Abstract
Background:
Mycobacterium bovis is a zoonotic member of the Mycobacterium tuberculosis complex with a wide range of hosts, mainly cattle. Molecular epidemiological studies should be conducted to determine the transmission route, zoonotic risk factors, and phylogenetic relationships of M. bovis strains. Aims: This study aimed to characterize bovine and human M. bovis isolates by molecular methods.
Methods:
Molecular characterization and clonal relationship of strains isolated from tissue and organ samples of 76 cattle with positive tuberculin tests were collected from a slaughterhouse, and four M. bovis strains isolated from clinical materials of patients with suspected pulmonary TB isolates were analyzed using 24-locus MIRU-VNTR and spoligotyping methods. QuantiFERON-TB Gold Plus (QFT-Plus; Qiagen) was used to determine the prevalence of latent TB infection among 21 slaughterhouse personnel including 7 veterinarians, 12 butchers, 1 caretaker, and 1 veterinary technician.
Results:
SB0288/SIT685 type was detected in both cattle and humans by the spoligotyping method. When evaluating MIRU-VNTR, the presence of a 100% compatible pattern between human and bovine isolates was not detected, but some human samples were found to be 91.6% similar to a bovine sample. In addition, 21 slaughterhouse workers were screened with the interferon gamma-released assay (IGRA) and a 23.8% positivity was detected.
Conclusion:
Clonal similarity was determined between the bovine and human isolates using the MIRU-VNTR and spoligotyping methods and IGRA positivity in the occupational group suggested that M. bovis might be associated with pulmonary tuberculosis in humans.
Key Words: IGRA, Molecular epidemiology, Mycobacterium bovis
Introduction
Mycobacterium bovis is the causative agent of bovine tuberculosis (TB), an infectious, zoonotic, and chronic disease that also affects other domestic animals and humans (Carneiro et al., 2020 ▶). Transmission occurs mostly through the gastrointestinal tract due to the consumption of contaminated dairy products, or to a lesser extent, contaminated meat. It can be transmitted by the inhalation of aerosols exhaled by infected animals or humans or through direct contact in the presence of an open wound (Djemal et al., 2017 ▶). Since the identification of M. bovis is not included in routine tests in pulmonary TB laboratories and clinical and pathological findings are similar to M. tuberculosis complex (MTBC) infection, data on the actual prevalence and incidence in patients with pulmonary tuberculosis is very limited (Jiang et al., 2015 ▶). The World Health Organization (WHO) states that there are 7,000 new cases of M. bovis in South America each year, but the incidence is estimated to be eight times higher (O’Reilly and Daborn, 1995 ▶). In the south subtropical climate zone, which is close to Turkey, due to a high rate of migration and immigration from countries like Syria, TB constitutes a major risk for such diseases (Ergönül et al., 2020 ▶). On the other hand, low income and poor socioeconomic and sociocultural status, close contact with animals, and failure to comply with hygiene rules in food production suggest that in addition to M. tuberculosis, M. bovis may be the cause of pulmonary TB in humans (Djibuti et al., 2014 ▶). Programs aiming to eliminate TB all over the world have revealed the necessity of identifying and monitoring strains isolated from patients with molecular epidemiological methods, such as RFLP-IS6110, MIRU-VNTR and spoligotyping. Thus, the concept of “One Medicine, One Health” has been adopted in the approach to pulmonary TB cases caused by the M. bovis agent. Molecular epidemiological studies should be conducted to determine the transmission route of zoonotic TB to humans, to define the dominant genotypes between humans and animals, and to understand the phylogenetic relationships of the strains (Thoen et al., 2016 ▶).
The risk of M. bovis infection is very high in groups that are in close contact with animals, such as veterinarians and butchers, especially due to occupational exposure. The tuberculin skin test (TST) and the interferon gamma released assay (IGRA) have been available for many years to screen these professional groups (Vayr et al., 2018 ▶). However, the sensitivity of TST, which is most commonly used in screening studies, is only 70-85% in latent infection (Menzies et al., 2009). In recent years, The IGRA test has been used to evaluate the cellular immune response (IFN-γ) against ESAT-6 and CFP-10 antigens belonging to MTBC. The sensitivity and specificity of this test are reported to be 98.9 and 98.1%, respectively (Takasaki et al., 2017 ▶).
In this study, we aimed to investigate the clonal relationship of strains isolated from animals and humans using the 24-locus MIRU-VNTR and spoligotyping methods and to determine the presence and rates of latent TB in at-risk occupational groups using the QuantiFERON-TB Gold Plus (QFT-Plus) test.
Materials and Methods
Statement of ethics
Animal experiments and animal management procedures were designed in accordance with the requirements of the animal health and ethics committee of Çukurova University Faculty of Veterinary Medicine and applied after receiving the committee approval. Human sputum samples were included in the study with the permission of the Ethics Committee of Çukurova University Faculty of Medicine.
Sample recruitment
In order to determine the epidemiological characteristics of M. bovis in our region, the tissue and organ samples of 76 slaughtered cattle with positive tuberculin tests were collected from a slaughterhouse between October 2018 and December 2019. In addition, the study included four M. bovis strains isolated from the clinical materials of patients with suspected pulmonary TB, who were presented to Cukurova University Tropical Disease Research and Application Center (TDRAC) and the Turkish Ministry of Health Adana Regional Tuberculosis Laboratory, as well as blood samples collected from 21 slaughterhouse personnel including seven veterinarians, 12 butchers, one caretaker and one veterinary technician. Individuals who agreed to participate were asked to sign a consent form and complete a questionnaire including questions on sociodemographic factors.
Histopathological and microbiological examina-tions
Lung and mediastinal lymph node tissues were fixed in 10% formaldehyde. After the routine tissue follow-up procedure, the tissues were embedded in paraffin. Four micron-thick sections were cut from each paraffin block. The slides were stained with hematoxylin and eosin (HE) and Ehrlich-Ziehl-Neelsen (EZN). The stained sections were examined under a light microscope. EZN staining was performed to examin existing acid-fast stained bacilli in various clinical samples collected from the lung and lymph nodes of cattle with a positive tuberculin test in slaughterhouses and the sputum samples of patients with suspected pulmonary TB.
Tissue samples taken from the lungs and lymph nodes of cattle with a positive tuberculin test and the sputum samples of the patients with suspected pulmonary TB were subjected to the decontamination-homogenization procedure. 3 g of samples taken from the lungs and lymph nodes were dissected in 10 ml 0.85% physiological saline. 1.0 ml of 4% NaOH was added to 1.0 ml of the homogenizer, and the sample was incubated at 37°C for 20 min. It was then centrifuged at 1000 × g for 20 min before PBS was added.
The samples were inoculated in Lowenstein-Jensen and MGIT 960 broth (Becton Dickinson Diagnostic System, Sparks, MD). The isolates grown in the medium from clinical specimens were confirmed to be MTBC using the immunochromatographic MPT64 card test (Bioline, Standard Diagnostics, Seoul, South Korea) following microscopic examinations with EZN staining.
Genomic DNA isolation
Mycobacterial DNA was extracted from the cultures grown on Lowenstein-Jensen and BACTEC-MGIT 960 using a Mickle cell disruptor (The Mickle Lab. Engineering Co. Ltd., Gamshall, Surrey, UK) and immediately stored at -20°C to be used in various molecular methods.
Hsp65-PCR
TB11 and TB12 primers were used in the PCR method targeting the hsp65 gene region in which strains belonging to the genus Mycobacterium were detected among the isolates grown on the media as previously described (Brunello et al., 2001 ▶).
Spoligotyping
Spoligotyping was used to identify the genotype of the MTBC strains at the DR locus as previously described (Kamerbeek et al., 1997). All chromosomal DNA was amplified with primary DRa (5´-CCG AGA GGG GAC GGA AAC-3´) and DRb (5´-GGT TTT GGT CTG ACG AC-3´). The amplicons were hybridized after PCR. After hybridization, the products were made visible by adding streptavidin alkaline phosphatase enzyme and phosphate chemifluorescent substrate (Kremer et al., 1999 ▶; Sola et al., 2003 ▶). The presence of spacer regions was evaluated considering the blotting resulting from PCR product hybridization. The results were converted into “Octal code” format consisting of 15 digits between 0-7 using the octal coding key. The data was evaluated using various databases (Couvin et al., 2019 ▶).
MIRU-VNTR
The 24-locus MIRU-VNTR method was performed to identify and determine clonal relationships between the Mycobacterium bovis isolates. Primers were used for the targeted MIRU loci as described in a previous study (Table 1) (Supply et al., 2006 ▶). PCR reactions were performed to determine the VNTR number of the 24 MIRU loci specific to each strain. The number of allele repeats at each MIRU locus was determined based on the band size (Weniger et al., 2010 ▶).
Table 1.
HGDI values of 24-locus MIRU-VNTR primers
| Locus | Copy numbers (%) | Allelic diversity |
|---|---|---|
| ETRA | 4(7), 5(7), 6(8) | 0.69 |
| Qub26 | 3(4), 5(5), 4(13) | 0.59 |
| Mtub21 | 1(9), 3(13) | 0.50 |
| Qub11b | 2(9), 4(13) | 0.50 |
| MIRU27 | 2(9), 3(13) | 0.50 |
| ETRC | 3(7), 5(15) | 0.45 |
| ETRB | 6(5), 5(17) | 0.36 |
| MIRU31 | 2(2), 4(2), 3(18) | 0.32 |
| MIRU23 | 5(3), 4(19) | 0.24 |
| Mtub04 | 1(2), 2(20) | 0.17 |
| Mtub34 | 2(2), 3(20) | 0.17 |
| Mtub39 | 3(2), 2(20) | 0.17 |
| MIRU40 | 3(2), 2(20) | 0.17 |
| MIRU26 | 6(2), 5(20) | 0.17 |
PncA-PCR
The pncA-PCR method was applied to the strains analyzed by spoligotyping and MIRU-VNTR methods, as previously described (Spositto et al., 2014 ▶). Various primers were used to support the identification of M. bovis. The pncAMTB-1,2F and pncAMT primers for M. tuberculosis and the pncAMTB-1,2F and pncAMB primers for M. bovis were used.
QuantiFERON-TB Gold Plus Test
Blood samples were collected from 21 slaughterhouse workers including seven veterinarians, 12 butchers, one caretaker and one veterinary technician, and placed in lithium-heparin tubes. The samples were then transferred to four distinct tubes: Nil tube as a negative control, TB-antigen tube 1 (TB1), TB-antigen tube 2 (TB2), and mitogen tube as a positive control. The QFT-TB Gold Plus tubes were incubated at 37°C for 16-24 h. After incubation, the plasma samples were separated by centrifugation and the amount of IFN-gamma (IU/ml) was measured using the ELISA method according to the instructions of the commercial kit (QFT-Plus; Qiagen, Germantown, MD).
Results
This study included 71 lymph node and 5 lung samples of 76 cattle with positive tuberculin test in farm tuberculosis screening. Also, 7830 suspicious human sputum, pleural and gastric fluid samples were sent to the TDRAC Regional Tuberculosis Laboratory for Diagnosis. The bovine and human isolates were identified using conventional and molecular methods, and M. bovis was further analyzed with the 24-locus MIRU-VNTR, spoligotyping, and PncA-PCR methods. Twenty-five M. bovis were detected by conventional and molecular methods in cattle. In humans, 524 were identified as members of the MTB complex, while four isolates were identified as M. bovis.
MTBC detection in cattle
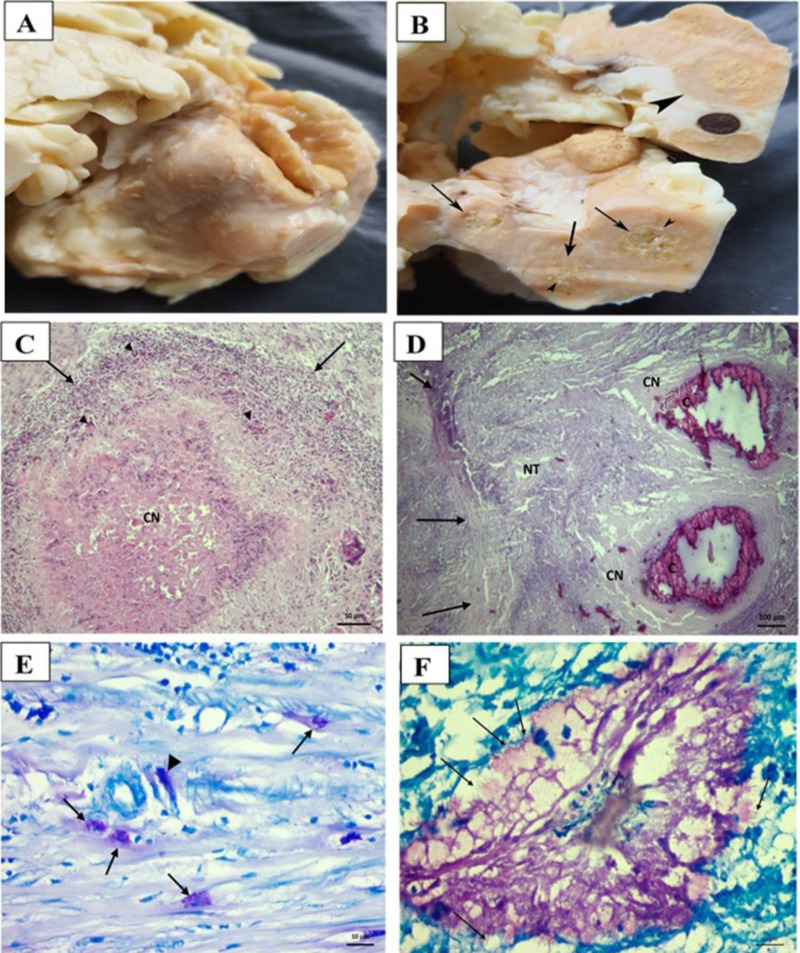
In the microbiological and histopathological examinations, the presence of acid resistant bacteria was determined in 20 of 76 cattle tissue samples by EZN and HE staining. Macroscopically, granulomas were observed in lung and mediastinal lymph node tissues. When cutting the lesioned area, it felt as if the blade was being pulled through sand. The central part of the cross-sectional surface of the lesions in the lung and lymph nodes was hard and friable. Gray-white mineralization foci were detected in the center of caseification necrosis in some granulomas. Histopathological examination revealed typical lesions in both lungs and mediastinal lymph nodes. The typical lesions were granulomas composed of epithelioid histiocytes, Langhan’s multinucleated giant cells, and mononuclear inflammatory cells, surrounded by fibrous tissue. The central part of the granulomas was observed to have caseification necrosis and some exhibited mineralization. In addition, conglomerated tubercles were found in the lymph nodes. Atelectasis areas were seen in the lungs where the granuloma was compressing on the alveoli. In EZN staining, Mycobacterium bacilli were observed in the alveoli and the cytoplasm of histiocyte and macrophages in inflammatory areas. Free and scattered bacilli were also detected in the connective tissue (Fig. 1). In the Lowenstein-Jensen and MGIT960 broth, growth was observed in 25 of the bovine samples, which was confirmed by EZN staining.
Fig. 1.
Histopathological and microbiological examination of the cattle lymph nodes. (A) Mediastinal lymph node enlarged with granulomas in cattle, (B) Sectional face of the mediastina lymph node, granuloma (large arrowhead), caseification necrosis (arrows), and gray-white mineralization foci (small arrowheads) in cattle, (C) A granuloma (tubercle) structure, caseification necrosis in the middle (CN), the area around the necrosis consisting of mononuclear inflammatory cells (arrows), and Langhan’s giant cells (arrowheads), (H&E, scale bar, 50 µm), (D) Conglomerated tubercle, capsule (arrows) formed from outer to inner connective tissue, newly formed tubercle (NT), caseification necrosis (CN), and mineralization areas (C), (H&E, scale bar, 100 µm), (E) Acid-resistant bacilli in the cytoplasm of macrophages (arrows), and free-form bacilli in connective tissue (arrowhead), (ZN, scale bar, 10 µm), and (F) Free acid-fast bacilli in the lumen of the alveoli (arrows), (ZN, scale bar, 10 µm)
With the MTBC genomic DNA isolated from MGIT broth, using the Mickle extraction method and amplified with the primers of the hsp65 gene region, the strains from the 25 bovine samples were identified as Mycobacterium spp.
MTBC detection in humans
In human sputum samples, EZN staining, inoculation to Lowenstein-Jensen and MGIT960 broth, and post-growth EZN staining processes were applied. DNA extraction from the MGIT960 broth of isolates was performed using the Mickle extraction method and the 524 strains were determined to belong to the MTB complex with the hsp65-PCR.
M. bovis detection in cattle and human by spoligotyping, MIRU-VNTR and PncA-PCR
Spoligotyping and 24-loci MIRU-VNTR methods were used to investigate the molecular epidemiological relationship between cattle and human isolates. Eighteen of the bovine isolates and all human samples were evaluated by molecular epidemiological methods. Seven of the bovine isolates were not used because they could not be amplified in the pre-stages of the MIRU-VNTR and spoligotyping methods.
Spoligotyping
The clonal group distribution of the 18 bovine and all human isolates included was examined with the spoligotyping method. The spoligopattern of all 18 bovine isolates and the four 524 MTBC isolates included in the study belonged to M. bovis. In addition, 22 M. bovis isolates were clustered in a total of four spoligotypes.
Spoligotypes determined in cattle were SB0120/ SIT482 (40.9%) for nine isolates, SB0288/SIT685 (31.81%) for seven isolates, and SB0140/SIT683 (9.09%) for two isolates. When the spoligotypes of the human isolates were examined, we found that two isolates were SB0989/SIT1118 (9.09%) and the other two isolates were SB0140/SIT683 (9.09%) (Table 2).
Table 2.
Spoligotype distribution of the bovine and human samples
| Spoligotypes | Bovine isolates | Human isolates | Incidence (%) |
|---|---|---|---|
| SB0120/SIT482 | 9 (40.9%) | - | 40.9% |
| SB0288/SIT685 | 7 (31.81%) | 2 (9.09%) | 40.9% |
| SB0989/SIT1118 | - | 2 (9.09%) | 9.09% |
| SB0140/SIT683 | 2 (9.09%) | - | 9.09% |
When we compared the spoligotyping and 24-locus MIRU-VNTR methods in terms of clonal relationship, it was determined that the latter had more discriminatory power than the former, with the number of clusters being determined as 8 and 4, respectively (Table 3). Although the same spoligotypes were determined in the bovine and human isolates, there was no 100% similarity among the total 22 isolates’ patterns according to the 24-locus MIRU-VNTR method.
Table 3.
Spoligotype and 24-locus MIRU-VNTR type distributions of the human and bovine samples
| Mıru 02 | Mtub 04 | EtrC | Mıru04 | Mıru40 | Mıru10 | Mıru16 | Mtub21 | Mıru20 | Qub11b | EtrA | Mtub29 | Mtub30 | EtrB | Mıru23 | Mıru24 | Mıru26 | Mıru27 | Mtub34 | Mıru31 | Mtub39 | Qub26 | Qub4156 | Mıru39 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CATTLE | SB0120/SIT482 | 4 isolates | 2 | 2 | 5 | 3 | 2 | 2 | 3 | 1 | 2 | 4 | 4 | 3 | 4 | 5 | 4 | 2 | 5 | 2 | 3 | 3 | 2 | 4 | 1 | 2 |
| 2 isolates | 2 | 2 | 5 | 3 | 2 | 2 | 3 | 1 | 2 | 4 | 4 | 3 | 4 | 5 | 5 | 2 | 5 | 2 | 3 | 3 | 2 | 4 | 1 | 2 | ||
| 1 isolate | 2 | 2 | 5 | 3 | 2 | 2 | 3 | 1 | 2 | 2 | 4 | 3 | 4 | 5 | 5 | 2 | 5 | 2 | 3 | 3 | 2 | 4 | 1 | 2 | ||
| 2 isolates | 2 | 2 | 5 | 3 | 2 | 2 | 3 | 1 | 2 | 4 | 5 | 3 | 4 | 5 | 4 | 2 | 6 | 2 | 3 | 4 | 2 | 4 | 1 | 2 | ||
| SB0288/SIT685 | 2 isolates | 2 | 1 | 3 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 6 | 3 | 4 | 6 | 4 | 2 | 5 | 3 | 3 | 2 | 2 | 5 | 1 | 2 | |
| 3 isolates | 2 | 2 | 3 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 5 | 3 | 4 | 6 | 4 | 2 | 5 | 3 | 3 | 3 | 2 | 5 | 1 | 2 | ||
| 2 isolates | 2 | 2 | 3 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 6 | 3 | 4 | 6 | 4 | 2 | 5 | 3 | 3 | 2 | 2 | 5 | 1 | 2 | ||
| SB0140/SIT683 | 1 isolate | 2 | 2 | 5 | 3 | 2 | 2 | 3 | 3 | 2 | 4 | 6 | 3 | 4 | 5 | 4 | 2 | 5 | 3 | 3 | 3 | 3 | 3 | 1 | 2 | |
| 1 isolate | 2 | 2 | 5 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 6 | 3 | 4 | 5 | 4 | 2 | 5 | 3 | 3 | 3 | 3 | 3 | 1 | 2 | ||
| HUMAN ISOLATES |
SB0288/ SIT685 |
2 isolates | 2 | 2 | 3 | 3 | 2 | 2 | 3 | 3 | 2 | 2 | 5 | 3 | 4 | 5 | 4 | 2 | 5 | 3 | 3 | 3 | 2 | 4 | 1 | 2 |
| SB0989/ SIT1118 |
2 isolates | 2 | 2 | 5 | 3 | 3 | 2 | 3 | 3 | 2 | 4 | 6 | 3 | 4 | 5 | 4 | 2 | 5 | 3 | 2 | 3 | 2 | 3 | 1 | 2 |
24-loci MIRU-VNTR
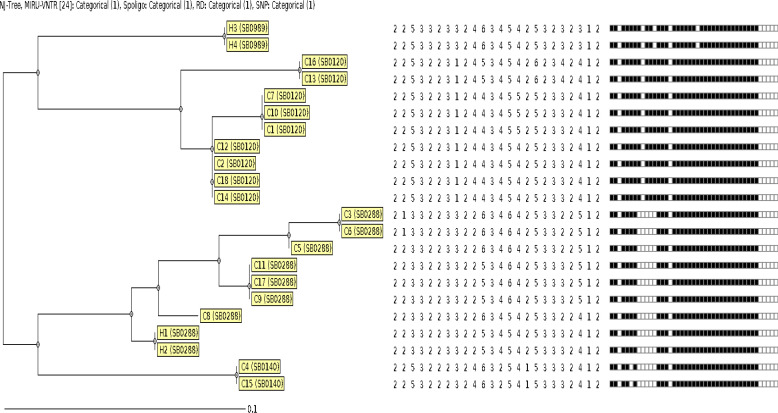
When 18 bovine and all human isolates were evaluated by the MIRU-VNTR method, it was determined that all 18 bovine isolates and four 524 MTBC isolates were M. bovis. According to the 24-locus MIRU-VNTR method, there were eight clusters harboring 20 isolates, and two unique profiles were detected (Fig. 2).
Fig. 2.
N-J tree of M. bovis isolates according to the 24-locus MIRU-VNTR method
When the number of the repetitions of 24 locus was examined with the MIRU-VNTR method, there was no difference in MIRU02, MIRU04, MIRU10, MIRU16, MIRU20, Mtub29, Mtub30, MIRU24, Qtub4156, and MIRU39 locus repeats, but Mtub04, Mtub34, Mtub39, ETRA, ETRB, ETRC, Qub11b, Qub26, MIRU40, MIRU21, MIRU23, MIRU26, MIRU27, and MIRU31 locus repeats showed variability (Table 3). When we calculated the discriminatory powers of the primers used in MIRU-VNTR, we found that the highest values (P≥0.50) belonged to ETRA (0.69), Qub26 (0.59), Qub11b (0.50), Mtub21 (0.50), and Miru27 (0.50) (Table 1).
PncA-PCR
Using the pncA-PCR method used to support M. bovis identification, 185 bp bands were obtained from 25 bovine and 4 human isolates based on amplification with M. bovis-specific primers pncAMTB-1,2F and pncAMB2R.
QFT-TB Gold-Plus
A total of 21 slaughterhouse workers, including 12 butchers, seven veterinarians, one caretaker, and one veterinary technician, were enrolled for the analysis of the QFT-TB Gold-Plus status. The distribution of the samples according to gender was 19 (90.5%) males and two (9.5%) females. The age of the study population ranged from 27 to 51 years (mean age 38.3 ± 8.07 years). According to the results of QFT-TB Gold-Plus, of the 21 individuals, five (23.8%) tested positive, of whom four butchers and one was a veterinary technician (Table 4).
Table 4.
Risk factors in the IGRA test group
| Risk factor | QFT-positive (n=5) |
QFT-negative (n=16) |
|---|---|---|
| N (%) | N (%) | |
| Age | 42.2 ± 9.68 | 37.1 ± 7.44 |
| Gender | ||
| Male | 5 | 14 |
| Female | - | 2 |
| Occupation | ||
| Butcher | 4 | 8 |
| Veterinarian | - | 7 |
| Veterinary technician | 1 | - |
| Caretaker | - | 1 |
Discussion
In this study, we used the spoligotyping and MIRU-VNTR methods for the molecular epidemiological determination of M. bovis isolated from humans and cattle, and we determined four clusters harboring 22 isolates with the spoligotyping method. The major spoligotype was SB0120/SIT482 with 9/22 (40.9%) isolates, which was frequently detected in countries such as Iran, Spain, Portugal, Italy, France, Germany, Algeria, Zambia, and Morocco in previous studies (Kubica et al., 2003 ▶; Duarte et al., 2008 ▶; Munyeme et al., 2009 ▶; Sahraoui et al., 2009 ▶; Rodriguez et al., 2010 ▶; Yahyaoui-Azami et al., 2017 ▶; Ghavidel et al., 2018 ▶). It has been suggested that the reason for the high rate of detection of strains belonging to the SB0120/SIT482 type in Middle Eastern, European and African countries may be the trade of live animals or processed meat products between these countries or homoplasy. In addition, in another spoligotyping study conducted in our region, it was reported that the SB0120/SIT482 pattern was encountered most frequently at a rate of 42.85% (Tuzcu et al., 2020 ▶). In our opinion, this spoligotype represents the major M. bovis genotypes all over the world.
In our study, SB0288/SIT685 (31.81%) was the second frequent spoligotype among the 18 (81.8%) bovine isolates, which is similar to the situation reported in Iran and West Azerbaijan (Tadayon et al., 2009 ▶; Tadayon et al., 2013 ▶). This similarity may be related to the widespread cattle breeding and trade carried out in the study area bordering these countries. In Turkey, it was previously reported that six M. bovis strains, four of which were isolated from cattle and two from goats, sent to the laboratory for suspected TB from six slaughterhouses in Aydın, Manisa, Muğla, and Izmir between 2010 and 2015, genotypically belonged to the spoligotype SB0288/SIT685 (Avsever et al., 2017 ▶). The authors noted that this spoligotype also showed homology with the spoligotype patterns of M. bovis strains isolated from humans in the Aegean Region. In our study, two (50%) of the four human isolates belonged to this family, namely the SB0288/SIT685 spoligotype. In a previous study evaluating 482 MTBC strains isolated from clinical samples sent for a routine mycobacteriological examination, the authors determined that 13 isolates were M. bovis, of which nine (63.6%) belonged to the ST685 (SB0288) family (Çavuşoğlu et al., 2017 ▶).
In the bovine samples, the lowest number of spoligotypes belonged to SB0140/SIT683 detected in two isolates (9.09%). This clade has been found in studies conducted in many countries such as Iran, Portugal, South Africa, Mozambique, Northern Ireland, Mexico, and Brazil (Cobos-Marín et al., 2005 ▶; Duarte et al., 2010 ▶; Skuce et al., 2010 ▶; Parreiras et al., 2012 ▶; Çavuşoğlu et al., 2017 ▶; Machado et al., 2018 ▶; Sichewo et al., 2020 ▶). In another study undertaken in Turkey, the rate of the SB0140/SIT683 spoligotype was reported to be 26.19% (Tuzcu et al., 2020 ▶). Intense activity in the international livestock trade; i.e., animal imports from many countries, may be the reason for the differences between studies in relation to the percentages of M. bovis genotypes. Our minor genotype being the second largest genotype in the previous study, can be attributed to a periodic change.
We determined that the remaining two (9.09%) M. bovis strains isolated from the human clinical samples belonged to the SB0989/SIT1118 spoligotype. In a study which evaluated only samples isolated from clinical human materials, Çavuşoğlu et al. (2017) ▶ reported that 7.7% of the M. bovis isolates belonged to the SB0989/SIT1118 spoligotype. The number of human-derived M. bovis isolates is insufficient for statistical evaluation and proportional calculations. However, the collection of isolates of human origin from two spoligotypes and the isolation of these spoligotypes from human samples in Turkey will guide further studies to be conducted with more isolates obtained from the region.
Considering the diversity of our spoligotyping profile, we found that SB0288/SIT685 was the only common type observed in both the bovine and human groups. However, SB0120/SIT482 and SB0140/SIT683 were detected only in the bovine samples, and SB0989/SIT1118 in two of the four human samples.
In our study, we used the 24-locus MIRU-VNTR method instead of a 12-14-15 locus due to its higher discriminatory power in detecting clonal relationship. We found that the ETRA, Qub26, Qub11b, Mtub21, and Miru27 primers were highly discriminative in identifying the MIRU-VNTR locus. Similarly, many previous studies that used the 24-locus MIRU-VNTR method revealed that the highest discriminatory power was obtained from the ETRA, Qub26, and Qub11b locus (Yang et al., 2015 ▶; Carvalho et al., 2016 ▶; Elsayed et al., 2019 ▶).
In the MIRU-VNTR method, our isolates were divided into eight clusters with two unique profiles. When the similarities between the locus patterns of the human and bovine isolates were evaluated, 100% similarity was not determined. However, the H1 and H2 human samples were found to be 91.6% similar to a bovine sample. In a previous study, 32 bovine and 10 human M. bovis isolates were evaluated with the 12-locus MIRU-VNTR and spoligotyping methods, and the MIRU-VNTR patterns of five human isolates were 100% compatible with the bovine isolates (Skuce et al., 2010 ▶). Accordingly, the authors emphasized that bovine isolates could cause infections in humans. Although our data did not provide statistically significant results due to the low number of human strains, it was hypothesized that cattle-originated M. bovis could play a role in active pulmonary TB in humans. Due to this possibility, we also examined slaughterhouse workers as a sampling group that had the most contact with pulmonary TB-suspected animals with a positive tuberculin skin test. However, there were no clinical symptoms in this group. Therefore, we decided to screen for latent TB infection in the blood samples collected from the 21 individuals. MTBC infection was screened using the QFT-TB Gold-Plus assay, and 23.8% positivity (5/21) was detected. In a similar study involving 311 farm and slaughterhouse workers and households, blood samples were taken to screen for latent TB, and tuberculin skin test (TST) and IGRA positivity were found at the rates of 76.2% and 58.5%, respectively (Torres-Gonzalez et al., 2013 ▶).
The presence of M. bovis in risk groups was proven by culture and molecular methods, although it could not be determined at the level of Mycobacterium species using TST and IGRA. In a previous study, M. bovis was isolated from the bronchoalveolar lavage of a person who had worked as a butcher for 35 years in Australia (Ingram et al., 2010 ▶). In Pakistan, M. bovis was detected from sputum samples in slaughterhouse workers, butchers and veterinarians using the PCR analysis (Khattak et al., 2016 ▶). In another case reported from Turkey, M. bovis was isolated from a butcher with a persistent skin lesion and pulmonary infection in Turkey (Mertoğlu et al., 2018 ▶). Additionally, M. bovis SB0271 spoligotype was isolated from the sputum sample of a female veterinary nurse in England, while latent TB was also detected in her daughter with the QuantiFERON-TB Gold test. In addition, the same spoligotype was present in the tracheal mucus sample collected from the nurse’s dog. Interestingly, when the spoligotype archives on cattle were screened, the same spoligotype (SB0271) was found in an animal tested for tuberculin by an animal nurse years ago. This animal was considered as a possible source of infection (Shrikrishna et al., 2009 ▶).
In conclusion, although zoonotic TB is not a new disease, it has been neglected for many years. With molecular epidemiological studies, the importance of M. bovis and zoonotic TB has been better understood, and the eradication of this disease globally by 2030 has become one of the United Nations Sustainable Development Goals. Determining the causative agent and breaking the zoonotic chain are essential to the eradication of the disease. Spoligotyping and MIRU-VNTR should always be used as key methods to identify the source of isolates and monitor transmissions in humans and animals. Risk groups should be monitored continuously and the risk of infection of the most exposed group should be eliminated (WHO, 2017 ▶).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was financially supported by Cukurova University Scientific Research Projects (TSA-2018-10909), Adana, Turkey. We would like to thank the staff of “Meat and Dairy Institution Adana Meat Combination Directorate” and “Adana Metropolitan Municipality Slaughterhouse” for whom we collected clinical samples from cattle and blood samples from veterinarians, butchers and technicians in our study.
References
- Avsever M, Çavuşoğlu C, Yazıcıoğlu Ö, Eskiizmirliler S, Erdal G, Günen M, Tunalıgil S, Alparslan B, Aksoy A. New spoligotyping pattern of Mycobacterium bovis isolates from farm animals in Turkey. Ankara Univ. Vet. Fak. Derg. 2017;64:37–43. [Google Scholar]
- Brunello F, Ligozzi M, Cristelli E, Bonora S, Tortoli E, Fontana R. Identification of 54 mycobacterial species by PCR-Restriction Fragment Length Poly-morphism analysis of the hsp65 Gene. J. Clin. Microbiol. 2001;39:2799–2806. doi: 10.1128/JCM.39.8.2799-2806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro PAM, Pasquatti TN, Takatani H, Zumárraga MJ, Marfil MJ, Barnard C, Fitzgerald SD, Abramovitch RB, Araujo FJ, Kaneene JB. Molecular characterization of Mycobacterium bovis infection in cattle and buffalo in Amazon Region, Brazil. Vet. Med. Sci. 2020;6:133–141. doi: 10.1002/vms3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RCT, Vasconcellos SEG, Issa MA, Filho PMS, Mota PMPC, de Araújo FR, Carvalho ACS, Gomes HM, Suffys PN, Figueiredo EES, Paschoalin VMF. Molecular typing of Mycobacterium bovis from cattle reared in Midwest Brazil. 2016:PloS One. 1–16. doi: 10.1371/journal.pone.0162459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çavuşoğlu C, Yılmaz FF. Molecular epidemio-logy of human Mycobacterium bovis infection in Aegean Region, Turkey. Mikrobiyol. Bul. 2017;51:165–170. doi: 10.5578/mb.53963. [DOI] [PubMed] [Google Scholar]
- Cobos-Marín L, Montes-Vargas J, Zumarraga M, Cataldi A, Romano MI, Estrada-Garcia I, Gonzalez-y-Merchand JA. Spoligotype analysis of Mycobacterium bovis isolates from Northern México. Can. J. Microbiol. 2005;51:996–1000. doi: 10.1139/w05-083. [DOI] [PubMed] [Google Scholar]
- Couvin D, David A, Zozio T, Rastogi N. Macro-geographical specificities of the prevailing tuberculosis epidemic as seen through SITVIT2, an updated version of the Mycobacterium tuberculosis genotyping database. Infect. Genet. Evol. 2019;72:31–43. doi: 10.1016/j.meegid.2018.12.030. [DOI] [PubMed] [Google Scholar]
- Djemal SE, Siala M, Smaoui S, Kammoun S, Marouane C, Bezos J, Messadi-Akrout F, Romero B, Gdoura R. Genetic diversity assessment of Tunisian Mycobacterium bovis population isolated from cattle. BMC Vet. Res. 2017;13:393. doi: 10.1186/s12917-017-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djibuti M, Mirvelashvili E, Makharashvili N, Magee JM. Household income and poor treatment outcome among patients with tuberculosis in Georgia: a cohort study. BMC Pub. Health. 2014;14:88. doi: 10.1186/1471-2458-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte EL, Domingos M, Amado A, Botelho A. Spoligotype diversity of Mycobacterium bovis and Mycobacterium caprae animal isolates. Vet. Microbiol. 2008;130:415–421. doi: 10.1016/j.vetmic.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Duarte EL, Domingos M, Amado A, Cunha MV, Botelho A. MIRU-VNTR typing adds discriminatory value to groups of Mycobacterium bovis and Mycobacterium caprae strains defined by spoligotyping. Vet. Microbiol. 2010;143:299–306. doi: 10.1016/j.vetmic.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Elsayed MSA. A first insight into the application of high discriminatory MIRU-VNTR typing using QIAxcel technology for genotyping Mycobacterium bovis isolated from the Delta area in Egypt. Inf. Gen. Evol. 2019;71:211–214. doi: 10.1016/j.meegid.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Ergönül Ö, Tülek N, Kayı I, Irmak I, Erdem O, Dara M. Profiling infectious diseases in Turkey after the influx of 3 5 million Syrian refugees. Clin. Mic. Inf. 2020;26:307–312. doi: 10.1016/j.cmi.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavidel M, Mansury D, Nourian K, Ghazvini K. The most common spoligotype of Mycobacterium bovis isolated in the world and the recommended loci for VNTR typing; A systematic review. Microb. Pathog. 2018;118:310–315. doi: 10.1016/j.micpath.2018.03.036. [DOI] [PubMed] [Google Scholar]
- Ingram PR, Bremner P, Inglis TJ, Murray RJ, Cousins DV. Zoonotic tuberculosis: on the decline Communicable Diseases Intelligence. 2010;34:339–344. doi: 10.33321/cdi.2010.34.35. [DOI] [PubMed] [Google Scholar]
- Jiang G, Wang G, Chen S, Yu X, Wang X, Zhao L, Ma Y, Dong L, Huang H. Pulmonary tuberculosis caused by Mycobacterium bovis in China. Sci. Rep. 2015;4:8538. doi: 10.1038/srep08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak I, Mushtaq MH, Ahmad MU, Khan MS, Haider J. Zoonotic tuberculosis in occupationally exposed groups in Pakistan. Occup. Med. 2016;66:371–376. doi: 10.1093/occmed/kqw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer K, van Soolingen D, Frothingham R, Haas WH, Hermans PW, Martín C, Palittapongarnpim P, Plikaytis BB, Riley LW, Yakrus MA, Musser JM, van Embden JD. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: inter-laboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 1999;37:2607–2618. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubica T, Rusch-Gerdes S, Niemann S. Mycobacterium bovis subsp caprae caused one-third of human M bovis-associated tuberculosis cases reported in Germany between 1999 and 2001. J. Clin. Microbiol. 2003;41:3070–3077. doi: 10.1128/JCM.41.7.3070-3077.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Rito T, Ghebremichael S, Muhate N, Maxhuza G, Macuamule C, Moiane I, Macucle B, Marranangumbe AS, Baptista J, Manguele J, Koivula T, Streciher EM, Warren RM, Kallenius G, van Helden P, Correia-Neves M. Genetic diversity and potential routes of transmission of Mycobacterium bovis in Mozambique. PLOS Neg. Trop. Dis. 2018;12:e0006147. doi: 10.1371/journal.pntd.0006147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies, D. Immune-based tests for tuberculosis. In: Zumla, AS, editor. Tuberculosis. 2nd Edn. Elsevier Health Sciences, W. B. Saunders.; 2009. pp. 179–196. [Google Scholar]
- Mertoğlu A, Biçmen C, Karaarslan S, Buğdayci MH. Pulmonary tuberculosis due to Mycobacterium bovis revealed by skin lesion in slaughterhouse worker. Clin. Respir. J. 2018;12:317–321. doi: 10.1111/crj.12485. [DOI] [PubMed] [Google Scholar]
- Munyeme M, Rigouts L, Shamputa IC, Muma JB, Tryland M, Skjerve E, Djønne B. Isolation and characterization of Mycobacterium bovis strains from indigenous Zambian cattle using Spacer oligonucleotide typing technique. BMC Microbiol. 2009;9:144. doi: 10.1186/1471-2180-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber. Lung. Dis. 1995;76:1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- Parreiras PM, Andrade GI, Nascimento TF, Oelemann MC, Gomes HM, Alencar AP, Assis RA, Mota PMPC, Pereira MAS, Lobato FCF, Lage AP, Suffys PN. Spoligotyping and variable number tandem repeat analysis of Mycobacterium bovis isolates from cattle in Brazil. Mem. Inst. Oswaldo Cruz. 2012;107:64–73. doi: 10.1590/s0074-02762012000100009. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Romero B, Bezos J, de Juan L, Alvarez J, Castellanos E, Moya N, Lozano F, Gonzales S, Saez-Lorente JL, Mateos A, Dominquez L, Aranaz A. High spoligotype diversity within a Mycobacterium bovis population: clues to understanding the demography of the pathogen in Europe. Vet. Microbiol. 2010;141:89–95. doi: 10.1016/j.vetmic.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Sahraoui N, Muller B, Guetarni D, Boulahbal F, Yala D, Ouzrout R, Berg S, Smith NH, Zinsstag J. Molecular characterization of Mycobacterium bovis strains isolated from cattle slaughtered at two abattoirs in Algeria. BMC Vet. Res. 2009;5:4. doi: 10.1186/1746-6148-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrikrishna D, de la Rua-Domenech R, Smith NH, Colloff A, Coutts I. Human and canine pulmonary Mycobacterium bovis infection in the same household: re-emergence of an old zoonotic threat? Thorax. 2009;64:89–91. doi: 10.1136/thx.2008.106302. [DOI] [PubMed] [Google Scholar]
- Sichewo PR, Hlokwe TM, Etter EMC, Michel AL. Tracing cross species transmission of Mycobacterium bovis at the wildlife/livestock interface in South Africa. BMC Microbiol. 2020;20:49. doi: 10.1186/s12866-020-01736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuce RA, Mallon TR, McCormick CM, McBride SH, Clarke G, Thompson A, Couzens C, Gordon AW, McDowell SWJ. Mycobacterium bovis genotypes in Northern Ireland: herd level surveillance (2003 to 2008) Vet. Rec. 2010;167:684–689. doi: 10.1136/vr.c5108. [DOI] [PubMed] [Google Scholar]
- Sola C, Filliol I, Legrand E, Lesjean S, Locht C, Supply P, Rastogi N. Genotyping of the Mycobacterium tuberculosis complex using MIRUs: association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Inf. Genet. Evol. 2003;3:125–133. doi: 10.1016/s1567-1348(03)00011-x. [DOI] [PubMed] [Google Scholar]
- Spositto FLE, Campanerut PAZ, Ghiraldi LD, Leite CQF, Hirata MH, Hirata RDC, Siqueira VLD, Fressatti CR. Multiplex-PCR for differentiation of Mycobacterium bovis from Mycobacterium tuberculosis complex. Braz. J. Mic. 2014;45:841–843. doi: 10.1590/s1517-83822014000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, Savine E, de Haas P, van Deutekom H, Roring S, Bifani P, Kurepina N, Kreiswirth B, Sola C, Rastogi N, Vatin V, Gutierrez MC, Fauville M, Niemann S, Skuce R, Kremer K, Locht C, van Soolingen D. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 2006;44:4498–4510. doi: 10.1128/JCM.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayon K, Mosavari N, Feizabadi MM. An epidemiological perspective on bovine tuberculosis spotlighting facts and dilemmas in Iran; a historically zebu-dominant farming country. Iran J. Mic. 2013;5:1–13. [PMC free article] [PubMed] [Google Scholar]
- Tadayon K, Mosavari N, Sadeghi F, Forbes KJ. Mycobacterium bovis infection in Holstein Friesiancattle; Iran. Emerg. Infect. Dis. 2009;14:1919–1921. doi: 10.3201/eid1412.070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki J, Manabe T, Morino E, Muto Y, Hashimoto Y, Likura M, Izumi S, Sugiyama H, Kudo K. Sensitivity and specificity of QuantiFERON-TB Gold Plus compared with QuantiFERON-TB Gold In-Tube and T-SPOT B on active tuberculosis in Japan. J. Inf. Chem. 2017;24:188–192. doi: 10.1016/j.jiac.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Thoen CO, Kaplan B, Thoen TC, Gilsdorf MJ, Shere JA. Zoonotic tuberculosis A comprehensive one health approach. Medicina (B Aires) 2016;76:159–165. [PubMed] [Google Scholar]
- Torres-Gonzalez P, Soberanis-Ramos O, Martinez-Gamboa A, Chavez-Mazari B, Barrios-Herrera MT, Torres-Rojas M, Cruz-Hervert LP, Garcia-Garcia L, Singh M, Gonzalez-Aguirre A, de Leon-Garduño AP, Sifuentes-Osornio J, Bobadilla-Del-Valle M. Prevalence of latent and active tuberculosis among dairy farm workers exposed to cattle infected by Mycobacterium bovis. PLOS Neg. Trop. Dis. 2013;7:e2177. doi: 10.1371/journal.pntd.0002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzcu N, Köksal F. Genetic evaluation of Mycobacterium bovis isolates with MIRU-VNTR and spoligotyping. Turk. J. Med. Sci. 2020;50:2017–2023. doi: 10.3906/sag-1910-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayr F, Martin-Blondel G, Savall F, Soulat JM, Deffontaines G, Herin F. Occupational exposure to human Mycobacterium bovis infection: A systematic review. PLoS Negl. Trop. Dis. 2018;12:e0006208. doi: 10.1371/journal.pntd.0006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger T, Krawczyk J, Supply P, Niemann S, Harmsen D. MIRU-VNTRplus: a web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Res. 2010;38:326–331. doi: 10.1093/nar/gkq351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. End TB by 2030. Framework for implementing the “End TB Strategy” In the African region 2016-2020. 2017. Cited Dec.20.2020. https://apps.who.int/iris/bitstream/handle/10665/259636/TBstrateng.pdf?sequence=1.
- Yahyaoui-Azami H, Aboukhassib H, Bouslikhane M, Berrada J, Rami S, Reinhard M, Gagneux S, Feldmann J, Borrell S, Zinsstag J. Molecular characterization of bovine tuberculosis strains in two slaughterhouses in Morocco. BMC Vet. Res. 2017;13 doi: 10.1186/s12917-017-1165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang C, Wang H, Meng Q, Wang Q. Evaluation of MIRU-VNTR for typing of Mycobacterium bovis isolated from Sika deer in Northeast China. BMC Vet. Res. 2015;11:93. doi: 10.1186/s12917-015-0402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]