ABSTRACT
The number of patients ≥65 years of age suffering from advanced chronic kidney disease and transitioning to end-stage kidney disease (ESKD) is increasing. However, elderly patients often have poor outcomes once haemodialysis is initiated, including high mortality within the first year as well as fast cognitive and functional decline and diminished quality of life. The question is how we can smooth this transition to ESKD in older patients who also exhibit much higher proportions of frailty when compared with community-dwelling non-dialysis older adults and who are generally more vulnerable to invasive treatment such as kidney replacement therapy. To avoid early death and poor quality of life, a carefully prepared smooth transition should precede the initiation of treatment. This involves pre-dialysis physical and educational care, as well as mental and psychosocial preparedness of the patient to enable an informed and shared decision about the individual choice of treatment modality. Communication between a healthcare professional and patient plays a pivotal role but can be challenging given the high rate of cognitive impairment in this particular population. In order to practise patient-centred care, adapting treatment tailored to the individual patient should include comprehensive conservative care. However, structured treatment pathways including multidisciplinary teams for such conservative care are still rare and may be difficult to establish outside of large cities. Generally, geriatric nephrology misses data on the comparative effectiveness of different treatment modalities in this population of old and very old age on which to base recommendations and decisions.
Keywords: death, dialysis modality, older adults, quality of life, transition
TRANSITIONS IN NEPHROLOGY—MEANING
Transition is the ‘process or period of changing from one state or condition to another’ [1]. In nephrology, patients with chronic kidney disease (CKD) are prone to several transitions during their ‘disease career’. Many transit through several stages of CKD before some of them experience one of the main transitions from CKD Stage 4 to 5, end-stage kidney disease (ESKD), where most receive kidney replacement therapy (KRT). In the majority of cases worldwide, KRT is haemodialysis (HD), less frequently peritoneal dialysis (PD) and least often a kidney transplant. The minority of dialysis patients who eventually receive a kidney transplant undergo a second transition from being a dialysis patient to becoming a kidney transplant recipient. Some of these transplant organs fail before the recipient dies, leading to a third transition, albeit backwards in direction to KRT again. This review concentrates on the transition from CKD Stage 4 to 5 in older, frail adults whose health state does not allow transplantation, as this is the population in which such a transition comes along with difficult questions and decisions.
TRANSITION TO HD IN OLD AGE: A STATISTICAL PERSPECTIVE ON EARLY DEATH RATES
Despite improvement in dialysis treatment over the past decades, mortality rates remain high, especially among older patients. Older patients with advanced CKD are biologically older compared with patients their age without CKD, and frailty is reported to be 32% and 79%, respectively, among incident older dialysis patients, much higher compared with 7% in the general older population without CKD [2]. Thus they are at high risk for complications and death after initiation of HD. Landmark publication data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) demonstrated high mortality rates worldwide after dialysis initiation, especially within the first 3–6 months, in a huge sample of almost 87 000 individuals. When stratified by age, the data further demonstrated that this phenomenon is mostly driven by patients ≥65 years of age [3], with a mortality rate (deaths/100 person-years) of 40 in this age group. The United States Renal Data System (USRDS) annual data report from 2017 confirms these results with a first-year 30% mortality rate in patients ≥65 years of age, probably even underestimating the true number, as those who die before an outpatient dialysis provider enters them into the registry are not included [4]. Other data show that adjusted mortality among dialysis patients ≥75 years of age is 4-fold higher compared with age-matched non-dialysis Medicare beneficiaries [5, 6]. Also, when compared with non-dialysis patients with similar life-limiting illnesses such as cancer or congestive heart failure, rates of hospitalization, intensive treatments and dying in an intensive care setting are much higher among dialysis patients [7, 8].
TRANSITION TO DIALYSIS IN OLD AGE: THE PATIENT'S PERSPECTIVE
For the huge majority of patients, transitioning from CKD Stage 4 to 5 under nephrology care means the initiation of HD. Dialysis is a life saving procedure, but it comes with life-altering changes affecting both body and soul. This involves physical constraints and psychological stress, as many experience the transition as frightening and stressful [9, 10]. As much as uraemic symptoms improve, many patients also experience potential side effects, especially with HD, such as drops in blood pressure, muscle cramps, a significant decrease in or even a cessation of diuresis and fatigue. The ability to engage in social life is naturally limited by dialysis sessions three times per week and further diminished by session fatigue. In addition, losing autonomy by depending on a treatment where one is literally bound to a machine can reduce well-being and alters a patient's body image. All this reaches even greater complexity in old and frail incident dialysis patients with frequent cognitive and functional decline after dialysis initiation [11]. In particular, people starting dialysis at ≥80 years of age seem to lose their independence quickly [12]. Physical functional impairment as well as burdensome psychosocial factors diminish quality of life (QoL) immensely [13].
HOW DO WE SMOOTH THE TRANSITION?
With dialysis offering a marginal survival benefit while adversely affecting QoL among the elderly, we apparently need to understand better how to smooth this transition and adapt treatment tailored to a patient's age and needs and accompanying chronic complex conditions. We also need to acknowledge that there are cases where our traditional KRT repertoire should be expanded by supportive care or even replaced by conservative management. The aim is to do no physical and mental harm to a population that is more vulnerable compared with younger patients. Indicators of a successful transition can be hospitalization and survival rates, healthcare costs and a patient's QoL. This review will focus on death and QoL and factors that can positively impact them.
What determines early death among older adults on dialysis?
It has been known for some time that timely and appropriate nephrology care for Stage 4 CKD patients [14–16] as well as the use of an arteriovenous fistula positively influences first-year mortality in incident HD patients [17, 18]. In addition, Karaboyas et al. [19] calculated the attributable fractions for common mortality risk factors among almost 16 000 incident HD patients (<60 days vintage) across 21 countries in the framework of DOPPS. The mean age of the population under study was 63 years, and a quarter did not experience pre-dialysis nephrology care. The largest fractions of early dialysis mortality were attributed to malnutrition (low serum albumin and low creatinine; 29%), catheter use (22%), lack of pre-dialysis nephrology care (9%) and lack of residual urine volume (9%). Other common measures such as systolic blood pressure, haemoglobin and phosphorus contributed to a lesser extent. These estimates may provide information on the potentially beneficial impact of pre-dialysis physical care interventions, although data with a special focus on old and very old incident HD adults are still scarce. Existing prediction models hardly target older adults and have proven to perform disappointingly if externally validated [20]. A recent early mortality prediction model developed in a large sample of US veterans with a mean age of 69 years demonstrated consistently acceptable C-statistics, also in external validation. In patients ≥65 years, however, C-statistics appeared to be lower [21]. Thus some decisions remain difficult in old age; placing an arteriovenous fistula under general anaesthesia instead of a catheter in an 84-year-old, frail patient with limited life expectancy who chose HD to provide the best of care is not an easy decision.
Incremental dialysis as an adaptive approach
The thought behind ‘incremental’ is that the start of standard dialysis often happens abruptly, ignoring a longer and insidious process of declining kidney function over months or years. Also, it has been argued that the rather fast loss of residual kidney function often experienced after initiation of standard thrice-weekly HD could contribute to the high early mortality rate [22–25]. The slope of kidney function decline can be heterogeneous. Especially among older adults, a serious fraction exhibit slower progression of CKD [26], potentially making them good candidates for incremental HD. An incremental, stepped HD regimen with a scheduled transition from twice- to thrice-weekly is believed to offer the body more time to adapt to the new treatment compared with the sudden start of standard HD, the prescription of which is in itself fundamentally empirical [27]. However, barriers lie in the risk of ‘under dialysis’, leading to a lasting uraemic milieu with all hazardous implications. Also, patients’ adherence when it comes to increased dialysis frequency is questionable and requires closer monitoring by the dialysis staff. Evidence so far comparing the clinical safety and effectiveness of incremental HD versus standard HD relies mainly on observational studies. A meta-analysis of 22 cohort studies compared all-cause mortality and residual kidney function in patients receiving incremental HD or incremental PD versus standard HD or standard PD [28]. Overall, incremental dialysis allowed longer preservation of residual kidney function and deferred standard dialysis by approximately 1 year with no increase in mortality risk. However, the studies included, however, did not specifically target older adults, demonstrated high heterogeneity and, due to their observational design, disclosed methodological pitfalls such as residual confounding. In order to determine if especially older patients would benefit most from an incremental regime compared with standard dialysis, data from randomized controlled trials (RCTs) comparing early death rates are needed. A recent feasibility RCT including 55 patients with a mean age of 62 years, randomized to either incremental (twice-weekly) or standard dialysis (thrice-weekly), demonstrated similar outcomes in terms of deaths, residual kidney function and QoL in both arms, justifying a larger trial [29], which will then hopefully include more older adults.
Another incremental approach is once-weekly HD combined with a low protein diet. A recent review summarized four articles, albeit all in younger or middle-aged patients and either uncontrolled or of limited sample sizes [30]. Results indicate that this approach could be of benefit in specific motivated patients with good adherence, an assessment confirmed by a Japanese study of 112 patients with a mean age of 63 years [31]. There is one trial in uraemic patients older than 70 years and without diabetes that demonstrated that a very low protein diet was effective and safe when postponing dialysis treatment. However, the results should be interpreted with great caution, as there were severe methodological limitations (i.e. the estimated sample size could not be reached) [32]. Principally, in old and frail patients, in whom low muscle mass is frequently present, a low protein diet between HD sessions should be applied with caution, as it will potentially worsen sarcopenia.
DIALYSIS MODALITY IN OLD AGE: COMPARING MORTALITY IN EARLY PHASES OF HD VERSUS PD
A related topic of debate has been the choice of the classic dialysis modalities in older age, namely HD or PD. The USRDS annual report [4] compares mortality during the first year of dialysis depending on the choice of dialysis modality among patients ≥65 years of age. It demonstrates a steeper increase in mortality during the first months followed by a generally higher mortality (300 deaths per 1000 patient-years) among HD patients compared with PD patients (200 deaths per 1000 patient-years). Despite multiple adjustments, these results may be influenced by selection bias, as patients on PD are potentially healthier compared with patients on HD. Again, RCTs comparing survival rates of these two modalities among older adults are unfortunately missing.
What determines QoL among older adults in early phases of dialysis?
As stated above and to reflect the patient's perspective, QoL among incident older dialysis patients is, apart from mortality, an indicator of a smooth transition.
DIALYSIS MODALITY IN OLD AGE: COMPARING QOL IN EARLY PHASES OF HD VERSUS PD
As with mortality, there are no RCTs comparing QoL among patients in the early phases of HD versus PD treatment, only prospective cohort studies [33–41]. As these studies report on various patient scales and focus on different QoL domains, they are difficult to compare. Overall, results are inconclusive: some studies favour PD, some HD and others do not see significant differences at all. Only two studies specifically target older adults but are quite small in size, with 174 [34] and 206 [38] patients. One uses the Kidney Disease Quality of Life (KDQOL) scale [34], whereas the other one applies five different scales, including the Hospital Anxiety and Depression Scale, the 12-item Short-Form Health Survey, symptom score illness scale, Barthel score and Renal Treatment Satisfaction Questionnaire [38]. None of the two studies demonstrates significant differences in any scoring system. The biggest study uses data from DOPPS comparing approximately 4500 HD patients with 3200 PD patients, stratified by dialysis vintage over a wide age range. Here PD patients reported a lower burden of disease score than HD patients using the KDQOL questionnaire, with a stable burden over 12 months. When stratified by age, these PD-favourable results were especially visible in older adults [41].
QOL: PRE-ESKD PHYSICAL CARE IN OLD AGE
To a large extent, QoL is determined by physical constraints. A recent study from the Japanese DOPPS analysed QoL in almost 900 prevalent HD patients ≥60 years of age and observed that physical QoL assessed by the Physical Component Summary (PCS) score became worse with increasing dialysis vintage [42]. Also, the PCS deteriorated with increasing age. In patients with advanced CKD, there is a multiplicity of symptoms, including pruritus, exhaustion, sleep disorders, anorexia, soreness of muscles, faintness or dizziness, numbness in the hands or feet, nausea, vomiting or loss of appetite, constipation, diarrhoea, dyspnea, chest pain, depression and pain [43]. Altogether, these symptoms need treatment during pre-ESKD care and constitute a large burden for patients and caregivers. Physical constraints also cause declines in physical functioning, like residual urine volume and sexual or cognitive function. This multiplicity of symptoms becomes multidimensional in older adults in whom frailty and serious cognitive impairment aggravate chronic conditions. Thus, to smooth the transition to ESKD, it is important that patients with advanced CKD are regularly questioned about their physical constraints. Regular symptom assessment and management, including symptoms of depression, have been identified by patients as crucial in order to practice patient-centred care [44, 45]. It also provides an opportunity to discuss supportive care options [43]. Once again, this underscores the need for a timely referral to regular nephrology care in order to accompany the patient in an organized way from Stage 4 to 5. However, USRDS data show that only 22–36% of new ESKD cases receive little or no pre-ESKD care, numbers that have improved over the years, but are still too high. These numbers may be even higher in old age when a huge number of patients primarily consult their general practitioners.
QOL: PRE-ESKD EDUCATIONAL CARE IN OLD AGE
Pre-ESKD educational care should go hand in hand with pre-ESKD physical care. Even if there is only limited robust evidence of the causal effects of health literacy on hard patient outcomes such as death, time to KRT or cardiovascular events [46], it plays a pivotal role in decision-making. Patients need to be educated about their condition and about renal replacement therapy before they start it and receive all the information they need in a way and in a language they understand in order to make an informed decision and to avoid regret. Data on the proportion of dialysis patients who regret their decision to start have shown heterologous results: 61% in a Canadian cohort with a mean age of 68 years [47], 21% in a US cohort with a mean age of 59 years [48] and only 7% in a nationwide Dutch survey where 64.5% of participants were ≥65 years of age [49]. Here, older age was even associated with less regret. Overall, data on dialysis regret in old and very old age are limited, and the wide range of results may also be explained by differences in practice patterns across countries and regions, dialysis providers, mode of dialysis, vintage, frailty status, method of data collection and publication year.
Education should involve a concrete description of treatment modality and frequency, differences between modalities, dialysis access and pros and cons against the background of personal circumstances, as well as prognosis and life expectancy. To explain rather complex treatment options and their implications, especially to old or very old patients, can be a communication challenge that often needs to involve relatives.
PRE-ESKD EDUCATIONAL CARE AND THE PROBLEM OF COGNITIVE IMPAIRMENT
In addition, cognitive impairment in older adults with advanced CKD exacerbates communication. CKD is found to be an independent risk factor for cognitive impairment [50] and aggravates the already existing problem of cognitive decline in old age. In a study of 374 US prevalent HD patients ≥55 years of age, only 13% had normal function, whereas 14% were classified with mild, 36% with moderate and 37% with severe cognitive impairment [51]. Several studies show that the prevalence is already high in individuals transitioning to KRT. Using the Mini-Mental State Examination tool, a British study of 132 patients with a mean age of 58 years showed that 20% were cognitively impaired and those with impairment were significantly older [52]. A Canadian study using the Montreal Cognitive Assessment [53] uncovered a high proportion of 61% of unrecognized cognitive impairment in 385 patients with CKD Stages 4 and 5 and a mean age of 68 years. Several studies have found associations of dementia with an increased mortality in older patients reaching ESKD [11, 54–57]. Cognitive changes occur early in CKD, and orientation and attention, as well as language, are particularly affected domains likely to diminish a patient's ability to make autonomous healthcare decisions, as shown in a systematic review [58]. Given the high prevalence of sometimes unrecognized cognitive impairment in older patients with advanced CKD and the impact this has on advance care planning, healthcare professionals advocate the incorporation of cognitive function assessment in routine diagnostics in these individuals [59]. In many cases, this will require a sensitive approach, as some patients may feel overwhelmed by unexpected cognitive testing when they in fact expected kidney function tests.
Especially in old and frail patients with limited life expectancy, these conversations should automatically involve the option of conservative treatment, discussed in more detail below.
QOL: PRE-ESKD MENTAL AND PSYCHOSOCIAL CARE IN OLD AGE
As indicated above, patients with advanced kidney disease experience a high burden of psychosocial problems, reflecting an interplay of physical and mental chronic health conditions. Typical psychosocial themes commonly affecting patients with ESKD are adjustment to illness, death and dying, depression and anxiety, perception of loss, family and social functioning, employment and financial stress [13]. Digestion of such existential subjects needs help and coping strategies. Patients who received psychosocial support from social workers had a better QoL, lower depression scores and fewer clinic visits [13]. Studies from other medical specialties such as oncology or gastroenterology have also shown that psychosocial care enhances QoL [60, 61]. In particular, oncology has gained a role model function by turning this into a speciality of its own, psycho-oncology. Psycho-oncology addresses the psychological responses of patients and the psychological, behavioural and social factors that influence the disease process. Since the life expectancy of most older dialysis patients is approximately as limited as that of patients with metastasized cancer, comprehensive care should include timely psycho-nephrology to support patients to learn coping strategies and to adjust mentally to the new circumstances.
COMMUNICATION IS KEY
Addressing psychosocial stressors needs sensitivity, time and communication skills. Having end-of-life conversations with older adults in advanced stages of life-limiting illness requires specialized training of health professionals. A ‘serious illness conversation guide’ may give some advice on the most important topics as well as on how to open and close a conversation [62]. Most patients have a great need for information regarding their illness itself, future symptoms and their management, life expectancy and information about clinical treatment options. However, some prefer less information. Fewer than 10% of patients on dialysis report having had a conversation addressing their preferences [47]. Generally, patients often find themselves between wanting to know what to expect and fearing the news. They prefer a trusted health professional who shows empathy and honesty and who feels comfortable talking about death and dying [63].
Communication is also important when it comes to discussing advance care planning and explaining various treatment options that all impact a patient's life differently. However, patients, care partners and physicians hold discordant views about the responsibility for discussing advance care planning [64]. Despite the best intentions, it can be challenging for physicians to balance objective clinical assessment, intuition and subjective perception. Thus it was observed that implicit persuasion among physicians is common when discussing different treatment modalities [65]. Good communication is a healthy blend of empathy, common sense, commitment and time to listen to the patient and relatives. It cannot be valued highly enough.
ADAPTING TREATMENT TAILORED TO INDIVIDUAL PATIENT'S NEEDS
Over time it has become increasingly apparent that treatment modalities that apply in younger patients do not necessarily apply in older patients. Functional and cognitive decline as well as frailty are extremely common in this age group and strongly associated with adverse outcomes [66]. In such a highly heterogeneous population of older adults, risk stratification based on functional decline or frailty can be difficult. Instead, individualized care has been propagated, offering treatment tailored to individual patient needs. This includes better advance care planning [67], which is still found to be much lower in ESKD patients compared with cancer patients, although symptom rates are similarly high and treatment intensity is even higher in ESKD patients [8]. Figure 1 demonstrates the multiple factors that potentially influence the choice of treatment modality in old age. It also comprises comprehensive conservative care as a non-dialysis treatment option.
FIGURE 1:
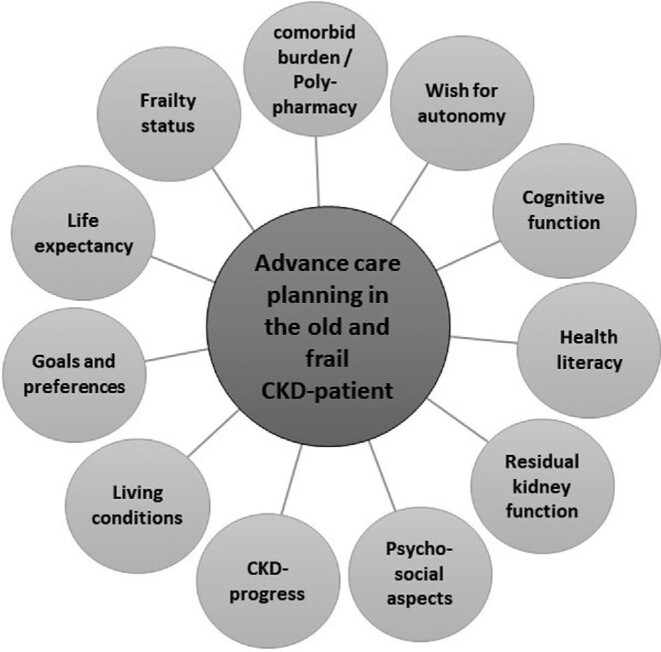
Multidimensional factors that influence the choice of treatment modality in old age.
COMPREHENSIVE CONSERVATIVE CARE
In old, frail and morbid patients with multiple health conditions, many agree that dialysis should not be the default option. Instead, conservative management has been increasingly advocated in recent years as an alternative treatment option [43], especially since we know that many older patients prioritize QoL over extending life [63, 67]. Comprehensive conservative care is understood as a ‘planned holistic patient-centred care’ approach that includes ‘a full range of treatment and support, but not dialysis’ [43]. Despite a growing body of literature, conservative management is still not being routinely offered as a standard of care in many centres, and if it is, it is not necessarily of the highest quality [68]. Quality indicators of conservative kidney management are multidisciplinary teams; shared decision-making; symptom management; psychological, cultural and spiritual support and healthcare provider training. The Global Kidney Health Atlas survey from 2018 shows that availability and accessibility differ immensely between countries and are lower in low-income countries [68]. Applying conservative management requires a structured treatment pathway; it requires knowledge and training and infrastructure to enable a multidisciplinary and intersectoral team approach and to create a framework in which these quality indicators do not remain simple buzzwords. A multidisciplinary team would ideally be comprised of a nephrologist, geriatrician, palliative care doctor, nurse, dietician and psychologist. To offer easy access to such complex services across several settings (hospital, practice, hospice, home or nursing home) can be difficult, especially in rural areas. In the near future, the implementation of telehealth measures will hopefully be able to support and complement access to conservative care. To advance comprehensive conservative kidney care, it also needs a comprehensive policy approach. It needs incorporation of patient-centred care into the curriculum of medical students and nurses, including communication training, more educational programmes for health professionals who would like to specialize in this field, better demand planning for conservative care, better reimbursement and more healthcare research. A recent review found 11 observational studies comparing conservative management to dialysis in older adults, demonstrating the potential to achieve similar health-related QoL in patients receiving conservative care, but all studies were susceptible to selection bias and confounding [69]. The Prepare for Kidney Care study is a rare example of an RCT that aims at comparing preparation for dialysis versus preparation for conservative care [70]. By focusing on the preparation phase, it explicitly addresses the treatment transition phase in multi morbid, frail and older patients with Stage 5 CKD. The primary outcomes are quality-adjusted life years, and the results can hopefully be expected within the next couple of years.
SUMMARY
The number of older adults receiving dialysis continues to increase, although the survival advantage, especially among frail individuals, is not clear and QoL is often diminished. This review concentrated on the question of how the transition from CKD Stage 4 to ESKD in older adults can be smoothed, including treatment adaptation, death and QoL as indicators for a successful transition. After reviewing the literature, the following four messages can be summarized: (1) More research is needed, particularly targeting older adults with advanced kidney disease. This involves the collection of primary data to compare the clinical effectiveness of different treatment modalities in old and frail ESKD patients and help choosing a modality based on solid risk stratification. (2) In old and frail ESKD patients, dialysis should not be the default treatment option. Instead, clinicians should also focus on conservative management. To be able to do so, knowledge, frameworks and infrastructure for conservative care pathways have to be expanded and established, which will also need strong political will. It also requires timely referral to nephrology care in order to understand the progression of CKD, define treatment goals and prepare the patient accordingly. (3) Health communication is key, but challenging, in older patients suffering from complex chronic conditions and especially if cognitive impairment is present. More awareness of the huge burden of unrecognized cognitive impairment in older patients with advanced CKD should prompt nephrologists to integrate cognitive testing in their routine programme to guide communication and advance care planning. (4) The high burden of comorbidities and psychosocial factors in older patients with advanced CKD points to the need for patient-centred care. The growing integration of patient-reported outcome measures in medical education and patient care is an important step in the right direction.
CONFLICT OF INTEREST STATEMENT
E.S. has received lecture honorarium from Fresenius Medical Care. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1. Oxford Learner's Dictionary. Transition. https://www.oxfordlearnersdictionaries.com/us/definition/english/transition_1?q=transition (August 2021, date last accessed)
- 2. Fried LP, Tangen CM, Walston Jet al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156 [DOI] [PubMed] [Google Scholar]
- 3. Robinson BM, Zhang J, Morgenstern Het al. . Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 2014; 85: 158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saran R, Robinson B, Abbott KCet al. . US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2018; 71(3 Suppl 1): A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wachterman MW, O'Hare AM, Rahman OKet al. . One-year mortality after dialysis initiation among older adults. JAMA Intern Med 2019; 179: 987–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saran R, Robinson B, Abbott KCet al. . US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019; 73(3 Suppl 1): A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong SP, Kreuter W, O'Hare AM. Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med 2012; 172: 661–663; discussion 663–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wachterman MW, Lipsitz SR, Lorenz KAet al. . End-of-life experience of older adults dying of end-stage renal disease: a comparison with cancer. J Pain Symptom Manage 2017; 54: 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor DM, Bradley JA, Bradley Cet al. . Limited health literacy in advanced kidney disease. Kidney Int 2016; 90: 685–695 [DOI] [PubMed] [Google Scholar]
- 10. Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 2009; 4: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurella Tamura M, Covinsky KE, Chertow GMet al. . Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361: 1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med 2009; 361: 1612–1613 [DOI] [PubMed] [Google Scholar]
- 13. Hansen MS, Tesfaye W, Sewlal Bet al. . Psychosocial factors affecting patients with end-stage kidney disease and the impact of the social worker. J Nephrol 2021; doi: 10.1007/s40620-021-01098-8 [DOI] [PubMed] [Google Scholar]
- 14. Hasegawa T, Bragg-Gresham JL, Yamazaki Set al. . Greater first-year survival on hemodialysis in facilities in which patients are provided earlier and more frequent pre-nephrology visits. Clin J Am Soc Nephrol 2009; 4: 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slinin Y, Guo H, Gilbertson DTet al. . Meeting KDOQI guideline goals at hemodialysis initiation and survival during the first year. Clin J Am Soc Nephrol 2010; 5: 1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang SJ, Yang WC, Lin MYet al. . Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant 2010; 25: 2616–2624 [DOI] [PubMed] [Google Scholar]
- 17. Bradbury BD, Fissell RB, Albert JMet al. . Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2007; 2: 89–99 [DOI] [PubMed] [Google Scholar]
- 18. Ng LJ, Chen F, Pisoni RLet al. . Hospitalization risks related to vascular access type among incident US hemodialysis patients. Nephrol Dial Transplant 2011; 26: 3659–3666 [DOI] [PubMed] [Google Scholar]
- 19. Karaboyas A, Morgenstern H, Li Yet al. . Estimating the fraction of first-year hemodialysis deaths attributable to potentially modifiable risk factors: results from the DOPPS. Clin Epidemiol 2020; 12: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramspek CL, Voskamp PW, van Ittersum FJet al. . Prediction models for the mortality risk in chronic dialysis patients: a systematic review and independent external validation study. Clin Epidemiol 2017; 9: 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Obi Y, Nguyen DV, Zhou Het al. . Development and validation of prediction scores for early mortality at transition to dialysis. Mayo Clin Proc 2018; 93: 1224–1235 [DOI] [PubMed] [Google Scholar]
- 22. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis 2009; 53: 1068–1081 [DOI] [PubMed] [Google Scholar]
- 23. Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial 2011; 24: 487–494 [DOI] [PubMed] [Google Scholar]
- 24. Shafi T, Jaar BG, Plantinga LCet al. . Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) study. Am J Kidney Dis 2010; 56: 348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obi Y, Rhee CM, Mathew ATet al. . Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol 2016; 27: 3758–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Totoli C, Carvalho AB, Ammirati ALet al. . Associated factors related to chronic kidney disease progression in elderly patients. PLoS One 2019; 14: e0219956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murea M, Moossavi S, Garneata Let al. . Narrative review of incremental hemodialysis. Kidney Int Rep 2020; 5: 135–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garofalo C, Borrelli S, De Stefano Tet al. . Incremental dialysis in ESRD: systematic review and meta-analysis. J Nephrol 2019; 32: 823–836 [DOI] [PubMed] [Google Scholar]
- 29. Vilar E, Kaja Kamal RM, Fotheringham Jet al. . A multicenter feasibility randomized controlled trial to assess the impact of incremental versus conventional initiation of hemodialysis on residual kidney function. Kidney Int 2021; doi: 10.1016/j.kint.2021.07.025 [DOI] [PubMed] [Google Scholar]
- 30. Bolasco P, Cupisti A, Locatelli Fet al. . Dietary management of incremental transition to dialysis therapy: once-weekly hemodialysis combined with low-protein diet. J Ren Nutr 2016; 26: 352–359 [DOI] [PubMed] [Google Scholar]
- 31. Nakao T, Kanazawa Y, Takahashi T. Once-weekly hemodialysis combined with low-protein and low-salt dietary treatment as a favorable therapeutic modality for selected patients with end-stage renal failure: a prospective observational study in Japanese patients. BMC Nephrol 2018; 19: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brunori G, Viola BF, Parrinello Get al. . Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: a prospective randomized multicenter controlled study. Am J Kidney Dis 2007; 49: 569–580 [DOI] [PubMed] [Google Scholar]
- 33. Frimat L, Durand PY, Loos-Ayav Cet al. . Impact of first dialysis modality on outcome of patients contraindicated for kidney transplant. Perit Dial Int 2006; 26: 231–239 [PubMed] [Google Scholar]
- 34. Harris SA, Lamping DL, Brown EAet al. . Clinical outcomes and quality of life in elderly patients on peritoneal dialysis versus hemodialysis. Perit Dial Int 2002; 22: 463–470 [PubMed] [Google Scholar]
- 35. Manns B, Johnson JA, Taub Ket al. . Quality of life in patients treated with hemodialysis or peritoneal dialysis: what are the important determinants? Clin Nephrol 2003; 60: 341–351 [DOI] [PubMed] [Google Scholar]
- 36. Wu AW, Fink NE, Marsh-Manzi JVet al. . Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol 2004; 15: 743–753 [DOI] [PubMed] [Google Scholar]
- 37. Hiramatsu T, Okumura S, Asano Yet al. . Quality of life and emotional distress in peritoneal dialysis and hemodialysis patients. Ther Apher Dial 2020; 24: 366–372 [DOI] [PubMed] [Google Scholar]
- 38. Iyasere O, Brown E, Gordon Fet al. . Longitudinal trends in quality of life and physical function in frail older dialysis patients: a comparison of assisted peritoneal dialysis and in-center hemodialysis. Perit Dial Int 2019; 39: 112–118 [DOI] [PubMed] [Google Scholar]
- 39. Neumann D, Mau W, Wienke Aet al. . Peritoneal dialysis is associated with better cognitive function than hemodialysis over a one-year course. Kidney Int 2018; 93: 430–438 [DOI] [PubMed] [Google Scholar]
- 40. Jung HY, Jeon Y, Park Yet al. . Better quality of life of peritoneal dialysis compared to hemodialysis over a two-year period after dialysis initiation. Sci Rep 2019; 9: 10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown EA, Zhao J, McCullough Ket al. . Burden of kidney disease, health-related quality of life, and employment among patients receiving peritoneal dialysis and in-center hemodialysis: findings from the DOPPS program. Am J Kidney Dis 2021; 78: 489–500.e1 [DOI] [PubMed] [Google Scholar]
- 42. Ishiwatari A, Yamamoto S, Fukuma Set al. . Changes in quality of life in older hemodialysis patients: a cohort study on dialysis outcomes and practice patterns. Am J Nephrol 2020; 51: 650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davison SN, Levin A, Moss AHet al. . Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int 2015; 88: 447–459 [DOI] [PubMed] [Google Scholar]
- 44. O'Hare AM, Armistead N, Schrag WLet al. . Patient-centered care: an opportunity to accomplish the “Three Aims” of the National Quality Strategy in the Medicare ESRD program. Clin J Am Soc Nephrol 2014; 9: 2189–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Manns B, Hemmelgarn B, Lillie Eet al. . Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 2014; 9: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taylor DM, Fraser S, Dudley Cet al. . Health literacy and patient outcomes in chronic kidney disease: a systematic review. Nephrol Dial Transplant 2018; 33: 1545–1558 [DOI] [PubMed] [Google Scholar]
- 47. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saeed F, Ladwig SA, Epstein RMet al. . Dialysis regret: prevalence and correlates. Clin J Am Soc Nephrol 2020; 15: 957–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Berkhout-Byrne N, Gaasbeek A, Mallat MJKet al. . Regret about the decision to start dialysis: a cross-sectional Dutch national survey. Neth J Med 2017; 75: 225–234 [PubMed] [Google Scholar]
- 50. Etgen T, Chonchol M, Forstl Het al. . Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 2012; 35: 474–482 [DOI] [PubMed] [Google Scholar]
- 51. Murray AM, Tupper DE, Knopman DSet al. . Cognitive impairment in hemodialysis patients is common. Neurology 2006; 67: 216–223 [DOI] [PubMed] [Google Scholar]
- 52. Nulsen RS, Yaqoob MM, Mahon Aet al. . Prevalence of cognitive impairment in patients attending pre-dialysis clinic. J Ren Care 2008; 34: 121–126 [DOI] [PubMed] [Google Scholar]
- 53. Foster R, Walker S, Brar Ret al. . Cognitive impairment in advanced chronic kidney disease: the canadian frailty observation and interventions trial. Am J Nephrol 2016; 44: 473–480 [DOI] [PubMed] [Google Scholar]
- 54. Couchoud CG, Beuscart JB, Aldigier JCet al. . Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly patients with end-stage renal disease. Kidney Int 2015; 88: 1178–1186 [DOI] [PubMed] [Google Scholar]
- 55. Dusseux E, Albano L, Fafin Cet al. . A simple clinical tool to inform the decision-making process to refer elderly incident dialysis patients for kidney transplant evaluation. Kidney Int 2015; 88: 121–129 [DOI] [PubMed] [Google Scholar]
- 56. Park JY, Kim MH, Han SSet al. . Recalibration and validation of the Charlson comorbidity index in Korean incident hemodialysis patients. PLoS One 2015; 10: e0127240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rakowski DA, Caillard S, Agodoa LYet al. . Dementia as a predictor of mortality in dialysis patients. Clin J Am Soc Nephrol 2006; 1: 1000–1005 [DOI] [PubMed] [Google Scholar]
- 58. Berger I, Wu S, Masson Pet al. . Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med 2016; 14: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scott J, Owen-Smith A, Tonkin-Crine Set al. . Decision-making for people with dementia and advanced kidney disease: a secondary qualitative analysis of interviews from the conservative kidney management assessment of practice patterns study. BMJ Open 2018; 8: e022385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carlson LE, Bultz BD. Benefits of psychosocial oncology care: improved quality of life and medical cost offset. Health Qual Life Outcomes 2003; 1: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reiss M, Sandborn WJ. The role of psychosocial care in adapting to health care reform. Clin Gastroenterol Hepatol 2015; 13: 2219–2224 [DOI] [PubMed] [Google Scholar]
- 62. Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol 2017; 12: 854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Parker SM, Clayton JM, Hancock Ket al. . A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage 2007; 34: 81–93 [DOI] [PubMed] [Google Scholar]
- 64. Ladin K, Neckermann I, D'Arcangelo Net al. . Advance care planning in older adults with CKD: patient, care partner, and clinician perspectives. J Am Soc Nephrol 2021; 32: 1527–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Dulmen S, Peereboom E, Schulze Let al. . The use of implicit persuasion in decision-making about treatment for end-stage kidney disease. Perit Dial Int 2021; doi: 10.1177/08968608211027019 [DOI] [PubMed] [Google Scholar]
- 66. Kallenberg MH, Kleinveld HA, Dekker FWet al. . Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD—a systematic review. Clin J Am Soc Nephrol 2016; 11: 1624–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Higginson IJ, Gomes B, Calanzani Net al. . Priorities for treatment, care and information if faced with serious illness: a comparative population-based survey in seven European countries. Palliat Med 2014; 28: 101–110 [DOI] [PubMed] [Google Scholar]
- 68. Lunney M, Bello AK, Levin Aet al. . Availability, accessibility, and quality of conservative kidney management worldwide. Clin J Am Soc Nephrol 2020; 16: 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verberne WR, van den Wittenboer ID, Voorend CGNet al. . Health-related quality of life and symptoms of conservative care versus dialysis in patients with end-stage kidney disease: a systematic review. Nephrol Dial Transplant 2021; 36: 1418–1433 [DOI] [PubMed] [Google Scholar]
- 70. Murphy E, Burns A, Murtagh FEMet al. . The prepare for kidney care study: prepare for renal dialysis versus responsive management in advanced chronic kidney disease. Nephrol Dial Transplant 2021; 36: 975–982 [DOI] [PMC free article] [PubMed] [Google Scholar]


