Abstract
Mild encephalopathy/encephalitis with a reversible splenial lesion (MERS) and longitudinally extensive transverse myelitis (LETM) are neuroinflammatory conditions related to the brain and spinal cord, respectively. Most cases of MERS and LETM are related to a secondary autoimmune process in response to an initial insult (i.e., infection, immunization, etc.). The case of an 18-year-old female who developed a three-day history of fever, quadriplegia, cough, and mild encephalopathy is reported here. The patient tested positive for influenza B by nasopharyngeal swab with polymerase chain reaction (PCR). Initial magnetic resonance imaging (MRI) revealed the presence of a diffusion-restricted non-enhancing lesion confined to the splenium of the corpus callosum (MERS type I) and longitudinally extensive non-enhancing T2 hyperintensities from C1 to C5. The patient was managed with a five-day course of 1,000 mg of intravenous methylprednisolone (IVMP). Additionally, five days of therapeutic plasmapheresis (PLEX) was completed. The patient showed significant improvement with medical management and physical therapy. At the one-year follow-up, her motor symptoms had resolved and endorsed only mild paresthesia in the upper extremities. A repeat MRI revealed a reversal of the splenium lesion and moderate improvement in T2 hyperintensities of the cervical cord. Assessing neuroinvasion of the influenza virus is difficult, and diagnostic challenges arise in determining primary infectious versus autoimmune-mediated neuroinflammation. A review of the literature on influenza infection with radiographic findings of MERS and LETM is included.
Keywords: neurotropism, splenium, mild encephalopathy/encephalitis with reversible splenial lesion, neuroinfectious diseases, corpus callosum, influenza virus, influenza b, encephalomyelitis, longitudinally extensive transverse myelitis, infectious encephalitis
Introduction
Influenza B is a negative single-stranded RNA virus that belongs to the Orthomyxoviridae family. Both influenza A and B are primarily viral respiratory infections; however, cases of neurological sequelae secondary to influenza have been reported. This is of particular concern given the annual seasonal spike of cases seen around the world from these viruses. Altered mental status has been commonly reported and is termed influenza-associated encephalopathy (IAE). Cases of IAE have been reported more frequently in children. Although cases of encephalopathy have been reported, influenza is not considered a virus with considerable neuroinvasive potential. In fact, only a few cases of IAE with the detection of influenza RNA in cerebrospinal fluid (CSF) have been reported [1].
Mild encephalitis with a reversible splenial lesion (MERS) is a clinical radiographic syndrome defined by encephalopathy and a reversible lesion in the splenium of the corpus callosum. Typically, cases have been reported from East Asia and Japan. Here, a case is reported from the United States. Longitudinally extensive transverse myelitis (LETM) is defined as a longitudinal lesion that occurs at three or more consecutive spinal levels. LETM has been associated with demyelinating diseases such as neuromyelitis optica spectrum disorder (NMOSD) and myelin oligodendrocyte glycoprotein-associated disease (MOGAD). Clinical etiology also includes bacterial, fungus, and viral myelitis.
The case of an 18-year-old female with clinical and radiographic findings consistent with encephalomyelitis is reported. Lesions consistent with mild encephalitis with a reversible splenial lesion (MERS) and longitudinally extensive transverse myelitis (LETM) from C1 to C5 are rarely observed together [2].
Case presentation
An 18-year-old right-handed female presented during the northern hemisphere influenza season (October-April) with a three-day history of progressively worsening cough, dyspnea, and weakness. Prior medical and family history was unremarkable. Vital signs at presentation were significant for a temperature of 38.9°C. Initial neurological examination was significant for profuse muscle weakness. The Medical Research Council (MRC) Scale for Muscle Strength grading (0-5) revealed 1/5 muscle strength in the bilateral lower extremities both proximally and distally. The patient was unable to maintain neck flexion against gravity. Muscle strength in the upper extremities was 3/5. Reflexes were 3+ on the bilateral patella and Achilles tendon, with two beats of clonus, a mute Babinski reflex, and a positive Hoffman reflex on the right. All other reflexes were 2+ and symmetric. She was oriented to person, place, and time and exhibited some memory loss consistent with mild encephalopathy. The rectal tone was normal, and she was continent of urine. Formal neurocognitive testing was not completed on the initial presentation. A chest radiograph demonstrated hypoinflated lungs. The complete blood count and comprehensive metabolic panel revealed no leukocytosis or lactic acidosis. The patient began to develop progressive bradypnea and was subsequently intubated and brought to the intensive care unit (ICU) for further treatment. She tested positive for influenza B through polymerase chain reaction (PCR) cultured from a nasopharyngeal swab. A magnetic resonance imaging (MRI) of the brain with and without gadolinium contrast (Gd+) revealed an isolated 11-mm diffusion-restricting lesion within the splenium of the corpus callosum that is hyperintense on T2-weighted imaging (T2W1) and hypointense on T1-weighted imaging (T1WI) (Figure 1). An MRI of the cervical spine with and without Gd+ showed a predominantly gray matter lesion from C1 to C5 segments of the cervical spinal cord consistent with longitudinally extensive transverse myelitis (LETM) (Figure 2). None of the identified lesions were contrast-enhancing.
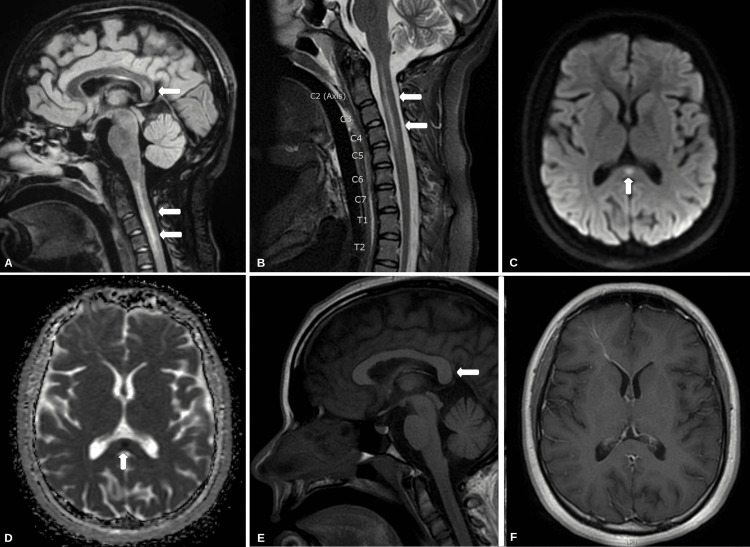
Figure 1. MRI of the brain and cervical spinal cord.
(A) T2 FLAIR sagittal sequence shows a non-enhancing lesion in the splenium of the corpus callosum (arrows). (B) T2 FLAIR sagittal sequence of the cervical spinal cord with hyperintensities from C1 to C5 (arrows). (C) DWI sequence depicts diffusion restriction within the splenium of the corpus callosum (arrow). (D) ADC sequence confirming the aforementioned diffusion restriction (arrow). (E and F) T1-weighted imaging with only mild hypointensity in the splenium of the corpus callosum (arrow) and without enhancement.
MRI: magnetic resonance imaging, FLAIR: fluid-attenuated inversion recovery, DWI: diffusion-weighted imaging, ADC: apparent diffusion coefficient
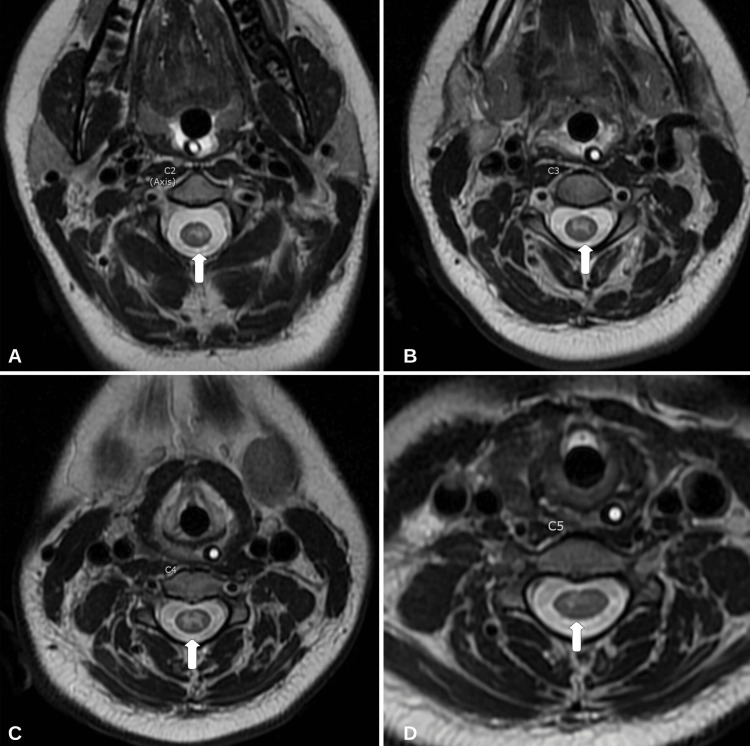
Figure 2. MRI (axial view) of the cervical spine.
(A-D) T2 FLAIR (axial view) of spinal segments C2-C5 reveals primarily gray matter hyperintensities (arrows).
MRI: magnetic resonance imaging, FLAIR: fluid-attenuated inversion recovery
Cerebrospinal fluid (CSF) analysis for influenza B was negative. CSF chemistry revealed an albumin level of 9 mg/dL (normal range: 0-35 mg/dL), glucose of 66 mg/dL (normal range: 50-80 mg/dL), and immunoglobulin G (IgG) of 1.4 mg/dL (normal range: 0-6 mg/dL). CSF analysis for oligoclonal bands, aquaporin-4 (AQP4) antibody/neuromyelitis optica (NMO), and myelin oligodendrocyte glycoprotein (MOG) antibody was also negative. Serum evaluation for NMO, MOG, and autoimmune panel was also negative. The serum IgG level was elevated at 1,760 mg/dL (normal range: 768-1,632 mg/dL). Concerned about an infectious or para-autoimmune process, high-dose intravenous methylprednisolone (IVMP) was administered at 1,000 mg for five days. Due to difficulties with weaning from mechanical ventilation, five days of therapeutic plasmapheresis (PLEX) was completed after IVMP therapy. Extubation was achieved on the 10th day of admission, and moderate improvement in muscle weakness was observed. On the 15th day of admission, the patient was discharged to a rehabilitation facility and had achieved 4/5 muscle strength in her lower extremities. Upper extremity strength and neck flexion were 5/5. After completing three weeks of intensive inpatient rehabilitation, the patient was sent home but still required a cane for assistance with walking. At the one-year follow-up appointment, she endorsed only mild upper extremity paresthesia and had regained full motor strength. A repeat MRI of the brain and cervical spine was performed (Figure 3).
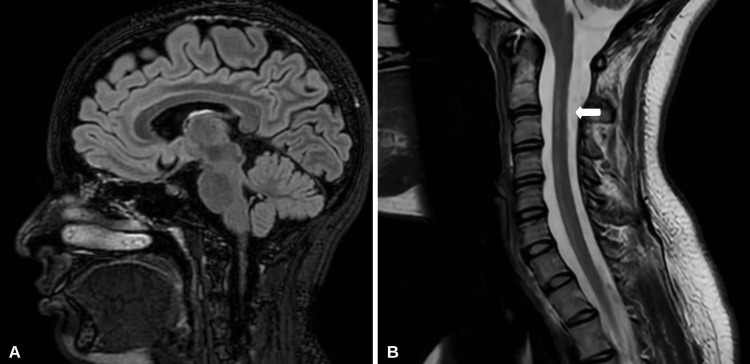
Figure 3. Repeat MRI of the brain and cervical spine.
(A) MRI of the brain T2/FLAIR sagittal sequence reveals resolution of previous splenium lesion on the corpus callosum. (B) MRI of the cervical spine T2/FLAIR sagittal sequence depicts improvement in hyperintense lesions (arrow).
MRI: magnetic resonance imaging, FLAIR: fluid-attenuated inversion recovery
Discussion
Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS), not to be confused with the Middle Eastern respiratory virus, is a clinical radiographic syndrome characterized by the presence of mild encephalopathy and a transient lesion within the splenium of the corpus callosum (MERS type I) or frontal white matter (MERS type II). The condition is more prevalent among children and less often reported in adults. The isolated splenial lesion is hypothesized to be secondary to rapid cytotoxic and intramyelinic edema secondary to neuroinflammation that resolves. The reported etiologies of MERS include metabolic abnormalities, infection, autoimmune conditions, toxins, and drug withdrawal [3,4]. The case reported here is that of MERS secondary to acute influenza infection. The patient had a significant improvement in her symptoms with high-dose IVMP and therapeutic plasmapheresis. The resolution of the splenium lesion on subsequent MRI and the resolution of mild encephalopathy are consistent with this diagnosis. Acute disseminated encephalomyelitis (ADEM) can be considered a differential diagnosis of MERS. However, ADEM typically presents with gadolinium enhancement (depending on the acuity) and without diffusion restriction. Furthermore, MRI findings of ADEM include asymmetric white matter disease less often involving the corpus callosum. In this case, the presence of an isolated non-enhancing diffusion-restricted lesion within the splenium of the corpus callosum points away from ADEM and toward MERS [5].
Longitudinally extensive transverse myelitis (LETM) is defined as a longitudinal lesion that extends through three or more spinal segments. LETM is typically associated with neuromyelitis optica spectrum disorder (NMOSD) and myelin oligodendrocyte glycoprotein-associated disease (MOGAD), where the latter condition has a more favorable outcome. The diagnosis of LETM is supported by the presence of bilateral symptoms, evidence of active neuroinflammation (i.e., pleocytosis, high IgG index, etc.), sensory level of the trunk, and the lack of a more appropriate diagnosis [2]. In the presented case, bilateral symptoms with weakness of the lower extremities and hyperreflexia were observed. The diagnosis was supported by a para-infectious process of an active influenza B infection.
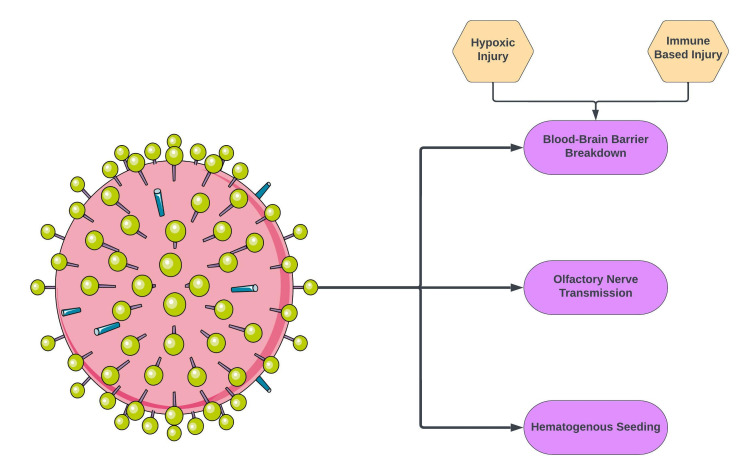
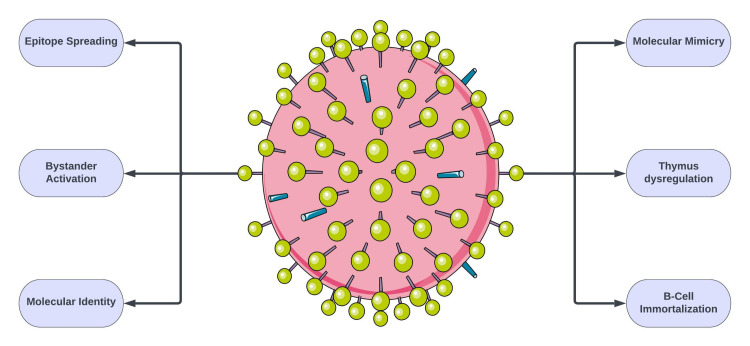
Infection of nervous tissue is observed in neurotropic viruses such as rabies, herpes simplex, and poliovirus. Radiographic findings of these viruses can vary. One study across two influenza seasons identified nine patients with influenza-associated encephalopathy (IAE). No evidence of influenza neuroinvasion was observed. Similarly, in this reported case, significant central nervous system (CNS) disease is clearly observed; however, neuroinvasion through influenza B CSF analysis is non-contributory [6]. One hypothesis is that active infection leads to overstimulation of the immune system and causes secondary neuroinflammation. Such events have been recorded in the past with immunization-related transverse myelitis and encephalitis. Furthermore, in this reported case, the utilization of known immunomodulators such as high-dose IVMP and PLEX led to improvement in symptoms. The lack of evidence on CSF is not limited only to the influenza virus. There have been multiple instances of significant CNS disease from a virus without direct neuroinvasive CSF evidence (i.e., monkeypox virus and coronavirus) [7]. The mechanisms by which viruses exert neurotropism include blood-brain barrier (BBB) breakdown, retrograde axonal transport from a peripheral nerve, and direct hematogenous seeding (Figure 4) [8]. The proposed mechanisms of autoimmunity include molecular mimicry, bystander activation, and B-cell immortalization, among others (Figure 5) [9].
Figure 4. Proposed mechanisms of viral neurotropism.
Image credits: Bahadar S. Srichawla
Figure 5. Proposed mechanisms of viral autoimmunity.
Image credits: Bahadar S. Srichawla
Literature review
A literature search was conducted utilizing PubMed/PubMed Central/Medical Literature Analysis and Retrieval System Online (MEDLINE) database to identify similar cases of influenza-mediated encephalitis and myelitis. Records identifying mild encephalitis with a reversible splenial lesion (MERS) and longitudinally extensive transverse myelitis (LETM) were included. A gray literature search was conducted reviewing the first 100 results of Google Scholar. Backward and forward citation was utilized to further expand the literature search. Records without the full text available, non-English, and not peer-reviewed were excluded. Data extracted includes reference, study type, sample size, patient age, gender, clinical symptoms, neuroimaging, diagnostic modality, management, and outcomes. A total of eight records are identified and included in Table 1.
Table 1. Records identified from literature search.
F: female, M: male, MRI: magnetic resonance imaging, PCR: polymerase chain reaction, LETM: longitudinally extensive transverse myelitis, MERS: mild encephalitis with a reversible splenial lesion, Gd+: gadolinium enhancement, AQP: aquaporin-4 antibody, NMO: neuromyelitis optica, T2WI: T2-weighted imaging, T1WI: T1-weighted imaging, IVIG: intravenous immunoglobulin, IVMP: intravenous methylprednisolone, MOG: myelin oligodendrocyte glycoprotein, MOGAD: myelin oligodendrocyte glycoprotein-associated disease, ANA: antinuclear antibody
| Reference | Study type | Extracted sample size | Age | Gender | Clinical symptom(s) | Diagnostics | Neuroimaging | Diagnosis | Management | Outcomes |
| Ka et al. (2015) [10] | Case series | 1 | 5 | F | Fever, lethargy, ataxia, mildly decreased consciousness | Influenza B via PCR | MRI: focal diffusion restriction in the corpus callosum | Influenza B MERS | Supportive | Recovery within 72 hours, discharged on day 5 |
| Takahashi et al. (2020) [11] | Case report | 1 | 50 | M | Fever, altered mental status, incoherent speech, upper extremity myoclonus, + Kernig sign | Influenza B via rapid antigen test | MRI: focal diffusion restriction within the splenium of the corpus callosum | Influenza B MERS | Supportive | Resolution |
| Takatsu et al. (2017) [12] | Case report | 1 | 31 | F | Mild encephalopathy, dysosmia | Influenza A via rapid antigen test | MRI: T2WI hyperintensity with diffusion restriction and T1WI hypointensity within the splenium of the corpus callosum | Influenza A MERS | Oseltamivir and supportive | Resolution of splenium lesion by day 14, full recovery |
| Imataka et al. (2020) [13] | Case report | 1 | 6 | F | Encephalopathy | Influenza A via rapid antigen test | MRI: T2WI hyperintensity with diffusion restriction and T1WI hypointensity within the splenium of the corpus callosum | Influenza A MERS | High-dose IVMP, IVIG | Recovery |
| Fluss et al. (2010) [14] | Case report | 1 | 2 | F | Irritable, absent speech, truncal ataxia | Influenza A via PCR | MRI: T2WI hyperintensity with diffusion restriction and T1WI hypointensity within the splenium of the corpus callosum | Influenza A MERS | Supportive | Recovery |
| Amano et al. (2014) [15] | Case report | 1 | 32 | M | Fever, dysesthesia, lower extremity muscle weakness, urinary retention | Influenza A via PCR, ANA titer 1:320, MOG titer 65,536 | MRI: non-enhancing T2WI hyperintensities from C2 to conus | Influenza A-positive MOGAD LETM | High-dose IVMP for five days | MOG titers decreased to 16,384 on day 7 and decreased to 4,096 on day 30 |
| Márquez et al. (2022) [16] | Case report | 1 | 30 | F | Urinary retention, lower extremity paraparesis, upper extremity weakness, optic nerve and macular edema | Influenza A rapid antigen test | MRI: T2WI hyperintensities from C4 to T12 | Influenza A LETM | High-dose IVMP for three days and six cycles of monthly intravenous cyclophosphamide | Recovery |
| Nakamura et al. (2011) [17] | Case report | 1 | 15 | F | Visual disturbance, dysuria, dysesthesia, hyperreflexia in the lower extremities | Influenza A via PCR, AQP4/NMO negative | MRI: T2WI hyperintensities from T2 to T5, Gd+ enhancing lesion in the right parietal lobe and hyperintense lesion in the left occipital and parietal white matter | Influenza A-associated NMO LETM | High-dose IVMP for three days | Recovery |
Of the eight identified patients, 6/8 (75%) are identified as females. The average age is 21.3 years. Of the cases, 4/8 (50%) are documented in the pediatric population (<18 years of age). Five of the eight identified patients (62.5%) of the reported cases are that of MERS and three (37.5%) of LETM. Influenza A was diagnosed in 6/8 (75%) of cases and influenza B in 2/8 (25%). Amano et al. reported the case of a 32-year-old male with fever, weakness of the lower extremities, and urinary retention that was positive for influenza A by PCR. Antinuclear antibody (ANA) and MOG antibody titers were significantly elevated at 1:320 and 65,536, respectively [15]. This case points to an autoimmune condition that developed secondary to influenza A infection rather than direct neuroinflammation. The patient reported by Márquez et al. had a concurrent history of systemic lupus erythematosus [16]. The case of Nakamura et al. had negative serum tests for AQP4/NMO antibodies, but the diagnosis was made based on revised criteria [17].
Edet et al. also reported the case of a 32-year-old male presenting with a subacute onset of cough and confusion. He was diagnosed with an acute influenza A infection, and neuroimaging was consistent with significant periventricular hyperintensities with a diffusion-restricting lesion in the corpus callosum. The diagnosis of IAE was made, and radiographic findings were more closely associated with ADEM. Despite aggressive treatment, the patient could not be weaned from mechanical ventilation and was subsequently terminally extubated [18].
Conclusions
The case of an 18-year-old female diagnosed with influenza B virus infection with clinical and radiographic findings of encephalomyelitis was discussed. This is the first reported case of influenza B with findings consistent with both mild encephalitis with a reversible splenial lesion (MERS) and longitudinally extensive transverse myelitis (LETM). Determining viral neurotropism remains difficult with negative CSF findings. Neuroinflammation from direct infectious involvement or secondary autoimmune reaction is discussed. A literature review on cases of influenza-associated MERS and LETM is included.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Neurologic complications of influenza B virus infection in adults, Romania. Popescu CP, Florescu SA, Lupulescu E, et al. Emerg Infect Dis. 2017;23:574–581. doi: 10.3201/eid2304.161317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myelitis and other autoimmune myelopathies. Lopez Chiriboga S, Flanagan EP. Continuum (Minneap Minn) 2021;27:62–92. doi: 10.1212/CON.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 3.Mild encephalitis/encephalopathy with reversible splenial lesion: a little-known entity with favourable prognosis. Gómez Iglesias P, López Valdés E, Vega Bayoll M, Gómez Ruíz MN. Neurologia (Engl Ed) 2020;35:581–583. doi: 10.1016/j.nrl.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Mild encephalitis/encephalopathy with a reversible splenial lesion: five cases and a literature review. Pan JJ, Zhao YY, Lu C, Hu YH, Yang Y. Neurol Sci. 2015;36:2043–2051. doi: 10.1007/s10072-015-2302-2. [DOI] [PubMed] [Google Scholar]
- 5.The spectrum of acute disseminated encephalomyelitis and mild encephalopathy with reversible splenial lesion. Sáenz-Farret M, Cansino-Torres MA, Sandoval-Rodríguez V, Navarro-Ibarra R, Zúñiga-Ramírez C. Case Rep Neurol Med. 2019;2019:9272074. doi: 10.1155/2019/9272074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Influenza-associated encephalopathy: no evidence for neuroinvasion by influenza virus nor for reactivation of human herpesvirus 6 or 7. van Zeijl JH, Bakkers J, Wilbrink B, Melchers WJ, Mullaart RA, Galama JM. Clin Infect Dis. 2005;40:483–485. doi: 10.1086/427027. [DOI] [PubMed] [Google Scholar]
- 7.Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. Zhou Z, Kang H, Li S, Zhao X. J Neurol. 2020;267:2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evidence of coronavirus (CoV) pathogenesis and emerging pathogen SARS-CoV-2 in the nervous system: a review on neurological impairments and manifestations. Jha NK, Ojha S, Jha SK, et al. J Mol Neurosci. 2021;71:2192–2209. doi: 10.1007/s12031-020-01767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses. 2019;11 doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mild encephalopathy with reversible splenial lesion: an important differential of encephalitis. Ka A, Britton P, Troedson C, et al. Eur J Paediatr Neurol. 2015;19:377–382. doi: 10.1016/j.ejpn.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) following influenza virus infection. Takahashi I, Yano H, Kinjo M. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-235461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mild encephalitis/encephalopathy with a reversible splenial lesion in an adult patient with influenza. Takatsu H, Ishimaru N, Ito M, Kinami S. Intern Med. 2017;56:3093–3095. doi: 10.2169/internalmedicine.8997-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Typical MRI imaging with clinically mild encephalitis/encephalopathy of a reversible splenial lesion (MERS) caused by influenza A virus. IM G, YO S. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7152623/pdf/IJPH-49-191.pdf. Iran J Public Health. 2020;49:191–192. [PMC free article] [PubMed] [Google Scholar]
- 14.Mild influenza-associated encephalopathy/encephalitis with a reversible splenial lesion in a Caucasian child with additional cerebellar features. Fluss J, Ferey S, Menache-Starobinski C, Delavelle J, Van Bogaert P, Vargas MI. Eur J Paediatr Neurol. 2010;14:97–100. doi: 10.1016/j.ejpn.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Influenza-associated MOG antibody-positive longitudinally extensive transverse myelitis: a case report. Amano H, Miyamoto N, Shimura H, et al. BMC Neurol. 2014;14:224. doi: 10.1186/s12883-014-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Extensive longitudinal transverse myelitis after influenza A virus infection in a patient with systemic lupus erythematosus. Márquez S, Vilá LM. Case Rep Rheumatol. 2022;2022:9506733. doi: 10.1155/2022/9506733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Influenza-associated monophasic neuromyelitis optica. Nakamura Y, Ikeda K, Yoshii Y, et al. Intern Med. 2011;50:1605–1609. doi: 10.2169/internalmedicine.50.5027. [DOI] [PubMed] [Google Scholar]
- 18.Acute influenza encephalitis/encephalopathy associated with influenza A in an incompetent adult. Edet A, Ku K, Guzman I, Dargham HA. Case Rep Crit Care. 2020;2020:6616805. doi: 10.1155/2020/6616805. [DOI] [PMC free article] [PubMed] [Google Scholar]