Abstract
Postural orthostatic tachycardia syndrome (POTS) is a cardiovascular autonomic disorder characterized by excessive heart rate increase on standing, leading to debilitating symptoms with limited therapeutic possibilities. Proteomics is a large-scale study of proteins that enables a systematic unbiased view on disease and health, allowing stratification of patients based on their protein background. The aim of the present study was to determine plasma protein biomarkers of POTS and to reveal proteomic pathways differentially regulated in POTS. We performed an age- and sex-matched, case–control study in 130 individuals (case–control ratio 1:1) including POTS and healthy controls. Mean age in POTS was 30 ± 9.8 years (84.6% women) versus controls 31 ± 9.8 years (80.0% women). We analyzed plasma proteins using data-independent acquisition (DIA) mass spectrometry. Pathway analysis of significantly differently expressed proteins was executed using a cutoff log2 fold change set to 1.2 and false discovery rate (p-value) of < 0.05. A total of 393 differential plasma proteins were identified. Label-free quantification of DIA-data identified 30 differentially expressed proteins in POTS compared with healthy controls. Pathway analysis identified the strongest network interactions particularly for proteins involved in thrombogenicity and enhanced platelet activity, but also inflammation, cardiac contractility and hypertrophy, and increased adrenergic activity. Our observations generated by the first use a label-free unbiased quantification reveal the proteomic footprint of POTS in terms of a hypercoagulable state, proinflammatory state, enhanced cardiac contractility and hypertrophy, skeletal muscle expression, and adrenergic activity. These findings support the hypothesis that POTS may be an autoimmune, inflammatory and hyperadrenergic disorder.
Subject terms: Biomarkers, Biomarkers, Cardiology, Medical research
Introduction
Postural orthostatic tachycardia syndrome (POTS) is a cardiovascular autonomic disorder characterized by an excessive heart rate increase of > 30 beats per minute (bpm) or a heart rate of > 120 bpm and symptoms of orthostatic intolerance when assuming upright posture1. The syndrome affects predominantly young women (70–80%) within a range of 15–40 years old. It is estimated that POTS affects approximately 3 million people in the United States2, and since the start of COVID-19 pandemic it has been observed that individuals may also develop POTS following a SARS-CoV-2 infection3,4 which implies an even greater clinical burden.
The heterogenous and multifactorial etiology, poses substantial challenges for physicians striving to provide targeted treatment options for patients, and few studies have identified promising biomarkers5,6. A greater mechanistic understanding and clinical phenotyping, as well as detection of novel POTS biomarkers are required to gauge disease activity, severity and response to therapy.
Data independent acquisition (DIA) is a mass spectrometric technique, also known as sequential window acquisition of all theoretical mass spectra (SWATH-MS), offering a powerful proteomic approach for unbiased discovery, generating comprehensive, digital proteome maps and highly reproducible analysis of hundreds of proteins in the plasma7. SWATH is a specific variant of DIA technology, coupled with peptide spectral library match enables precise label-free quantification of the proteome8. Compared with conventional mass spectrometry, DIA is based on the fragmentation of all precursor ions identified in a scan rather than only acquiring fragmentation data from a predefined set of selected precursor ions. This improves the depth of sample analysis, reproducibility of the protein identification and both accuracy and precision of the data quantification. In the present study, we aimed to identify the proteomic footprint of POTS versus healthy controls using the unbiased proteomics DIA label-free quantification method.
Methods
Ethical approval
The study has been approved by the Regional Ethical Review Board in Lund, Sweden (no 82/2008), with amendment in 2017. All study participants provided informed written consent. All procedures were carried out in line with relevant current guidelines and regulations.
Study population and design
We performed a sex- and age-matched case–control study, analyzing plasma samples of 130 individuals (case–control ratio 1:1). Sixty-five POTS patients with a sustained heart rate increase of ≥ 30 bpm during head-tilt (HUT) and chronic symptoms for ≥ 6 months without orthostatic hypotension1,2, were randomly selected from the Syncope Study of Unselected Population in Malmö (SYSTEMA) cohort (including moderate to severe POTS with maximum delta heart rate at 5 min ranging from 30 to 73 bpm, regardless of history of complete loss of consciousness. SYSTEMA enrolled over 2200 patients investigated for syncope at the Skåne University Hospital in Malmö, Sweden between 2008 and 2020. Details of the SYSTEMA cohort are described elsewhere9. Only participants with available plasma samples were included.
Sixty-five healthy age- and sex-matched controls (without CVD, diabetes autoimmune disease, immunosuppressive treatment, history of cancer or other proliferative disease, and psychiatric disorder) were included. Healthy controls were recruited through personal invitation, among healthy medical students, hospital staff and younger participants of parallel population-based epidemiological programs in Malmö, Sweden. No financial incentive was given. Controls completed a self-administered questionnaire regarding their past and current medical history, and were examined with ECG, blood pressure measurements and active standing test at the Clinical Research Unit of Dept. of Internal Medicine, Skåne University Hospital, Malmö, Sweden. Controls had no history of syncope, POTS, orthostatic intolerance, cancer, cardiovascular or endocrine disease.
Investigation protocol
Cases and controls were examined in a dedicated clinical research unit at Skåne University Hospital, Malmö. All cardiovascular pharmacological agents were discontinued 72 h prior to examination. Study participants performed an active standing test, with 10-min rest in the supine position prior to standing. Blood pressure and heart rate were measured twice in the supine position by a validated automated oscillometer device (Omron, Kyoto, Japan), and then after 1, 3, 5, and 10 min of standing. An average of two measurements was used for group comparisons.
Mass spectrometric analysis
The mass spectrometry proteomics data have been deposited at the ProteomeXchange Consortium via the PRIDE10 partner repository with the dataset identifier PXD031458. Details about the plasma sample preparation can be found in the Supplement. Twenty µL of each sample were loaded into Evosep tip for the LC–MS/MS analysis11. The plasma samples were analyzed on a timsTOF Pro mass spectrometer (Bruker Daltonik, Germany) coupled with Evosep One (Evosep, Odense, Denmark) with 60 samples per day (SPD) and DIA-PASEF long gradient method. Solvent A (0.1% FA in water) and solvent B (0.1% FA in ACN) were used to create gradient and the analytical column, EV1109 (150 μm × 8 cm, 1.5 µm) used for the sample analysis. Details on data analysis using DIA method is outlined in the Supplement.
Protein pathway analysis
Protein pathways were analyzed using the bioinformatic tool STRING (Search Tool for Retrieval of Interacting Genes/Proteins) to acquire protein–protein interaction (PPI) networks12. The STRING database aims to integrate all known and predicted associations between proteins, including both physical interactions as well as functional associations. The significantly up- or down-regulated proteins were submitted separately to full STRING network search with medium interaction score (0.4) and a false discovery rate (FDR) ≤ 0.05 was used when classifying the cellular component (Gene Ontology) of each protein. For each obtained network, PPI enrichment p-value was reported. p-values are corrected for multiple testing within each category using the Benjamini–Hochberg procedure.
Statistical analyses
Continuous data are shown as mean ± standard deviation (all variables were normally distributed), whereas frequencies are used to describe categorical data. Continuous variables were compared using the paired Student’s t test for matched couples. Paired and multiple proportions were compared using Pearson’s chi-square test.
DIA data were analyzed using Spectronaut (version 15, Biognosys, Switzerland) using the directDIA workflow. The data was based on extracted maximum intensities of both precursors and fragment ions. Default settings of the Spectronaut method were applied for both identification and quantification of peptides and proteins. Data was filtered by q-value and an automatic strategy was used for the cross-run normalization. The protein quantitative report was exported from Spectronaut and downstream statistical analysis was performed with Perseus 1.6.14.013. Label-free quantification (LFQ) of DIA data was log2-transformed and normalized to median value of each sample.
Statistical analyses were performed in IBM SPSS Statistics 27 (IBM Corporation, Armonk, NY, USA), two-sample test (unpaired Student’s t test) was performed for the proteomics data within the Perseus Software, and data were plotted with R Studio (Boston, MA, USA).
Results
Study characteristics of the population are shown in Table 1. The mean age of POTS and healthy controls were 30 ± 9.8 years (84.6% women) and 31 ± 9.8 years (80.0% women), receptively. Supine heart rate (70.5 ± 11.6 vs. 62.9 ± 10.1 bpm, p < 0.001) and heart rate after 5 min of standing were significantly higher in POTS versus healthy controls (108.7 ± 17.4 bpm vs. 82.7 ± 12.7 bpm, p < 0.001).
Table 1.
Study characteristics of the population.
| Characteristics | POTS (n = 65) | Healthy controls (n = 65) | p-value |
|---|---|---|---|
| Mean age, years ± SD (age range) | 30 ± 9.8 (17–61) | 31 ± 9.8 (16–62) | 0.37 |
| Female, n (%) | 55 (84.6) | 52 (80.0) | 0.49 |
| Systolic BP supine, mmHg ± SD | 116.1 ± 12.6 | 112.8 ± 10.3 | 0.11 |
| Diastolic BP supine, mmHg ± SD | 71.7 ± 8.8 | 67.9 ± 7.9 | 0.01 |
| Heart rate supine, bpm ± SD | 70.5 ± 11.6 | 62.9 ± 10.1 | < 0.001 |
| Heart rate 5 min standing, bpm ± SD | 108.7 ± 17.4 | 82.7 ± 12.7 | < 0.001 |
BP blood pressure, bpm beats per minute, POTS postural orthostatic tachycardia syndrome, SD standard deviation.
Explorative proteomic analysis
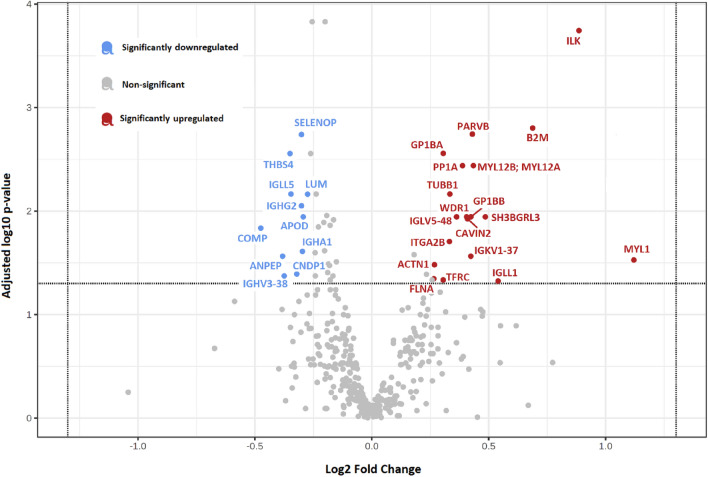
In the explorative analysis, a total of 393 unbiased plasma proteins were detected. The complete list of proteins can be found in the Supplementary Table S1. Overall, label-free protein quantification identified proteins significantly differently expressed in POTS, 19 upregulated and 11 downregulated proteins (Fig. 1, Table 2) using a cutoff fold change set to 1.2 and FDR (q-value) of < 0.05.
Figure 1.
Proteomic footprint of significantly dysregulated proteins in POTS. Volcano plot illustrating 30 significantly dysregulated proteins in POTS. Eleven downregulated proteins in blue and 19 upregulated proteins in red. Complete names of proteins are found in Table 2.
Table 2.
Significantly dysregulated proteins in POTS identified using data-independent acquisition (DIA) label-free quantification mass spectrometry.
| Protein name | Gene name | Fold change | Log2 Fold change | p-value |
|---|---|---|---|---|
| Upregulated proteins | ||||
| Alpha-actinin-1 | ACTN1 | 1.20 | 0.27 | 0.003 |
| Beta-2-microglobulin | B2M | 1.61 | 0.69 | < 0.001 |
| Beta-parvin | PARVB | 1.35 | 0.43 | < 0.001 |
| Caveolae-associated protein 2 | CAVIN2 | 1.33 | 0.41 | 0.001 |
| Filamin-A | FLNA | 1.20 | 0.27 | 0.006 |
| Immunoglobulin lambda-like polypeptide 1 | IGLL1 | 1.45 | 0.54 | 0.006 |
| Integrin alpha-IIb | ITGA2B | 1.26 | 0.33 | 0.001 |
| Integrin-linked protein kinase | ILK | 1.85 | 0.89 | < 0.001 |
| Myosin light chain 1/3, skeletal muscle isoform | MYL1 | 2.17 | 1.12 | 0.003 |
|
Myosin regulatory light chain 12B Myosin regulatory light chain 12A |
MYL12B MYL12A |
1.35 | 0.43 | < 0.001 |
| Peptidyl-prolyl cis–trans isomerase A | PP1A | 1.31 | 0.39 | < 0.001 |
| Platelet glycoprotein Ib alpha chain | GP1BA | 1.23 | 0.30 | < 0.001 |
| Platelet glycoprotein Ib beta chain | GP1BB | 1.34 | 0.42 | 0.001 |
| Probable non-functional immunoglobulin lambda variable 5–48 | IGLV5-48 | 1.29 | 0.36 | 0.001 |
| Probable non-functional immunoglobulin kappa variable 1–37 | IGKV1-37 | 1.34 | 0.42 | 0.002 |
| SH3 domain-binding glutamic acid-rich-like protein 3 | SH3BGRL3 | 1.40 | 0.49 | 0.001 |
| Transferrin receptor protein 1 | TFRC | 1.24 | 0.31 | 0.006 |
| Tubulin beta-1 chain | TUBB1 | 1.26 | 0.34 | < 0.001 |
| WD repeat-containing protein 1 | WDR1 | 1.32 | 0.41 | 0.001 |
| Downregulated proteins | ||||
| Aminopeptidase N | ANPEP | 0.77 | − 0.38 | 0.002 |
| Apolipoprotein D | APOD | 0.82 | − 0.29 | 0.001 |
| Beta-Ala-His dipeptidase | CNDP1 | 0.80 | − 0.32 | 0.005 |
| Cartilage oligomeric matrix protein | COMP | 0.72 | − 0.48 | 0.001 |
| Immunoglobulin heavy constant alpha 1 | IGHA1 | 0.81 | − 0.30 | 0.002 |
| Immunoglobulin heavy constant gamma 2 | IGHG2 | 0.82 | − 0.29 | < 0.001 |
| Immunoglobulin lambda-like polypeptide 5 | IGLL5 | 0.79 | − 0.35 | < 0.001 |
| Lumican | LUM | 0.83 | − 0.26 | < 0.001 |
| Probable non-functional immunoglobulin heavy variable 3–38 | IGHV3-38 | 0.77 | − 0.37 | 0.005 |
| Selenoprotein P | SELENOP | 0.81 | − 0.30 | < 0.001 |
| Thrombospondin-4 | THBS4 | 0.78 | − 0.35 | < 0.001 |
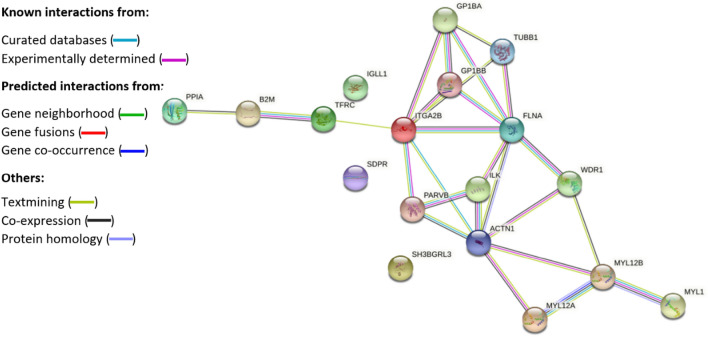
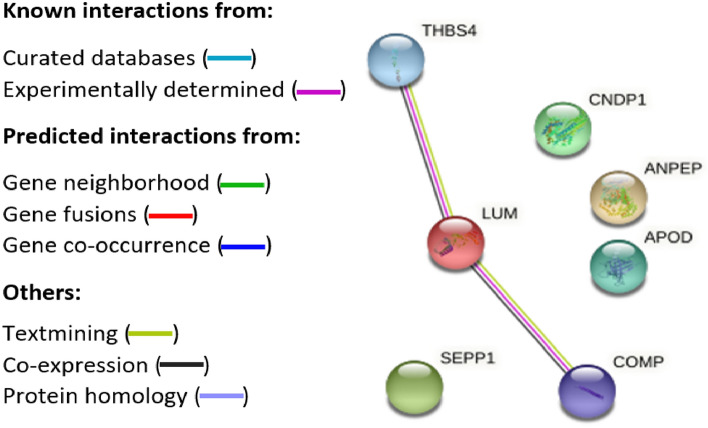
Label-free protein quantification identified 30 significantly dysregulated proteins in POTS, 19 upregulated and 11 downregulated proteins using a cutoff log2 fold change set to 1.2 and false discovery rate (p-value) of < 0.05. Proteins in bold displayed a strong protein–protein interaction in the STRING pathway analysis (Figs. 2, 3).
Pathway analysis
STRING pathway analysis of proteins provided a network of upregulated (protein–protein interaction (PPI) enrichment p-value: < 1.0e−16, Fig. 2) and downregulated (PPI enrichment p-value: 0.0085, Fig. 3) proteins in POTS. The strongest network interaction among upregulated proteins was particularly found for proteins related to platelet aggregation (FDR 6.88e−06, Supplementary Table S2) and activation (FDR 1.39e−05, Supplementary Table S2). Proteins with significant p-value (< 0.05) without application of fold-change are displayed in the pathway analysis in the Supplementary Fig. 1. As shown, the proteins did not differ much compared with Fig. 2 which identified 19 proteins versus 21 proteins. We also noted network interactions for proteins involved in proinflammatory states, enhanced cardiac contractility and hypertrophy, as well as skeletal muscle expression and adrenergic activity.
Figure 2.
Protein–protein pathway analysis illustrating significantly upregulated proteins in POTS. Protein–protein interaction analysis of 18 out of 19 upregulated proteins with association networks detected by STRING in POTS. In the network figures, each protein is represented by a colored node while protein–protein interaction and association are represented by a line. The strongest network interactions in POTS were particularly associated with a hypercoagulable state and upregulated expression of proteins related to platelet activity, but also enhanced inflammation, cardiac contractility and hypertrophy, skeletal muscle expression, and adrenergic activity. Complete names of proteins are found in Table 2.
Figure 3.
Protein–protein pathway analysis illustrating significantly downregulated proteins in POTS. Protein–protein interaction analysis of 7 out of 11 upregulated proteins with association networks detected by STRING in POTS. In the network figures, each protein is represented by a colored node while protein–protein interaction and association are represented by a line. The strongest network interactions in POTS were particularly associated with a hypercoagulable state, inflammation, and enhanced cardiac contractility and hypertrophy. Complete names of proteins are found in Table 2.
Discussion
In this case–control study, an unbiased DIA proteomic analysis identified 30 plasma proteomic biomarkers that were differentially expressed in postural orthostatic tachycardia syndrome (POTS) compared with healthy, age- and sex-matched controls. In particular, we identified significant up- and downregulated expression of proteins related to platelet activity, cardiac contractility, hypertrophy, proinflammation, skeletal muscle expression, and adrenergic activity.
Procoagulant changes and enhanced platelet activity in POTS
We observed significant upregulation of platelet-related proteins, such as glycoproteins 1B (GP1BA and GP1BB) which serve as receptors for von Willebrand factor (vWF) and mediate vWF-dependent platelet adhesion to injured vascular surfaces in the arterial circulation, facilitate hemostasis14. Also, filamin A (FLNA), which interacts with GP1BA, strengthens adhesion of platelets onto VWF, promoting platelet activation15. A previous study has similarly found that patients with syncope and orthostatic hypotension have increased levels of vWF during orthostasis16. Tubulin beta-1 (TUBB1), which is required for optimal platelet assembly, was also upregulated in POTS. Deficiency in TUBB1 leads to thrombocytopenia, whereas overexpression is associated with formation of large platelets and platelet hyperaggregation17.
Cartilage oligomeric matrix protein (COMP) is extracellular matrix protein is secreted by platelets and inhibits thrombin-induced platelet aggregation and activation18. Integrin-linked kinase (ILK) facilitates rapid platelet activation and is essential for the formation of stable thrombi19, and beta-parvin (PARVB), is known to play a regulatory role in integrin-signaling via ILK. Our study demonstrates that COMP was significantly downregulated in POTS, which may result in defective hemostasis and thrombosis. Similarly, ILK and PARVB were upregulated in POTS, enhancing platelet aggregation and thrombus formation.
There is limited data regarding specific complications arising from POTS and possible rates of thromboembolism20. As such, interventions targeting thrombogenicity in POTS, e.g., thromboprophylaxis with low-molecular-weight heparin, merit further investigation focusing on proteins associated with the von Willebrand complex, such as the involvement of GP1B.
Cardiac contractility and hypertrophy in POTS
In addition to its prothrombic function, ILK together with PARVB also form a critical complex responsible for regulation of cardiac contractility21. ILK regulates diverse signal transduction pathways implicated in cardiac hypertrophy and contractility22. We noted that both ILK and PARVB were upregulated in POTS. To date, few studies have thoroughly assessed the cardiac morphology by echocardiography in POTS with only one small study in 19 patients have shown that individuals with POTS exhibited smaller left ventricular mass compared with 16 healthy controls23. Larger case–control studies with echocardiographic evaluation in POTS are warranted to elucidate whether these patients exhibit possible cardiac hypertrophy.
Thrombospondin-4 (TSP-4) is involved in regulation and remodeling of the cardiac extracellular matrix and myocyte contractility. We observed downregulated expression of TSP-4 in POTS. In vivo mouse models have shown that TSP-4 deficient mice develop pronounced cardiac hypertrophy, fibrosis together with left ventricular dilatation, and depressed systolic function24. These defects in adaptation to chronic pressure overload, result in chamber dilation, reduced cardiac function, and increased cardiac mass24.
Proinflammatory state in POTS
Notably, the most upregulated plasma protein within the proinflammatory pathway in POTS was beta-2-microglobulin (B2M), which is a component of the major histocompatibility complex class I. Ongoing inflammation is associated with elevated levels of β2M25, likewise, increased levels of B2M have been reported in several autoimmune diseases. Previous studies have also found that B2M-specific autoantibodies cause platelet aggregation26, thereby facilitating thrombosis and providing a link between thrombogenicity and inflammation. Downregulation of WD repeat-containing protein 1 (WDR1) can cause autoinflammation and thrombocytopenia27, in this study, we observed enhanced expression of WDR1 in POTS.
Growing evidence points toward POTS being an autoimmune disease, oftentimes triggered by a viral or bacterial infection28. Lumican (LUM) interacts with CD14 and CD18 on macrophage and neutrophils to promote innate immune response, and normally helps to restrict autoimmunity, antiviral, bacterial and inflammatory responses29. We noted downregulated expression of LUM in POTS and hypothesized that it may result in an impaired anti-inflammatory response, unable to inhibit viral replication in these patients.
A previous study tried to elucidate the inflammatory proteomic signature of POTS using antibody-based Proximity Extension Assay (PEA) technique in nearly 400 patients, measuring simultaneously 57 inflammatory protein biomarkers30. Only one plasma protein, proconvertase furin, was found to be downregulated compared with individuals without POTS. Unlike our current study, the previous one did not include healthy controls, but merely individuals with a normal orthostatic response. Moreover, the proteomic methods differed between both studies. In the current study, we used a mass spectrometry-based technique, offering a more detailed molecular characterization of proteins. Rather than just detection, like the PEA, mass spectrometry can reveal unique chemical fragments of proteins, such as specific biochemical interactions31.
Increased expression of skeletal muscle myosin in POTS
We observed significantly increased expression of myosin light chain 1/3, skeletal muscle isoform (MYL1) expressed only in fast skeletal muscles in adults and required for proper formation and maintenance of myofibers, and muscle function32. Faulty regulation in myogenesis leads to deconditioning-related changes characterized by decreased muscle mass33. These findings are in line with the previous identification of upregulation of myoglobin in POTS34. In POTS, differences in sympathetic nerve discharge and fiber loss from skeletal muscles is observed35, which could putatively explain muscle fatiguability and deconditioning associated with POTS.
Upregulated alpha-adrenergic activity in POTS
We noted significant upregulation of myosin regulatory light chain 12B (MYL12B) protein in POTS, which triggers polymerization of vascular smooth muscle36. Vascular smooth muscle cells are predominantly innervated by the sympathetic α1 adrenergic receptors and play an important role in maintaining cardiovascular homeostasis by regulating vascular tone, blood flow and blood pressure37. In POTS, increased sympathetic activation causes activation of the adrenergic receptors and a surge in norepinephrine levels, and approximately 89% of patients with POTS exhibit elevated levels of autoantibodies against the adrenergic α1 receptor38.
Strengths and limitations of the study
Our study is the first to use an unbiased proteomics analysis by mass spectrometry in one of the larger studies of POTS populations. To date, only a single mass spectrometry study has been conducted in POTS, with limited number of participants (10 POTS patients and seven healthy controls), which explored autoantibodies in POTS as potential novel biomarkers39. Here, we have thoroughly matched POTS cases with healthy controls according to age and sex, improving statistical precision and validity. Moreover, the use of mass spectrometric analysis offers several advantages compared with other methods, including high sensitivity, good proteome coverage, rapid method setup, accessibility of post-translational modifications, rendering it very attractive for unbiased biomarker discovery40. The recently developed DIA method achieves even higher reproducibility making technical replicates unnecessary and rendering it highly suitable for large scale population studies7.
Although mass spectrometry-based proteomics is an evolving technique, the sensitivity of the instruments and the high dynamic range of plasma samples does not allow detection of low-abundance proteins in plasma. The proteomic markers identified in this study ought to be replicated and validated in an independent cohort. Detection of these biomarkers does not reveal what effects they may have on target end-organs which must be the subject of further study. Since POTS is more prevalent in women than men, it is expected that the number of male patients with POTS were fewer, explaining in part why we did not observe any differences in proteomic expression between male POTS patients and healthy male controls. Moreover, the observational design of our study precludes conclusions on causality and cannot exclude unknown confounding.
Clinical perspectives and implications
Few studies have identified promising biomarkers in POTS, and therapeutic possibilities are mainly focused on symptom relief, posing substantial challenges to physicians41. Our study identified novel proteomic footprints in POTS linked to increased platelet activity, proinflammatory states, and hyperadrenergic activity, facilitating a greater mechanistic understanding of the syndrome. Further investigations are warranted into the underlying molecular mechanisms leading to POTS and development of targeted therapeutic strategies.
Conclusions
Our observations are the first to use a label-free unbiased MS-based quantification approach to elucidate the proteomic footprint of postural orthostatic tachycardia syndrome (POTS). The strongest network interaction in POTS was notably associated with a hypercoagulable state, an upregulated expression of proteins related to platelet activity. In addition, we observed proteomic patterns involved in proinflammatory states, enhanced cardiac contractility and hypertrophy, as well as adrenergic activity. These findings support the hypothesis that POTS may be an autoimmune, inflammatory and hyperadrenergic disorder.
Supplementary Information
Acknowledgements
We thank Dysautonomia International, USA for supporting this research project.
Abbreviations
- B2M
β-2-Microglobulin
- COMP
Cartilage oligomeric matrix protein
- DIA
Data independent acquisition
- FLNA
Filamin-A
- GP1BA
Platelet glycoprotein Ib alpha chain
- GP1BB
Platelet glycoprotein Ib beta chain
- ILK
Integrin-linked kinase
- LUM
Lumican
- MYL12A
Myosin regulatory light chain 12A
- MYL12B
Myosin regulatory light chain 12B
- PARVB
β-Parvin
- POTS
Postural orthostatic tachycardia syndrome
- PP1A
Peptidyl-prolyl cis–trans isomerase A
- THBS4
Thrombospondin-4
- TUBB1
Tubulin beta-1 chain
- WDR1
WD repeat-containing protein 1
Author contributions
M.J. and A.F. contributed to the study design. M.J. and A.F. acquired the data. M.J., C.W., H.Y., A.V. conducted the analyses. M.J. drafted the manuscript. All authors were involved in data interpretation and critically reviewed and approved the manuscript.
Funding
Open access funding provided by Lund University. This study was funded by Dysautonomia International, USA.
Data availability
All authors had full access to all the data in the study and takes responsibility for its integrity and the data analysis. The mass spectrometry proteomics data have been deposited at the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD031458.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Madeleine Johansson and Artur Fedorowski.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-24729-x.
References
- 1.Brignole M, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018;39:1883–1948. doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- 2.Vernino S, et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Auton. Neurosci. 2021;235:102828. doi: 10.1016/j.autneu.2021.102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson M, et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: The Swedish experience. JACC Case Rep. 2021 doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raj SR, et al. Long-COVID postural tachycardia syndrome: An American Autonomic Society statement. Clin. Auton. Res. 2021;31:365–368. doi: 10.1007/s10286-021-00798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raj SR, et al. Postural orthostatic tachycardia syndrome (POTS): Priorities for POTS care and research from a 2019 National Institutes of Health Expert Consensus Meeting—Part 2. Auton. Neurosci. 2021;235:102836. doi: 10.1016/j.autneu.2021.102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boris JR, Huang J, Bernadzikowski T. Orthostatic heart rate does not predict symptomatic burden in pediatric patients with chronic orthostatic intolerance. Clin. Auton. Res. 2020;30:19–28. doi: 10.1007/s10286-019-00622-y. [DOI] [PubMed] [Google Scholar]
- 7.Bichmann L, et al. DIAproteomics: A multifunctional data analysis pipeline for data-independent acquisition proteomics and peptidomics. J. Proteome Res. 2021;20:3758–3766. doi: 10.1021/acs.jproteome.1c00123. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig C, et al. Data-independent acquisition-based SWATH-MS for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2018;14:e8126. doi: 10.15252/msb.20178126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedorowski A, Burri P, Struck J, Juul-Moller S, Melander O. Novel cardiovascular biomarkers in unexplained syncopal attacks: The SYSTEMA cohort. J. Intern. Med. 2013;273:359–367. doi: 10.1111/joim.12043. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Riverol Y, et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bache N, et al. A novel LC system embeds analytes in pre-formed gradients for rapid ultra-robust proteomics. Mol. Cell. Proteom. 2018;17:2284–2296. doi: 10.1074/mcp.TIR118.000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szklarczyk D, et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyanova S, et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 14.Firbas C, Siller-Matula JM, Jilma B. Targeting von Willebrand factor and platelet glycoprotein Ib receptor. Expert Rev. Cardiovasc. Ther. 2010;8:1689–1701. doi: 10.1586/erc.10.154. [DOI] [PubMed] [Google Scholar]
- 15.Lopez JJ, Jardin I, Rosado JA. Filamin A modulates platelet function. Aging (Albany, NY) 2018;10:3052–3053. doi: 10.18632/aging.101635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamrefors V, Fedorowski A, Strandberg K, Sutton R, Isma N. Procoagulatory changes induced by head-up tilt test in patients with syncope: Observational study. Thromb. J. 2017;15:16. doi: 10.1186/s12959-017-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoupa A, et al. TUBB1 mutations cause thyroid dysgenesis associated with abnormal platelet physiology. EMBO Mol. Med. 2018;10:e9569. doi: 10.15252/emmm.201809569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y, et al. Cartilage oligomeric matrix protein is a natural inhibitor of thrombin. Blood. 2015;126:905–914. doi: 10.1182/blood-2015-01-621292. [DOI] [PubMed] [Google Scholar]
- 19.Jones CI, et al. Integrin-linked kinase regulates the rate of platelet activation and is essential for the formation of stable thrombi. J. Thromb. Haemost. 2014;12:1342–1352. doi: 10.1111/jth.12620. [DOI] [PubMed] [Google Scholar]
- 20.Quan W, Wang Y, Chen S, Du J. Orthostatic intolerance and coagulation abnormalities: An update. Neurosci. Bull. 2019;35:171–177. doi: 10.1007/s12264-018-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannigan GE, Coles JG, Dedhar S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circ. Res. 2007;100:1408–1414. doi: 10.1161/01.RES.0000265233.40455.62. [DOI] [PubMed] [Google Scholar]
- 22.Traister A, et al. ILK induces cardiomyogenesis in the human heart. PLoS One. 2012;7:e37802. doi: 10.1371/journal.pone.0037802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Q, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 2010;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cingolani OH, et al. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ. Res. 2011;109:1410–1414. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amighi J, et al. Beta 2 microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke. 2011;42:1826–1833. doi: 10.1161/STROKEAHA.110.600312. [DOI] [PubMed] [Google Scholar]
- 26.Falus A, et al. Beta-2-microglobulin-specific autoantibodies cause platelet aggregation and interfere with ADP-induced aggregation. Clin. Exp. Immunol. 1982;47:103–109. [PMC free article] [PubMed] [Google Scholar]
- 27.Standing AS, et al. Autoinflammatory periodic fever, immunodeficiency, and thrombocytopenia (PFIT) caused by mutation in actin-regulatory gene WDR1. J. Exp. Med. 2017;214:59–71. doi: 10.1084/jem.20161228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thieben MJ, et al. Postural orthostatic tachycardia syndrome: The Mayo clinic experience. Mayo Clin. Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 29.Maiti G, et al. Matrix lumican endocytosed by immune cells controls receptor ligand trafficking to promote TLR4 and restrict TLR9 in sepsis. Proc. Natl. Acad. Sci. U.S.A. 2021;118:e2100999118. doi: 10.1073/pnas.2100999118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spahic JM, et al. Proconvertase furin is downregulated in postural orthostatic tachycardia syndrome. Front. Neurosci. 2019;13:301. doi: 10.3389/fnins.2019.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Gool A, et al. Analytical techniques for multiplex analysis of protein biomarkers. Expert Rev. Proteom. 2020;17:257–273. doi: 10.1080/14789450.2020.1763174. [DOI] [PubMed] [Google Scholar]
- 32.Schiaffino S, Rossi AC, Smerdu V, Leinwand LA, Reggiani C. Developmental myosins: Expression patterns and functional significance. Skelet. Muscle. 2015;5:22. doi: 10.1186/s13395-015-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benavides Damm T, Egli M. Calcium's role in mechanotransduction during muscle development. Cell Physiol. Biochem. 2014;33:249–272. doi: 10.1159/000356667. [DOI] [PubMed] [Google Scholar]
- 34.Medic Spahic J, et al. Proteomic analysis reveals sex-specific biomarker signature in postural orthostatic tachycardia syndrome. BMC Cardiovasc. Disord. 2020;20:190. doi: 10.1186/s12872-020-01465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carew S, et al. A review of postural orthostatic tachycardia syndrome. Europace. 2009;11:18–25. doi: 10.1093/europace/eun324. [DOI] [PubMed] [Google Scholar]
- 36.Park I, et al. Myosin regulatory light chains are required to maintain the stability of myosin II and cellular integrity. Biochem. J. 2011;434:171–180. doi: 10.1042/BJ20101473. [DOI] [PubMed] [Google Scholar]
- 37.Ringvold HC, Khalil RA. Protein kinase C as regulator of vascular smooth muscle function and potential target in vascular disorders. Adv. Pharmacol. 2017;78:203–301. doi: 10.1016/bs.apha.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunning WT, 3rd, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural orthostatic tachycardia syndrome is associated with elevated g-protein coupled receptor autoantibodies. J. Am. Heart Assoc. 2019;8:e013602. doi: 10.1161/JAHA.119.013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang XL, et al. Autoimmunoreactive IgGs from patients with postural orthostatic tachycardia syndrome. Proteom. Clin. Appl. 2012;6:615–625. doi: 10.1002/prca.201200049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angel TE, et al. Mass spectrometry-based proteomics: Existing capabilities and future directions. Chem. Soc. Rev. 2012;41:3912–3928. doi: 10.1039/c2cs15331a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones PK, Shaw BH, Raj SR. Clinical challenges in the diagnosis and management of postural tachycardia syndrome. Pract. Neurol. 2016;16:431–438. doi: 10.1136/practneurol-2016-001405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All authors had full access to all the data in the study and takes responsibility for its integrity and the data analysis. The mass spectrometry proteomics data have been deposited at the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD031458.