Abstract
The external auditory canal is less susceptible to infections than the sensitive middle-ear cavity. Since recent research has provided insight to the production of potent antimicrobial peptides from various surface epithelia, we wanted to investigate whether protection of the external auditory canal in part could be explained by the production of human β-defensin-1 (HBD-1). This particular peptide is known to be constitutively expressed in various surface epithelia, such as airway, skin, and urogenital tissues. By reverse transcriptase PCR we demonstrate HBD-1 mRNA in the pars tensa and pars flaccida of the tympanic membrane and in the meatal skin. In situ hybridization studies localized the HBD-1 mRNA to the epidermal layer of these tissues. The HBD-1 transcripts were also evident in the sebaceous glands and in hair follicles of the meatal skin. In contrast, HBD-1 mRNA was not detected in the tympanal epithelium of the eardrum. The widespread presence of mRNA encoding for this broad-spectrum antimicrobial peptide in the meatal skin and tympanic membrane suggests that HBD-1 participates in the innate antimicrobial defense of the external auditory canal and middle-ear cavity.
The external auditory canal (EAC) is a special anatomic region, forming a “cul-de-sac” with the thin tympanic membrane (TM) separating the EAC from the sterile environment of the middle-ear cavity. It is well known that organisms potentially pathogenic to the middle ear can be found in the EAC (4, 5, 25). These circumstances make it obvious that the intact TM provides an effective physical barrier, preventing the penetration and entrance of pathogenic microorganisms from the EAC into the sensitive middle-ear cavity. The external surface of the intact TM is covered with a stratified and keratinized surface epithelium, which participates in preventing EAC microorganisms from reaching the middle ear cavity. In addition, cerumen, which is a combination of ceruminous and sebaceous secretions, exerts antimicrobial properties that tend to eliminate pathogenic organisms (28).
During recent years, endogen antimicrobial peptides, active against gram-positive and gram-negative bacteria, have attracted much interest (2, 3, 8, 13, 20). Defensins, a subgroup of these substances, are small peptides of 29 to 47 amino acids which have been classified into the two families, α-defensins and β-defensins. Whereas some α-defensins are expressed in the Paneth cells of the gastrointestinal tract, others have been demonstrated in neutrophil granulocytes where these peptides constitute 5 to 7% of the total protein content (9). These polypeptides function in both phagocytic and extracellular killing of microbes and are therefore important for host defense (9). Human β-defensin-1 (HBD-1) was recently isolated from plasma (1) and was shown to be constitutively expressed in salivary glands (30), in gingival cells (12), in urogenital tissues (29, 30), in the pancreas (23, 30), in the respiratory epithelium of the lower airways (18), and in keratinocytes of the skin (7).
The purpose of the present study was to investigate whether the effective impediment against infections in the inner part of the EAC could in part be explained by evidence for HBD-1 peptide production. The pars tensa and pars flaccida from eardrums, and meatal skin tissue were investigated by using reverse transcriptase PCR (RT-PCR) and in situ hybridization techniques. We found that the surface epithelium of the eardrum and meatal skin contained mRNA for the antimicrobial peptide HBD-1. Considering both this widespread presence of the HBD-1 mRNA in the region and its known broad-spectrum antibiotic activity, we suggest that HBD-1 contributes significantly to the antimicrobial protection of the EAC, the TM, and the middle-ear cavity.
MATERIALS AND METHODS
Tissue sampling.
TMs with adjacent skin were dissected at autopsy from 10 ears within 36 h postmortem with an endoscope. No signs of ear disease were observed at inspection prior to excision. After removal, tissues from the pars tensa and the pars flaccida of the eardrum and meatal skin were separated. In addition, small meatal skin pieces were excised during otosclerosis surgery of five patients. Special care was made to sample skin fragments uninvolved by the disease for which the patients were operated. The specimens were either prepared for RT-PCR or in situ hybridization studies. The study was approved by the Medical Ethics Committee of the University of Tromsø, Tromsø, Norway.
RT-PCR.
Total RNA from the tissue fragments (1 mm3) was isolated by the single-step guanidium thiocyanate-phenol-chloroform extraction method (6). The RT reaction was performed mainly as recently described (17). In brief, 5 μl of RNA (1 μg) was added to 55 μl of an RT mixture containing 60 U of RNasin (Promega), 0.63 mM concentrations of each deoxynucleotide from a deoxynucleoside triphosphate mix (Pharmacia Biotech), 0.3 ng of pd(N)6 Random Hexamer primers (Pharmacia Biotech), 600 U of Molony leukemia virus RT (Gibco/Life Technologies) in 1× RT buffer (Gibco), and incubated for 1 h at 37°C. For negative control of the RT reaction, 5 μl of DEPC-H2O was added to the RT mixture. The reaction products were heated (100°C) for 10 min, chilled on ice, and spun briefly before 240 μl of DEPC-H2O was added to each sample. The primers used for the PCR were the forward primer A1 (5′-TTGTCTGAGATGGCCTCAGGTGGTAAC-3′ [30]) and the reverse primer R3 (5′-TTTCACTTCTGCGTCATTTCTTCTGG-3′). The A1 primer corresponds to the nucleotides 753 to 771 in exon 1 and includes sequence 7734 to 7741 in exon 2 (numbering according to Liu et al. [16]). The reverse primer R3 is complementary to nucleotides 7890 to 7916 in exon 2. The expected size of the PCR product generated from these primers was 200 bp. The primers 5′-CCCGAGGCTTCCTCTTTGGC-3′ and 5′-CCTCGCTTAAGGGCAGGGAG-3′ for the housekeeping gene adenosylphosphoribosyltransferase (APRT) were run simultaneously (the expected size of this PCR product was 300 bp). The primers correspond to the sequence 1940 to 1959 in exon 3 and 2725 to 2744 in exon 5 of the APRT gene, respectively (GenBank access number Y00486). The primers were purchased from Eurogenetec. The samples were denatured at 94°C for 5 min and amplified by 35 cycles at 94°C for 1 min, at 60°C for 1 min, and at 72°C for 1 min. For a final elongation step, the samples were incubated for 5 min at 72°C. The PCR products were analyzed by gel electrophoresis.
In situ hybridization.
The cDNA used for the preparation of the sense and antisense HBD-1 probes has been described recently (29). RNA-probes for in situ hybridization were prepared by using the DIG RNA Labeling Kit (Boehringer Mannheim). The dissected specimens were fixed by immersion overnight in 4% paraformaldehyde (pH 7.4) at 4°C. Paraffin sections were mounted on glass slides which had been pretreated with 1% 3-aminopropyl-trietoxysilane (ICN). Some sections were stained with hematoxylin and eosin for histological examination. Parallel sections were deparaffinized and hydrated prior to an incubation for 15 min in phosphate-buffered saline (PBS)–0.3% Triton X-100. Digestion was performed for 45 min with 25 μg of proteinase K (Boehringer Mannheim) per ml in 0.1 M Tris-HCl and 0.05 M EDTA (pH 8.0) at 37°C. The proteinase K activity was stopped by incubating the slides in PBS–4% paraformaldehyde for 5 min. The slides were washed twice for 3 min in sterile water, and the sections were acetylated by soaking them into 0.1 M triethanolamine-HCl (Sigma), to which 0.375 ml of acetic anhydride (Sigma) was added twice (with 5 min of incubation after each addition). After dehydration in sequential ethanol baths and drying (20°C, 60 min), hybridization solution (100 μl) containing 0.3 M NaCl, 20 mM Tris-HCl (pH 7.2), 5 mM EDTA, Denhardt solution (Sigma), 50% deionized formamide, 10% dextran sulfate, 0.25 mg of yeast tRNA per ml, and 250 ng of DIG-labeled RNA probe per ml was applied onto each section. The hybridization solution was overlaid with parafilm, and all samples were incubated overnight at 42°C in a moisture chamber. The sections were incubated in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 50°C for 45 min and in 0.2× SSC at 50°C for 30 min and then briefly immersed in sterile water. The sections were then incubated twice for 10 min in Tris-HCl–NaCl buffer (pH 7.5) and for 20 min in blocking buffer (Tris-HCl–NaCl buffer with 1% bovine serum albumin and 2% sheep serum). The sections were subsequently incubated for 2 h with the anti-DIG-AP Fab fragment antibody (Boehringer Mannheim) diluted to 1:300 in a 1:1 mixture of the Tris-HCl–NaCl buffer (pH 7.5) and the blocking buffer. Cells with hybridized probe were visualized by incubation for 12 h (4°C) with nitroblue tetrazolium, 5-bromo-4-chloro-3-indolyl phosphate, and levamisole (Sigma). The specimens were examined in a Zeiss Axioplan microscope (Oberkochen, Germany).
RESULTS
RT-PCR studies.
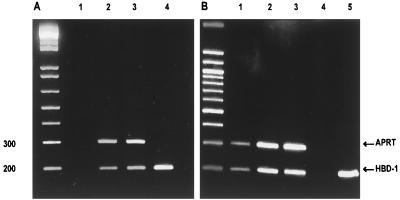
The RT-PCR examination showed that biopsied skin from the EAC and autopsied tissue pieces of the pars tensa and the pars flaccida of the eardrum (Fig. 1) contained mRNA encoding for HBD-1 (Fig. 2). The PCR products extracted from all of the anatomical sites had the size (200 bp) which was expected from the selected primers. For a control, the linearized pCR3.1 vector containing the full-length HBD-1 cDNA was amplified with the same primers, which also generated a PCR product of 200 bp. Sequencing of the RT-PCR products confirmed 100% sequence homology to the HBD-1 (not shown). The primers used for the housekeeping gene (APRT, see Materials and Methods for specifications), produced a PCR product (300 bp) from each of the tissue samples. The APRT primers gave no signal when tested with the linearized HBD-1 cDNA vector.
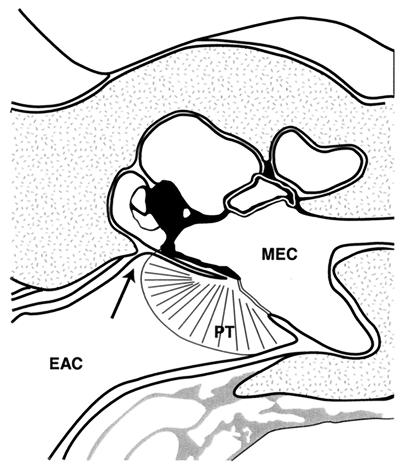
FIG. 1.
Illustration of the TM separating the middle-ear cavity (MEC) from the EAC. The pars tensa (PT) of the TM is shown, and the arrow demonstrates the location of the pars flaccida.
FIG. 2.
RT-PCR products of mRNA from biopsied (A) and autopsied (B) tissues, as demonstrated by gel electrophoresis. The 200-bp fragments represent the presence of mRNA for HBD-1, and the 300-bp fragments represent the control APRT mRNA product. (A) Lanes: 1, negative control (running the RT procedure with sterile water); 2, RT-PCR of mRNA collected from an EAC skin sample; 3, control tissue sample from skin; 4, PCR product obtained by amplification of the cDNA for HBD-1 inserted in the pCR3.1 plasmid. (B) Lanes: 1, RT-PCR products from an autopsied tissue fragment of EAC skin; 2, pars tensa of the TM; 3, pars flaccida of the TM; 4, negative control; 5, amplification product of the HBD-1 cDNA.
Light microscopy studies of the eardrum and adjacent skin.
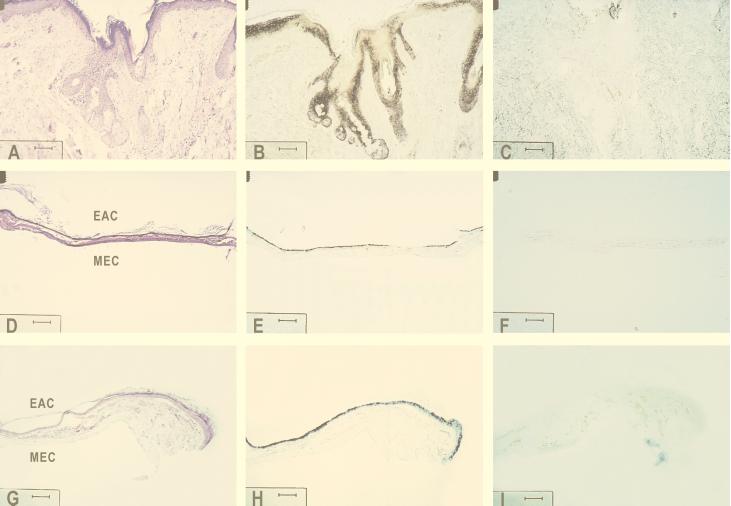
Light microscopy studies showed that the skin of the EAC (Fig. 3A to C) possessed a well-defined epidermis and dermis. Sebaceous and ceruminous glands originating from the epidermal layer were observed in the dermis. The TM (Fig. 3D to F) had an outer meatal side consisting of keratinizing, stratified, squamous epithelium that was three to seven cells thick. Underlying the epithelium, the thin layer of connective tissue is arranged with two layers of loose connective tissue, covering each side of a middle layer of collagen fibers as described previously (14, 15, 26). In the pars flaccida (Fig. 3G to I), the keratin layer of the meatal epithelium was more distinct than in the pars tensa (Fig. 3D). The single-layered tympanal epithelium did not differ from that lining the pars tensa. The connective tissue portion of the pars flaccida (Fig. 3G) was somewhat thicker than that of the pars tensa (Fig. 3D). As seen from Fig. 3D and G, the autopsied material showed minor postmortem changes.
FIG. 3.
In situ hybridization studies showing the localization of the HBD-1 mRNA in the epidermal layer of the EAC skin, pars tensa, and pars flaccida. Parallel micrographs from the meatal skin (A to C), from the pars tensa (D to F), and from the pars flaccida (G to I) are shown. The sections presented in panels A, D, and G were stained with hematoxylin and eosin; panels B, E, and H show parallel sections hybridized with DIG-labeled antisense RNA probe for HBD-1, and panels C, F, and I were from control sections hybridized with DIG-labeled sense transcript for potential nonspecific labeling. MEC, middle-ear cavity. Bars, 80 μm.
In situ hybridization studies.
The keratinocytes of the meatal skin epidermis, as well as keratinocytes of pars tensa and pars flaccida, stained positive for HBD-1 mRNA (Fig. 3B, E, and H). In contrast, the flat monolayered tympanal epithelium facing the MEC and the connective tissue of the skin (dermis) and the TM showed no staining (Fig. 3B, E, and H). In the meatal skin epidermis, it seemed to be mainly the keratinocytes of the middle layers (stratum granulosum) that were stained (Fig. 3B). A striking observation was also the pronounced staining of cells in the sebaceous glands and hair follicles (Fig. 3B). There was no specific localization with the DIG-labeled sense transcript for HBD-1 (Fig. 3C, F, and I), confirming the specificity of the antisense HBD-1 probe. The same patterns of staining were observed in biopsy and in autopsy material.
DISCUSSION
Clinical observations suggest that the EAC, which is frequently exposed to microbial pathogens, actually is less disposed to infections than the middle-ear cavity. Being unique in that it separates the two gas-filled environments (Fig. 1), the TM provides a physical barrier which prevents EAC pathogens from reaching the middle ear. To fulfill its prime function as a vibrating membrane, the eardrum is so thin that structures such as the ossicles of the middle ear and the promontory with the round window niche can be discerned behind it. The morphology of the human TM with adjacent skin is well described (14, 15, 26), and our study confirmed earlier findings.
Recent research has demonstrated that various epithelial surfaces express a variety of effective antibiotic peptides. We therefore presumed that these peptides also could be present in the eardrum surface epithelium and the adjacent EAC skin. Antimicrobial peptides produced by the TM could prevent bacterial microorganisms, normally colonizing the EAC, from reaching the vulnerable middle-ear cavity. Under normal conditions, Staphylococcus epidermidis, Corynebacterium spp., Micrococcus spp., and alpha-hemolytic and nonhemolytic streptococci, but also small numbers of Staphylococcus aureus and pseudomonas aeruginosa, are normal commensals of the EAC (4, 21, 25). The most common bacteria causing acute otitis media, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, are not normally found in the EAC when the eardrum is intact (11). In contrast, S. aureus and P. aeruginosa are well-known pathogens in chronically inflamed, draining ears.
We found that keratinocytes of the EAC epidermis and eardrums were the site of mRNA transcription for the HBD-1 peptide. HBD-1 has previously been shown constitutively expressed in keratinocytes of the skin (7), and our findings of mRNA for HBD-1 in the EAC keratinocytes are consistent with these results. On the other hand, we did not find evidence of HBD-1 production in the tympanal epithelium. The defensins, when tested in vitro, exhibit potent antimicrobial effects against gram-positive and gram-negative bacteria (13). Specifically, HBD-1 has microbicidal effects against the gram-negative P. aeruginosa (24). As reviewed by Lehrer et al. (13), defensins are also able to kill some fungi, among them Candida spp. Important questions regarding the mode of action of β-defensins on microorganisms in the EAC still exist. Is the peptide transported extracellularly, or is it only acting on bacteria that penetrate into the epithelial cells? It seems reasonable to believe that HBD-1 acts mainly extracellularly, as it may in bronchoalveolar lavage fluid (24) and in the urogenital tract (29). Another important question is whether additional amounts of other peptide antibiotics, e.g., HBD-2 (10, 24), can be induced in keratinocytes of the EAC. The fact that HBD-1 is transcribed in the cells of the sebaceous glands (Fig. 3H) suggests that the antimicrobial peptide is expressed and secreted with the cerumen of the EAC. Cerumen from sebaceous and ceruminous glands contains fatty acids and lysozyme and has previously been shown to exert bacteriostatic or bactericidal effects (28). The function of cerumen is controversial since it also can serve as an excellent substrate for Pityrosporum ovale (27), a lipophilic yeast that is the etiologic agent of dandruff (19) and folliculitis (22). Further studies are needed to reveal whether or not HBD-1 is able to kill P. ovale. On the basis of our findings we speculate that HBD-1, produced by the secretory glands and keratinocytes of the EAC, contributes to the bactericidal activity of cerumen in addition to lysozyme (28). These findings, taken together, suggest that HBD-1 contributes to the antimicrobial defense of the TM and the EAC.
ACKNOWLEDGMENTS
We are grateful to Wenche Helen Bakkelund and Anja Inkeri Vepsä for excellent technical assistance. We are also indebted to Marijke Van Ghelue for the DNA sequencing.
The work was supported by grants from the Norwegian Research Council (Norges Forskningsråd).
REFERENCES
- 1.Bensch K W, Raida M, Magert H J, Schulz Knappe P, Forssmann W G. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 2.Boman H G. Gene-encoded peptide antibiotics and the concept of innate immunity: an updated review. Scand J Immunol. 1998;48:15–25. doi: 10.1046/j.1365-3083.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 3.Boman H G, Nilsson I, Rasmuson B. Inducible antibacterial defence system in Drosophila. Nature. 1972;237:232–235. doi: 10.1038/237232a0. [DOI] [PubMed] [Google Scholar]
- 4.Brook I. Microbial studies of the bacterial flora of the external auditory canal in children. Acta Otolaryngol. 1981;91:285–287. doi: 10.3109/00016488109138509. [DOI] [PubMed] [Google Scholar]
- 5.Brooks G F, Butel J S, Ornson L N, Jawetz E, Melnick J L, Adelberg E A. Jawetz, Melnick, and Adelberg’s medical microbiology. 19th ed. London, United Kingdom: Prentice-Hall Inc.; 1991. [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single step method of RNA isolation by guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Fulton C, Anderson G M, Zasloff M, Bull R, Quinn A G. Expression of natural peptide antibiotics in human skin. Lancet. 1997;350:1750–1751. doi: 10.1016/S0140-6736(05)63574-X. [DOI] [PubMed] [Google Scholar]
- 8.Ganz T, Selsted M E, Lehrer R I. Defensins. Eur J Haematol. 1990;44:1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 9.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 10.Harder J, Bartels J, Christophers E, Scröder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 11.Kamme C, Lundgren K, Mårdh P-A. The aetiology of acute otitis media in children. Scand J Infect Dis. 1971;3:217–223. doi: 10.3109/inf.1971.3.issue-3.07. [DOI] [PubMed] [Google Scholar]
- 12.Krisanaprakornkit S, Weinberg A, Perez C N, Dale B A. Expression of the peptide antibiotic human β-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun. 1998;66:4222–4228. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 14.Lim D J. Tympanic membrane. I. Pars tensa. Acta Otolaryngol. 1968;66:181–198. doi: 10.3109/00016486809126286. [DOI] [PubMed] [Google Scholar]
- 15.Lim D J. Tympanic membrane. II. Pars flaccida. Acta Otolaryngol. 1968;66:47–56. doi: 10.3109/00016486809126316. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Zhao C, Heng H H Q, Ganz T. The human beta-defensin-1 and alpha-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics. 1997;43:316–320. doi: 10.1006/geno.1997.4801. [DOI] [PubMed] [Google Scholar]
- 17.Loennechen T, Moens U, Kildalsen H, Andersen A, Rekvig O P, Aarbakke A. Effects of 3-deazaadenosine on apoptosis-related gene transcripts in HL-60 cells. Pharmacol Toxicol. 1997;81:199–204. doi: 10.1111/j.1600-0773.1997.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 18.McCray P B, Jr, Bentley L. Human airway epithelia express a beta-defensin. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 19.McGinley K J, Leyden J J, Marples R R, Path M R C, Kligman A M. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrhoeic dermatitis. J Invest Dermatol. 1975;64:401–405. doi: 10.1111/1523-1747.ep12512335. [DOI] [PubMed] [Google Scholar]
- 20.Nissen-Meyer J, Nes I F. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch Microbiol. 1997;167:67–77. [PubMed] [Google Scholar]
- 21.Ostfeld E, Rubinstein E, Gazit E. Effect of systemic antibiotics on the microbial flora of the external ear canal in hospitalized children. Pediatrics. 1977;60:364–368. [PubMed] [Google Scholar]
- 22.Potter B S, Burgoon C F, Johnson J E. Pityrosporum folliculitis. Arch Dermatol. 1973;107:388–391. doi: 10.1001/archderm.107.3.388. [DOI] [PubMed] [Google Scholar]
- 23.Schnapp D, Reid C J, Harris A. Localization of expression of human beta defensin-1 in the pancreas and kidney. J Pathol. 1998;186:99–103. doi: 10.1002/(SICI)1096-9896(199809)186:1<99::AID-PATH133>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Singh P K, Jia H P, Wiles K, Hesselberth J, Liu L D, Conway B A D, Greenberg E P, Valore E V, Welsh M J, Ganz T, Tack B F, McCray P B., Jr Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sipilä P, Jokipii A M M, Jokipii L, Karma P. Bacteria in the middle ear and ear canal of patients with secretory otitis media and noninflamed ears. Acta Otolaryngol. 1981;92:123–130. doi: 10.3109/00016488109133246. [DOI] [PubMed] [Google Scholar]
- 26.Stenfors L-E, Blom G D, Hellström S. The tympanic membrane. Acta Otolaryngol. 1984;414(Suppl.):28–30. doi: 10.3109/00016488409122877. [DOI] [PubMed] [Google Scholar]
- 27.Stenfors L-E, Räisänen S. Is Pityrosporum ovale a pathogen of the external auditory meatus? Acta Otolaryngol. 1991;111:943–945. doi: 10.3109/00016489109138434. [DOI] [PubMed] [Google Scholar]
- 28.Stone M, Fulghum R S. Bactericidal activity of wet cerumen. Ann Otol Rhinol Laryngol. 1984;93:183–186. doi: 10.1177/000348948409300217. [DOI] [PubMed] [Google Scholar]
- 29.Valore E V, Park C H, Quale A J, Wiles K R, McCray P B, Jr, Ganz T. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao C, Wang I, Lehrer R I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]