | 0.047 | |
| Pandemic timelined; Ref: March–June 2020 | Jul–Sep 2020 | –2.1 (–6.1 to 1.9) | 0.298 | 2.2 (–5.2 to 9.6) | 0.559 | 0.4 (0.2–0.8) | 0.008 |
| Oct–Dec 2020 | –11.0 (–19.1 to –2.8) | 0.008 | 1.6 (–9.7 to 13.0) | 0.779 | 1.6 (0.2–11.6) | 0.656 | |
| Jan–Mar 2021 | 1.8 (–5.1 to 8.6) | 0.613 | 7.1 (–3.1 to 17.4) | 0.172 | 3.4 (0.7–17.5) | 0.142 | |
| Procedure locationd; Ref: ICU | Operating room | 0.6 (–6.1 to 7.2) | 0.865 | 4.2 (–6.3 to 14.7) | 0.265 | 0.4 (0.04–3.1) | 0.354 |
| Other | –5.6 (–23.4 to 12.2) | 0.536 | 31.1 (10.6–51.7) | < 0.001 | 36.2 (3.5–374.6) | 0.003 | |
| Techniqued; Ref: percutaneous | Open | 2.0 (–1.9 to 5.9) | 0.312 | –1.9 (–9.8 to 6.1) | 0.650 | 1.0 (0.6–1.7) | 0.904 |
| Aerosol reductiond; Ref: standard | Additional methods used | –6.4 (–12.4 to –0.5) | 0.035 | –7.1 (–16.6 to 2.4) | 0.141 | < 0.1 (0.0–0.4) | 0.012 |
Ref = reference.
aDifferences in ventilator-dependent days and ICU length of stay by tracheostomy timing were analyzed by either Student t test (bivariate) or linear regression (multivariable).
bDifferences in 30-d survival by tracheostomy timing were analyzed using Cox proportional hazards modeling for both bivariate and multivariable analyses; data are shown as hazard ratio (95% CI).
cBivariate comparison between early and late tracheostomy cohorts for ventilator-dependent days and ICU length of stay shown as days (interquartile range); unadjusted Cox proportional hazard modeling for 30-d survival shown as hazard ratio (95% CI).
dResults from multivariable analyses adjusting for age, country of origin, number of comorbidities, pretracheostomy Pao2:Fio2 ratio, pandemic timeline, technique, and use of aerosol-reducing methods shown as estimate (95% CI).
Analyses of ventilator dependence, ICU length of stay, and survival in COVID-19 positive patients undergoing tracheostomy within 14 d of intubation (“early”) compared with “late” tracheostomy. Early tracheostomy patients demonstrate reduced ventilator dependence and experience shorter lengths of stay, but they also demonstrate worse 30-d survival.
ICU Length of Stay
Most patients were discharged from the ICU alive (n = 380/549, 69%). In bivariate analysis, early versus late tracheostomy patients spent a median of 25 days (IQR, 19–41) in the ICU compared with 38 days (IQR, 30–48; p < 0.001), respectively. In multivariable analysis, early tracheostomy was associated with 15.7 fewer days in the ICU (95% CI, –22.5 to –8.9; p < 0.001). Among other covariates, only tracheostomies performed in a location other than the ICU or operating room were significantly associated with a longer ICU LOS (31.1 d; 95% CI, 10.6–51.7; p < 0.001) (Table 3).
Hospital Length of Stay
Sixty-six percent of patients (n = 365/549) were discharged from the hospital alive. Early tracheostomy patients spent less time in the hospital (42 d [IQR, 28–54 d] vs 53 d [IQR, 36–70 d]; p < 0.001). In multivariable analysis, early tracheostomy was associated with 21 fewer days in the hospital (95% CI, –31 to –12; p < 0.001; Supplemental Table 1, http://links.lww.com/CCX/B88).
Survival
A total of 130 patients (25%) died within 30 days of hospital admission, whereas 229 (55%) died within 90 days of hospital admission. The proportion of inpatient death did not differ between cohorts (early: 88% [n = 42/48] vs late: 91% [n = 164/181]; p = 0.723). Cause of death was available for 39% of patients who died (n = 89/229) and not differ by tracheostomy timing (Supplemental Table 2, http://links.lww.com/CCX/B88).
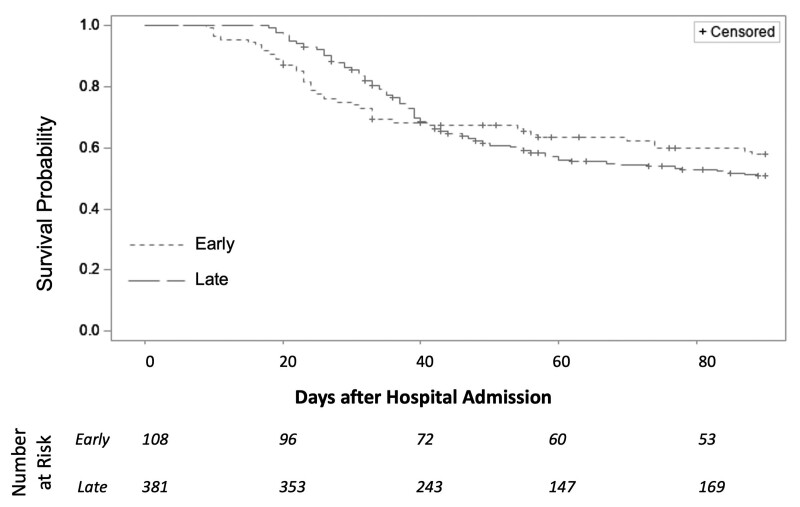
Survival at 30-day posthospital admission was lower among patients who underwent early tracheostomy in unadjusted analysis (hazard ratio [HR], 2.1; 95% CI, 1.3–3.3; p = 0.002; Fig. 1). Survival at 90-day posthospital admission did not differ between the two cohorts (HR, 0.9; 95% CI, 0.7–1.3; p = 0.565); however, the data violated Cox proportional hazards assumptions in that timeframe.
Figure 1.
Survival in COVID-19 patients undergoing early or late tracheostomy. Kaplan-Meier curves of survival up to 90 d after hospital admission. Survival at 30 d post-hospital admission was significantly lower among patient who underwent early tracheostomy vs late tracheostomy in bivariable Cox Proportional Hazards analysis (hazard ratio [HR], 2.1; 95% CI, 1.3–3.3; p = 0.002). However, survival at 90 d post-hospital admission did not differ between tracheostomy cohorts (HR, 0.9; 95% CI, 0.7–1.3; p = 0.565).
A total of 344 patients were included in multivariable survival analysis due to missing observations for covariates. Early tracheostomy remained associated with worse survival (HR, 3.0; 95% CI, 1.8–5.2; p < 0.001; Table 3). Age greater than 65 (HR, 1.9; 95% CI, 1.1–3.0; p = 0.014), location in Brazil (HR, 12.6; 95% CI, 2.9–54.6; p < 0.001), and P:F ratio less than 100 (HR, 4.6; 95% CI, 1.7–12.5; p = 0.003) were also associated with worse survival.
Complications
Periprocedural complications, including significant desaturation, bleeding, and loss of airway, were reported but did not differ between early and late tracheostomy cohorts (p = 0.639; Table 2). Postprocedural airway-related complications occurring within 30 days after tracheostomy were reported for 8% of patients. Neither frequency of airway-related complications (early: 11% [9/79] vs late: 6% [12/198]; p = 0.130) nor frequency of non-airway-related events (early: 57% [45/79] vs late: 68% [134/198]; p = 0.092) differed by tracheostomy timing (Supplemental Table 3, http://links.lww.com/CCX/B88).
DISCUSSION
In this study of 549 hospitalized COVID-19 patients across 13 hospitals in four countries, patients who underwent tracheostomy within 14 days of intubation spent 12 fewer days on the mechanical ventilator, 15 fewer days in the ICU, and 22 fewer days in the hospital relative to those undergoing later tracheostomy. However, early tracheostomy patients experienced worse survival at 30-day postadmission. Tracheostomy technique was not associated with differences in ventilator dependence, LOS, or survival. In contrast, country, age, pretracheostomy P:F ratio, procedure location, and use of aerosol-reducing methods were significantly associated with outcome differences.
Initially, professional societies produced guidelines recommending delaying or avoiding tracheostomy in COVID-19 patients (14–17). Nonetheless, many providers continued to perform early tracheostomies. Our data suggest that performing early tracheostomies helps reduce ventilator utilization in COVID-19 patients. This is consistent with other studies that demonstrated reduced ventilator dependence in Spain (20), the United Kingdom (24), and the US (21–23, 25). Reduced ventilator dependence has been reported for various early tracheostomy timeframes, ranging from within 7 days (20, 28) to within 21-day postintubation (22). However, many prior studies demonstrated an effect only in unadjusted analyses, likely due to smaller study sizes (21, 22, 24, 25, 29). Our study strengthens the existing literature by confirming the association between tracheostomy timing and ventilator dependence when adjusting for other factors.
Consistent with existing literature, early tracheostomy patients had reduced ICU and hospital LOS. Early tracheostomy has been associated with up to 20 fewer days in the ICU (20, 24, 26) and up to 15 fewer days in the hospital (20, 23). The shorter ventilator dependence and LOS suggest that early tracheostomy may assist with resource management. Furthermore, reductions in ventilator use and LOS have other beneficial effects, such as decreased sedation and analgesia requirements. Early tracheostomy patients require less opiate medication relative to later tracheostomy patients (34) and may return to physical activity more quickly (35, 36).
Overall mortality postadmission was 25% at 30 days and 55% at 90 days, despite 66% of patients being alive at discharge. Early tracheostomy patients demonstrated worse survival at 30-day postadmission, although 90-day survival did not appear to differ. The literature is mixed regarding the relationship between tracheostomy timing and survival in COVID-19 patients (21, 23, 26, 29, 37–39). However, these findings should be understood with consideration that division of “early” versus “late” cohorts is arbitrary rather than biological, and that the definition of “early” can vary widely.
Early tracheostomy patients may have undergone intervention before the trajectory of disease was clear and thus been poorly selected. Though not reflected in the available data, early tracheostomy patients may have been less stable at the time of the procedure, whereas late tracheostomy patients had time to “declare” their illness. Additionally, fewer obese patients underwent early tracheostomy. Obesity, although associated with increased risk of severe COVID-19 (1, 40), may improve survival in critically ill COVID-19 populations (41, 42). Thus, greater obesity in the late tracheostomy cohort could contribute to improved survival. COVID-19 clearance data were not available for this study, but disease clearance could also affect survival. Future studies comparing early and late tracheostomy patients to intubated patients who never underwent tracheostomy and/or to non-COVID-19 patients could help evaluate these possibilities.
The higher mortality in Brazil is consistent with publicly available data (30). During the study timeline, 2.9% of confirmed COVID-19 cases resulted in death in Brazil, compared with 1.8% in the United States, 2.2% in Spain, and 4.0% in Bolivia. Differences in testing availability and reporting norms must be considered when interpreting these data. Peak test positivity in Bolivia was as high as 63% on July 18, 2020, compared with peaks of 43% in Spain (March 26, 2020) and 21% in the United States (April 6, 2020; Brazil testing data unavailable). Low- and middle-income countries, such as Brazil and Bolivia, have had disproportionately high case fatality rates (30). Thus, whether country-specific survival differences in our cohort are related to the tracheostomy procedure cannot be determined.
The results of this study should be considered hypothesis-generating considering several of the study’s limitations, most notably missing observations for laboratory values, anthropometrics, and race. Given the retrospective nature of the study and inclusion of data from diverse settings with a wide variety of record-keeping practices, the level of missing data is unsurprising though unfortunate. Although we enhanced our understanding of disease severity with multiple indicators, such as SAPS3, SOFA, P:F ratio, and vasopressor use, missing data pertaining to these indicators could confound the survival results identified. Additionally, reventilation—that is, a return to invasive mechanical ventilation in patients who previously achieved a minimum of 24 hours off ventilation—was not captured. Early tracheostomy patients could have achieved ventilator-free time earlier than late tracheostomy patients but required return to ventilation more frequently. This should be evaluated in future studies.
Race-related data were most complete for the U.S. population. Race as a cultural context is perceived and recorded differently in the United States relative to other countries, which may explain this discrepancy (43). The fact that Black and African American patients represented over 40% of the known racial makeup of the study population is important, given the growing body of evidence indicating potential disparities in COVID-19 prevalence and outcomes by race (44, 45).
Although the multinational nature of the study is a strength, cases from the United States and Brazil dominated the study. This distribution resembles the case distribution across these countries. During the study timeline, the United States accounted for 60% of the nearly 56 million confirmed cases in the four participating countries, whereas Brazil accounted for 32%, Spain 7%, and Bolivia 1% (30). The slight overrepresentation of Bolivia and Brazil relative to their COVID-19 case burden is a strength of the article. These countries are otherwise underrepresented in the literature on tracheostomies in COVID-19 patients. However, the fact that Brazilian data were collected from a single large institution whereas U.S. data were collected from 10 institutions may indicate that institution-specific practices and population impact the overall findings.
CONCLUSIONS
This study suggests that COVID-19 patients undergoing early tracheostomy have reduced ventilator dependence, ICU LOS, and hospital LOS, but may suffer increased 30-day mortality. Future research should seek to identify those patients most likely to benefit from early tracheostomy while accounting for location-specific factors and capacity.
ACKNOWLEDGMENTS
We thank for the assistance of our volunteer translators, including Daniela Farchi, Bahaa Kazzi, Eliana Lillivek, and Gustavo Moraes. We thank for the contributions to this article and the dedication to improving patient care during the pandemic demonstrated by the following members of the PTS-COVIDTrach Collaborative: Felix Fernandez, Seth Force, Wissam S. Jaber, Renee’ Moore, Manu Sancheti, Alejandro Sardi, Limeng Wan, Cindy L. Austin, Tracy Haertling, Hannah Kim, Jeffrey Coughenour, Jennifer Randolph, Juan Pablo Merida, and Keriann Van Nostrand.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by an Emory University School of Medicine “Imagine, Innovate, Inspire” Nexus Award and the Fogarty International Center of the National Institutes of Health (D43 TW009337). Use of Research Electronic Data Capture (REDCap) was supported by the Library Information Technology Services grant (UL1 TR000424) for REDCap.
Dr. Harrell Shreckengost discloses financial support from the Lifebox Foundation as well as a Fogarty Global Health Fellowship. Dr. Nguyen discloses financial support from Prytime, Biomet, and Teleflex (honoraria for educational lectures). The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Monteiro AC, Suri R, Emeruwa IO, et al. : Obesity and smoking as risk factors for invasive mechanical ventilation in COVID-19: A retrospective, observational cohort study. PLoS One 2020; 15:e0238552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potere N, Valeriani E, Candeloro M, et al. : Acute complications and mortality in hospitalized patients with coronavirus disease 2019: A systematic review and meta-analysis. Crit Care 2020; 24:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer AJ, Morley EJ, Meyers K, et al. : Cohort of four thousand four hundred four persons under investigation for COVID-19 in a New York hospital and predictors of ICU care and ventilation. Ann Emerg Med 2020; 76:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandon P, Leibner ES, Hackett A, et al. : The third wave: Comparing seasonal trends in COVID-19 patient data at a large hospital system in New York City. Crit Care Explor 2022; 4:e0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Alencar JCG, Sternlicht JM, Veiga ADM, et al. : Timing to intubation COVID-19 patients: Can we put it off until tomorrow? Healthc Basel Switz 2022; 10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dochi H, Nojima M, Matsumura M, et al. : Effect of early tracheostomy in mechanically ventilated patients. Laryngoscope Investig Otolaryngol 2019; 4:292–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bösel J, Schiller P, Hook Y, et al. : Stroke-related early tracheostomy versus prolonged orotracheal intubation in neurocritical care trial (SETPOINT). Stroke 2013; 44:21–28 [DOI] [PubMed] [Google Scholar]
- 8.Adly A, Youssef TA, El-Begermy MM, et al. : Timing of tracheostomy in patients with prolonged endotracheal intubation: A systematic review. Eur Arch Oto-Rhino-Laryngol 2018; 275:679–690 [DOI] [PubMed] [Google Scholar]
- 9.Scales DC, Thiruchelvam D, Kiss A, et al. : The effect of tracheostomy timing during critical illness on long-term survival. Crit Care Med 2008; 36:2547–2557 [DOI] [PubMed] [Google Scholar]
- 10.Herritt B, Chaudhuri D, Thavorn K, et al. : Early vs. late tracheostomy in intensive care settings: Impact on ICU and hospital costs. J Crit Care 2018; 44:285–288 [DOI] [PubMed] [Google Scholar]
- 11.Liu CC, Rudmik L: A cost-effectiveness analysis of early vs late tracheostomy. JAMA Otolaryngol 2016; 142:981–987 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention: Scientific Brief: SARS-CoV-2 Transmission. Atlanta, GA, Centers for Disease Control and Prevention, 2021 [PubMed] [Google Scholar]
- 13.World Health Organization: Prevention, Identification and Management of Health Worker Infection in the Context of COVID-19: Interim Guidance [Internet]. Geneva, Switzerland, World Health Organization, 2020 [Google Scholar]
- 14.Bertroche JT, Pipkorn P, Zolkind P, et al. : Negative-pressure aerosol cover for COVID-19 tracheostomy. JAMA Otolaryngol Head Neck Surg 2020; 146:672–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosling AF, Bose S, Gomez E, et al. : Perioperative considerations for tracheostomies in the era of COVID-19. Anesth Analg 2020; 131:378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb CR, Desai NR, Angel L, et al. : Use of tracheostomy during the COVID-19 pandemic: American College of Chest Physicians/American Association for Bronchology and Interventional Pulmonology/Association of Interventional Pulmonology program directors expert panel report. Chest 2020; 158:1499–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima DS, Ribeiro Junior MF, Vieira, et al. : Alternatives for establishing a surgical airway during the COVID-19 pandemic. Rev Col Bras Cir 2020; 47:e20202549. [DOI] [PubMed] [Google Scholar]
- 18.Niroula A, Van Nostrand KM, Khullar OV, et al. : Percutaneous tracheostomy with apnea during coronavirus disease 2019 era: A protocol and brief report of cases. Crit Care Explor 2020; 2:e0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angel L, Kon ZN, Chang SH, et al. : Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann Thorac Surg 2020; 110:1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez G, Ramos FJ, Añon JM, et al. : Early tracheostomy for managing ICU capacity during the COVID-19 outbreak: A propensity-matched cohort study. Chest 2022; 161:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmood K, Cheng GZ, Van Nostrand K, et al. : Tracheostomy for COVID-19 respiratory failure: Multidisciplinary, multicenter data on timing, technique, and outcomes. Ann Surg 2021; 274:234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao TN, Harbison SP, Braslow BM, et al. : Outcomes after tracheostomy in COVID-19 patients [Internet]. Ann Surg 2020; 272:e181–e186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwak PE, Connors JR, Benedict PA, et al. : Early outcomes from early tracheostomy for patients with COVID-19. JAMA Otolaryngol Head Neck Surg 2021; 147:239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glibbery N, Karamali K, Walker C, et al. : Tracheostomy in the coronavirus disease 2019 patient: Evaluating feasibility, challenges and early outcomes of the 14-day guidance. J Laryngol Otol 2020; 134:688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farlow JL, Park PK, Sjoding MW, et al. : Tracheostomy for COVID-19 respiratory failure: Timing, ventilatory characteristics, and outcomes. J Thorac Dis 2021; 13:4137–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battaglini D, Missale F, Schiavetti I, et al. : Tracheostomy timing and outcome in severe COVID-19: The WeanTrach multicenter study. J Clin Med 2021; 10:2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Wu Y, Zhu F, et al. : Tracheostomy in 80 COVID-19 patients: A multicenter, retrospective, observational study. Front Med 2020; 7:615845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livneh N, Mansour J, Kassif Lerner R, et al. : Early vs. late tracheostomy in ventilated COVID-19 patients - a retrospective study. Am J Otolaryngol 2021; 42:103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angel LF, Amoroso NE, Rafeq S, et al. : Percutaneous dilational tracheostomy for coronavirus disease 2019 patients requiring mechanical ventilation. Crit Care Med 2021; 49:1058–1067 [DOI] [PubMed] [Google Scholar]
- 30.Mathieu E, Ritchie H, Rodés-Guirao L, et al. : Coronavirus Pandemic (COVID-19). 2020. Published online at OurWorldInData.org. Available at: https://ourworldindata.org/coronavirus. Accessed October 3, 2022 [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent JL, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 33.Moreno RP, Metnitz PGH, Almeida E, et al. : SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005; 31:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapp CM, Latifi A, Feller-Kopman D, et al. : Sedation and analgesia in patients undergoing tracheostomy in COVID-19, a multi-center registry. J Intensive Care Med 2022; 37:240–247 [DOI] [PubMed] [Google Scholar]
- 35.Sutt AL, Tronstad O, Barnett AG, et al. : Earlier tracheostomy is associated with an earlier return to walking, talking, and eating. Aust Crit Care 2020; 33:213–218 [DOI] [PubMed] [Google Scholar]
- 36.Smailes S, Spoors C, da Costa FM, et al. : Early tracheostomy and active exercise programmes in adult intensive care patients with severe burns. Burns J Int Soc Burn Inj 2021; 48:1599–1605 [DOI] [PubMed] [Google Scholar]
- 37.Martin-Villares C, Perez Molina-Ramirez C, Bartolome-Benito M, et al. : Outcome of 1890 tracheostomies for critical COVID-19 patients: A national cohort study in Spain [Internet]. Eur Arch Otorhinolaryngol 2020; 278:1605–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed Y, Cao A, Thal A, et al. : Tracheotomy outcomes in 64 ventilated COVID-19 patients at a high-volume center in Bronx, NY. Laryngoscope 2021; 131:E1797–E1804 [DOI] [PubMed] [Google Scholar]
- 39.Polok K, Fronczek J, van Heerden PV, et al. : Association between tracheostomy timing and outcomes for older critically ill COVID-19 patients: Prospective observational study in European intensive care units. Br J Anaesth 2021; 128:482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kompaniyets L, Goodman AB, Belay B, et al. : Body Mass Index and Risk for COVID-19–Related Hospitalization, Intensive Care Unit Admission, Invasive Mechanical Ventilation, and Death — United States, March–December 2020. 2021. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/mmwr/volumes/70/wr/mm7010e4.htm?s_cid=mm7010e4_w. Accessed October 3, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dana R, Bannay A, Bourst P, et al. : Obesity and mortality in critically ill COVID-19 patients with respiratory failure. Int J Obes 2021; 45:2028–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daviet F, Guilloux P, Hraiech S, et al. : Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: Results from an ambispective observational cohort. Ann Intensive Care 2021; 11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Travassos C, Williams DR: The concept and measurement of race and their relationship to public health: A review focused on Brazil and the United States. Cad Saude Publica 2004; 20:660–678 [DOI] [PubMed] [Google Scholar]
- 44.Mude W, Oguoma VM, Nyanhanda T, et al. : Racial disparities in COVID-19 pandemic cases, hospitalisations, and deaths: A systematic review and meta-analysis. J Glob Health 2021; 11:05015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackey K, Ayers CK, Kondo KK, et al. : Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: A systematic review. Ann Intern Med 2021; 174:362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.