Abstract
Introduction
Today, colon cancer is one of the most common types of gastrointestinal cancer worldwide. CD133 as a known cancer stem cell marker has been found effective in cell proliferation and differentiation in various cancers, including colon cancer. We aimed to investigate the relationship between CD133 expression in colon cancer with prognostic factors and survival rate of patients with colon cancer by immunohistochemistry.
Methods
Formalin-fixed paraffin-embedded (FFPE) tissue was taken from patients with colon cancer. Histopathology examination was done using hematoxylin and eosin staining. Immunohistochemistry was performed to determine CD133 expression. Association between CD133 expression and clinicopathological profile was then assessed.
Results
There was a statistically significant association between CD133 protein expression and sex , cancer stage, and lymphatic invasion (p = 0.044, p = 0.131, and p = 0.002, respectively). However, no significant correlation was identified between CD133 expression and other factors, including age of patients with colorectal cancer (CRC) (p = 0.267), tumor location (p = 0.494), tumor differentiation grade (p = 0.263), neural tissue invasion, and 5-year survival (p = 0.054).
Conclusion
CD133 is a useful predictive or prognostic biomarker for CRC in clinical assessment and may serve as a potential therapeutic target for CRC.
Keywords: Carcinoma, Colon, Immunohistochemistry, Neoplastic stem cells, Rectum
Key Summary Points
| Colon cancer is one of the most common types of gastrointestinal cancer. |
| CD133 as a known cancer stem cell marker has been found effective in cell proliferation and differentiation in various cancers, including colon cancer. |
| Statistically significant association between CD133 expression and gender, tumor stage III, and lymphovascular invasion was shown. |
| CD133 expression has potential as a colorectal cancer (CRC) prognostic stem cell marker for colon cancer. |
Introduction
Colon cancer is one of the most prevalent cancers in the world and a major cause of cancer-related mortality [1–3]. In Iran, colon cancer is the third most common cancer in men and fourth most common cancer in women [4]. It is suggested that the highly tumorigenic, chemotherapy-resistant nature of colon cancer adversely affects survival rate [5–8]. Targeted therapies have promising potential improvement of survival in patients with colon cancer [9]. Cancer stem cells (CSCs) are defined by their ability to self-renew and self-repair and their role in the tumor initiation, metastasis, therapeutic resistance, and recurrence [10–12]. Different markers have also been found to be expressed on the surface of CSCs, among which CD133 has been paid special attention. CD133/prominin-1 is a 120-kDa 5-transmembrane domain antigen on chromosome 4p15.32 mainly localized in membrane protrusions [13, 14]. CD133 was first discovered in hematopoietic stem and progenitor cells by Yin et al. [15]. Previous studies identified CD133 as a putative CSC marker in cancer cells [7, 16] with ability to initiate tumor growth [7, 17]. This CSC marker now widely used to identify cancer stem cells in many cancers, including brain, colon, and lung cancers [18, 19]. Furthermore, CD133 is currently one of the best markers to characterize colon CSCs [20]. CD133 is normally expressed by primitive hematopoietic stem cells from adult blood and bone narrow, human fetal liver and stem cells in cord blood and peripheral blood, endothelial progenitor cells, epithelial cells, and neural and glial stem cells [21, 22]. Several studies have suggested that high CD133 expression is associated with clinicopathologic characteristics such as poor prognosis, distant metastasis, survival rate, and even chemotherapy resistance in patients with colon cancer. Therefore, as a CRC-initiating CSC marker, CD133 expression can be used for developing novel therapeutic approaches in colon cancer [20, 23–25]. In this study, we investigated the association between the expression and prognostic significance of the CSC marker CD133 with clinicopathologic features of patients with CRC including survival rate, using the immunohistochemistry (IHC) method.
Methods
Tissue Samples
In this study, 34 formalin-fixed paraffin-embedded (FFPE) tumor tissues were selected from 34 patients who underwent surgical resection from 2008 to 2019 in Shahid Beheshti Hospital in Kashan, Iran. Written consent was obtained from all subjects, and the study was approved by the Research Ethical Committee of Kashan University of Medical Sciences, Iran. All clinicopathologic data were verified using hematoxylin and eosin (H&E)-stained pathologic slides, patients’ medical files, and pathology record. Tumors were classified according to the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) TNM criteria [26, 27]. Clinicopathologic parameters included age, gender, tumor location, tumor stage, tumor differentiation grade, lymphatic and neural tissue invasion, 5-year survival, and tumor recurrence. Colon cancer with detected positive immunostaining for CD133 was considered as a positive control. Cases and controls were examined by two expert pathologists. Positive reaction was defined by the presence of at least 10% of cancer cells displaying membranous and/or cytoplasmic staining [28–32]. A number of tumor samples were excluded from the study owing to inappropriate paraffin blocks, low-quality staining, and unavailable data. Also, the small-sized specimens prepared by biopsy that were inadequate for performing histopathological examinations were excluded [33–35].
CD133 Immunohistochemical Staining
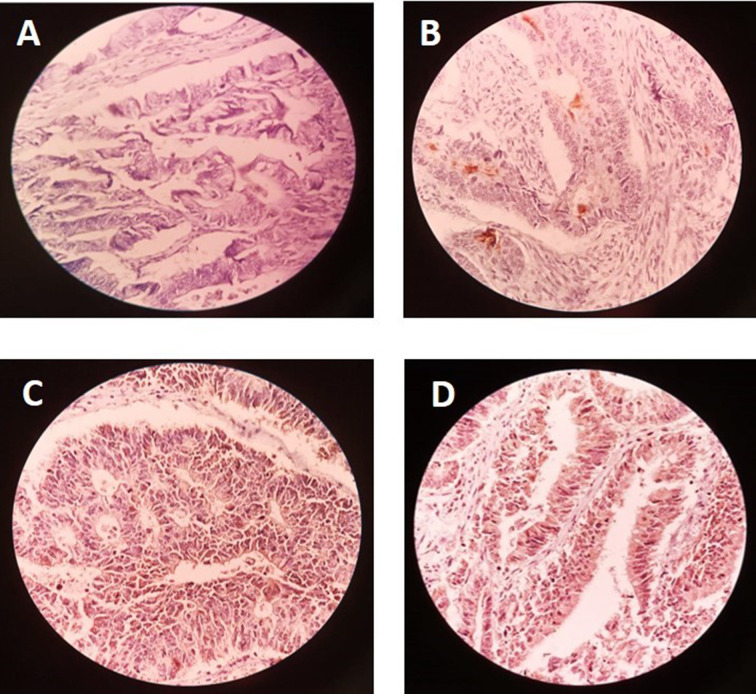
The expression level of CD133 was determined in CRC sections by the immunohistochemical staining (IHC) method as follows: formalin-fixed paraffin-embedded CRC tissue blocks were sectioned at 5-μm thickness for IHC staining. All slides were deparaffinized with xylene, and rehydrated in a graded ethylic alcohol (50%, 70%, 80%, and 100%) followed by distilled water. After washing three times with phosphate-buffered saline (PBS) buffer every 5 min, endogenous peroxidase was inactivated by immersing the sections in 1–0.1% H2O2 for 5 min. Then, for heat-induced antigen unmasking, sample tissues were treated in sodium citrate buffer (pH 6.0) at 95 °C for 5 min in a microwave oven and allowed to cool for 1 h at room temperature. CD133 expression was identified and visualized after reaction with primary anti-CD133 antibody (1:100) at 4 °C for one night. After washing the slides three times with PBS, a secondary antibody (peroxide-conjugated) was used to detect the primary antibody, and slides were incubated for 30 min in room temperature. Again, after three times washing in PBS every 5 min, samples were incubated with 3, 3′-diaminobenzidine tetrahydrochloride-chromogen (DAB) for 1–5 min for visualizing immunostaining and finally counterstained with hematoxylin dye and dehydrated in ethylic alcohol serial dilution (60%, 80%, and 100%) and cleared in xylene (100%). Our negative control was tissue covered with just buffer without the primary antibody. Prepared specimens were studied using light microscope. Imaging analysis of immunohistochemical staining of the specimens was evaluated by one pathologist without knowledge about the patients’ clinicopathological characteristics. CD133 expression was assessed by observing the appearance of yellow to brown color in the cytoplasm and membrane of glandular epithelial cells. A score of less than 10% CD133 cells (low CD133 expression) was considered as negative and more than 10% CD133 cells (high CD133 expression) as positive, respectively. Staining intensity was evaluated by immunohistochemical method (Fig. 1).
Fig. 1.
Evaluation of CD133 expression by immunohistochemical method: A no staining (CD133 was not expressed), B +1 (weak staining), C +2 (moderate staining), D +3 (strong staining)
Statistical Analysis
All statistical analyses were performed using SPSS software version 20 (SPSS Inc., Chicago, IL., USA). The results were expressed as mean ± standard deviation. The statistical significance of differences between immunohistochemical CD133 expression levels and clinicopathological features of patients with CRC were evaluated using chi-square and one-way analysis of variance (ANOVA), or Mann–Whitney test or Kruskal–Wallis parametric tests (based on the results of Kolmogorov–Smirnov test), as appropriate. Survival was evaluated using the Kaplan–Meier method with a log-rank test. p-Values of < 0.05 were considered statistically significant.
Results
This study included 34 tissue samples from 15 (44.1) male patients and 19 (55.9%) female patients with a median age of 62 (± 15.35) years at the time of diagnosis. The distribution of patients with CRC according to demographic features is presented in Table 1.
Table 1.
Characteristics of 38 patients with CRC and their prognostic factors
| Variable [number (%)] | |
|---|---|
| Gender | |
| Male | 15 (44.1) |
| Female | 19 (55.9) |
| Age (mean) (± SD) (years) | 62 (± 15.35) |
| Tumor location | |
| Ascendance colon | 14 (429.4%) |
| Transverse | 1(3.0%) |
| Sekom | 6 (18.2%) |
| Sigmoid 8 (2.2%) | 8(24.2%) |
| Descending | 1 (3.0%) |
| Rectum colon | 2 (6.1%) |
| Ileocecal valve | 2(6.1%) |
| Histopathology grade | |
| Well differentiated (G1) | 21 (61.8%) |
| Moderately differentiated (G2) | 11 (32.4%) |
| Poorly differentiated (G3) | 2 (5.8%) |
| CD133 expression | |
| High level | 12 (35.3%) |
| Low level | 9 (26.5%) |
| 5-Year survival | |
| Positive | 26 (76.5%) |
| Negative | 8 (23.5%) |
| Lymphatic invasion | |
| Positive | 20 (58.8%) |
| Negative | 14 (41.2%0 |
| Tumor stage | |
| 1 | 9 (26.5%) |
| 2a | 7 (20.6) |
| 2b | 4 (11.8%) |
| 3a | 1 (2.9%) |
| 3b | 8 (23.5%) |
| 3c | 5 (14.7%) |
| Neuronal invasion | |
| Positive | 4 (11.8%) |
| Negative | 30 (88.2%) |
The pathological results showed (Table 1) that most tumors were located in ascendance colon 13 (39.4%) and in sigmoid 8 (2.2%), while the fewest tumors were located in descending 1 (3.0%) and transverse 1 (3.0%). On the basis of tumor differentiation degree, there were 21 (61.8%) well-differentiated (G1), 11 (32.4%) moderately differentiated (G2), and 2 (5.8%) poorly differentiated (G3) tumors. The tumor stages with the highest prevalence were stage I (26.5%) and stage 3b (23.5%), while the lowest frequency was observed at stage 3a (2.9%). Moreover, lymphatic vascular invasion was detected in 20 patients (58.8%) and neuronal invasion in 4 (11.8%), while 26 (76.5%) patients had 5-year survival.
Correlation between CD133 Expression and Clinicopathological Factor Features
On the basis of our pathologic findings, expression of CD133 marker was positive in 25 (73.5) patients and negative in 9 (26.5) patients. Associations between CD133 expression rate and clinicopathological variables are presented in Table 2.
Table 2.
Association of CD133 expression with clinicopathological variables
| CD133 expression (%) | ||||||
|---|---|---|---|---|---|---|
| Variables | No | 0 | 1+ | 2+ | 3+ | p-Value |
| Gender | ||||||
| Male | 15 | 4 (55.6) | 0 | 6 (75.0) | 4 (33.3) | 0.044 |
| Female | 19 | 8 (66.7) | 2 (25.0) | 5 (100) | 4 (44.4) | |
| Age | 34 | 55.77 ± 17.15 | 470 ± 14.47 | 67.25 ± 10.52 | 59.83 ± 16.114 | 0.267 |
| Tumor location | ||||||
| Colon | 16 | 4 (44.4) | 2 (40.0) | 6 (75.0) | 4 (33.3) | 0.182 |
| Rectum | 10 | 2 (22.3) | 3 (60.0) | 2 (25.0) | 3 (25.0) | |
| Sekom | 8 | 3 (33.3) | 0 | 0 | 5 (41.7) | |
| Tumor stage | ||||||
| I | 9 | 3 (33.3) | 4 (80.0) | 2 (25.0) | 0 | 0.011 |
| II | 11 | 4 (44.4) | 1 (20.0) | 3 (37.5) | 3 (25.0) | |
| III | 14 | 9 (75.0) | 3 (37.5) | 0 | 2 (22.3) | |
| Tumor grade | ||||||
| High | 4 | 0 | 0 | 0 | 4 ( 33.3) | 0.05 |
| Low | 30 | 8 (66.7) | 8 (100.0) | 5 (100.0) | 9 (100.0) | |
| Lymphatic invasion | ||||||
| Positive | 20 | 2 (22.2) | 2 (40.0) | 4 (50.0) | 12 (100.0) | 0.002 |
| Negative | 14 | 7 (77.8) | 3 (60.0) | 4(50.00 | 0 | |
| Neural invasion | ||||||
| Positive | 4 | 0 | 0 | 1 (12.5) | 3 (25.0) | 0.273 |
| Negative | 30 | 9 (75.0) | 7 (87.5) | 5 (100.0) | 9 (100.0) | |
| 5-Year survival | ||||||
| Positive | 26 | 9 (100.0) | 2 (40.0) | 7 (87.5) | 8 (66.7) | 0.054 |
| Negative | 8 | 4 (33.3) | 1 (12.5) | 3 (60.0) | 0 | |
According to the results (Table 2), there was a significant association between CD133 expression and gender, tumor stage, and lymphatic vascular invasion in the studied patients (p = 0.044, p = 0.011, and p = 0.002, respectively). The highest CD133 expression was observed in eight females (66.7%). Also, in all cases with high CD133 expression, lymphatic invasion was detected (p = 0.002). In addition, the highest CD133 expression was observed in CRC cases with advanced tumor stage III . However, no significant correlation was found between CD133 expression and other clinical factors, including age of patients with CRC (p = 0.267), tumor location (p = 0.182), tumor differentiation grade (p = 0.05), neural tissue invasion (p = 0.002), and 5-year survival (p = 0.054). The 5-year survival in 26 (66.7%) patients with high CD13 expression showed no significant differences with patients without 5-year survival and low CD133 expression (p = 0.054).
Immunohistochemical Results
Stained slides were examined by immunohistochemical method in terms of CD133 protein occurrence by observing the appearance of yellow to brown color in the cytoplasm of glandular cells. The samples were classified into negative (less than 10% cells stained) and positive (10% or more cells stained) categories. Positive samples were classified according to the intensity of cytoplasmic color as follows:
The staining intensity ranged from unstained (negative) to strong (positive): 0, no staining; +1, weak staining; +2, moderate staining; +3, strong staining.
Discussion
Colon cancer, the leading cause of cancer-related death, is a significant public health concern due to its increasing rate in Iran [36–38]. Many specific immunohistochemical and PCR markers have been identified for detecting colon primary and metastatic tumors. CD133 is one of the immunohistochemical markers that was first observed in stem cells [23, 39, 40]. This marker has been suggested to be effective in cell proliferation and differentiation of various cancers, including colon cancer [40, 41]. Therefore, in this study, we evaluated the expression of CD133 using immunohistochemical staining, its relationship with clinicopathological features, and the prognostic indicators of colon cancer . Our study demonstrated that CD133 expression was significantly correlated with CRC histopathology, including gender, tumor stage, and lymphatic vascular invasion (p = 0.044, p = 0.011, and p = 0.002, respectively). Higher CD133 expression was found at advanced tumor stage (III). However, there was no association with other factors such as age, 5-year survival, and tumor location in this study . In line with our study, several previous studies also showed no significant difference between positive and negative CD133 and tumor location [7, 42, 43]. However, in contrast to our study, a previous study reported that CD133 expression was higher in rectum colon [39]. Similar to our results, Kojima et al., analyzing samples from 189 patients with different stages of CRC using IHC, concluded that CD133 overexpression occurred mainly in well- to moderately differentiated tumors and was not correlated with recurrence-free survival [40]. However, there were results conflicting with our study regarding the expression of CD133 IHC staining in CRC and its relationship with clinicopathological factors [44]. Many studies have demonstrated that CD133 expression is correlated with survival, recurrence, metastases, and chemotherapy resistance, and most studies support the hypothesis that high CD133 expression is a poor prognostic marker [45, 46]. In addition, in contrast to our results, Pitule et al. showed that patients with high CD133 expression had longer disease-free survival interval [23], while our study as well as some other studies reported that CD133 was not significantly correlated with survival time (5 years) [47–49]. Another study performed by Park on 303 patients with CRC using immunohistochemical staining showed that CD133 expression in CRC was significantly associated with 5-year survival and tumor stage, but not with other clinical factors such as gender that were significantly associated in our study. Moreover, they suggested CD133 expression may be considered as a biomarker with greater potential for prognosis of high risk in patients with stage II CRC [50]. This result was not consistent with our results. Contrary to our results, the results of a meta-analysis of 37 studies conducted by Huang in 2018 demonstrated that higher CD133 expression was positively correlated with shorter overall survival, lymphatic vascular invasion, distant metastasis, and poorer prognosis in patients with CRC. They also assumed that this low 5-year survival rate that might play an important role in the progression of colorectal cancer in patients with CRC was due to sex hormone, genetic, and epigenetic factors affected by the environment and lifestyle and a variety of therapies [43]. In Rey study on 118 patients in 2020, CD133 expression was significantly associated with the tumor location (p = 0.002), but not with other clinicopathological factors such as gender, age, or body mass. Also, tumor location had an impact on the survival of patients with CRC. This was in line with two previous studies [39, 41, 51] but not with our study. Kazama , in an immunohistochemistry study of 200 endoscopically resected colorectal polyps and 20 normal mucosae, demonstrated that CD133 expression was associated with only the degree of tumor differentiation and tumor size but not with gender, age, or tumor location. Apart from two factors, i.e., age and tumor location, the statistical result of the gender parameter was consistent with our results. Thus, CD133 might play an important role in tumor development. Finally, the discrepancies between our results and those of other research may be due to differences in geographical area or race.
There was a limitation in our study. A larger number of cases is needed to achieve higher statistical power to detect significant differences. However, owing to financial issues, we could not to perform a large project to cover precise aspects of the subjects.
Conclusions
Our findings reveal a statistically significant association between CD133 expression and gender, tumor stage III, and lymphovascular invasion, but no other factors, although there were some nonsignificant associations . Therefore, CD133 expression has potential as a CRC prognostic stem cell marker for colon cancer.
Acknowledgements
At the end of this study, authors of the current study represent their most appreciation to officials and nurses of Shahid Beheshti Hospital affiliated at Kashan University of Medical Sciences, officials of microbial laboratories in special.
Funding
This work was supported by Kashan University of Medical Science, Kashan, Iran. We also thank the Deputy of Research and Technology, Ministry of Health and Medical Education of Iran for research grant support. No funding or sponsorship was provided for the publication of this article.
Author Contributions
HE, MT and FA were responsible for the study conception and design. MT, FA and HE performed data collection. HE, EGH and HHK preparing the first draft of the manuscript. FA and HHK made critical revisions to the paper for important intellectual content. All authors approved the final version of the manuscript draft.
Disclosures
Mohammad Tolouee, Hassan Ehteram, Hamed Haddad Kashani and Fatemeh Aslanbeigi confirm that they have no conflicts of interest to declare.
Compliance with Ethics Guidelines
This study was approved by Ethical Committee of Kashan University of Medical Sciences (Reference number: IR.KAUMS.MEDNT.REC.1400.034). All procedures performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. All study process was presented to patients and they were reassured about confidentiality of their records, they were requested to present their written consent of participation in the study.
Data Availability
The primary data for this study is available from the authors on direct request.
Contributor Information
Hassan Ehteram, Email: ehteram.kaums@gmail.com.
Fatemeh Aslanbeigi, Email: f.aslanbeigi1995@gmail.com.
Ebrahim Ghoochani Khorasani, Email: ebgh1360@gmail.com.
Mohammad Tolouee, Email: tolouee.kaums@gmail.com.
Hamed Haddad Kashani, Email: hamedir2010@gmail.com.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends global patterns of cancer. Cancer Epidemiol Biomark Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Kolah DS, Sajadi A, Radmard AR, Khademi H. Five common cancers in Iran. 2010. [PubMed]
- 5.Munro MJ, Wickremesekera SK, Peng L, Tan ST, Itinteang T. Cancer stem cells in colorectal cancer: a review. J Clin Pathol. 2018;71(2):110–116. doi: 10.1136/jclinpath-2017-204739. [DOI] [PubMed] [Google Scholar]
- 6.Dalerba P, Dylla SJ, Park I-K, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Xia L, Wang H, Oyang L, Su M, Liu Q, Lin J, Tan S, Tian Y, Liao Q. Cancer stem cells in progression of colorectal cancer. Oncotarget. 2018;9(70):33403. doi: 10.18632/oncotarget.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 10.Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol. 2009;27(1):44–46. doi: 10.1038/nbt0109-44. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Huang EH. The colon cancer stem cell microenvironment holds keys to future cancer therapy. J Gastrointest Surg. 2014;18(5):1040–1048. doi: 10.1007/s11605-014-2497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangemi R, Paleari L, Orengo AM, Cesario A, Chessa L, Ferrini S, Russo P. Cancer stem cells: a new paradigm for understanding tumor growth and progression and drug resistance. Curr Med Chem. 2009;16(14):1688–1703. doi: 10.2174/092986709788186147. [DOI] [PubMed] [Google Scholar]
- 13.Corbeil D, Röper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275(8):5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 14.Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18(3):506–514. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 15.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood J Am Soc Hematol. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- 16.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Can Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 18.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 19.Cheng J-X, Liu B-L, Zhang X. How powerful is CD133 as a cancer stem cell marker in brain tumors? Cancer Treat Rev. 2009;35(5):403–408. doi: 10.1016/j.ctrv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99(8):1285–1289. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonanno G, Perillo A, Rutella S, De Ritis DG, Mariotti A, Marone M, Meoni F, Scambia G, Leone G, Mancuso S. Clinical isolation and functional characterization of cord blood CD133+ hematopoietic progenitor cells. Transfusion. 2004;44(7):1087–1097. doi: 10.1111/j.1537-2995.2004.03252.x. [DOI] [PubMed] [Google Scholar]
- 22.Bonanno G, Mariotti A, Procoli A, Corallo M, Rutella S, Pessina G, Scambia G, Mancuso S, Pierelli L. Human cord blood CD133+ cells immunoselected by a clinical-grade apparatus differentiate in vitro into endothelial- and cardiomyocyte-like cells. Transfusion. 2007;47(2):280–289. doi: 10.1111/j.1537-2995.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 23.Pitule P, Cedikova M, Daum O, Vojtisek J, Vycital O, Hosek P, Treska V, Hes O, Kralickova M, Liska V. Immunohistochemical detection of cancer stem cell related markers CD44 and CD133 in metastatic colorectal cancer patients. BioMed Res Int. 2014 doi: 10.1155/2014/432139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim ST, Sohn I, Do I-G, Jang J, Kim SH, Jung SH, Park JO, Park YS, Talasaz A, Lee J. Transcriptome analysis of CD133-positive stem cells and prognostic value of survivin in colorectal cancer. Cancer Genomics Proteomics. 2014;11(5):259–266. [PubMed] [Google Scholar]
- 25.Wang K, Xu J, Zhang J, Huang J. Prognostic role of CD133 expression in colorectal cancer: a meta-analysis. BMC Cancer. 2012;12(1):1–6. doi: 10.1186/1471-2407-12-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116(24):5592–5598. doi: 10.1002/cncr.25550. [DOI] [PubMed] [Google Scholar]
- 27.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women's Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327. doi: 10.1200/JCO.2012.48.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saba MA, Valeh T, Ehteram H, Kashani HH, Zahedi MG. Diagnostic value of neuron-specific enolase (NSE) and cancer antigen 15-3 (CA 15-3) in the diagnosis of pleural effusions. Asian Pacific J Cancer Prevent. 2017;18(1):257. doi: 10.22034/APJCP.2017.18.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seyed Hosseini E, Alizadeh Zarei M, Babashah S, Nakhaei Sistani R, Sadeghizadeh M, Haddad Kashani H, Amini Mahabadi J, Izadpanah F, Atlasi MA, Nikzad H. Studies on combination of oxaliplatin and dendrosomal nanocurcumin on proliferation, apoptosis induction, and long non-coding RNA expression in ovarian cancer cells. Cell Biol Toxicol. 2019;35(3):247–266. doi: 10.1007/s10565-018-09450-8. [DOI] [PubMed] [Google Scholar]
- 30.Ferdosian M, Khatami MR, Malekshahi ZV, Mohammadi A, Kashani HH, Shooshtari MB. Identification of immunotopes against Mycobacterium leprae as immune targets using PhDTm-12mer phage display peptide library. Trop J Pharm Res. 2015;14(7):1153–1159. [Google Scholar]
- 31.Kashani HH, Moshkdanian G, Atlasi MA, Taherian AA, Naderian H, Nikzad H. Expression of galectin-3 as a testis inflammatory marker in vasectomised mice. Cell J (Yakhteh) 2013;15(1):11. [PMC free article] [PubMed] [Google Scholar]
- 32.Nikzad H, Kashani HH, Kabir-Salmani M, Akimoto Y, Iwashita M. Expression of galectin-8 on human endometrium: molecular and cellular aspects. Iran J Reprod Med. 2013;11(1):65. [PMC free article] [PubMed] [Google Scholar]
- 33.Hamed HK, Zohre F, Hosein N, Kazem P, Mohammad SM, Mohsen N. The effect of aqueous extract of Salep prepared from root-tubers of Dactylorhiza maculate (Orchidaceae) on the testes and sexual hormones of immature male mice. J Med Plants Res. 2012;6(24):4102–4106. [Google Scholar]
- 34.Kamani M, Hosseini ES, Kashani HH, Atlasi MA, Nikzad H. Protective effect of Lepidium sativum seed extract on histopathology and morphology of epididymis in diabetic rat model. Int J Morphol. 2017;35(2):603–610. [Google Scholar]
- 35.Saeedi Sadr A, Ehteram H, Seyed Hosseini E, Alizadeh Zarei M, HassaniBafrani H, Haddad Kashani H. The effect of irisin on proliferation, apoptosis, and expression of metastasis markers in prostate cancer cell lines. Oncol Ther. 2022 doi: 10.1007/s40487-022-00194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pahlavan PS, Jensen K. A short impact of epidemiological features of colorectal cancer in Iran. Tumori J. 2005;91(4):291–294. doi: 10.1177/030089160509100401. [DOI] [PubMed] [Google Scholar]
- 37.Foroutan M, Rahimi N, Tabatabaeifar M, Darvishi M, Hashemi M, Hossein-Panah F, Zali MR. Clinical features of colorectal cancer in Iran: a 15-year review. J Dig Dis. 2008;9(4):225–227. doi: 10.1111/j.1751-2980.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- 38.Ansari R, Mahdavinia M, Sadjadi A, Nouraie M, Kamangar F, Bishehsari F, Fakheri H, Semnani S, Arshi S, Zahedi M-J. Incidence and age distribution of colorectal cancer in Iran: results of a population-based cancer registry. Cancer Lett. 2006;240(1):143–147. doi: 10.1016/j.canlet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Hong I, Hong SW, Chang YG, Lee WY, Lee B, Kang YK, Kim YS, Paik IW, Lee H. Expression of the cancer stem cell markers CD44 and CD133 in colorectal cancer: an immunohistochemical staining analysis. Ann Coloproctol. 2015;31(3):84. doi: 10.3393/ac.2015.31.3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kojima M, Ishii G, Atsumi N, Fujii S, Saito N, Ochiai A. Immunohistochemical detection of CD133 expression in colorectal cancer: a clinicopathological study. Cancer Sci. 2008;99(8):1578–1583. doi: 10.1111/j.1349-7006.2008.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider M, Huber J, Hadaschik B, Siegers GM, Fiebig H-H, Schüler J. Characterization of colon cancer cells: a functional approach characterizing CD133 as a potential stem cell marker. BMC Cancer. 2012;12(1):1–11. doi: 10.1186/1471-2407-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest. 2009;27(8):844–850. doi: 10.1080/07357900902744502. [DOI] [PubMed] [Google Scholar]
- 43.Huang R, Mo D, Wu J, Ai H, Lu Y. CD133 expression correlates with clinicopathologic features and poor prognosis of colorectal cancer patients: an updated meta-analysis of 37 studies. Medicine. 2018;97(23):e10446. doi: 10.1097/MD.0000000000010446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nosrati A, Naghshvar F, Maleki I, Salehi F. Cancer stem cells CD133 and CD24 in colorectal cancers in Northern Iran. Gastroenterol Hepatol Bed Bench. 2016;9(2):132. [PMC free article] [PubMed] [Google Scholar]
- 45.Iinuma H, Watanabe T, Mimori K, Adachi M, Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes' stage B and C colorectal cancer. J Clin Oncol. 2011;29(12):1547–1555. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- 46.Jao S-W, Chen S-F, Lin Y-S, Chang Y-C, Lee T-Y, Wu C-C, Jin J-S, Nieh S. Cytoplasmic CD133 expression is a reliable prognostic indicator of tumor regression after neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol. 2012;19(11):3432–3440. doi: 10.1245/s10434-012-2394-3. [DOI] [PubMed] [Google Scholar]
- 47.Choi D, Lee HW, Hur KY, Kim JJ, Park G-S, Jang S-H, Song YS, Jang K-S, Paik SS. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J Gastroenterol. 2009;15(18):2258. doi: 10.3748/wjg.15.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lugli A, Iezzi G, Hostettler I, Muraro M, Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L, Zlobec I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103(3):382–390. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou F, Mu YD, Liang J, Liu ZX, Chen HS, Zhang JF. Expression and prognostic value of tumor stem cell markers ALDH1 and CD133 in colorectal carcinoma. Oncol Lett. 2014;7(2):507–512. doi: 10.3892/ol.2013.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park YY, An CH, Oh ST, Chang ED, Lee J. Expression of CD133 is associated with poor prognosis in stage II colorectal carcinoma. Medicine. 2019;98(32):e16709. doi: 10.1097/MD.0000000000016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S, Song X, Chen Z, Li X, Li M, Liu H, Li J. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLoS One. 2013;8(2):e56380. doi: 10.1371/journal.pone.0056380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data for this study is available from the authors on direct request.