Abstract
Neuroendocrine tumors can lead to carcinoid heart disease with subsequent development of severe tricuspid regurgitation due to thickening and restriction of the tricuspid leaflets. We present a patient who underwent successful heterotopic transcatheter tricuspid valve replacement for torrential tricuspid regurgitation due to carcinoid heart disease. (Level of Difficulty: Intermediate.)
Key Words: carcinoid heart disease, right-side heart failure, tricuspid regurgitation, tricuspid valve replacement
Abbreviations and Acronyms: CT, computed tomography; MRI, magnetic resonance imaging; NYHA, New York Heart Association; TR, tricuspid regurgitation; TV, tricuspid valve
Central Illustration
A 70-year-old female patient was referred to our clinic from an outside hospital with right-sided heart failure due to torrential tricuspid regurgitation (TR) for further diagnostics and treatment planning. The patient reported exertional dyspnea (New York Heart Association [NYHA] functional class IV), occasional thoracic discomfort, and a history of syncope. Physical examination revealed distinct peripheral edema, ascites, pleural effusion, and jugular vein distension. Auscultation revealed a 2/6 systolic murmur in position of the tricuspid valve (TV) and attenuated breath sounds over the right lung. Laboratory testing showed elevated N-terminal pro–B-type natriuretic peptide levels, as well as cardiorenal1 and cardiohepatic syndrome2 (Table 1). Electrocardiography at admission revealed regular sinus rhythm with a known left bundle branch block. At the time of admission, the patient was being treated with the maximum tolerated dose of diuretic medication (60 mg/d torasemide and 10 mg/d xipamide).
Learning Objectives
-
•
To understand the difficulties regarding the interventional treatment of tricuspid regurgitation with carcinoid heart disease.
-
•
To keep heterotopic transcatheter tricuspid valve replacement in mind as a treatment option for patients with tricuspid regurgitation due to carcinoid heart syndrome.
Table 1.
Laboratory Evaluation
| eGFR, mL/min (≥60 mL/min) | 23 |
| Creatinine, mg/dL (0.5-1.0 mg/dL) | 2.1 |
| Urea, mg/dL (17-49 mg/dL) | 94 |
| Bilirubin, mg/dL (≤1.2 mg/dL) | 2.9 |
| AST, U/L (≤34 U/L) | 23 |
| ALT, U/L (≤34 U/L) | 16 |
| GGT, U/L (≤39 U/L) | 323 |
| Alkaline phosphatase, U/L (35-105 U/L) | 189 |
| NT-proBNP, pg/mL (≤623 pg/mL) | 905 |
| Chromogranin A, ng/mL (≤94 ng/mL) | 544 |
| Serotonin, ng/mL (117-192 ng/mL) | 71 |
Normal values for each parameter are indicated in parentheses.
ALT = alanine transaminase; AST = aspartate transaminase; GGT = gamma glutamyltransferase; eGFR = estimated glomerular filtration rate; N-terminal pro–B-type natriuretic peptide.
Medical History
The patient had recently undergone laparoscopic surgery for removal of an indeterminate ovarian tumor. Pathologic examination of the surgical specimen (15 × 10 × 6 cm) revealed the presence of a cystic ovarian teratoma with portions of a carcinoid tumor (positive staining for chromogranin and synaptophysin). After surgery, the patient was transferred to the external cardiologic department owing to progressive right-side heart failure. Echocardiographic evaluation revealed the torrential TR. In the presence of a severe cardiorenal syndrome, the patient needed intermittent hemodialysis. Significant coronary artery disease was excluded by coronary angiography. Left ventricular systolic function was normal. Right heart catheterization showed no evidence of pulmonary hypertension (systolic pulmonary arterial pressure 25 mmHg; inferior vena cava v-wave 17 mmHg). Furthermore, the patient reported a history of paroxysmal atrial fibrillation and heparin-induced thrombocytopenia type II. Abdominal ultrasound showed signs of venous hepatic congestion with beginning cirrhosis.
Investigations
In our department, echocardiographic evaluation (transthoracic and transesophageal) revealed torrential TR with a false-low triangular continuous-wave Doppler signal. TR quantification by means of the proximal isovelocity surface area method was not applicable owing to atrioventricular pressure equalization3 (Figures 1 and 2). TR led to dilation of the inferior vena cava and flow reversal within the hepatic veins. All 3 TV leaflets were severely restricted, leading to a large coaptation gap (3-dimensional gap area 5.5 cm2, 2-dimensional coaptation gap in the septolateral direction 3.2 cm [Figure 1]). Right ventricular (RV) function was preserved, while end-diastolic RV dimensions indicated significant RV enlargement (RV ejection fraction 50.8%). RV to pulmonary artery coupling was preserved (0.84 mm/mmHg). Echocardiographic imaging further showed a circular pericardial effusion (15 mm).
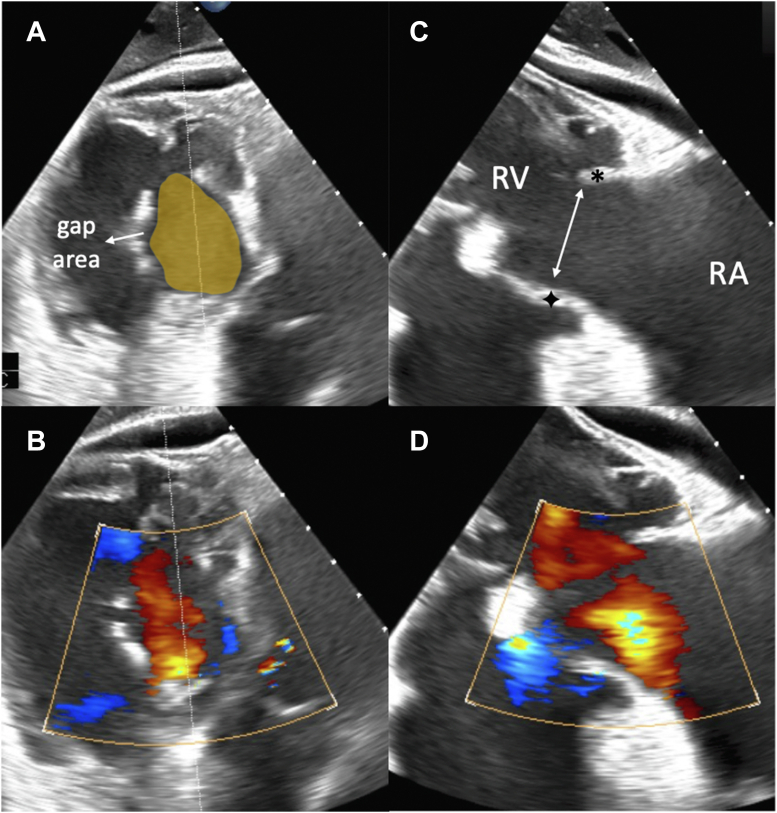
Figure 1.
Transesophageal Echocardiography
Transesophageal echocardiographic evaluation reveals a large coaptation gap during systole with subsequent torrential tricuspid regurgitation. (A and B) Transgastric view: severely restricted tricuspid valve leaflets with large coaptation gap. (C and D) Cross-sectional view derived from the transgastric angulation: severe restriction of the septal (∗) and anterior (+) tricuspid valve leaflets leading to a large coaptation gap. RA = right atrium; RV = right ventricle.
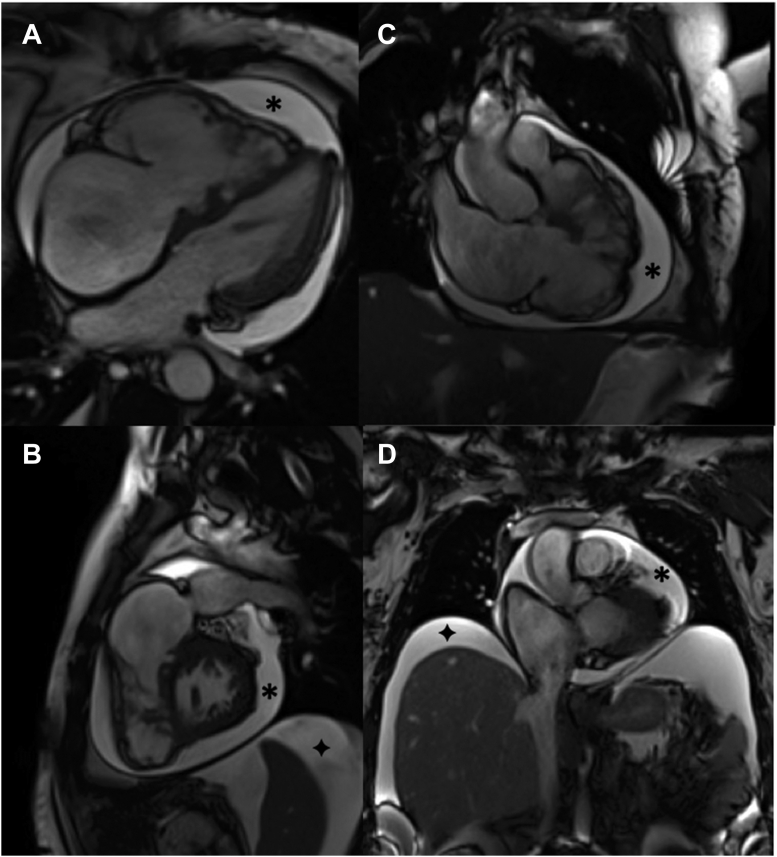
Figure 2.
Magnetic Resonance Imaging
Cardiac magnetic resonance imaging of the heart in (A) 4-chamber, (B) 3-chamber, and (C) short-axis views. The patient presented with concomitant pericardial effusion (∗) and ascites (+).
Positron emission tomography (Ga-68 DOTA-TOC) and abdominal magnetic resonance imaging (MRI) revealed no evidence of local tumor recurrence or lymphogenic or distant metastases. Cardiac MRI confirmed significant RV dilation and TR (regurgitant volume 84 mL (Figure 2). Furthermore, TR was accompanied by perihepatic and perisplenic ascites, as well as right-side pleural effusion (Figure 2). For further therapy planning, full-cycle computed tomography (CT) of the TV was performed.
Management
After discussion in the interdisciplinary heart team consisting of cardiac surgeons, interventional cardiologists, and heart failure specialists, the decision for an interventional TR treatment was made. TRI Score (8 points, 48% in-hospital mortality) and LaPar Score (12% mortality risk, 38% major morbidity risk) were indicative for advanced surgical risk.4,5 Owing to the large coaptation gap, severely restricted TV leaflets, and significant TV annular dilation, neither an “edge-to-edge” tricuspid valve repair nor an orthotopic TV replacement (as compassionate use) were suitable. After anatomy and hemodynamics were found to be suitable for a heterotopic TV replacement, treatment of TR was successfully performed using the TricValve (P&F) system (Central Illustration). As previously described, the implantation of 2 biological valves made of bovine pericardium placed inside self-expanding nitinol-covered stents in the superior and inferior vena cava was performed with the use of inguinal venous access under fluoroscopic and transthoracic echocardiographic guidance as previously described.6,7 Video 1 shows an RV angiogram after device placement. Chest X-ray and transthoracic echocardiography confirmed the regular valve positioning (Figure 3). Another 5 days later, the patient was discharged home.
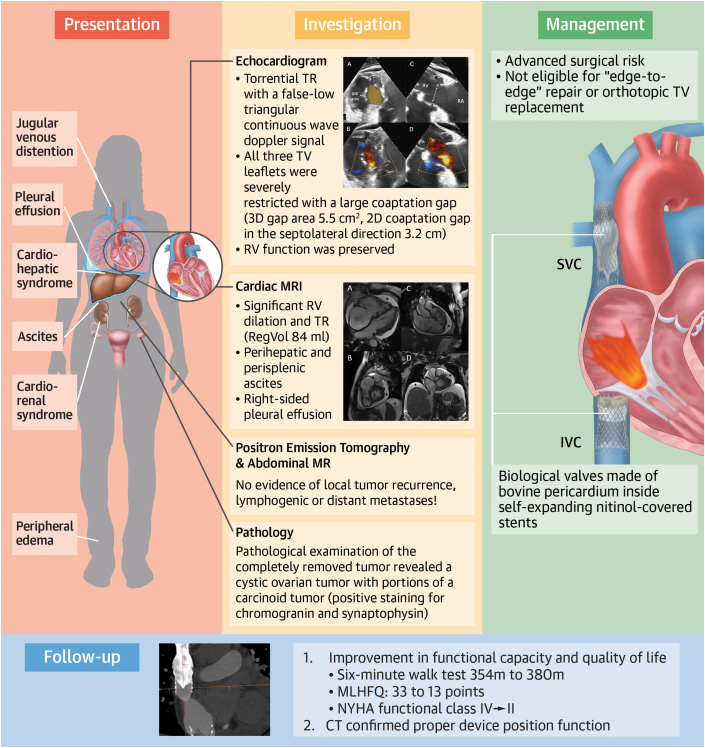
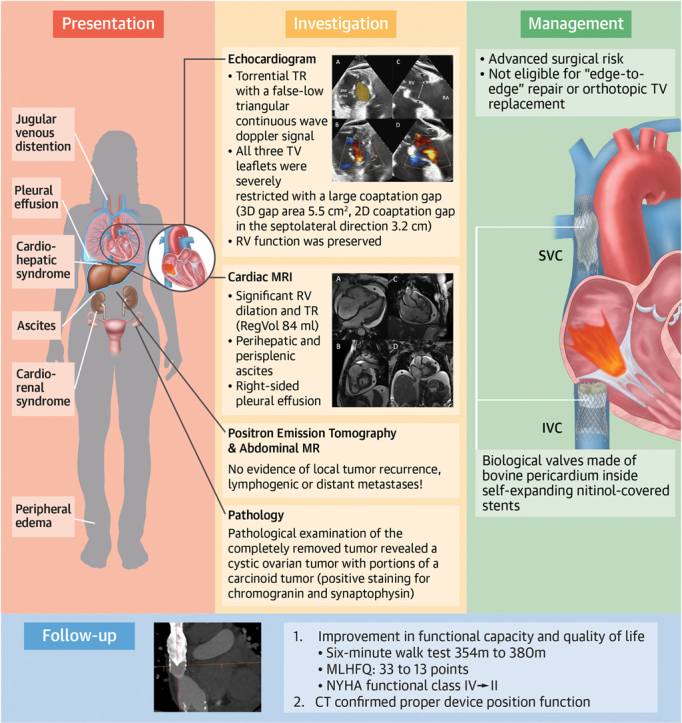
Central Illustration.
Heterotopic TTVR in a Patient With TR and Carcinoid Heart Disease
Presentation, investigation, management, and follow-up of a patient with carcinoid heart disease who received heterotopic transcatheter tricuspid valve replacement. CT = computed tomography; IVC = inferior vena cava; MLHFQ = Minnesota Living With Heart Failure Questionnaire; MR = magnetic resonance; MRI = magnetic resonance imaging; NYHA = New York Heart Association; SVC = superior vena cava; TR = tricuspid regurgitation; TTVR = transcatheter TV replacement; TV = tricuspid valve.
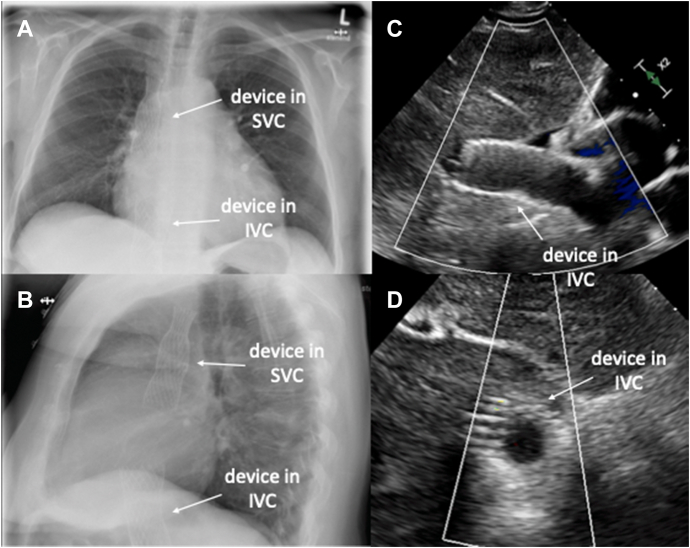
Figure 3.
Postinterventional Imaging
(A and B) Caval stents within the superior (SVC) and inferior (IVC) venae cavae in a chest X-ray. (C and D) View derived from transthoracic echocardiography, showing the device located in the IVC.
Discussion
Given the presence of torrential TR due to severely restricted TV leaflets combined with the history of a histologically proven neuroendocrine tumor, the most likely diagnosis in the present case was carcinoid heart disease. The latter is a rare condition with a prevalence of 1 to 2 per 100,000 people per year8 being associated with neuroendocrine tumors. It is currently thought that serotonin and other neuroendocrine substances produced by the respective neoplasms lead to fibrosis, thickening, and immobilization of the TV leaflets, chordae tendineae, and papillary muscles.9, 10, 11 Unlike in our case, further progression of the disease can also lead to the development of pulmonary stenosis.12 Surgical removal of the ovarian tumor might have prevented such disease progression in our patient. Owing to inactivation of endocrine substances within the pulmonary microvasculature, carcinoid heart disease usually does not affect the mitral or aortic valve.13 Of note, the gynecologic surgery was curative in our patient, with comprehensive oncologic work-up (MRI, positron emission tomography–CT) showing no evidence of either local tumor residues or metastases. This was of particular importance because the bicaval stents of the replacement system contain biological valves, which are known to degenerate quickly in the presence of an active neuroendocrine malignancy.14
Torrential TR as seen in the present case is associated with severe symptoms of right-side heart failure, secondary organ damage [eg, cardiohepatic and cardiorenal syndrome] impaired quality of life and limited life expectancy. Surgical tricuspid valve replacement is known to be associated with high periprocedural mortality.15 Especially in old patients with advanced or prohibitive surgical risk, alternative interventional treatment options can be considered.16 Owing to the complicated anatomy with restricted, almost immobile, leaflets and severely dilated RV dimensions, transcatheter tricuspid valve edge-to-edge repair and orthotopic transcatheter TV replacement (TTVR) are often inapplicable in the context of carcinoid heart disease. By definition, heterotopic transcatheter TV replacement does not depend on the anatomy of the TV itself. The bicaval stents of the TV replacement system prevent backflow from the volume-overloaded right atrium into the systemic circulation by implanting valve-containing stents into the superior and inferior venae cavae.6 Early outcome data after heterotopic TV replacement showed high rates of procedural success (up to 94%), low complication rates, and significant reduction of heart failure–related symptoms accompanied by significant quality of life improvement.7,17,18 In accordance with these results, our patient presented with a significant improvement of heart failure–related symptoms after 6 months (no edema, no pleural effusion, no ascites). Diuretic medication at follow-up comprised torasemide (20 mg/d) and spironolactone (25 mg/d). Similar to pre-existing literature, RV dimensions and function remained unchanged during follow-up.6 To assess the durability of transcatheter heterotopic TV replacement in the setting of carcinoid heart disease, the patient will undergo regular follow-up visits including cardiologic and oncologic monitoring.
Follow-Up
At 6-month follow-up, the patient presented in a significantly improved health status (NYHA II) without clinical evidence of peripheral edema or ascites. She denied dizziness, syncope, thoracic discomfort, and nycturia. Both 6-minute walking test distance and quality of life according to the Minnesota Living With Heart Failure Questionnaire improved (6MWD: 354 m to 380 m; MLHFQ: 33 to 13 points). Echocardiographic (Videos 2A and 2B) and CT follow-up examinations (Video 3) revealed no significant changes in RV function or dimensions (Table 2). Pericardial effusion was constant in size. Laboratory follow-up revealed no significant changes in renal or hepatic function.
Table 2.
Baseline and Follow-Up Imaging
| Baseline | Follow-Up | |
|---|---|---|
| Echocardiography | ||
| RV end-diastolic area, cm2 (8-20 cm2) | 15.0 | 16.9 |
| RV end-systolic area, cm2 (3-11 cm2) | 30.3 | 30.8 |
| RV fractional area change, % (≥35%) | 50.0 | 45.1 |
| RV ejection fraction (3D), % (≥45%) | 50.8 | 47.2 |
| RV free wall longitudinal strain, % (≤−20%) | -15.4 | -19.2 |
| TV annular diameter, mm (<40 mm) | 46.4 | 48.5 |
| TR max PG, mm Hg (≤30 mm Hg) | 14.3 | 15.1 |
| IVC diameter, mm (≤21 mm) | 25.0 | 18.1 |
| RA area, mm2 (≤18 mm2) | 25.8 | 32.5 |
| TAPSE, mm (≥17mm) | 21.0 | 23.2 |
| LV ejection fraction, % (≥60%) | 63.0 | 60.2 |
| Magnet resonance imaging | ||
| RV end-diastolic volume, mL (<176 mL) | 263 | a |
| RV end-systolic volume, mL (<104 mL) | 133 | a |
| RV ejection fraction, % (≥45%) | 49 | a |
| RegVol, mL | 84 | a |
| RV forward SV, mL (>50 mL) | 46 | a |
| Computed tomography | ||
| RV end-diastolic volume, mL (<177 mL) | 256 | 252 |
| RV end-systolic volume, mL (<78 mL) | 128 | 122 |
| RV ejection fraction, % (≥45%) | 48 | 51 |
Normal values for each parameter are indicated in parentheses.
3D = 3-dimensional; IVC = inferior vena cava; LV = left ventricle; RegVol = regurgitant volume; PG = pressure gradient; RA = right atrial; RV = right ventricular; SV = stroke volume; TAPSE = tricuspid annular plane systolic excursion; TR = tricuspid regurgitation; TV = tricuspid valve; VTI = velocity time integral; normal values for each parameter are indicated in brackets.
No MRI at follow-up performed.
Conclusions
TR in the context of carcinoid heart syndrome is often associated with challenging TV anatomy, which a few years ago would have lacked appropriate therapeutic options. As demonstrated in the present case, heterotopic tricuspid valve replacement is a reasonable interventional treatment approach for these patients and may reduce heart failure symptoms.
Funding Support and Author Disclosures
Dr Hausleiter has received research support and speaker honoraria from Edwards Lifesciences. Dr Näbauer has received speaker fees from Abbott Vascular and Edwards Lifesciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Diana Rösler, Andrea Englmaier, Patricia Lempert, and Tobias Reithmayer for their extensive support over the course of this study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
RV Angiography After Heterotopic Tricuspid Valve Replacement. Angiography of the right ventricle with caval stents in the inferior and superior venae cavae in situ. Biological valves within the stents prevent backflow of regurgitant blood into the systemic circulation.
Echocardiographic Imaging of the Inferior Vena Cava. (A) Dilated inferior vena cava before device implantation, with backflow into the hepatic veins, which is prevented by (B) placement of a caval stent.
Echocardiographic Imaging of the Inferior Vena Cava. (A) Dilated inferior vena cava before device implantation, with backflow into the hepatic veins, which is prevented by (B) placement of a caval stent.
Full-Cycle Computed Tomography of the Implanted Devices. Dynamic function of the devices being positioned in the superior and inferior venae cava.
References
- 1.Jentzer J.C., Bihorac A., Brusca S.B., et al. Contemporary management of severe acute kidney injury and refractory cardiorenal syndrome: JACC Council perspectives. J Am Coll Cardiol. 2020;76:1084–1101. doi: 10.1016/j.jacc.2020.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stolz L., Orban M., Besler C., et al. Cardiohepatic syndrome is associated with poor prognosis in patients undergoing tricuspid transcatheter edge-to-edge valve repair. J Am Coll Cardiol Intv. 2022;15:179–189. doi: 10.1016/j.jcin.2021.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Lurz P., Orban M., Besler C., et al. Clinical characteristics, diagnosis, and risk stratification of pulmonary hypertension in severe tricuspid regurgitation and implications for transcatheter tricuspid valve repair. Eur Heart J. 2020;41:2785–2795. doi: 10.1093/eurheartj/ehaa138. [DOI] [PubMed] [Google Scholar]
- 4.Dreyfus J., Audureau E., Bohbot Y., et al. TRI-SCORE: a new risk score for in-hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J. 2022;43:654–662. doi: 10.1093/eurheartj/ehab679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaPar D.J., Likosky D.S., Zhang M., et al. Development of a risk prediction model and clinical risk score for isolated tricuspid valve surgery. Ann Thorac Surg. 2018;106:129–136. doi: 10.1016/j.athoracsur.2017.11.077. [DOI] [PubMed] [Google Scholar]
- 6.Lauten A., Figulla H.R., Unbehaun A., et al. Interventional treatment of severe tricuspid regurgitation: early clinical experience in a multicenter, observational, first-in-man study. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.117.006061. [DOI] [PubMed] [Google Scholar]
- 7.Estévez-Loureiro R., Sánchez-Recalde A., Amat-Santos I.J., et al. 6-month outcomes of the tricvalve system in patients with tricuspid regurgitation: the TRICUS EURO study. J Am Coll Cardiol Intv. 2022;15:1366–1377. doi: 10.1016/j.jcin.2022.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Modlin I.M., Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya S., Toumpanakis C., Burke M., Taylor A.M., Caplin M.E., Davar J. Features of carcinoid heart disease identified by 2- and 3-dimensional echocardiography and cardiac MRI. Circ Cardiovasc Imaging. 2010;3 doi: 10.1161/CIRCIMAGING.109.886846. [DOI] [PubMed] [Google Scholar]
- 10.Roth B.L. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharyya S., Schapira A.H., Mikhailidis D.P., Davar J. Drug-induced fibrotic valvular heart disease. Lancet. 2009;374:577–585. doi: 10.1016/S0140-6736(09)60252-X. [DOI] [PubMed] [Google Scholar]
- 12.Ghukasyan H. Hedinger syndrome: a rare cardiac manifestation of carcinoid syndrome. Cureus. 2022;14 doi: 10.7759/cureus.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundin L., Norheim I., Landelius J., Oberg K., Theodorsson-Norheim E. Carcinoid heart disease: relationship of circulating vasoactive substances to ultrasound-detectable cardiac abnormalities. Circulation. 1988;77:264–269. doi: 10.1161/01.cir.77.2.264. [DOI] [PubMed] [Google Scholar]
- 14.Connolly H.M., Schaff H.V., Abel M.D., et al. Early and late outcomes of surgical treatment in carcinoid heart disease. J Am Coll Cardiol. 2015;66:2189–2196. doi: 10.1016/j.jacc.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Wong W.K., Chen S.W., Chou A.H., et al. Late outcomes of valve repair versus replacement in isolated and concomitant tricuspid valve surgery: a nationwide cohort study. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doldi P.M., Näbauer M., Massberg S., Hausleiter J. Interventional tricuspid valve repair after failed surgical tricuspid valve reconstruction. Can J Cardiol. 2020;36:1832.e5–1832.e6. doi: 10.1016/j.cjca.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 17.Muntané-Carol G., Taramasso M., Miura M., et al. Transcatheter tricuspid valve intervention in patients with right ventricular dysfunction or pulmonary hypertension: insights from the TriValve Registry. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.009685. [DOI] [PubMed] [Google Scholar]
- 18.Figulla H.R., Kiss K., Lauten A. Transcatheter interventions for tricuspid regurgitation—heterotopic technology: TricValve. EuroIntervention. 2016;12:Y116–Y118. doi: 10.4244/EIJV12SYA32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RV Angiography After Heterotopic Tricuspid Valve Replacement. Angiography of the right ventricle with caval stents in the inferior and superior venae cavae in situ. Biological valves within the stents prevent backflow of regurgitant blood into the systemic circulation.
Echocardiographic Imaging of the Inferior Vena Cava. (A) Dilated inferior vena cava before device implantation, with backflow into the hepatic veins, which is prevented by (B) placement of a caval stent.
Echocardiographic Imaging of the Inferior Vena Cava. (A) Dilated inferior vena cava before device implantation, with backflow into the hepatic veins, which is prevented by (B) placement of a caval stent.
Full-Cycle Computed Tomography of the Implanted Devices. Dynamic function of the devices being positioned in the superior and inferior venae cava.