Abstract
Cigarette smoking is the main risk factor for head and neck cancer (HNC) and many HNC patients are active smokers at diagnosis. We conducted a systematic literature review and meta-analysis to quantify the survival impact of smoking cessation at or around the time of HNC diagnosis. We searched studies published until December 31, 2021, and used random-effects meta-analysis to pool study-specific estimates into summary hazard ratio (SHR) and corresponding 95% confidence intervals (CI). Sixteen studies were published between 1983 and 2021, and over 2300 HNC patients were included. Studies were diverse in terms of design, patients, tumours and treatment characteristics, and criteria used to discriminate quitters from continued smokers. HNC patients who quit smoking at or around diagnosis had significantly better overall survival than continued smokers (SHR 0.80, 95% CI 0.70–0.91, n studies = 10). A beneficial effect of post-diagnosis smoking cessation was suggested for other survival endpoints as well, but the results were based on fewer studies (n = 5) and affected by publication bias. Cessation counselling should be offered to all smokers who start a diagnostic workup for HNC and should be considered standard multidisciplinary oncological care for HNC patients. PROSPERO registration number CRD42021245560.
Subject terms: Head and neck cancer, Head and neck cancer
Background
Head and neck cancer (HNC), mostly in the form of squamous cell carcinoma (SCC), represents the sixth most common malignancy worldwide. HNC encompasses cancers at several sites (e.g. the oral cavity, oropharynx, nasopharynx, hypopharynx and larynx) that have been classically associated with smoking habits and alcohol intake, although differences across sites exist in terms of other risk factors, age at onset, and survival [1]. Overall, tobacco smoking, alcohol abuse, and human papillomavirus (HPV) infection represent the major risk factors for HNC development and account for the majority of cases globally [1, 2]. HNC incidence rates are on the rise in Western Europe and the USA, mainly because of HPV-related oropharyngeal tumours [3]. In developing countries, smoked and smokeless tobacco consumption still represent the main contributors [4]. The 5-year overall survival (OS) and disease-specific survival of HNC patients are around 46% and 63%, respectively [5], and improvements in survival have been slow to achieve in the past decades.
Quitting smoking after cancer diagnosis is thought to be associated with longer patients’ survival, however convincing evidence supporting this belief is still lacking except for lung cancer [6, 7]. This is a severe yet unappreciated knowledge gap considering that smoking status after diagnosis and during treatment is a clinically actionable item unlike a patient’s history of past tobacco exposure. For HNC, the studies that attempted to quantify the prognostic impact of post-diagnosis smoking cessation yielded conflicting results. For instance, Chen et al. found a favourable effect of smoking cessation on the risk of disease progression but not on patients’ OS; Warren et al. observed an improvement in OS among HNC patients who stopped smoking upon diagnosis, but limited to those of male sex; and Sandoval and colleagues did not detect any positive impact of post-diagnosis smoking cessation on any survival endpoint [8–10]. Despite the fact that the existing articles have found conflicting results on this important topic, no formal meta-analysis has been conducted to date.
In recent years, multidisciplinary therapeutic refinements have not led to appreciable outcome gains, whereby the prognosis of most patients with head and neck cancer remains dismal. Thus, comprehensive strategies centred on modifying preventable risk factors may have a relevant impact. Here, we conducted a systematic review and meta-analysis of the studies that quantified the effect of tobacco smoking cessation at or around the time of the diagnosis on the survival of HNC patients.
Methods
This meta-analysis is consistent with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [11]. The protocol was registered in the PROSPERO database (registration number CRD42021245560) [12].
Search strategy
MEDLINE and EMBASE databases were searched for articles published up to December 31, 2021 that investigated the effect on survival of quitting smoking at or around diagnosis in smokers diagnosed with HNC. In order to ensure greater sensitivity and minimise the probability to miss eligible articles, we opted to use a search string that would yield all existing articles focusing on the association between smoking cessation and cancer survival, regardless of the cancer site, and to remove all the articles focusing on cancers other than HNC during subsequent steps. Namely, the following search string was used: (smok*) AND (cease OR cessation OR quit* OR stop*) AND (cancer OR carcinoma OR tumour OR malignancy) AND (survival OR prognos* OR outcome OR mortality). No language, time or geographical restrictions were applied as long as an English abstract was provided.
Study selection
Titles and abstracts were screened by three independent researchers after removing duplicates. The articles considered as potentially eligible for inclusion were reviewed independently by two authors; in case of disagreement, a consensus choice was reached with a third author. Further eligible articles were searched for by means of backward citation chaining, i.e. by looking into the reference list of all articles read in full text and of previously published reviews and meta-analyses. HNC were defined as cancers of the lip, oral cavity, and pharynx (topography codes C00 through C14 according to the International Classification of diseases for Oncology (ICD-O), 3rd edition, 1st revision), and cancer of the larynx (ICD-O topography code -C33). Studies were eligible if they provided a hazard ratio (HR) and a measure of the statistical uncertainty (e.g., 95% confidence intervals (CI), standard errors, variance, or exact P values) quantifying the effect of quitting smoking at or around diagnosis on the survival of HNC patients (any of: overall survival; HNC-specific survival; disease-, progression- or distant recurrence-free survival; and locoregional control (LRC)). When no HR was provided in the article but a Kaplan–Meier survival curve was available, an unadjusted HR was calculated using Parmar’s method [13]. No inclusion criterion was set based on the minimum or median/mean duration of patients’ follow-up.
For this systematic review, quitters were defined as HNC patients who quit smoking from up to 12 months before diagnosis, at diagnosis, or afterwards. Studies failing in identifying the precise moment in which patients quit were excluded.
Data extraction
The following information was retrieved from all studies: country/region and year(s) in which the study was conducted; design; total number of smoking HNC patients and their breakdown into quitters and continued smokers; exact definition of quitters and continued smokers; distribution of patients in terms of sex and age, HNC anatomical subsite, histology (SCC vs. non-SCC), TNM stage at diagnosis, and treatments received; follow-up length; details on statistical methods and variables used for adjustment. The HR and 95% CI for the association between at/around diagnosis smoking status and patients' survival were inverted—if necessary— to have the “continued smokers” as the reference group.
Quality assessment
The assessments of possible biases and quality of each included study were performed by using the Quality in Prognosis Studies (QUIPS) tool, as recommended by the Prognosis Methods Group of the Cochrane Collaboration [14]. For each included paper, the QUIPS tool allowed to rate the risk of bias as high, moderate, or low, for each of the following six domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding and statistical analysis and reporting.
Statistical analysis
Study-specific HRs and corresponding 95% CI were transformed into log(HR) and corresponding variance using Greenland’s formula [15]. Meta-analysis was conducted when there were log(HR) from at least five independent papers for a given endpoint: this occurred for patients’ OS, “event-free” survival (which encompasses disease-, progression- and distant recurrence-free survival) [16], and LRC. Summary hazard ratios (SHRs) were calculated through random-effects models with maximum likelihood estimation [17]. The between-studies were quantified by the I2 statistics: [18] when this exceeded 50% (denoting substantial heterogeneity) [19], we used subgroup analysis, meta-regression, and leave-one-out sensitivity analysis to identify possible sources of the observed heterogeneity. Study characteristics that were used in subgroup analysis and meta-regression were the study country and publication year, patients’ demographics, the definition of quitters (strictly at or after diagnosis vs. up to 12 months before diagnosis), and whether the HR estimates were from multivariable regression models or were unadjusted. The Egger’s and Begg’s test were used to evaluating the presence of publication bias [20, 21], and the “trim and fill” method was applied (when publication bias was detected) to estimate the number of missing studies and provide an adjusted SHR based on the filled studies [22]. Finally, we conducted a sensitivity analysis considering the quality of the studies as assessed by means of the QUIPS tool.
Statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc, Cary, NC, USA) and R software, version 4.1.1. All tests were two-sided and statistical significance was set at P values below 0.05.
Results
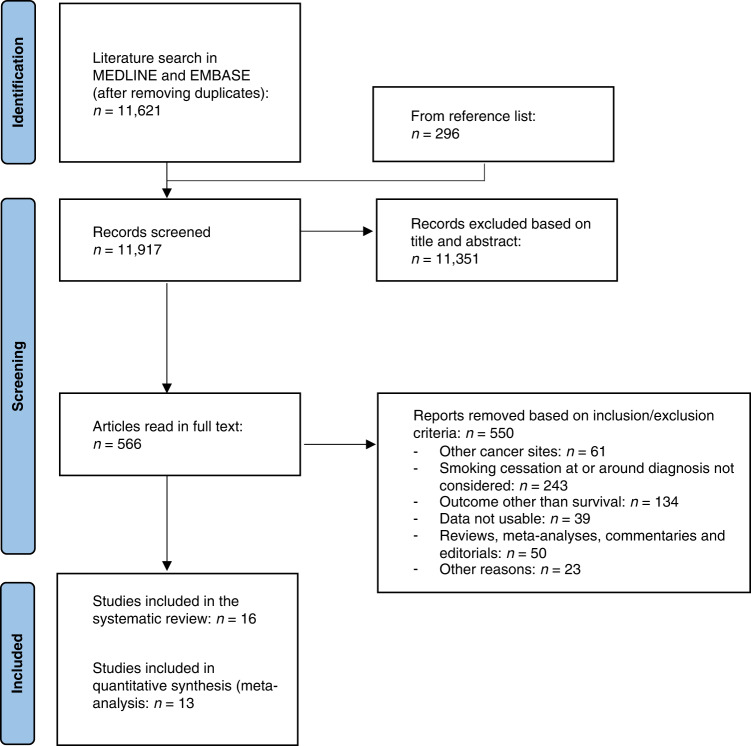
The literature search produced 11,621 non-duplicate entries, and an additional 296 articles were found by backward citation chaining (Fig. 1). Out of the 11,917 articles that were screened, 10,783 were removed based on their title, and an additional 568 articles were discarded after reading their abstract. Of the 566 articles that were read in full text, 550 were removed for not considering smoking cessation at/around diagnosis (n = 243), focusing on outcomes other than survival (n = 134), enrolling patients with cancer other than HNC (n = 61), reporting study results in a way that did not match our inclusion criteria (n = 39), reviews, meta-analyses, commentaries and editorials (n = 50) or other reasons (n = 23; Fig. 1). The complete list of all the articles that were removed upon reading the full text (alongside the reason for discarding) is available as Supplementary File S1. Eventually, 16 studies (published between 1983 and 2021) were found eligible and included in the systematic review: in thirteen articles (whose data were used for meta-analysis calculations), quitters and continued smokers were compared directly in terms of survival [8, 10, 23–32], while in the remaining three papers, two separate HRs were provided that compared the survival of either group to the third group of patients (e.g. never- or long former smokers), taken as the reference group [33–35]. These three studies were not used in meta-analysis but their main results were presented in a dedicated table and commented in the text.
Fig. 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
PRISMA flowchart of the literature search and articles selection for the systematic review and meta-analysis on the effect of quitting smoking at or around diagnosis on the survival of head and neck cancer patients. Adapted from Page MJ et al. [51]. For more information, visit: http://www.prisma-statement.org/.
The 16 articles that were included in the review were conducted in North America (n = 9), Europe (n = 5), and Asia (n = 2), and included over 3000 HNC patients (Table 1). The mean/median age at HNC diagnosis ranged between 55 and 70 years, and the follow-up length ranged from up to 2 years to 27.7 years (in only four studies, a minimum follow-up length was set as a criterion for inclusion of patients, ranging from 1 year in Koshiaris et al. to 12 years in Warren et al.). The studies by Tatekawa et al. and Benninger et al. were entirely based on patients with glottic cancer [23, 30], while the totality of patients included in the studies by Garden et al. and Ritoe et al. had oropharyngeal or, respectively, laryngeal cancers [27, 28]. The remaining studies encompassed patients with cancers at various anatomical head and neck subsites. The studies differed also in terms of stage distribution (from only early-stage HNC to a totality of Stage III/IV cancers). In eleven papers, the totality of HNC cases was SCC, while the remaining five papers did not provide details about whether non-SCC HNC cases were included. Quitters were mostly defined as patients who stopped smoking strictly at diagnosis or at some point thereafter, except in the studies by Warren et al. (from 12 months before to 1 month upon HNC diagnosis) and by Browman et al. (1993) (up to 12 weeks before) [9, 31]. The assessment of smoking status relied on the measurement of exhaled carbon monoxide (CO) in the two studies by Tatekawa et al. and Chen et al., and on self-reporting in the remaining studies.
Table 1.
Main characteristics of the articles included in the systematic review on the prognostic effect of quitting smoking at or around diagnosis on the survival of head and neck cancer patients.
| Author | Country | Distribution by subsite | Stage | Age (years) | Follow-up (years) | Treatment | Timing of quitting | Number of patientsa |
|---|---|---|---|---|---|---|---|---|
| Tatekawa et al. [23] | Japan | Glottis (100%) | Early stage (100%) | Median 70 range 48–86 | Up to 9.2 median 5.0 | RT only (65%), RT + CHT (35%) | Throughout treatment (confirmed by exhaled CO measurements) | 103 |
| Day et al. [24] | USA | Oral cavity (58%), HPV-neg oropharynx (5%), hypopharynx (8%), larynx (29%) | I (22%), II (15%), III (19%), IV (44%) | Median 57 IQR 50-61 | Up to 5.0 | surgery only (27%), surgery + RT (20%), surgery + CHT (21%), RT only (9%), CHT only (23%) | At 9 months after diagnosis | 117 |
| Fazel et al. [33]b | Germany | Oral cavity (8%), oropharynx (39%), nasopharynx (8%), hypopharynx (15%), larynx (25%), other (5%) | nr | Mean 64.5 range 40–98 | Up to 4.4 mean 1.6 | Surgery only (49%), primary RT + CHT (17%), primary RT (4%), adjuvant RT + CHT (19%), adjuvant RT (11%) | After diagnosis | 349 |
| Chen et al. [8] | Taiwan | Oral cavity (25%), oropharynx (42%), hypopharynx (21%), larynx (6%), other (6%) | I (5%), II (10%), III (17%), IV (68%) | Median 56 range 30–70 | Up to 3.9 median 2.7 | RT + CHT (100%) | Throughout treatment (confirmed by exhaled CO measurements) | 63 |
| Koshiaris et al. [25] | UK | nr | nr | Mean 60.1 | At least 1.0 up to 15 | Surgery (28%), RT (24%), CHT (17%) | Within the first year after diagnosis | 757 |
| Choi et al. [34]b | USA | Oral cavity (23%), oropharynx (39%), hypo/nasopharynx (6%), larynx (23%), other (9%) | 0 (2%), I (11%), II (9%), III (15%), IV (63%) | Mean 58.2 range 21–92 | Up to 8.5 median 4.6 | Surgery (53%), RT (87%), CHT (68%) | After diagnosis | 245 |
| Eichler et al. [35]b | Germany | Hypopharynx (7%), larynx (42%), hypopharynx/larynx (4%), other/missing (47%) | nr | Mean 58.0 | Up to 13.1 mean 5.4 | Total laryngectomy (100%) | After diagnosis | 82 |
| Deutschmann et al. [26] | USA | Oral cavity (37%), oropharynx (28%), larynx (29%), other (6%) | I (17%), II (9%), III (17%), IV (57%) | Mean 60 range 24–96 | Up to 5.0 mean 3.8 | Surgery only (20%), RT only (3%), surgery + RT (20%), CHT only (32%), surgery + CHT (26%) | After diagnosis | 282 |
| Garden et al. [27] | USA | Oropharynx (100%) | III (18%), IV-A (62%), IV-B (21%) | Median 55 range 35–80 | Up to 10.8 median 4.8 | RT alone (34%), RT + CHT (43%), induction CHT, then RT (12%), induction CHT, then RT + CHT (12%) | After diagnosis | 242 |
| Warren et al. [9] | USA | nr | Local (32%), regional (55%), distant (13%) | Mean 61 | Up to 27.7 at least 12.0 | nr | Between 12 months before and 1 month after diagnosis | 191 |
| Sandoval et al. [10] | Spain | Oral cavity (79%), oropharynx (21%) | nr | <60 (53%) ≥60 (47%) | At least 2.0 | Surgery with or without RT (63%), RT with or without CHT (29%), other (8%) | After diagnosis | 85 |
| Ritoe et al. [28] | Netherlands | Larynx (100%) | I (38%) II–IV (62%) | nr | Median 5.5 | Surgery alone, RT alone, surgery + RT (% not given) | After diagnosis | nr |
| Browman et al. [29] | USA and Canada | Oral cavity (17%), oropharynx (36%), hypopharynx (11%), larynx (36%) | III (58%) IV (42%) | Mean 60 range 18–72 | Up to 3.0 | RT (100%) | Throughout RT (confirmed by measuring blood cotinine) | 146 |
| Benninger et al. [30] | USA | Glottis (100%) | Early stage (100%) | Range 43–81 | At least 3.0 | RT (100%) | During or after completion of RT | 57 |
| Browman et al. [31] | USA and Canada | nr | III (49%) IV (51%) | Mean 62 | Up to 2.0 | RT (100%), concomitant CHT (49.6%) | Less than 12 weeks before diagnosis | 85 |
| Stevens et al. [32] | USA | Oral cavity (42%), pharynx (18%), larynx (40%) | Early stage (45%), late stage (55%) | mean 62 range 17–89 | Up to 22.0 | Surgery only (12%), RT only (48%), surgery + RT (35%), CHT (4%), none (1%) | After diagnosis | 154 |
CHT chemotherapy, CO carbon monoxide, HPV human papillomavirus, IQR interquartile range, RT radiotherapy, nr not reported.
aThis is the number of patients included in the analysis aiming at evaluating the prognostic effect of quitting smoking at or around diagnosis. For some studies, it is lower than the total number of patients (since the study may include never- and former smokers, as well as patients who were smokers at diagnosis and whose smoking status afterwards was unknown).
bNot included in the meta-analysis.
The results of the quality assessment conducted using the QUIPS tool are reported in Supplementary Table S1. Generally, the quality of the papers was considered as fair, since the risk of bias was never rated as “high” for any domain in any of the papers that were included. The most frequently encountered limitations (which account for the risk of bias being rated as “moderate” in some papers) were the lack of details on the proportion of eligible patients that did not agree to join the study (domain “study participation”); the fact that smoking status was self-reported (domain “prognostic factor measurement”); the lack of information on prognostic factors other than smoking (domain “study confounding”); and the lack of specification on important details of the statistical analysis (e.g. the moment in which the observation time started for each patient), the failure to integrate smoking status as a time-varying exposure in the statistical models, and the inability to adjust for potential confounders and to report results stratified by other prognostic factors (domain “statistical analysis and reporting”).
Quitting smoking at or around diagnosis was associated with a significant 20% gain in OS among HNC patients (SHR 0.80, 95% CI 0.70–0.91, n = 10) (Supplementary Table S2 and Fig. 2). The substantial heterogeneity of HRs across studies (I2 = 58.8%) was entirely attributable to the highly divergent HR for men (0.47, 95% CI 0.31–0.70) and women (1.96, 95% CI 1.09–3.45) reported by Warren et al. [9]: once these estimates were removed, the SHR became 0.80 (95% CI 0.70–0.93), with no heterogeneity (I2 = 0%). No other study provided HR stratified by sex. We conducted a subgroup analysis by dividing the studies into those in which the proportion of male patients was above or, respectively, below 79.5% (median value calculated across all studies): the SHR was 0.57 (95% CI 0.41–0.78, n = 6) in the former group of studies, and 0.85 (95% CI 0.75–0.97, n = 5) in the latter, the difference being statistically significant (QM test P = 0.007). Other subgroup analyses and meta-regression did not detect any variability of SHR according to study country (QM test P = 0.468), publication year (QM test P = 0.711), patients’ age (QM test P = 0.487) and timing at smoking cessation (QM test P = 0.908), nor when comparing studies that reported adjusted vs. unadjusted HR estimates (QM test P = 0.373). There was no evidence of publication bias (P = 0.978 for Egger’s test and P = 0.758 for Begg’s test). An HR for HNC-specific survival was available in only four studies and was generally very close to the corresponding HR for OS (Supplementary Table S3).
Fig. 2. Forest plot for the association between quitting smoking at or around the diagnosis and overall survival of patients with head and neck cancer.
HR hazard ratio, CI confidence intervals.
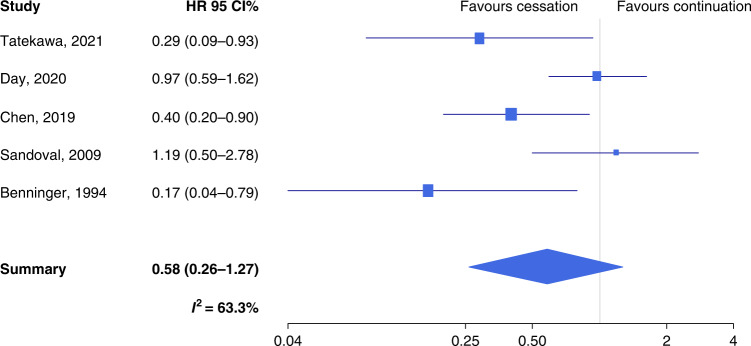
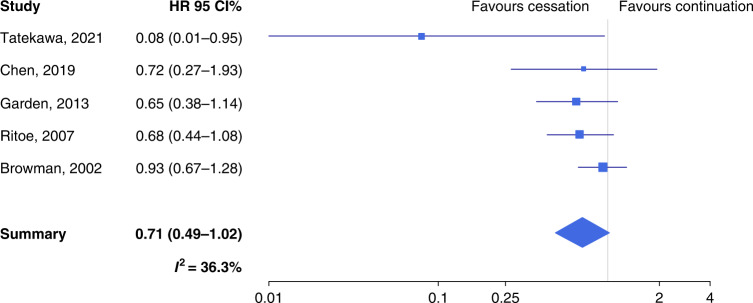
Quitters had a non-significantly longer event-free survival than continued smokers (SHR 0.58, 95% CI 0.26–1.27, n = 5) (Supplementary Table S3 and Fig. 3), with substantial heterogeneity (I2 = 63.3%). A suggestion for publication bias emerged from Egger’s test (P = 0.061). The trim-and-fill method detected two missing studies: once these were inputted, the SHR rose to 0.78 (95% CI 0.39–1.53). Also, LRC was longer among quitters than continued smokers (SHR 0.71, 95% CI 0.49–1.02), with acceptable heterogeneity (I2 = 36.3%) (Supplementary Table S3 and Fig. 4). Both the Begg’s (P = 0.021) and the Egger’s test (P = 0.008) detected significant publication bias, and the trim-and-fill method suggested that there were two missing studies. Upon imputation, the SHR changed only slightly (SHR 0.77) and remained close to statistical significance (95% CI 0.54–1.09). Similar to OS, also for event-free survival and LRC, the SHR was significantly lower when the proportion of male patients was above vs. below the median value (the QM test P for the difference was 0.020 and 0.033, respectively).
Fig. 3. Forest plot for the association between quitting smoking at or around diagnosis and event-free survival of patients with head and neck cancer.
HR hazard ratio, CI confidence intervals.
Fig. 4. Forest plot for the association between quitting smoking at or around the diagnosis and locoregional control among patients with head and neck cancer.
HR hazard ratio, CI confidence intervals.
In sensitivity analysis, the studies were split into two groups, depending on whether the risk of bias was rated as “moderate” in 0–2 vs. ≥3 domains of the QUIPS tool, and meta-analysis models were then separately fitted in the two groups of articles. There was no evidence that the SHRs differed between the two groups of articles: the P value for the difference was 0.94 for OS, 0.70 for event-free survival, and 0.10 for LRC.
As already mentioned, three studies could not be used for the meta-analysis because of the way the results were reported. In the study by Fazel et al. [33], the OS and HNC-specific survival of quitters were worse than among never smokers (HR were 2.43 and 1.33, respectively), but better than among continued smokers (HR were 4.52 and 4.77, respectively) (Table 2). Instead, Choi et al. did not detect any substantial differences between quitters and continued smokers in terms of both OS (HR for comparison to never smokers were 2.38 and 2.71, respectively) and HNC-specific survival (HR 2.38 and 2.07, respectively) [34]. Quitters appeared to have a mildly better prognosis than continued smokers in the study by Eichler et al. [35]: the HR comparing the OS to that of long former smokers (taken as reference) was 1.06 among quitters and 1.33 among continued smokers (Table 2).
Table 2.
Main results of the studies that reported the effect (hazard ratio, HR, and 95% confidence intervals, CI) of quitting or continuing smoking after diagnosis on the survival of head and neck cancer patients by using a third group of patients as reference.
| Author | Type of survival and category of reference | Smoking status | HR | 95% CI | Adjusting variables |
|---|---|---|---|---|---|
| Fazel et al. [33] | OS (ref: never smokers) | Quit | 2.43 | (1.04–5.69) | nr |
| Continued | 4.52 | (2.34–8.73) | |||
| H&N cancer-specific survival (ref: never smokers) | Quit | 1.33 | (0.84–6.70) | ||
| Continued | 4.77 | (1.84–12.18) | |||
| Choi et al. [34] | OS (ref: never smokers) | Quit | 2.38 | (1.29–4.36) | Age, sex, socio-economic status, alcohol intake, stage, comorbidities, other |
| Continued | 2.71 | (1.48–4.98) | |||
| H&N cancer-specific survival (ref: never smokers) | Quit | 2.38 | (1.13–5.01) | ||
| Continued | 2.07 | (0.96–4.47) | |||
| Eichler et al. [35] | OS (ref: former smokers who quit within two years before diagnosis) | Quit | 1.06 | (0.69–1.61) | Age, sex, socio-economic status, alcohol intake, other |
| Continued | 1.33 | (0.86–2.07) |
H&N head and neck, OS overall survival.
Discussion
We conducted a systematic review and meta-analysis of articles that investigated whether smoking cessation at or around diagnosis positively affects the survival of HNC patients. The 16 articles that were included were diverse under several regards, which translated into moderate-to-substantial heterogeneity of summary estimates, and publication bias also affected meta-analysis estimates for event-free survival and LRC. Despite these limitations, the meta-analysis showed a statistically significant ≈20% reduction in the hazard of death from any cause among quitters vs. continued smokers, and survival gains of comparable magnitude for the other survival endpoints that were considered.
HNC management is complicated because of the usually elderly age at diagnosis and the high prevalence of comorbidities [36]. In the last decades, much research focused on identifying the major positive prognostic an predictive factors in locally advanced HNC. A patient’s smoking status and cumulative tobacco exposure at diagnosis heavily affect treatment response, risk of recurrence and second primary tumours, and eventually survival of HNC patients [37–40]. However, only smoking cessation shortly before, at, or following a cancer diagnosis is an actionable item for clinicians, who can deliver smoking cessation support only during times of diagnostic workup and treatment. This is why we restricted the focus of our analysis to smokers who either quit or continued smoking at or around HNC diagnosis, which endows our results with immediate translational value. Besides their clinical value, our findings have important public health implications as well. On the one hand, the integration of smoking cessation programmes into diagnostic protocols may help prevent some of the several high-incidence and life-threatening health consequences of cigarette smoking also among those who eventually turn out to be free from HNC, with substantial gains in terms of life expectancy and quality of life for these subjects, as well as a reduction in healthcare costs associated with the treatment of those diseases. On the other hand, the successful implementation, among cancer patients, of an intervention that has the potential to reduce the hazard of death by 20% may progressively lead (along with improvements in systemic therapies) to an increase in the demand for follow-up medical care that the health services are called to meet.
Several mechanisms may explain our findings. Ongoing smoking exposure is associated with a higher incidence of late side effects during radiotherapy (RT) [41]. Smoking may also reduce the efficacy of RT through the mechanism of hypoxia’s induction [42, 43]. Moreover, actively smoking HNC patients undergoing primary surgery may be at increased risk of postoperative complications [44, 45]. Finally, active tobacco use may induce an ongoing suppression of immune response through the inhibition of cytotoxic T lymphocytes [46].
The positive impact of smoking cessation on HNC prognosis was more evident in studies with a higher proportion of men. We do not have a convincing explanation for it, yet a few tentative hypotheses can be made. Men are generally stronger smokers than women, thus cessation may have a greater impact for the former [47]. In addition, women relapse more often than men after a period of abstinence [48]. Finally, male and female HNC patients differ in terms of the prevalence of risk factors other than smoking and driver genes [49], and these might interact with smoking, through mechanisms that remain to be elucidated, in affecting survival.
To the best of our knowledge, this is the first meta-analysis investigating the impact of smoking cessation at or around diagnosis on survival outcome of smokers with HNC. Some of the limitations of our work originate from those affecting the studies that were included in it. The studies were largely heterogeneous in terms of design, patients characteristics, and the primary treatment strategy adopted. In only four papers, a minimum duration of follow-up was set as a criterion for inclusion of patients into the study, which may be suboptimal for endpoints whose incidence is low in the first months upon diagnosis. Only one included study reported information about HPV status, thus preventing from conducting stratified sub-analyses. None of the included papers positively mentioned to have included nonsquamous HNC cases (which differ from SCC cases under many regards, including prognosis), thus our results should be applied to SCC HNC patients only. Most studies provided no or limited data on tobacco lifetime exposure (e.g. pack/years). Moreover, smoking status after diagnosis was mostly self-reported, and the studies were inconsistent in the way the category of quitters was defined. Importantly, some important details were missing regarding how the statistical analysis of data was conducted, and in no article did the Authors explicitly mentioned that smoking status was entered as a time-varying exposure in the survival analyses (although in some studies, it was reported that smoking status was repeatedly assessed over time in an attempt to prevent misclassification of the exposure). Despite these several potential sources of bias, however, the results were confirmed in the sensitivity analysis carried out by splitting the available studies into lower- and higher-quality ones, which is reassuring as far as it concerns the reliability of the results of the meta-analysis. Finally, because of the lack of site-specific measures of association it was not possible to calculate SHR stratified by HNC subsite.
The survival of HNC patients remains unsatisfactory, and therapeutical advances are long overdue. Our findings warrant confirmation in well-designed and adequately sized investigations, and the effectiveness of smoking cessation programmes in the oncological setting, as well as the identification of factors associated with sustained cessation, remain to be fully determined. According to the GRADE framework [50], and also while taking into account the fact that our research question would be difficult to address by setting up a formal RCT (for ethical reasons, a smoking cessation programme should be proposed to all patients), we would rate the strength of the evidence as no more than “moderate” (i.e. “we are moderately confident in the effect estimate, as there is a possibility that the true effect is substantially different”), given the limitations of the currently available studies that we listed in the previous paragraphs. Yet, the strength of the recommendation needs to be rated as “strong”, given the known, broad health benefits that smoking cessation can bring to any smoker. Moreover, concerning our specific research question, the currently available data suggest that it is indeed never too late to quit smoking, and that cessation at or around diagnosis may significantly improve the survival of HNC patients. Thus, smoking cessation counselling ought to be fully recognised as a non-optional part of standard multidisciplinary oncological care.
Supplementary information
Author contributions
SC: conceptualisation, data curation, investigation, methodology, project administration, resources, supervision, validation and writing—original draft. MDR: conceptualisation, data curation, investigation, methodology, project administration and writing—original draft. VV: conceptualisation, data curation, investigation, project administration, validation and writing—original draft. OD’E: data curation, formal analysis, investigation, methodology, software, visualisation, writing—review and editing. PB: conceptualisation, investigation, methodology, resources, supervision, writing—original draft. LGL: investigation, methodology and writing—original draft. VS: data curation, investigation, validation and writing—original draft. OG: conceptualisation, investigation, resources, supervision and writing—review & editing. MT: data curation, formal analysis, software, visualisation and writing—review and editing. SR: formal analysis, funding acquisition, methodology, software, visualisation and writing—review and editing. CS: investigation, resources, supervision and writing—review and editing. FC: data curation, investigation, validation and writing—original draft. BB: investigation, methodology, supervision and writing—review and editing. SG: conceptualisation, formal analysis, funding acquisition, methodology, project administration, resources, software, supervision, visualisation and writing—review & editing.
Funding
This work was supported by the Italian Ministry of Health (Ricerca Corrente and 5 ×1000 funds), no grant number applicable. The Funder had no role in designing and conducting the study, in the writing of the manuscript, and in the decision to submit it in its present form.
Data availability
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01945-w.
References
- 1.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow LQM. Head and neck cancer. Reply. N Engl J Med. 2020;382:e57. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 3.Menezes FDS, Fernandes GA, Antunes JLF, Villa LL, Toporcov TN. Global incidence trends in head and neck cancer for HPV-related and -unrelated subsites: a systematic review of population-based studies. Oral Oncol. 2021;115:105177. doi: 10.1016/j.oraloncology.2020.105177. [DOI] [PubMed] [Google Scholar]
- 4.Hashim D, Genden E, Posner M, Hashibe M, Boffetta P. Head and neck cancer prevention: from primary prevention to impact of clinicians on reducing burden. Ann Oncol. 2019;30:744–56. doi: 10.1093/annonc/mdz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiwana MS, Wu J, Hay J, Wong F, Cheung W, Olson RA. 25 year survival outcomes for squamous cell carcinomas of the head and neck: population-based outcomes from a Canadian province. Oral Oncol. 2014;50:651–6. doi: 10.1016/j.oraloncology.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Sitas F, Weber MF, Egger S, Yap S, Chiew M, O’Connell D. Smoking cessation after cancer. J Clin Oncol. 2014;32:3593–5. doi: 10.1200/JCO.2014.55.9666. [DOI] [PubMed] [Google Scholar]
- 7.Caini S, Del Riccio M, Vettori V, Scotti V, Martinoli C, Raimondi S. Quitting Smoking At or Around Diagnosis Improves the Overall Survival of Lung Cancer Patients: A Systematic Review and Meta-Analysis. J Thorac Oncol. 2022;17:623–36. doi: 10.1016/j.jtho.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Chen JL, Shen CW, Wang CC, Huang YS, Chen JP, Chiang CH, et al. Impact of smoking cessation on clinical outcomes in patients with head and neck squamous cell carcinoma receiving curative chemoradiotherapy: a prospective study. Head Neck. 2019;41:3201–10. doi: 10.1002/hed.25814. [DOI] [PubMed] [Google Scholar]
- 9.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–10. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 10.Sandoval M, Font R, Mañós M, Dicenta M, Quintana MJ, Bosch FX, et al. The role of vegetable and fruit consumption and other habits on survival following the diagnosis of oral cancer: a prospective study in Spain. Int J Oral Maxillofac Surg. 2009;38:31–39. doi: 10.1016/j.ijom.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health Research. PROSPERO International prospective register of systematic reviews. Accessed 28 April 2021. https://www.crd.york.ac.uk/PROSPERO/.
- 13.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 15.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 16.Michiels S, Le Maître A, Buyse M, Burzykowski T, Maillard E, Bogaerts J, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: meta-analyses of individual patient data. Lancet Oncol. 2009;10:341–50. doi: 10.1016/S1470-2045(09)70023-3. [DOI] [PubMed] [Google Scholar]
- 17.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Cochrane Handbook for Systematic Reviews of Interventions. Chapter 10: Analysing data and undertaking meta-analyses. Accessed 21 December 2021. https://training.cochrane.org/handbook/current/chapter-10.
- 20.Egger M, Davey, Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Tatekawa S, Shimamoto S, Miyata Y, Yoshino Y, Hirata T, Tamari K, et al. Monitoring expiratory carbon monoxide to study the effect of complete smoking cessation on definitive radiation therapy for early stage glottic carcinoma. Acta Oncol. 2021;60:582–8. doi: 10.1080/0284186X.2020.1865563. [DOI] [PubMed] [Google Scholar]
- 24.Day AT, Dahlstrom KR, Lee R, Karam-Hage M, Sturgis EM. Impact of a tobacco treatment program on abstinence and survival rates among current smokers with head and neck squamous cell carcinoma. Head Neck. 2020;42:2440–52. doi: 10.1002/hed.26268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshiaris C, Aveyard P, Oke J, Ryan R, Szatkowski L, Stevens R, et al. Smoking cessation and survival in lung, upper aero-digestive tract and bladder cancer: cohort study. Br J Cancer. 2017;117:1224–32. doi: 10.1038/bjc.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutschmann MW, Sykes KJ, Harbison J, Cabrera-Muffly C, Shnayder Y. The impact of compliance in posttreatment surveillance in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141:519–25. doi: 10.1001/jamaoto.2015.0643. [DOI] [PubMed] [Google Scholar]
- 27.Garden AS, Kies MS, Morrison WH, Weber RS, Frank SJ, Glisson BS, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol. 2013;8:21. doi: 10.1186/1748-717X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritoe SC, Verbeek AL, Krabbe PF, Kaanders JH, van den Hoogen FJ, Marres HA. Screening for local and regional cancer recurrence in patients curatively treated for laryngeal cancer: definition of a high-risk group and estimation of the lead time. Head Neck. 2007;29:431–8. doi: 10.1002/hed.20534. [DOI] [PubMed] [Google Scholar]
- 29.Browman GP, Mohide EA, Willan A, Hodson I, Wong G, Grimard L, et al. Association between smoking during radiotherapy and prognosis in head and neck cancer: a follow-up study. Head Neck. 2002;24:1031–7. doi: 10.1002/hed.10168. [DOI] [PubMed] [Google Scholar]
- 30.Benninger MS, Gillen J, Thieme P, Jacobson B, Dragovich J. Factors associated with recurrence and voice quality following radiation therapy for T1 and T2 glottic carcinomas. Laryngoscope. 1994;104:294–8. doi: 10.1288/00005537-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Browman GP, Wong G, Hodson I, Sathya J, Russell R, McAlpine L, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N. Engl J Med. 1993;328:159–63. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 32.Stevens MH, Gardner JW, Parkin JL, Johnson LP. Head and neck cancer survival and life-style change. Arch Otolaryngol. 1983;109:746–9. doi: 10.1001/archotol.1983.00800250040009. [DOI] [PubMed] [Google Scholar]
- 33.Fazel A, Quabius ES, Gonzales-Donate M, Laudien M, Herzog A, Kress K, et al. Alteration of smoking habit at time of first diagnosis influences survival of patients with HNSCC. Mol Clin Oncol. 2020;13:50. doi: 10.3892/mco.2020.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi SH, Terrell JE, Bradford CR, Ghanem T, Spector ME, Wolf GT, et al. Does quitting smoking make a difference among newly diagnosed head and neck cancer patients? Nicotine Tob Res. 2016;18:2216–24. doi: 10.1093/ntr/ntw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eichler M, Keszte J, Meyer A, Danker H, Guntinas-Lichius O, Oeken J, et al. Tobacco and alcohol consumption after total laryngectomy and survival: a German multicenter prospective cohort study. Head Neck. 2016;38:1324–9. doi: 10.1002/hed.24436. [DOI] [PubMed] [Google Scholar]
- 36.Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–83. doi: 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 37.Do KA, Johnson MM, Doherty DA, Lee JJ, Wu XF, Dong Q, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States) Cancer Causes Control. 2003;14:131–8. doi: 10.1023/A:1023060315781. [DOI] [PubMed] [Google Scholar]
- 38.Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, Benner SE, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–50. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 39.Meyer F, Bairati I, Fortin A, Gélinas M, Nabid A, Brochet F, et al. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: a randomized trial among head and neck cancer patients. Int J Cancer. 2008;122:1679–83. doi: 10.1002/ijc.23200. [DOI] [PubMed] [Google Scholar]
- 40.Duffy SA, Ronis DL, McLean S, Fowler KE, Gruber SB, Wolf GT, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27:1969–75. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith J, Nastasi D, Tso R, Vangaveti V, Renison B, Chilkuri M. The effects of continued smoking in head and neck cancer patients treated with radiotherapy: a systematic review and meta-analysis. Radiother Oncol. 2019;135:51–57. doi: 10.1016/j.radonc.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Chen AM, Chen LM, Vaughan A, Farwell DG, Luu Q, Purdy JA, et al. Head and neck cancer among lifelong never-smokers and ever-smokers: matched-pair analysis of outcomes after radiation therapy. Am J Clin Oncol. 2011;34:270–5. doi: 10.1097/COC.0b013e3181dea40b. [DOI] [PubMed] [Google Scholar]
- 43.Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126:1131–4. doi: 10.1001/archsurg.1991.01410330093013. [DOI] [PubMed] [Google Scholar]
- 44.Fiorini FR, Deganello A, Larotonda G, Mannelli G, Gallo O. Tobacco exposure and complications in conservative laryngeal surgery. Cancers (Basel) 2014;6:1727–35. doi: 10.3390/cancers6031727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO). WHO tobacco knowledge summaries: tobacco and postsurgical outcomes. Accessed 28 January 2022. https://apps.who.int/iris/handle/10665/330485.
- 46.de la Iglesia JV, Slebos RJC, Martin-Gomez L, Wang X, Teer JK, Tan AC, et al. Effects of tobacco smoking on the tumor immune microenvironment in head and neck squamous cell carcinoma. Clin Cancer Res. 2020;26:1474–85. doi: 10.1158/1078-0432.CCR-19-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters SA, Huxley RR, Woodward M. Do smoking habits differ between women and men in contemporary Western populations? Evidence from half a million people in the UK Biobank study. BMJ Open. 2014;4:e005663. doi: 10.1136/bmjopen-2014-005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith PH, Bessette AJ, Weinberger AH, Sheffer CE, McKee SA. Sex/gender differences in smoking cessation: a review. Prev Med. 2016;92:135–40. doi: 10.1016/j.ypmed.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mundi N, Ghasemi F, Zeng PYF, Prokopec SD, Patel K, Kim HAJ, et al. Sex disparities in head & neck cancer driver genes: an analysis of the TCGA dataset. Oral Oncol. 2020;104:104614. doi: 10.1016/j.oraloncology.2020.104614. [DOI] [PubMed] [Google Scholar]
- 50.The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Accessed 5 July 2022. https://gdt.gradepro.org/app/handbook/handbook.html.
- 51.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.