ABSTRACT
Background
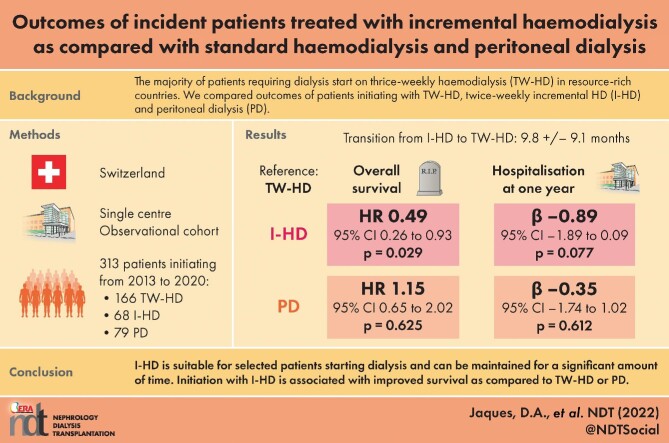
Residual kidney function is considered better preserved with incremental haemodialysis (I-HD) or peritoneal dialysis (PD) as compared with conventional thrice-weekly HD (TW-HD) and is associated with improved survival. We aimed to describe outcomes of patients initiating dialysis with I-HD, TW-HD or PD.
Methods
We conducted a retrospective analysis of a prospectively assembled cohort in a single university centre including all adults initiating dialysis from January 2013 to December 2020. Primary and secondary endpoints were overall survival and hospitalization days at 1 year, respectively.
Results
We included 313 patients with 234 starting on HD (166 TW-HD and 68 I-HD) and 79 on PD. At the end of the study, 10 were still on I-HD while 45 transitioned to TW-HD after a mean duration of 9.8 ± 9.1 months. Patients who stayed on I-HD were less frequently diabetics (P = .007). Mean follow-up was 33.1 ± 30.8 months during which 124 (39.6%) patients died. Compared with patients on TW-HD, those on I-HD had improved survival (hazard ratio 0.49, 95% confidence interval 0.26-0.93, P = .029), while those on PD had similar survival. Initial kidney replacement therapy modality was not significantly associated with hospitalization days at 1 year.
Conclusions
I-HD is suitable for selected patients starting dialysis and can be maintained for a significant amount of time before transition to TW-HD, with diabetes being a risk factor. Although hospitalization days at 1 year are similar, initiation with I-HD is associated with improved survival as compared with TW-HD or PD. Results of randomized controlled trials are awaited prior to large-scale implementation of I-HD programmes.
Keywords: incremental haemodialysis, mortality, outcomes, peritoneal dialysis, survival
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Residual kidney function has been associated with improved survival in dialysis patients and is considered better preserved with twice-weekly incremental haemodialysis (I-HD) or peritoneal dialysis (PD) as compared with conventional thrice-weekly HD (TW-HD).
Despite current guidelines supporting an incremental approach to HD initiation, the vast majority of patients requiring kidney replacement therapy (KRT) still begin on conventional TW-HD in resource-rich countries.
What this study adds?
In selected patients initiating dialysis, I-HD is a suitable KRT modality that can be maintained for a significant amount of time before transitioning to a conventional TW-HD regimen.
In the appropriate clinical setting, I-HD initiation is associated with improved survival as compared with TW-HD or PD.
What impact this may have on practice or policy?
An I-HD regimen can be safely implemented in patients initiating dialysis when carefully selected.
These results reinforce current guidelines and call for long-awaited randomized controlled trials prior to large-scale implementation of I-HD programmes.
INTRODUCTION
In Europe, 100 000 patients initiated kidney replacement therapy (KRT) in 2016, corresponding to an overall incidence of 132 per million population. In-centre haemodialysis (HD) is the most commonly used modality [1]. Most incident patients still have a significant residual kidney function (RKF) when starting HD, with an estimated glomerular filtration rate (eGFR) of 6–10 mL/min/1.73 m2 [2]. The importance of RKF preservation in dialysed patients is increasingly recognized as it has been associated with improved survival and quality of life, as well as decreased inflammation and erythropoietin use in observational studies [3]. A dogma of thrice-weekly HD (TW-HD) has been implemented based on urea kinetic modelling and dialysis adequacy targets, as evaluated by small molecule dialytic clearance (Kt/V). This metric was first formalized in the 1980s and later updated in the 2000s in large randomized controlled trials (RCTs) [4, 5]. Results from those seminal studies have defined the concept of ‘dialysis adequacy’ with a target sessional delivered single pool Kt/V of 1.2 still recommended in current guidelines [6]. However, most included patients did not have significant RKF. Incremental HD (I-HD) is generally defined as a once- or twice-weekly HD regimen prescribed in incident end-stage kidney disease (ESKD) patients in order to achieve the target weekly small molecule clearance while accounting for RKF. I-HD has been associated with greater preservation of RKF in incident patients as compared with TW-HD in observational studies [7]. Despite current guidelines supporting the choice of an incremental approach for HD prescription, the vast majority of ESKD patients still begin on a TW-HD regimen in resource-rich countries [6, 8, 9]. Although not universal, incremental prescription considering RKF is more commonly accepted in peritoneal dialysis (PD) [10]. Moreover, PD has also been associated with better RKF preservation as compared with TW-HD in observational reports [11].
As an incremental approach to dialysis prescription is gaining wider acceptance, our aim is to describe the impact of initial KRT modality (I-HD, TW-HD or PD) on overall mortality and other selected outcomes in incident ESKD patients.
MATERIALS AND METHODS
Participants and study design
We conducted a retrospective analysis of a prospectively assembled cohort in a single university centre (Geneva University Hospitals, Geneva, Switzerland). The Swiss renal registry and quality assessment programme was established in 2006 by the Swiss Society of Nephrology on a voluntary basis and on a legal obligation since 2013. Therefore, all patients in Switzerland on chronic dialysis are included in this registry and provide informed consent for their anonymized data to be used for quality control and clinical research purpose. In the present study, we included all adults initiating dialysis (incident patients) at our centre, from January 2013 to December 2020 with a follow-up to December 2021. Patients already on dialysis (prevalent patients) or those requiring dialysis for acute kidney injury (functional alteration in kidney function lasting <3 months) were not included. Demographic, clinical and laboratory data were retrieved from electronic medical records. eGFR at KRT initiation was calculated with the 2012 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using serum creatinine measured at the very beginning of the first HD session [12]. Urine output at KRT initiation was derived from a urine sample collected over 24 h during the first interdialytic interval after three initial HD sessions and prior to the fourth regular HD session. Residual kidney urea clearance (KRU) was calculated based on this same urine collection using a 0.9 correction factor for time-averaged concentration of pre-HD urea [13]. The primary endpoint was overall survival during follow-up according to initial KRT modality (I-HD, T-HD or PD). Secondary endpoints were hospitalization days and decline in urine output at 1 year as well as factors associated with transition from I-HD to TW-HD.
Dialysis prescription
Every incident ESKD patient at our centre is offered a pre-dialysis educational programme regarding KRT modality (HD, PD and transplantation). Initial KRT modality is then decided with a major emphasis on patient preferences. Patients attributed to HD are systematically offered I-HD if urine output is ≥500 mL/day, KRU ≥2 mL/min and interdialytic weight gain (IDWG) ≤2.5 kg [14]. Patients fulfilling those criteria begin I-HD with twice-weekly 3-h sessions, with ultrafiltration (UF) rate not exceeding 10 mL/kg/h. Patients on I-HD have 24-h urine collected every 2 months routinely. If urine output, KRU or IDWG are not in the required range, patients are transitioned to TW-HD. As urine output has showed a stronger association with clinical prognosis as compared with KRU, we empirically put more emphasis on this parameter to decide transition to TW-HD [15]. Patients can also transition to TW-HD for other clinical reasons at the discretion of the attending physician, including overall clinical condition, uremic symptoms, volume control (UF >10 mL/kg/h), blood pressure control, anaemia, electrolytes and acid–base status. Kt/V monitoring is not routinely done at our centre but rather obtained when clinically indicated. Patients were dialyzed using either HD or haemodiafiltration (HDF) with postdilution reinjection according to the choice of attending physician. High-flux polysulfone dialysers were used with Braun (Braun, Melsungen, Germany) or Fresenius (Fresenius AG, Bad Homburg, Germany) dialysis machines. Patients choosing PD started with a continuous ambulatory PD (CAPD) incremental prescription typically consisting of two glucose dwells during the day as well as one icodextrin dwell overnight. Urine output and KRF are then monitored regularly in order to adapt PD prescription with a target total weekly Kt/V ≥1.7.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median (interquartile range) according to distribution and categorical ones as number and relative frequencies (%). Between-groups comparison was conducted using the t-test, and ANOVA and Chi-square for continuous and categorical variables, respectively.
For primary endpoint, all-cause mortality was considered as the outcome and initial KRT modality as the main predictor. Kaplan–Meier curves and log-rank tests were performed to compare survival. Cox proportional hazard models were used with KRT modality (TW-HD, I-HD and PD) as a three-level categorical variable. Proportional hazard assumption was tested using log–log plots. As we aimed to describe the impact of initial KRT modality on further clinical outcomes, status from patients transitioning from I-HD to TW-HD was not updated over time and only initial dialysis modality was considered. Patients transplanted during follow-up were censored. Multivariate analyses included the following variables as potential confounders selected on prior scientific knowledge: age, gender, Charlson comorbidity score, presence of arteriovenous fistula (AVF) (as opposed to catheter), and urgent KRT initiation. Charlson score comprised diabetes and cardiovascular disease, as well as other items according to the standard description [16, 17]. Urgent KRT initiation was defined as the absence of contact with a nephrologist within 1 month prior to dialysis initiation. Late referral was defined as <3 months follow-up by a nephrologist prior to dialysis initiation. Cardio-renal syndrome was defined as chronic heart failure participating in kidney function impairment and volume overload [18].
For secondary endpoint, hospitalization days were considered as the outcome and initial KRT modality as the main predictor. Multivariate linear regression was used to adjust for above-described potential confounders. Normality of residuals and homoscedasticity were tested graphically.
Variables were transformed when appropriate. Specifically, square-root transformation was used for hospitalization days in linear models. Data were considered to be missing completely at random and therefore patients with any missing variables were excluded from the multivariate analyses. P-values <.05 were considered significant. Statistical analyses were conducted using STATA version 15 (StataCorp, 4905 Lakeway Drive, College Station, TX 77845, USA).
Ethics
This study was approved by the local ethics committee ‘Commission cantonale d’éthique de la recherche’ (CCER), Geneva, Switzerland, and was performed according to the Declaration of Helsinki.
RESULTS
From January 2013 to March 2021, 313 patients started chronic dialysis at our institution. Among those, 234 started on HD with 166 on TW-HD and 68 on I-HD, while 79 started on PD. Absolute and relative numbers of incident I-HD patients according to study year are depicted in Supplementary data, Figure S1a and b. Baseline characteristics of patients according to initial KRT modality are described in Table 1. Mean age was 62.0 ± 16.2 with 213 (68%) men, without significant differences across KRT modalities. As compared with patients initiating with I-HD or PD, those on TW-HD had a higher prevalence of diabetes and a lower prevalence of glomerulonephritis. They also had higher Charlson scores, a lower prevalence of AVF, lower serum albumin and lower KRU, as well as lower urine output as compared with I-HD and PD patients. As compared with patients initiating with I-HD or TW-HD, those on PD had a higher prevalence of cardiorenal syndrome and a lower prevalence of urgent KRT initiation as well as late referral. They also had higher serum bicarbonate, higher calcium, lower phosphate and higher eGFR at KRT initiation as compared with TW-HD and I-HD patients. Overall, 78 patients later received a kidney transplant during follow-up with 39 (23.4%) in the TW-HD group, 17 (25.0%) in the I-HD group and 22 (27.8%) in the PD group (P = .762).
Table 1.
Baseline characteristics according to initial KRT modality
| TW-HD (N = 166) | I-HD (N = 68) | PD (N = 79) | P-value | |
|---|---|---|---|---|
| Age (years) | 62.8 ± 15.7 | 59.7 ± 16.9 | 62.5 ± 16.8 | 0.422 |
| Gender (men) | 113 (68.0%) | 46 (67.6%) | 54 (68.3%) | 0.996 |
| Smoker | 45 (27.6%) | 22 (35.4%) | 16 (20.7%) | 0.155 |
| Diabetes | 84 (50.6%) | 21 (30.8%) | 28 (35.4%) | 0.007 |
| HT | 145 (87.3%) | 52 (76.4%) | 66 (83.5%) | 0.118 |
| CV disease | 74 (44.5% | 27 (39.7%) | 31 (39.2%) | 0.656 |
| ESKD causeDM and/or HTGlomerulonephritisOther |
88 (53.3%) 18 (10.9%) 59 (35.7%) |
27 (39.7%) 16 (23.5%) 25 (36.7%) |
42 (53.1%) 17 (21.5%) 20 (25.3%) |
0.034 |
| Cardiorenal syndrome | 5 (3.0%) | 3 (4.4%) | 9 (11.3%) | 0.024 |
| Charlson score | 7.3 ± 3.0 | 6.3 ± 2.6 | 6.8 ± 3.0 | 0.047 |
| AVF | 26 (15.6%) | 25 (36.7%) | NA | <0.001 |
| Urgent KRT initiation | 56 (33.7%) | 28 (41.1%) | 9 (11.3%) | <0.001 |
| Late referral | 57 (34.3%) | 26 (38.2%) | 12 (15.1%) | 0.003 |
| Sodium (mmol/l) | 137.4 ± 4.7 | 138.4 ± 3.7 | 137.2 ± 5.3 | 0.226 |
| Potassium (mmol/l) | 4.5 ± 0.8 | 4.6 ± 0.9 | 4.4 ± 0.7 | 0.318 |
| Bicarbonate (mmol/l) | 20.4 ± 4.3 | 20.6 ± 5.3 | 22.5 ± 4.4 | 0.007 |
| PTH (pmol/l) | 37.4 ± 40.2 | 38.1 ± 27.5 | 37.4 ± 28.5 | 0.992 |
| Calcium (mmol/l) | 2.21 ± 0.26 | 2.12 ± 0.22 | 2.29 ± 0.21 | <0.001 |
| Phosphate (mmol/l) | 2.08 ± 0.66 | 2.09 ± 0.58 | 1.85 ± 0.56 | 0.024 |
| Albumin (g/l) | 30.9 ± 6.1 | 35.8 ± 6.7 | 34.8 ± 6.1 | <0.001 |
| eGFR at KRT initiation (mL/min/1.73 m2) | 7.7 ± 3.5 | 6.6 ± 3.0 | 8.3 ± 4.7 | 0.021 |
| KRU (mL/min) | 2.2 ± 1.9a | 3.1 ± 2.1 | 4.1 ± 2.3 | <0.001 |
| Urine output at KRT initiation (mL) | 1220 ± 717a | 1851 ± 759 | 1732 ± 691 | <0.001 |
aAvailable in a subgroup of 75 patients only.
Abbreviations: CV, cardiovascular; DM, diabetes; HT, hypertension; PTH, parathormone. Bold values indicate P <0.05.
Over time, four patients initiating with I-HD eventually recovered sufficient KRF to become HD-independent after a mean duration of 7.0 ± 3.5 months. No patients in the TW-HD and PD groups became dialysis-independent. Among the 64 remaining patients on I-HD, 5 were transplanted and 4 died while still on I-HD. Among the 55 remaining patients, 10 were still on I-HD at the end of follow-up, while 45 transitioned to TW-HD after a mean duration of 9.8 ± 9.1 months. The rate of persistence of I-HD at 1 year was 28.8%. The patient flowchart is provided as Supplementary data, Figure S2.
Mean follow-up time was 33.1 ± 30.8 months during which 124 (39.6%) patients died. Kaplan–Meier survivor function according to initial KRT modality is illustrated in Fig. 1. Patients initiating with I-HD had increased survival as compared with those on TW-HD or PD (P = .009 for log-rank test). In univariate Cox proportional hazard model, patients on I-HD had increased survival as compared with those on TW-HD [hazard ratio (HR) 0.41, 95% confidence interval (95% CI) 0.22–0.76; P = .005], while those on PD had similar survival to those on TW-HD (HR 1.05, 95% CI 0.69–1.59; P = .798). Multivariate analyses included 307 patients without any missing values on considered covariates. In the multivariate Cox proportional hazard model, patients on I-HD had increased survival as compared with those on TW-HD (HR 0.49, 95% CI 0.26–0.93; P = .029), while those on PD had similar survival compared with those on TW-HD (HR 1.15, 95% CI 0.65–2.02; P = .625) (Table 2). Cox survivor function according to initial KRT modality is illustrated in Fig. 2. Charlson score was positively associated with mortality (HR 1.17, 95% CI 1.08 1.26; P < .001). Other considered covariates were not significantly associated with mortality (Table 2).
Figure 1:
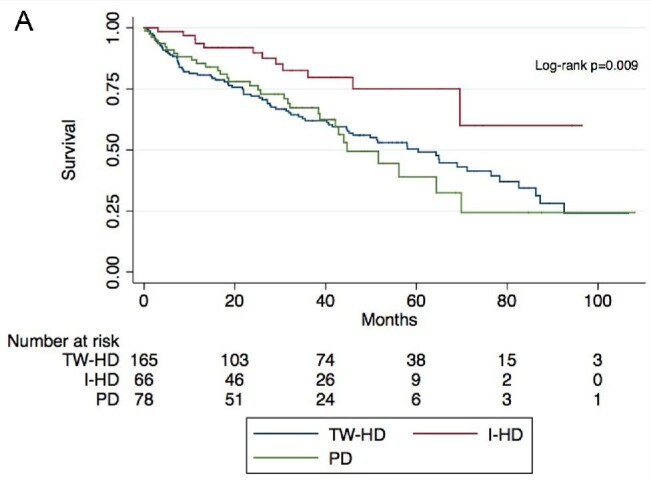
Kaplan–Meier survivor function according to initial KRT modality.
Table 2.
Association with overall mortality using multivariate Cox proportional hazard model
| HR (95% CI) | P-value | |
|---|---|---|
| Initial KRT modalityTW-HDI-HDPD |
Ref 0.49 (0.26–0.93) 1.15 (0.65–2.02) |
0.029 0.625 |
| Age (years) | 1.02 (1.00–1.04) | 0.016 |
| Gender (men) | 0.94 (0.63–1.39) | 0.769 |
| Charlson score | 1.17 (1.08–1.26) | <0.001 |
| Urgent KRT initiation | 1.21 (0.79–1.85) | 0.375 |
| Access typeHD catheterAVFPD catheter |
Ref 1.14 (0.61–2.14) 0.94 (0.45–1.95) |
0.668 0.885 |
Bold values indicate P <0.05.
Figure 2:
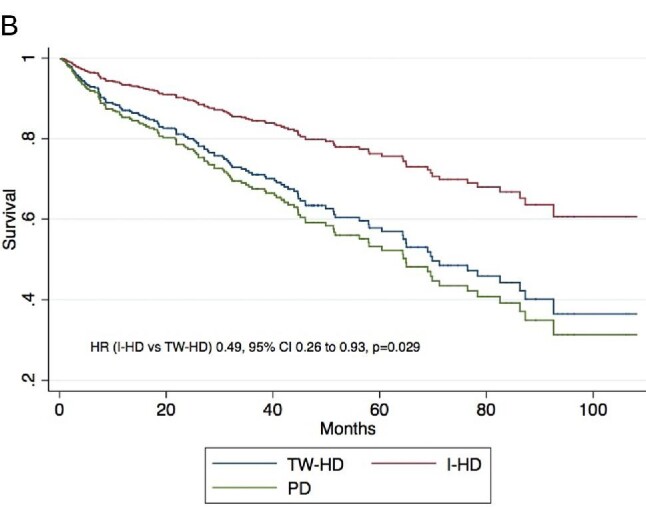
Multivariate Cox survivor function according to initial KRT modality. Multivariate model is adjusted for the following covariates: age, gender, Charlson score, urgent KRT initiation and access type.
Among patients initiating dialysis with I-HD, 45 transitioned to TW-HD, while 19 stayed on I-HD during follow-up. Prevalence of diabetes was lower in patients who stayed on I-HD as compared with those who transitioned to TW-HD. Prevalence of hypertension tended to be lower and urine output at KRT initiation higher in patients who maintained I-HD, although not reaching statistical significance. Other considered variables were similar between those groups (Table 3).
Table 3.
Baseline characteristics according to transition from I-HD to TW-HD
| Stayed on I-HD (N = 19) | Transitioned to TW-HD (N = 45) | P-value | |
|---|---|---|---|
| Age (years) | 58.9 ± 15.9 | 61.1 ± 16.7 | 0.634 |
| Gender (men) | 13 (68.4%) | 32 (71.1%) | 0.830 |
| Smoker | 4 (21.0%) | 19 (33.3%) | 0.164 |
| Diabetes | 1 (5.2%) | 17 (38.6%) | 0.007 |
| HT | 13 (68.4%) | 38 (86.3%) | 0.096 |
| CV disease | 6 (31.5%) | 19 (43.1%) | 0.388 |
| Charlson score | 5.3 ± 2.1 | 6.5 ± 2.9 | 0.112 |
| AVF | 10 (52.6%) | 15 (34.8%) | 0.106 |
| Urgent KRT initiation | 6 (31.5%) | 15 (33.3%) | 0.891 |
| eGFR at KRT initiation (mL/min/1.73 m2) | 5.5 ± 2.2 | 6.6 ± 2.5 | 0.105 |
| KRU (mL/min) | 3.8 ± 1.7 | 3.1 ± 2.2 | 0.331 |
| Urine output at KRT initiation (mL) | 2144 ± 776 | 1780 ± 791 | 0.096 |
Four patients on I-HD who recovered from dialysis during follow-up were excluded from this analysis (see Supplementary data, Fig. S2).
Abbreviations: CV, cardiovascular; HT, hypertension. Bold values indicate P <0.05.
Selected outcomes at 1 year are presented in Table 4. As compared with patients initiating with I-HD or PD, those on TW-HD had higher mortality and hospitalization days. As compared with PD patients, those on I-HD had similar urine output at 1 year but higher decline in urine output from baseline. Multivariate analyses included 311 patients without missing values on considered covariates. In multivariate linear regression, initial KRT modality was not significantly associated with hospitalization days at 1 year. Charlson score was positively associated with hospitalization days at 1 year (HR 0.43, 95% CI 0.24–0.62, P < .001). Other considered covariates were not significantly associated with hospitalization days at 1 year (Table 5).
Table 4.
Selected outcomes at 1 year
| TW-HD (N = 166) | I-HD (N = 68) | PD (N = 79) | P-value | |
|---|---|---|---|---|
| Mortality | 36 (21.6%) | 5 (7.3%) | 11 (13.9) | 0.021 |
| Hospitalization days | 27 (11–69) | 18 (3–46) | 16 (3–48) | 0.003 |
| Urine output (mL) | NA | 1291 ± 810 | 1392 ± 656 | 0.429 |
| Decline in urine output from baselinea (%) | NA | 33.3 ± 31.2 | 23.3 ± 24.1 | 0.041 |
aPatients who increased their urine output between baseline and 1-year follow-up were considered to have a decline of 0%. Bold values indicate P <0.05.
Table 5.
Association with hospitalization daysa at 1 year using multivariate linear regression
| β coefficient (95% CI) | P-value | |
|---|---|---|
| Initial KRT modalityTW-HDI-HDPD |
Ref −0.89 (−1.89 to 0.09) −0.35 (−1.74 to 1.02) |
0.077 0.612 |
| Age (years) | −0.02 (−0.05 to 0.01) | 0.252 |
| Gender (men) | −0.19 (−0.99 to 0.61) | 0.643 |
| Charlson score | 0.43 (0.24 to 0.62) | <0.001 |
| Urgent KRT initiation | −0.11 (−1.01 to 0.77) | 0.793 |
| Access typeHD catheterAVFPD catheter |
Ref −1.08 (−2.19 to 0.02) −1.27 (−2.84 to 0.28) |
0.054 0.108 |
aSquare-root transformation was used. Bold values indicate P <0.05.
In sensitivity analysis, PD patients were excluded and analyses repeated including only I-HD and TW-HD patients. In multivariate Cox proportional hazard model, patients on I-HD had increased survival as compared with those on TW-HD (HR 0.50, 95% CI 0.26–0.94; P = .034). Associations between mortality and considered covariates are presented in Supplementary data, Table S1. In multivariate linear regression, initial KRT modality was not significantly associated with hospitalization days at 1 year. Associations between hospitalization days at 1 year and considered covariates are presented in Supplementary data, Table S2.
DISCUSSION
In this retrospective analysis of a prospectively assembled cohort, we found that ESKD patients initiating KRT with I-HD had improved overall survival as compared with patient initiating on standard TW-HD or PD while accounting for potential confounders. Although patients could be maintained on I-HD for several months, transition to TW-HD was often necessary, especially when diabetes was present.
Conceptually, I-HD is designed as a KRT modality aiming at decreasing dialysis doses by taking into account patient RKF in an attempt to improve clinical outcomes. Practically, patients on I-HD are thus dialysed twice a week instead of the traditional TW-HD regimen. Suitability for I-HD initiation is based on expert recommendations, and consideration is given to general metrics of dialysis adequacy with a particular emphasis on RKF and urine output [19]. Although growing over the past decade, the body of evidence on I-HD is surprisingly small [19]. Moreover, as clinical trials are yet to be published, the literature on I-HD is exclusively observational. RKF and its maintenance are central to I-HD implementation and have been linked to important benefits. Shafi et al. [3] showed that the presence of RKF was associated with improved survival, quality of life, inflammation and erythropoietin use in a cohort of 734 incident HD patients. Similarly, Obi et al. [20] found that RKF decline during the first year after HD initiation was associated with decreased survival in a cohort of 6500 patients starting maintenance HD. Those results offer a strong rational for I-HD implementation where endogenous RKF partially substitute for dialytic clearance. As both I-HD and PD are thought to better preserve RKF over time as compared with TW-HD, one could wonder whether those incremental approaches would provide measurable clinical benefits over conventional HD regimen [7, 11]. In 2016, Obi et al. compared 8068 patients treated with conventional TW-HD to 351 matched patients on twice-weekly I-HD initiating dialysis. In their analysis, I-HD regimen was associated with increased mortality in patients with baseline KRU <3.0 mL/min or urine output <600 mL/day but not in those with higher RKF [7]. Similarly, Mathew et al. [21] found similar overall survival when comparing 434 I-HD with 50 162 TW-HD patients initiating dialysis. In a recent meta-analysis including more than 75 000 ESKD patients starting dialysis, I-HD was associated with better RKF preservation with no increase in mortality as compared with TW-HD [22]. Finally, two pilot multicentric RCTs focusing on feasibility of I-HD programmes were recently published. A first study by Vilar et al. [23] in the UK enrolled 55 incident HD patients with 29 receiving I-HD and 26 TW-HD. Although not primarily designed to describe clinical outcomes, the authors reported a lower hospitalization rate in the I-HD group. Similarly, Murea et al. [24] included 23 I-HD and 25 TW-HD patients in the USA and described a trend towards lower hospitalization and mortality rates for I-HD patients, although results did not reach statistical significance. Taken globally, those observations suggest that I-HD does not increase mortality risk as compared with traditional TW-HD regimen when patients are carefully selected with sufficient RKF. However, I-HD is not harmless in uncontrolled settings as the large-scale application of once- or twice-weekly HD in resource-limited environments has been associated with poor survival [25]. Moreover, twice-weekly HD has also been found detrimental in a Korean cohort, but suboptimal nutritional status in the I-HD group as well as censoring at RKF disappearance and TW-HD transition could have influenced the results [26].
In our cohort, mortality rate was overall comparable to that of prior reports on incident patients from other Swiss centres [27, 28]. However, we found that patients initiating with I-HD regimen had improved survival as compared with those treated with conventional TW-HD or PD. A longer follow-up as compared with prior comparable studies could potentially participate in this positive finding. While reduced exposition to the harmful effects of HD procedure could theoretically improve outcomes in I-HD patients, reverse causality and residual confounding inherent to observational studies cannot be excluded. Among such possibilities, lead-time bias is particularly important in studies comparing dialysis regimen as time to death is measured from dialysis initiation. As patients on I-HD could start HD earlier than those on TW-HD, they could benefit from lead-time, artificially decreasing their mortality risk while on dialysis. This phenomenon is, however, very unlikely in our cohort as patients on I-HD actually had slightly lower eGFR at dialysis initiation as compared with those on TW-HD or PD. Comorbid conditions are obviously a major potential confounder in such studies, with healthier patients preferentially attributed to incremental strategies. Thus, in our analysis, patients initiating I-HD had slightly lower Charlson scores and that score itself was a strong predictor of overall mortality. However, I-HD patients maintained lower mortality risk despite adjustment for this factor in multivariate analysis suggesting an independent effect of the HD regimen itself. Overall, our results reinforce findings from recently published pilot RCTs, suggesting potential clinical benefits of I-HD in incident patients [23, 24].
Prior major studies on I-HD regimen were conducted on large national registries with a vast majority of patients treated with TW-HD. Thus, it is difficult to infer what proportion of incident patients would actually be suitable for I-HD [7, 21]. As I-HD was routinely considered as a KRT modality in our prospectively assembled cohort, we could observe that almost one-third of all incident HD patients were offered twice-weekly HD, suggesting that a large-scale application of incremental strategy is conceivable. We also observed that the proportion of patients initiating with I-HD rather that TW-HD increased over time, potentially reflecting a progressive change in clinical practice. Moreover, while a significant proportion of I-HD patients had eventually to transition to TW-HD, twice-weekly HD could be maintained for numerous months. Importantly, diabetes was found to be an important risk factor for TW-HD rather than I-HD initiation as well as for transition from I-HD to TW-HD during follow-up despite similar RKF between groups at baseline. In a recent publication, Torreggianni et al. [29] compared 53 patients starting dialysis with TW-HD with 91 patients initiating with I-HD. While the mean duration of I-HD to TW-HD transition was not provided, the rate of persistence on I-HD at 1 year was around 50%. In another large observational study including 434 incident patients on I-HD, 155 transitioned to TW-HD after a median duration of 9 months [21]. In the present study, we report a mean longevity on I-HD of 9.8 months corresponding to a 1-year persistence rate of 29%. Comparison of maintenance on I-HD between different cohorts is inherently difficult owing not only to differences in patients’ characteristics but also to heterogeneity in clinical practice regarding dialysis initiation and criteria for transition to TW-HD.
PD is thought to allow better RKF preservation as compared with TW-HD [11, 22]. Moreover, an early survival advantage of PD as compared with HD has been reported [30]. However, direct comparison between incident PD and I-HD patients has not been previously reported. In our cohort, patients initiating with PD had mortality rates comparable to that of TW-HD but higher than that of I-HD patients. This association holds true despite less frequent urgent KRT initiation in the PD group. Of note, preservation of RKF seemed superior with PD as compared with I-HD, with a slightly lower decline in urine output at 1 year.
Significant limitations apply to our findings. First, adjustment in multivariate models was based on variables measured at baseline. Thus, factors potentially influencing transition from I-HD to TW-HD during follow-up could not be taken into account. Second, dialysis Kt/V was not included in our analyses, as it was not consistently calculated in our cohort. However, qualitative modification of our results is very unlikely, as patients treated with I-HD would be expected to have lower dialysis Kt/V as compared with TW-HD. Third, for patients initiating with TW-HD, data on RKF were available in a subgroup only, as urine collection was often not ordered when patients had obvious reasons to initiate with a TW-HD regimen. Fourth and most importantly, confounding by indication, residual confounding and reverse causality cannot be excluded in this observational study.
CONCLUSION
In this observational study, we report that a significant proportion of ESKD patients starting dialysis are suited to a twice-weekly I-HD regimen. Incremental dialysis can be maintained for a significant period of time in selected patients before transition to conventional TW-HD, with diabetes being a major risk factor. When comparing KRT modality, patients initiating dialysis with I-HD have increased survival as compared with those initiating with either TW-HD or PD. Urine output at 1 year is slightly better preserved on PD as compared with I-HD. Globally, these findings show that I-HD can be safely implemented in incident patients when carefully selected. Results of RCTs are eagerly awaited prior to large-scale implementation of I-HD programs.
Supplementary Material
Contributor Information
David A Jaques, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Belen Ponte, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Fadi Haidar, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Anne Dufey, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Sebastian Carballo, Division of Internal Medicine, Geneva University Hospitals, Geneva, Switzerland.
Sophie De Seigneux, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
Patrick Saudan, Division of Nephrology, Geneva University Hospitals, Geneva, Switzerland.
FUNDING
This study required no specific source of funding.
AUTHORS’ CONTRIBUTIONS
D.A.J. analysed the data, interpreted the data and wrote the manuscript. B.P. analysed and interpreted the data. F.H. collected and interpreted the data. A.D. collected and interpreted the data. S.C. interpreted the data and revised the manuscript. S.D.S. collected the data and revised the manuscript. P.S. designed the study, collected, analysed and interpreted the data, as well as revising the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Stel VS, de Jong RW, Kramer A.et al. Supplemented ERA-EDTA Registry data evaluated the frequency of dialysis, kidney transplantation, and comprehensive conservative management for patients with kidney failure in Europe. Kidney Int 2021; 100: 182–95. Available from: https://pubmed.ncbi.nlm.nih.gov/33359055/ [DOI] [PubMed] [Google Scholar]
- 2. Tattersall J, Dekker F, Heimbürger O.et al. When to start dialysis: updated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) Study. Nephrol Dial Transplant 2011; 26: 2082–6. Available from: https://pubmed.ncbi.nlm.nih.gov/21551086/ [DOI] [PubMed] [Google Scholar]
- 3. Shafi T, Jaar BG, Plantinga LC.et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis 2010; 56: 348–58. Available from: https://pubmed.ncbi.nlm.nih.gov/20605303/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eknoyan G, Beck GJ, Cheung AK.et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002; 347: 2010–19. Available from: https://pubmed.ncbi.nlm.nih.gov/12490682/ [DOI] [PubMed] [Google Scholar]
- 5. Gotch FA, Sargent JA.. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int 1985; 28: 526–34. Available from: https://pubmed.ncbi.nlm.nih.gov/3934452/ [DOI] [PubMed] [Google Scholar]
- 6. Rocco M, Daugirdas JT, Depner TA.et al. KDOQI Clinical Practice Guideline for Hemodialysis adequacy: 2015 update. Am J Kidney Dis 2015; 66: 884–930. Available from: https://pubmed.ncbi.nlm.nih.gov/26498416/ [DOI] [PubMed] [Google Scholar]
- 7. Obi Y, Streja E, Rhee CM.et al. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. Am J Kidney Dis 2016; 68: 256–65. Available from: https://pubmed.ncbi.nlm.nih.gov/26867814/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanson JA, Hulbert-Shearon TE, Ojo AO.et al. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol 1999; 19: 625–33. Available from: https://pubmed.ncbi.nlm.nih.gov/10592355/ [DOI] [PubMed] [Google Scholar]
- 9. Couchoud C, Kooman J, Finne P.et al. From registry data collection to international comparisons: examples of haemodialysis duration and frequency. Nephrol Dial Transplant 2009; 24: 217–24. Available from: https://pubmed.ncbi.nlm.nih.gov/18678560/ [DOI] [PubMed] [Google Scholar]
- 10. Cheetham MS, Cho Y, Krishnasamy R.et al. Incremental versus standard (full-dose) peritoneal dialysis. Kidney Int Rep 2022; 7: 165–76. Available from: 10.1016/j.ekir.2021.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jansen MAM, Hart AAM, Korevaar JC.et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62: 1046–53. Available from: https://pubmed.ncbi.nlm.nih.gov/12164889/ [DOI] [PubMed] [Google Scholar]
- 12. Inker LA, Schmid CH, Tighiouart H.et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Obi Y, Kalantar-Zadeh K, Streja E.et al. Prediction equation for calculating residual kidney urea clearance using urine collections for different hemodialysis treatment frequencies and interdialytic intervals. Nephrol Dial Transplant 2018; 33: 530–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marshall MR. Observations of twice a week hemodialysis. Kidney Int 2016; 90: 936–38. Available from: 10.1016/j.kint.2016.06.040 [DOI] [PubMed] [Google Scholar]
- 15. Lee MJ, Park JT, Park KS.et al. Prognostic value of residual urine volume, GFR by 24-hour urine collection, and eGFR in patients receiving dialysis. Clin J Am Soc Nephrol 2017; 12: 426–34. Available from: https://pubmed.ncbi.nlm.nih.gov/28228465/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quan H, Li B, Couris CM.et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–82. Available from: https://pubmed.ncbi.nlm.nih.gov/21330339/ [DOI] [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL.et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. Available from: https://pubmed.ncbi.nlm.nih.gov/3558716/ [DOI] [PubMed] [Google Scholar]
- 18. Rangaswami J, Bhalla V, Blair JEA.et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019; 139: E840–78. Available from: http://ahajournals.org [DOI] [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Unruh M, Zager PG.et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis 2014; 64: 181–6. Available from: https://pubmed.ncbi.nlm.nih.gov/24840669/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obi Y, Rhee CM, Mathew AT.et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol 2016; 27: 3758–68. Available from: www.jasn.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathew A, Obi Y, Rhee CM.et al. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int 2016; 90: 1071–9. Available from: https://pubmed.ncbi.nlm.nih.gov/27528548/ [DOI] [PubMed] [Google Scholar]
- 22. Garofalo C, Borrelli S, De Stefano T.et al. Incremental dialysis in ESRD: systematic review and meta-analysis. J Nephrol 2019; 32: 823–36. Available from: https://pubmed.ncbi.nlm.nih.gov/30604150/ [DOI] [PubMed] [Google Scholar]
- 23. Vilar E, Kaja Kamal RM, Fotheringham J.et al. A multicenter feasibility randomized controlled trial to assess the impact of incremental versus conventional initiation of hemodialysis on residual kidney function. Kidney Int 2022; 101: 615–25. Available from: www.kidney-international.org [DOI] [PubMed] [Google Scholar]
- 24. Murea M, Patel A, Highland BR.et al. Twice-weekly hemodialysis with adjuvant pharmacotherapy and transition to thrice-weekly hemodialysis: a pilot study. Am J Kidney Dis 2021; S0272-6386(21): 01040-4. doi: 10.1053/j.ajkd.2021.12.001 [DOI] [PubMed] [Google Scholar]
- 25. Stankuviene A, Žiginskiene E, Kuzminskis V.et al. Impact of hemodialysis dose and frequency on survival of patients on chronic hemodialysis in Lithuania during 1998-2005. Medicina 2010; 46: 516–21. Available from: https://www.mdpi.com/1648-9144/46/8/516 [PubMed] [Google Scholar]
- 26. Hwang HS, Hong YA, Yoon HE.et al. Comparison of clinical outcome between twice-weekly and thrice-weekly hemodialysis in patients with residual kidney function. Medicine 2016; 95: e2767. Available from: https://journals.lww.com/00005792-201602150-00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breidthardt T, Moser-Bucher CN, Praehauser C.et al. Morbidity and mortality on chronic haemodialysis: a 10-year Swiss single centre analysis. Swiss Med Wkly 2011; 141: w13150. Available from: https://smw.ch/article/doi/smw.2011.13150 [DOI] [PubMed] [Google Scholar]
- 28. Winzeler R, Ambuhl PM. MO818. Survival on dialysis: Switzerland in comparison with other countries – a follow up. Nephrol Dial Transplant 2021; 36 (Suppl 1). Available from: https://academic.oup.com/ndt/article/36/Supplement_1/gfab098.0010/6289277 [Google Scholar]
- 29. Torreggiani M, Fois A, Chatrenet A.et al. Incremental and personalized hemodialysis start: a new standard of care. Kidney Int Rep 2022; 7: 1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marshall MR. The benefit of early survival on PD versus HD—Why this is (still) very important. Perit Dial Int 2020; 40: 405–18. Available from: https://www.who.int/gho/mortality_burden_ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.