Abstract
Objective:
Lung disease associated with Systemic Juvenile Idiopathic Arthritis (sJIA-LD) is a potentially life threating complication in children with sJIA. Although high resolution computed tomography (HRCT) is considered the gold standard imaging modality for evaluating interstitial lung disease (ILD), lung ultrasound (LUS) has shown utility for ILD screening in adults with connective tissue diseases (CTD) at lower cost and without utilizing ionizing radiation. The goals of this pilot study were to describe LUS features in children with known SJIA-LD and to assess the feasibility of LUS in this population.
Methods:
Children <18 years with sJIA-LD and healthy controls were enrolled. LUS acquisition was performed at 14 lung positions. Demographic, clinical, and HRCT data were collected and reviewed. Feasibility was assessed through patient surveys. LUS findings were qualitatively and semi-quantitatively assessed and compared to HRCT findings.
Results:
LUS was performed in 9 children with sJIA-LD and 6 healthy controls and took 12 minutes on average to perform. LUS findings in sJIA-LD included focal to diffuse pleural irregularity, granularity, and thickening, with associated scattered or coalesced B-lines, and subpleural consolidations. LUS findings appeared to correspond to HRCT findings.
Conclusion:
LUS in sJIA-LD reveals highly conspicuous abnormalities in the pleura and sub-pleura that appear to correlate with peripheral lung findings on HRCT. LUS is a feasible imaging tool in children even from an early age. This study suggests a potential role of LUS in sJIA-LD screening, diagnosis, and/or prognostication.
Keywords: lung ultrasound, systemic juvenile idiopathic arthritis, interstitial lung disease
INTRODUCTION
Patients with systemic juvenile idiopathic arthritis (sJIA), a chronic inflammatory disease, represent 10–20% of the total JIA population[1]. In recent years, there is increasing recognition of a severe and potentially life threating chronic lung disease associated with sJIA (sJIA-LD) that is characterized by a spectrum of pulmonary alveolar proteinosis (PAP) and endogenous lipoid pneumonia (ELP) at biopsy, histopathology rarely encountered in other rheumatologic diseases[2, 3]. Why some pediatric sJIA patients develop sJIA-LD continues to be a topic of much investigation. The severity of sJIA-LD and its potential treatability necessitates early detection and monitoring. Unfortunately, clinical features of sJIA-LD may lag behind findings on high resolution computed tomography (HRCT) and biopsy which may result in delayed diagnosis and management with potentially detrimental outcomes[2, 3]. HRCT is the gold standard imaging modality for interstitial lung disease (ILD) detection. HRCT is more sensitive in detecting ILD changes than plain chest radiograph[4]. However, the significant cumulative radiation and the costs related to serial HRCT precludes its routine use for screening and monitoring of sJIA-LD progression and response to therapy in patients with sJIA.
Lung ultrasound (LUS) is a non-invasive, ionizing radiation-free, low-cost imaging technique traditionally used for the evaluation of the pleura or for US guided procedures. Recently, LUS has also been explored for the screening of connective tissue disease (CTD)-ILD in adults[5]. Three key sonographic findings that have been associated with ILD: presence and number of B-lines (vertical reverberation artifact), pleural line thickening, and pleural line irregularity. A meta-analysis looking to evaluate the diagnostic performance of LUS compared to HRCT in CTD-ILD reported LUS sensitivity for ILD of 91.5% and specificity of 81.3%[6]. This study also reported an area under the curve of 0.915, suggesting high diagnostic accuracy. Fairchild et al[7] proposed a novel and feasible LUS interpretation criteria which is highly sensitive and specific for systemic sclerosis associated ILD (SSc-ILD) and had perfect agreement among sonographic and non-sonographic readers.
The role of LUS for the assessment of ILD in childhood rheumatic diseases like sJIA has yet to be established. Given the severity and long-term complications faced by children with sJIA-LD, we conducted a pilot study to describe LUS findings in sJIA-LD and to evaluate the feasibility of performing LUS in the pediatric rheumatology clinic.
PATIENTS AND METHODS
Study population
We enrolled patients age < 18 years with a diagnosis of sJIA according to the International League of Associations for Rheumatology classification with known sJIA-LD. Patients were enrolled at both Lucille Packard Children’s Hospital (LPCH) and Cincinnati Children’s Hospital Medical Center (CCHMC), two large tertiary care centers with internationally recognized pediatric rheumatology experts in sJIA. The study was approved by the Stanford University and CCHMC Institutional Review Board. Informed consent from the patient’s parent and/or guardian and patient assent, where applicable, was obtained for all participants.
Clinical, imaging, and laboratory assessment
Standard demographic data and clinical data variables collected included sJIA disease duration, sJIA-LD duration, clinical signs and symptoms, HRCT findings, lung tissue biopsy findings, pulmonary function tests (PFT) if performed, current and prior therapies. HRCT was reviewed by one radiologist expert in pediatric ILD (AS).
LUS assessment
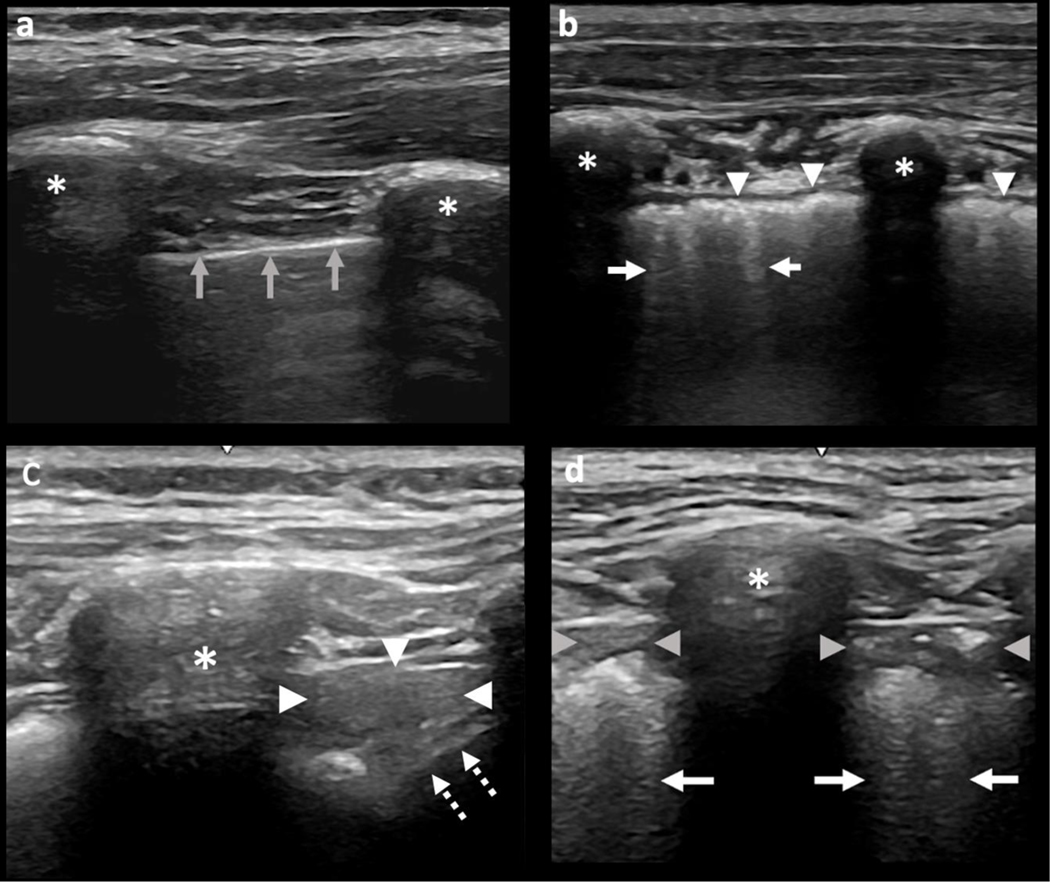
LUS evaluations were performed using a GE Logiq E system equipped with a 5–12 MHz linear array probe with standard B-mode settings similar to our prior work[7]. Various probe types (linear, convex, cardiac) can be used in CTD-ILD with good correlation between linear and cardiac probes previously being demonstrated in CTD-ILD[5]. Experienced ultrasonographers at LPCH (RF) and CCHMC (PVF and TT) performed all LUS examinations. Protocols previously described by our group[7] and Gutierrez et al [8] were used for image acquisition. In brief, we evaluated 14 lung positions including 6 anterior, 2 axillary (patient supine), and 6 posterior positions (patient upright) (Supplementary Table 2). The US probe was angulated in a sagittal or pseudo-sagittal orientation, spanning adjacent ribs to allow pleural and subpleural feature tracking with respiration. Superior to inferior lung positions were examined, and a 4 second cine clip of the most representative findings in each of the 14 lung zones was recorded during normal respiration (Supplementary Media 1–4). Cine clips were obtained at 12MHz frequency except where body habitus required a lower frequency for increased tissue penetration. Sonographers were blinded to HRCT findings at the time of LUS examination. Deidentified LUS cine clips and HRCT studies were stored in a secured research platform managed by the Imaging Research Center and Radiology Informatics team at CCHMC. All de-identified LUS videos were evaluated by 2 pediatric (PVF, TT) and 2 adult (DM, RF) ultrasonographers. All ultrasonographers first reviewed all imaging to assure clear visualization of the pleural surface and high image quality. Next, all LUS images were descriptively evaluated to identify common findings in SJIA-LD. Subsequently, each lung window was evaluated for the presence or absence of B-lines, pleural irregularity, and consolidations. Pleural irregularity was noted as either a focal (scattered and/or non-confluent) or diffuse (continuous and/or confluent) loss of smoothness/regularity in the pleural surface, often associated with thickening and granularity (noted as fine or coarse) (Figure 1b and Figure 2). B-lines were defined as “a vertical hyperechoic reverberation artifact that arises from the pleural line, extends to the bottom of the screen without fading, and moves synchronously with lung sliding” as described by OMERACT[9]. Consolidations were defined as areas of non-aerated hypoechoic lung superficially bounded at the pleural line, with a non-artifactual pattern deep to the pleural surface, and occasionally appearing as hepatization (appearance similar to that of the liver)[10] (Figure 1c, 1d). LUS feasibility in this pediatric population was assessed by recording LUS exam duration and by feasibility survey provided to the patients/parents after LUS examination.
Figure 1.
Normal and sJIA-LD on Lung Ultrasound. LUS in a healthy control (a) and sJIA-LD (b-d). (a) Control patient with normal pleural surface denoted by a thin hyperechoic pleural line (grey arrows). (b) Diffuse pleural irregularity, coarse granularity, and thickening (arrowheads), and associated B-lines (arrows). (c) Consolidation at the posterior lung base seen as hepatization (arrowheads) adjacent to the diaphragm (dotted arrows). (d) Subpleural consolidations (arrowheads) and associated dense B-lines (arrows). Asterisk = ribs. LUS = lung ultrasound.
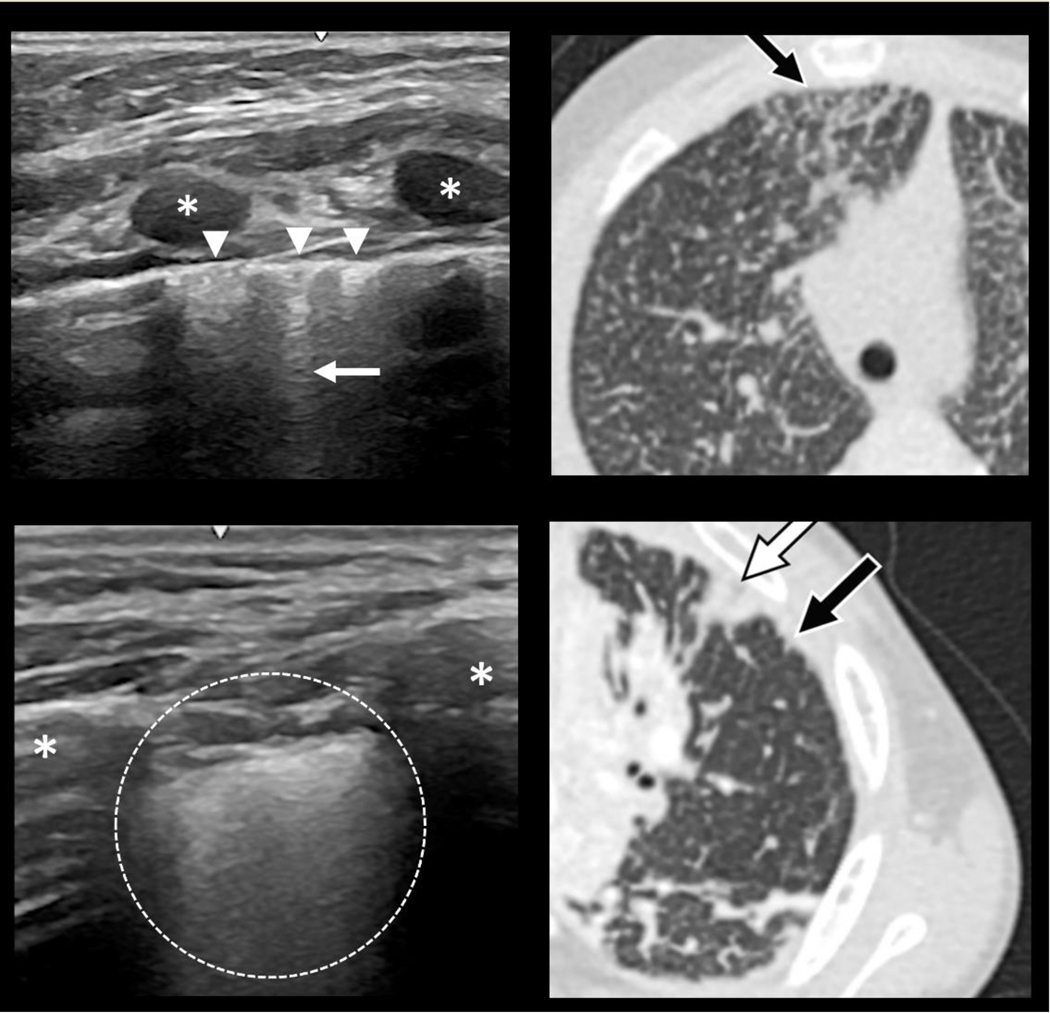
Figure 2.
Paired LUS and HRCT findings in and sJIA-LD. Lung ultrasound (LUS) and high-resolution computer tomography (HRCT) images of the lungs in sJIA-LD Top: Focal pleural irregularities (arrowheads) and scattered B-lines (arrow) on LUS (left) that correspond to subpleural interlobular septal thickening and reticulation (arrow) on HRCT (right). Bottom. Diffuse pleural irregularity with a fine granular pattern and dense B-line confluence on LUS (left) that corresponds to an area of minimal subpleural consolidation (black arrow) adjacent to an area of more pronounced subpleural consolidation (white arrow) on HRCT (right). Asterisk = ribs.
Statistical analysis
Descriptive statistics were calculated as median and interquartile range or mean and standard deviation for continuous variables, and frequency and percentages for categorical variables.
RESULTS
General clinical features
Between May of 2020 and October of 2021, 9 patients with sJIA-LD and 6 healthy control patients (Table 1) were enrolled. Patients with sJIA-LD were 3 to 10 years of age (median 5, IQR 4–8) compared to a median age of 7.5 years (IQR 4.75–8.75) for the control participants. The mean disease duration of sJIA was 61 months (± 25) and mean duration of sJIA-LD was 29 months (± 27). A detailed listing of current and prior medication exposure, disease history, and symptomatology is available in Table 1, supplementary Table 1.
Table 1.
Patient Characteristics
| sJIA | (n = 9) |
| Age, median (IQR) | 5 (4 – 8) |
| Female, n (%) | 5 (55.6) |
| Race/Ethnicity, n (%) | |
| Caucasian | 8 (88.9) |
| Hispanic | 1 (11.1) |
| sJIA disease duration, mean months (SD) | 61 (25) |
| LD disease duration, mean months (SD) | 29 (27) |
| Controls | (n = 6) |
| Age, median (IQR) | 7.50 (4.75–8.75) |
| Female, n (%) | 3 (50) |
sJIA = systemic juvenile idiopathic arthritis; LD = lung disease; IQR = inter-quartile range; SD = standard deviation
HRCT findings
Seven patients had a HRCT available for analysis (Supplementary Table 2). Consolidation was present on all of the HRCTs except for one, was always bilateral, and was in the following distributions: lower lobe subpleural (6 patients), lower lobe peribronchovascular (6 patients), anterior right middle lobe and/or lingula subpleural (4 patients), right middle lobe and/or lingula peribronchovascular (3 patients), anterior upper lobe subpleural (4 patients), and/or upper lobe peribronchovascular (4 patients). Less frequently, additional findings included ground-glass opacity (GGO) (3 patients), interlobular septal thickening (3 patients), and cysts (2 patients). The GGO and interlobular septal thickening occurred together in either a peribronchovascular, subpleural, or diffuse distribution. Notably, the only patient without subpleural consolidation had subpleural GGO and interlobular septal thickening suggesting that all patients had subpleural disease visible to LUS. The consolidation, GGO, and interlobular septal thickening in the above-described distributions are typical of sJIA-LD[2, 3]. In one patient with cysts, the cysts were small and scattered throughout all lobes in a predominantly subpleural location in association with diffuse GGO and interlobular septal thickening. In the other patient with cysts, the cysts were located in a subpleural location in the paraspinal and apical aspects of both upper lobes.
LUS findings
Of the possible 126 lung zones in patients with sJIA-LD 122 (97%) could be evaluated, with 4 precluded by the cardiac footprint. Of the 122 evaluated lung zones, 106 (87%) were abnormal, often with severely abnormal pleural-subpleural architecture on LUS. These findings often spared the axillary zones, favoring anterior and posterior fields similar to HRCT. Typical abnormalities on LUS included thickened, irregular, fine and/or coarse granular hyperechoic pleural surface with associated scattered or coalesced B-lines with or without consolidations (Figure 1 and 2, Supplementary Media 1–4). Across all lung fields in our sJIA-LD cohort, focal pleural irregularities were seen in 20 (16%) fields, diffuse pleural irregularities in 86 (70%), B-lines in 106 (87%), and subpleural consolidations in 44 (36%) of lung fields. All types of LUS abnormalities were encountered in all sJIA-LD patients. Occasional trace pleural effusions were observed. When comparing location specific LUS findings to HRCT we noted focal areas of pleural irregularity and associated scattered B-lines corresponding with subpleural reticulation and interlobular septal thickening on HRCT, diffuse irregularity and coalesced B-lines associated with minimal subpleural consolidation on HRCT, and consolidative features on LUS matching more substantial subpleural consolidation on HRCT (Figure 2).
In contrast to patients with sJIA-LD, control participants demonstrated a normal thin pleural surface with normal gliding motion with respiration (Figure 1). Scattered, non-confluent B-lines were occasionally observed (3 or less per lung field) and were occasionally associated with small < 1-millimeter pleural irregularities. Although a direct comparison was not made with a healthy adult population, in our experience, the number of B-lines and associated small focal pleural irregularities in healthy children are more frequent compared to healthy adults. No pleural thickening, granularity, effusions, or consolidations were observed in our healthy pediatric controls.
LUS feasibility
Across all sJIA-LD and control participants LUS examinations were brief, taking an average of 12 (±4) minutes to complete. Surveys filled out by all participants after examination noted excellent tolerability and feasibility (Supplementary Table 3). All subjects approached to be part of the study agreed to participate.
DISCUSSION
In this study, we provide the first description of LUS findings encountered in sJIA-LD, an increasingly recognized complication in children with sJIA[2, 3, 11]. A protocol for LUS acquisition previously used by our group[7] in adults with CTD-ILD was feasible to apply to children as young as 3 years of age. Similar to the use of LUS in adults with CTD-ILD, LUS examination in this pediatric sJIA population was of short-duration (mean of 12 minutes), well-tolerated, and revealed highly conspicuous abnormalities of the pleura and subpleural lung in sJIA-LD. In comparison with adults, the smaller and thinner body habitus of these children allowed for a wider field of view with greater pleural and subpleural detail.
Recent reports indicate that both acute and chronic lung findings can be advanced by the time of initial clinical presentation with lung involvement (i.e., dyspnea, clubbing)[2, 3] suggesting the need for techniques that can screen for subclinical disease. sJIA-LD can present as young as 2 years of age[2, 3], when children are unable to perform recommended screening tests for sJIA-LD including the 6-minute walk test, PFT, or a non-sedated HRCT[1]. These considerations along with the substantial cost and ionizing radiation exposure accrued with repeated HRCT provide a compelling rationale to further investigate the potential use of LUS as a screening tool of sJIA-LD. Such research has the potential to address one of the key priorities of the sJIA community: early identification of sJIA-LD using diagnostic imaging techniques with minimal or no ionizing radiation[12].
Similar to adult systemic sclerosis (SSc)-ILD (most commonly nonspecific interstitial pneumonia, NSIP and usual interstitial pneumonia, UIP)[5, 7], we found sJIA-LD in our cohort typically demonstrates peripheral lung disease that is easily visualized at the lung surface by LUS[2, 3]. In both SSc-ILD and sJIA-LD peripheral lung findings on HRCT may include GGO, reticulation, interlobular septal thickening, and/or subpleural cysts. However, consolidation is an additional prominent finding on HRCT in sJIA-LD that is not seen with NSIP and UIP of SSc-ILD[2, 3, 7]. Paralleling this, LUS shows a combination of pleural irregularity, thickening, and granularity in both SSc-ILD and our cohort of sJIA-LD. However, consolidation is a unique feature to sJIA-LD (Figure 1d). We observed that the distribution of subpleural consolidation (Figure 2) was primarily in the anterior and posterior lung distributions with relative sparing of the axillary line similar to HRCT.
In adults CTD-ILD, focal scattered pleural irregularities and associated scattered B-lines on LUS were previously found to be associated with HRCT findings of interlobular septal thickening and reticulation[6]. Associations between these LUS and HRCT findings were similarly seen in our pediatric sJIA-LD cohort (Figure 2). Diffuse irregularity was seen in the majority of lung windows of our sJIA-LD cohort and varied in quality from a fine granular pattern with dense reverberations (confluent B-lines) to a coarser and more nodular pattern with either confluent or scattered B-lines. The precise associations between these various appearances are difficult to ascertain. However, in adult ILD, a fine granular pattern with dense B-line reverberations is often associated with GGO on HRCT, while a more coarse pattern is often associated with fibrosis and honeycombing[13]. Histopathology of sJIA-LD in prior studies and in several of the patients in our cohort showed subpleural airspace filling, inflammatory infiltrate, and fibrosis within the spectrum of PAP and ELP[2, 3]. The intermixing of these findings and residual subpleural aerated alveoli may provide an additional explanation for LUS findings of dense reverberations noted in association with a fine granular pattern of pleural irregularity in our sJIA-LD cohort[14]. Our small cohort prevented a quantitative comparative analysis between features on LUS and HRCT, however at face value, LUS findings did appear to grossly correlate with findings on HRCT. Similarly in this pilot, we did not attempt to assess for correlations in severity between LUS and HRCT given the small sample size, although patients with a greater number of LUS windows with normal pleura also showed milder overall HRCT findings. Lastly, only scattered B-lines were seen in controls compared to prominent and diverse LUS abnormalities encountered in sJIA-LD cohort, supporting a potential role of LUS as a screening modality in sJIA-LD, although validation is needed.
While we were limited by our small sample size which precluded in-depth statistical analyses, the diffuse nature of lung disease in our sJIA-LD cohort and assessment of 14 lung windows provided ample data to provide an initial description of LUS findings in this disease. Importantly, we recognize that our small cohort may not be representative of the entire pathologic spectrum of sJIA-LD. Additionally, LUS findings in our cohort might be more severe than the general sJIA population as we recruited patients with known sJIA-LD. However, for our purpose of describing LUS findings in sJIA-LD in comparison with HRCT it was necessary to recruit patients with known disease; further studies evaluating mild and subclinical disease would provide additional insights.
Our findings indicate that LUS may be a useful imaging tool to evaluate for sJIA-LD even from an early age. These preliminary results suggest further investigations into LUS for sJIA-LD screening, surveillance, and response to therapy are warranted. Larger studies aimed at determining LUS sensitivity for sJIA-LD detection, imaging to histopathologic correlates, LUS change over time, and response to therapy would shed further light on the role of this technique as a relatively more benign alternative to HRCT, bronchoscopy, and/or biopsy. Validated LUS image acquisition techniques and scoring protocols are needed to enhance the evaluation and description of sJIA-LD.
Supplementary Material
SIGNIFICANCE & INNOVATION.
First study to describe lung ultrasound findings in a pediatric systemic Juvenile Idiopathic Arthritis patient population with lung disease (sJIA-LD)
Bedside lung ultrasound is well tolerated and takes an average 12 minutes to perform
Lung ultrasound could provide a low-cost and safe modality for screening and monitoring sJIA-LD
ACKNOWLEDGEMENT
We thank the patients and families for participating in this study, the Systemic Juvenile Idiopathic Arthritis Foundation for facilitating patient recruitment. We also acknowledge Drs. Tzielan Lee MD, Joyce Hsu MD, Elizabeth Mellins MD, and Christopher Towe MD for assistance with this project.
Dr. Vega-Fernandez’s work was supported by the Center for Clinical & Translational Science & Training (CCTST) at the University of Cincinnati funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant 2UL1TR001425–05A1 and KL2 (2KL2TR001426–05A). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Erkens R, et al. , Pathogenesis and Treatment of Refractory Disease Courses in Systemic Juvenile Idiopathic Arthritis: Refractory Arthritis, Recurrent Macrophage Activation Syndrome and Chronic Lung Disease. Rheum Dis Clin North Am, 2021. 47(4): p. 585–606. [DOI] [PubMed] [Google Scholar]
- 2.Saper VE, et al. , Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis, 2019. 78(12): p. 1722–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulert GS, et al. , Systemic Juvenile Idiopathic Arthritis-Associated Lung Disease: Characterization and Risk Factors. Arthritis Rheumatol, 2019. 71(11): p. 1943–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Lauretis A, Veeraraghavan S, and Renzoni E, Review series: Aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron Respir Dis, 2011. 8(1): p. 53–82. [DOI] [PubMed] [Google Scholar]
- 5.Ferro F and Delle Sedie A, The use of ultrasound for assessing interstitial lung involvement in connective tissue diseases. Clin Exp Rheumatol, 2018. 36 Suppl 114(5): p. 165–170. [PubMed] [Google Scholar]
- 6.Song G, Bae SC, and Lee YH, Diagnostic accuracy of lung ultrasound for interstitial lung disease in patients with connective tissue diseases: a meta-analysis. Clin Exp Rheumatol, 2016. 34(1): p. 11–6. [PubMed] [Google Scholar]
- 7.Fairchild R, et al. , Development and Assessment of a Novel Lung Ultrasound Interpretation Criteria for the Detection of Interstitial Lung Disease in Systemic Sclerosis. Arthritis Care Res (Hoboken), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez M, et al. , Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders--preliminary results. Arthritis Res Ther, 2011. 13(4): p. R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedie AD, et al. , FRI0304 DEFINITION AND STANDARDIZATION OF INTERSTITIAL LUNG DISEASE ASSESSMENT BY ULTRASOUND: RESULTS FROM A DELPHI PROCESS AND WEB-RELIABILITY EXERCISE BY THE OMERACT ULTRASOUND WORKING GROUP (WG). Annals of the Rheumatic Diseases, 2019. 78(Suppl 2): p. 834–834. [Google Scholar]
- 10.Lichtenstein DA, et al. , Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med, 2004. 30(2): p. 276–281. [DOI] [PubMed] [Google Scholar]
- 11.Kimura Y, et al. , Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken), 2013. 65(5): p. 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canna SW, et al. , Proceedings from the 2(nd) Next Gen Therapies for Systemic Juvenile Idiopathic Arthritis and Macrophage Activation Syndrome symposium held on October 3–4, 2019. Pediatr Rheumatol Online J, 2020. 18(Suppl 1): p. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manolescu D, et al. , The reliability of lung ultrasound in assessment of idiopathic pulmonary fibrosis. Clin Interv Aging, 2018. 13: p. 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soldati G, Copetti R, and Sher S, Sonographic interstitial syndrome: the sound of lung water. J Ultrasound Med, 2009. 28(2): p. 163–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.